Abstract
Airway smooth muscle (ASM) cells are known to switch from a contractile to a proliferative and synthetic phenotype in culture in response to serum and growth factors. Phenotype switching in response to contractile agonists, however, is poorly characterised, despite the possible relationship between ASM phenotype and airway remodelling in asthma.
To investigate the effects of muscarinic receptor stimulation on ASM phenotype, we used organ-cultured bovine tracheal smooth muscle (BTSM) strips, in which contractile responsiveness, contractile protein expression and proliferation were measured after pretreatment with methacholine.
Long-term methacholine pretreatment (8 days) decreased maximal contraction and sensitivity to methacholine as well as to histamine and KCl. This decrease was dose-dependent (pEC50=5.2±0.1). Pretreatment with the highest concentration of methacholine applied (100 μM) could suppress maximal histamine-induced contraction to 8±1% of control. In addition, contractile protein expression (myosin, actin) was downregulated two-fold. No concomitant increase in proliferative capacity was observed.
The M3/M2 muscarinic receptor antagonist DAU 5884 (0.1 μM) completely inhibited the observed decrease in contractility. In contrast, the M2/M3 muscarinic receptor antagonist gallamine (10 μM) was ineffective, demonstrating that M2 receptors were not involved.
Pretreatment (8 days) with 60 mM KCl could mimick the strong decreases in contractility. This was completely prevented by pretreatment with verapamil (1 μM).
Regulation of contractility was not affected by protein kinase C inhibition, whereas inhibitors of phosphatidyl inositol 3-kinase and p42/p44 mitogen activated protein kinase were partially effective.
These results show that long-term methacholine pretreatment (8 days) induces an M3 receptor-dependent decrease in BTSM contractility without increased proliferative capacity.
Keywords: Airway smooth muscle, contractility, phenotype, bovine, muscarinic M3 receptor, phosphatidylinositol 3-kinase, mitogen activated protein kinase, protein kinase C, proliferation
Introduction
Serum and growth factors are known to induce a switch in airway smooth muscle (ASM) phenotype, which is associated with a decreased contractile responsiveness and an increased proliferative capacity (Halayko & Solway, 2001; Gosens et al., 2002). Recently, we showed that long-term pretreatment up to 8 days of organ-cultured bovine tracheal smooth muscle (BTSM) strips with growth factors decreases contractility in a time-dependent manner. In addition, it was shown that the extent to which growth factors modulate contractility is linearly related to their proliferative response, which is in agreement with the concept that phenotypic changes occur in intact smooth muscle (Gosens et al., 2002). The mechanisms involved in this phenotypic shift are still not fully clear, although considerable evidence exists to support the hypothesis that the p42/p44 mitogen-activated protein kinase (MAPK)-pathway is involved in modulation toward a less contractile phenotype (Hayashi et al., 1999; Roy et al., 2001; Gosens et al., 2002). PI 3-kinase activity, however, has both been associated with a decrease in contractility (Gosens et al., 2002) and an increase in contractility in smooth muscle cells (Hayashi et al., 1998).
Muscarinic receptor stimulation may also play a role in phenotypic modulation. Both the Gi-coupled M2 receptor and the Gq-coupled M3 receptor are known to activate p42/p44 MAP kinase (Wylie et al., 1999) and PI 3-kinase (Murga et al., 1998) and may as such modulate ASM phenotype. ASM proliferation is dependent on the same pathways (Ammit & Panettieri, 2001), yet muscarinic receptor agonists are poor mitogens (Ediger & Toews, 2000; Gosens et al., 2003). Since proliferative response and modulation of contractility are linearly correlated, this would imply a relatively small role for nonmitogenic GPCR agonists in phenotypic modulation. However, proliferation synergy between growth factors and GPCR agonists has been reported on multiple occasions (Panettieri et al., 1998; Ediger & Toews, 2000; Krymskaya et al., 2000; Gosens et al., 2003). Hence, it can be imagined that muscarinic receptor agonists may augment growth factor-induced phenotypic modulation to a proliferative, less contractile state without large effects on phenotype by themselves. It has been reported, however, that the Rho/Rho-kinase pathway is involved in the activation of smooth muscle specific gene transcription through regulation of the nuclear localisation of serum response factor (SRF) (Liu et al., 2003). By activating RhoA, muscarinic receptor agonists might increase contractility or reduce the growth factor-induced decrease in contractility.
It has been postulated that ASM phenotype switching may be involved in airway remodelling in patients with chronic asthma, characterised by increased smooth muscle mass and irreversible airflow obstruction (Hirst, 1996). Since exaggerated cholinergic reflex mechanisms contribute to bronchial hyperreactivity in asthmatics (Adamko et al., 2001) and in allergen challenged guinea pigs (Santing et al., 1995), and since dysfunctional inhibitory M2 autoreceptors contribute to increased vagal acetylcholine in asthmatics (Adamko et al., 2001) and in guinea pigs (ten Berge et al., 1995), the long-term effects of muscarinic receptor agonists on ASM phenotype may be relevant for asthma. Since extracellular matrix proteins may influence ASM phenotype (Hirst et al., 2000; Gosens et al., 2002; Tao et al., 2003), we used organ-cultured BTSM strips with intact endogenous matrix. It was found that pretreatment with the muscarinic receptor agonist methacholine induces a less contractile phenotype with no concomitant increase in proliferative capacity, which was mediated by muscarinic M3 receptors.
