Abstract
Diosmectite is a natural silicate effectively used in the treatment of infectious diarrhoea. Its antidiarrhoeal properties involve adsorption of toxins and bacteria and modifications of the rheological characteristics of gastrointestinal mucus. Hence, the aim of this study was to test the intestinal anti-inflammatory activity of diosmectite.
Diosmectite (500 mg kg−1 day−1, p.o.) was administered as a post-treatment to rats with chronic trinitrobenzene sulphonic acid colitis. Colonic status was checked 1 and 2 weeks after colitis induction by macroscopic, histological and biochemical examination.
Diosmectite post-treatment resulted in amelioration of the morphological signs (intestinal weight, macroscopic damage, necrosed area, histology) and biochemical markers (myeloperoxidase activity, glutathione levels, MUC2 expression, inducible nitric oxide synthase and interleukin-1β (IL-1β) and leukotriene B4 synthesis), as well as in the reduction of the severity of diarrhoea. The effect of the clay was comparable to that of sulphasalazine (50 mg kg−1 day−1).
Diosmectite exhibited a dose-dependent capacity to adsorb proteins in vitro as well as a dose-dependent inhibitory effect on the basolateral secretion of IL-8 by lipopolysaccharide (LPS)-stimulated HT29 cells.
Diosmectite had a dose-dependent inhibitory effect on IL-1β production by LPS-stimulated THP-1 cells.
The effect of diosmectite on MUC2 was post-transcriptional, since mRNA levels were unaffected. However, diosmectite is able to upregulate MUC2 mRNA levels in HT29-MTX cells.
Diosmectite has anti-inflammatory activity administered as a post-treatment. Possible mechanisms include adsorption of luminal antigens, increase of colonic mucin levels and possibly a direct modulatory action of cytokine production by mucosal cells.
Keywords: Diosmectite, experimental colitis, inflammatory bowel disease, trinitrobenzenesulphonic acid
Introduction
Inflammatory bowel disease (IBD) is a denomination that refers essentially to two different but closely related conditions, ulcerative colitis and Crohn's disease. Both are chronic disorders that have a substantial prevalence in the developed world (Sandler, 1994) and are currently difficult to cure pharmacologically (Sands, 2000). In fact, IBD represents one of the pathological disorders posing the most compelling need for new therapeutic strategies. Glucocorticoids, immunosuppressants and aminosalicylates comprise the mainstay of pharmacological therapy in IBD (Sands, 2000). However, despite their proven efficacy in managing these conditions, these drugs have a significant profile of side effects that are all the more important in the context of continued administration. IBD (Brennan et al., 1995; Jung et al., 1995) is one of the gastrointestinal conditions, including infectious diarrhoea (Powell, 1994; Mathan et al., 1995) and food allergy (Heyman & Desjeux, 1992; Heyman et al., 1994), in which the intestinal barrier is weakened by the release of proinflammatory cytokines due to the abnormal activation of the epithelial cells (Jung et al., 1995) and the underlying immune system (Adams et al., 1993; Castro & Powell, 1994).
Barrier disruption by inflammation leads to increased stimulation by luminal antigens, such as food antigens, bacterial toxins and microorganisms, of the different types of cells in the lamina propria, including immune cells, nerve plexus cells, mast cells and mesenchymal cells. In this connection, mucosal inflammation can be considered a self-perpetuating process in which the disruption of the epithelial layer plays a central role (Heyman & Desjeux, 1992). In the case of IBD, namely Crohn's disease, a mucosal permeability defect has been reported to precede inflammation (Hollander et al., 1986). Restoration of the epithelial barrier might be achieved by acting on the basal or lamina propia side of the epithelium; for instance, antibodies directed against tumour necrosis factor (TNF) have been found to prevent the effect of TNF in vitro (Heyman et al., 1994) and have a therapeutic value in vivo (Bell & Kamm, 2000). An alternative approach would be to act on the luminal side of the epithelium by removing the offending antigens or restoring the integrity of the epithelial barrier. Diosmectite is a natural clay consisting of an insoluble double silicate of aluminium and magnesium that has been effectively used in the treatment of several gastrointestinal diseases, like infectious diarrhoea and food allergy (Dupont et al., 1992; Theodorou et al., 1994). In acute diarrhoea in children, this beneficial effect was manifested by reductions in the duration of diarrhoea, the frequency of liquid stools and the number of cases of prolonged diarrhoea (Madkour et al., 1993). The mechanism of action of diosmectite has been claimed to involve adsorption of toxins and bacteria and modifications of the gastrointestinal mucus that reinforces the mucosal barrier (Fioramonti et al., 1990). In fact, diosmectite treatment induces changes in the chemical nature of mucopolysaccharides (More et al., 1987), thus modifying their rheological properties (Droy et al., 1985). These changes strongly reduce the penetration of toxins through the mucus layer, since a protective action of diosmectite against toxin-induced digestive disturbances has been observed only after several days of treatment and when toxins were given at a time when the last dose of diosmectite had been eliminated from the digestive tract (Fioramonti et al., 1987). Diosmectite treatment may also repair mucosal integrity, as suggested by the normalization of the urinary lactulose/mannitol ratio found in children with acute diarrhoea (Dupont et al., 1992) and by the restoration of luminal surface integrity in experimental bacterial infection (Rateau et al., 1982; Fioramonti et al., 1987). In addition, diosmectite has been shown to increase the effectiveness of the mucus barrier against mucosal damage by pepsin in vivo (Leonard et al., 1994), and to protect against the allergic digestive disturbances induced in guinea-pigs sensitized to cow's milk (Theodorou et al., 1994). Recently, it has been demonstrated that diosmectite restores the epithelial barrier defect induced by the proinflammatory cytokine TNF in the intestinal cell line HT29-19A, as shown by the significant decrease in ionic conductance and the decreases in mannitol and horseradish peroxidase permeabilities (Mahraoui et al., 1997), by an unknown mechanism. These results suggest that diosmectite may reduce the consequences of intestinal inflammation by acting from the luminal side of the epithelium. It should be noted that, despite the aforementioned pieces of evidence, diosmectite has not gained wide acceptance in gastrointestinal pharmacotherapy. This should be regarded as a consequence of causes beyond scientific proof, since both the antidiarrhoeal activity of diosmectite and its remarkable absence of adverse effects have been demonstrated by large controlled studies (Madkour et al., 1993; Guarino et al., 2001).
Here, we present evidence that diosmectite, administered as a post-treatment, is effective in controlling hapten-induced colonic inflammation with an efficacy comparable to that of sulphasalazine, through a mechanism that may involve adsorption of proteins, mucus protection and possibly a direct modulation of the production of proinflammatory mediators by the colonic mucosa.
Methods
Animals
Female Wistar rats (250–300 g) obtained from the Laboratory Animal Service of the University of Granada were used, housed in makrolon cages and maintained in our laboratory in air-conditioned animal quarters with a 12 h light–dark cycle. Animals were provided with free access to tap water and food (Panlab A04, Panlab, Barcelona, Spain). This study was carried out in accordance with the Directive for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes of the European Union (86/609/EEC).
Induction of colitis
Colitis was induced by the method of Morris et al. (1989) with minor modifications. Animals were fasted overnight and anaesthetized with halothane. Under these conditions, animals were given 10 mg of trinitrobenzene sulphonic acid (TNBS) dissolved in 0.25 ml of 50% ethanol (v v−1) by means of a Teflon cannula inserted 8 cm through the anus.
