Abstract
We have investigated the in vitro interaction between chloride ions and endothelium as revealed by alterations in vascular contractility and smooth muscle cell membrane potential in isolated pulmonary arteries from Dahl salt-resistant normotensive and salt-sensitive hypertensive rats.
Exposure to nitro-L-arginine methyl ester (L-NAME) of tissues from normotensive but not hypertensive rats augmented contractions to cirazoline. While chloride removal did not alter cirazoline-induced contractions, it completely abolished the augmentation by L-NAME in normotensive rats. However, in hypertensive rats, removal of chloride ions significantly attenuated contractions elicited by cirazoline, and L-NAME effectively reversed this inhibition.
Methacholine-induced endothelium-dependent relaxations of the same magnitude were evident in both normotensive and hypertensive rats. However, basal cyclic GMP levels were found to be significantly higher (7.8-fold) in blood vessels of normotensive rats compared to hypertensive rats.
The resting membrane potential in pulmonary arteries of hypertensive rats (−52.1±1.04 mV) revealed a significant hyperpolarisation when compared with that of normotensive rats (−46.4±1.58 mV). Cirazoline did not produce a significant depolarisation in blood vessels of either normotensive or hypertensive rats. Perfusion with chloride-free solution resulted in a modest but significant hyperpolarisation (−8.0 mV) in the blood vessels of hypertensive but not in normotensive rats.
We conclude that salt-dependent hypertension in Dahl rats is accompanied by functional and biochemical changes in low-pressure blood vessels. These changes can, in part, be attributed to impairment in the basal, but not methacholine-stimulated, release of nitric oxide, and to altered chloride ion handling.
Keywords: Chloride ions, membrane potential, pulmonary artery, contraction, relaxation, salt-induced hypertension, Dahl rats
Introduction
Chloride ions play a critical role in the control of blood vessel tone and blood flow (Criddle et al., 1996; 1997; He & Tabrizchi, 1997; Parai & Tabrizchi, 2002). In particular, agonist-mediated depolarisation in vascular smooth muscle has been linked to stimulation of a calcium-activated chloride current subsequent to the release of intracellular calcium, with the resulting depolarisation and opening of L-type calcium channels leading to contraction of vascular smooth muscle (for a review, see Large & Wang, 1996). It has been inferred that noradrenaline stimulation of chloride flux in blood vessels, in part, is dampened by tonically released nitric oxide (Lamb & Barna, 1998a, 1998b). Evidence from our laboratory suggests that chloride ion handling and endothelial regulation of chloride ions undergo pathophysiological alterations in systemic blood vessels of Dahl rats with established salt-induced hypertension (Tabrizchi & Duggan, 2000).
Alteration in endothelial cell function accounts at least in part for changes in blood vessel contractility in established models of salt-induced hypertension (Lüscher et al., 1987a; Boegehold, 1992; Boulanger, 1999). Basal levels of 3′,5′-cyclic monophosphate (cGMP) in the aortic tissue of Dahl salt-sensitive (DSS) hypertensive rats are significantly lower than those of Dahl salt-resistant (DSR) normotensive rats (Tabrizchi & Duggan, 2000). As well, the nitric oxide/nitric oxide synthase pathway appears to be less active in hypertensive salt-sensitive than in normotensive salt-resistant Dahl rats, as evidenced by the failure of nitric oxide synthase inhibition to increase aortic contractility. In the salt-resistant normotensive rats, however, both nitric oxide synthase inhibition and removal of extracellular chloride ions augmented aortic contractility in a non-additive manner (Tabrizchi & Duggan, 2000). To date, these relationships have been investigated only in the aorta, that is, the high-pressure segment of the circulatory system.
According to the available literature, extracellular chloride replacement does not lead to significant depolarisation of the membrane potential of smooth muscle cells (for a review, see Chipperfield & Harper, 2000). In fact, rabbit pulmonary artery myocytes bathed in isethionate containing chloride-free medium showed a moderate hyperpolarisation (Gerstheimer et al., 1987). Smooth muscle hyperpolarisation is not easily reconciled with increased contractility, as seen in rat aorta following substitution of extracellular chloride ions with propionate, a relatively impermeant anion (Tabrizchi & Duggan, 2000). However, this increase is manifested only in tissues from normotensive salt-resistant Dahl rats, inhibition being evident in the tissue from hypertensive salt-sensitive Dahl rats (Tabrizchi & Duggan, 2000).
According to the prevailing view, hypertension-associated increases in vascular contractility and deficits in vasorelaxation reflect an impaired interaction between vascular endothelium and smooth musculature (Nishida et al., 1998; Boulanger, 1999). Increased physical stress acting upon the arterial vascular wall is a primary causative factor (Lee & Triggle, 1986; Lee, 1987; Lüscher et al., 1987a, 1987b). The absence of an elevated mean pressure in the pulmonary circulation should protect this functional interrelationship between endothelium and smooth musculature in a state of experimentally induced systemic hypertension. Based on this rationale, we have attempted to characterise the interactions between chloride ions and endothelium operating in the low-pressure segment of the circulatory system (i.e., pulmonary artery) of DSR normotensive and DSS hypertensive rats. Our basic approach was to assess the effect of extracellular chloride ion substitution, alone or in combination with nitric oxide synthase inhibition, on mechanical and electrical activity in pulmonary artery branches obtained from salt-resistant normotensive and salt-sensitive hypertensive rats kept on a 4% salt diet for 7 weeks. The specific aims of our study were: (a) to determine how replacing chloride ions with propionate alters α-adrenoceptor-mediated contractions and muscarinic receptor-mediated relaxations in intact and denuded blood vessels; (b) to evaluate the influence of chloride ion removal upon the effect of nitric oxide synthase inhibition on these parameters; and (c) to assess parallel effects on the membrane potential of vascular smooth muscle cells. Finally, it appeared of interest to determine whether the previously observed changes in cGMP levels in aortic tissue also occur in the low-pressure vascular bed during salt-induced hypertension.
Methods
Surgical procedures
Female RAPP Dahl rats (salt-resistant and salt-sensitive; 6–7 weeks of age) housed (two per cage) with 12 h light/dark cycles were given free access to 4% salt diet and tap water ad libitum.
