Abstract
Gastro-oesophageal acid reflux may cause airway responses such as cough, bronchoconstriction and inflammation in asthmatic patients. Our previous results suggest that microvascular leakage induced, in the guinea-pig airways, by intra-oesophageal hydrochloric acid (HCl) infusion was mainly dependent on the release of tachykinins. Nociceptin, an endogenous ligand of the opioid receptor NOP, has been shown to inhibit bronchoconstriction and cough in guinea-pig or cat by inhibiting tachykinin release.
The purpose of this study was to investigate the effects of nociceptin on the intra-oesophageal HCl-induced airway microvascular leakage evaluated by Evans blue dye extravasation measurement in anaesthetised guinea-pigs pretreated with propranolol, atropine and phosphoramidon.
Infusion of intra-oesophageal HCl led to a significant increase in plasma extravasation in the main bronchi and trachea. This increase was abolished when animals underwent a bilateral vagotomy.
Airway microvascular leakage was inhibited by nociceptin (3–30 μg kg−1 i.v.) in a dose-dependent manner (maximal inhibition at the dose of 30 μg kg−1: 19.76±1.13 vs 90.92±14.00 ng mg−1 tissue for nociceptin and HCl infusion, respectively, in the main bronchi, P<0.01). The NOP receptor agonist [Arg14,Lys15]N/OFQ mimicked the inhibitory effect of nociceptin, but at a 10-fold lower dose (3 μg kg−1 i.v). The NOP receptor antagonist J-113397 had no effect on plasma protein extravasation by itself, but was able to block the inhibitory effect of nociceptin.
Morphine (1 mg kg−1) had a similar inhibitory effect as that of nociceptin. Naloxone pretreatment abolished the effect of morphine, but was enable to block the inhibitory effect of nociceptin.
Under similar conditions, nociceptin, in the previous range of concentration, was unable to counteract the airway microvascular leakage induced by substance P (SP).
These results suggest that airway plasma extravasation induced by intra-oesophageal HCl instillation might be inhibited by specific stimulation of the NOP receptor with nociceptin. Nociceptin is likely to act at a pre-junctional level, by inhibiting tachykinin release, since it was unable to prevent SP-induced airway plasma extravasation.
Keywords: Nociceptin, NOP receptor, airways, inflammation, microvascular leakage
Introduction
Gastro-oesophageal reflux disease (GORD) is a common clinical disorder associated with a variety of respiratory symptoms, including bronchoconstriction and chronic cough (Spaulding et al., 1982; Irwin et al., 1989; Ing et al., 1991). It has been shown that GORD is more frequent in asthmatic patients (Harding & Sontag, 2000), and that drugs which inhibit acid secretion, such as proton pump inhibitors, are able to improve the peak expiratory flow in GORD-related asthmatic patients (Tsugeno et al., 2003). Experimental studies have shown that oesophageal hydrochloric acid (HCl) perfusion induces a slight bronchoconstriction in dogs (Mansfield et al., 1981), cats (Tuchman et al., 1984) and rabbits (Gallelli et al., 2003), as well as in asthmatic patients (Spaulding et al., 1982; Hervé et al., 1986). Oesophageal HCl perfusion also induces airway microvascular leakage in guinea-pigs and rabbits (Hamamoto et al., 1997; Daoui et al., 2002; Gallelli et al., 2003).
The mechanisms by which GORD might enhance asthmatic symptoms remain unclear. A reflux theory based on the microaspirations of acid contents in the airways has been suggested. Although instillation of acid into the airways produces bronchospasm in animals (Tuchman et al., 1984; Ishikawa et al., 1999; Ricciardolo, 2001), pulmonary aspiration of refluxed stomach content has not been convincingly demonstrated in clinical or isotopic studies of large groups of asthmatic patients with gastro-oesophageal reflux (Ghaed & Stein, 1979; Berquist et al., 1981). A reflex theory involving vagus and sensory nerves has also been suggested (Mansfield & Stein, 1978; Kjellen et al., 1981; Hervé et al., 1986). The role of vagus nerves and of cholinergic transmission has been demonstrated in both animals and humans. In dogs (Mansfield & Stein, 1978; Mansfield et al., 1981) and guinea-pigs (Hamamoto et al., 1997; Advenier et al., 2002), both bilateral vagotomy and atropine treatment inhibit airway resistance increase or microvascular leakage induced by oesophagus HCl infusion. Colson et al. (1990) and Hervé et al. (1986) found that, in asthmatic patients, reduction in forced expiratory volume in 1 s or maximal expiratory flow at 50% of the vital capacity and oxygen saturation induced by oesophageal acid was abolished by atropine pretreatment. In animals, sensory nerves and tachykinins are involved in the airway effects of HCl infusion. Indeed, airway plasma extravasation or pulmonary resistance increase induced by intra-oesophageal HCl infusion in guinea-pigs or rabbits are abolished either by a pretreatment with capsaicin, which induces a depletion of tachykinins from sensory nerves, or by treatment with neurokinin NK1 (FK 888, SR 140333) or NK3 (SR 142801) receptor antagonists (Hamamoto et al., 1997; Daoui et al., 2002; Gallelli et al., 2003).
