Abstract
The opening of cardiac plasma-membrane ATP-sensitive K+ channels (pmKATP) can protect the heart against ischaemia/reperfusion injury. We recently demonstrated that the resting membrane potential (Em) of ventricular myocytes strongly modulates reoxygenation-induced Ca2+ overload. This led to the hypothesis that activation of pmKATP can influence the extent of chemically induced hypoxia (CIH)/reoxygenation Ca2+ overload via hyperpolarization of the diastolic membrane potential of ventricular myocytes.
The membrane potential (Em) of isolated rat myocytes was determined using the perforated patch-clamp technique and DiBac4(3) imaging. Intracellular Ca2+ ([Ca2+]i) was monitored using FURA-2 imaging.
CIH/reoxygenation caused a significant depolarization of Em and a substantial increase in [Ca2+]i. The KATP opener pinacidil (100 μM) and the pmKATP opener P-1075 (100 μM) hyperpolarized the Em of normoxic myocytes. Pinacidil (100 μM) and P-1075 (10 and 100 μM), applied during reoxygenation, hyperpolarized Em and prevented reoxygenation-induced increases in [Ca2+]i.
Myocyte hypercontracture and death increased in parallel with an Em depolarization of 10–15 mV and increases in [Ca2+]i. Under these conditions, the selective pmKATP channel inhibitor HMR 1098 further depolarized myocyte membrane potential and increased hypercontracture.
In conclusion, activation of pmKATP channels can prevent CIH/reoxygenation-induced Ca2+ overload via a mechanism that is dependent on hyperpolarization of diastolic membrane potential. Hyperpolarization toward normal resting membrane potential favours the Ca2+ extrusion mode of Na+/Ca2+ exchange.
Keywords: ATP-sensitive potassium channels, calcium overload, membrane potential, sodium/calcium exchange, heart
Introduction
In the time period following the discovery of ATP-sensitive potassium (pmKATP) channels in cardiac tissue (Noma, 1983), many studies have demonstrated that pharmacological activation of KATP channels under hypoxic or ischaemic conditions can result in protection of the myocardium (Cole et al., 1991; McPherson et al., 1993; Hearse, 1995; Light et al., 2001). In the mammalian myocardium, two distinct classes of KATP channels are expressed in (i) the plasma membrane (pm) and the (ii) mitochondrium (mito). There is an ongoing debate regarding the mechanism(s) of action of potassium channel openers that results in cardioprotection. There is also uncertainty and controversy surrounding the putative roles of pm and mitoKATP channels in affording this protection (for recent reviews, see Gross & Fryer, 1999; Gross & Peart, 2003). Activation of mitoKATP channels is thought to improve mitochondrial function via regulation of mitochondrial volume, calcium-handling and/or mitochondrial membrane potential (see the review by O'Rourke, 2000). However, recent work by Das et al. (2003) argues against a significant functional role of a sulphonylurea-inhibitable mitoKATP channel. Several compelling lines of evidence indicate that activation of pmKATP channels is also cardioprotective. For example, pmKATP channel-deficient (KO) mouse hearts failed to exhibit preconditioning. In wild-type hearts, this ‘compromised' state could be mimicked by the selective pmKATP channel inhibitor HMR1098 (Suzuki et al., 2002). Also, a recent work by Suzuki et al. (2003) suggests that the cardioprotective effects of diazoxide, long considered to be a selective mitoKATP opener, is in fact mediated mainly by the activation of pmKATP channels. Our recent findings demonstrate that activation of pmKATP channels can significantly reduce hypoxia-induced calcium overload in rat ventricular myocytes (Light et al., 2001). Despite these findings, the cellular ionic mechanisms by which pharmacological activation of pmKATP in the heart elicits a cardioprotective effect remain to be established.
Significant and maintained increases in intracellular calcium (Ca2+ overload) are generally thought to be a key determinant of the severity of myocardial damage during ischaemia/reperfusion (IR) (Marbán et al., 1989; Park & Lucchesi, 1999; Piper & Garcia-Dorado, 1999). Dysfunctional intracellular Ca2+ homeostasis during reperfusion is thought to represent a major determinant of the early phase of reperfusion injury, including stunning and cell hypercontracture/death (Marbán et al., 1989; Tani, 1990; Bolli & Marbán, 1999). However, many of the underlying mechanisms responsible for the observed calcium overload have not been demonstrated. Recent findings strongly suggest that alterations in the activity of the plasma-membrane Na+/Ca2+ exchanger contributes to abnormal Ca2+ regulation during ischaemia/reperfusion (Eigel & Hadley, 2001; Schäfer et al., 2001), and it is known that the severity of myocyte calcium overload is dependent on transmembrane electrochemical gradients for Na+ and Ca2+ (Blaustein & Lederer, 1999). Our previous results have shown that: (i) pmKATP channel activation by protein kinase C reduces reoxygenation-induced calcium overload (Light et al., 2001) and (ii) hyperpolarization of the diastolic/resting membrane potential reduces the magnitude of calcium overload mediated by reverse-mode Na+/Ca2+ exchange (Baczkó et al., 2003a).
Accordingly, the main goal of the present study was to determine the relationship between: (i) pharmacological activation of the repolarizing K+ current pmKATP, (ii) the diastolic membrane potential, (iii) calcium overload and myocyte hypercontracture, in the setting of chemically induced hypoxia (CIH)/reoxygenation. Our findings confirm that activation of pmKATP hyperpolarizes the diastolic membrane potential, and demonstrate that this is essential for reducing hypoxia/reoxygenation-induced calcium overload and irreversible myocyte injury.
Part of this work has been recently presented in abstract form (Baczkó et al., 2003b).
