Abstract
The renal medulla is a major source of plasminogen activators (PA), recently shown to induce vasodilation in vitro. Treatment with PA inhibitors has been associated with renal dysfunction, suggesting compromised renal microvasculature. We investigated the impact of the PA inhibitor epsilon amino-caproic acid (EACA) upon vascular tone in vitro, and studied the effect of both tPA and EACA upon intrarenal hemodynamics in vivo.
In vitro experiments were carried out in isolated aortic rings and with cultured vascular smooth muscle cells. Studies of renal microcirculation and morphology were conducted in anesthetized Sprague–Dawley rats.
In isolated aortic rings, EACA (but not the other inhibitors of the fibrinolytic system PAI-1 or α-2 antiplasmin) reduced the half-maximal effective concentration of phenylephrine (PE) required to induce contraction (from 32 nM in control solution to 2 and 0.1 nM at EACA concentrations of 1 and 10 μM, respectively). Using reteplase (retavase) in the same model, we also provide evidence that the vasoactivity of tPA is in part kringle-dependent. In cultured vascular smooth muscle cells, Ca2+ internalization following PE was enhanced by EACA, and retarded by tPA.
In anesthetized rats, EACA (150 mg kg−1) did not affect systemic blood pressure, total renal or cortical blood flow. However, the outer medullary blood flow declined 12±2% below the baseline (P<0.03). By contrast, tPA (2 mg kg−1), transiently increased outer medullary blood flow by 8±5% (P<0.02). Fibrin microthrombi were not found within the renal microvasculature in EACA-treated animals.
In conclusion, both fibrinolytic and antifibrinolytic agents modulate medullary renal blood flow with reciprocal effects of vasodilation (PA) and vasoconstriction (EACA). In vitro studies suggest that these hemodynamic responses are related to direct modulation of the vascular tone.
Keywords: Kidney, medulla, rat, tissue plasminogen activator, vascular smooth muscle, calcium, aminocaproic acid, reteplase, hemodynamics, laser Doppler, microcirculation
Introduction
An intact intrarenal fibrinolytic system, counter-balancing local procoagulants, is essential for maintaining the patency of renal capillaries and for the prevention of plugging of the urinary collecting system by blood clots. Renal tissues are a major source of plasminogen activators (PAs). Tissue plasminogen activator (tPA) mRNA is abundant in renal vascular endothelium, while urinary plasminogen activator (uPA), produced by tubular cells, is secreted into the urine (Xu et al., 1996) and perhaps also acts on adjacent microcirculation. The delicate balance between plasma procoagulant activity and these paracrine systems, also controlled by locally produced plasminogen-activator inhibitors type 1 (PAI-1), is compromised in multiple situations, such as tissue hypoxemia (Gertler et al., 1993), glomerulonephritis (Rondeau et al., 1990; Colucci et al., 1991), renal irradiation injury (Oikawa et al., 1997; Robbins et al., 1998) and kidney graft rejection (Malyszko et al., 1996). Enhanced formation of microthrombi within the renal vasculature is a morphologic hallmark of disseminated coagulopathy (Clarkson et al., 1969) and in certain disease entities such as thrombotic thrombocytopenic purpura (Dunea et al., 1966). Microthrombi in the renal vasculature are identified in 12% of autopsies, underscoring the high prevalence of altered intrarenal fibrinolysis antemortum (Myhre-Jensen et al., 1972).
The fibrinolytic system may exert an additional direct effect upon the renal microcirculation. We have recently shown that urokinase and urokinase-derived peptides regulate vascular smooth muscle contraction (Haj-Yehia et al., 2000). tPA also relaxes isolated aortic rings incubated under normoxic conditions and induces cerebral vasodilation in vivo (Nassar et al., 2004). This vasoactive effect, mediated by kringle, a molecular locus remote from the serine protease domain (Nassar et al., 2002b; 2004), may explain some of the immediate circulatory effects of PAs noted in the clinical practice.
The renal outer medulla is prone to hypoxic injury, resulting from the very low ambient pO2 under normal physiologic conditions. This phenomenon reflects intensive regional oxygen consumption for tubular reabsorptive work, which is barely matched by the limited oxygen supply (Brezis & Rosen, 1995; Heyman et al., 1995). We have speculated that an intact thrombolytic system may be especially important within the outer medulla, since hypoxia-induced shift of the anticoagulant/procoagulant balance to favor activation of coagulation (Gertler et al., 1993; Pinsky et al., 1998) may predispose to a further compromise of medullary oxygenation. The observation that the renal medulla is the principal source of renal PAs (Smokovitis et al., 1991) further supports this hypothesis.
The safety of inhibitors of the fibrinolytic system that are used to counteract bleeding diathesis (Royston, 1998) has been a concern (Dobkowski & Murkin, 1998). With this perspective, the current study was designed to explore the impact of the fibrinolytic system upon intrarenal microcirculation. Herein, we report that while tPA transiently increases medullary circulation, the lysine analogue plasminogen activation inhibitor epsilon amino-caproic acid (EACA) selectively compromises outer medullary microcirculation in vivo. We further demonstrate that EACA can directly intensify vascular smooth muscle contraction in vitro, associated with enhancement of Ca2+ internalization.
Methods
Animals and materials
Sprague–Dawley rats (mean weight 335 g) used for all experiments were fed on regular chow and allowed free access to drinking water. The study was conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals. Human umbilical vein vascular smooth muscle cells (SMC) were the kind gift of Dr D. Cines (Department of Pathology and Laboratory Medicine, University of Pennsylvania, U.S.A.). Reteplase was purchased from Roche Holding AG, Bern, Switzerland. All other materials were purchased from Sigma Co. (St Louis, MO, U.S.A.).
