Abstract
Background
Biological rhythmicity, particularly circadian rhythmicity, is considered to be a key mechanism in the maintenance of physiological function. Very little is known, however, about biological rhythmicity pattern in preterm and term neonates in neonatal intensive care units (NICU). In this study, we investigated whether term and preterm neonates admitted to NICU exhibit biological rhythmicity during the neonatal period.
Methods
Twenty-four-hour continuous recording of four physiological variables (heart rate: HR recorded by electrocardiogram; pulse rate: PR recorded by pulse oxymetry; respiratory rate: RR; and oxygen saturation of pulse oxymetry: SpO2) was conducted on 187 neonates in NICU during 0–21 days of postnatal age (PNA). Rhythmicity was analyzed by spectral analysis (SPSS procedure Spectra). The Fisher test was performed to test the statistical significance of the cycles. The cycle with the largest peak of the periodogram intensities was determined as dominant cycle and confirmed by Fourier analysis. The amplitudes and amplitude indexes for each dominant cycle were calculated.
Results
Circadian cycles were observed among 23.8% neonates in HR, 20% in PR, 27.8% in RR and 16% in SpO2 in 0–3 days of PNA. Percentages of circadian cycles were the highest (40%) at <28 wks of gestational age (GA), decreasing with GA, and the lowest (14.3%) at >= 37 wks GA within 3 days of PNA in PR and were decreased in the later PNA. An increase of the amplitude with GA was observed in PR, and significant group differences were present in all periods. Amplitudes and amplitude indexes were positively correlated with postconceptional age (PCA) in PR (p < 0.001). Among clinical parameters, oxygen administration showed significant association (p < 0.05) with circadian rhythms of PR in the first 3 days of life.
Conclusion
Whereas circadian rhythmicity in neonates may result from maternal influence, the increase of amplitude indexes in PR with PCA may be related to physiological maturity. Further studies are needed to elucidate the effect of oxygenation on physiological rhythmicity in neonates.
Background
Preterm neonates hospitalized in a neonatal intensive care unit (NICU) face many challenges to adapt to the new environment. Heat loss [1], weight loss [2], respiratory distress and cardiac instability [3] are very common features for them. An artificial environment in NICU is mandatory to support these neonates; however, external influences such as constant light, noise, and medical intervention may be stressful. Further, neonates are deprived of maternal influences, which is essential for their development. It has been thought that this environmental condition may influence the development of biological rhythm in preterm neonates [4-6].
Circadian rhythms are generated endogenously by a biological clock, which is located in the anterior hypothalamic suprachiasmatic nuclei (SCN) [7,8], and are modulated by exogenous factors [9,10]. Many physiological processes are now known to be cyclically organized [11]. They show different cycles: circadian cycles last approximately 24 hours, ultradian cycles shorter than 24 hours, and infradian cycles longer than 24 hours [12]. These rhythms interact mutually as well as with the outside fluctuating environment under the control of feedback systems providing an orderly function that enables life [11].
Circadian rhythms have been described in the human fetus [13-16] and have been attributed either to the maternal environment or to the maturation of the fetal nervous system [13,17,18]. The SCN has been detected as early as 18–20 weeks of gestational age [19], and primate studies indicated that the SCN is responsive to light at 24 weeks of gestational age [20]. In term neonates, circadian rhythms have been reported to be present immediately after birth but to eventually disappear [4,21], not being detected again until 3 to 4 weeks of postnatal life [22]. Some studies showed that circadian rhythms are predominant in preterm neonates [4,21,23], while others showed ultradian rhythms to be dominant in preterm neonates [22,24-27]. To elucidate the developmental process of physiological rhythmicities, we studied four physiological variables in preterm and term neonates.
Methods
Subjects and data collection
From January 2004 to March 2006, 520 neonates were admitted to the NICU at Miechuo Medical Center. All of them were monitored with electrocardiogram (ECG) for heart rate (HR), respiration rate (RR), and with pulse oxymetry on the wrist or the feet for saturation of pulse oxymetry oxygen (SpO2) and pulse rate (PR) throughout their stay in the NICU. Monitored physiological information was transformed as measurement variables at 10-second intervals by the Wave Achieving System (WAS-J; Philips Electronics Japan, Tokyo, Japan) through the local area network in the NICU. The data were recorded for 24 hours for the following postnatal periods: Period 1: days 0–3; Period 2: days 4–6; Period 3: days 7–13; and Period 4: days 14–21. Subjects with continuously disrupted data for more than 1 minute were excluded from the study. A total of 187 neonates (114 boys and 73 girls) were recorded from period 1 to period 4.