Methods
Tissue preparation and organ-culture procedure
Bovine tracheae were obtained from local slaughterhouses and rapidly transported to the laboratory in Krebs-Henseleit (KH) buffer of the following composition (mM): NaCl 117.5, KCl 5.60, MgSO4 1.18, CaCl2 2.50, NaH2PO4 1.28, NaHCO3 25.00 and glucose 5.50, pregassed with 5% CO2 and 95% O2; pH 7.4. After dissection of the smooth muscle layer and careful removal of mucosa and connective tissue, tracheal smooth muscle strips were prepared while incubated in gassed KH-buffer at room temperature. Care was taken to cut tissue strips with macroscopically identical length (1 cm) and width (2 mm). Tissue strips were washed once in sterile Dulbecco's modification of Eagle's medium (DMEM), supplemented with NaHCO3 (7 mM), HEPES (10 mM), sodium pyruvate (1 mM), nonessential amino-acid mixture (1 :1 00), gentamicin (45 μg ml−1), penicillin (100 U ml−1), streptomycin (100 μg ml−1) and amphotericin B (1.5 μg ml−1). Next, tissue strips were transferred into suspension culture flasks and a volume of 7.5 ml serum-free medium was added per tissue strip. Drugs were added in a small volume (7.5 μl). Strips were maintained in culture in an incubator shaker (37°C, 55 rpm) for 8 days, refreshing the medium on day 4.
Isometric tension measurements
Tissue strips, collected from suspension culture flasks, were washed with several volumes of KH-buffer pregassed with 5% CO2 and 95% O2, pH 7.4 at 37°C. Subsequently, strips were mounted for isometric recording (Grass force-displacement transducer FT03) in 20 ml water-jacked organ baths, containing KH-buffer at 37°C, continuously gassed with 5% CO2 and 95% O2, pH 7.4. During a 90-min equilibration period, with washouts every 30 min, resting tension was gradually adjusted to 3 g. Subsequently, muscle strips were precontracted with 20 and 30 mM isotonic KCl solutions. Following two washouts, basal smooth muscle tone was established by the addition of 0.1 μM isoprenaline and tension was readjusted to 3 g, immediately followed by two changes of fresh KH-buffer. After another equilibration period of 30-min cumulative concentration response curves (CRCs) were constructed to stepwise increasing concentrations of histamine (10 nM100 μM), isotonic KCl (5.6–50 mM) or methacholine (1 nM–100 μM). Only one CRC was constructed per tissue strip. When maximal tension was obtained, the strips were washed several times and basal tone was established using isoprenaline (10 μM).
[3H]thymidine-incorporation
To obtain a proper comparison with functional responses of tissue-cultured strips (with intact extracellular matrix), we used tissue slices instead of cells for [3H]thymidine-incorporation experiments, following essentially the same procedure as in BTSM cells (Gosens et al., 2003). After dissection of the smooth muscle layer, the muscle was chopped three times at a setting of 500 μM and three times at a setting of 100 μM (McIlwain tissue chopper). Tissue slices were then transferred to suspension culture flasks containing medium, supplemented as mentioned above. Methacholine (10 μM) was included in the medium for 8 days, refreshing the medium on day 4. Tissue slices were then washed extensively by centrifugation with phosphate-buffered saline (PBS, composition (mM) NaCl, 140.0; KCl, 2.6; KH2PO4, 1.4; Na2HPO4·2H2O, 8.1; pH 7.4) and transferred into six-well cluster plates at 50 mg wet weight ml−1 well−1. Slices were stimulated with medium with or without PDGF (10 ng ml−1) for 48 h in the presence of [3H]thymidine. After incubation the slices were washed twice with PBS at room temperature and once with ice-cold 5% trichloroacetic acid (TCA). Slices were treated with this TCA-solution on ice for 30 min and subsequently the acid-insoluble fraction was dissolved in 1 ml NaOH (1 M). Incorporated [3H]thymidine was quantified by liquid-scintillation counting using a Beckman LS1701 β-counter.
Western analysis of contractile protein expression
After pretreatment of tissue strips as described under organ culture procedures, homogenates were prepared by pulverising tissue under liquid nitrogen and subsequent sonification in homogenisation buffer (composition in mM: NaCl 150.0, Tris HCl 10.0, 2-glycerophosphoric acid 5.0, EGTA 2.0, DTT 2.0, PMSF 1.0, Na3VO4 1.0, NaF 1.0, pH 7.5, supplemented with 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 1% Triton X-100). Homogenates were stored at –80°C until further use. Protein content was determined according to Bradford (1976). In total, 20 μg of protein per lane was separated by SDS/PAGE on 6% (for sm-myosin heavy chain, sm-MHC) or 10% (for sm-α-actin) polyacrylamide gels. Proteins in the gel were then transferred onto nitrocellulose membranes, which were blocked overnight in blocking buffer (composition: Tris 50.0 mM; NaCl 150.0 mM; Tween-20 0.1%, dried milk powder 5%). After two washes in wash buffer (composition: Tris 50.0 mM; NaCl 150.0 mM; Tween-20 0.1%), membranes were incubated for 1 h at room temperature in primary antibodies (anti-sm-MHC or anti-sm-α-actin, both diluted 1 : 200 in blocking buffer). After three washes, membranes were incubated in horseradish peroxidase labelled secondary antibodies (dilution 1 : 1000 in blocking buffer) at room temperature for 1 h, followed by another three washes. Antibodies were then visualised by enhanced chemiluminescence. Blots were analysed by densitometry (Totallab tm).