Nociceptin (Meunier et al., 1995; Reinscheid et al., 1995), a 17 amino-acid peptide, is the endogenous ligand for the opioid receptor-like 1 receptor (Mollereau et al., 1994), recently named NOP receptor, which has an overall 60% homology with the classical MOP, DOP and KOP opioid receptors. The NOP receptor contains seven transmembrane domains and is a member of the Gi/o-coupled receptor superfamily. The cellular mechanisms of action of nociceptin (activation of K+ and inhibition of Ca2+ channels, inhibition of cyclic AMP production) are probably the same as for other opioid receptors (Meunier, 1997). In vivo experiments have demonstrated that nociceptin is involved in a wide variety of biological functions such as nociception, anxiety and memory processes, food intake, regulation of arterial blood pressure and spontaneous locomotor activity (Calò et al., 2000). In the airways, nociceptin was found to inhibit the contractions of the guinea-pig isolated bronchus induced by electrical field stimulation (EFS) (Fischer et al., 1998), an effect that is mediated by a pre-junctional mechanism not involving the classical opioid receptors. Other studies performed in guinea-pigs showed that nociceptin inhibits acetylcholine release in the trachea (Patel et al., 1997) and capsaicin-induced bronchoconstriction in isolated lungs (Corboz et al., 2000). In vivo, nociceptin was found to inhibit cough provoked by capsaicin in guinea-pig (McLeod et al., 2001) or by mechanical stimulation of intra-thoracic airways in the cat (Bolser et al., 2001). Taken altogether, these studies demonstrate that nociceptin may influence airway physiology by modulating cholinergic and/or tachykinergic neurotransmission.
The purpose of the present study was to investigate the effect of NOP receptor stimulation by nociceptin on airway microvascular leakage induced by intra-oesophageal HCl instillation in guinea-pigs.
Methods
Animal preparation
The study was approved by the committee of animal use and care of the university. Male Hartley guinea-pigs (N=97) weighing 250–350 g were used throughout this study. Animals were anaesthetised intraperitoneally with urethane (1.25 g kg−1). Additional injections were given as necessary. The left jugular vein was cannulated for injection of drugs. At 30 min before experimentation, animals were pretreated with atropine (3 mg kg−1 i.p.), propranolol (1 mg kg−1 i.p.) to block muscarinic and β-adrenergic receptors, and phosphoramidon (1 mg kg−1 i.p.) to reduce tachykinin metabolism. The oesophageal wall was partly sectioned at the level of the third to fifth tracheal cartilage ring and a catheter was placed in the mid-oesophagus. The oesophagus was ligated at the upper portion to avoid HCl leakage. The lower end of the oesophagus was then exposed from the abdomen and ligated to block communication between the oesophagus and the stomach. Thus, there was no leakage of fluid from the oesophagus.
Measurement of airway microvascular leakage
Vascular permeability was quantified by the extravasation of Evans blue dye (Rogers et al., 1988). Evans blue dye (30 mg kg−1) was injected into the jugular vein, followed 1 min later by intra-oesophageal HCl (1 N, 0.4 ml) or saline (0.9%) infusion. Previous experiments have determined HCl 1 N to produce the maximum of Evans blue extravasation (Daoui et al., 2002). At 10 min after completion of the intra-oesophageal HCl or saline infusion, the thorax was opened up. Animals were perfused with phosphate buffer via the left ventricle. The lungs were then removed. The connective tissues, vasculature and parenchyma were gently scraped away and the airways were divided into two components, trachea and main bronchi (Rogers et al., 1988). The tissues were blotted dry, weighed and their dye content was extracted in formamide at 37°C for 18 h. Dye concentration was quantified by light absorbance at 620 nm (DCP spectrophotometer; Vital, Dieren, Netherlands), and tissue content (ng dye mg−1 wet weight tissue) was calculated from a standard curve of dye concentrations in the 0.5–10 μg ml−1 range.
Evaluation of the effect of bilateral vagotomy on HCl-induced airway plasma protein extravasation
Vagus nerves were cut bilaterally at the fourth tracheal cartilage level. After 5 min, Evans blue dye (30 mg kg−1) was injected intravenously. After 1 min, 1 N HCl (0.4 ml) was infused into the oesophagus for 10 min and then the Evans blue dye leakage was measured.
Measurement of the effect of nociceptin on HCl-induced airway microvascular leakage
To evaluate the effect of nociceptin on airway plasma extravasation, either nociceptin (3, 10 and 30 μg kg−1) or its saline vehicle was injected intravenously 1 min before Evans blue dye. In similar conditions, a NOP receptor agonist, [Arg14,Lys15]N/OFQ, or its saline vehicle was tested (0.3 and 3 μg kg−1). In complementary experiments, J-113397, a nonpeptide NOP receptor antagonist (Kawamoto et al., 1999; Bigoni et al., 2000; Ozaki et al., 2000), or its vehicle (see Materials) was injected at the dose of 3 mg kg−1 10 min before nociceptin injection. In another set of experiments, nociceptin effect was compared to that of morphine: a single dose of morphine (1 mg kg−1) or its vehicle (see Materials) was administered intravenously 10 min before dye infusion. The MOP, DOP and KOP receptor antagonist naloxone (3 mg kg−1 i.v) or its saline vehicle were given 10 min before morphine (1 mg kg−1) or 19 min before nociceptin (30 μg kg−1) injection.
Evaluation of nociceptin effect on substance P (SP)-induced airway microvascular leakage
In order to assess the mechanism of action of nociceptin, an SP-induced airway plasma extravasation was performed. SP (0.3 μg kg−1) was injected intravenously 5 min before Evans blue dye injection. Leakage was measured 10 min later. Nociceptin was used at the dose of 30 μg kg−1.