Following 7 weeks on a 4% salt (NaCl) diet, each rat was anaesthetised with halothane (five in 100% oxygen for induction and 1.25 in 100% oxygen for maintenance), and a catheter (polyethylene tubing ID 0.58 mm, OD 0.965 mm) was inserted into the left femoral artery for the measurement of blood pressure. The catheter was filled with heparinised saline (25 i.u. ml−1 in 0.9% NaCl), and blood pressure and heart rate (HR) were measured for 30 min continuously. Arterial blood pressure was recorded with a pressure transducer (Gould Statham, U.S.A.; Model PD23B) connected to a Gould Universal amplifier and recorder (Gould, France; Model 8188-2202-00). HR was calculated from the blood pressure tracing. The animal was tracheotomised and artificially ventilated by positive force ventilation (Harvard Ventilator Model 683, Harvard Apparatus, Canada) at a volume of 1.0 ml per 100 g at 58 cycles min−1 with a mixture of halothane 1.25 and 100% oxygen. The chest cavity was then opened and pulmonary pressure was recorded via a hypodermic needle inserted into the main pulmonary artery (Tabrizchi et al., 1995). Pulmonary pressure was recorded with a pressure transducer (Gould Statham, U.S.A.; Model PD23B) connected to a Gould Universal amplifier and recorder (Gould, France; Model 8188-2202-00). Subsequently, each animal was killed and the pulmonary vasculature, heart, lungs and kidneys were removed. The weights of the left and right kidneys, right ventricle and left ventricle+septum were recorded.
Tissue isolation
The left and right pulmonary artery branches were isolated and dissected free of connective tissue at room temperature in Krebs buffer of the following composition (in mM): NaCl, 125; KCl, 4.6; glucose, 11; MgCl2, 1.2; CaCl2, 2.5; KH2PO4, 1.2; NaHCO3, 12.5. All experiments were carried out either in normal Krebs or chloride-free buffer of the following composition (in mM): C2H2COONa (sodium propionate) 125, C2H2COOK (potassium propionate) 4.6, glucose 11, MgSO4 1.2, Ca gluconate 2.5, KH2PO4 1.2 and NaHCO3 12.5. The pH of the buffers following saturation with a 95% O2 : 5% CO2 gas mixture was 7.4 at 36±1°C.
Experimental protocol
Mechanical force measurements
Pulmonary artery rings from each branch (∼2 mm in length) were mounted in 20 ml organ baths at 36–37°C under a force of 9.8 mN, and gassed continuously with a mixture of 95% O2 : 5% CO2 for mechanical studies. In some tissues, the endothelium was removed by gentle rubbing. The tissues were equilibrated for 60 min and isometric tension was measured using force displacement transducers (Model FT03, Grass Instruments Co., MA, U.S.A.) connected to a polygraph (Model 7PCPB, Grass Instruments Co., MA, U.S.A.). Tissues were initially contracted with a single concentration of cirazoline (1.0 μM) over a 60 min period after the equilibration. Subsequently, each tissue was washed with fresh normal Krebs solution, and allowed to equilibrate for 60 min (washed once at 30 min with regular Krebs buffer), before the first cumulative concentration–response curve for cirazoline (1 nM–30 μM) was obtained. The tissues were then washed with regular Krebs buffer and allowed to equilibrate for 30 min. After this period, each tissue was either washed with regular Krebs or chloride-free buffer and a second cirazoline concentration–response curve was obtained in the presence of twice-distilled water (20 μl), or L-NAME (10 μM). Twice-distilled water and L-NAME were left in contact with each tissue for 25 min prior to and throughout the construction of the second concentration–response curve. This protocol was repeated in endothelium intact or denuded blood vessels. Concentration–response curves for the relaxant effects of methacholine (1 nM–30 μM) were obtained in separate tissues pre-contracted with 1 μM cirazoline (endothelium intact or denuded vessels). In denuded tissues, a concentration–response curve was obtained for sodium nitroprusside (1 nM–3 μM).
cGMP studies
Pulmonary artery branches (∼3 mm in length) were incubated in normal Krebs solution for 120 min and gassed continuously with a 95% O2 : 5% CO2 gas mixture kept at 37°C. The buffer was replaced with fresh buffer every 30 min. Subsequently, each tissue was rapidly frozen in liquid nitrogen and stored at −80°C until it was assayed for cGMP.
Levels of cGMP in pulmonary arteries were measured by the technique described by Delpy et al. (1996). Briefly, frozen pulmonary arteries were homogenised in 0.4 ml of cold trichloroacetic acid (6%) using an electric tissue homogeniser (Dyna-mix Model 43, Fisher Scientific, ON, Canada). The homogenate was centrifuged using an Eppendorf centrifuge (Model 5415C, Brinkman Instruments Inc., NY, U.S.A.) at 5000 g for approximately 1 min and the supernatant was decanted without disturbing the protein pellet. Subsequently, the supernatant was washed four times with 6 volumes of water-saturated diethyl ether to remove the trichloroacetic acid. The upper ether layer was discarded after each wash. Following the final extraction, any remaining ether was evaporated under air stream. Throughout these procedures, the samples were consistently stored at approximately 4°C in an ice-water bath. The aqueous phase was then evaporated to dryness with a Speed-vac system (Model SVC100H, Savant Instruments Inc., London, U.K.) for approximately 1.5 h and the dried extract dissolved in a suitable volume of Tris-EDTA assay buffer prior to analysis. cGMP content was determined by scintillation proximity assay kits (Amersham, ON, Canada). Protein concentration in the pellet was measured using Bradford's (1976) method (Bio-Rad Life Research Product, ON, Canada).
Membrane potential measurements
Pulmonary artery rings were held in place in a 5 ml sylgard lined tissue chamber perfused with 95% O2 : 5% CO2 pre-gassed physiological salt solution delivered at a rate of 3–4 ml min−1 and warmed to 34–35°C. Blood vessels were allowed to equilibrate for 60 min, and the membrane potential was recorded with borosilicate capillary microelectrodes filled with KCl (3.0 M) with a tip resistance of 10–20 MΩ. The Ag/AgCl reference half-cell containing KCl (3.0 M) was connected to the bath via an agar salt bridge containing 150 mM NaCl. Impalements were made by means of a Narishige x–y–z micropositioner, typically from the endothelial side at depths >50 μm below the surface. The criteria for impalement and measurement of smooth muscle cell membrane potential were an abrupt drop in voltage upon penetration of the cell membrane, a stable membrane potential for at least 3–4 min, and a sharp return of the membrane potential to zero upon withdrawal of the electrode from the cell. Voltage signals were recorded by means of an Electrometer Duo 773 (World Precision Instruments; input resistance 1015 Ω), the output of which was fed into a DigiData 1200 Series Interface (Axon Instruments Inc.). Data were acquired and displayed on AxoScope (Version 1.1) and stored on an IBM compatible microcomputer.
