Abstract
Despite the accumulating evidence that under various pathological conditions the extracellular elevation of adenine-based nucleotides and nucleosides plays a key role in the control of astroglial reactivity, how these signalling molecules interact in the regulation of astrocyte function is still largely elusive.
The action of the nucleoside adenosine in the modulation of the intracellular calcium signalling ([Ca2+]i) elicited by adenosine 5′-triphosphate (ATP)-induced activation of P2 purinoceptors was investigated on neocortical type-1 astrocytes in primary culture by using single-cell microfluorimetry.
Astrocyte challenge with ATP (1–10 μM) elicited biphasic [Ca2+]i responses consisting of an initial peak followed by a sustained elevation. The stable adenosine analogue 2-chloroadenosine (2-ClA) potentiated the transient [Ca2+]i rise induced by activation of metabotropic P2Y receptors. Among the various P1 receptor agonists tested, the nonselective agonist 5′-N-ethylcarboxamidoadenosine (NECA) mimicked the 2-ClA action, whereas the selective A1 R(−) N6-(2-phenylisopropyl)-adenosine (R-PIA), the A2A 2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine (CGS-21680) and A3 1-deoxy-1-(6-[([3-lodophenyl]methyl)-amino]-9H-purin-9-yl)-N-methyl-β-D-ribofuranuronamide (IB-MECA) agonists were ineffective.
Application of R-PIA>NECA⩾2-ClA depressed the [Ca2+]i plateau reversibly. Moreover, in the presence of R-PIA or 2-ClA, the prolonged [Ca2+]i signal was maintained by application of the A1 antagonist 1,3-diethyl-8-phenylxanthine (DPX). Finally, preincubation of the astrocytes with pertussis toxin abrogated the 2-ClA inhibition of the ATP-elicited sustained [Ca2+]i rise without affecting the transient [Ca2+]i potentiation.
Taken together, these findings indicate that stimulation of A1 and A2 adenosine receptors mediates a differential modulation of [Ca2+]i signalling elicited by P2 purinoceptors. Since variations in [Ca2+]i dynamics also affect cell proliferation and differentiation, our data suggest that tuning of the extracellular levels of adenosine may be relevant for the control of astrogliosis mediated by adenine nucleotides.
Keywords: Astroglia, P2 purinoceptors, adenosine receptors, microfluorimetry, calcium homeostasis
Introduction
Adenine-based purines are ubiquitous extracellular signalling molecules, which in mammals are involved in the functional regulation of virtually all tissues and organs (Ralevic & Burnstock, 1998). In the brain, under physiological conditions, the relative amount of extracellular adenosine 5′-triphosphate (ATP) and adenosine is dynamically controlled by ATP corelease from the cholinergic and catecholaminergic presynaptic terminals, its rate of metabolic degradation by ecto 5′-nucleotidases, as well as by the rate of release and uptake of adenosine by neuronal and glial cells (for a review, see Fredholm, 1997). Large amounts of adenine-based purine nucleotides and nucleosides are also released from neuronal and glial cells that are stressed or anoxic, injured and metabolically active (Parkinson et al., 2002). Whereas the roles of adenine purines in the control of neuronal activity is relatively well defined (for a review, see Ribeiro et al., 2002), their contribution to the regulation of the activity of astroglial cells in physiological as well as in pathophysiological conditions is just beginning to emerge (Neary et al., 1996; Abbracchio & Burnstock, 1998). In vitro and in situ evidence indicates that a rise in extracellular ATP promotes astroglial proliferation (Abbracchio et al., 1994, Franke et al., 1999). Conversely, high levels of adenosine cause astrocyte cell death through an apoptotic mechanism (Abbracchio et al., 1995; Di Iorio et al., 2002). However, how the adenine nucleotides and nucleosides signalling crosstalk affects the functional reaction of the astroglial syncytium to pathological stimuli still remains elusive (Rathbone et al., 1999).
In the central nervous system, ATP and adenosine interact with their plasma membrane receptors called P2 and P1, respectively, present on both neurons and glial cells (Lee et al., 1983; Cunha et al., 1994; Illes et al., 1996; King et al., 1996; Jiménez et al., 2000; Franke et al., 2001a, 2001b; Amadio et al., 2002; Fumagalli et al., 2003). The P2 purinoceptors comprise two subtypes, P2Y and P2X; P2Y receptors are seven transmembrane segment proteins coupled to G proteins cells, whereas P2X are ligand-gated ion channels (Ralevic & Burnstock, 1998). Activation of various subtypes of P2Y receptors leads to the elevation of free cytosolic Ca2+ concentration ([Ca2+]i) mediated by phosphoinositide turnover-dependent Ca2+ release from intracellular organelles. In different cell preparations, this P2Y-mediated Ca2+ mobilization is accompanied by a substantial Ca2+ influx from the extracellular milieu via a pathway that is commonly referred to as capacitative Ca2+ entry (CCE), which is mediated by the opening of store-operated Ca2+ channels (Parekh & Penner, 1997).
It has been suggested that in situ a significant amount of the ATP-evoked [Ca2+]i signal in astrocytes derives from the activation of ionotropic P2X7 receptors (James & Butt, 2002). Indeed, astroglial cells both in situ and in vitro possess P2X7 receptors, whose functional role, however, remains to be fully elucidated (Ballerini et al., 1996; Sun et al., 1999; Panenka et al., 2001; Franke et al., 2001a, 2001b; Hung & Sun, 2002; Fumagalli et al., 2003). In this context, we recently demonstrated that in cultured rat cortical type-1 astrocytes, the sustained [Ca2+]i increase observed following the transient [Ca2+]i signal elicited upon stimulation of P2Y purinoceptors with low micromolar concentrations of ATP is not mediated by CCE but is the result of extracellular Ca2+ influx through P2X7-like receptors (Nobile et al., 2003).