Materials
Atropine sulphate, Evans blue dye, formamide, naloxone, nociceptin, phosphoramidon and propranolol were obtained from Sigma, St Quentin Fallavier, France. HCl and urethane were obtained from Prolabo, Paris, France. SP was obtained from Bachem, Voisins-le-Bretonneux, France. The following drugs were dissolved in 0.9% NaCl: [Arg14,Lys15]N/OFQ, which was a gift from Dr Calò and Pr Regoli (University of Ferrara, Italy), atropine sulphate, Evans blue dye, naloxone, nociceptin, phosphoramidon, propranolol and SP. Morphine was dissolved in a mixture of HCl 1 N, ethanol and saline (1/12/87). Morphine and J-113397 were gifts from Dr Emonds-Alt, (Sanofi-Synthelabo Recherche, Montpellier, France). The chemical name of J-113397 is 1-[(3R,4R)]-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one. This compound was dissolved in a mixture of HCl 1 N, ethanol and saline (10/10/80).
Statistical analysis
Data are expressed as means±s.e.m. Statistical analysis of the results was performed by using Student's t-test for unpaired data. Differences among groups were considered significant when P<0.05.
Results
Effect of bilateral vagotomy on airway plasma extravasation induced by oesophageal stimulation with HCl
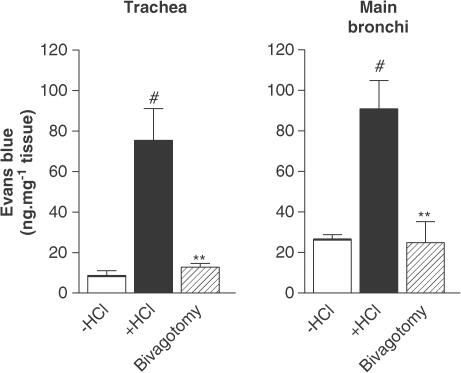
Infusion of 1 N HCl into the oesophagus significantly increased plasma extravasation in the trachea (8.74±2.31 vs 75.48±15.64 ng mg−1 tissue for saline and HCl infusion respectively, P<0.01) and main bronchi (24.68±2.02 vs 90.92±14.00 ng mg−1 tissue for saline and HCl infusion, P<0.01) (Figure 1). Plasma extravasation was significantly reduced after bilateral vagotomy performed 5 min before HCl infusion (Figure 1).
Figure 1.
Effect of intra-oesophageal HCl instillation on plasma protein extravasation in guinea-pig trachea and main bronchi. Comparison with the effect of bilateral vagotomy. Guinea-pigs were pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). Data are expressed as mean±s.e.m., n=3–4 per group. #P<0.01 vs −HCl, **P<0.01 vs +HCl.
Effect of NOP receptor activation on HCl-induced plasma extravasation. Comparison with morphine
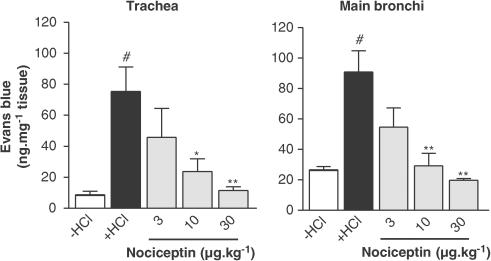
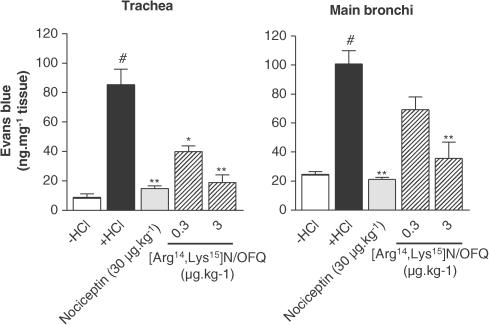
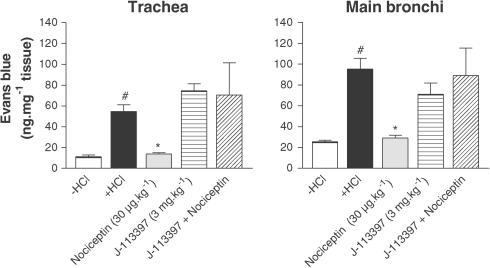
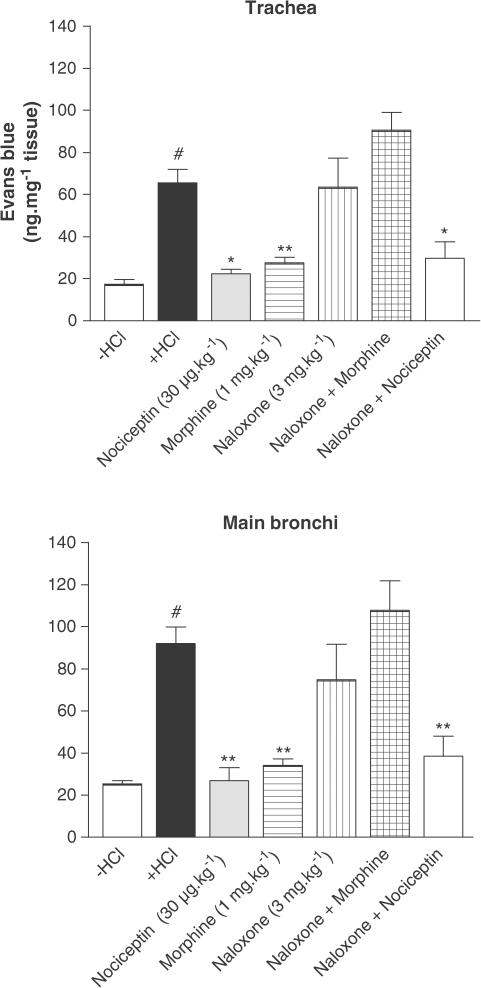
Nociceptin decreased the microvascular leakage in a dose-dependent manner, in the trachea and main bronchi (Figure 2). Maximal inhibition was reached at the dose of 30 μg kg−1 (19.76±1.13 vs 90.92±14.00 ng mg−1 tissue for nociceptin and HCl infusion, respectively, in the main bronchi, P<0.01) (Figure 2). The effect of [Arg14,Lys15]N/OFQ, a NOP receptor agonist, was tested on the model (Figure 3). This agonist produced an effect that was equivalent to that of nociceptin, but at a 10-fold lower dose (3 μg kg−1) than nociceptin. The NOP receptor antagonist J-113397 (3 mg kg−1) had no effect by itself on HCl-induced microvascular leakage, but was able to block the inhibitory effect of nociceptin (Figure 4). The inhibitory effect of nociceptin was mimicked by a dose of 1 mg kg−1 of morphine. This effect was abolished by naloxone (MOP, DOP and KOP receptor antagonist) pretreatment, whereas nociceptin effect was insensitive to such treatment (Figure 5).