Experimental design
Rats were randomly assigned to four groups: control (group C, n=12), which received 0.25 ml of phosphate-buffered saline (PBS); TNBS (group T, n=16), diosmectite (group D, n=16) and sulphasalazine (group S, n=16), which received the TNBS/ethanol enema and were given vehicle (1% methylcellulose p.o.), diosmectite (500 mg kg−1 day−1 p.o.) or sulphasalazine (50 mg kg−1 day−1 p.o.), respectively, starting 1 day after the TNBS challenge. Animals from all groups were killed by cervical dislocation 1 and 2 weeks after colitis induction. After diosmectite maximal effect was found to occur at 1 week, additional experiments (n=20–24 per group) were performed in order to obtain new samples for the determination of mucins, inducible nitric oxide synthase (iNOS) and interleukin-1β (IL-1β) expression, histological analysis and diarrhoeal scores. Some additional animals were used for glycoprotein determination. Animal body weight and total food intake for each group were recorded daily in each case. Diarrhoeal status was scored according to the criterion of Kerr et al. (1999).
Assessment of colonic damage
The colon was removed and placed on an ice-cold plate, cleaned of fat and mesentery and blotted on filter paper. Each specimen was weighed and its length measured under a constant load (2 g). The large intestine was longitudinally opened and scored for macroscopically visible damage on a 0–10 scale by an observer unaware of the treatment, according to the criterion previously proposed by Bell et al. (1995). The colon was subsequently divided longitudinally in several pieces for biochemical determinations. The fragments were immediately frozen at −30°C except for the sample for total glutathione content determination, which was immediately weighed and frozen in 1 ml of 5% (w v−1) trichloroacetic acid, and the fragments for leukotriene B4 (LTB4) synthesis and Western blot analysis (see below). Myeloperoxidase (MPO) activity was measured according to the technique described by Krawisz et al. (1984), using 0.5% hexadecyltrimethylammonium bromide in PBS (pH=6.0) for tissue homogenization and o-dianisidine dihydrochloride (533 μM) as chromogen. The results are expressed as MPO units (μmol min−1) per gram of wet tissue. The total glutathione content was quantitated with a recycling assay using 5,5′-dithiobis-2-nitrobenzoic acid as chromogen and glutathione reductase to regenerate oxidized glutathione (Akerboom & Sies, 1981). The results are expressed as nmol g wet tissue−1.
The colonic levels of IL-1β and iNOS were determined by immunoblotting. In addition, the expression of mucins 2, 3 and 4, as well as of trefoil factor 3 (TFF3) was examined by reverse transcription and polymerase chain reaction (RT–PCR) and also by immunoblotting where applicable. For MUC2, colonic specimens were immediately minced and homogenized for 1 min in 6 M guanidine hydrochloride with 100 mM dithiothreitol and protease inhibitors (1,10-phenanthroline, phenylmethylsulphonylfluoride, aprotinin), stirred for 24 h at 4°C, dialysed against PBS and centrifuged at 10,000 × g for 10 min (Tytgat et al., 1995). For MUC4 and iNOS, the colonic samples were homogenized in lysis buffer (0.1% SDS, 0.1% sodium deoxycholate, 1% Triton X-100 in PBS) with protease inhibitors, the supernatants were boiled for 4 min in Laemmli buffer, separated by SDS–PAGE, transferred to nitrocellulose membranes and immunoblotted with the corresponding antibodies. The bands were detected by enhanced chemiluminescence (NEN, Zaventem, Belgium) and quantitated with National Institutes of Health software (Scion Image).
For the RT–PCR analysis of MUC2, MUC3, MUC4 and TFF3, colonic fragments were frozen immediately post mortem in liquid nitrogen. Total RNA was extracted with Trizol (Life Technologies, Rockville, MD, U.S.A.). RNA (5 μg) per sample were subjected to reverse transcription using the First-strand cDNA synthesis kit (Amersham Pharmacia Biotech, Barcelona, Spain). Polymerase chain reaction (PCR) amplification was performed using 2 μl of cDNA for a final PCR reaction volume of 25 μl. The primers were: actin (sense, 5′-GGC CAA CCG TGA AAA GAT G-3′; antisense, 5′-GGA TCT TCA TGA GGT AGT CTG TC-3′); MUC2 (sense, 5′-GCT CAA TCT CAG AAG GCG ACA G-3′; antisense, 5′-CCA GAT AAC AAT GAT GCC AGA GC-3′); MUC3 (sense, 5′-CAC AAA GGC AAG AGT CCA GA-3′; antisense, 5′-ACT GTC CTT GGT GCT GAA TG-3′); MUC4 (sense, 5′-CGT ACT AGA GAA CTT GGA CAT G-3′; antisense, 5′-GGT AGG AGA ACT TGT TCA TGG-3′); TFF3 (sense, 5′-ATG GAG ACC AGA GCC TTC TG-3′; antisense, 5′-ACA GCC TTG TGC TGA CTG TA-3′).
For the determination of LTB4 synthesis, colonic samples (100–300 mg) were minced, diluted 1 : 10 (w v−1) in 10 mM PBS and incubated for 20 min in a shaking bath at 37°C. The samples were centrifuged at 9000 × g for 30 s at 4°C and the supernatants were kept at −80°C until assay. LTB4 levels were quantitated by enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Barcelona, Spain). IL-1β levels were assayed in samples obtained as above, boiled in Laemmli buffer, separated by 15% SDS–PAGE, electrotransferred onto nitrocellulose membranes and immunoblotted. Quantitation of band density was carried out as described above.
Histological analysis
The samples for the histological study were taken from the edge of the necrosed area, coded, fixed in formaldehyde and processed for routine analysis using haematoxylin/eosin staining. The sections were scored for microscopic damage by an investigator blinded to the sample identity as described previously (Stucchi et al., 2000).
Glycoprotein synthesis
The rate of colonic glycoprotein synthesis was estimated in a subgroup of rats (from groups T and D, at 1 week of colitis) by in vivo labelling with [6-3H]glucosamine, administered as an intraperitoneal dose (15 μCi) (Asfaha et al., 2001). The animals were killed after 4 h and the colonic mucosa was gently scraped and vortexed in PBS. This suspension was centrifuged at 1000 × g and the supernatant was mixed with an equal volume of 10% trichloroacetic acid/1% phosphotungstic acid for 30 min at 4°C to precipitate the proteins. The pellet obtained after centrifugation (2000 × g, 10 min) was resuspended in scintillation liquid (Optifase Hisafe 2, Wallac, Turku, Finland) for measurement of radioactivity. For the determination of glycoproteins in HT29-MTX cells, [6-3H]glucosamine was added to culture medium (1 μCi ml−1) with varying concentrations of diosmectite. After 24 h, the cells were washed to remove the excess marker and stimulated for 15 h with carbachol (50 μM), 3-isobutylmethylxanthine (100 μM) and PMA (1 μM). Both the culture medium and cells were processed as above to measure radioactivity.