Membrane potentials from a number of cells were initially recorded prior to exposure of the blood vessels to drugs or chloride-free solution. Subsequently, tissues were exposed for 20–30 min to solutions containing cirazoline (0.3 μM), methacholine (1.0 μM), L-NAME (10 μM) or chloride-free buffer and membrane potentials were recorded. Recordings were done continuously while perfusate solutions were being changed, except in the case of cirazoline where cells were sampled individually before and after the addition of the agonist. The membrane potentials recorded in chloride-free buffer were corrected for offset voltage (junction potentials, typically 7–9 mV), as measured from baseline shifts determined when pipettes were kept extracellularly during the switch from chloride-containing to chloride-free medium.
Data and statistical analysis
Results from the contraction/relaxation studies were calculated as a percentage of the maximum response induced by the agonists following the construction of the concentration–response curve to the agonists. Percent maximum, Hill coefficient (nH) and EC50 values were calculated for individual curves using a program executed on an IBM compatible microcomputer (Tabrizchi & Duggan, 2000). These parameters were determined by fitting the percent contractile response at increasing concentrations of the agonist ([A]) by nonlinear least squares to the relation Y=a+bX, where Y=response and X=[A]n/([A]n+[EC50]n), with n (nH) fixed at ‘floating' integral values to obtain the best fit. Analysis of variance was used for comparisons of percent maximum, nH, and pD2 (negative log of EC50 for an agonist). For multiple comparisons, Duncan's multiple range test was used to compare between means. Student's unpaired t-test was used for comparison between other values body weight (BW), blood pressure, pulmonary artery pressure, HR, ratio of right ventricle to left ventricle+septum and kidney weights and basal cGMP levels). The data for membrane potential were log transformed for the purpose of statistical analysis as their distribution was found not to be normal, and positively skewed. Student's unpaired t-test was used for comparison between log-transformed data. The data reported for membrane potentials of vascular smooth cells is the geometric mean±standard error of mean (the standard error of mean that is reported is the larger value, as the distribution was asymmetrical). For all cases, a probability of error of less than 0.05 was selected as the criterion for statistical significance.
Chemicals
Stock solutions of all drugs were made in twice-distilled water. Cirazoline, methacholine and L-NAME were purchased from Sigma Chemical Company/Research Biochemical International (St Louis, MO, U.S.A.).
Results
Systemic blood pressure (systolic and diastolic) and the HR of DSS hypertensive rats were significantly higher than those of DSR rats, while pulmonary artery pressure of the two strains was the same 7 weeks after they were placed on 4% salt diet (Table 1). The weight of the left ventricle plus the septum but not the right ventricle (corrected for BW) was significantly greater in DSS hypertensive rats in comparison to DSR normotensive rats (Table 2). In addition, the ratio of the right ventricle to the left ventricle plus the septum was found to be significantly greater in salt-resistant normotensive rats in comparison to salt-sensitive hypertensive rats. The weights of the left and right kidneys (corrected for BW) were also found to be significantly lower in DSS hypertensive rats than in DSR normotensive rats (Table 2).
Table 1.
Systolic blood pressure (SBP), diastolic blood pressure (DPB), mean pulmonary artery pressure (MPAP) and heart rate (HR)
| Strains | SBP (mmHg) | DBP (mmHg) | MPAP (mmHg) | HR (beats min−1) |
|---|---|---|---|---|
| DSR | 103±2 | 78±2 | 11.90±0.25 | 383±7 |
| DSS | 176±2a | 137±2a | 12.4±0.30 | 399±7a |
Each value represents the mean of 25 measurements±s.e.m.; Dahl salt-resistant (DSR); Dahl salt-sensitive (DSS).
Significantly different from Dahl SR rats, P<0.05.
Table 2.
Weights of body, kidneys and heart for Dahl salt-resistant (DSR) normotensive and salt-sensitive (DSS) hypertensive rats
| Strains | BW (g) | LKa (g × 10−3) | RKa (g × 10−3) | RVa (g × 10−3) | LVSa (g × 10−3) | RV/LVS |
|---|---|---|---|---|---|---|
| DSR | 239±3 | 5.3±0.19 | 5.3±0.16 | 0.65±0.02 | 2.30±0.02 | 0.28±0.01 |
| DSS | 272±4b | 4.2±0.10b | 4.3±0.10b | 0.78±0.02 | 3.42±0.10b | 0.23±0.01b |
Body weight (BW), right kidney (RK); left kidney (RV); right ventricle (RV); left ventricle plus septum (LVS). Each value represents the mean of 25 measurements±s.e.m.
Value corrected for body weight.
Significantly different from Dahl SR rats, P<0.05.