Figure 2.
Effect of nociceptin (3–10 or 30 μg kg−1) on the microvascular leakage induced by intra-oesophageal HCl infusion in guinea-pig trachea and main bronchi. Guinea-pigs were pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). Data are expressed as mean±s.e.m., n=3–4 per group. #P<0.01 vs −HCl, *P<0.05 vs +HCl, **P<0.01 vs +HCl.
Figure 3.
Comparison of the effects of [Arg14,Lys15]N/OFQ (0.3–3 μg kg−1), an agonist of NOP receptor, and of nociceptin (30 μg kg−1) on the airway plasma extravasation in guinea-pigs pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). Data are expressed as mean±s.e.m., n=3–4 per group. #P<0.01 vs −HCl, *P<0.05 vs +HCl, **P<0.01 vs +HCl.
Figure 4.
Effect of J-113397 (3 mg kg−1), a NOP receptor antagonist, on the inhibitory effect of nociceptin (30 μg kg−1). Guinea-pigs were pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). J-113397 was administered 10 min before nociceptin. Data are expressed as mean±s.e.m., n=3–5 per group. #P<0.01 vs −HCl, *P<0.05 vs +HCl.
Figure 5.
Effect of morphine (1 mg kg−1) on airway plasma exudation in guinea-pigs pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). Morphine was given intravenously 10 min before dye infusion. Naloxone was injected at the dose of 3 mg kg−1, 10 min before morphine or 19 min before nociceptin. Data are expressed as mean±s.e.m., n=2–5 per group. #P<0.01 vs −HCl, *P<0.05 vs +HCl, **P<0.01 vs +HCl.
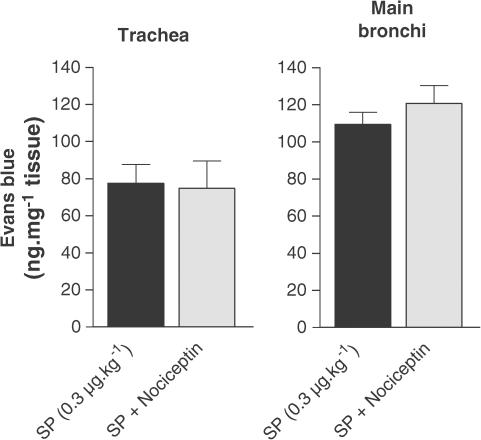
Effect of nociceptin on SP-induced airway microvascular leakage
Nociceptin had no significant influence on SP (0.3. μg kg−1)-induced plasma extravasation either in trachea or in the main bronchi (Figure 6).
Figure 6.
Effect of nociceptin on SP-induced plasma extravasation on the trachea and main bronchi of guinea-pigs pretreated with atropine (3 mg kg−1), propranolol (1 mg kg−1) and phosphoramidon (1 mg kg−1). SP (0.3 μg kg−1) was injected 5 min before dye infusion. Data are expressed as mean±s.e.m., n=4 per group.
Discussion
In the present study, we found that, in guinea-pigs pretreated with atropine, propranolol and phosphoramidon, oesophageal stimulation by HCl infusion causes plasma extravasation in the trachea and main bronchi. Airway microvascular leakage, a manifestation of neurogenic inflammation, was abolished either by nociceptin (10–30 μg kg−1), by the NOP receptor agonist [Arg14, Lys15]N/OFQ (0.3–3 μg kg−1) or by morphine (1 mg kg−1). The protective effect of nociceptin was abolished by pretreatment with J-113397, a NOP receptor antagonist, but not by naloxone, an antagonist of classical opioid receptors, showing that the MOP, KOP and DOP opioid receptors are not involved in the suppressant activity of nociceptin. In addition, we have shown that nociceptin did not inhibit SP-induced microvascular leakage in airways, suggesting that nociceptin does not act via a postsynaptic inhibitory effect at the level of the vasculature. Our present results, combined with previous results of our group (Daoui et al., 2002) showing that tachykinins play an important role in this model of plasma extravasation induced in the airways by intra-oesophageal HCl infusion (see introduction), suggest that nociceptin could exert its preventative effect on sensory nerves at a presynaptic level.