Cell culture
HT29 cells, a human colonic epithelial cell line, were used as a model intestinal epithelium (passages 136–142). The cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (Boëhringer Mannheim, Barcelona, Spain), 2 mM L-glutamine, 50,000 U l−1 penicillin/streptomycin and 2.5 mg l−1 amphotericin B, in a humidified 5% CO2 atmosphere at 37°C. THP-1 cells, which have a human monocytic phenotype, were cultured in RPMI medium with inactivated foetal bovine serum, antibiotics and amphotericin B as above. HT29-MTX cells (passages 13–16), a kind gift from Dr Thecla Lesuffleur (INSERM), were cultured like HT29 cells but with inactivated foetal bovine serum. HT29 and THP-1 cells were obtained from the cell culture facility of the University of Granada.
Effect of diosmectite on cytokine and mucin production in vitro
For the cytokine production studies, HT29 cells were seeded at 50,000 cells cm−2 on polycarbonate Transwell® filters with a pore diameter of 0.4 μm and a surface area of 4.7 cm2 (Costar, Brumath, France). At 3–4 days after confluence, intestinal cells were incubated on their apical side with 1, 10, 50 or 100 mg ml−1 diosmectite and stimulated on their basolateral side with 10 μg ml−1 lipopolysaccharide (LPS) (from Escherichia coli serotype 055:B5). After 20 h, supernatants were harvested, cleared at 7000 × g for 10 min and stored at −80°C for the determination of IL-8 levels by enzyme-linked immunosorbent assay (Biosource International, Nivilles, Belgium) and L-lactate dehydrogenase (EC 1.1.1.27, LDH) activity (as an index of cytotoxicity). LDH activity was measured by the sodium pyruvate-dependent disappearance of β-NADH and expressed as mU ml−1. THP-1 cells, a human monocytic cell line, were used to assess the effects of diosmectite on IL-1β production. In order to separate the clay from the cells, the latter were placed in the upper chamber of Transwell® inserts, while diosmectite was added to the medium in the lower reservoir. THP-1 cells (106 ml−1) were stimulated with LPS (10 μg ml−1) and 4-α-phorbol 12-myristate 13-acetate (PMA, 1 μM) for 1 h and then washed in regular medium prior to exposure to diosmectite (1–50 mg ml−1). This protocol prevents the possibility of direct binding of LPS to diosmectite. The levels of IL-1β were examined in cell lysates and culture medium 16 h after cell stimulation.
The effect of diosmectite on mucin expression was examined in HT29-MTX cells, which display a goblet cell phenotype. HT29-MTX cells were cultured as confluent monolayers for 3 weeks and exposed to diosmectite (1–100 mg ml−1) for 24 h, after which RNA was obtained as above. The primers were: β-actin (sense, 5′-CCA TGG ATG TGA TAT CGC CG-3′; antisense, 5′-GCC TAG AAG CAT TTG CGG TGG-3′); MUC2 (sense, 5′-CTG CAC CAA GAC CGT CCT CAT G-3′; antisense, 5′-GCA AG G ACT GAA CAA AGA CTC AGA-3′); MUC3 (sense, 5′-GCA AGT CAG TAA CGA GCC TCA G-3′; antisense, 5′-GGG GAA AGG ATA CAG GAA TGA G-3′); MUC4 (sense, 5′-GTT CTT CGG CAT CTT CTT TGG GGC-3′; antisense, 5′-CAC CTT CCC TTT TCC AGT CTC CCA-3′). Samples were electrophoresed on a 2% agarose gel.
Protein binding
Diosmectite (1–100 mg ml−1) was incubated for 24 h at 37°C in culture medium (DMEM) containing one of the following: LDH (2000 mU ml−1), horseradish peroxidase (EC 1.11.1.7, 2000 mU ml−1), glutathione transferase (EC 2.5.1.18, 500 mU ml−1), powdered nonfat milk (1% w v−1) or a concentrated lactoserum (1% w v−1), a kind gift from Dr Emilia Guadix. At the end of this period, the samples were centrifuged at 7000 × g for 10 min at 4°C and the supernatants were assayed for protein content (Bradford, 1976) or enzyme activity. Glutathione transferase was quantitated using 1-chloro-2,4-dinitrobenzene as a substrate (Warholm et al., 1985). Horseradish peroxidase was measured using o-dianisidine as the electron donor, in the same manner as MPO. The results are expressed as the percentage of concentration or enzyme activity cleared by centrifugation.
Materials and reagents
Diosmectite was supplied by Beaufour IPSEN Industrie (Dreux, France). Except where indicated, all reagents were obtained from Sigma (Madrid, Spain). The polyclonal antibody against murine MUC2 was a generous gift from Dr Jan Dekker. The MUC4 monoclonal antibody developed by Dr Kermit L Carraway was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA, U.S.A. The iNOS antibody was purchased from Transduction Laboratories, now BD Biosciences (Franklin Lakes, NJ, U.S.A.). All other antibodies were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The composition of the Ringers solution was (in mM): 115 NaCl, 25 NaHCO3, 1.2 CaCl2, 1.2 MgCl2, 2.4 K2HPO4, 0.4 KH2PO4, 10 glucose.
Statistical analysis
The results are expressed as mean±s.e.m. Differences among means were tested for statistical significance by one-way analysis of variance and a posteriori least significance tests on preselected pairs. Nonparametric data (colonic damage, histological and diarrhoeal status scores) were expressed as median (25–75% quartiles) and analysed by Kruskal–Wallis analysis of variance on ranks or by the Mann–Whitney test. Differences in the incidence of diarrhoea were analysed by the χ2 test. Data from the control groups were not significantly different and were therefore pooled together and presented as a single group. All statistical analyses were carried out with the SigmaStat program (Jandel Corporation, San Rafael, CA, U.S.A.). Statistical significance was set at P<0.05.