Functional studies in endothelium intact pulmonary arteries
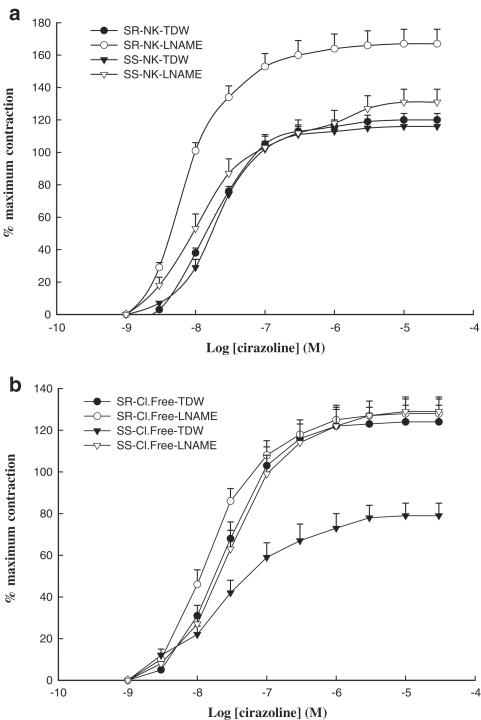
Cirazoline elicited a concentration-dependent contraction in arteries of DSR normotensive and DSS hypertensive rats with a maximal tension of 290±13 mN (mean±s.e.m.; n=48), and 296±14 mN (mean±s.e.m.; n=48), respectively. Maximal contractions obtained with cirazoline did not significantly differ in normotensive and hypertensive animals. Addition of L-NAME alone to the bath did not elicit contractions in rings of either DSR normotensive or DSS hypertensive rats. However, exposure of blood vessels from DSR normotensive rats to L-NAME augmented contraction to cirazoline, and significantly increased maximal response (%Emax: 162±10 vs 117±4; mean±s.e.m.; n=6; P<0.05), when compared to responses of vessels from the same strain treated with twice-distilled water (Figure 1a). In contrast to this observation, L-NAME failed to augment cirazoline-mediated contractions in pulmonary vessels obtained from salt-sensitive hypertensive rats (Figure 1a). While removal of extracellular chloride ions did not affect cirazoline-induced contractions in pulmonary arteries obtained from salt-resistant normotensive rats, it abolished the facilitatory effects of L-NAME on these tissues (Figure 1b). In contrast, in pulmonary arteries from DSS hypertensive rats, removal of chloride ions attenuated contractions elicited by cirazoline, significantly reducing the maximal response (%Emax: 79±6 vs 113±5; mean±s.e.m.; n=6; P<0.05) to this agonist; pretreatment with L-NAME was able to reverse the inhibitory actions of chloride removal (%Emax: 128±7 vs 79±6; mean±s.e.m.; n=6; P<0.05) in these blood vessels (Figure 1b).
Figure 1.
Concentration–contraction curves for cirazoline in endothelium-intact pulmonary artery rings (a) from DSR normotensive rats in normal Krebs solution (NK) plus twice-distilled water (TDW; closed circles), in normal Krebs solution containing L-NAME (10 μM; open circles), or from DSS hypertensive rats in normal Krebs solution plus twice-distilled water (closed triangles), in normal Krebs solution containing L-NAME (10 μM; open triangles), and (b) from salt-resistant normotensive rats in chloride-free buffer (Cl.Free) plus twice-distilled water (closed circles), in chloride-free buffer containing L-NAME (10 μM; open circle), or from salt-sensitive hypertensive rats in chloride-free buffer plus twice-distilled water (closed triangles), and in chloride-free buffer containing L-NAME (10 μM; open triangle). Each point represents a mean of six experiments±s.e.m.
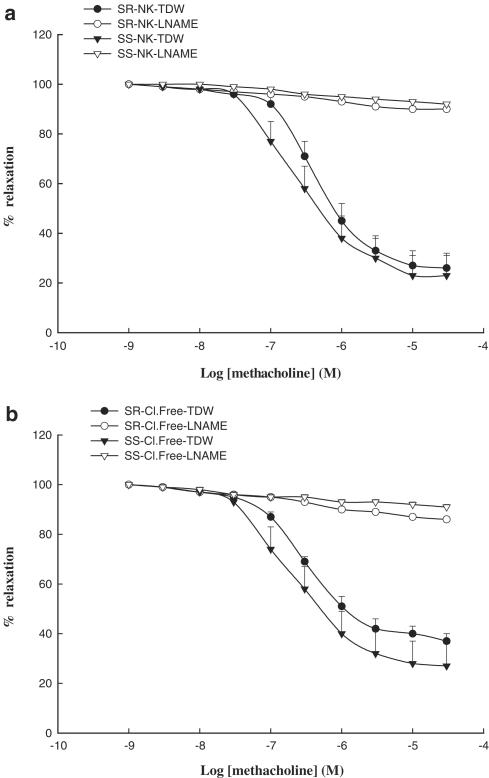
Methacholine produced concentration-dependent relaxations with similar efficacy and potency in pulmonary arteries of either normotensive or hypertensive rats (Figure 2a). Treatment of vessels with L-NAME completely abolished vasorelaxation induced by methacholine in pulmonary arteries (Figure 2a). Chloride removal neither affected the relaxation produced by methacholine, nor did it alter its inhibition by L-NAME (Figure 2b).
Figure 2.
Concentration–relaxation curves for methacholine in endothelium-intact pulmonary artery rings (a) from DSR normotensive rats in normal Krebs solution (NK) plus twice-distilled water (TDW; closed circles; pD2=6.33±0.06a; nH=1.22±0.15a; %Emax=75±6a), in normal Krebs solution containing L-NAME (10 μM; open circles; pD2=6.79±0.16; nH=0.6±0.04; %Emax=11±1; P<0.05a), or from DSS hypertensive rats in normal Krebs solution plus twice-distilled water (closed triangles; pD2=6.49±0.17b; nH=1.49±0.20b; %Emax=77±8b), in normal Krebs containing L-NAME (10 μM; open triangles; pD2=5.81±0.12; nH=0.53±0.02; %Emax=9±1; P<0.05b), and (b) from salt-resistant normotensive rats in chloride-free buffer (Cl.Free) plus twice-distilled water (closed circles), in chloride-free buffer containing L-NAME (10 μM; open circles; nH=0.56±0.09; %Emax=14±3; P<0.05b), or from salt-sensitive hypertensive rats in chloride-free buffer plus twice-distilled water (closed triangles), and in chloride-free buffer containing L-NAME (10 μM; open triangles; nH=0.43±0.09; %Emax=12±1; P<0.05b). Each point represents a mean of six experiments±s.e.m.