Hemodynamic studies
As previously described (Heyman et al., 2000a), rats were anesthetized with Inactin (100 mg kg−1 IP), and catheters were placed in the trachea (PE-250), external jugular vein, femoral artery and vein (PE-50). Normal saline, supplemented with bovine serum albumin (4 g l−1), was infused throughout the experiment at a rate of 5 ml h−1 and the mean arterial blood pressure was continuously monitored by a pressure transducer system connected to the arterial line. The left kidney was exposed through a midline incision, decapsulated and mechanically fixed. The rat core and renal temperatures were monitored and maintained at approximately 37°C with a heating lamp and intermittent dripping of warm saline and paraffin oil. The urinary bladder was incised to prevent urinary retention.
A perivascular transonic ultrasonic volume flow sensor (T-106, Transonic Systems Inc., Ithaca, NY, U.S.A.) monitored the renal blood flow, with the probe mounted on the left renal artery. Regional blood flows were measured by laser-Doppler probes connected to flowmeters (Perimed, Stockholm, Sweden). A superficial probe (1 mm diameter) monitored the cortical blood flow, while a needle probe (0.45 mm diameter) was inserted at a depth of 4.5–5.0 mm, for the determination of changes in outer medullary blood flow. Its proper placement within the outer medulla was confirmed macroscopically at the conclusion of the experiment.
On-line recordings of hemodynamic studies were stored, displayed and analyzed using a computerized system (MacLab/8, Analog Digital Instruments, Pty LTD, Castle Hill, NSW, Australia). The mean values for each determinant were analyzed over 1–5-min periods. All values are expressed as the percentage of baseline measurements.
Changes in total renal and regional vascular resistances were extrapolated from the relative changes in blood pressure divided by the relative changes in blood flow.
Experimental design
In the first set of experiments, following a baseline period of 30 min, eight rats were infused intravenously over 2–3 min with EACA (150 mg kg−1). In a second set of experiments, following a baseline equilibrium period, tPA was administered over 2 min to 12 rats (2 mg kg−1). In both sets of experiments, the hemodynamic parameters were monitored for up to 30 min after the manipulation of the fibrinolytic system.
Complementary studies
EACA-induced selective reduction in the outer medullary blood flow (see Results) could be caused by clot formation at the tip of the penetrating needle probe. In order to exclude such an artifact, in a separate set of experiments EACA was administered to six rats with the needle probe inserted into the renal cortex, at a depth of about 1–1.5 mm.
In vitro experiments: aortic ring studies
A direct effect of EACA upon vascular tone was explored in isolated aortic rings. Under ether anesthesia, rats were killed by bleeding. As previously detailed (Kyong et al., 1992; Haj-Yehia et al., 2000), the thoracic aortas were removed with care to avoid damage to the endothelium, dissected free of fat and connective tissue and cut into transverse rings 5 mm in length. The aortic rings were mounted in a 10 ml bath containing Krebs Henselite solution under continuous aeration and were contracted by phenylephrine (PE), added in stepwise increments (from 0.1 nM to 100 μM). EACA (1 or 10 μM) was administered 15 min before the addition of PE. In control experiments, aortic rings subjected to buffer solution alone were exposed to PE. Isometric tension was measured with a force displacement transducer and recorded online, using a computerized system (Experimentia). The half-maximal effective concentration (EC50), defined as the PE concentration that induces 50% of the maximal effect, was calculated from the determination of the response of the aortic rings to increasing concentration of PE. In additional studies designed to define the molecular basis for the vasoactive properties of tPA, we conducted the same experiment using the tPA variant reteplase (retavase) which lacks kringle-1 and finger domains.
In vitro experiments: radiolabeled Ca2+ uptake studies
Complementary studies were directed to explore the effect of EACA and tPA on intracellular Ca2+ in vascular smooth muscle cells (SMC). As previously described (Kasir et al., 1999), human umbilical vein SMC were grown in 60-mm dishes. 45Ca2+ uptake was determined by overlaying the cells with KH buffer containing 25 μM 45Ca2+ at 37°C. PE (0.1 μM) was then added alone or 5 min following tPA or EACA. After 15 min, the cells were washed and solubilized and their 45Ca2+ content was determined as in (Kasir et al., 1999).
Renal morphological evaluation
To evaluate the potential impact of EACA-induced intrarenal microthrombi formation on renal hemodynamics, anesthetized rats were killed as previously detailed (Heyman et al., 2000b) for morphological evaluation 15–20 min after the injection of hexacaprone (150 mg kg−1 i.v.). The left kidney was removed and the right kidney was perfusion fixed in vivo with 3% formalin (in PBS pH 7.4), through a 17 g needle inserted into the aorta. The superior mesenteric artery and the aorta above the renal arteries and below the needle insertion site were tied, and the right ureter and vena cava were cut to enable perfusion fixation under a constant pressure of 110 mmHg.
Both the left (non-perfusion fixed) and the right kidneys were processed for HE staining and analyzed for the detection of microthrombi within the renal vascular bed. Perfusion-fixed and non-perfused kidneys of intact rats served as controls.
Statistical analysis
Data are presented as mean±s.e.m. One-way analysis of variance (ANOVA) with post hoc Newman–Keuls analysis and paired t-test were performed for the evaluation of repeated within-group hemodynamic measurements. Statistical significance was set at P<0.05.