Results
Effect of diosmectite on TNBS-induced colitis
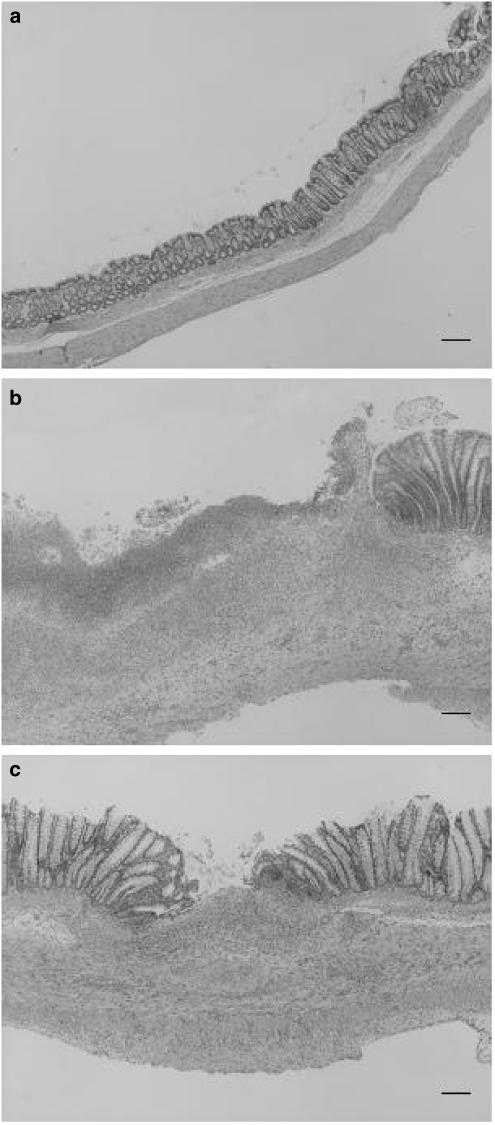
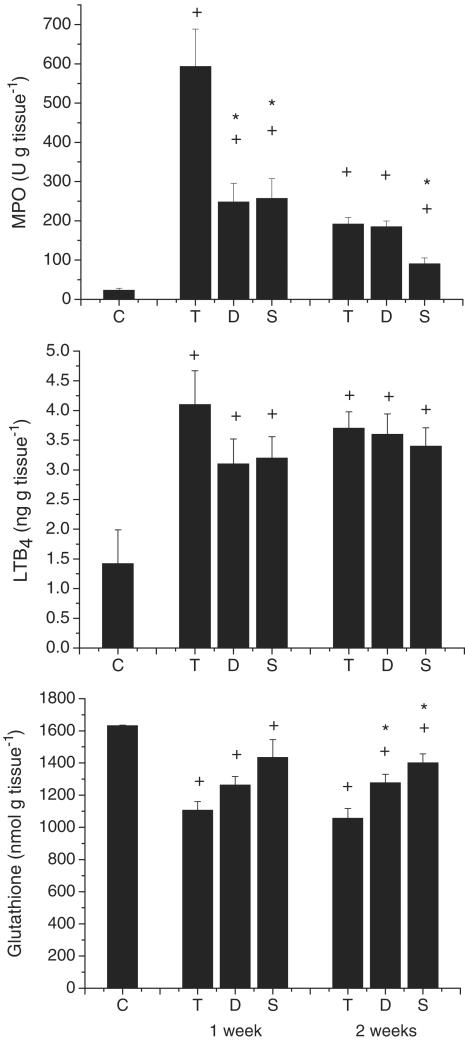
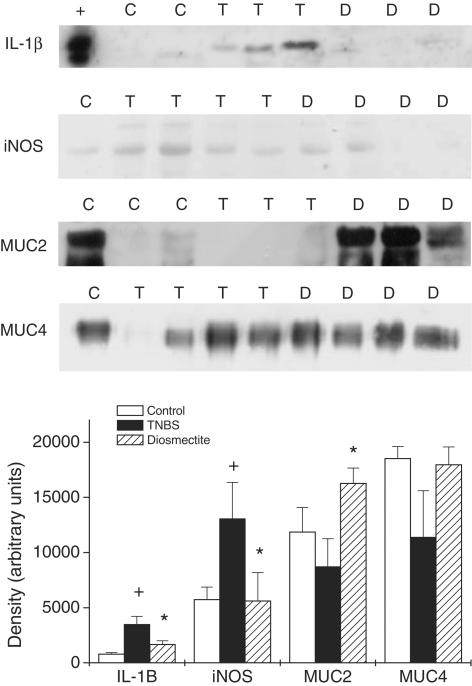
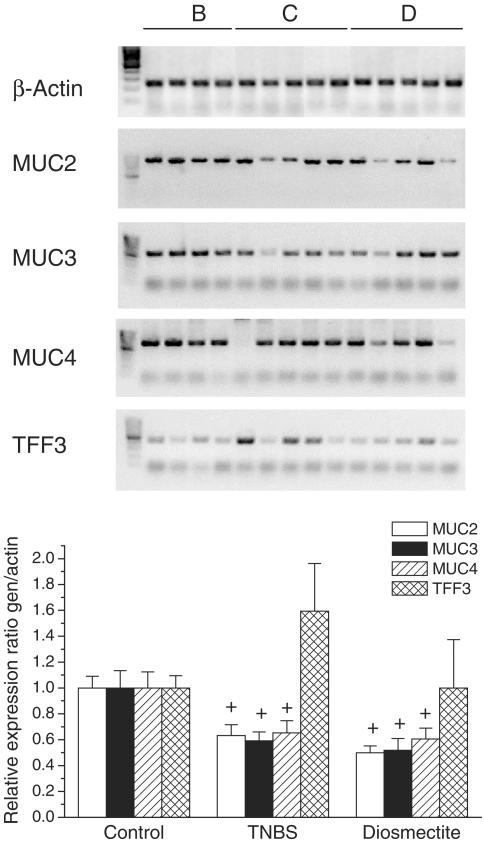
As expected, TNBS administration elicited an intense inflammatory response in the rat colon. At 1 week postchallenge, this was characterized by extensive epithelial necrosis, oedema, bowel wall thickening and adhesions with neighbouring tissue (Table 1 and Figure 1). The severity of the inflammatory reaction decreased at the second week, particularly with regard to the extension of the necrotic lesion (Table 1), but healing was far from complete. Experimental colitis was accompanied by marked anorexia and loss of body weight during the first few days after TNBS challenge (data not shown). Diarrhoea, as evidenced indirectly by perianal fur soiling, was prominent among colitic animals at 1 week, but generally was absent at the second week. Histologically, TNBS colitis was characterized by mucosal ulceration and intense leucocytic infiltration in the mucosa and submucosa, sometimes extending to the muscular layers and serosa (Figure 1). The infiltrate was composed mainly of granulocytes (neutrophils, eosinophils) as well as macrophages. Neutrophilic infiltration was also evidenced by the 21-fold increase in MPO activity at 1 week (Figure 2). The synthesis of LTB4, one of the main chemoattractants in the inflamed intestine, was similarly elevated (Figure 2). On the other hand, the total levels of glutathione in the rat colon were significantly decreased up to 2 weeks after administration of the hapten (Figure 2). Further examination of the status of rats at 1 week of colitis revealed that the production of IL-1β and iNOS was markedly upregulated (Figure 3). On the contrary, colitic rats showed decreased mRNA levels of mucins (Figure 4). The corresponding mucin protein levels (MUC2 and MUC4) were lower in the TNBS group, but the difference did not reach statistical significance (Figure 3). TFF3 expression was not modified significantly, although the level was higher in colitic rats.
Table 1.
Evolution of the parameters of macroscopic damage in TNBS colitic rats
| Damage score | Extension of necrosis (cm) | Colonic weight/length ratio (mg cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (week) | 2 (week) | 1 (week) | 2 (week) | 1 (week) | 2 (week) | ||||
| Control | 0 (0–0) | 0 | 69.1±3.5 | ||||||
| TNBS | 5 (5–6) | 4 (3–4) | 2.3±0.2 | 0.8±0.1 | 170.2±13.4+ | 92.3±3.9+ | |||
| Diosmectite | 5 (1.8–5)* | 3 (2–4) | 1.0±0.2* | 0.7±0.2 | 119.0±9.9+* | 94.8±6.0+ | |||
| Sulphasalazine | 4 (4–6) | 4 (2–4) | 1.5±0.3 | 0.7±0.2 | 154.0±9.6+ | 83.8±4.9+ | |||
Results are expressed as median (25–75% quartiles) and mean±s.e.m., respectively.
P<0.05 compared with TNBS group. Groups TNBS, diosmectite and sulphasalazine were significantly different from the control group at all time points (not shown).
Figure 1.
Histological sections of TNBS colitic rats at 1 week. (a) Control group. (b). TNBS group. (c) Diosmectite group. Bar: 200 μm.
Figure 2.