The extracellular actions of adenine nucleosides are mediated through P1 receptors (Schubert et al., 1997). These are G protein-coupled receptors, which have been classified into A1, A2A, A2B and A3 types, based on their effects on adenylyl cyclase (AC), different affinities for adenosine and the potency of selective agonists and antagonists. A1 and A3 subtypes are negatively and A2 is positively coupled to AC, respectively (Ralevic & Burnstock, 1998). Other studies have also suggested the coupling of some subtypes to different effectors, including phospholipase C (PLC) and ion channels (Linden, 1991; Schubert et al., 1994). Moreover, since adenosine acts as an ubiquitous cell modulator, it is likely that it functionally interacts with the signalling of other transmitters. In this context, in cultured astroglial cells adenosine was shown to cooperate with metabotropic glutamate receptor agonists in mobilising [Ca2+]i (Ogata et al., 1994; Cormier et al., 2001), to potentiate the transient [Ca2+]i responses evoked by stimulation of metabotropic ATP receptors (Jiménez et al., 1998; Jiménez et al., 1999) and muscarinic acetylcholine receptors (Ferroni et al., 2002).
Given our previous findings indicating that astroglial ATP-induced [Ca2+]i responses are mediated by the differential activation of P2 receptors, here we have addressed the issue whether the stimulation of P1 receptors could differently affect the various components of the [Ca2+]i signal. We provide pharmacological evidence that whereas adenosine potentiates the [Ca2+]i peak, it downregulates the [Ca2+]i plateau. We also show that the two effects are mediated by different P1 receptors. Owing to the relevance of the [Ca2+]i dynamics in the regulation of astroglial functions (for a review, see Verkhratsky et al., 1998), these findings provide a possible molecular frame to explain the variable effects of adenine-based purines in astroglial cells under physiological and pathophysiological conditions.
Methods
Cell cultures
Primary cultures of cortical rat astrocytes were prepared as previously described (Nobile et al., 2003) with the approval of the Committee on Animal Research of our institution. Briefly, cerebral cortices of 2-day-old pups devoid of meninges were triturated and placed in cell culture flasks containing Dulbecco's modified Eagle's medium with 15% fetal calf serum and penicillin–streptomycin (100 U ml−1 and 100 μg ml−1, respectively). Culture flasks were maintained in a humidified incubator with 5% CO2 for 2–5 weeks. At confluence, astroglial cells were enzymatically dispersed (trypsin-EDTA, 0.5–0.2 g) and were replated in 20-mm glass coverslips at a density of 5 × 103 per coverslip. All experiments were performed at room temperature (20–22°C) at days 3–8 after re-seeding. Immunostaining for glial fibrillary acidic protein (GFAP) and the flat, polygonal morphological phenotype of the cultured cells indicated that > 95% were type-1 cortical astrocytes (Ferroni et al., 1995).
Solutions
The extracellular bath solution contained (mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes and 10 glucose (pH adjusted to 7.4 with NaOH). The calcium-free extracellular saline was prepared by replacing CaCl2 with equimolar amounts of MgCl2 and by adding 0.5 mM EGTA. All the salts and chemicals used for the fluorimetric determinations of the [Ca2+]i signals were obtained from Sigma (Sigma-Aldrich S.r.L., Italy); for the preparation of the astrocyte cultures, all the materials were from Gibco-BRL (Invitrogen S.r.L., Italy).
Imaging and data analysis
Intracellular calcium measurements were performed by using the fluorescent Ca2+ indicator fura-2 AM. Rat astrocytes were loaded with 10 μM fura-2 AM dissolved in extracellular solution for 45 min at 37°C. The microperfusion chamber containing the cell coverslip was placed on the stage of an inverted fluorescence microscope Nikon TE200 (Nikon, Tokyo, Japan) equipped with a dual excitation fluorometric calcium imaging system (Hamamatsu, Sunayama-Cho, Japan). Low-density seeded astrocytes were continuously perfused at a rate of about 2.5 ml min−1. Emission florescence of selected astrocytes was passed through a narrow-band filter and recorded with a digital CCD camera Hamamatsu C4742-95-12ER. Monochromator settings, chopper frequency and complete data acquisition were controlled by Aquacosmos Ratio software U7501-01 (Hamamatsu). The sampling rate was 0.25 or 0.5 Hz. Fura-2-loaded astrocytes were excited at 340 and 380 nm, and fluorescence was measured at 510 nm. The fluorescence ratio F340/F380 was used to monitor [Ca2+]i changes.