Methods
Resting membrane potential measurements using perforated patch-clamp and DiBac4(3) fluorescence-imaging techniques
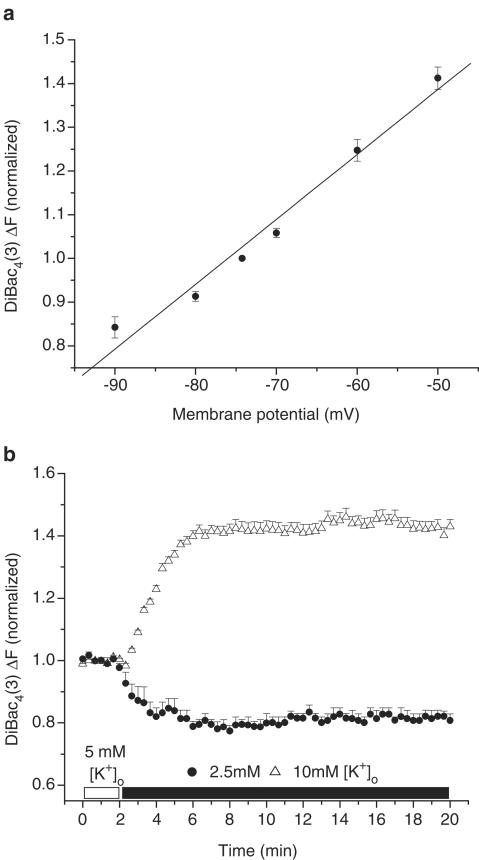
Adult rats were euthanized with pentobarbital (150 mg kg−1, i.p.) according to the University of Alberta Animal Policy and Welfare Committee and the Canadian Council on Animal Care (CCAC) Guidelines. The hearts were removed under complete anaesthesia and right ventricular myocytes were then obtained by enzymatic dissociation using the standard protocols described previously (Bouchard et al., 1993; Light et al., 1998). Changes in the resting membrane potential of freshly isolated myocytes were estimated by monitoring changes in fluorescence intensity of the potentiometric bisoxonol dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol (DiBac4(3)), an anionic probe that, upon changes in membrane potential, partitions into the myocyte sarcolemma and exhibits enhanced fluorescence on depolarization (Epps et al., 1994). Conversely, hyperpolarization leads to efflux of the probe and a decrease in fluorescence intensity. Right ventricular myocytes were incubated with 2 μM DiBac4(3) for 20 min to achieve homogenous probe distribution in the myocyte sarcolemma. After incubation, cells were placed on coverslips for observation at × 200 with an inverted microscope (Olympus, CK40) and were superfused with the control solution containing 2 μM DiBac4(3) and (in mM): NaCl 140, KCl 5, HEPES 10, CaCl2 2.0, MgCl2 1.4, glucose 10. A Photon Technology International (PTI, Lawrenceville, NJ, U.S.A.) imaging system with PTI Imagemaster software (version 1.49) was used for data acquisition and analysis. DiBac4(3) was excited with 470 nm wavelength light and the emitted light intensity at 525 nm was digitized and stored. Normalized values were expressed as the ratio of F(test)/F(control), where F(test)=525 nm wavelength fluorescence intensity at a given time point in test conditions and F(control) is the mean 525 nm fluorescence intensity during the stable 2 min control period, before the application of test solutions. The resting membrane potentials of DiBac4(3)-loaded right ventricular myocytes were measured using the perforated-patch technique similar to our previous study (Baczkó et al., 2003a). The cardiomyocytes were then voltage-clamped at selected potentials (–90 to –50 mV, every 10 mV) and simultaneous DiBac4(3) fluorescence intensity measurements were carried out on the same cells at 35°C. The DiBac4(3) fluorescence intensity at the resting membrane potential was defined as 1.0 during normalization (Figure 1a).
Figure 1.
Effects of diastolic membrane potential (Em) and selected [K+]o on DiBac4(3) fluorescence in right ventricular myocytes isolated from adult rat hearts. (a) Cardiomyocytes were voltage-clamped using the perforated patch-clamp technique. At selected membrane potentials, changes in DiBac4(3) fluorescence intensity (ΔF) were assessed and ΔF was used as an estimate of relative Em changes in subsequent imaging experiments. ΔF was normalized in each cell to F measured at the resting membrane potential, which averaged –74.25±0.86 mV (n=5 cells). The line was fitted by linear regression. (b) Note that 2.5 mM [K+]o hyperpolarized, and 10 mM [K+]o depolarized the Em of quiescent, normoxic myocytes.
To demonstrate that the changes in Em observed using DiBac4(3) imaging were maintained for the duration of our experimental protocols, ventricular myocytes were superfused with 2.5, 10 mM [K+]o for 18 min and their DiBac4(3) fluorescence was monitored (Figure 1b). Membrane potential measurements and DiBac4(3) imaging experiments were performed at 35±1°C.
Measurements of intracellular Ca2+ ([Ca2+]I)
A sustained increase in [Ca2+]i has been reported to correlate closely with cell mortality in the setting of ischaemia/reperfusion (for a review, see Bolli & Marbán, 1999). Accordingly, continuous measurements of [Ca2+]i have been used as an indicator of calcium homeostasis in models of CIH/reoxygenation (Jovanovic et al., 1998). Right ventricular myocytes from adult rats were loaded for 15 min with the esterified form of the Ca2+-sensitive fluorescent probe FURA-2-AM (2 μM, dissolved in a 50% dimethyl sulphoxide and a 50% v v−1 pluronic acid mixture (Molecular Probes, Eugene, OR, U.S.A.)). After loading, cells were washed and placed on coverslips for observation at × 200 with an inverted microscope (Olympus, CK40), while being superfused with the control solution containing (in mM): NaCl 140, KCl 5, HEPES 10, CaCl2 2.0, MgCl2 1.4, or with selected test solutions. A Photon Technology International (PTI, Lawrenceville, NJ, U.S.A.) imaging system with PTI Imagemaster software (version 1.49) was used for data acquisition and analysis. FURA-2 was excited with alternating 340 and 380 nm wavelengths of light, and the emitted light intensity at 520 nm was digitized and stored. An estimate of [Ca2+]i was obtained from the calculated 340/380 nm ratio. Normalized values were expressed as the ratio F(test)/F(control), where F(test)=the image ratio of 340/380 nm at a given time point in test conditions and F(control) is the mean image ratio of 340/380 nm measured during the stable 2 min normoxic period, before the application of the solutions which resulted in hypoxic insult. In each successful protocol, myocytes were subjected to 8 min of CIH (5 mM 2-deoxy-glucose and 4 mM NaCN) and then 8 min of reoxygenation in the normal solution. All experimental solutions contained 100 μM probenecid, an inhibitor of the uric acid transporter system, to slow FURA-2-AM leakage from the cells (Di Virgilio et al., 1990). Results from only those myocytes that did not develop hypercontracture during CIH/reoxygenation are included in the figures, which illustrate changes in [Ca2+]i during CIH or reoxygenation. All Ca2+-imaging experiments were carried out at 35±1°C.
Drugs and chemicals
1-[[5-[2-(5-chloro-o-anisamido)ethyl]-2-methoxyphenyl]sulphonyl]-3-methylthiourea, sodium salt (HMR 1098) (Aventis, Frankfurt, Germany) was dissolved as a 5 mM stock solution in distilled water. Pinacidil (Sigma Chemical Co., St Louis, MO, U.S.A.), P-1075 (Tocris, Ellisville, MO, U.S.A.) and Probenecid (Sigma Chemical Co., St Louis, MO, U.S.A.) were made up as 10, 10 and 100 mM stock solutions in dimethyl sulphoxide, respectively. Pluronic F-127 (Molecular Probes, Eugene, OR, U.S.A.) was prepared as a 20% w v−1 stock solution in dimethyl sulphoxide. Each stock solution was diluted to the required concentration immediately before use.