Data analysis
All data represent means±s.e. mean from n separate experiments. The statistical significance of differences between data was determined by the Student's t-test for paired observations or one-way ANOVA, where appropriate. Differences were considered to be statistically significant when P<0.05.
Materials
DMEM and methacholine hydrochloride were obtained from ICN Biomedicals (Costa Mesa, CA, U.S.A.). Foetal bovine serum, NaHCO3 solution (7.5%), HEPES solution (1 M), sodium pyruvate solution (100 mM), nonessential amino-acid mixture, gentamycin solution (10 mg ml−1), penicillin/streptomycin solution (5000 U ml−1/5000 μg ml−1) and amphotericin B solution (250 μg ml−1) (Fungizone) were obtained from Gibco BRL Life Technologies (Paisley, U.K.). Mouse monoclonal anti sm-MHC was from Neomarkers (Fremont, CA, U.S.A.). Platelet-derived growth factor AB (PDGF-AB, human recombinant), insulin (from bovine pancreas), mouse monoclonal anti sm-α-actin, rabbit anti-mouse IgG (peroxidase conjugate), sodium-dodecyl sulphate, aprotinin, leupeptin, apotransferrin (human), soybean trypsin inhibitor, gallamine triethiodide, histamine dihydrochloride and (−)isoprenaline hydrochloride were from Sigma (St Louis, MO, U.S.A.). Enhanced chemiluminescence reagents were from Pierce (Rockford, IL, U.S.A.). PD 98059 and LY 294002 were obtained from Tocris Cookson Ltd. (Bristol, U.K.). DAU 5884 was a kind gift from Dr HN Doods (Dr Karl Thomae GmbH, Biberach, Germany). L(+)ascorbic acid was from Merck (Darmstadt, Germany). [methyl-3H]thymidine (specific activity 25 Ci mmol−1) was obtained from Amersham (Buckinghamshire, U.K.) All other chemicals were of analytical grade.
Results
Effects of pretreatment with methacholine on BTSM phenotype
Long-term pretreatment (8 days) of BTSM strips with 10 μM methacholine resulted in a decline in contractility, as determined using both KCl, histamine and methacholine-induced contraction (Figure 1, Table 1). Apart from a diminished maximal contraction (Emax), sensitivity (pEC50) to all agonists used was decreased as well. This decrease in sensitivity was the most profound for methacholine itself (Table 1). Pretreatment with 10 ng ml−1 PDGF also induced a decline in Emax of histamine, though considerably smaller as compared to methacholine pretreatment, but no effect on sensitivity was observed (Figure 2). Remarkably, combined pretreatment with 10 μM methacholine and 10 ng ml−1 PDGF depressed maximal contraction in an additive rather than synergistic manner (Figure 2).
Figure 1.
Effect of 8-day methacholine pretreatment on BTSM contractility. BTSM strips were organ cultured for 8 days in the absence (open symbols) or presence (closed symbols) of 10 μM methacholine, after which contraction to (a) KCl, (b) histamine and (c) methacholine-induced was determined. Data represent means±s.e. mean from four (methacholine) to eight (KCl, histamine) experiments, each performed in duplicate.
Table 1.
Maximal contraction (Emax) and sensitivity of BTSM strips for KCl, histamine and methacholine after 8-day pretreatment in the absence or presence of 10 μM methacholine
| Control | Methacholine (10 μM) pretreated | |||
|---|---|---|---|---|
| Sensitivity | Emax (%) | Sensitivity | Emax (%) | |
| KCl | 20.8±1.1 | 100 | 25.7±0.9* | 37±5** |
| Histamine | 5.9±0.1 | 100 | 5.7±0.1* | 46±3** |
| Methacholine | 7.3±0.1 | 100 | 6.5±0.1* | 48±6* |
Sensitivity to KCl expressed as EC50 (mM); sensitivity to histamine and methacholine expressed as pEC50 (−log M). Data represent means±s.e.mean from four (methacholine) to eight (KCl, histamine) experiments, each performed in duplicate.
P<0.05;
P<0.001 compared to control.
Figure 2.
Interaction of methacholine and PDGF pretreatment on histamine-induced BTSM contraction. BTSM strips were organ cultured for 8 days in the absence or presence of methacholine (10 μM), PDGF (10 ng ml−1) and the combination hereof. Data represent means±s.e. mean from five experiments, each performed in duplicate.