Results
Hemodynamic studies
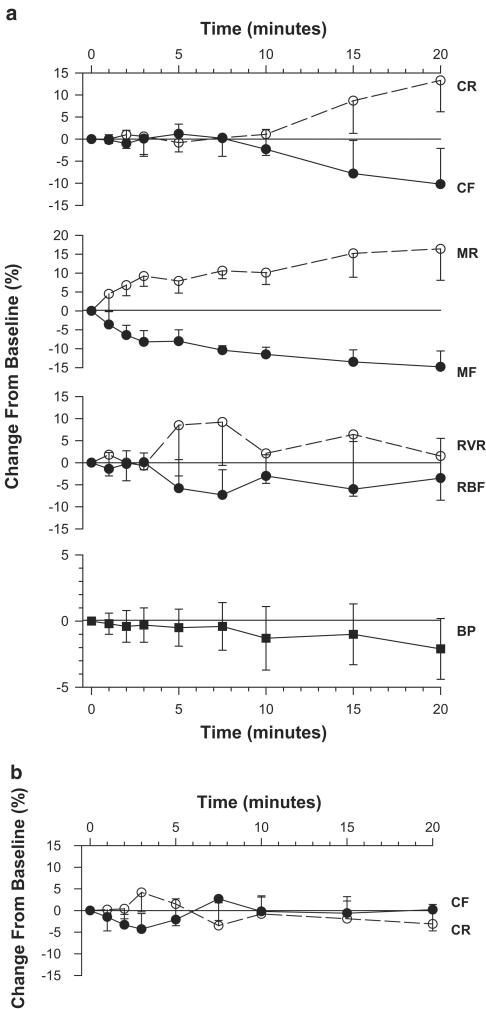
As shown in Figure 1, the administration of EACA was well tolerated and did not affect systemic blood pressure, total or cortical blood flow (Figure 1a). By contrast, a consistent decline in medullary blood flow was noted as early as 2 min after the initiation of treatment, reaching 11.5±1.9% below baseline by 10 min, and persisting throughout a 30-min observation period (P<0.03, Newman–Keuls post hoc ANOVA). Calculated outer medullary vascular resistance reciprocated, increasing by 10.1±3.1% above baseline at 10 min. In a complementary set of experiments, cortical flow, determined with the penetrating needle probe rather than the superficial nontraumatizing probe, was again unaffected by EACA (Figure 1b). These later findings confirm the validity of the medullary readings, excluding artificial microvascular compromise due to clot formation at the tip of the needle probe.
Figure 1.
Renal hemodynamic response to EACA. (a) Changes in systemic blood pressure (BP), total renal blood flow (RBF) and selective cortical (CF) and outer medullary (MF) flows are displayed as filled circles, while the calculated corresponding total renal (RVR), cortical (CR) and medullary vascular resistance (MR) are presented as empty symbols. Medullary flow significantly declines, with the null hypothesis rejected at a P-value of 0.007. Values at 2′–10′ are significantly lower than baseline measurements (n=8, P<0.03, Newman–Keuls post hoc test). The slope of reciprocal rise in medullary resistance falls short of statistical significance, but values at 2′–10′ are significantly higher than baseline (P<0.05, paired t-test). Blood pressure, total renal and cortical flows (determined with a superficial nonpenetrating probe) are unaffected by EACA. (b) Cortical flow (CF) and resistance (CR), determined with a penetrating needle probe inserted into the superficial cortex, are again unaffected by EACA (n=6).
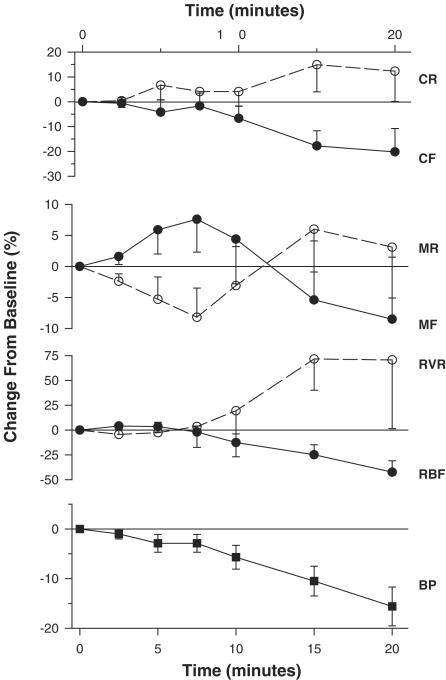
Figure 2 illustrates the hemodynamic changes induced by tPA. Blood pressure remained stable during the initial 5–7.5 min following tPA infusion, but subsequently the animals developed hemodynamic instability, with blood pressure gradually declining to 22.5±5.2% below baseline values by 30 min. The fall in blood pressure was absent in an additional group of animals given tPA infusion without laparotomy (−2.7±3.9% below baseline at 30 min, n=9), probably indicating a progressive plasma and blood loss invoked by this procedure. Total and renal blood flow gradually declined, in parallel with the progressive fall in blood pressure, with total renal and selective cortical vascular resistance rising reciprocally. By contrast, the outer medullary blood flow initially increased during the early hemodynamically stable phase, reaching 7.6±5.3% above baseline by 7.5 min (P<0.02 vs baseline, paired t-test), reflecting a reciprocal decline in the calculated regional vascular resistance. Subsequently, as hypotension developed and intensified, the outer medullary blood flow declined as well and the reduction in calculated regional resistance was blunted and reversed, probably reflecting renal hypoperfusion beyond the aotoregulatory range (Brezis et al., 1994).
Figure 2.
Renal hemodynamic response to tPA. Symbols and abbreviations are similar to those of Figure 1. While blood pressure, total renal and selective cortical blood flows remain unchanged during the first 7.5′, medullary flow rises and regional resistance falls (n=12, P<0.02 for the 7.5′ time point as compared to baseline values, paired t-test). Subsequently, blood pressure declines with renal microcirculatory deterioration both in the cortex and outer medulla (P<0.01, ANOVA).