Statistical analysis
Data are presented as means±s.e.m. Statistical significance was evaluated using Student's unpaired and paired t-test, as appropriate, with Origin 6.0 software. Myocyte hypercontracture data were analysed using the χ2 method with Yates' correction. A level of P<0.05 was considered to be statistically different.
Results
Effects of Em and selected [K+]o on DiBac4(3) fluorescence
Measurements using the perforated patch-clamp technique from five adult rat ventricular myocytes loaded with 2 μM DiBac4(3) yielded the following resting membrane potential: −74.25±0.86 mV. So that changes in DiBac4(3) fluorescence in our imaging experiments could be correlated with changes in membrane potential, these myocytes were voltage-clamped and changes in DiBac4(3) fluorescence were assessed (Figure 1a).
To show that we were able to qualitatively monitor changes in resting membrane potential by measuring changes in DiBac4(3) fluorescence for the duration of our experiments, myocytes were superfused with Tyrode's solution containing 10 mM [K+]o to elicit depolarization, and 2.5 mM [K+]o to induce hyperpolarization of the resting membrane potential. Our data demonstrate that the changes in resting membrane potential of ventricular myocytes caused by the application of selected [K+]o are well maintained and monitored by DiBac4(3) imaging (Figure 1b).
Effect of pharmacological activation and inhibition of IK-ATP on the Em of adult rat ventricular myocytes
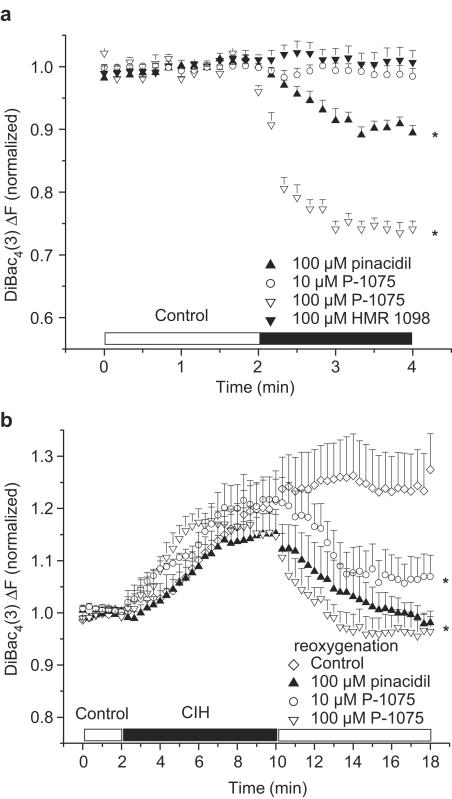
Pharmacological activation of plasma-membrane KATP by 100 μM pinacidil and P-1075 significantly hyperpolarized the resting membrane potential of normoxic rat ventricular myocytes (Figure 2a). However, 10 μM P-1075 induced only a transient and very limited hyperpolarization in normoxic myocytes. The time course of reaching the maximal hyperpolarization (approximately 1 min) was similar in 100 μM pinacidil- and 100 μM P-1075-treated groups (Figure 2a). As expected, HMR 1098 (100 μM) did not change the resting membrane potential of ventricular myocytes in normoxic conditions (Figure 2a).
Figure 2.
Effect of pharmacological activation and inhibition of pmKATP on resting membrane potential in right ventricular myocytes isolated from adult rat hearts. (a) Pinacidil (100 μM) and P-1075 hyperpolarized, while 10 μM P-1075 and 100 μM HMR 1098 did not change the Em of normoxic myocytes. (b) Pinacidil and the pmKATP selective P-1075 hyperpolarized the chemical hypoxia-induced depolarized Em of myocytes towards EK. Pinacidil, P-1075 and HMR 1098 were applied during reoxygenation only. *P<0.05 vs baseline (a) or vs control (b), n=6 experiments, 2–5 cells per experiment.
In the next set of experiments, myocytes were subjected to 8 min of CIH and 8 min reoxygenation with test solutions. All groups of myocytes were superfused with a Tyrode's solution with the same composition until the end of the CIH period. CIH was followed by reoxygenation by one of the test solutions. Test solutions were control solution containing the KATP openers pinacidil and P-1075, the pmKATP inhibitor HMR 1098 or the combination of P-1075 and HMR 1098. The control group was reoxygenated with control solution only. Our rationale for administering these compounds only in reoxygenation was to pharmacologically activate or inhibit KATP only upon reoxygenation, since our previous work suggested a distinct protective role for pmKATP during reoxygenation (Light et al., 2001).
CIH caused a similar, progressive depolarization of myocytes in each group (Figure 2b)
No significant change in resting membrane potential was observed in myocytes superfused with the control solution during reoxygenation; the myocytes remained depolarized (increase in normalized F was 5.1±1.28% by the end of reoxygenation vs the end of CIH, P>0.05; Figure 2b). However, application of the KATP openers pinacidil and the pmKATP selective P-1075 (10 and 100 μM) in the reoxygenation solution significantly hyperpolarized the Em of myocytes close to baseline values (normalized ΔF compared to baseline was 0.98±0.02, 1.07±0.05 and 0.96±0.03 vs 1.25±0.07 in controls at the end of reoxygenation, respectively, P<0.05; Figure 2b).
Effect of depolarization and hyperpolarization of the resting membrane potential on Ca2+ loading during CIH and reoxygenation
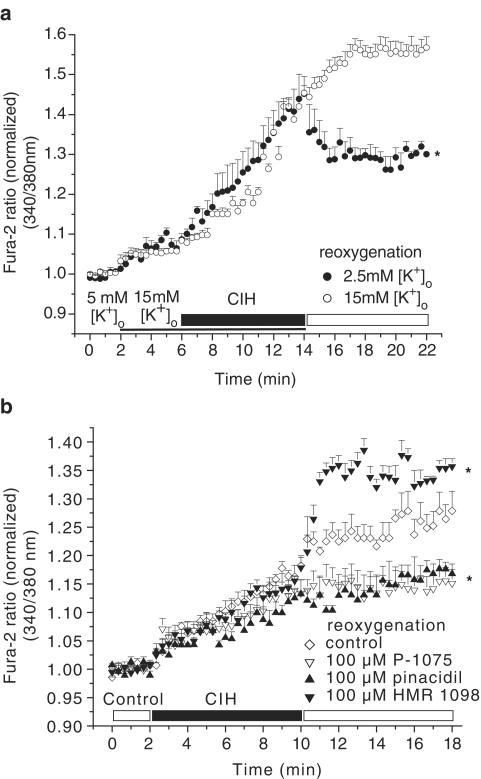
CIH induced a progressive increase in [Ca2+]i (increase in Fura-2 ratio was 16.9±0.36%; Figure 3b, control group). This was followed by a further rise in [Ca2+]i during reoxygenation (increase in Fura-2 ratio was 9.8±0.18%; Figure 3b, control group).