The decrease in contractile capacity after pretreatment with methacholine was not accompanied by an increase in proliferative capacity. Basal incorporation of [3H]thymidine was not changed after pretreatment with methacholine. Interestingly, stimulated (PDGF-induced) incorporation of [3H]thymidine was decreased rather than increased (P<0.05, Figure 3). It should be noted that methacholine was present during pretreatment only and was washed away thoroughly before the start of the [3H]thymidine-incorporation experiment, to prevent a functional interaction with PDGF.
Figure 3.
Basal and PDGF-stimulated (10 ng ml−1) [3H]thymidine incorporation in BTSM tissue slices, organ cultured for 8 days in the absence or presence of 10 μM methacholine. After the culture period, tissue slices were thoroughly washed and stimulated thereafter. Data represent means±s.e. mean from four experiments, each performed in duplicate. *P<0.05 compared to control.
A concentration response curve could be constructed for the inhibitory effect of methacholine pretreatment on maximal histamine-induced contraction, that was characterised by a pEC50 of 5.2±0.1. The maximal contraction to histamine was almost abolished by pretreatment with the highest concentration of methacholine tested (Figure 4). A similar concentration dependency was observed for contractile protein expression in BTSM strips, pretreated with methacholine (Figure 5). Both sm-α-actin and sm-MHC were considerably downregulated to 41±12 and 50±13% of control, respectively, at the highest concentration of methacholine applied (100 μM).
Figure 4.
Concentration dependency of the methacholine-induced pretreatment effects. (a) Histamine-induced contraction of BTSM strips, organ cultured for 8 days in the absence or presence of increasing concentrations of methacholine. (b) Maximal contraction (Emax) of histamine of BTSM strips, organ cultured with increasing concentrations of methacholine. Data in (b) represent maximal contractions measured under (a). Data represent means±s.e. mean from four experiments, each performed in duplicate.
Figure 5.
Western analysis of contractile protein expression (sm-MHC, sm-α-actin) in BTSM strips, organ cultured for 8 days in the absence (C) or presence of increasing concentrations of methacholine. Blots shown are representative for four experiments. Graph shows the means±s.e. mean obtained after densitometry scans of the blots.
Role of M2 and M3 receptors
To establish the muscarinic receptor subtype(s) involved in these responses to methacholine, we measured the inhibitory effects of selective receptor antagonists DAU 5884 and gallamine. Since tracheal smooth muscle expresses M3 and M2 muscarinic receptors only (Zaagsma et al., 1997), DAU 5884 was applied in a concentration selective for M3 over M2 receptors and gallamine in a concentration selective for M2 over M3 receptors (Roffel et al., 1988; Gosens et al., 2003). The antagonists were applied during the entire pretreatment period. Interestingly, combined pretreatment with DAU 5884 could completely prevent the strong methacholine (10 μM)-induced decline in Emax of histamine. In contrast, gallamine was fully ineffective (Figure 6).
Figure 6.
Role of M2 and M3 receptors in the methacholine-induced effects on BTSM contractility. BTSM strips were organ cultured for 8 days in the absence (open bars) or presence (closed bars) of 10 μM methacholine. During the entire culture period, BTSM strips were also incubated with DAU 5884 (0.1 μM, DAU) or gallamine (10 μM, Gall) where indicated. Untreated preparations served as controls (C). Data shown represent maximal contractile responses to histamine and are means±s.e. mean from four experiments, each performed in duplicate. *P<0.05.
Effect of prolonged [Ca2+]i increases
Long-term pretreatment with methacholine may induce a prolonged rise in intracellular [Ca2+]. To investigate a possible role for Ca2+ in long-term regulation of contractility, strips were pretreated for 8 days with medium suppplemented with CaCl2 (2.5 mM) and KCl (60 mM) to induce a prolonged increase in intracellular [Ca2+] by opening of L-type Ca2+-channels. This pretreatment strongly decreased histamine-induced contraction when compared to control strips. Combined pretreatment with the Ca2+-entry blocker verapamil (1 μM) completely prevented this 60 mM K+-induced decrease (Figure 7).
Figure 7.
Effects of prolonged increases in intracellular [Ca2+] on BTSM contractility. BTSM strips were organ cultured for 8 days in serum-free medium (open circles), in medium supplemented with CaCl2 and KCl to produce final concentrations of 2.5 mM Ca2+ and 60 mM K+, respectively (closed circles), or in medium supplemented with verapamil (1 μM) and CaCl2 and KCl (open triangles). Data shown represent contractile responses to histamine and are means± s.e. mean from three experiments, each performed in duplicate.