Functional studies in denuded pulmonary arteries
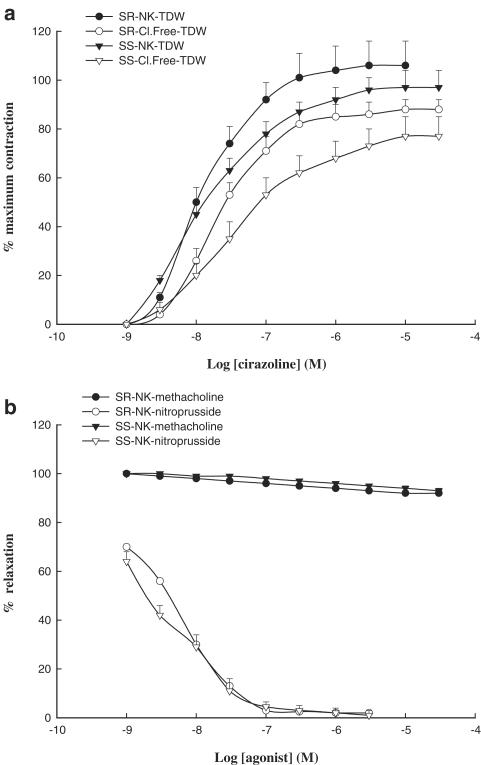
In the absence of chloride ions, contractile responses to cirazoline were attenuated in blood vessels obtained from both normotensive (%Emax: 86±5 vs 103±11; mean±s.e.m.; n=6) and hypertensive (%Emax: 73±8 vs. 94±4; mean±s.e.m.; n=6) animals, as evidenced by a significant (P<0.05) reduction in maximal response to cirazoline (Figure 3a).
Figure 3.
(a) Concentration–contraction curves for cirazoline in endothelium-denuded pulmonary artery rings from DSR normotensive rats in normal Krebs (NK) plus twice-distilled water (TDW; closed circles), in chloride-free buffer (Cl.Free) plus twice-distilled water (open circles), or from DSS hypertensive rats in normal Krebs plus twice-distilled water (closed triangles), chloride-free buffer plus twice-distilled water (open triangles). (b) Concentration–response curves for methacholine in endothelium-denuded pulmonary artery rings from salt-resistant normotensive (closed circle) or salt-sensitive hypertensive (closed triangles) rats, and concentration–relaxation curves for sodium nitroprusside in endothelium-denuded pulmonary artery rings from salt-resistant normotensive rats (open circles; pD2=8.4±0.05; nH=0.76±0.06; n=6; mean±s.e.m.), and salt-sensitive hypertensive (open triangles; pD2=8.7±0.09; nH=0.81±0.09; n=6; mean±s.e.m.) in normal Krebs solution. Each point represents a mean of six experiments±s.e.m.
Removal of the endothelium abolished methacholine-mediated relaxation, but not that induced by sodium nitroprusside (Figure 3b). The vasorelaxant effects of sodium nitroprusside were of the same magnitude and sensitivity in blood vessels from hypertensive and normotensive rats (Figure 3b).
cGMP levels in pulmonary arteries
The basal levels of cGMP in tissue from DSR salt normotensive and DSS hypertensive rats were 166.2±12.2 and 21.3±1.8 pmol per mg protein (mean±s.e.m.; n=7), respectively. The basal levels of cGMP were found to be significantly (P<0.05) lower in the pulmonary arteries of hypertensive when compared to normotensive rats.
Membrane potential studies
The resting membrane potential of smooth muscle cells in pulmonary arteries of DSR normotensive rats (−46.4±1.58 mV; mean±s.e.m.; n=73 cells; n=25 animals) was significantly (P<0.05) different from that of DSS hypertensive rats (−52.1±1.04 mV; mean±s.e.m.; n=73 cells; n=25 animals).
Exposure of blood vessels of salt-resistant normotensive and salt-sensitive hypertensive rats to Krebs buffer containing cirazoline produced a modest (+5.4 and +6.5 mV, respectively), but insignificant positive shift in the membrane potential (Table 3). When blood vessels from either normotensive or hypertensive rats were bathed in Krebs containing either methacholine or L-NAME, membrane potentials remained virtually unchanged (Table 3). Upon exposure to chloride-free solution, smooth muscle cells of pulmonary arteries from salt-resistant normotensive rats showed an insignificant hyperpolarisation (−5.5 mV), while those of salt-sensitive hypertensive rats became significantly hyperpolarised by approximately −8.0 mV (Table 3).
Table 3.
Membrane potential (Em) in vascular smooth muscle cells of pulmonary artery ring preparation with intact endothelium
| Em (mV) | ||
|---|---|---|
| Group | DSR | DSS |
| No cirazoline (NK) | −46.50±3.74 (15) (6) | −56.50±2.23 (15) (6) |
| Cirazoline (0.3 μM) (NK) | −41.10±2.93 (15) (6) | −49.50±4.00 (15) (6) |
| No methacholine (NK) | −45.52±3.19 (11) (6) | −50.00±2.11 (9) (8) |
| Methacholine (1.0 μM) (NK) | −47.00±3.27 (11) (6) | −50.00±2.09 (9) (8) |
| No L-NAME (NK) | −46.80±5.15 (6) (3) | −50.10±3.11 (8) (6) |
| L-NAME (10 μM) (NK) | −42.45±4.88 (6) (3) | −52.10±1.04 (8) (6) |
| NK | −45.50±2.59 (11) (9) | −52.00±2.18 (11) (5) |
| w/o Cl | −51.00±4.53 (11) (9) | −61.06±2.70a (11) (5) |
Each value represents the geometric mean±s.e.m., with the number of cells and number of animals indicated in parentheses. Dahl salt-resistant normotensive (DSR); Dahl hypertensive salt-sensitive (DSS); normal Krebs (NK); chloride-free buffer (w/o Cl).
Significantly different from NK within their respective group; P<0.05.
Discussion
As shown in this study, chloride removal does not produce uniform changes in the mechanical and electrical responsiveness of pulmonary artery smooth musculature. Dichotomous effects were observed depending on the presence of salt-induced systemic hypertension. These effects did not result from alteration in α1-agonist-induced active force generation or muscarinic agonist-evoked relaxation; rather, they point to changes in the interaction between vascular endothelium and smooth muscle as the underlying mechanism. Based on the differences in basal levels of cGMP, it is evident that there is significant reduction in levels of NO in the pulmonary arteries of hypertensive vs normotensive rats.