Several types of protective effect against sensory nerve activation involving nociceptin have been reported in airways, such as inhibition of the contractions of the guinea-pig isolated bronchus induced by EFS (Fischer et al., 1998), or inhibition of capsaicin-induced bronchoconstriction in guinea-pig isolated lung (Corboz et al., 2000). In addition, it has been shown that nociceptin can inhibit capsaicin-induced bronchoconstriction via an activation of an inward-rectifier K+ channel (Jia et al., 2002). In human isolated bronchi, nociceptin was shown to inhibit airway hyper-responsiveness induced by fenoterol (Faisy et al., 2003). In the gastro-intestinal tract, nociceptin was described to inhibit in vitro the high-frequency EFS-mediated nonadrenergic noncholinergic (NANC) contractions of the longitudinal muscle of the guinea-pig ileum (Nicholson et al., 1998; Osinski & Brown, 2000).
Under our experimental conditions, nociceptin exerts its protective effect against airway microvascular leakage at very low doses. [Arg14,Lys15]N/OFQ effects were observed at doses 10-fold lower than that of nociceptin, a ratio of dose in agreement with the reported results by Rizzi et al. (2002). This range of doses contrasts with that used by McLeod et al. (2001) for inhibition of cough in guinea-pig. These authors have shown that nociceptin significantly reduced cough induced by aerosolised capsaicin only at 3 mg kg−1 (i.v., immediately before cough test). However, in our study, the protective effect of nociceptin was observed in the same range of doses as that described in other in vivo studies in the gastro-intestinal tract (Osinski & Brown, 2000), suggesting that the mechanism and/or site of action of nociceptin for inhibition of cough induced by capsaicin and for airway microvascular leakage induced by intra-oesophageal HCl are different.
The low doses necessary to block airway plasma extravasation induced by intra-oesophageal HCl instillation firstly suggest that nociceptin effects may involve peripheral nerves. In this way, specific nociceptin-positive fibres have been identified in guinea-pig bronchi by double-labelling immunohistochemistry experiments (Fischer et al., 1998). In addition, several published in vitro evidences in guinea-pig airways (Patel et al., 1997; Nemeth et al., 1998; Corboz et al., 2000) and gut (Osinski & Brown, 2000) suggest that nociceptin is able to mediate effects locally. Among the possible sites of action, nociceptin can acts on nerve endings or on nerve connections between the oesophagus and airways in the autonomic ganglia (Myers & Undem, 1993; Myers et al., 1996), where anatomical studies have demonstrated the presence of axon collaterals from visceral afferent fibres (gastro-intestinal tract, bladder, airways) (Coleridge et al., 1989; Mawe, 1995; Canning et al., 1996; Myers et al., 1996). Interestingly, Canning et al. (1998) have reported in an in vitro preparation of guinea-pig trachea and oesophagus with their respective nerves, and in the presence of a combination of propranolol, atropine, indomethacin and SR 48968 (a NK2 receptor antagonist), that capsaicin elicited concentration-dependent relaxations of the trachea only when the adjacent oesophagus was intact. The effect of capsaicin was abolished by tetrodotoxin. On the basis of these results and of additional immunochemistry studies, it was concluded that the NANC responses of the guinea-pig trachea induced by oesophagus stimulation were mediated by nerve fibres from parasympathetic ganglia that are intrinsic to the adjacent oesophagus (Canning et al., 1998). Similarly, our results suggest that stimulation by HCl of capsaicin-sensitive fibres in the oesophagus may elicit microvascular leakage in airways through intermediate neurons, including parasympathetic ganglion neurons.
However, while a peripheral effect of nociceptin can be suggested because of low doses needed to achieve protection against HCl-induced microvascular leakage, a central effect of nociceptin may also be proposed since, in agreement with Hamamoto et al. (1997), we have shown that HCl-induced airway microvascular leakage was abolished in guinea-pigs after bilateral vagotomy. A peripheral reflex would not have been affected by this manipulation. Thus, the evoked plasma leakage in airways may be a reflex, involving the central integration of afferent information in response to oesophageal acidification. Indeed, several reports have suggested that tachykinins are present in the nucleus of the solitary tract (NTS) and might be involved in the central control of cardiovascular and respiratory functions related to other C-fibre-mediated reflexes (Mazzone & Geraghty, 1999, 2000). It has also been shown, in guinea-pigs, that stimulation of the C-fibres induced either by systemic injections of capsaicin (Mutoh et al., 2000), bradykinin, histamine (Canning et al., 2001), or by laryngeal mucosal application of capsaicin or bradykinin (Mazzone & Canning, 2002) induces changes in blood pressure, heart rate and airways. These changes are potentiated by injection in the NTS of either SP, an effect mediated through an activation of NK1 and/or NK3 receptors (Mutoh et al., 2000; Mazzone & Canning, 2002). Tachykinin receptors in the NTS are also activated following intra-gastric challenge with HCl (Jocic et al., 2001; Michl et al., 2001). Since NOP receptors are abundantly expressed in the NTS (Bunzow et al., 1994; Anton et al., 1996; Mao & Wang, 2000a), and since their activation is able to reduce the responsiveness of NTS neurons to baroreceptor inputs (Mao & Wang, 2000a, 2000b), we may thus suggest that nociceptin is able to either inhibit the tachykinin release in the NTS or reduce sensory nerve excitability (Dong et al., 2002) in this area, and consequently inhibit the reflex involved in the airway plasma extravasation evoked following oesophageal acidification.