Figure 3.
Effect of depolarization and hyperpolarization of Em on CIH/reoxygenation (CIH/Reox) Ca2+ overload. (a) Depolarization of myocytes (in 15 mM [K+]o) caused a progressive increase in [Ca2+]i during CIH/Reox. Hyperpolarization of the myocyte (following superfusion with 2.5 mM [K+]o) prevented the reoxygenation-induced increase in [Ca2+]i. (b) Pharmacological activation of pmKATP by 100 μM pinacidil and P-1075 reduced, pmKATP inhibition by 100 μM HMR 1098 enhanced reoxygenation-induced increase in [Ca2+]i. All compounds were applied during reoxygenation only. *P<0.05 vs control, n=6 experiments, 2–5 cells per experiment.
As expected, superfusion of myocytes with Tyrode's solution containing 15 mM [K+]o caused a depolarization, while subsequent application of 2.5 mM [K+]o caused hyperpolarization of the resting membrane potential, with respect to the Em estimated during superfusion with 5 mM [K+]o (Baczkó et al., 2003a; Figure 1b). In the next set of experiments, the influence of resting membrane potential on [Ca2+]i was evaluated. A 4 min exposure to 15 mM [K+]o significantly increased [Ca2+]i (increase in Fura-2 ratio was 7.0±0.58% vs baseline, P<0.01; Figure 3a). The CIH-induced Ca2+ loading was significantly larger in the myocytes depolarized with 15 mM [K+]o (36.1±0.94% vs 16.9±0.36% in controls superfused with 5 mM [K+]o during CIH, P<0.001; Figure 3a, b). Importantly, reoxygenation-induced elevation of [Ca2+]i was reduced markedly in cardiac myocytes hyperpolarized with Tyrode's solution containing 2.5 mM [K+]o (change in Fura-2 ratio was –13.3±0.97 vs 13.5±1.1% in myocytes reoxygenated with 15 mM [K+]o, P<0.001; Figure 3a). Note that the Fura-2 ratio at the end of the reoxygenation period was reduced compared to that at the end of the CIH period (1.31±0.009 vs 1.44±0.053, P<0.05; Figure 3a). These results are in agreement with our recent study showing that the resting membrane potential of cardiac myocytes is a critical determinant of CIH/reoxygenation-induced Ca2+ overload (Baczkó et al., 2003a). In the course of the imaging experiments, some myocytes developed hypercontracture either during CIH or reoxygenation, which was preceded by a rapid increase in [Ca2+]i. These myocytes were no longer viable; thus, they were excluded from evaluation of [Ca2+]i, but were included in the evaluation of cell hypercontracture (Table 1).
Table 1.
Percentage of cardiomyocyte hypercontracture during reoxygenation
| Drug in reoxygenation | n | Hypercontracted myocytes (%) |
|---|---|---|
| Control | 23 | 44 |
| 100 μM pinacidil | 28 | 11* |
| 10 μM P-1075 | 32 | 13* |
| 100 μM P-1075 | 27 | 7* |
| 100 μM HMR 1098 | 24 | 75* |
| 10 μM P-1075+100 μM HMR 1098 | 22 | 47 |
n is the number of cardiomyocytes subjected to reoxygenation.
P<0.05 vs control group.
Pinacidil and P-1075 prevent and HMR 1098 increases reoxygenation-induced Ca2+ loading via a mechanism involving hyperpolarization of the diastolic resting membrane potential
In addition to causing resting membrane potential hyperpolarization upon reoxygenation (Figure 2b), pinacidil and P-1075 (10 and 100 μM) significantly reduced reoxygenation-induced increase in [Ca2+]i (increases in normalized Fura-2 ratio were 4.9±0.78, 0.4±0.26 and 1.4±0.29 vs 9.8±0.18% in controls, respectively, P<0.05; Figure 3b and 4b). HMR 1098 (100 μM) significantly increased reoxygenation-induced Ca2+ loading (increase in normalized Fura-2 ratio was 20.5±0.61 vs 9.8±0.18% in controls, P<0.05; Figure 3b).
Both 2.5 mM [K+]o and pmKATP activation resulted in hyperpolarization of the resting membrane potential (Figures 1b and 2b) and consequent protection against increases in [Ca2+]i during reoxygenation. A difference between the findings shown in Figures 3a and b can be noted, however. Reoxygenation with 2.5 mM [K+]o caused a significant decrease in [Ca2+]i compared to the end of the CIH period (normalized Fura-2 ratios were 1.31±0.01 at the end of reoxygenation vs 1.44±0.05 at the end of CIH, P<0.05; Figure 3a). Reoxygenation with 100 μM P-1075 prevented further increase of [Ca2+]i during reoxygenation (normalized Fura-2 ratios were 1.15±0.02 at the end of reoxygenation vs 1.14±0.02 at the end of CIH, P>0.05; Figure 3b). We calculate that in myocytes superfused with 15 mM [K+]o during CIH and with 2.5 mM [K+]o during reoxygenation (Figure 3a), the change in membrane potential is approximately 30 mV by the end of reoxygenation (EK at 15 mM [K+]o minus EK at 2.5 [K+]o). On the other hand, in myocytes superfused with 5 mM [K+]o both during CIH and reoxygenation (Figure 3b), 100 μM P-1075 hyperpolarizes the cell by approximately 8–10 mV (Em during CIH (Figure 2b) minus EK of –85 mV at 5 mM [K+]o). These results further suggest and are in agreement with our previous study (Baczkó et al., 2003a) showing that changes in resting membrane potential are critical in the development of reoxygenation-induced Ca2+ loading.