The NICU was maintained under a light-dark cycle. The light was dimmed (less than 30 lux) during the night from 21:00 pm to 07:00 am, while it was maintained at a higher level (300–580 lux) during the daytime. NICU staff also varied according to time of day: the number of attendants at night was one third that of attendants during daytime hours. Parent's visitations were allowed three times a day (11:00 to 12:00 in the morning, 14:00 to 15:00 in the afternoon, and 17:00 to 21:00 in the evening). Bathing and measurement of body weight were conducted daily in the morning. Medical examinations, such as blood sampling, radiography, or ultrasonography, were mostly provided in the morning if necessary.
Written informed consent was obtained from the parents, and the study was approved by the ethical committee of the institute. Demographics and health status information's were obtained from the medical records.
Analysis of rhythms
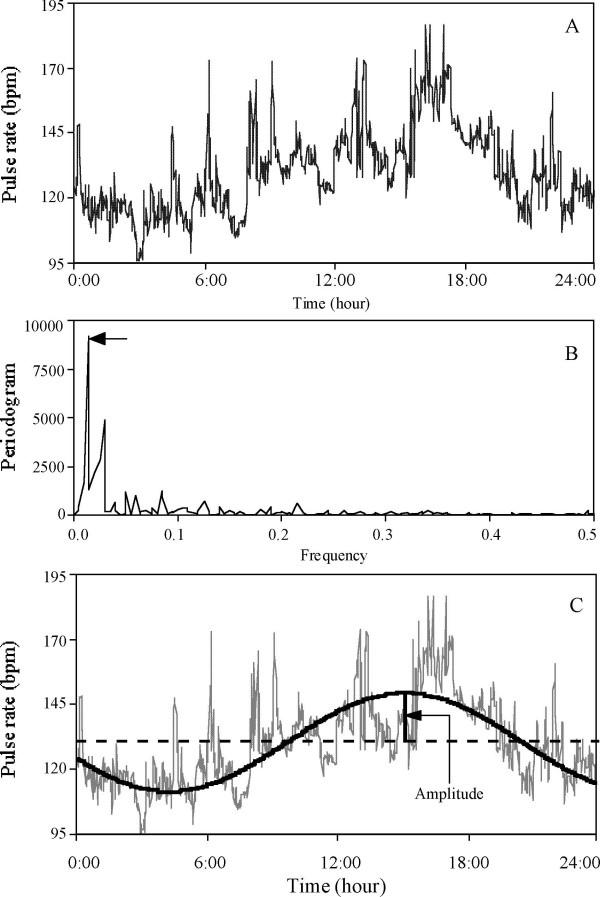
Physiological rhythmicity was analyzed for HR, PR, RR and SpO2 with spectral analysis (periodogram) with SPSS 11.5 software (SPSS Inc. Chicago, IL), as previously reported [28]. Briefly, 24 hours sessions were run in 10-second intervals and were aggregated into 1-minute time blocks. Periodogram analysis was performed with a time series of 1440 minutes (N = 1440 observations). The Fisher test was used to test the statistical significance of the cyclic components (N = 1440, α = 0.05) [28,29]. Among the significant cycles, the cycle with the largest peak in the periodogram was considered to be the dominant cycle for each time series data and was used for further analysis [28]. All dominant cycles were confirmed by Fourier analysis, and further circadian cycles were confirmed by cosinor analysis with a significance of p < 0.05 by least square analysis (Figure 1). The amplitude, the distance between mesor and the highest value of the cosine curve, was calculated for each dominant cycle. In addition, an amplitude index was calculated as follows:
Figure 1.
Brief description of steps to determine the dominant cycle using spectral analysis. A: Plot of original data for pulse rate (PR). PR was measured once every 10 seconds and averaged into 1 minute time block for 1440 minutes; N = 1440 observation. B: Periodogram intensities for PR (plotted on linear scale). The largest peak of the periodogram was selected (arrow) as representative cyclic component that represent the largest amount of variance. C: The corresponding cycle of the largest peak in the periodogram intensities was reconstructed from the FFT coefficient to fit the sinusoidal function: χt = μ + Acos(ω t) + Bsin(ω t). The bold line is the detected cycle (period: 1440 minutes = 24 hours) superimposed on the original data.