α1-agonist force generation in salt-sensitive hypertensive and salt-resistant normotensive rats
We have previously reported that left ventricular and vascular hypertrophy (in systemic blood vessels) occurs in DSS hypertensive rats on a salt diet following development of hypertension (Tabrizchi & Duggan, 2000; Duggan & Tabrizchi, 2002). Changes in their functional behaviour are associated with morphological changes in systemic blood vessels (Boegehold, 1992; Sudir et al., 1993; Nishida et al., 1998; Duggan & Tabrizchi, 2002). As demonstrated in the present investigation, the development of the salt-induced hypertensive state was not accompanied by an altered capability of the low-pressure pulmonary vascular bed to generate active force. Indeed, the responsiveness of rings from DSS hypertensive rats was not increased, despite the finding of lower cGMP levels. This anomaly suggests a secondary alteration in function that prevents the development of increased responsiveness or increased maximal force generation, such as receptor desensitisation. However, discrete functional changes were evident in this segment of the circulation. Thus, the presence of the nitric oxide synthase inhibitor L-NAME significantly potentiated mechanical force development elicited by the activation of α1-adrenoceptors in isolated blood vessels obtained from salt-resistant normotensive rats, whereas no such effects were observed with the same protocol in blood vessels obtained from salt-sensitive hypertensive rats. Contrary to expectation, mechanical removal of the endothelium failed to duplicate the effects of L-NAME on contractility. Inadvertent damage to the smooth muscle layer appears to be an inadequate explanation, because endothelium-denuded tissue from DSR normotensive and DSS hypertensive rats behaved in an identical manner. An alternative explanation is that L-NAME treatment may produce effects unrelated to NOS inhibition. Despite these caveats, it appears reasonable to infer that an L-NAME-sensitive process plays a critical role in modulating the actions of cirazoline in pulmonary arteries of DSR normotensive rats, but this mechanism seems to be inoperative in DSS hypertensive rats.
It is recognised that α-adrenoceptor-mediated depolarisation in blood vessels is linked to stimulation of a calcium-activated chloride current subsequent to the release of intracellular calcium, resulting in depolarisation and the opening of L-type calcium channels leading to contraction (for a review, see Large & Wang, 1996). In the present study, we would have expected the role of chloride ions to be diminished in chloride-free medium, leading to an attenuation of cirazoline-mediated contraction (He & Tabrizchi, 1997). Indeed, chloride ion removal resulted in an inhibitory effect that, however, was confined to pulmonary arteries of endothelium-denuded vessels of either strain and endothelium-intact vessels of salt-sensitive hypertensive rats. These observations are consistent with an impairment of pulmonary arterial endothelial cell function in salt-sensitive hypertensive rats. Contrary to expectation, nitric oxide synthase inhibition with L-NAME reversed the depressant effect of chloride removal on cirazoline contractions in salt-sensitive hypertensive rats. Barring other non-nitric oxide synthase actions of L-NAME, the re-appearance of an L-NAME-sensitive component after chloride removal implies the presence of a ‘basal' nitric oxide release from an endothelium abnormally responsive to chloride ions.
Endothelium-dependent and -independent relaxation in salt-sensitive hypertensive and salt-resistant normotensive rats
Since endothelium-dependent relaxations by methacholine and endothelium-independent relaxations by sodium nitroprusside showed comparable magnitude and sensitivity in blood vessels of DSS hypertensive rats relative to DSR normotensive rats, we infer that neither endothelium-dependent relaxation nor relaxation per se was affected by the development of hypertension in this conduit blood vessel. This contrasts with evidence obtained in aortic rings where endothelium-dependent but not endothelium-independent relaxations were found to be impaired in DSS hypertensive rats (Lüscher et al., 1987a). Our present findings could be taken to suggest that high pressure (i.e., mechanical stress impinging on the aortic luminal surface) is a major determinant in altering endothelium-dependent relaxation in systemic arterial vessels of DSS hypertensive rats. However, it is clear that mechanical stress (such as increased intraluminal pressure in the pulmonary artery) is not the major factor in impeding basal endothelial cell function in Dahl hypertensive rats. Thus, our present observations indicate a significant reduction in basal cGMP levels in the low-pressure bed (i.e., the pulmonary artery) of hypertensive rats.
Vascular electrical response in salt-sensitive hypertensive and salt-resistant normotensive rats
Surprisingly, the resting membrane potential of vascular smooth muscle cells in the pulmonary arteries of salt-sensitive hypertensive rats was significantly more negative than that of salt-resistant normotensive rats. There is conflicting evidence regarding alterations in the membrane potential of vascular smooth muscle cells of arteries from DSS hypertensive and DSR normotensive rats. It has been suggested that there is no significant difference between the resting membrane potential of vascular smooth muscle cells in caudal arteries (Abel et al., 1981) and mesenteric arteries (Fujii et al., 1997) of DSS hypertensive and DSR normotensive rats, respectively. However, more recently, Wellman et al. (2001) have reported that the resting membrane potential of vascular smooth muscle cells of cerebral arteries of DSS hypertensive rats (−36.8 mV) was significantly more depolarised relative to that of DSR normotensive rats (−49.7 mV). The reason for our divergent observation that vascular smooth muscle cells are more hyperpolarised in pulmonary blood vessels of hypertensive rats vs normotensive rats is unclear. The absence of increased mechanical stress in the pulmonary circulation would implicate humoral/hormonal factors that act to alter vascular smooth muscle function in the low-pressure bed, possibly by promoting hyperpolarisation in the pulmonary bed.
Our observations support the view that the actions of both cirazoline (contraction) and methacholine (relaxation) occur in the absence of significant changes in the resting membrane potential. It is believed that endothelium-dependent relaxation in blood vessels involves the release of nitric oxide, prostacyclin and/or hyperpolarising factor(s) (Busse et al., 2002). In our present investigation, the endothelium-dependent hyperpolarising factor(s) did not seem to play a role in methacholine-induced relaxation in blood vessels from either Dahl salt-insensitive normotensive or salt-sensitive hypertensive rats. Consistent with this interpretation, methacholine-induced relaxations were nearly completely abolished in the presence of L-NAME. In addition, it is clear that the nitric oxide synthase inhibitor had no obvious effects on the resting membrane potential of vascular smooth muscle cells in these blood vessels. Therefore, it would seem unlikely that basal tonic release of nitric oxide has an influence on the resting membrane potential of vascular smooth muscle cells in pulmonary arteries of either DSS hypertensive or DSR normotensive rats. Clearly, this inference agrees with the finding that neither methacholine nor L-NAME had a significant effect on the membrane potential of smooth muscle cells.