Role of protein kinase C, PI 3-kinase and p42/p44 MAP kinase
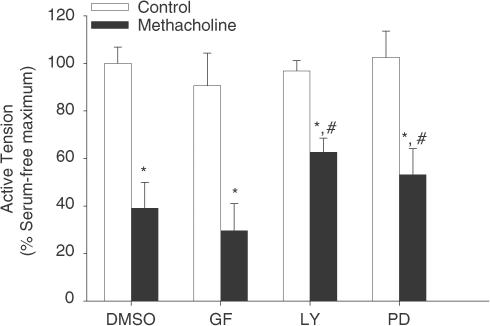
To investigate the contribution of protein kinase C (PKC), PI 3-kinase and p42/p44 MAP kinase to the methacholine-induced decline in contractility, selective inhibitors were used: GF 109203X (10 μM) for PKC, LY 294002 (10 μM) for PI 3-kinase and PD 98059 (30 μM) for p42/p44 MAP kinase. All kinase inhibitors were dissolved in dimethylsulphoxide (DMSO) at a final concentration of 0.1% (vehicle), which did not affect the effect of methacholine pretreatment (Emax=34±11 and 39±11% in the absence and presence of DMSO, respectively). With GF 109203X present during the culture period, no reduction in the methacholine-induced effects was observed. In contrast, both PD 98059 and LY 294002 were partially but significantly effective (Figure 8).
Figure 8.
Role of PKC, p42/p44 MAP kinase and PI 3-kinase in the methacholine-induced effects on BTSM contractility. BTSM strips were organ cultured for 8 days in the absence (open bars) or presence (closed bars) of 10 μM methacholine. During the entire culture period, GF 109203X (10 μM, GF) PD 98059 (30 μM, PD) or LY 294002 (10 μM, LY) were also present where indicated. Vehicle treated (0.1% DMSO) preparations served as controls. Data shown represent maximal contractile responses to histamine and are means±s.e. mean from four experiments, each performed in duplicate. *P<0.05 compared to controls, # P<0.05 compared to methacholine (10 μM) and DMSO (0.1%)-pretreated.
Discussion
In the present study we found that pretreatment with methacholine dramatically decreases contractile responsiveness of organ-cultured BSTM strips towards both receptor- and nonreceptor-mediated stimuli. Since contractility of 8 days serum-free pretreated strips is only slightly higher compared to fresh tissue (Gosens et al., 2002), it can be concluded that the methacholine-induced phenotype is also less contractile when compared to fresh tissue. This less contractile state induced by methacholine appeared to be different of nature from that induced by growth factors and serum: pretreatment with methacholine could almost completely abrogate contractile responses, whereas maximal modulation of contractility induced by the highly mitogenic growth factor PDGF amounts only 33–37% (Gosens et al., 2002).
Furthermore, sensitivities (EC50/pEC50) to KCl, histamine and methacholine were all decreased after pretreatment with methacholine, which is not observed after pretreatment with serum and growth factors. Not surprisingly, this shift in sensitivity was the largest for methacholine itself, indicating homologous desensitisation of muscarinic M3 receptors as well. Hypothetically, histamine H1 receptors and voltage-dependent Ca2+ channels may also have been downregulated or desensitised, since Gq coupled receptors may induce both homologous and heterologous desensitisation (Bundey & Nahorski, 2001). Nevertheless, it is unlikely that changes in receptor density or coupling efficiency account for the observed decreases in maximal contraction. First, maximal KCl, histamine and methacholine-induced contraction were influenced similarly by methacholine pretreatment, both qualitatively and quantitatively. This implies the involvement of a common mechanism. However, methacholine and histamine induce contraction through stimulation of PI-turnover and subsequent release of Ca2+ from intracellular stores followed by capacitative Ca2+-entry (Zaagsma et al., 1997). In contrast, KCl-induced contraction is fully dependent on voltage-dependent Ca2+-influx and does not require GPCRs. Therefore, quantitative similarities between KCl, histamine and methacholine can be achieved only by affecting contraction downstream of intracellular Ca2+-increases. Moreover, contractile protein expression (sm-α-actin, sm-MHC) was dramatically decreased after methacholine pretreatment with a similar concentration dependency as observed for the decrease in contractility. Taken together, this clearly indicates that changes in contractile protein expression rather than changes in receptor density are responsible for the observed decrease in maximal contraction.
Basal incorporation of [3H]thymidine was not changed after pretreatment with methacholine, whereas stimulated (PDGF) incorporation of [3H]thymidine was decreased, which might indicate desensitisation of receptor tyrosine kinases. Previously, we showed that acute costimulation with similar concentrations of methacholine and PDGF synergistically increases [3H]thymidine incorporation (Gosens et al., 2003). Note that the present experiments were performed in tissue slices rather than in cells to avoid loss of cell culture-induced muscarinic receptor expression (Widdop et al., 1993). However, PDGF-induced incorporation of [3H]thymidine produced quantitatively similar results in tissue slices and cells. The observation that the decrease in contractility induced by methacholine was not accompanied by an increase in proliferative capacity further indicates that the phenotype change induced by methacholine is different from the classical switch induced by serum and growth factors. The decline in contractility induced by growth factors is accompanied by an increase in proliferation, which represents a shift in smooth muscle function for optimal adaptation to a mitogenic environment.