Biochemical parameters of TNBS colitic rats. The colonic levels of MPO, LTB4 and glutathione are shown. +P<0.05 vs group C; *P<0.05 vs group T.
Figure 3.
Colonic expression of IL-1β, iNOS, MUC and MUC4. Representative Western blots and densitometric analysis (+, positive control; C, control group; T, TNBS group; D, diosmectite group). +P<0.05 vs control group; *P<0.05 vs TNBS group.
Figure 4.
mRNA levels of mucins and TFF3 in TNBS colitis. RT–PCR analysis and quantification are shown. +P<0.05 vs control group.
Diosmectite administration to TNBS-challenged rats resulted in a markedly lower degree of colonic inflammation. Thus at 1 week, animals from the diosmectite group exhibited a 30 and 65% reduction in colonic weight length ratio (Table 1) and MPO activity (Figure 2), respectively (P<0.05), as well as a significant decrease in colonic damage score and a 57% reduction in the extension of the necrotic area (Table 1). At the histological level, diosmectite-treated animals presented a lower severity of ulceration and a less marked degree of leucocyte infiltration in the mucosa and submucosa (Figure 1). The histological score was significantly reduced by diosmectite treatment (15.0 (13.2–19.5) vs 21.0 (17.5–22.0), P<0.05). The incidence of diarrhoea was lower in the diosmectite-treated group (20.0 vs 60.0%, P<0.05). A separate experiment using individually housed rats in which diarrhoea was scored for the severity and presence of mucus and blood was performed, confirming the beneficial effect of diosmectite on diarrhoea, which reached statistical significance at 5–7 days (2 (1–2.3) vs 4 (2–4), P<0.05). Glutathione depletion was also partially counteracted by diosmectite treatment, although this effect was significant only at 2 weeks (Figure 2). In addition, the levels of IL-1β and iNOS were significantly lower than in the TNBS controls at 1 week (Figure 3). On the other hand, diosmectite treatment was associated with a dramatic increase in colonic MUC2 expression level (Figure 3). The level of MUC4 was comparable to that of the control, but there were no significant differences overall. Interestingly, the corresponding mRNA levels of MUC2, MUC4 and also MUC3 were unaffected by the treatment (Figure 4). In a separate experiment, total glycoprotein synthesis, as assessed lay [6-3H]glucosamine, was unchanged by diosmectite treatment (not shown). TFF3 expression was comparable to that of the control rats (Figure 4). There were no significant changes in LTB4 synthesis, body weight, food intake or incidence of adhesions (Figure 2 and data not shown).
The therapeutic effect of diosmectite on TNBS colitis was comparable to that of sulphasalazine, one of the drugs currently used to treat IBD. Thus in the first week sulphasalazine inhibited MPO increase as effectively as the clay and counteracted glutathione depletion (Figure 2). However, diosmectite appeared to be more effective on the macroscopic parameters (Table 1), as well as in reducing the incidence of diarrhoea (37.5%, P>0.05 vs control). Unlike diosmectite, the beneficial effect of sulphasalazine on neutrophil infiltration extended to the second week postchallenge, since it significantly inhibited MPO activity compared to the TNBS group (Figure 2).
In vitro experiments
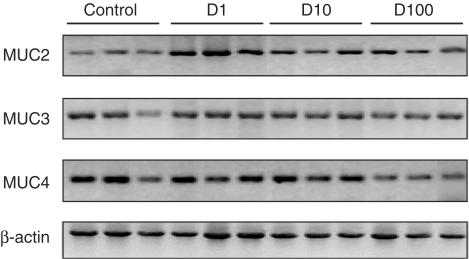
In order to ascertain the mechanisms involved in the anti-inflammatory activity of diosmectite, the HT29 human epithelial cell line was used as a model for the interaction between the clay and the colonic epithelium. Our results show that diosmectite dose dependently inhibits the secretion of IL-8 by intestinal epithelial cells stimulated by LPS (Figure 5). This was not attributable to a deleterious effect on the cells, because the levels of LDH in the culture medium were similar in the control and diosmectite groups (data not shown). In separate experiments, diosmectite did not show a significant impact on cell proliferation or viability (data not shown). On the other hand, diosmectite inhibited the production of IL-1β by THP-1 cells, suggesting that it may have similar effects if granted access to the lamina propria (Figure 5). Finally, the addition of clay to HT29-MTX cells, which are a model of goblet cells, for 24 h modulated significantly the mucin expression pattern (Figure 6). Thus, diosmectite increased specifically the expression of MUC2 at 1 mg ml−1 (P<0.05), but had little effect on MUC3 or MUC4. Again, diosmectite exposure did not modify total glycoprotein synthesis, since both the amount of tritium in the cells and that secreted to the culture medium in response to a stimulating drug cocktail were similar in the control and diosmectite groups (data not shown).
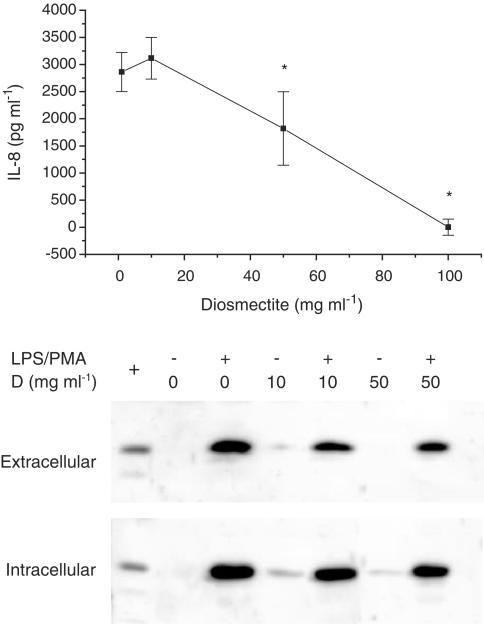
Figure 5.
Effect of diosmectite on HT29 and THP-1 cells. (Upper panel) Dose–response curve of the effect of apically added diosmectite on LPS-stimulated IL-8 secretion by HT29 cells. *P<0.05 vs no addition of diosmectite. The effect of the clay was not attributable to a deleterious effect on the cells, because the levels of LDH in the culture medium were similar in the control and diosmectite groups (data not shown). Values are mean±s.e.m. No significant effects were detected. (Bottom panel) Effect of diosmectite on IL-1β expression in THP-1 cells. D: diosmectite. The clay reduced the extracellular and intracellular levels of the cytokine. The positive control (left lane) was the supernatant from activated THP-1 cells from a different experiment.
Figure 6.
Effect of diosmectite on mucin mRNA levels in HT29-MTX cells. The gels shown are representative of at least quadruplicate experiments. Diosmectite strongly augments MUC2 expression at 1 mg ml−1.