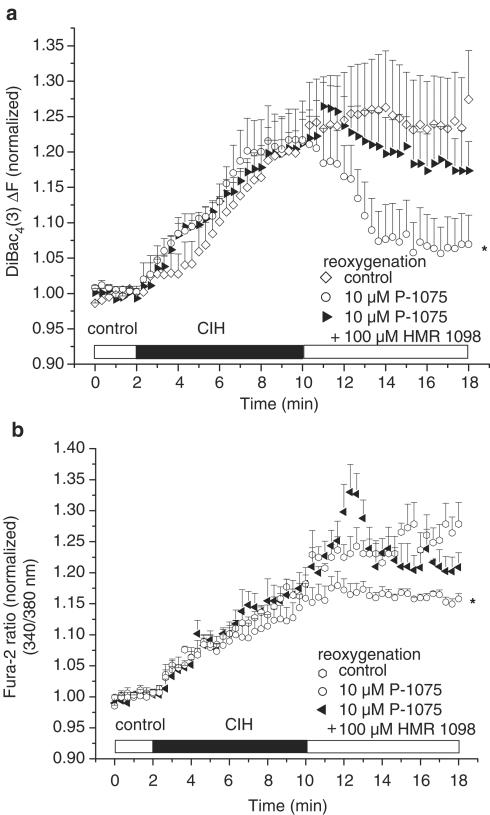
HMR 1098 blocks the protective effect of P-1075 during reoxygenation
The presence of 100 μM HMR 1098 in the reoxygenation solution prevented the hyperpolarizing effect of 10 μM P-1075 (Figure 4a), and blocked the protective effect of 10 μM P-1075 on reoxygenation-induced Ca2+ overload (Figure 4b).
Figure 4.
(a) HMR 1098 (100 μm) prevents the hyperpolarization of resting membrane potential produced by 10 μM P-1075 during reoxygenation. (b) HMR 1098 (100 μM) blocks the protective effect of 10 μM P-1075 on reoxygenation-induced Ca2+ overload. All compounds were applied during reoxygenation only. *P<0.05 vs control, n=6 experiments, 2–5 cells per experiment.
Activation and inhibition of pmKATP and reoxygenation-induced myocyte hypercontracture
The resting membrane depolarization and increased Ca2+ overload showed good correlation with the increased number of hypercontracted cells in DiBac4(3) and Fura 2 imaging experiments. We use the term hypercontracture to describe myocytes exhibiting completely irreversible pathophysiological behaviour; this included dramatic cell shortening (>80% of original cell length), and/or losing their rod-shaped cellular morphology (rounding up), and blebbing of the sarcolemma. These morphological changes were accompanied by a massive, irreversible membrane potential depolarization and large increases in [Ca2+]i. Pharmacological activation of pmKATP channels by 100 μM pinacidil, 10 and 100 μM P-1075 significantly decreased the number of ventricular myocytes that hypercontracted during reoxygenation (Table 1). Conversely, reoxygenation of myocytes with Tyrode's solution containing 100 μM of the pmKATP inhibitor HMR 1098 (Gögelein et al., 2000) significantly increased cell hypercontracture and death (Table 1). The incidence of cell death in the group of ventricular myocytes reoxygenated with the combination of 10 μM P-1075 and 100 μM HMR 1098 was not statistically significant from controls (Table 1). This result was in agreement with those showing that 100 μM HMR 1098 prevented the resting membrane potential hyperpolarizing effect (Figure 4a), and blocked the protective effect of 10 μM P-1075 on reoxygenation-induced Ca2+ overload (Figure 4b).
Discussion
Summary
Our findings provide important new evidence that activation of the plasma-membrane KATP current in mammalian ventricle can reduce reoxygenation-induced increases in [Ca2+]i, and show that this is due to hyperpolarization of the resting membrane potential. This hyperpolarization of the diastolic membrane potential by pmKATP activation markedly reduces the number of myocytes that hypercontract and die. In contrast, inhibition of pmKATP increases both Ca2+ loading and the incidence of myocyte hypercontracture in the setting of hypoxia/reoxygenation.
Rationale of findings
The potentiometric dye DiBac4(3), which functions as a qualitative monitor of membrane potential (Em) and provides reproducible estimates of changes in Em, was employed in this study. To correlate changes in membrane potential and DiBac4(3) fluorescence, DiBac4(3)-loaded rat ventricular myocytes were voltage-clamped and the DiBac4(3) fluorescence was measured (Figure 1a). To test the hypothesis that the deleterious effects of increases in intracellular calcium concentration [Ca2+]i resulting from CIH and subsequent reoxygenation can be reduced by hyperpolarization of the diastolic membrane potential following pmKATP activation, changes in the membrane potentials of rat ventricular myocytes were followed by DiBac4(3) imaging during CIH/reoxygenation in the presence or absence of pharmacological pmKATP activation. Our rationale (Pandit et al., 2001) is that this manoeuvre moves the membrane potential into a range where the Na+/Ca2+ exchanger can more effectively extrude Ca2+ in the presence of a normal electrochemical gradient for Na+ (Bouchard et al., 1993; Pandit et al., 2001).
Our data confirm that DiBac4(3) can qualitatively monitor depolarizations and hyperpolarizations from the normal resting potential in rat ventricular myocytes (Figure 1a, b), and that these changes in Em are maintained for the duration of the experimental protocols used in this study (Figure 1b). In mammalian ventricular myocardium, this finding is important for two reasons:
(i) A hyperpolarization (approximately 8–10 mV) to a stable membrane potential is essential for this experimental design, since reductions in [K+]o to 2.5 mM may, in isolated myocytes (e.g. which are compromised as a result of the enzymatic dispersion procedure), lead to oscillations between two levels of Em. This is due to the ion–ion interaction in the background inwardly rectifying K+ current which controls the resting membrane potential (Pandit et al., 2001). A significant stable hyperpolarization is a requirement for the manoeuvres, which yield data that test our working hypothesis.
(ii) DiBac4(3) reports changes in membrane potential through time-dependent partitioning into the myocyte sarcolemma. For this reason, it may be toxic. Our experimental design involves subtle, voltage-dependent interactions between potassium channels (Kir 2.1) and antiporter mechanisms (Na+/Ca2+ exchanger). Our findings (Figure 1a, b), however, provide no evidence for any toxic effects of DiBac4(3) when used in isolated cardiomyocytes, under the conditions described in this paper.
Pharmacological activation and inhibition of plasma-membrane KATP
The main goal of this study was to determine whether pharmacological activation of the population of ATP-sensitive potassium channels which are expressed predominantly in the sarcolemma can ameliorate myocyte damage due to elevated [Ca2+]i, following CIH/reoxygenation. For this reason, it was essential to activate sarcolemmal KATP channels as opposed to those expressed in the mitochondria. The available literature suggests that P-1075 functions in this capacity (Liu et al., 2001), and our findings are consistent with this supposition. Liu et al. have shown that P-1075, even in the highest concentration used in this study (100 μM), does not activate native mitoKATP channels. We also used pinacidil, a nonselective KATP channel opener with a chemical structure very similar to P-1075, for further confirmation of the effect of KATP channel activation on reoxygenation-induced Ca2+ overload.