Finally, nociceptin may act on vagus nerves at the level of jugular ganglion neurons, which play an important role in the regulation of sensory nerve activity (Fischer et al., 1996) and where Fischer et al. (1998) have localised NOP receptor mRNA.
In summary, our results suggest that airway plasma extravasation induced by intra-oesophageal HCl instillation might be inhibited by nociceptin-induced stimulation of NOP receptor. In this way, nociceptin, which plays a physiological role in regulating tachykinergic transmission in the airways, may represent an interesting pharmacological tool to study the physiopathology of gastro-oesophageal reflux-induced asthma.
Acknowledgments
We are very grateful to Professor Regoli and Dr Calò for their advices and for having kindly provided us the NOP receptor agonist [Arg14,Lys15]N/OFQ.
Abbreviations
- EFS
electrical field stimulation
- GORD
gastro-oesophageal reflux disease
- HCl
hydrochloric acid
- NANC
nonadrenergic noncholinergic
- NTS
nucleus of the solitary tract
- SP
substance P
References
- ADVENIER C., D'AGOSTINO B., CUI Y.Y., EUTAMENE H., ROUGET C., BARDOU M., BUENO L.Animal models for investigating gastroesophageal reflux disease-induced asthma Experimental and Clinical Pharmacology of Gastroesophageal Reflux-induced Asthma 2002Pisa: Pacini Editore; 51–54.ed. Dal Negro, R.W., Geppetti P. & Morice A.H. pp [Google Scholar]
- ANTON B., FEIN J., TO T., LI X., SILBERSTEIN L., EVANS C.J. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- BERQUIST W.E., RACHELEFSKY G.S., KADDEN M., SIEGEL S.C., KATZ R.M., FONKALSRUD E.W., AMENT M.E. Gastroesophageal reflux-associated recurrent pneumonia and chronic asthma in children. Pediatrics. 1981;68:29–35. [PubMed] [Google Scholar]
- BIGONI R., CALÒ G., RIZZI A., GUERRINI R., DE RISI C., HASHIMOTO Y., HASHIBA E., LAMBERT D.G., REGOLI D. In vitro characterization of J-113397, a non-peptide nociceptin/orphanin FQ receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:565–568. doi: 10.1007/s002100000220. [DOI] [PubMed] [Google Scholar]
- BOLSER D.C., MCLEOD R.L., TULSHIAN D.B., HEY J.A. Antitussive action of nociceptin in the cat. Eur. J. Pharmacol. 2001;430:107–111. doi: 10.1016/s0014-2999(01)01244-4. [DOI] [PubMed] [Google Scholar]
- BUNZOW J.R., SAEZ C., MORTRUD M., BOUVIER C., WILLIAMS J.T., LOW M., GRANDY D.K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- CALÒ G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANNING B.J., FISCHER A., UNDEM B.J. Pharmacological analysis of the tachykinin receptors that mediate activation of nonadrenergic, noncholinergic relaxant nerves that innervate guinea pig trachealis. J. Pharmacol. Exp. Ther. 1998;284:370–377. [PubMed] [Google Scholar]
- CANNING B.J., REYNOLDS S.M., MAZZONE S.B. Multiple mechanisms of reflex bronchospasm in guinea pigs. J. Appl. Physiol. 2001;91:2642–2653. doi: 10.1152/jappl.2001.91.6.2642. [DOI] [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J., KARAKOUSIS P.C., DEY R.D. Effects of organotypic culture on parasympathetic innervation of guinea pig trachealis. Am. J. Physiol. 1996;271:L698–L706. doi: 10.1152/ajplung.1996.271.5.L698. [DOI] [PubMed] [Google Scholar]
- COLERIDGE H.M., COLERIDGE J.C., SCHULTZ H.D. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol. Ther. 1989;42:1–63. doi: 10.1016/0163-7258(89)90021-1. [DOI] [PubMed] [Google Scholar]
- COLSON D.J., CAMPBELL C.A., WRIGHT V.A., WATSON B.W. Predictive value of oesophageal pH variables in children with gastro-oesophageal reflux. Gut. 1990;31:370–373. doi: 10.1136/gut.31.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBOZ M.R., RIVELLI M.A., EGAN R.W., TULSHIAN D., MATASI J., FAWZI A.B., BENBOW L., SMITH-TORHAN A., ZHANG H., HEY J.A. Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur. J. Pharmacol. 2000;402:171–179. doi: 10.1016/s0014-2999(00)00505-7. [DOI] [PubMed] [Google Scholar]
- DAOUI S., D'AGOSTINO B., GALLELLI L., ALT X.E., ROSSI F., ADVENIER C. Tachykinins and airway microvascular leakage induced by HCl intra-oesophageal instillation. Eur. Respir. J. 2002;20:268–273. doi: 10.1183/09031936.02.00250902. [DOI] [PubMed] [Google Scholar]
- DONG X.W., WILLIAMS P.A., JIA Y.P., PRIESTLEY T. Activation of spinal ORL-1 receptors prevents acute cutaneous neurogenic inflammation: role of nociceptin-induced suppression of primary afferent depolarization. Pain. 2002;96:309–318. doi: 10.1016/S0304-3959(01)00460-2. [DOI] [PubMed] [Google Scholar]