Amplitude index = amplitude ÷ mean of variables × 100.
Statistical analysis
Data were analyzed with SPSS and Statview. ANOVA was used to evaluate the differences between gestational age groups. The Pearson correlation coefficient was used to analyze the relationships between postconceptional age (PCA) and rhythmicity parameters. Univariate analysis using Mann-Whitney U-test for continuous variables or Fisher's exact test for categorical variables was used to compare clinical variables according to the development of physiological rhythmicity. A multiple logistic regression analysis was performed using a step-wise approach to determine the independent relationship of significant variables found in the univariate analysis.
Results
Sample characteristics
The demographics of neonates are shown in Table 1. The median gestational age (GA) was 34 weeks (range: 23–42 weeks), and the median birth weight was 1968 g (range: 454–4132 g). Among these neonates, 9.1% were born at < 28 weeks of gestation age and 14.4% had birth weight of less than 1000 g. The median age at hospitalization was 0 day (range: 0–9 day) and the median duration of hospitalization was 32 days (range: 5–182 days). One hundred eleven neonates (59.4%) were intubated and 72 neonates (38.5%) received oxygen.
Table 1.
Demographic characteristics of 187 preterm and term neonates.
| Variables/Categories | n (%) |
| Gender (boys/Girls) | 114 (61)/73 (39) |
| Gestational age (wks), median (range) | 34 (23–42) |
| < 28 | 17 (9.1) |
| 28–32 | 49 (26.2) |
| 33–36 | 58 (31) |
| ≥37 | 63 (33.7) |
| Birth Weight (g), median (range) | 1968 (454–4132) |
| < 1000 | 27 (14.4) |
| 1000–1499 | 31 (16.6) |
| 1500–1999 | 38 (20.3) |
| ≥2000 | 91 (48.7) |
| Apgar score 1 min/5 min, median (range) | 8 (0–10)/9 (2–10) |
| Age at hospitalization (day), median (range) | 0 (0–9) |
| Hospitalization (day), median (range) | 32 (5–182) |
| Caesarian Section | 96 (51.3) |
| Multiple gestation | 4 (2.3) |
| Intubation | 111(59.4) |
| Oxygenation | 72 (38.5) |
| Birth asphyxia | 27 (14.4) |
| Intrauterine growth retardation | 23 (12.3) |
| Respiratory distress syndrome | 31 (16.6) |
| Transient tachypnea of the newborn | 38 (20.3) |
Data are expressed as mean ± SD or n (%).
Rhythmicity analysis
Results of the analyses of rhythmicity are summarized in Table 2. To ensure the accuracy of rhythmicity analysis, parameters missing more than 7% of total data were excluded from the analysis in each study. Among 461 time series recorded for each parameters, eligible samples were obtained in 304 for HR, 379 for PR, 372 for RR, and 383 for SpO2 within the 4 periods. Among eligible samples, rhythmicity was observed in more than 90% of neonates in each period for HR, PR, RR and SpO2 (Table 2). The percentage was not much lower (HR: 89%, PR: 90%, RR: 79%, SpO2: 76%) after Bonferroni correction for multiple testing (p < 0.0001).
Table 2.
Descriptive profiles for significant cycles of HR, PR, RR and SpO2.
| Period | Period 1 | Period 2 | Period 3 | Period 4 | |
| Sampling | (0–3) | (4–6) | (7–13) | (14–21) | |
| n | 116 | 114 | 125 | 106 | |
| Eligible sample* | HR | 82 (70.7) | 64 (56.1) | 91 (72.8) | 67 (63.2) |
| PR | 101 (87.1) | 88 (77.2) | 106 (84.8) | 84 (79.2) | |
| RR | 99 (85.3) | 85 (74.6) | 104 (83.2) | 84 (79.2) | |
| SpO2 | 103 (88.8) | 89 (78.1) | 106 (84.8) | 85 (80.2) | |
| Significant cycle** | HR | 80 (98) | 64 (100) | 89 (98) | 67 (100) |
| PR | 100 (99) | 87 (99) | 104 (98.1) | 83 (99) | |
| RR | 90 (91) | 84 (99) | 97 (93.3) | 79 (94) | |
| SpO2 | 94 (91.3) | 86 (97) | 103 (97) | 78 (92) | |
| Circadian cycle*** | HR | 19 (23.8) | 11 (17.2) | 20 (22.5) | 13(19.4) |
| PR | 20 (20) | 16 (18.4) | 20 (19.2) | 16 (19.3) | |
| RR | 25 (27.8) | 28 (33.3) | 21 (21.6) | 11 (13.9) | |
| SpO2 | 15 (16) | 10 (11.6) | 17 (16.5) | 15 (19.2) |
Data are shown in n (%). Parentheses are percentages of * eligible samples in all samples, ** significant cycles in all eligible samples, and *** circadian cycles in significant cycles.