The time course of the onset of the effects of KATP openers on resting membrane potential and reoxygenation-induced Ca2+ overload averaged approximately 1 min, which makes it unlikely that the hyperpolarization observed following P-1075 application, even at this high concentration, was mediated by mitochondrial KATP channel activation. As shown by Sato et al. (2000), KATP channel openers elicit their effect on mitochondrial function in 5–10 min.
ATP-sensitive potassium channels consist of a hetero-octamer of four inwardly rectifying pore-forming potassium channel subunits (Kir6.2) together with four regulatory sulphonylurea receptor subunits (SUR) (Inagaki et al., 1995). Distinct isoforms of the SUR expressed in different tissues lead to well-defined pharmacological characteristics of the expressed KATP channel. In the plasma membrane of cardiac myocytes and skeletal muscle, SUR2A is the SUR isoform (Inagaki et al., 1996).
In the present study, HMR 1098, a cardioselective sulphonylurea, was used for the inhibition of ventricular myocyte KATP channels (Gögelein et al., 2000). The pharmacological profile of mitochondrial KATP closely resembles the Kir6.1/SUR1 (Liu et al., 2001). A recent study from our laboratory shows that HMR 1098 selectively inhibits the pmKATP isoform Kir6.2/SUR2A, as opposed to Kir6.1/SUR1 (Manning Fox et al., 2002). This same study also shows that HMR 1098 (10–1000 μM) did not have an effect on Kir2.1 current (Manning Fox et al., 2002). Also, evidence from recent work using pinacidil and [3H]P-1075 demonstrates that the target site for potassium channel opener binding and selectivity is the C-terminal of the sulphonylurea receptor complex (Schwanstecher et al., 1998). The results of these studies suggest that it is very unlikely that the resting membrane potential changes seen in our experiments were due to the effects of pinacidil, P-1075 or HMR 1098 on the background inwardly rectifying K+ current (IK1).
Activation of plasma-membrane IK-ATP hyperpolarizes the resting membrane potential and provides cardioprotection
It has been suggested that the activation of mitoKATP as opposed to pmKATP plays an important role in cardioprotection (reviewed by Gross & Fryer, 1999). This suggestion is based on pharmacological studies using diazoxide and 5-hydroxydecanoate (5-HD) as a mitoKATP opener and blocker, respectively (Garlid et al., 1997; Liu et al., 1998). However, diazoxide has been shown to cause flavoprotein oxidation through a mechanism independent of its mitoKATP activation (Grimmsmann & Rustenbeck, 1998; Hanley et al., 2002), and can open pmKATP at low concentration in the presence of ADP (D'Hahan et al., 1999), and to protect cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation (Lawrence et al., 2001). Also, 5-HD can be a substrate and/or inhibitor of fatty acid β-oxidation (Hanley et al., 2003). Accordingly, these effects of diazoxide and 5-HD could contribute to their ability to mimic and/or block the effects of ischaemic preconditioning.
We are aware that a number of papers have been published recently, suggesting a critical role for pmKATP in cardioprotection in the setting of ischaemia/hypoxia and reperfusion/reoxygenation. Cotransfection of COS-7 cells with the Kir6.2/SUR2A genes encoding the cardiac pmKATP isoform resulted in decreased hypoxia/reoxygenation-induced Ca2+ loading (Jovanovic et al., 1998). Also, Suzuki et al. (2002) have shown that the infarct size-reducing effect of ischaemic preconditioning is abolished in Kir6.2-deficient mice. Another recent study from their laboratory suggests that diazoxide exerts its cardioprotective effects via the pmKATP channels due to its ability to significantly improve post-ischaemic functional recovery only in mice hearts expressing the pmKATP channel as opposed to Kir6.2 knockout hearts (Suzuki et al., 2003).
Our results confirm that activation of pmKATP plays an important role in protecting ventricular myocytes against hypoxia/reoxygenation injury: in the present study, pharmacological activation of pmKATP by pinacidil and P-1075, a pmKATP selective pinacidil analogue, significantly decreased reoxygenation-induced Ca2+ overload (Figure 3b), an effect that was blocked by the pmKATP inhibitor HMR 1098 (Figure 4b). We also show that reoxygenation of myocytes in the presence of Tyrode's solution containing 2.5 mM [K+]o significantly reduced reoxygenation-induced Ca2+ overload in cardiac myocytes (Figure 3a). These results confirm our recent observations, which demonstrate the beneficial effects of hyperpolarization of the resting membrane potential on reoxygenation-induced Ca2+ overload by altering Na+/Ca2+ exchanger activity (Baczkó et al., 2003a). In agreement, the membrane potential hyperpolarizing effect of pmKATP openers observed in this study in reoxygenation after hypoxia-induced membrane depolarization (Figure 2b) is accompanied by a reduction in Ca2+ overload (Figure 3b). Based on our previous observations and on the present study, we propose that the modulation of Na+/Ca2+ exchanger-mediated Ca2+ entry by hyperpolarization of the resting membrane potential as a consequence of pmKATP activation is an essential component of the mechanism responsible for the cardioprotection offered by ATP-sensitive potassium channel opening in ventricular myocytes.
Implications for cardioplegia
The heart is subjected to elective global ischaemia during cardiac surgery or cardiac transplantation. The application of cardioplegic solutions arrests the heart and preserves its postoperative function. However, some aspects of the postoperative cardiac dysfunction observed in the majority of patients can, at least in part, be attributed to the use of hyperkalaemic cardioplegic solutions (Leung, 1993; Cohen et al., 1995). The results from our previous (Baczkó et al., 2003a) and present study (Figure 1b, 3a) show that the increased [K+]o, used in hyperkalaemic cardioplegic solutions (induction: 23 mM, maintenance: 13 mM; Fukuhiro et al., 2000),depolarizes myocytes. This diastolic depolarization can lead to increased Ca2+ overload and cell hypercontracture/death upon reperfusion. A number of strategies have been utilized to reduce Ca2+ overload during experimental cardioplegia. These include: (i) lowering [Ca2+]o in a Mg2+-containing solution (Fukuhiro et al., 2000), (ii) addition of the sodium channel blocker tetrodotoxin (Snabaitis et al., 1997), (iii) application of the Na+/H+ exchanger inhibitor HOE-642 (Fukuhiro et al., 2000) and (iv) addition of KATP openers. It is known that the KATP opener pinacidil can enhance cardioprotection compared to cardioplegic solution alone (Hosoda et al., 1994). The observation that another KATP opener, lemakalim, protected rat hearts undergoing hypothermic cardioplegia when given alone, but failed to improve post-ischaemic functional recovery when applied in a hyperkalaemic cardioplegic solution (Galiñanes et al., 1992), can be rationalized by our results. Thus, it is very likely that the protective effect of lemakalim was reduced due to depolarization of the resting membrane potential in hyperkalaemic milieu. Notably, recent studies on experimental cardioplegia are in agreement with our results: solutions hyperpolarizing the diastolic membrane potential in rat hearts are effective during cardioplegia (Chambers & Hearse, 1999).