Statistics
All data are given as means±s.e.m. The statistical significance of differences between mean values was assessed using Student's t-test. Differences were regarded as statistically significant for P<0.05.
Results
Adenosine potentiates the ATP-evoked [Ca2+]i transient in cultured astrocytes
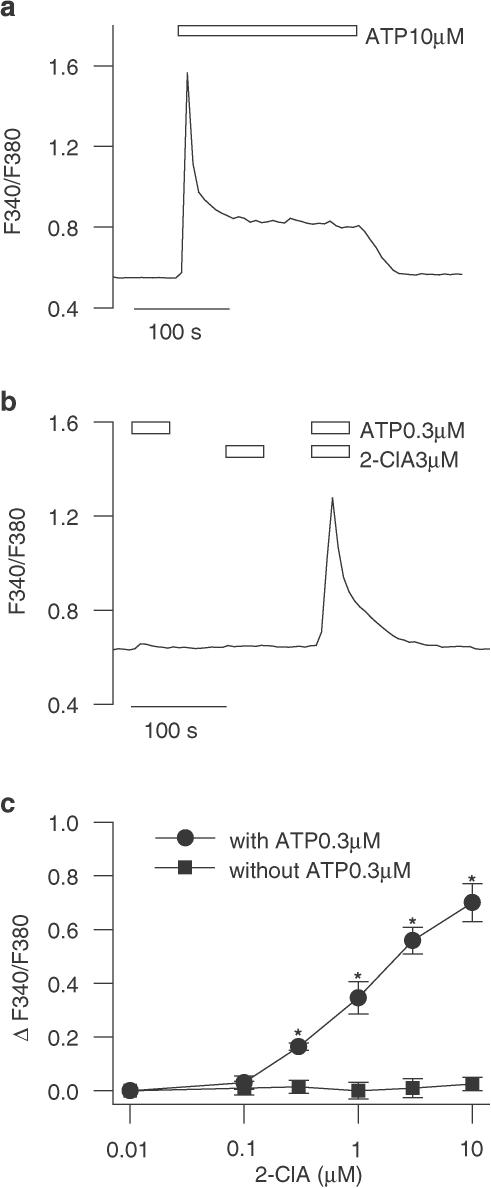
Consistent with our previous observations (Nobile et al., 2003), challenge of cultured rat cortical type-1 astrocytes with micromolar concentrations of ATP elicited biphasic [Ca2+]i responses (n=220; Figure 1a); the initial large transient component, mediated by activation of P2 metabotropic purinoceptors (P2Y), was followed by a smaller sustained phase caused by Ca2+ influx through ionotropic P2X7-like receptor. To test whether adenosine (P1) receptors could regulate the various components of the ATP-induced astroglial [Ca2+]i signalling, astrocytes were exposed to nanomolar concentrations of adenosine 5′-triphosphate (ATP, 0.3 μM) and to the stable adenosine analogue 2-chloroadenosine (2-ClA, 3 μM). Whereas application of ATP promoted only a low, nonsignificant [Ca2+]i rise above the basal level (n=36; P>0.05) and 2-ClA (n=28) was totally ineffective, their brief coapplication elicited a significant, transient [Ca2+]i elevation, which decayed to the basal level within 3 min (Figure 1b; n=33; P<0.01). The magnitude of the [Ca2+]i response was fairly unchanged upon removal of extracellular Ca2+, thereby indicating that the [Ca2+]i rise was mediated likely by Ca2+ release from intracellular stores (n=35; data not shown). The observation that 2-ClA up to 10 μM (n=42) did not change the basal [Ca2+]i level indicates that the synergistic [Ca2+]i response was due to a 2-ClA potentiation of ATP-evoked [Ca2+]i signal. In the presence of 0.3 μM ATP, a dose-dependent increase in [Ca2+]i could be observed upon exposure to 2-ClA concentrations above 0.1 μM (Figure 1c; n=88; P<0.05). Thus, the following experiments on the synergistic effect of 2-ClA and ATP on [Ca2+]i level were performed by using concentrations of 10 μM 2-ClA and 0.3 μM ATP.
Figure 1.
Potentiation of the ATP-evoked transient [Ca2+]i response by 2-ClA in rat cortical astroglial cells. (a) Representative change in [Ca2+]i signal illustrating the biphasic response upon a prolonged application of ATP (10 μM; horizontal bar) consisting of an initial [Ca2+]i peak followed by a lower but sustained [Ca2+]i plateau. (b) Representative trace of [Ca2+]i rise elicited by individual exposures or coapplication of 0.3 μM ATP and 3 μM 2-ClA. (c) Dose-dependent changes in [Ca2+]i elicited by various 2-ClA concentrations in the absence or presence of 0.3 μM ATP. Various concentrations of 2-ClA were applied for 40 s alone or in conjunction with 0.3 μM ATP; for each condition, the maximum fluorescence ratio of [Ca2+]i increase above the basal levels is shown and each point is the mean of at least seven astrocytes. Error bars indicate s.e.m. *, P<0.05 vs 2-ClA 0.1 μM.
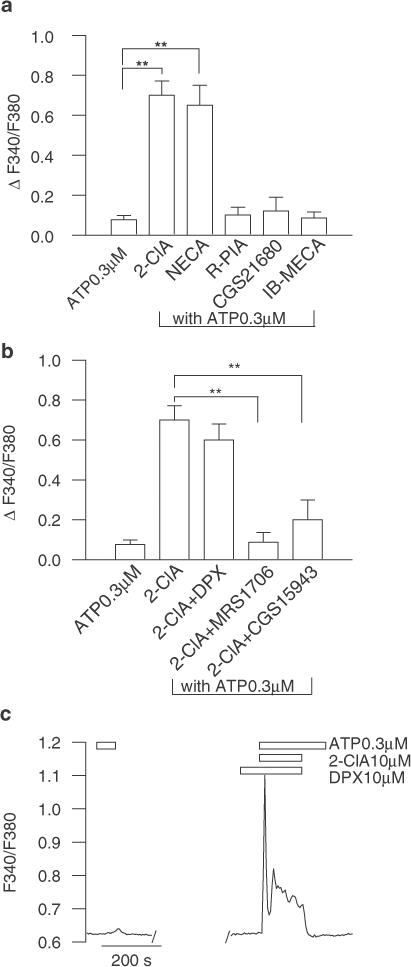
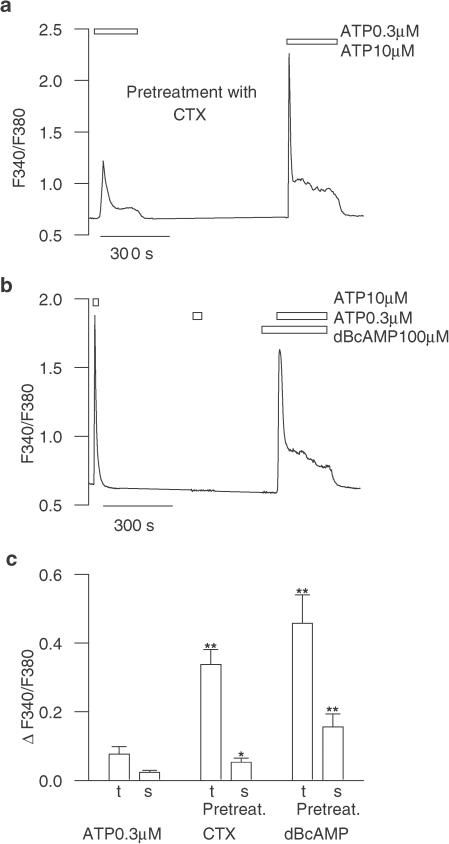