Given the inability of methacholine to induce BTSM cell proliferation by itself, and the strong correlations found for loss of contractility and proliferative responses induced by growth factors (Gosens et al., 2002), methacholine and growth factors are not likely to rely on the same mechanism to induce phenotype changes. Indeed, although PI 3-kinase and p42/p44 MAP kinase are involved, at least in part, in the growth factor-induced phenotype shift (Gosens et al., 2002), inhibitors of the p42/p44 MAP kinase pathway and PI 3-kinase only modestly inhibited the methacholine-induced decline in contractility. Inhibition of PKC using GF 109203X was even completely ineffective. It is noteworthy that the applied concentrations of GF 109203X and LY 294002 are known to almost completely inhibit PKC and PI 3-kinase, respectively (Walker et al., 1998), whereas the applied concentration of PD 98059 is known to fully inhibit p42/p44 MAPK activation in BTSM cells (Karpova et al., 1997). Therefore, it appears that p42/p44 MAPK, PI 3-kinase and PKC do not play a key role in the methacholine-induced effects, although p42/p44 MAPK and PI 3-kinase may contribute to some extent. (Togashi et al., 1998; Camoretti-Mercado et al., 2000; Mack et al., 2001)
The observed effects of methacholine pretreatment are dependent on muscarinic M3 receptors as determined using DAU 5884. In contrast, M2 receptors are not relevant in view of the lack of effect of gallamine. Importantly, BTSM contraction is fully dependent on M3 receptors (Roffel et al., 1988). Therefore, it could be envisaged that the presented regulation of contractility by methacholine is dependent on the contractile state of the muscle during the pretreatment period. Pretreatment with 1 μM methacholine has no effect at all, however, on any of the tested phenotype parameters (cf Figures 4 and 5), whereas BTSM strips acutely exposed to this concentration of methacholine contract almost maximally (88±6% of maximum). More specifically, the sensitivities of methacholine for the long-term effects on contractility (pEC50=5.2) and acute effects on BTSM contraction (pEC50=7.0) are too deviant. It could be envisaged that methacholine may have underwent some degree of metabolism during the incubation period, which would (in part) explain the difference in pEC50 values for contraction and for contractility changes. However, even the strips exposed to 1 μM methacholine were still contracted on day 4 when refreshing the medium and on day 8 when preparing for the experiment, demonstrating that a causal relationship between contractile status and regulation of contractility can be excluded. A better match is observed with the potency of methacholine on phosphoinositide metabolism (pEC50=5.6, Hoiting et al., 1996). Moreover, prolonged increases in [Ca2+]i are known to attenuate contractility in the organ-cultured rat tail artery (Hellstrand, 1998; Lindqvist et al., 1999) and of the organ-cultured guinea pig ileum (Gomez & Sward, 1997). In addition, induction of a sustained increase in [Ca2+]i using medium containing 60 mM K+ and 2.5 mM Ca2+ strongly decreased contractility of BTSM, which was fully dependent on Ca2+-entry in view of the effect of combined pretreatment with verapamil. Therefore, it seems reasonable to assume that the prolonged increases in [Ca2+]i caused by long-term M3 receptor stimulation is the cause of the observed decrease of contractility. The mechanisms behind these effects of [Ca2+]i remain to be established, however.
A regulatory mechanism in smooth muscle cells to adjust for prolonged increases in [Ca2+]i may be physiologically relevant. However, activation of the Rho/Rho-kinase/SRF-pathway may act as an opposing mechanism to increase contractility (Liu et al., 2003). It is not clear how the balance in these mechanisms relates to long-term regulation of contractility. The phenotypic starting-point may be of critical relevance: the highest SRF-mediated smooth muscle specific gene transcription is observed in synthetic, not contractile smooth muscle cells (Camoretti-Mercado et al., 2000). Since intact BTSM was used in our present study, which consists of contractile cells, studies using cultured synthetic cells may observe other effects of GPCR stimulation. The phenotypic status may therefore be very relevant for the effects of long-term stimulation with GPCR agonists and ultimately determining the effect on contractility in vivo. In addition, load applied to the muscle during culture may also interact with GPCR-induced effects on smooth muscle contractility or phenotype since mechanical strain is known to increase RhoA activation and ASM contractile protein expression (Smith et al., 1997, 2003). Since ASM is subjected to (some) load under physiological conditions, the in vivo response to prolonged GPCR agonist exposure may therefore be somewhat more complex.
In conclusion, prolonged treatment with methacholine strongly decreases BTSM contractility without a concomitant increase in proliferative capacity. The decreased contractility is explained by diminished contractile protein expression and depends on M3 receptor stimulation and subsequent increases in intracellular [Ca2+]. Since muscarinic M3 receptor stimulation is nonmitogenic by itself, this indicates that the phenotype change induced by methacholine is different from the classical switch induced by serum and growth factors.
Acknowledgments
This work was financially supported by the Netherlands Asthma Foundation, grants NAF 99.53 (RG) and 01.83 (DS).