In vitro experiments: aortic rings
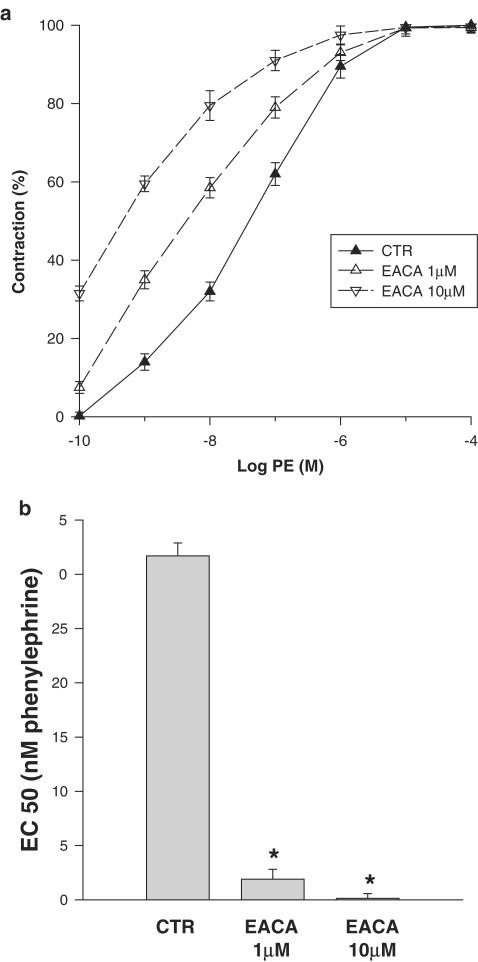
Figure 3 illustrates the PE concentration-dependent contraction of aortic rings under control conditions and with the addition of EACA. Isometric contraction of aortic rings was enhanced with EACA, with the PE concentration–contraction curve shifted to the left. As shown in Figure 4, half-maximal effective contraction (EC50) was reached at a PE concentration of 31.7 nM under control conditions. EACA reduced the EC50 of PE to 1.9 nM and to 0.14 nM when added at 1 and 10 μM concentrations, respectively. To verify the possibility that the effect of EACA on aorta rings contraction is unrelated to its inhibitory effect on the plasminogen activation system, we examined the effect of other inhibitors of the plasminogen activation system. Plasminogen activation inhibitor type 1 (PAI-1) or α2 antiplasmin had no effect on PE-induced contraction of isolated aorta rings (data not shown).
Figure 3.
Effect of EACA on isometric contraction of vascular smooth muscle. (a) Isometric tension was determined in isolated aortic rings incubated with PE. The PE concentration–contraction curve determined in control Krebs–Henselite solution is shifted to the left by the addition of EACA (1 and 10 μM). Each point along these curves represents the mean of 3–9 experiments. (b) Effect of EACA on half-maximal effective concentration (EC50) of PE in isometrically contracted aortic rings: EC50, defined as the PE concentration that induces 50% of the maximal contraction, extrapolated from (a), significantly falls with the addition of EACA (*P<0.001 vs controls for both concentrations of EACA).
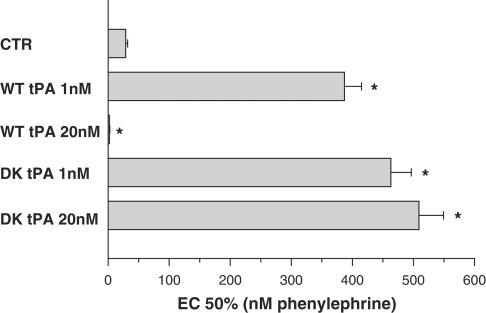
Figure 4.
Effect of tPA and reteplase on half-maximal effective concentration (EC50) of PE in isometrically contracted aortic rings: EC50 is defined as the PE concentration that induces 50% of the maximal contraction. While the full-length wild-type tPA (WT-tPA) exerts opposing pro-vasodilatory and vasoconstrictive effects at low (1 nM) and high (20 nM) concentrations, respectively (increasing and reducing EC50 of PE, respectively), the delta kringle reteplase (DK-tPA), a tPA variant lacking kringle-1 and a finger domain, exerts vasodilation at both concentrations (*P<0.001 vs control conditions – CTR).
We also examined the effect of the positively charged amino-acid lysine, a natural analog of EACA, on the contraction of the isolated aorta rings. Lysine, added at concentrations up to 10 μM, had no effect on PE-induced contraction of the aortic rings (data not shown).
In additional experiments, using isolated aortic rings, we further explored the molecular basis of the tPA vasoactive effect: Among other proteins, the fibrinolitic proteins (plasminogen, uPA, tPA) share a highly preserved domain (kringle), responsible for its interaction with fibrin clots. We have recently reported that the vasoconstrictive activity of uPA is kringle-dependent, since the uPA variant delta kringle uPA that lacks this domain has no vasoconstrictive effect (Haj-Yehia et al., 2000). tPA structure resembles that of uPA, though it has two rather than a single kringle domain (van Zonneveld et al., 1986). To determine which of the tPA kringles is involved in its vasoactivity, we examined the effect of the commercially available tPA variant reteplase that lacks kringle-1. Herein, we report that in contrast to the full-length tPA, which inhibits the PE-mediated vasoconstriction at low concentrations (1 nM) and at high concentrations (20 nM) induces a provasoconstricve effect (Nassar et al., 2004), the tPA variant reteplase exerts a pro-vasodilatory effect at both concentrations (Figure 4).