- FAISY C., NALINE E., ROUGET C., GUEROT E., FAGON J.Y., CHINET T., ADVENIER C. Nociceptin inhibits the in vitro fenoterol-induced neurosensitization of human bronchi. Am. J. Respir. Crit. Care Med. 2003;167:A148. doi: 10.1007/s00210-004-0974-x. [DOI] [PubMed] [Google Scholar]
- FISCHER A., FORSSMANN W.G., UNDEM B.J. Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea pig bronchus. J. Pharmacol. Exp. Ther. 1998;285:902–907. [PubMed] [Google Scholar]
- FISCHER A., MCGREGOR G.P., SARIA A., PHILIPPIN B., KUMMER W. Induction of tachykinin gene and peptide expression in guinea pig no dose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLELLI L., D'AGOSTINO B., MARROCCO G., DE ROSA G., FILIPPELLI W., ROSSI F., ADVENIER C. Role of tachykinins in the bronchoconstriction induced by HCl intraesophageal instillation in the rabbit. Life Sci. 2003;72:1135–1142. doi: 10.1016/s0024-3205(02)02372-x. [DOI] [PubMed] [Google Scholar]
- GHAED N., STEIN M.R. Assessment of a technique for scintigraphic monitoring of pulmonary aspiration of gastric contents in asthmatics with gastroesophageal reflux. Ann. Allergy. 1979;42:306–308. [PubMed] [Google Scholar]
- HAMAMOTO J., KOHROGI H., KAWANO O., IWAGOE H., FUJII K., HIRATA N., ANDO M. Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J. Appl. Physiol. 1997;82:738–745. doi: 10.1152/jappl.1997.82.3.738. [DOI] [PubMed] [Google Scholar]
- HARDING S.M., SONTAG S.J. Asthma and gastroesophageal reflux. Am. J. Gastroenterol. 2000;95:S23–S32. doi: 10.1016/s0002-9270(00)01075-3. [DOI] [PubMed] [Google Scholar]
- HERVÉ P., DENJEAN A., JIAN R., SIMONNEAU G., DUROUX P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am. Rev. Respir. Dis. 1986;134:986–989. doi: 10.1164/arrd.1986.134.5.986. [DOI] [PubMed] [Google Scholar]
- ING A.J., NGU M.C., BRESLIN A.B. Chronic persistent cough and gastro-oesophageal reflux. Thorax. 1991;46:479–483. doi: 10.1136/thx.46.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRWIN R.S., ZAWACKI J.K., CURLEY F.J., FRENCH C.L., HOFFMAN P.J. Chronic cough as the sole presenting manifestation of gastroesophageal reflux. Am. Rev. Respir. Dis. 1989;140:1294–1300. doi: 10.1164/ajrccm/140.5.1294. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA T., SEKIZAWA S.I., SANT'AMBROGIO F.B., SANT'AMBROGIO G. Larynx vs esophagus as reflexogenic sites for acid-induced bronchoconstriction in dogs. J. Appl. Physiol. 1999;86:1226–1230. doi: 10.1152/jappl.1999.86.4.1226. [DOI] [PubMed] [Google Scholar]
- JIA Y., WANG X., APONTE S.I., RIVELLI M.A., YANG R., RIZZO C.A., CORBOZ M.R., PRIESTLEY T., HEY J.A. Nociceptin/orphanin FQ inhibits capsaicin-induced guinea-pig airway contraction through an inward-rectifier potassium channel. Br. J. Pharmacol. 2002;135:764–770. doi: 10.1038/sj.bjp.0704515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOCIC M., SCHULIGOI R., SCHONINKLE E., PABST M.A., HOLZER P. Cooperation of NMDA and tachykinin NK(1) and NK(2) receptors in the medullary transmission of vagal afferent input from the acid-threatened rat stomach. Pain. 2001;89:147–157. doi: 10.1016/s0304-3959(00)00357-2. [DOI] [PubMed] [Google Scholar]
- KAWAMOTO H., OZAKI S., ITOH Y., MIYAJI M., ARAI S., NAKASHIMA H., KATO T., OHTA H., IWASAWA Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- KJELLEN G., TIBBLING L., WRANNE B. Bronchial obstruction after oesophageal acid perfusion in asthmatics. Clin. Physiol. 1981;1:285–292. doi: 10.1111/j.1475-097x.1981.tb00897.x. [DOI] [PubMed] [Google Scholar]
- MANSFIELD L.E., HAMEISTER H.H., SPAULDING H.S., SMITH N.J., GLAB N. The role of the vague nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Ann. Allergy. 1981;47:431–434. [PubMed] [Google Scholar]
- MANSFIELD L.E., STEIN M.R. Gastroesophageal reflux and asthma: a possible reflex mechanism. Ann. Allergy. 1978;41:224–226. [PubMed] [Google Scholar]
- MAO L., WANG J.Q. Microinjection of nociceptin (Orphanin FQ) into nucleus tractus solitarii elevates blood pressure and heart rate in both anesthetized and conscious rats. J. Pharmacol. Exp. Ther. 2000a;294:255–262. [PubMed] [Google Scholar]
- MAO L., WANG J.Q. Pharmacological activation of nociceptin receptors in the nucleus tractus solitarius inhibits baroreceptor reflex in pentobarbital-anesthetized rats. Neuroscience. 2000b;101:435–440. doi: 10.1016/s0306-4522(00)00370-5. [DOI] [PubMed] [Google Scholar]