Without correction for multiple testing, circadian cycle (1440 minutes) was observed among 23.8% neonates in HR, 20% in PR, 27.8% in RR and 16% in SpO2 in Period 1. Because many samples were excluded from HR analysis, and the percentage of eligible samples was consistently lower than for PR, further analysis of cardiac rhythmicity used PR instead of HR.
Rhythmicity and gestational age
Rhythmicity was analyzed in four gestational age groups: < 28 wks, 28–32 wks, 33–36 wks, ≥ 37 wks. The distribution of circadian cycles in each gestational age groups and periods is summarized in Table 3. In PR, the percentage of circadian cycles was highest (40%) at <28 wks of GA, decreasing with GA, and lowest (14.3%) at ≥ 37 wks of GA in Period 1. A similar tendency was observed in each period in PR; however, there was no consistent tendency in percentages of circadian cycle in RR and SpO2.
Table 3.
Distribution of circadian cycles according to gestational age groups in each period.
| Gestational age | Period 1 | Period 2 | Period 3 | Period 4 | |||||
| Groups | n | (0–3 d) | n | (4–6 d) | n | (7–13 d) | n | (14–21 d) | |
| PR | <28 wks | 10 | 4 (40) | 12 | 3 (25) | 12 | 5 (41.7) | 13 | 4 (30.8) |
| 28–32 wks | 26 | 6 (23.1) | 22 | 6 (27.3) | 42 | 11 (26.2) | 39 | 9 (23.1) | |
| 33–36 wks | 29 | 5 (17.2) | 26 | 5 (19.2) | 31 | 2 (6.5) | 23 | 3 (13.0) | |
| ≥37 wks | 35 | 5 (14.3) | 27 | 2 (7.4) | 19 | 2 (10.5) | 8 | 0 (0) | |
| RR | < 28 wks | 7 | 1 (14.3) | 11 | 1(9.1) | 13 | 5 (38.5) | 13 | 0 (0) |
| 28–32 wks | 24 | 8 (33.3) | 20 | 9 (45) | 38 | 9 (23.7) | 36 | 8 (22.2) | |
| 33–36 wks | 25 | 8 (32) | 27 | 9 (33.3) | 28 | 3 (10.7) | 22 | 2 (9.1) | |
| ≥37 wks | 34 | 8 (23.5) | 26 | 9 (34.6) | 18 | 4 (22.2) | 8 | 1(12.5) | |
| SpO2 | < 28 wks | 10 | 0 (0) | 12 | 3 (25) | 12 | 3 (25) | 13 | 2 (15.4) |
| 28–32 wks | 25 | 5 (20) | 20 | 3 (15) | 40 | 7 (17.5) | 37 | 9 (24.3) | |
| 33–36 wks | 26 | 5 (19.2) | 25 | 5 (20) | 32 | 4 (12.5) | 20 | 3 (15) | |
| ≥37 wks | 33 | 5 (15.2) | 29 | 4 (13.8) | 19 | 3 (15.8) | 8 | 1 (12.5) |
Data are shown in n (%).