In vitro experiments: radiolabeled Ca2+ uptake studies
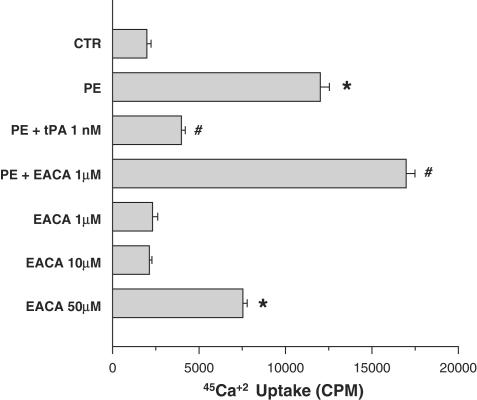
To investigate the mechanism by which EACA and tPA regulates SMC contractility, we examined the effect of tPA and EACA on PE-induced Ca2+ mobilization. As seen in Figure 5, 100 nM PE increased intracellular Ca2+ by about 600%. PE-mediated increase of intracellular Ca2+ was markedly inhibited by 50 nM tPA. By contrast, the addition of 1 μM of EACA stimulated PE-mediated 45Ca2+ internalization. Furthermore, EACA at high concentration induced 45Ca2+ internalization in the absence of PE (Figure 5).
Figure 5.
Effect of tPA and EACA on PE-induced Ca2+ mobilization in human umbilical vein SMC. SMC were incubated with buffer containing 45Ca2+. The experimental groups are: control group with no additives (CTR); 100 nM PE; 100 nM PE and 50 nM tPA (PE+tPA); 100 nM PE and 1 μM EACA (PE+EACA); and EACA (1 μM; 10 μM; 50 μM). While PE-mediated Ca2+ mobilization in vascular SMC is reduced by tPA, it is enhanced by EACA. Moreover, at high concentrations, EACA enhances Ca2+ mobilization in the absence of PE. (*P<0.01 vs CTR; #P<0.01 vs PE).
Morphological evaluation
In six animals, the entire renal microvasculature was found to be free of thrombi 15–20 min after the injection of EACA, the time period with established reduction of medullary blood flow. This negative finding was evident both in perfusion-fixed kidneys and in kidneys subjected to block fixation.
Discussion
Compound interactions exist between the vascular wall and the blood components, controlling blood flow and hemostasis. The current set of experiments indicates that within the outer medulla the fibrinolytic system follows this rule. Recombinant tPA induces a selective, transient enhancement of the regional microcirculation, while the plasminogen activation inhibitor EACA reduces it. The overall renal blood flow is hardly affected, as previously noted in healthy subjects given EACA (Swartz et al., 1966), since medullary blood flow comprises of only 10% of total renal blood flow (Jamison & Kriz, 1982). A direct effect of PAs and inhibitors upon vascular tone may be the principal mechanism for this response. PAs were shown in vitro to possess both vasoconstrictive and vasodilatory properties in a dose-dependent manner (Haj-Yehia et al., 2000; Nassar et al., 2004). In vivo plasmin activators enhance splanchnic (Gurll et al., 1978) and cerebral blood flow (Nassar et al., 2004). The transient nature of the vasodilatory response to tPA infusion in the current in vivo experiments probably reflects its short half-life (Biessen et al., 1997), in addition to the subsequent development of hemodynamic instability in this experimental group. Our previous reports (Haj-Yehia et al., 2000; Nassar et al., 2002a; Nassar et al., 2004) indicate that at low pharmacological concentrations urokinase, urokinase-derived peptide and recombinant tPA may directly induce vasodilation in isolated aortic rings. Nitric oxide seems to play a major role in this response (Stamler et al., 1992; Durante et al., 1993; Nassar et al., 2004), and our complementary studies indicate that tPA-related vasodilation is associated with inhibition of Ca2+ mobilization.
We report that, as opposed to tPA, EACA intensifies PE-induced vasoconstriction in aortic ring preparations. Our in vitro experiments further imply that this effect is not mediated by alteration of fibrin cleavage, since they were all conducted in an incubation medium devoid of fibrinogen or clotting factors. Moreover, PAI-1 and α2 antiplasmin, which also inhibit the fibrinolytic system, had no effect on the vascular tone. The mechanisms involved in EACA-mediated vasoconstriction are yet to be defined, but, as shown in our complementary studies, it involves an increase in [Ca2+] mobilization in vascular SMC.
While our manipulations of the fibrinolytic system in vitro clearly show a direct effect on vascular tone, our in vivo experiments indicate a heterogenous response within the renal microvasculature. Cortical flow was unaffected, while medullary flow selectively increased or declined with plasminogen activation or inhibition, respectively. The nature of the rather selective outer medullary response to manipulations of the fibrinolytic system in our in vivo experiments remains speculative. One possibility is diverse local expression of the plasminogen activation cascade components with consequent variation in fibrin generation and removal. Indeed, the internal milieu in the outer medulla favors coagulation by two mechanisms. First, low ambient medullary pO2, as found in the outer medulla, is known to induce plasminogen activator inhibitor-1 (PAI-1) and to inhibit plasmin activator gene expression in various vascular beds, hence promoting fibrin deposition (Gertler et al., 1993; Pinsky et al., 1998). Furthermore, the unique structure and low flow within the medullary microcirculation (Jamison & Kriz, 1982) may also promote coagulation. The importance of an efficient anticoagulant activity in the outer medulla is emphasized by the the predominant medullary expression of the fibrinolytic system (Smokovitis et al., 1991). There is a prominent expression of tPA mRNA within the renal venular endothelium, and tubular uPA antigen is abundantly found in the deep cortex and outer medulla (Xu et al., 1996). This distribution of renal PAs probably reflects the adaptation of the local fibrinolytic system for maintenance of regional vascular patency and low resistance.