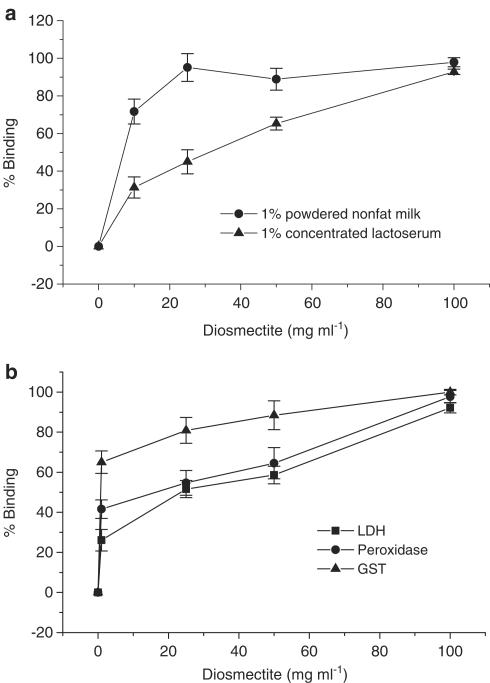
The binding capacity of diosmectite to different proteins was also studied (Figure 7). The clay was found to have a marked binding capacity for all the substrates tested. In addition, IL-8 was undetectable when cells were grown on nonpermeable supports, thus allowing direct contact between IL-8 and the clay (data not shown), suggesting that diosmectite may also have significant affinity for this cytokine.
Figure 7.
Binding curves of protein substrates to diosmectite. (a) Protein concentrates. (b) Purified enzymes. Proteins were incubated in HT29 culture medium with increasing concentrations of the clay at 4°C. The amount of protein cleared by centrifugation at 7000 × g was considered to bind diosmectite. Values are mean±s.e.m.
Discussion
Diosmectite is one of the clays long used in the treatment of diarrhoeal conditions (Madkour et al., 1993). The mechanism of action proposed involves the adsorption of toxins and bacteria as well as changes in the properties of secreted mucus (Dupont et al., 1992). However, its potential therapeutic value in chronic intestinal inflammation had not been examined to date. Therefore, we carried out the present study to test formally this hypothesis. The dose assayed (500 mg kg−1) is based on previous studies in animals (More et al., 1987; Fioramonti et al., 1987; Theodorou et al., 1994) and humans (Dupont et al., 1992). Our results demonstrate that diosmectite is effective in the treatment of established colonic inflammation in an experimental setting. The therapeutic role of diosmectite is based on amelioration of the morphological signs of the disease (intestinal weight, observable macroscopic damage, necrosed area, histology), as well as on the significant impact on several biochemical markers (MPO, glutathione levels, IL-1β, iNOS, mucins). Treatment of colitic animals with diosmectite also ameliorated colonic functional status in vivo, as evidenced by the significant decrease in the severity of diarrhoea, one of the capital signs of both IBD and TNBS colitis (Musch & Chang, 1994). It is important to note that the overall efficacy of diosmectite in the downregulation of the inflammatory response was comparable to that of a standard drug used in IBD therapy, namely sulphasalazine. In fact, diosmectite appeared to be better than sulphasalazine in terms of protection against macroscopic injury at the time of maximal inflammation, that is, 1 week. Conversely, the therapeutic effect of sulphasalazine but not diosmectite extended to the second week after the TNBS challenge, based on the significant inhibition of MPO activity (see below).
IBD, comprising Crohn's disease and ulcerative colitis, is a severe condition that is usually treated with corticoids, aminosalicylates and immunosuppressants (Sands, 2000). Although these drugs are generally effective in reducing the intensity of inflammatory bouts and in maintaining clinical remission, they have a wide profile of considerable side effects. Therefore, the search for new therapeutic strategies is much warranted. This objective is hampered to a great extent by the fact that the aetiology of IBD is largely unknown. Nevertheless, there is a growing consensus that IBD is the result of an exacerbated immune response to a stimulus that is innocuous for the general population (Fiocchi, 1998). This hypothesis is strongly supported by studies in which experimental colitic inflammation, including TNBS colitis, is significantly downregulated when animals are reared in a germ-free environment (Strober & Fuss, 1999). In fact, systemic endotoxaemia correlates positively with the presence and extent of intestinal ulceration and with the activity of the disease, and may contribute to the development of toxic megacolon, abnormalities of liver histology and other extraintestinal manifestations of IBD (Gardiner et al., 1993). This pathogenic role of intestinal flora in colonic inflammation should not be confused with an infectious aetiology, since immunosuppressive therapy ameliorates rather than worsens the progression of the disease, an effect just opposite to what would be expected in the latter case (Sands, 2000). In this regard, it should be noted that oral administration of adsorbents like kaolin and terra fullonica is able to reduce endotoxaemia in experimental colitis (Gardiner et al., 1993). Therefore, by adsorpting the antigenic load to the intestinal mucosa, diosmectite may be expected to reduce colonic inflammation. In fact, one of the main effects of diosmectite was a dramatic reduction of MPO and IL-1β, suggesting a diminished infiltration/activation of neutrophils and monocytes, respectively, as would be expected from a reduced antigenic load. The lower levels of iNOS and the histological analysis also are consistent with this scenario. The fact that both diosmectite and sulphasalazine failed to reduce significantly colonic LTB4 synthesis may reflect a relative lack of effect on neutrophils compared to macrophages. Alternatively, it may simply reflect a lack of statistical power to detect real differences, as suggested by the data in Figure 2. Our results confirm the adsorptive capacity of diosmectite, which binds in vitro to all the proteins tested to a great extent, albeit somewhat variably. It should be noted, however, that these in vitro data may not reflect an actual binding activity of diosmectite in vivo. As diosmectite binds to the mucus layer, its location in the intestine is ideal to provide protection for the colonic epithelium (Gardiner et al., 1993). In addition, diosmectite has been shown to confer protection to secreted mucus in vitro (Leonard et al., 1994). The main secretory, gel-forming mucin in the colon is MUC2, while MUC3 and MUC4 are important membrane-bound mucins in the large intestine and are involved in various functions such as cell signalling, adhesion and growth (Tytgat et al., 1995; Shirazi et al., 2000). MUC2 has been reported to be specifically inhibited in IBD (Tytgat et al., 1996). In our study, diosmectite treatment resulted in an increase in the colonic levels of MUC2. Thus, mucus protection from inflammatory damage may contribute to the therapeutic effects of diosmectite. While the clay appears to upregulate MUC2 in the HT29/MTX goblet cell model, the effect observed in vivo is clearly of post-transcriptional nature, since mRNA levels were unchanged. These results, together with the fact that overall glycoprotein synthesis is unchanged by the clay both in vivo and in vitro, suggest that diosmectite may exert its effect by binding mucins, protecting them from inflammatory damage.