To unveil the adenosine receptors mediating this effect, experiments were performed using different adenosine receptor agonists. The costimulation of individual astrocytes with 0.3 μM ATP and either the selective A1 receptor agonist R(−)N6-(2-phenylisopropyl)-adenosine (R-PIA; 10 μM; n=28), the selective A2A receptor agonist 2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine (CGS-21680; 10 μM; n=24) or the selective A3 receptor agonist 1-deoxy-1-(6-[([3-lodophenyl]methyl)-amino]-9H-purin-9-yl)-N-methyl-β-D-ribofuranuronamide (IB-MECA; 1 μM (n=42) did not cause any [Ca2+]i rise (Figure 2a). By contrast, 5′-N-ethylcarboxamidoadenosine (NECA), an A2-preferring agonist, mimicked the 2-ClA effect (n=18). We next examined the effect of specific antagonists on the 2-ClA-mediated potentiation of the transient [Ca2+]i response (Figure 2b). Whereas in the presence of the selective A1 receptor antagonist, 1,3-diethyl-8-phenylxanthine (DPX; 10 μM), the potentiating effect of 2-ClA was not significantly changed (n=17; P>0.05), the A2B receptor antagonists, N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide (MRS1706; 1 μM; n=24; P<0.01) and 9-chloro-2-(2-furyl)(1,2,4)-triazolo(1,5-c)quinazolin-5-amine (CGS15943; 10 μM; n=27; P<0.01) counteracted the 2-ClA action (Figure 2b). Interestingly, in the presence of DPX, coapplication of 2-ClA and ATP induced a biphasic [Ca2+]i signal composed of a peak and a plateau phase (Figure 2c), thus suggesting that 2-ClA, through the activation of A1 receptors, might downregulate the sustained [Ca2+]i elevation (see below). Collectively, these data support the tenet that the 2-ClA potentiation of ATP-induced [Ca2+]i signal is mediated by the A2B receptor subtype. Since it is well established that A2 receptor stimulation promotes intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) elevation in astroglial cells (van Calker et al., 1979), next we investigated whether astrocyte pretreatment with cholera toxin (CTX), which selectively stimulates the G protein positively coupled to AC (Gs), could recapitulate the 2-ClA potentiating action. Astrocytes pretreated for 2 h (1 μg ml−1 CTX) displayed a significant peak [Ca2+]i elevation upon application of a threshold concentration of ATP (Figure 3a; n=16; P<0.01) and a similar effect was also seen in astrocytes which have been challenged for 3 min with a membrane-permeable cAMP analogue, dibutyryl cyclic AMP (dBcAMP, 100 μM), before exposure to previously ineffective ATP (Figure 3b; n=26; P<0.01). Interestingly, under both experimental conditions, the [Ca2+]i signal had a biphasic behaviour consisting of transient and sustained components (Figure 3c), an observation suggesting that in cultured astrocytes the ATP-evoked sustained [Ca2+]i response is enhanced too by an increase in intracellular cAMP.
Figure 2.
Effects of P1 purinoceptor agonists on ATP-induced [Ca2+]i responses. (a) Among the several adenosine receptor agonists tested, only 2-ClA (10 μM) and NECA (10 μM) evoked a peak [Ca2+]i rise upon application of 0.3 μM ATP. Results are presented as increase in fluorescence ratio above the basal levels and representative of at least 18 astrocytes for each condition. **, P<0.01 vs 0.3 μM ATP alone. (b) Changes in fluorescence ratio depicting the 2-ClA potentiating action in the presence of various P1 antagonists; whereas in the presence of 2-ClA, the ATP-induced [Ca2+]i peak was not significantly altered by the selective A1 antagonist DPX (10 μM), it was potently depressed by A2B receptor antagonists MRS1706 (1 μM) and CGS15943 (10 μM). Mean±s.e.m. of at least 17 astrocytes for each condition. The bar graphs show the maximum fluorescence ratio of [Ca2+]i increase above the basal level. **, P<0.01 vs 2-ClA+ATP. (c) Representative [Ca2+]i responses obtained by coapplication of 0.3 μM ATP and 10 μM 2-ClA in the presence of 10 μM DPX showing the 2-ClA potentiation of the sustained [Ca2+]i rise.
Figure 3.
Potentiation of the ATP-evoked [Ca2+]i responses by elevation of intracellular cAMP. (a) In cortical astrocytes preincubated with CTX, a low threshold concentration of ATP (0.3 μM) induced a biphasic [Ca2+]i response, which had a temporal dynamics that was similar to that evoked by 10 μM ATP alone. (b) Representative [Ca2+]i signals in a cortical astrocyte challenged with individual applications of 10 μM and 0.3 μM ATP and after exposure to 0.3 μM ATP following a 3-min pretreatment with dBcAMP (100 μM). (c) Bar graph of the average transient (t) and sustained (s) [Ca2+]i increases above the basal levels, evoked by applications of 0.3 μM ATP alone, 0.3 μM ATP in the presence of CTX, or following dBcAMP pretreatment. Mean±s.e.m. of at least 16 astrocytes. **, P<0.01 and *, P<0.05 vs 0.3 μM ATP alone.