Limitations
The cellular events observed during metabolic inhibition in this study closely replicate those observed in the setting of ischaemia/reperfusion injury; however, they might not be the same. Therefore, our findings require further confirmation in future whole-heart studies.
In this study, DiBac4(3) proved to be a reliable qualitative reporter of changes in the resting membrane potential of ventricular myocytes. However, it seems that the dye is not an absolutely accurate measure of membrane potential at more negative potentials, where the relationship between normalized DiBac4(3) fluorescence intensity and actual membrane potential becomes less steep (Figure 1a). Therefore, the changes in DiBac4(3) fluorescence can be used only for estimates in changes in membrane potential as opposed to an exact voltage readout. It must be noted that this does not affect the main finding of this study, showing that the cardioprotective mechanism of pmKATP channel activation involves resting membrane potential hyperpolarization.
It is noted that there may be some additional effects of P-1075 at lower concentrations on cellular processes other than pmKATP channel activation (Jilkina et al., 2002). However, our finding that the selective pmKATP blocker HMR 1098 completely abolished the resting membrane potential hyperpolarizing as well as the protective effects of P-1075 strongly supports our interpretations regarding a mechanism of action of P-1075 being on the pmKATP in origin. Also, the findings of this paper confirm our previous observations regarding the important role of resting membrane potential hyperpolarization in the prevention of reoxygenation-induced Ca2+ loading (Baczkó et al., 2003a).
Conclusions
Results from this study show that the hyperpolarization of the resting membrane potential caused by pmKATP activation (Figure 2b) is sufficient to result in more effective Ca2+ extrusion in ventricular myocytes (Figure 3b). Recent findings from our laboratory (Baczkó et al., 2003a) suggest that hyperpolarization significantly reduces reverse-mode Na+/Ca2+ exchange activity, leading to a significant decrease in reoxygenation-induced Ca2+ overload. Therefore, we suggest that decreased Ca2+ loading via the Na+/Ca2+ exchanger may be the critical mechanism responsible for the beneficial effects of pmKATP activation on Ca2+ homeostasis during reoxygenation.
Acknowledgments
This work was supported by the Alberta Heritage Foundation for Medical Research (PEL, WRG), Heart and Stroke Foundation of Canada (WRG) and the Canadian Institutes for Health Research (PEL, WRG). PEL is a Scholar of the Alberta Heritage Foundation for Medical Research (AHFMR) and a Canadian Institutes of Health Research New Investigator. Salary support for Dr Baczkó was obtained from the Alberta Heart and Stroke Foundation Endowed Research Chair held by WRG, and a CIHR Strategic Training Initiative.
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- CIH
chemically induced hypoxia
- DiBac4(3)
bis-(1,3-dibutylbarbituric acid)trimethine oxonol
- EK
equilibrium potential for potassium
- Em
resting membrane potential
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- HMR 1098
1-[[5-[2-(5-chloro-o-anisamido)ethyl]-2-methoxyphenyl]sulphonyl]-3-methylthiourea, sodium salt
- KB-R7943
2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea mesylate
- KO
knockout
- mitoKATP channel
mitochondrial ATP-sensitive potassium channel
- P-1075
N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine
- pmKATP channel
plasma-membrane ATP-sensitive potassium channel
References
- BACZKÓ I., GILES W.R., LIGHT P.E. Resting membrane potential regulates Na+/Ca2+ exchange-mediated Ca2+ overload during hypoxia/reoxygenation in rat ventricular myocytes. J. Physiol. 2003a;550:889–898. doi: 10.1113/jphysiol.2003.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACZKÓ I., GILES W.R., LIGHT P.E. Activation of sarcolemmal KATP channels reduces hypoxia/reoxygenation-induced calcium overload via hyperpolarization of the resting membrane potential (abstract) Biophys. J. 2003b;84:422a. [Google Scholar]
- BLAUSTEIN M.P., LEDERER W.J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- BOLLI R., MARBÁN E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- BOUCHARD R.A., CLARK R.B., GILES W.R. Role of sodium–calcium exchange in activation of contraction in rat ventricle. J. Physiol. 1993;472:391–413. doi: 10.1113/jphysiol.1993.sp019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS D.J., HEARSE D.J. Developments in cardioprotection: ‘polarized' arrest as an alternative to ‘depolarized' arrest. Ann. Thorac. Surg. 1999;68:1960–1966. doi: 10.1016/s0003-4975(99)01020-6. [DOI] [PubMed] [Google Scholar]
- COHEN N.M., DAMIANO R.J., WECHSLER A.S. Is there an alternative to potassium arrest. Ann. Thorac. Surg. 1995;60:858–863. doi: 10.1016/0003-4975(95)00210-C. [DOI] [PubMed] [Google Scholar]
- COLE W.C., MCPHERSON C.D., SONTAG D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ. Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- D'HAHAN N., MOREAU C., PROST A.L., JACQUET H., ALEKSEEV A.E., TERZIC A., VIVAUDOU M. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAS M., PARKER J.E., HALESTRAP A.P. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J. Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI VIRGILIO F., STEINBERG T.H., SILVERSTEIN S.C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- EIGEL B.N., HADLEY R.W. Antisense inhibition of Na+/Ca2+ exchange during anoxia/reoxygenation in ventricular myocytes. Am. J. Physiol. 2001;281:H2184–H2190. doi: 10.1152/ajpheart.2001.281.5.H2184. [DOI] [PubMed] [Google Scholar]
- EPPS D.E., WOLFE M.L., GROPPI V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol (Dibac(4)(3)) in model systems and cells. Chem. Phys. Lipids. 1994;69:137–150. doi: 10.1016/0009-3084(94)90035-3. [DOI] [PubMed] [Google Scholar]
- FUKUHIRO Y., WOWK M., OU R., ROSENFELDT F., PEPE S. Cardioplegic strategies for calcium control: low Ca2+, high Mg2+, citrate, or Na+/H+ exchange inhibitor HOE-642. Circulation. 2000;102:III319–III325. [PubMed] [Google Scholar]
- GALIÑANES M., SHATTOCK M.J., HEARSE D.J. Effects of potassium channel modulation during global ischaemia in isolated rat heart with and without cardioplegia. Cardiovasc. Res. 1992;26:1063–1068. doi: 10.1093/cvr/26.11.1063. [DOI] [PubMed] [Google Scholar]