Abbreviations
- ASM
airway smooth muscle
- BTSM
bovine tracheal smooth muscle
- DMEM
Dulbecco's modification of Eagle's medium
- DMSO
dimethylsulphoxide
- FBS
foetal bovine serum
- GPCR
G-protein coupled receptor
- KH
Krebs-Henseleit
- MAPK
mitogen-activated protein kinase
- MHC
myosin heavy chain
- MLCK
myosin light chain kinase
- PBS
phosphate buffered saline
- PDGF
platelet-derived growth factor
- PI-3 kinase
phosphatidylinositol-3 kinase
- PI-turnover
phosphoinositide turnover
- PKC
protein kinase C
- SRF
serum response factor
- TCA
trichloroacetic acid
References
- ADAMKO D.J., FRYER A.D., JACOBY D.B.Dysfunction of prejunctional muscarinic M2 receptors: role of environmental factors Muscarinic receptors in airways diseases 2001Basel: Birhäuser; 107–120.ed. Zaagsma, J., Meurs, H. & Roffel, A.F., pp [Google Scholar]
- AMMIT A.J., PANETTIERI R.A., JR The circle of life: cell cycle regulation in airway smooth muscle. J. Appl. Physiol. 2001;91:1431–1437. doi: 10.1152/jappl.2001.91.3.1431. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUNDEY R.A., NAHORSKI S.R. Homologous and heterologous uncoupling of muscarinic M3 and α1B adrenoceptors to Gαq/11 in SH-SY5Y human neuroblastoma cells. Br. J. Pharmacol. 2001;134:257–264. doi: 10.1038/sj.bjp.0704229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMORETTI-MERCADO B., LIU H.W., HALAYKO A.J., FORSYTHE S.M., KYLE J.W., LI B., FU Y., MCCONVILLE J., KOGUT P., VIEIRA J.E., PATEL N.M., HERSHENSON M.B., FUCHS E., SINHA S., MIANO J.M., PARMACEK M.S., BURKHARDT J.K., SOLWAY J. Physiological control of smooth muscle-specific gene expression through regulated nuclear translocation of serum response factor. J. Biol. Chem. 2000;275:30387–30393. doi: 10.1074/jbc.M000840200. [DOI] [PubMed] [Google Scholar]
- EDIGER T.L., TOEWS M.L. Synergistic stimulation of airway smooth muscle cell mitogenesis. J. Pharmacol. Exp. Ther. 2000;294:1076–1082. [PubMed] [Google Scholar]
- GOMEZ M., SWARD K. Long-term regulation of contractility and calcium current in smooth muscle. Am. J. Physiol. 1997;273:C1714–C1720. doi: 10.1152/ajpcell.1997.273.5.C1714. [DOI] [PubMed] [Google Scholar]
- GOSENS R., MEURS H., BROMHAAR M.M., MCKAY S., NELEMANS S.A., ZAAGSMA J. Functional characterization of serum- and growth factor-induced phenotypic changes in intact bovine tracheal smooth muscle. Br. J. Pharmacol. 2002;137:459–466. doi: 10.1038/sj.bjp.0704889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSENS R., NELEMANS S.A., GROOTTE BROMHAAR M.M., MCKAY S., ZAAGSMA J., MEURS H. Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am. J. Resp. Cell Mol. Biol. 2003;28:257–262. doi: 10.1165/rcmb.2002-0128OC. [DOI] [PubMed] [Google Scholar]
- HALAYKO A.J., SOLWAY J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J. Appl. Physiol. 2001;90:358–368. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- HAYASHI K., SAGA H., CHIMORI Y., KIMURA K., YAMANAKA Y., SOBUE K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273:28860–28867. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- HAYASHI K., TAKAHASHI M., KIMURA K., NISHIDA W., SAGA H., SOBUE K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J. Cell Biol. 1999;145:727–740. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLSTRAND P. Long-term effects of intracellular calcium and growth factors on excitation and contraction in smooth muscle. Acta Physiol. Scand. 1998;164:637–644. doi: 10.1111/j.1365-201x.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- HIRST S.J. Airway smooth muscle cell culture: application to studies of airway wall remodelling and phenotype plasticity in asthma. Eur. Respir. J. 1996;9:808–820. doi: 10.1183/09031936.96.09040808. [DOI] [PubMed] [Google Scholar]
- HIRST S.J., TWORT C.H., LEE T.H. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am. J. Respir. Cell Mol. Biol. 2000;23:335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- HOITING B.H., MEURS H., SCHUILING M., KUIPERS R., ELZINGA C.R., ZAAGSMA J. Modulation of agonist-induced phosphoinositide metabolism, Ca2+ signalling and contraction of airway smooth muscle by cyclic AMP-dependent mechanisms. Br. J. Pharmacol. 1996;117:419–426. doi: 10.1111/j.1476-5381.1996.tb15207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARPOVA A.Y., ABE M.K., LI J., LIU P.T., RHEE J.M., KUO W.L., HERSHENSON M.B. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am. J. Physiol. 1997;272:L558–L565. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- KRYMSKAYA V.P., ORSINI M.J., ESZTERHAS A.J., BRODBECK K.C., BENOVIC J.L., PANETTIERI R.A., PENN R.B. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2000;23:546–554. doi: 10.1165/ajrcmb.23.4.4115. [DOI] [PubMed] [Google Scholar]