Adenosine A1 receptor stimulation downregulates the ATP-induced sustained [Ca2+]i response
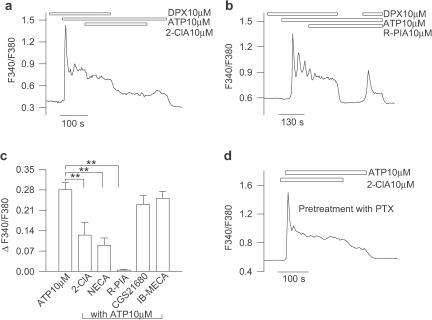
Owing to the effect of cAMP elevation on both the peak and plateau [Ca2+]i signals, next we sought to determine whether the selective potentiating action of 2ClA on the [Ca2+]i transient could be due to its ability to abrogate the ATP-mediated [Ca2+]i plateau through activation of A1 receptors. To this end, astrocytes were challenged with an ATP concentration (10 μM) able to evoke biphasic [Ca2+]i responses (see Figure 1a). Whereas coapplication of 2-ClA and ATP caused a transient [Ca2+]i elevation, which was of comparable magnitude of that evoked by ATP alone, the plateau phase of the [Ca2+]i signal elicited by a prolonged coapplication of effective concentrations of ATP and 2-ClA (1–10 μM) was depressed as depicted by the observation that under this condition only a transient [Ca2+]i rise that rapidly decayed towards the basal level could be measured (Figure 4a; n=55). Noteworthy, a similar effect was also seen upon astrocyte challenge with the A1 agonist R-PIA (n=34). This inhibition was reversible as upon removal of 2-ClA, the ATP-induced [Ca2+]i plateau recovered completely (Figure 4a). When 2-ClA was applied during the sustained [Ca2+]i rise, a large reversible depression of the [Ca2+]i signal occurred (Figure 4b; n=39). Importantly, 2-ClA (10 μM) also blocked with similar kinetics the [Ca2+]i plateau elicited by the P2X7 agonist 3′-O-(4-benzoyl)benzoyl-ATP (Bz-ATP; 10 μM; n=16, Figure 4c), which we previously identified to be one of the molecular component underlying the ATP-evoked [Ca2+]i plateau (Nobile et al., 2003). In order to confirm that A1 receptor subtype was involved in the downregulation of the sustained [Ca2+]i elevation, experiments were carried out using three different adenosine receptor antagonists. In the presence of the A2B receptor antagonists MRS 1706 (10 μM) or CGS-15943 (10 μM), the inhibitory effect of 2-ClA was still depicted (n=44; data not shown). By contrast, the selective A1 receptor antagonist DPX (10 μM) counteracted the blocking effect of 2-ClA (n=35; P<0.05), as illustrated by the finding that in the presence of 2-ClA, removal of DPX caused a sustained and reversible reduction of the ATP-induced [Ca2+]i plateau (Figure 5a). Notably, upon application of 10 μM R-PIA, instead of 2-ClA, the DPX washout produced a complete block of the sustained phase induced by 10 μM ATP (Figure 5b; n=34; P<0.01). A similar behaviour was also observed by using lower 2-ClA concentrations (1 μM; n=12; P<0.05) and R-PIA (0.1 μM; n=14; P<0.05). Figure 5c illustrates the effects on the sustained ATP-evoked [Ca2+]i responses in the presence of 10 μM of either 2-ClA (n=30), NECA (n=15), R-PIA (n=29), CGS21680 (n=18) or IB-MECA (n=27) compared with [Ca2+]i rises elicited by ATP alone (n=89). The data indicate that among the various P1 agonists tested, only the selective A1 receptor agonist R-PIA caused a significant reduction of the sustained [Ca2+]i response. Since A1 receptors are coupled to Gi/Go subtypes of G proteins, we finally investigated whether astrocyte preincubation with pertussis toxin (PTX), which selectively downregulates Gi/Go-coupled receptors, could abrogate the partial depression of the [Ca2+]i plateau caused by coapplication of 2-ClA and ATP. Whereas the costimulation of PTX-treated (24 h; 200 ng ml−1) astrocytes with ATP (0.3 μM) and 2-ClA (3 μM) was still able to cause a transient [Ca2+]i elevation (n=37; data not shown), under this condition 2-ClA (10 μM) did not reduce the [Ca2+]i plateau elicited by 10 μM ATP (Figure 5d; n=17; P>0.05). Altogether, these data strongly suggest that a lowering in intracellular cAMP through the A1-mediated depression of AC underlies the inhibitory action of adenosine on the sustained [Ca2+]i signal.
Figure 4.
Effects of P1 purinoceptor stimulation on ATP-induced sustained [Ca2+]i signal. (a) Coapplication of 10 μM ATP and 10 μM 2-ClA evoked a transient [Ca2+]i rise that decayed towards the basal level in about 3 min. Note that the sustained [Ca2+]i elevation recovered upon removal of 2-ClA. (b) Application of 2-ClA during the ATP-induced [Ca2+]i plateau, caused a reversible decrease in the steady-state [Ca2+]i level of about 60% (56±15%; n=23; P<0.05). (c) Astrocyte exposure to 2-ClA (10 μM) reduced by the same extent, and with comparable kinetics the [Ca2+]i plateau induced by 10 μM Bz-ATP.
Figure 5.
The A1 receptor mediates the adenosine-induced depression of the sustained [Ca2+]i response. (a) In the presence of the selective A1 receptor antagonist DPX (10 μM), 2-ClA (10 μM) did not cause a reduction of the ATP-evoked [Ca2+]i plateau; after DPX removal, 2-ClA caused a sustained decrease of the steady-state [Ca2+]i signal, which recovered upon 2-ClA washout. (b) In another representative astrocyte, the washing of DPX applied in conjunction with 10 μM R-PIA promoted a complete and reversible depression of the ATP-evoked sustained [Ca2+]i rise. (c) Among the several P1 agonists tested with the same procedure of panel a, the broad-spectrum agonists NECA and 2-ClA as well as the A1 selective agonist R-PIA caused a significant depression of the sustained [Ca2+]i responses, which instead were unchanged in the presence of the selective A2A agonist CGS-21680 or the selective A3 agonist IB-MECA. Mean±s.e.m. of at least 15 astrocytes for each condition. **, P<0.01 vs ATP 10 μM alone. (d) Coapplication of 10 μM ATP and 10 μM 2-ClA on a representative astrocyte from cultures, which have been preincubated for 24 h with PTX (200 ng ml−1), evoked a [Ca2+]i signal that was kinetically similar to that induced by ATP alone.