- GARLID K.D., PAUCEK P., YAROV-YAROVOY V., MURRAY H.N., DARBENZIO R.B., D'ALONZO A.J., LODGE N.J., SMITH M.A., GROVER G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- GÖGELEIN H., ENGLERT H.C., KOTZAN A., HACK R., LEHR K.H., SEIZ W., BECKER R.H.A., SULTAN E., SCHOLKENS B.A., BUSCH A.E. HMR 1098: an inhibitor of cardiac ATP-sensitive potassium channels. Cardiovasc. Drug Rev. 2000;18:157–174. [Google Scholar]
- GRIMMSMANN T., RUSTENBECK I. Direct effects of diazoxide on mitochondria in pancreatic β-cells and on isolated liver mitochondria. Br. J. Pharmacol. 1998;123:781–788. doi: 10.1038/sj.bjp.0701663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS G.J., FRYER R.M. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- GROSS G.J., PEART J.N. KATP channels and myocardial preconditioning: an update. Am. J. Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- HANLEY P.J., GOPALAN K.V., LAREAU R.A., SRIVASTAVA D.K., VON MELTZER M., DAUT J. Oxidation of 5-hydroxydecanoate, a putative blocker of mitochondrial ATP-sensitive potassium channels. J. Physiol. 2003;547:387–393. doi: 10.1113/jphysiol.2002.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANLEY P.J., MICKEL M., LOFFLER M., BRANDT U., DAUT J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEARSE D.J. Activation of ATP-sensitive potassium channels: a novel pharmacological approach to myocardial protection. Cardiovasc. Res. 1995;30:1–17. [PubMed] [Google Scholar]
- HOSODA H., SUNAMORI M., SUZUKI A. Effect of pinacidil on rat hearts undergoing hypothermic cardioplegia. Ann. Thorac. Surg. 1994;58:1631–1636. doi: 10.1016/0003-4975(94)91649-7. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., NAMBA N., INAZAWA J., GONZALEZ G., AGUILAR-BRYAN L., SEINO S., BRYAN J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., WANG C.Z., AGUILAR-BRYAN L., BRYAN J., SEINO S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- JILKINA O., KUZIO B., GROVER G.J., KUPRIYANOV V.V. Effects of KATP channel openers, P-1075, pinacidil, and diazoxide, on energetics and contractile function in isolated rat hearts. J. Mol. Cell. Cardiol. 2002;34:427–440. doi: 10.1006/jmcc.2001.1524. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC A., JOVANOVIC S., LORENZ E., TERZIC A. Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance to chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- LAWRENCE C.L., BILLUPS B., RODRIGO G.C., STANDEN N.B. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. Br. J. Pharmacol. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG J.M. Clinical evidence of myocardial stunning in patients undergoing CABG surgery. J. Cardiac. Surg. 1993;8 Suppl:220–223. doi: 10.1111/j.1540-8191.1993.tb01310.x. [DOI] [PubMed] [Google Scholar]
- LIGHT P.E., KANJI H.D., MANNING FOX J.E., FRENCH R.J. Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J. 2001;15:2586–2594. doi: 10.1096/fj.01-0188com. [DOI] [PubMed] [Google Scholar]
- LIGHT P.E., SHIMONI Y., HARBISON S., GILES W.R., FRENCH R.J. Hypothyroidism decreases the ATP sensitivity of KATP channels from rat heart. J. Membr. Biol. 1998;162:217–223. doi: 10.1007/s002329900359. [DOI] [PubMed] [Google Scholar]
- LIU Y.G., REN G.F., O'ROURKE B., MARBÁN E., SEHARASEYON J. Pharmacological comparison of native mitochondrial KATP channels with molecularly defined surface KATP channels. Mol. Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- LIU Y.G., SATO T., O'ROURKE B., MARBÁN E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- MANNING FOX J.E., KANJI H.D., FRENCH R.J., LIGHT P.E. Cardioselectivity of the sulphonylurea HMR 1098: studies on native and recombinant cardiac and pancreatic KATP channels. Br. J. Pharmacol. 2002;135:480–488. doi: 10.1038/sj.bjp.0704455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARBÁN E., KORETSUNE Y., CORRETTI M., CHACKO V.P., KUSUOKA H. Calcium and its role in myocardial cell injury during ischemia and reperfusion. Circulation. 1989;80:IV17–IV22. [PubMed] [Google Scholar]
- MCPHERSON C.D., PIERCE G.N., COLE W.C. Ischemic cardioprotection by ATP-sensitive K+ channels involves high-energy phosphate preservation. Am. J. Physiol. 1993;265:H1809–H1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- NOMA A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- O'ROURKE B. Pathophysiological and protective roles of mitochondrial ion channels. J. Physiol. 2000;15:23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDIT S.V., CLARK R.B., GILES W.R., DEMIR S.S. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys. J. 2001;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J.L., LUCCHESI B.R. Mechanisms of myocardial reperfusion injury. Ann. Thorac. Surg. 1999;68:1905–1912. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- PIPER H.M., GARCIA-DORADO D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann. Thorac. Surg. 1999;68:1913–1919. doi: 10.1016/s0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- SATO T., SASAKI N., SEHARASEYON J., O'ROURKE B., MARBÁN E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal KATP channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- SCHÄFER C., LADILOV Y., INSERTE J., SCHAFER M., HAFFNER S., GARCIA-DORADO D., PIPER H.M. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc. Res. 2001;51:241–250. doi: 10.1016/s0008-6363(01)00282-6. [DOI] [PubMed] [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DORSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C., BRYAN J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNABAITIS A.K., SHATTOCK M.J., CHAMBERS D.J. Comparison of polarized and depolarized arrest in the isolated rat heart for long-term preservation. Circulation. 1997;96:3148–3156. doi: 10.1161/01.cir.96.9.3148. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., SAITO T., SATO T., TAMAGAWA M., MIKI T., SEINO S., NAKAYA H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., SASAKI N., MIKI T., SAKAMOTO N., OHMOTO-SEKINE Y., TAMAGAWA M., SEINO S., MARBÁN E., NAKAYA H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANI M. Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu. Rev. Physiol. 1990;52:543–559. doi: 10.1146/annurev.ph.52.030190.002551. [DOI] [PubMed] [Google Scholar]