Taken together, it has been logical to associate the selective reduction of medullary blood flow with EACA-induced local thrombogenesis. However, our morphological studies did not reveal microthrombi within the medullary microvasculature at the time their presence would have been anticipated. Thus, the rather selective medullary microcirculatory effects of tPA and EACA may reflect the diverse response of vascular smooth muscle at different sites. Perhaps it is related to the difference in tissue oxygenation, since low ambient pO2 may modulate vascular tone by changing nitric oxide bioavailability (Heyman et al., 1999). Indeed, it has been recently reported that tPA-related vasodilation in isolated cerebral blood vessels is markedly intensified by hypoxia (Cipolla et al., 2000). In addition, PAI-1, predominantly generated in the hypoxic outer medulla, may alter the vasoactivity of tPA (Oikawa et al., 1997; Nassar et al., 2002a). In addition, preliminary findings in our laboratories indicate that changes in oxygen tension might markedly affect the cerebral vascular response to PA (Higazi & Heyman, unpublished data). Finally, the medullary vascular response to various physiologic systems often opposes the effects in other vascular beds, including the renal cortex, due to a peculiar intrarenal distribution of receptor subtypes, for instance, for adenosine (Dinour et al., 1993) or endothelin (Gurbanov et al., 1996). Thus, for the time being, our understanding of the reason for the selective outer medullary vascular response to tPA and EACA remains speculative.
Regarding the bimodal vasoactivity of tPA, we have previously reported that in uPA the kringle domain is responsible for its vasoconstrictive effect (Haj-Yehia et al., 2000). Similarly, we now report that tPA kringle-1 that shares about 80% of its amino-acid sequence with the uPA kringle (Li et al., 1994) also determines the vasoconstrictive effect of tPA; our conclusion is supported by the finding that reteplase, a tPA variant that lacks kringle-1, lacks the vasoconstrictive effect ex vivo, but exerts a vasodilatory effect at low and high concentrations. Thus, together with our recent report (Nassar et al., 2004), our findings strongly suggest that the vasodilatory effect of tPA observed in our in vivo model is cringle-independent.
Irrespective of the mechanisms involved, EACA-related alteration of outer medullary microcirculation might predispose to hypoxic medullary injury (Heyman et al., 1995). EACA administration has indeed been associated with the development of acute renal failure. In some cases, kidney failure has been attributed to renal artery thrombosis (Tubbs et al., 1979), to blood clots obliterating the urinary collecting system (Pitts et al., 1986), or to EACA-induced rhabdomyolysis (Seymour & Rubinger, 1997). Few cases, however, may have resulted from glomerular thrombi and tubular necrosis (Charytan & Purtilo, 1969; Clarkson et al., 1969), or from a direct vasoactive effect of EACA in cases where deposition of microthrombi was not detected. EACA is currently widely used during cardiovascular surgical procedures, in order to minimize the required blood transfusions (Dobkowski & Murkin, 1998). Renal deterioration is often encountered following these procedures (Andersson et al., 1993; Chertow et al., 1998). Although EACA administration was not found to contribute to renal dysfunction in one retrospective study (Stafford-Smith et al., 2000), the potential of renal microvascular compromise with lysine analogues or other inhibitors of fibrinolysis such as aprotenin is yet to be defined (Eaton & Deeb, 1998; Royston, 1998).
In conclusion, outer medullary microcirculation is affected by the manipulation of the fibrinolytic system. Exogenous pharmacological doses of recombinant tPA enhance the outer medullary blood flow, while plasmin activator inhibition with EACA reduces it. A mechanism of microthrombi formation and lysis seems unlikely as the basis for this effect, but a direct action on vascular tone is suggested from the in vitro experiments reported in the present communication, and supported by our recent report where a tPA vasoactive effect in vitro, as well as in vivo, has been abolished by a physiologic inhibitor (PAI-1) or a PAI-1-drived peptide (Nassar et al., 2004). As opposed to tPA, which induces vascular relaxation, EACA directly intensifies aortic smooth muscle contraction in response to PE. These responses are mediated through changes in [Ca2+] within vascular SMC, and are unrelated to the fibrinolytic/hemostatic actions of these agents. Inhibitors of the fibrinolytic system may compromise the medullary microcirculation, predisposing to medullary hypoxic damage.
Acknowledgments
This work, previously presented in part as an abstract (J. Am. Soc. Nephrd., 13: 336A, 2002), was supported by the Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical center, Boston MA.
Abbreviations
- EACA
epsilon amino-caproic acid
- EC50
half-maximal effective concentration
- PA
plasminogen activators
- PAI-1
plasminogen activator inhibitor-1
- PE
phenylephrine
- SMC
smooth muscle cells
- tPA
tissue plasminogen activator
- uPA
urinary plasminogen activator
References