Discussion and conclusions
In this study, we investigated the functional interplay of adenine-based purine nucleotides and nucleosides in the regulation of [Ca2+]i dynamics in cultured astroglial cells. We provide pharmacological evidence that the activation of various adenosine receptors (P1) has differential effects on the peak and sustained [Ca2+]i signals elicited by stimulation of ATP metabotropic receptors (P2), likely through P2Y1,2,4 subtypes (Fumagalli et al., 2003; Nobile et al., 2003). The results show that the ATP-evoked [Ca2+]i peak is potentiated by A2B subtype of P1 receptors, as among the different A1, A2 and A3 receptor agonists tested (R-PIA, CGS21680, NECA, 2-ClA, IB-MECA) only the A2-preferring agonist NECA and 2-ClA caused a large elevation of [Ca2+]i in the presence of threshold levels of ATP, and 2-ClA was ineffective upon a prior application of A2B antagonists. Accordingly, astrocyte pretreatment with CTX, which selectively stimulates G proteins positively coupled to AC (Gs), also positively shifted the sensitivity of the ATP-induced [Ca2+]i response. The synergistic 2-ClA action was also depicted in the absence of extracellular Ca2+, indicating a purely metabotropic mechanism. The involvement of cAMP elevation in the A2B-mediated potentiation of the [Ca2+]i signal was corroborated by the observation that a brief astrocyte exposure to dBcAMP was sufficient to generate a biphasic [Ca2+]i response in astrocytes challenged with previously ineffective ATP concentrations. By contrast, activation of A1 receptor subtype, which is negatively coupled to AC, did not have any effect on the threshold ATP-evoked [Ca2+]i peak, but produced a pronounced, PTX sensitive-depression of the sustained [Ca2+]i signal evoked by stimulation of P2X7-like receptors (Nobile et al., 2003). Likewise, the [Ca2+]i plateau elicited by the P2X7 agonist Bz-ATP was also inhibited by A1 receptor stimulation. Hence, these data indicate that variations in intracellular cAMP levels are critical for the regulation of the [Ca2+]i response in cultured astroglial cells. The possibility that the adenosine potentiation of the [Ca2+]i dynamics is differently affected by oscillations of intracellular cAMP is a further confirmation of the emerging complex crosstalk between cAMP-regulated signalling pathways and calcium homeostasis (for a review, see Zaccolo & Pozzan, 2003).
The crosstalk between adenosine and ATP metabotropic receptors has been demonstrated in a variety of cellular models including muscle cells (Gerwins & Fredholm, 1992). However, in that cellular context, adenosine synergized with ATP in promoting [Ca2+]i mobilization through an A1-mediated mechanism. In astroglial cells, adenosine was shown to potentiate the [Ca2+]i transients evoked by metabotropic glutamate and muscarinic acethylcholine receptors (Ogata et al., 1994; Ogata et al., 1996; Ferroni et al., 2002) and in both cases the effects were through A1 receptors. A PTX-sensitive potentiation of the [Ca2+]i signal by adenosine was also observed upon stimulation of tachykinin receptors (Delumeau et al., 1991). By contrast, in cerebellar astrocytes, the ATP-induced [Ca2+]i rise was reported to be upregulated through A2B receptors (Jiménez et al., 1999). However, in that study the adenosine effect apparently was analysed only on the [Ca2+]i peak. Our data support the hypothesis that a βγ subunits-dependent mechanism does not play a major role in the potentiating effect of adenosine on ATP-induced [Ca2+]i transient, a result which is in striking contrast with the findings described in cerebellar astrocytes (Jiménez et al., 1999). The reasons for this discrepancy are unknown, but it can be envisaged that the different cellular context and/or culture conditions might contribute significantly. However, it is noteworthy that in cultured cerebellar astrocytes elevation of intracellular cAMP was shown to cause an increase in the [Ca2+]i response by the upregulation of CCE (Wu et al., 1999). It has also been reported that A2B and P2Y2 receptor signalling converge to stimulate mitogen-activated protein kinases (MAPK) in HEK293 cells (Gao et al., 1999). Whether MAPK are also involved in the synergistic effect of adenosine and ATP on [Ca2+]i transients remains to be ascertained.
The signal transduction pathway underlying the A1-mediated reduction in sustained [Ca2+]i signal may depend on the ability of A1 receptors to depress the basal cAMP levels. This possibility is indirectly corroborated by the observation that the exogenously induced intracellular cAMP rise also augmented the [Ca2+]i plateau upon exposure to ineffective ATP. However, the contribution of a βγ-dependent mechanism cannot be ruled out as in another cellular system A1 receptors were shown to differently affect cAMP levels and PLC activation through α and βγ dimers, respectively (Tomura et al., 1997). In this context, further studies are necessary to clarify the contribution to other signal transduction pathways to the A1-mediated downregulation of the sustained [Ca2+]i response. It remains also to be ascertained why stimulation of A3 receptors, which also downregulate AC (Zhou et al., 1992), was unable to depress the [Ca2+]i plateau.
The physiological adenosine concentration in the brain is below 1 μM (Ballarin et al., 1991), but during ischaemia extracellular levels of adenosine increase up to 85-fold (Parkinson et al., 2000). Therefore, under this condition, the potentiation of the [Ca2+]i peak and the depression of ATP-induced [Ca2+]i plateau would be particularly relevant.
A crucial issue that remains to be addressed is the functional significance of such purine nucleotide and nucleoside crosstalk on [Ca2+]i dynamics. In the brain, adenine-based purines are involved in the regulation of a variety of physiological and pathophysiological processes, ranging from CNS development to nervous tissue remodelling following trauma, ischaemia or neurodegenerative disorders (for a review, see Rathbone et al., 1999). In astroglial cells, whereas adenine nucleotides seem to mainly trigger and maintain astrogliosis, adenosine has been suggested to play a crucial role in the control of astroglial proliferation and to promote apoptosis, thus tuning the elimination of useless cells during brain development or damaged cells during repair (Neary et al., 1996). However, whether changes in astroglial [Ca2+]i dynamics play a role in such processes remains to be ascertained. Notably, several lines of evidence indicate that the dynamics of [Ca2+]i signals play pivotal roles in the control of cell proliferation, cell differentiation and apoptosis in various cellular systems (for a review, see Berridge et al., 2000). Moreover, there is an indication that activation of different transcription factors depends on the amplitude and duration of the [Ca2+]i responses (Dolmetsch et al., 1997). Thus, it can be envisaged that some of the regulatory effects mediated by the activation of adenosine receptors in astrocytes may reside in its ability to regulate the [Ca2+]i patterning, elicited by stimulation of P2 receptors. In conclusion, we have shown that ATP and adenosine signalling can differently interact to regulate the cellular mechanisms controlling the [Ca2+]i response in cultured astrocytes. Owing to the importance of [Ca2+]i dynamics in several cellular functions, these data suggest that, compared to individual stimulation, coactivation of different adenine purinoceptor subfamilies may result in variable intracellular responses that may add a degree of specificity in the presence of complex extracellular inputs.
Acknowledgments
This study was supported by FIRB grants (MIUR) to M.N. and S.F.