Apart from the mechanisms explained above, we investigated the possibility that diosmectite exerted a direct modulatory effect on intestinal cells. Owing to the large size of the diosmectite particles (sheets 1.2–1.5 nm thick) (Albengres et al., 1985), the clay is expected to be confined to the luminal side of the epithelium, at least in the absence of ulcers or epithelial erosions. Therefore enterocytes, in addition to goblet cells, would be the main cell type directly exposed to diosmectite. Enterocytes are involved in the immunological response of the intestine by expressing major histocompatibility complex class II molecules and secreting a number of cytokines and chemokines (Hershberg & Mayer, 2000). We used the HT29 cell line as a model epithelium in vitro. Intestinal epithelial cells, including HT29 cells, respond to LPS stimulation with an increased production and release of IL-8. As LPS receptors are located basolaterally, this is interpreted as a proinflammatory signal in response to the mucosal penetration of endotoxin- or endotoxin-bearing microorganisms, generally as a consequence of an epithelial permeability defect. Since initial experiments suggested that diosmectite binds IL-8 avidly, cells were seeded on Transwell® filters, which allow separate apical exposure to diosmectite and basolateral exposure to LPS. Under these conditions, diosmectite showed a dose-dependent inhibitory effect on the basolateral secretion of IL-8. This effect was not attributable to cytotoxicity, since basolateral LDH levels were unchanged. Furthermore, diosmectite was shown to have no effect on cell viability or proliferation in parallel experiments. Although the mechanism, whereby diosmectite exerts this modulatory effect on enterocytes, is unknown, our data suggest the existence of novel, direct actions of this drug on the inflamed intestine. Of course, direct extrapolation is not possible and it should be kept in mind that at present we cannot demonstrate that such mechanisms are actually operative in vivo. At any rate, there is a precedent of such a direct action reported by Mahraoui et al. (1997), who showed that, using a similar experimental protocol, HT29-19A cells apically exposed to diosmectite were protected from the alterations in barrier function elicited by basolaterally added TNF and interferon-γ. However, the authors were unable to establish a putative mechanism of action, owing to the difficult traceability of the clay. The inhibition of IL-8 synthesis by the epithelium is in accordance with the diminished leucocyte infiltration (as determined by MPO activity) observed in the diosmectite group, since IL-8 is a powerful chemoattractant in the intestinal tissue.
On the other hand, the hypothesis that an ulcerated mucosa can be accessible to diosmectite led us to examine its effects on the model human monocytic cell line THP-1. Our results show that the levels of IL-1β, one of the main cytokines produced by monocytes/macrophages, are lowered by exposure to diosmectite in this experimental system. This opens up the possibility that the clay may exert more powerful anti-inflammatory effects when the integrity of the epithelium is affected. In fact, such a circumstance could explain why its effects are prominent at 1 week but not 2 weeks, since epithelial barrier function is restored in a few days in this model (Bell et al., 1995; Sanchez de Medina et al., 2002a, 2002b). The mechanisms of the effects of diosmectite on HT29 and THP-1 cells in vitro are unknown, but may be related to the binding of molecules involved in the autocrine/paracrine regulation of these cells. For example, IL-1β has been shown to exert such effects in THP-1 cells, creating a positive feedback loop that may be interrupted by the clay (Toda et al., 2002).
In summary, we have demonstrated that diosmectite has anti-inflammatory activity in experimental chronic colitis administered as a post-treatment, which is comparable to that of a established treatment like sulphasalazine. At least three different mechanisms may account for this effect: (1) adsorption of luminal antigens; (2) protection of colonic mucin; and (3) a possible modulation of the mucosal inflammatory response.
We thank Dr Mercedes Gonzalez and Dr María Elena Rodríguez for their technical assistance. HT29-MTX cells were a generous gift from Dr Thecla Lesuffleur (INSERM). This study was supported by the Spanish Ministry of Education and Science with CICYT (SAF2002-02592) and Instituto de Salud Carlos III (PI021732) funds and by the Ramón y Cajal program of the Ministry of Science and Technology, as well as by Beaufour IPSEN Industrie. Part of these data were presented in the National Meeting of the Spanish Society of Pharmacology (2000 and 2002) and the Falk Symposium No. 119 on immunosuppression in inflammatory bowel diseases (2000).
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- IBD
inflammatory bowel disease
- IL-1β
interleukin-1β
- IL-8
interleukin-8
- iNOS
inducible nitric oxyde synthase
- LTB4
leukotriene B4
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- PMA
4-α-phorbol 12-myristate 13-acetate
- RT–PCR
reverse transcription and polymerase chain reaction
- TNBS
trinitrobenzenesulphonic acid
- TNF
tumour necrosis factor
References
- ADAMS R.B., PLANCHON S.M., ROCHE J.K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J. Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- AKERBOOM T.P., SIES H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- ALBENGRES E., URIEN S., TILLEMENT J.P., OURY P., DECOURT S., FLOUVAT B., DRIEU K. Interactions between smectite, a mucus stabilizer, and acidic and basic drugs. In vitro and in vivo studies. Eur. J. Clin. Pharmacol. 1985;28:601–605. doi: 10.1007/BF00544074. [DOI] [PubMed] [Google Scholar]
- ASFAHA S., MACNAUGHTON W.K., APPLEYARD C.B., CHADEE K., WALLACE J.L. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G635–G644. doi: 10.1152/ajpgi.2001.281.3.G635. [DOI] [PubMed] [Google Scholar]
- BELL C.J., GALL D.G., WALLACE J.L. Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- BELL S., KAMM M.A. Antibodies to tumour necrosis factor alpha as treatment for Crohn's disease. Lancet. 2000;355:858–860. doi: 10.1016/S0140-6736(99)00442-0. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRENNAN F.M., MAINI R.N., FELDMANN M. Cytokine expression in chronic inflammatory disease. Br. Med. Bull. 1995;51:368–384. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- CASTRO G.A., POWELL D.W.The physiology of the mucosal immune system and immune-mediated responses in the gastrointestinal tract Physiology of the Gastrointestinal Tract 1994New York: Raven Press; 709–750.ed. Johnson, L.R., Alpers, D.A., Christensen, J., Jacobson, E.D. & Walsh, J.H. pp [Google Scholar]
- DROY M.T., DROUET Y., GERAUD G., SCHATZ B. Spinnability: a new approach to intestinal stress and its therapy. Gastroenterol. Clin. Biol. 1985;9:119–121. [PubMed] [Google Scholar]
- DUPONT C., MORENO J.L., BARAU E., BARGAOUI K., THIANE E., PLIQUE O. Effect of diosmectite on intestinal permeability changes in acute diarrhea: a double-blind placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 1992;14:413–419. doi: 10.1097/00005176-199205000-00007. [DOI] [PubMed] [Google Scholar]