The issue of agonist-induced changes in the membrane potential of vascular smooth muscle cells remains controversial. For example, it has been shown in the rabbit pulmonary artery that noradrenaline-mediated contraction occurs without a significant change in the membrane potential of the smooth muscle cells (Su et al., 1964). In addition, 5-hydroxytryptamine has also been reported to induce contraction in pig coronary arteries without any change in the membrane potential of smooth muscle cells (Frieden & Bény, 1995). In contrast, Haeusler (1978) and Haeusler & De Peyer (1989) have demonstrated using rabbit pulmonary arteries and aorta that noradrenaline induced a depolarisation of ∼16 mV. The modest depolarisation induced by the selective α1-adrenoceptor agonist cirazoline in the present investigation was too variable to reach statistical significance. In our present investigation, we cannot readily rule out the occurrence of significant transient depolarisation in pulmonary arteries of Dahl rats immediately following the application of cirazoline.
Electrical responses to extracellular chloride removal
Removal of chloride ions from the extracellular space should acutely move the chloride equilibrium potential towards zero and thus accentuate membrane depolarisation, assuming chloride channel opening is induced by cirazoline. After the chloride gradient dissipates, its contribution to the membrane potential should become minimal. Chloride-free buffer did not produce any depolarising effect on the membrane potential at time intervals of 20–30 min after chloride washout. Instead, cells showed a modest hyperpolarisation that reached significance although this was seen only in vessels from hypertensive salt-sensitive rats. Thus, in this tissue, transmembrane chloride ion gradients do not appear to make a theoretically predicted contribution to membrane potential. It is recognised that both sodium and chloride ions exert a depolarising influence on the resting membrane potential in smooth muscle cells (for a review, see Chipperfield & Harper, 2000). Furthermore, the depolarising effects of chloride ions on membrane potential appear to be greatly enhanced by active accumulation of chloride ions inside smooth muscle cells (for a review, see Chipperfield & Harper, 2000). However, hypertension induced by salt in Dahl rats reportedly does not result in redistribution of ions (e.g. Na+, K+ or Cl−) across the extracellular and intracellular space in vascular smooth muscles (Abel et al., 1981).
Increased chloride ion permeability has been implicated in vascular smooth muscle cell depolarisation. For instance, in the rat femoral artery, noradrenaline has been found to depolarise the smooth muscle cells from −64 to −54 mV, this depolarisation being accompanied by an increase in intracellular concentration of chloride from 31 to 43 mM (Davis et al., 1997). Moreover, in a chloride-free medium, the noradrenaline-induced depolarisation was found to be abolished (Davis et al., 1997). As shown here in the rat pulmonary artery, removal of chloride resulted in significant hyperpolarisation of vascular smooth muscle (by 6–8 mV) and attenuation of cirazoline-induced contractility in salt-sensitive hypertensive, but not salt-resistant normotensive Dahl rats.
In conclusion, our data provide evidence for functional and biochemical changes that are manifested in salt-induced hypertension in the Dahl rat and involve the pulmonary low-pressure vasculature. Some of these changes appear attributable to impaired basal, but not methacholine-stimulated, release of nitric oxide and a modified responsivity to chloride ion removal. The chloride removal-induced decrease in contractility may represent an indicator of altered endothelial function in salt-induced hypertension.
Acknowledgments
This work was supported by a grant-in-aid from the Natural Sciences and Engineering Research Council of Canada. We wish to thank Carol Ann Ford for technical assistance.
Abbreviations
- BW
body weight
- cGMP
3′,5′-cyclic monophosphate
- DBP
diastolic blood pressure
- DSR
Dahl salt-resistant
- DSS
Dahl salt-sensitive
- Em
membrane potential
- %Emax
percent maximum response
- EC50
effective concentration (molar) of agonist producing 50% of maximal response
- HR
heart rate
- LK
left kidney
- L-NAME
nitro-L-arginine methyl ester
- LVS
left ventricle plus septum
- MPAP
mean pulmonary artery pressure
- nH
Hill coefficient
- N
Newton
- Ω
Ohm
- pD2
negative log of EC50 for an agonist
- RK
right kidney
- RV
right ventricle
- SBP
systolic blood pressure
- V
volt
References
- ABEL P.W., TRAPANI A., MATSUKI N., INGRAM M.J., INGRAM F.D., HERMSMEYER K. Unaltered membrane properties of arterial muscle in Dahl strain genetic hypertension. Am. J. Physiol. 1981;241:H224–H227. doi: 10.1152/ajpheart.1981.241.2.H224. [DOI] [PubMed] [Google Scholar]
- BOEGEHOLD M.A. Reduced influence of nitric oxide on arteriolar tone in hypertensive Dahl rats. Hypertension. 1992;19:290–295. doi: 10.1161/01.hyp.19.3.290. [DOI] [PubMed] [Google Scholar]