Abbreviations
- AC
adenylyl cyclase
- ATP
adenosine 5′-triphosphate
- Bz-ATP
3′-O-(4-benzoyl)benzoyl-ATP
- [Ca2+]i
free cytosolic Ca2+ concentration
- cAMP
adenosine 3′,5′-cyclic monophosphate
- CCE
capacitative Ca2+ entry
- CGS-15943
9-chloro-2-(2-furyl)(1,2,4)-triazolo(1,5-c)quinazolin-5-amine
- CGS-21680
2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine
- 2-ClA
2-chloroadenosine
- CTX
cholera toxin
- dBcAMP
dibutyryl cyclic AMP
- DPX
1,3-diethyl-8-phenylxanthine
- IB-MECA
1-deoxy-1-(6-[([3-lodophenyl]methyl)-amino]-9H-purin-9-yl)-N-methyl-β-D-ribofuranuronamide
- IP3
inositol triphosphate
- MAPK
mitogen-activated protein kinase
- MRS 1706
N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide
- NECA
5′-N-ethylcarboxamidoadenosine
- R-PIA
R(−)N6-(2-phenylisopropyl)-adenosine
- PLC
phospholipase C
- PTX
pertussis toxin
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., CERUTI S., BARBIERI D., FRANCESCHI C., MALORNI W., BIONDO L., BURNSTOCK G., CATTABENI F. A novel action for adenosine: apoptosis of astroglial cells in rat brain primary cultures. Biochem. Biophys. Res. Commun. 1995;213:908–915. doi: 10.1006/bbrc.1995.2215. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., SAFFREY M.J., HOPKER V., BURNSTOCK G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- AMADIO S., D'AMBROSI N., CAVALIERE F., MURRA B., SANCESARIO G., BERNARDI G., BURNSTOCK G., VOLONTE C. P2 receptor modulation and cytotoxic function in cultured CNS neurons. Neuropharmacology. 2002;42:489–501. doi: 10.1016/s0028-3908(01)00197-6. [DOI] [PubMed] [Google Scholar]
- BALLARIN M., FREDHOLM B.B., AMBROSIO S., MAHY N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- BALLERINI P., RATHBONE M.P., DI IORIO P., RENZETTI A., GIULIANI P., D'ALIMONTE I., TRUBIANI O., CACIAGLI F., CICCARELLI R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7:2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., LIPP P., BOOTMAN M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- CORMIER R.J., MENNERICK S., MELBOSTAD H., ZORUMSKI C.F. Basal levels of adenosine modulate mGluR5 on rat hippocampal astrocytes. Glia. 2001;33:24–35. doi: 10.1002/1098-1136(20010101)33:1<24::aid-glia1003>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., JOHANSSON B., VAN DER PLOEG I., SEBASTIAO A.M., RIBEIRO J.A., FREDHOLM B.B. Evidence for functionally important adenosine A2A reeptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- DELUMEAU J.C., PETITET F., CORDIER J., GLOWINSKI J., PREMONT J. Synergistic regulation of cytosolic Ca2+ concentration in mouse astrocytes by NK1 tachykinin and adenosine agonists. J. Neurochem. 1991;57:2026–2035. doi: 10.1111/j.1471-4159.1991.tb06418.x. [DOI] [PubMed] [Google Scholar]
- DI IORIO P., KLEYWEGT S., CICCARELLI R., TRAVERSA U., ANDREW C.M., Crocker C.E., WERSTIUK E.S., RATHBONE M.P. Mechanisms of apoptosis induced by purine nucleosides in astrocytes. Glia. 2002;38:179–190. doi: 10.1002/glia.10055. [DOI] [PubMed] [Google Scholar]
- DOLMETSCH R.E., LEWIS R.S., GOODNOW C.C., HEALY J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- FERRONI S., MARCHINI C., SCHUBERT P., RAPISARDA C. Two distinct inwardly rectifying conductances are expressed in long term dibutyryl-cyclic-AMP treated rat cultured cortical astrocytes. FEBS Lett. 1995;367:319–325. doi: 10.1016/0014-5793(95)00588-z. [DOI] [PubMed] [Google Scholar]
- FERRONI S., MARCHINI C., OGATA T., SCHUBERT P. Recovery of deficient cholinergic calcium signaling by adenosine in cultured rat cortical astrocytes. J. Neurosci. Res. 2002;68:615–621. doi: 10.1002/jnr.10248. [DOI] [PubMed] [Google Scholar]
- FRANKE H., GROSCHE J., SCHADLICH H., KRUGEL U., ALLGAIER C., ILLES P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001a;108:421–429. doi: 10.1016/s0306-4522(01)00416-x. [DOI] [PubMed] [Google Scholar]
- FRANKE H., KRUGEL U., ILLES P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- FRANKE H., KRUGEL U., SCHMIDT R., GROSCHE J., REICHENBACH A., ILLES P. P2 receptor-types involved in astrogliosis in vivo. Br. J. Pharmacol. 2001b;134:1180–1189. doi: 10.1038/sj.bjp.0704353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B. Adenosine and neuroprotection. Int. Rev. Neurobiol. 1997;40:259–280. [PubMed] [Google Scholar]
- FUMAGALLI M., BRAMBILLA R., D'AMBROSI N., VOLONTE C., MATTEOLI M., VERDERIO C., ABBRACCHIO M.P. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- GAO Z., CHEN T., WEBER M.J., LINDEN J. A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells cross-talk between cyclic AMP and protein kinase c pathways. J. Biol. Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- GERWINS P., FREDHOLM B.B. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J. Biol. Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- HUNG A.C., SUN S.H. The P2X(7) receptor-mediated phospholipase D activation is regulated by both PKC-dependent and PKC-independent pathways in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal. 2002;14:83–92. doi: 10.1016/s0898-6568(01)00230-3. [DOI] [PubMed] [Google Scholar]
- ILLES P., NORENBERG W., GEBICKE-HAERTER P.J. Molecular mechanisms of microglial activation. B. Voltage- and purinoceptor-operated channels in microglia. Neurochem. Int. 1996;29:13–24. doi: 10.1016/0197-0186(95)00133-6. [DOI] [PubMed] [Google Scholar]