- MAWE G.M.Tachykinins as mediators of slow EPSPs in guinea-pig gall-bladder ganglia: involvement of neurokinin-3 receptors J. Physiol. 1995485513–524.Part 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., CANNING B.J. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br. J. Pharmacol. 1999;127:473–481. doi: 10.1038/sj.bjp.0702522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Characterization and regulation of tachykinin receptors in the nucleus tractus solitarius. Clin. Exp. Pharmacol. Physiol. 2000;27:939–942. doi: 10.1046/j.1440-1681.2000.03365.x. [DOI] [PubMed] [Google Scholar]
- MCLEOD R.L., PARRA L.E., MUTTER J.C., ERICKSON C.H., CAREY G.J., TULSHIAN D.B., FAWZI A.B., SMITH-TORHAN A., EGAN R.W., CUSS F.M., HEY J.A. Nociceptin inhibits cough in the guinea-pig by activation of ORL(1) receptors. Br. J. Pharmacol. 2001;132:1175–1178. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur. J. Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER L., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MICHL T., JOCIC M., SCHULIGOI R., HOLZER P. Role of tachykinin receptors in the central processing of afferent input from the acid-threatened rat stomach. Regul. Pept. 2001;102:119–126. doi: 10.1016/s0167-0115(01)00309-3. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MUTOH T., BONHAM A.C., JOAD J.P. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1215–R1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- MYERS A., UNDEM B., KUMMER W. Anatomical and electrophysiological comparison of the sensory innervation of bronchial and tracheal parasympathetic ganglion neurons. J. Auton. Nerv. Syst. 1996;61:162–168. doi: 10.1016/s0165-1838(96)00081-1. [DOI] [PubMed] [Google Scholar]
- MYERS A.C., UNDEM B.J. Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J. Physiol. 1993;470:665–679. doi: 10.1113/jphysiol.1993.sp019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEMETH J., HELYES Z., OROSZI G., THAN M., PINTER E., SZOLCSANYI J. Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur. J. Pharmacol. 1998;347:101–104. doi: 10.1016/s0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- NICHOLSON J.R., PATERSON S.J., MENZIES J.R., CORBETT A.D., MCKNIGHT A.T. Pharmacological studies on the ‘orphan' opioid receptor in central and peripheral sites. Can. J. Physiol. Pharmacol. 1998;76:304–313. [PubMed] [Google Scholar]
- OSINSKI M.A., BROWN D.R. Orphanin FQ/nociceptin: a novel neuromodulator of gastrointestinal function. Peptides. 2000;21:999–1005. doi: 10.1016/s0196-9781(00)00240-0. [DOI] [PubMed] [Google Scholar]
- OZAKI S., KAWAMOTO H., ITOH Y., MIYAJI M., AZUMA T., ICHIKAWA D., NAMBU H., IGUCHI T., IWASAWA Y., OHTA H. In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur. J. Pharmacol. 2000;402:45–53. doi: 10.1016/s0014-2999(00)00520-3. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., GIEMBYCZ M.A., SPICUZZA L., BARNES P.J., BELVISI M.G. Naloxone-insensitive inhibition of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by the novel opioid, nociceptin. Br. J. Pharmacol. 1997;120:735–736. doi: 10.1038/sj.bjp.0701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L. Mechanisms of citric acid-induced bronchoconstriction. Am. J. Med. 2001;111 Suppl 8A:18S–24S. doi: 10.1016/s0002-9343(01)00816-6. [DOI] [PubMed] [Google Scholar]
- RIZZI D., RIZZI A., BIGONI R., CAMARDA V., MARZOLA G., GUERRINI R., DE RISI C., REGOLI D., CALÒ G. Arg(14),Lys(15)]nociceptin, a highly potent agonist of the nociceptin/orphanin FQ receptor: in vitro and in vivo studies. J. Pharmacol. Exp. Ther. 2002;300:57–63. doi: 10.1124/jpet.300.1.57. [DOI] [PubMed] [Google Scholar]
- ROGERS D.F., BELVISI M.G., AURSUDKIJ B., EVANS T.W., BARNES P.J. Effects and interactions of sensory neuropeptides on airway microvascular leakage in guinea-pigs. Br. J. Pharmacol. 1988;95:1109–1116. doi: 10.1111/j.1476-5381.1988.tb11745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAULDING H.S., Jr, MANSFIELD L.E., STEIN M.R., SELLNER J.C., GREMILLION D.E. Further investigation of the association between gastroesophageal reflux and bronchoconstriction. J. Allergy Clin. Immunol. 1982;69:516–521. doi: 10.1016/0091-6749(82)90176-2. [DOI] [PubMed] [Google Scholar]
- TSUGENO H., MIZUNO M., FUJIKI S., OKADA H., OKAMOTO M., HOSAKI Y., ASHIDA S., MITSUNOBU F., TANIZAKI Y., SHIRATORI Y. A proton-pump inhibitor, rabeprazole, improves ventilatory function in patients with asthma associated with gastroesophageal reflux. Scand. J. Gastroenterol. 2003;38:456–461. doi: 10.1080/00365520310002490. [DOI] [PubMed] [Google Scholar]
- TUCHMAN D.N., BOYLE J.T., PACK A.I., SCWARTZ J., KOKONOS M., SPITZER A.R., COHEN S. Comparison of airway responses following tracheal or esophageal acidification in the cat. Gastroenterology. 1984;87:872–881. [PubMed] [Google Scholar]