- BOULANGER C.M. Secondary endothelial dysfunction: hypertension and heart failure. J. Mol. Cell. Cardiol. 1999;31:39–49. doi: 10.1006/jmcc.1998.0842. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FÉLÉTOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concept together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CHIPPERFIELD A.R., HARPER A.A. Chloride in smooth muscle. Prog. Biophys. Mol. Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- CRIDDLE D.N., DE MOURA R.S., GREENWOOD I.A., LARGE W.A. Effect of niflumic acid on noradrenaline-induced contraction of the rat aorta. Br. J. Pharmacol. 1996;118:1065–1071. doi: 10.1111/j.1476-5381.1996.tb15507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDDLE D.N., DE MOURA R.S., GREENWOOD I.A., LARGE W.A. Inhibitory action of niflumic acid on noradrenaline- and 5-hydroxytryptamine-induced pressor responses in the isolated mesenteric vascular bed of rat. Br. J. Pharmacol. 1997;120:813–818. doi: 10.1038/sj.bjp.0700981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS J.P.L., HARPER A.A., CHIPPERFIELD A.R. Stimulation of intracellular chloride accumulation by noradrenaline potentiates its depolarisation of rat arterial smooth muscle in vitro. Br. J. Pharmacol. 1997;122:639–642. doi: 10.1038/sj.bjp.0701431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPLY E., COSTE H., GOUVILLE A.C. Effects of cyclic GMP elevation on isoprenaline-induced increase in cyclic AMP and relaxation in rat aortic smooth muscle: role of phosphodiesterase 3. Br. J. Pharmacol. 1996;119:471–478. doi: 10.1111/j.1476-5381.1996.tb15696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGGAN J.A., TABRIZCHI R. Effect of nitric oxide synthase inhibitor, Nω nitro-L-arginine methyl ester on relaxant responses to calcium channel antagonists in isolated aortic rings from Dahl normotensive ad hypertensive rats. J. Cardiovasc. Pharmacol. 2002;39:354–362. doi: 10.1097/00005344-200203000-00006. [DOI] [PubMed] [Google Scholar]
- FRIEDEN M., BÉNY J.-L. Effect of 5-hydroxytryptamine on the membrane potential of endothelial and smooth muscle cells in the pig coronary artery. Br. J. Pharmacol. 1995;115:95–100. doi: 10.1111/j.1476-5381.1995.tb16325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., ONAKA U., OHYA Y., OHMORI J., TOMINAGA M., ABE I., TAKATA Y., FUJISHIMA M. Role of eicosanoids in alteration of membrane electrical properties in isolated mesenteric arteries of salt-loaded, Dahl salt-sensitive rats. Br. J. Pharmacol. 1997;120:1207–1214. doi: 10.1038/sj.bjp.0701023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSTHEIMER F.P., MÜHLEISEN M., NEHRING D., KREYE V.A.W. A chloride–bicarbonate exchanging anion carrier in vascular smooth muscle of the rabbit. Pflügers Arch. 1987;409:60–66. doi: 10.1007/BF00584750. [DOI] [PubMed] [Google Scholar]
- HAEUSLER G. Relationship between noradrenaline-induced depolarization and contraction in vascular smooth muscle. Blood Vessels. 1978;15:46–54. doi: 10.1159/000158152. [DOI] [PubMed] [Google Scholar]
- HAEUSLER G., DE PEYER J.-E. Rabbit aorta: electrical properties and agonist-induced depolarization. Eur. J. Pharmacol. 1989;166:175–182. doi: 10.1016/0014-2999(89)90057-5. [DOI] [PubMed] [Google Scholar]
- HE Y., TABRIZCHI R. Effects of niflumic acid on α1-adrenoceptor-induced vasoconstriction in mesenteric artery in vitro and in vivo in two-kidney one-clip hypertensive rats. Eur. J. Pharmacol. 1997;328:191–199. doi: 10.1016/s0014-2999(97)83045-2. [DOI] [PubMed] [Google Scholar]
- LAMB F.S., BARNA T.J. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. Am. J. Physiol. 1998a;275:H151–H160. doi: 10.1152/ajpheart.1998.275.1.H151. [DOI] [PubMed] [Google Scholar]
- LAMB F.S., BARNA T.J. The endothelium modulates the contribution of chloride currents to norepinephrine-induced vascular contraction. Am. J. Physiol. 1998b;275:H161–H168. doi: 10.1152/ajpheart.1998.275.1.H161. [DOI] [PubMed] [Google Scholar]
- LARGE W.A., WANG Q. Characteristics and physiological role of the Ca2+ activated Cl− conductance in smooth muscle. Am. J. Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- LEE R.M. Structural alterations of blood vessels in hypertensive rats. Can. J. Physiol. Pharmacol. 1987;65:1528–1535. doi: 10.1139/y87-241. [DOI] [PubMed] [Google Scholar]
- LEE R.M., TRIGGLE C.R. Morphometric study of mesenteric arteries from genetically hypertensive Dahl strain rats. Blood Vessels. 1986;23:199–224. doi: 10.1159/000158642. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., RAIJ L., VANHOUTTE P.M. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension. 1987a;9:157–163. doi: 10.1161/01.hyp.9.2.157. [DOI] [PubMed] [Google Scholar]
- LüSCHER T.F., VANHOUTTE P.M., RAIJ L. Antihypertensive treatment normalizes decreased endothelium-dependent relaxation in rats with salt-induced hypertension. Hypertension. 1987b;9 Part 2:III-193–III-197. doi: 10.1161/01.hyp.9.6_pt_2.iii193. [DOI] [PubMed] [Google Scholar]
- NISHIDA Y., DING J., ZHOU M.S., CHEN Q.H., MURAKAMI H., WU X.Z., KOSAKA H. Role of nitric oxide in vascular hyper-responsiveness to norepinephrine in hypertensive Dahl rats. J. Hypertens. 1998;16:1611–1618. doi: 10.1097/00004872-199816110-00007. [DOI] [PubMed] [Google Scholar]
- PARAI K., TABRIZCHI R. A comparative study of the effects of Cl− channel blockers on mesenteric vascular conductance in anaesthetized rat. Eur. J. Pharmacol. 2002;448:59–66. doi: 10.1016/s0014-2999(02)01895-2. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J.A., URSILLO R.C. Electrical quiescence of pulmonary artery smooth muscle during sympathomimetic stimulation. Circ. Res. 1964;15:20–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- SUDIR K., KURTZ T.W., YOCK P.G., CONNOLLY A.J., MORRIS R.C.J.R. Potassium preserves endothelial function and enhances aortic compliance in Dahl rats. Hypertension. 1993;22:315–322. doi: 10.1161/01.hyp.22.3.315. [DOI] [PubMed] [Google Scholar]
- TABRIZCHI R., DUGGAN J.A. The interrelationship between chloride ions and endothelium on α1-adrenoceptor-mediated contractions in aortic rings from Dahl normotensive and hypertensive rats. Cardiovasc. Res. 2000;48:393–401. doi: 10.1016/s0008-6363(00)00193-0. [DOI] [PubMed] [Google Scholar]
- TABRIZCHI R., MACNICOL B.J., LIN B.P. Effects of calcium channel antagonists and pertussis toxin on noradrenaline-induced contractions in pulmonary artery from pulmonary hypertension rats. Life Sci. 1995;56:1173–1185. doi: 10.1016/0024-3205(95)00056-c. [DOI] [PubMed] [Google Scholar]
- WELLMAN G.C., CARTIN L., ECKMAN D.M., STEVENSON A.S., SAUNDRY C.M., LEDERER W.J., NELSON M.T. Membrane depolarization, elevated Ca2+ entry, and gene expression in cerebral arteries of hypertensive rats. Am. J. Physiol. 2001;281:H2559–H2567. doi: 10.1152/ajpheart.2001.281.6.H2559. [DOI] [PubMed] [Google Scholar]