- FIOCCHI C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- FIORAMONTI J., DROY-LEFAIX M.T., BUENO L. Changes in gastro intestinal motility induced by cholera toxin and experimental osmotic diarrhoea in dogs: effects of treatment with an argillaceous compound. Digestion. 1987;36:230–237. doi: 10.1159/000199423. [DOI] [PubMed] [Google Scholar]
- FIORAMONTI J., NAVETAT H., DROY-LEFAIX M.T., BUENO L.Antidiarrheal properties of clay minerals: pharmacological and clinical studies Veterinary Pharmacology, Toxicology and Therapy in Food Producing Animals 1990Budapest: Unipharma; 245–251.ed. Simon, F., Less, P. & Semjen, G. pp [Google Scholar]
- GARDINER K.R., ANDERSON N.H., MCCAIGUE M.D., ERWIN P.J., HALLIDAY M.I., ROWLANDS B.J. Adsorbents as antiendotoxin agents in experimental colitis. Gut. 1993;34:51–55. doi: 10.1136/gut.34.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUARINO A., BISCEGLIA M., CASTELLUCCI G., IACONO G., CASALI L.G., BRUZZESE E., MUSETTA A., GRECO L. Smectite in the treatment of acute diarrhea: a nationwide randomized controlled study of the Italian Society of Pediatric Gastroenterology and Hepatology (SIGEP) in collaboration with primary care pediatricians. SIGEP Study Group for Smectite in Acute Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001;32:71–75. doi: 10.1097/00005176-200101000-00019. [DOI] [PubMed] [Google Scholar]
- HERSHBERG R.M., MAYER L.F. Antigen processing and presentation by intestinal epithelial cells–polarity and complexity. Immunol. Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- HEYMAN M., DARMON N., DUPONT C., DUGAS B., HIRRIBAREN A., BLATON M.A., DESJEUX J.F. Mononuclear cells from infants allergic to cow's milk secrete tumour necrosis factor alpha, altering intestinal function. Gastroenterology. 1994;106:1514–1523. doi: 10.1016/0016-5085(94)90405-7. [DOI] [PubMed] [Google Scholar]
- HEYMAN M., DESJEUX J.F. Significance of intestinal food protein transport. J. Pediatr. Gastroenterol. Nutr. 1992;15:48–57. [PubMed] [Google Scholar]
- HOLLANDER D., VADHEIM C.M., BRETTHOLZ E., PETERSEN G.M., DELAHUNTY T., ROTTER J.I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- JUNG H.C., ECKMANN L., YANG S.K., PANJA A., FIERER J., MORZYCKA-WROBLEWSKA E., KAGNOFF M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR S.W., WOLYNIEC W.W., FILIPOVIC Z., NODOP S.G., BRAZA F., WINQUIST R.J., NOONAN T.C. Repeated measurement of intestinal permeability as an assessment of colitis severity in HLA-B27 transgenic rats. J. Pharmacol. Exp. Ther. 1999;291:903–910. [PubMed] [Google Scholar]
- KRAWISZ J.E., SHARON P., STENSON W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- LEONARD A., DROY-LEFAIX M.T., ALLEN A. Pepsin hydrolysis of the adherent mucus barrier and subsequent gastric mucosal damage in the rat: effect of diosmectite and 16,16 dimethyl prostaglandin E2. Gastroenterol. Clin. Biol. 1994;18:609–616. [PubMed] [Google Scholar]
- MADKOUR A.A., MADINA E.M., EL-AZZOUNI O.E., AMER M.A., EL-WALILI T.M., ABBASS T. Smectite in acute diarrhea in children: a double-blind placebo-controlled clinical trial. J. Pediatr. Gastroenterol. Nutr. 1993;17:176–181. doi: 10.1097/00005176-199308000-00008. [DOI] [PubMed] [Google Scholar]
- MAHRAOUI L., HEYMAN M., PLIQUE O., DROY-LEFAIX M.T., DESJEUX J.F. Apical effect of diosmectite on damage to the intestinal barrier induced by basal tumour necrosis factor-alpha. Gut. 1997;40:339–343. doi: 10.1136/gut.40.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHAN M.M., CHANDY G., MATHAN V.I. Ultrastructural changes in the upper small intestinal mucosa in patients with cholera. Gastroenterology. 1995;109:422–430. doi: 10.1016/0016-5085(95)90329-1. [DOI] [PubMed] [Google Scholar]
- MORE J., BENAZET F., FIORAMONTI J., DROY-LEFAIX M.T. Effects of treatment with smectite on gastric and intestinal glycoproteins in the rat: a histochemical study. Histochem. J. 1987;19:665–670. doi: 10.1007/BF01676173. [DOI] [PubMed] [Google Scholar]
- MORRIS G.P., BECK P.L., HERRIDGE M.S., DEPEW W.T., SZEWCZUK M.R., WALLACE J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- MUSCH M.W., CHANG E.B.Diarrhea in inflammatory bowel diseases Inflammatory Bowel Disease: From Bench to Bedside 1994Baltimore: Williams & Wilkins; 239–254.ed. Targan, S.R. & Shanahan, F. pp [Google Scholar]
- POWELL D.W. New paradigms for the pathophysiology of infectious diarrhea. Gastroenterology. 1994;106:1705–1707. doi: 10.1016/0016-5085(94)90430-8. [DOI] [PubMed] [Google Scholar]
- RATEAU J.G., MORGANT G., DROY-PRIOT M.T., PARIER J.L. A histological, enzymatic and water–electrolyte study of the action of smectite, a mucoprotective clay, on experimental infectious diarrhoea in the rabbit. Curr. Med. Res. Opin. 1982;8:233–241. doi: 10.1185/03007998209109772. [DOI] [PubMed] [Google Scholar]
- SANCHEZ DE MEDINA F., PEREZ R., MARTINEZ-AUGUSTIN O., GONZALEZ R., LORENTE M.D., GALVEZ J., ZARZUELO A. Disturbances of colonic ion secretion in inflammation: role of the enteric nervous system and cAMP. Pflugers Arch. 2002a;444:378–388. doi: 10.1007/s00424-002-0807-z. [DOI] [PubMed] [Google Scholar]
- SANCHEZ DE MEDINA F., VERA B., GALVEZ J., ZARZUELO A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002b;70:3097–3108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- SANDLER R.S.Epidemiology of inflammatory bowel diseases Inflammatory Bowel Disease: From Bench to Bedside 1994Baltimore: Williams & Wilkins; 5–30.ed. Targan, S.R. & Shanahan, F. pp [Google Scholar]
- SANDS B.E. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- SHIRAZI T., LONGMAN R.J., CORFIELD A.P., PROBERT C.S. Mucins and inflammatory bowel disease. Postgrad. Med. J. 2000;76:473–478. doi: 10.1136/pmj.76.898.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROBER W., FUSS I.J.Experimental models of inflammation and their relation to human inflammatory bowel disease Intestinal Mucosa and its Diseases. Pathophysiology and Clinics 1999Dordrecht, The Netherlands: Kluwer Academic Publishers BV; 457–470.ed. Domschke, W., Stoll, R., Brasitus, T.A. & Kagnoff, M.F. pp [Google Scholar]
- STUCCHI A.F., SHOFER S., LEEMAN S., MATERNE O., BEER E., MCCLUNG J., SHEBANI K., MOORE F., O'BRIEN M., BECKER J.M. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- THEODOROU V., FIORAMONTI J., DROY-LEFAIX M.T., PLIQUE O., BUENO L. Protective action of diosmectite treatment on digestive disturbances induced by intestinal anaphylaxis in the guinea-pig. Aliment. Pharmacol. Ther. 1994;8:295–299. doi: 10.1111/j.1365-2036.1994.tb00291.x. [DOI] [PubMed] [Google Scholar]
- TODA Y., TSUKADA J., MISAGO M., KOMINATO Y., AURON P.E., TANAKA Y. Autocrine induction of the human pro-IL-1beta gene promoter by IL-1beta in monocytes. J. Immunol. 2002;168:1984–1991. doi: 10.4049/jimmunol.168.4.1984. [DOI] [PubMed] [Google Scholar]
- TYTGAT K.M., BOVELANDER F.J., OPDAM F.J., EINERHAND A.W., BULLER H.A., DEKKER J. Biosynthesis of rat MUC2 in colon and its analogy with human MUC2. Biochem. J. 1995;309:221–229. doi: 10.1042/bj3090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYTGAT K.M., VAN DER WAL J.W., EINERHAND A.W., BULLER H.A., DEKKER J. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochem. Biophys. Res. Commun. 1996;224:397–405. doi: 10.1006/bbrc.1996.1039. [DOI] [PubMed] [Google Scholar]
- WARHOLM M., GUTHENBERG C., VON BAHR C., MANNERVIK B. Glutathione transferases from human liver. Methods Enzymol. 1985;113:499–504. doi: 10.1016/s0076-6879(85)13065-x. [DOI] [PubMed] [Google Scholar]