- LINDQVIST A., NORDSTROM I., MALMQVIST U., NORDENFELT P., HELLSTRAND P. Long-term effects of Ca(2+) on structure and contractility of vascular smooth muscle. Am. J. Physiol. 1999;277:C64–C73. doi: 10.1152/ajpcell.1999.277.1.C64. [DOI] [PubMed] [Google Scholar]
- LIU H.W., HALAYKO A.J., FERNANDES D.J., HARMON G.S., MCCAULEY J.A., KOCIENIEWSKI P., MCCONVILLE J., FU Y., FORSYTHE S.M., KOGUT P., BELLAM S., DOWELL M., CHURCHILL J., LESSO H., KASSIRI K., MITCHELL R.W., HERSHENSON M.B., CAMORETTI-MERCADO B., SOLWAY J. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am. J. Respir. Cell Mol. Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- MACK C.P., SOMLYO A.V., HAUTMANN M., SOMLYO A.P., OWENS G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- MURGA C., LAGUINGE L., WETZKER R., CUADRADO A., GUTKIND J.S. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinasegamma. J. Biol. Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- PANETTIERI R.A., TAN E.M., CIOCCA V., LUTTMANN M.A., LEONARD T.B., HAY D.W. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction In vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am. J. Respir. Cell Mol. Biol. 1998;19:453–461. doi: 10.1165/ajrcmb.19.3.2999. [DOI] [PubMed] [Google Scholar]
- ROFFEL A.F., ELZINGA C.R., VAN AMSTERDAM R.G., DE ZEEUW R.A., ZAAGSMA J. Muscarinic M2 receptors in bovine tracheal smooth muscle: discrepancies between binding and function. Eur. J. Pharmacol. 1988;153:73–82. doi: 10.1016/0014-2999(88)90589-4. [DOI] [PubMed] [Google Scholar]
- ROY J., KAZI J.M., HEDIN U., THYBERG J. Phenotypic modulation of arterial smooth muscle cells is associated with prolonged activation of ERK 1/2. Differentiation. 2001;67:50–58. doi: 10.1046/j.1432-0436.2001.067001050.x. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., PASMAN Y., OLYMULDER C.G., ROFFEL A.F., MEURS H., ZAAGSMA J. Contribution of a cholinergic reflex mechanism to allergen-induced bronchial hyperreactivity in permanently instrumented, unrestrained guinea-pigs. Br. J. Pharmacol. 1995;114:414–418. doi: 10.1111/j.1476-5381.1995.tb13242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P.G., MORENO R., IKEBE M. Strain increases airway smooth muscle contractile and cytoskeletal proteins in vitro. Am. J. Physiol. 1997;272:L20–L27. doi: 10.1152/ajplung.1997.272.1.L20. [DOI] [PubMed] [Google Scholar]
- SMITH P.G., ROY C., ZHANG Y.N., CHAUDURI S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2003;28:432–436. doi: 10.1165/rcmb.4754. [DOI] [PubMed] [Google Scholar]
- TAO F., CHAUDRY S., TOLLOCZKO B., MARTIN J.G., KELLY S.M. Modulation of smooth muscle phenotype in vitro by homologous cell substrate. Am. J. Physiol. Cell Physiol. 2003;284:C1531–C1541. doi: 10.1152/ajpcell.00264.2002. [DOI] [PubMed] [Google Scholar]
- TEN BERGE R.E., SANTING R.E., HAMSTRA J.J., ROFFEL A.F., ZAAGSMA J. Dysfunction of muscarinic M2 receptors after the early allergic reaction: possible contribution to bronchial hyperresponsiveness in allergic guinea-pigs. Br. J. Pharmacol. 1995;114:881–887. doi: 10.1111/j.1476-5381.1995.tb13286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOGASHI H., EMALA C.W., HALL I.P., HIRSHMAN C.A. Carbachol-induced actin reorganization involves Gi activation of Rho in human airway smooth muscle cells. Am. J. Physiol. 1998;274:L803–L809. doi: 10.1152/ajplung.1998.274.5.L803. [DOI] [PubMed] [Google Scholar]
- WALKER T.R., MOORE S.M., LAWSON M.F., PANETTIERI R.A., JR, CHILVERS E.R. Platelet-derived growth factor-BB and thrombin activate phosphoinositide 3-kinase and protein kinase B: role in mediating airway smooth muscle proliferation. Mol. Pharmacol. 1998;54:1007–1015. doi: 10.1124/mol.54.6.1007. [DOI] [PubMed] [Google Scholar]
- WIDDOP S., DAYKIN K., HALL I.P. Expression of muscarinic M2 receptors in cultured human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 1993;9:541–546. doi: 10.1165/ajrcmb/9.5.541. [DOI] [PubMed] [Google Scholar]
- WYLIE P.G., CHALLISS R.A., BLANK J.L. Regulation of extracellular-signal regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem. J. 1999;338:619–628. [PMC free article] [PubMed] [Google Scholar]
- ZAAGSMA J., ROFFEL A.F., MEURS H. Muscarinic control of airway function. Life Sci. 1997;60:1061–1068. doi: 10.1016/s0024-3205(97)00048-9. [DOI] [PubMed] [Google Scholar]