- JAMES G., BUTT A.M. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- JIMÉNEZ A.I., CASTRO E., COMMUNI D., BOEYNAEMS J.M., DELICADO E.G., MIRAS-PORTUGAL M.T. Coexpression of several types of metabotropic nucleotide receptors in single cerebellar astrocytes. J. Neurochem. 2000;75:2071–2079. doi: 10.1046/j.1471-4159.2000.0752071.x. [DOI] [PubMed] [Google Scholar]
- JIMÉNEZ A.I., CASTRO E., DELICADO E.G., MIRAS-PORTUGAL M.T. Potentiation of adenosine 5′-triphosphate calcium responses by diadenosine pentaphosphate in individual rat cerebellar astrocytes. Neurosci. Lett. 1998;246:109–111. doi: 10.1016/s0304-3940(98)00227-4. [DOI] [PubMed] [Google Scholar]
- JIMÉNEZ A.I., CASTRO E., MIRABET M., FRANCO R., DELICADO E.G., MIRAS-PORTUGAL M.T. Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia. 1999;26:119–128. [PubMed] [Google Scholar]
- KING B.F., NEARY J.T., ZHU Q., WANG S., NORENBERG M.D., BURNSTOCK G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- LEE K.S., SCHUBERT P., REDDINGTON M., KREUTZBERG G.W. Adenosine receptor density and the depression of evoked neuronal activity in the rat hippocampus in vitro. Neurosci. Lett. 1983;37:81–85. doi: 10.1016/0304-3940(83)90508-6. [DOI] [PubMed] [Google Scholar]
- LINDEN J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., RATHBONE M.P., CATTABENI F., ABBRACCHIO M.P., BURNSTOCK G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- NOBILE M., MONALDI I., ALLOISIO S., CUGNOLI C., FERRONI S. ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: evidence for a non-capacitative, P2X7-like-mediated calcium entry. FEBS Lett. 2003;538:71–76. doi: 10.1016/s0014-5793(03)00129-7. [DOI] [PubMed] [Google Scholar]
- OGATA T., NAKAMURA Y., SCHUBERT P. Potentiated cAMP rise in metabotropically stimulated rat cultured astrocytes by a Ca2+-related A1/A2 adenosine receptor cooperation. Eur. J. Neurosci. 1996;8:1124–1131. doi: 10.1111/j.1460-9568.1996.tb01280.x. [DOI] [PubMed] [Google Scholar]
- OGATA T., NAKAMURA Y., TSUJI K., SHIBATA T., KATAOKA K., SCHUBERT P. Adenosine enhances intracellular Ca2+ mobilization in conjunction with metabotropic glutamate receptor activation by t-ACPD in cultured hippocampal astrocytes. Neurosci. Lett. 1994;170:5–8. doi: 10.1016/0304-3940(94)90225-9. [DOI] [PubMed] [Google Scholar]
- PANENKA W., JIJON H., HERX L.M., ARMSTRONG J.N., FEIGHAN D., WEI T., YONG V.W., RANSOHOFF R.M., MACVICAR B.A. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J. Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PARKINSON F.E., SINCLAIR C.J., OTHMAN T., HAUGHEY N.J., GEIGER J.D. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43:836–846. doi: 10.1016/s0028-3908(02)00083-7. [DOI] [PubMed] [Google Scholar]
- PARKINSON F.E., ZHANG Y.W., SHEPEL P.N., GREENWAY S.C., PEELING J., GEIGER J.D. Effects of nitrobenzylthioinosine on neuronal injury, adenosine levels, and adenosine receptor activity in rat forebrain ischemia. J. Neurochem. 2000;75:795–802. doi: 10.1046/j.1471-4159.2000.0750795.x. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RATHBONE M.P., MIDDLEMISS P.J., GYSBERS J.W., ANDREW C., HERMAN M.A., REED J.K., CICCARELLI R., DI IORIO P., CACIAGLI F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- RIBEIRO J.A., SEBASTIAO A.M., DE MENDONCA A. Adenosine receptors in the nervous system: pathophysiological implications. Prog. Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- SCHUBERT P., OGATA T., MARCHINI C., FERRONI S., RUDOLPHI K. Protective mechanisms of adenosine in neurons and glial cells. Ann. N. Y. Acad. Sci. 1997;825:1–10. doi: 10.1111/j.1749-6632.1997.tb48409.x. [DOI] [PubMed] [Google Scholar]
- SCHUBERT P., RUDOLPHI K.A., FREDHOLM B.B., NAKAMURA Y. Modulation of nerve and glial function by adenosine: role in the development of ischemic damage. Int. J. Biochem. 1994;26:1227–1236. doi: 10.1016/0020-711x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- SUN S.H., LIN L.B., HUNG A.C., KUO J.S. ATP-stimulated Ca2+ influx and phospholipase D activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J. Neurochem. 1999;73:334–343. doi: 10.1046/j.1471-4159.1999.0730334.x. [DOI] [PubMed] [Google Scholar]
- TOMURA H., ITOH H., SHO K., SATO K., NAGAO M., UI M., KONDO Y., OKAJIMA F. Betagamma subunits of pertussis toxin-sensitive G proteins mediate A1 adenosine receptor agonist-induced activation of phospholipase C in collaboration with thyrotropin. A novel stimulatory mechanism through the cross-talk of two types of receptors. J. Biol. Chem. 1997;272:23130–23137. doi: 10.1074/jbc.272.37.23130. [DOI] [PubMed] [Google Scholar]
- VAN CALKER D., MULLER M., HAMPRECHT B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- VERKHRATSKY A., ORKAND R.K., KETTENMANN H. Glial calcium: homeostasis and signaling function. Physiol. Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- WU M.L., CHEN W.H., LIU I.H., TSENG C.D., WANG S.M. A novel effect of cyclic AMP on capacitative Ca2+ entry in cultured rat cerebellar astrocytes. J. Neurochem. 1999;73:1318–1328. doi: 10.1046/j.1471-4159.1999.0731318.x. [DOI] [PubMed] [Google Scholar]
- ZACCOLO M., POZZAN T. cAMP and Ca2+ interplay: a matter of oscillation patterns. TRENDS Neurosci. 2003;26:53–55. doi: 10.1016/s0166-2236(02)00017-6. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.Y., LI C., OLAH M.E., JOHNSON R.A., STILES G.L., CIVELLI O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]