- ANDERSSON L.G., EKROTH R., BRATTEBY L.E. Acute renal failure after coronary surgery – a study of incidence and risk factors in 2009 consecutive patients. Thorac. Cardiovasc. Surg. 1993;41:237–241. doi: 10.1055/s-2007-1013861. [DOI] [PubMed] [Google Scholar]
- BIESSEN E.A., VAN TEIJLINGEN M., VIETSCH H., BARRETT-BERGSHOEFF M.M., BIJSTERBOSCH M.K., RIJKEN D.C., VAN BERKEL T.J., KUIPER J. Antagonists of the mannose receptor and the LDL receptor-related protein dramatically delay the clearance of tissue plasminogen activator. Circulation. 1997;95:46–52. doi: 10.1161/01.cir.95.1.46. [DOI] [PubMed] [Google Scholar]
- BREZIS M., HEYMAN S.N., EPSTEIN F. Determinants of intrarenal oxygenation: 2. Hemodynamic effects. Am. J. Physiol. 1994;267:F1063–F1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- BREZIS M., ROSEN S. Hypoxia of the renal medulla – its implications for disease. N. Engl. J. Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- CHARYTAN C., PURTILO D. Glomerular capillary thrombosis and acute renal failure after epsilon-amino caproic acid therapy. N. Engl. J. Med. 1969;280:1102–1104. doi: 10.1056/NEJM196905152802006. [DOI] [PubMed] [Google Scholar]
- CHERTOW G.M., LEVY E.M., HAMMERMEISTER K.E., GROVER F., DALEY J. Independent association between acute renal failure and mortality following cardiac surgery. Am. J. Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- CIPOLLA M.J., LESSOV N., CLARK W.M., HALEY E.C. Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- CLARKSON A.R., SAGE R.E., LAWRENCE J.R. Consumption coagulopathy and acute renal failure due to gram-negative septicemia after abortion. Complete recovery with heparin therapy. Ann. Intern. Med. 1969;70:1191–1199. doi: 10.7326/0003-4819-70-6-1191. [DOI] [PubMed] [Google Scholar]
- COLUCCI M., SEMERARO N., MONTEMURRO P., CHIUMARULO P., TRIGGIANI R., MORRONE L.F., SCHENA F.P. Urinary procoagulant and fibrinolytic activity in human glomerulonephritis. Relationship with renal function. Kidney Int. 1991;39:1213–1217. doi: 10.1038/ki.1991.153. [DOI] [PubMed] [Google Scholar]
- DINOUR D., AGMON Y., BREZIS M. Adenosine: an emerging role in the control of renal medullary oxygenation. Exp. Nephrol. 1993;1:152–157. [PubMed] [Google Scholar]
- DOBKOWSKI W.B., MURKIN J.M. A risk–benefit assessment of aprotenin in cardiac surgical procedures. Drug Saf. 1998;18:21–41. doi: 10.2165/00002018-199818010-00003. [DOI] [PubMed] [Google Scholar]
- DUNEA G., MEUHREKE R.C., NAKAMOTO S., SCHWARTZ F.D. Thrombotic thrombocytopenic purpura with acute anuric renal failure. Am. J. Med. 1966;41:1000–1006. doi: 10.1016/0002-9343(66)90057-x. [DOI] [PubMed] [Google Scholar]
- DURANTE W., SCHINI V.B., CATOVSKY S., KROLL M.H., VANHOUTTE P.M., SCHAFER A.I. Plasmin potentiates induction of nitric oxide synthesis by the interleukine-1 beta in vascular smooth muscle cells. Am. J. Physiol. 1993;264:H617–H624. doi: 10.1152/ajpheart.1993.264.2.H617. [DOI] [PubMed] [Google Scholar]
- EATON M.P., DEEB G.M. Aprotenin versus epsilon-aminocaproic acid for aortic surgery using deep hypothermic circulatory arrest. J. Cardiothorac. Vasc. Anesth. 1998;12:548–552. doi: 10.1016/s1053-0770(98)90099-4. [DOI] [PubMed] [Google Scholar]
- GERTLER J.P., PENY L., L'ITALIEN G., CHUNG-WELCH N., CAMBRIA R.P., ORKIN R., ABBOTT W.M. Ambient oxygen tension modulates endothelial fibrinolysis. J. Vasc. Surg. 1993;18:939–945. [PubMed] [Google Scholar]
- GURBANOV K., RUBINSTEIN I., HOFFMAN A., ABASSI Z., BETTER O.S., WINAVER J. Differential regulation of renal regional blood flow by endothelin-1. Am. J. Physiol. 1996;271:F1166–F1172. doi: 10.1152/ajprenal.1996.271.6.F1166. [DOI] [PubMed] [Google Scholar]
- GURLL N., ZINNER M.J., TURTINEN L., REYNOLDS D.G. Vasodilation, fibrinolysis and thrombosis with intraarterial infusion of urokinase in the canine superior mesenteric artery. Gastroenterology. 1978;75:425–431. [PubMed] [Google Scholar]
- HAJ-YEHIA A., NASSAR T., SACHAIS B., KUO A., BADEIR K., AL-MEHDI A.B., MAZAR A., CINES D., HIGAZI A.A.R. Urokinase-derived peptides regulate vascular smooth muscle contraction in vitro and in vivo. FASEB J. 2000;14:1411–1422. doi: 10.1096/fj.14.10.1411. [DOI] [PubMed] [Google Scholar]
- HEYMAN S.N., DARMON D., GOLDFARB M., BITZ H., SHINA A., ROSEN S, BREZIS M. Endotoxin-induced renal failure: I. A role for altered renal microcirculation. Exp. Nephrol. 2000a;8:266–274. doi: 10.1159/000020678. [DOI] [PubMed] [Google Scholar]
- HEYMAN S.N., FUCHS S., BREZIS M. The role of medullary ischemia in acute renal failure. New Horizons. 1995;3:597–607. [PubMed] [Google Scholar]
- HEYMAN S.N., GOLDFARB M., DARMON D., BREZIS M. Tissue oxygenation modifies nitric oxide bioavailability. Microcirculation. 1999;6:199–203. [PubMed] [Google Scholar]
- HEYMAN S.N., ROSEN S., DARMON D., GOLDFARB M., BITZ H., SHINA A., BREZIS M. Endotoxin-induced renal failure: II. A role for tubular hypoxic damage. Exp. Nephrol. 2000b;8:275–282. doi: 10.1159/000020679. [DOI] [PubMed] [Google Scholar]
- JAMISON R.L., KRIZ W.Structure of the medulla as a whole Urinary Concentration Mechanism. Structure and Function 1982New York: Oxford University Press; 55–76.ed. Jamison, R.L. & Kriz, W. pp [Google Scholar]
- KASIR J., REN X., FURMAN I., RHAMIMOFF H. Truncation of the C terminus of the rat brain Na–Ca exchanger RBE-1 (NCX1.4) impairs surface expression of the protein. J. Biol. Chem. 1999;274:24873–24880. doi: 10.1074/jbc.274.35.24873. [DOI] [PubMed] [Google Scholar]
- KYONG S.K., CHANG K.S., STEVENS W. Endothelium-dependent increase in vascular sensitivity to phenylephrine in long-term streptozotocin diabetic rat aorta. Br. J. Pharmacol. 1992;107:983–990. doi: 10.1111/j.1476-5381.1992.tb13395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X., BOKMAN A.M., LLINAS M., SMITH R.A., DOBSON C.M. Solution structure of the kringle domain from urokinase-type plasminogen activator. J. Mol. Biol. 1994;235:1548–1559. doi: 10.1006/jmbi.1994.1106. [DOI] [PubMed] [Google Scholar]
- MALYSZKO J., MALYSZKO J.S., PAWLAK K. The coagulo-lytic system and endothelial function in cyclosporine-treated kidney allograft recipients. Transplantation. 1996;62:828–830. doi: 10.1097/00007890-199609270-00021. [DOI] [PubMed] [Google Scholar]
- MYHRE-JENSEN O., HANSEN E.S., BUTRAGO B. Renal microthrombosis. Incidence in 500 consecutive autopsies. Clinico-pathological relations. Acta Pathol. Microbiol. Scand. 1972;80:403–411. [PubMed] [Google Scholar]
- NASSAR T., AKKAWI S., SHINA A., HAJ-YEHIA A., BDEIR K., TARSHIS M., HEYMAN S.N., HIGAZI A.A.R. In vitro and in vivo effect of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- NASSAR T., BAR-SHAVIT R., HAJ-YEHIA A., AKKAWI S., BDEIR K., AL-MEHDI A.B., TARSHIS M., HIGAZI A.A.R. Human alpha-defensin regulates smooth muscle cell contraction. A role for low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Blood. 2002a;100:4026–4032. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- NASSAR T., HAJ-YEHIA A., AKKAWI S., KUO K., BDEIR K., MAZAR A., CINES D., HIGAZI A.A.R. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J. Biol. Chem. 2002b;277:40499–40504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- OIKAWA T., FREEMAN M., LO W., VAUGHAN D.E., FOGO A. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin–angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- PINSKY D.J., LIANO H., LaAWSON C.A., VAN S.F., CHEN J., CARMELIET P., LOSKUTOFF D.J., STERN D.M. Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J. Clin. Invest. 1998;102:919–928. doi: 10.1172/JCI307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS T.O., SPERO J.A., BONTEMPO F.A., GREENBERG A. Acute renal failure due to high grade obstruction following therapy with epsilon-aminocaproic acid. Am. J. Kidney Dis. 1986;8:441–444. doi: 10.1016/s0272-6386(86)80172-x. [DOI] [PubMed] [Google Scholar]
- ROBBINS M.E., STEPHENS L.C., JOHNSTON D.A., THAMES H.D., PETERS L.J., HOPEWELL J.W., ANG K.K. A morphometric analysis of glomerular and tubular alterations following fast-neutron irradiation of the pig and monkey kidney. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:1149–1156. doi: 10.1016/s0360-3016(98)00161-8. [DOI] [PubMed] [Google Scholar]
- RONDEAU E., MOUGENOT B., LACAVE R., PERALDI M.N., KRUITHOF E.K., SRAER J.D. Plasminogen activator inhibitor 1 in renal fibrin deposits of human nephropathies. Clin. Nephrol. 1990;33:55–60. [PubMed] [Google Scholar]
- ROYSTON D. Aprotenin versus lysine analogues: the debate continuous. Ann. Thorac. Surg. 1998;65:S9–S19. doi: 10.1016/s0003-4975(98)00071-x. [DOI] [PubMed] [Google Scholar]
- SEYMOUR B.D., RUBINGER M. Rhabdomyolysis induced by epsilon-aminocaproic acid. Ann. Pharmacother. 1997;31:56–58. doi: 10.1177/106002809703100109. [DOI] [PubMed] [Google Scholar]
- SMOKOVITIS A., KOKOLIS N., TAITZOGLOU I. Sex-related differences in plasminogen activator activity and plasminogen activator inhibition of human and animal kidneys: effect of orchidectomy or ovariectomy. Haemostasis. 1991;21:305–312. doi: 10.1159/000216241. [DOI] [PubMed] [Google Scholar]
- STAFFORD-SMITH M., PHILLIPE-BUTE B., REDDAN D.N., BLACK J., NEWMAN M.F. The association of åepsilon-aminocaproic acid with postoperative decrease in creatinine clearance in 1502 coronary bypass patients. Anesth. Analg. 2000;91:1085–1090. doi: 10.1097/00000539-200011000-00008. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SIMON D.I., JARAKI O., OSBORNE J.A., FRANCIS S., MULLINS M., SINGEL D., LOSCALZO J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZ C., ONESTI G., RAMIREZ O., SHAH N., BREST A.N. Cardiac and renal hemodynamic effects of the antifibrinolytic agent, epsilon aminocaproic acid. Curr. Ther. Res. Clin. Exp. 1966;8:336–342. [PubMed] [Google Scholar]
- TUBBS R.R., BENJAMIN S.P., DOHN D.E. Recurrent subarachnoid hemorrhage associated with aminocaproic acid therapy and acute renal artery thrombosis. Case report. J. Neurosurg. 1979;51:94–97. doi: 10.3171/jns.1979.51.1.0094. [DOI] [PubMed] [Google Scholar]
- XU Y., HAGEGE J., MOUGENOT B., SRAER J.D., RONNE E., RONDEAU E. Different expression of the plasminogen activation system in renal thrombotic microangiopathy and the normal human kidney. Kidney Int. 1996;50:2011–2019. doi: 10.1038/ki.1996.523. [DOI] [PubMed] [Google Scholar]
- VAN ZONNEVELD A.J., VEERMAN H., MACDONALD M.E., VAN MOURIK J.A., PANNEKOEK H. Structure and function of human tissue-type plasminogen activator (t-PA) J. Cell. Biochem. 1986;32:169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]