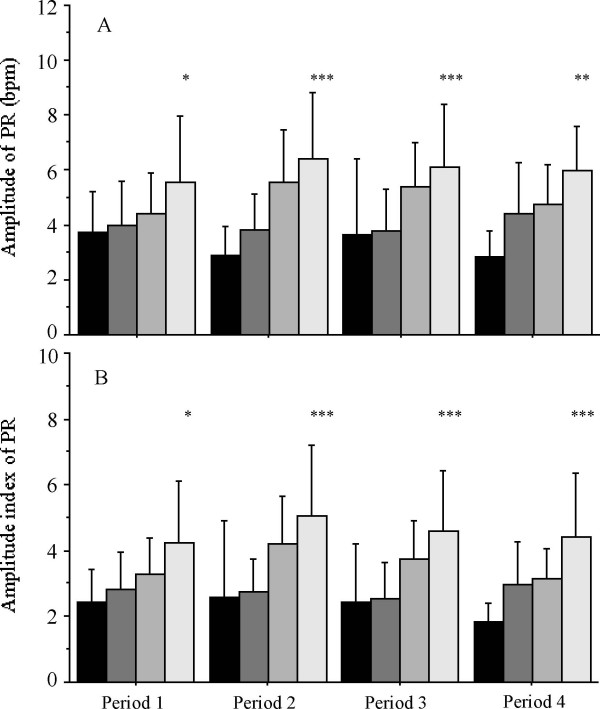
Amplitudes and amplitude indexes of all detected cycles in PR in each period are shown in Figure 2. An increase of circadian amplitude with gestational age was observed in PR. and significant differences were present among gestational age groups in all periods (Figure 2A). These changes were not observed in RR and SpO2 (data not shown). Amplitude indexes showed similar tendency to amplitudes in PR (Figure 2B). There were no significant associations between cycles and amplitudes in any parameter in each period (data not shown).
Figure 2.
Amplitudes (A) and amplitude indexes (B) of all detected cycle of PR over the 4 periods for 4 gestational age groups infants. Data are shown in Mean ± SD. The dark bar is for < 28 wks, the gray bar is for 28–32 wks, the light gray bar is for 33–36 wks, and white bar is for ≥ 37 wks. * p < 0.01, ** p < 0.001, *** p < 0.0001, according to ANOVA. The sample size for each gestational age group is shown in Table 2.
Relationship between rhythmicity and postconceptional age
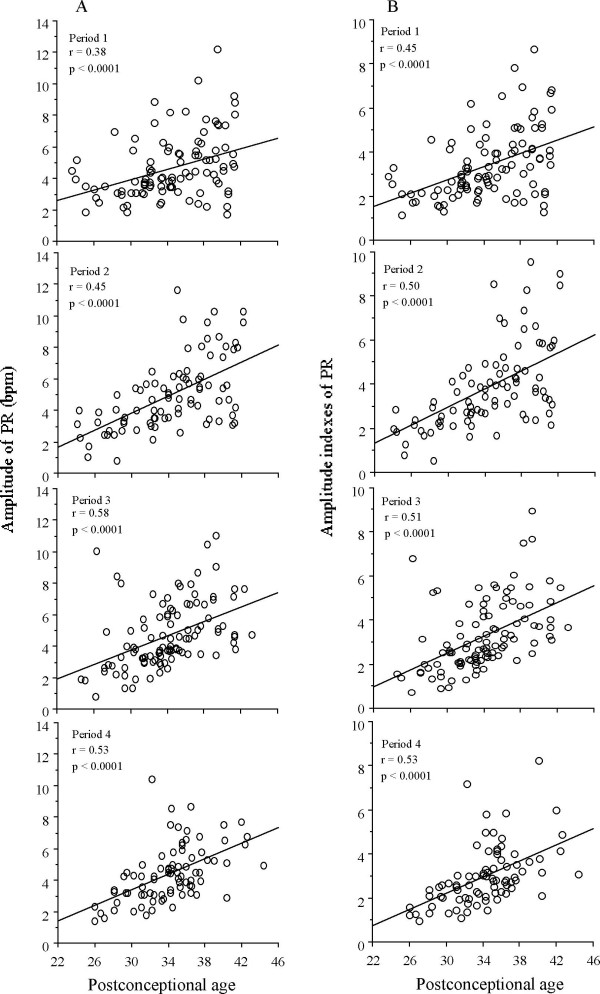
In examining the relationship with postconceptional age (PCA), correlation of coefficient was performed using amplitudes and amplitude indexes in each period for all parameters. Amplitudes and amplitude indexes of PR were positively correlated with PCA in all four periods (Figure 3).
Figure 3.
Linear regression (and coefficients of correlation) for amplitudes and amplitude indexes of PR as functions of postconceptional age. A significant increase in amplitudes and amplitude indexes with postconceptional age is present in all period in PR.
Clinical conditions associated with rhythmicity
To determine whether clinical conditions may affect the emergence and development of rhythmicity, clinical factors were determined according to cycle length with circadian cycles (1440 minutes) or ultradian cycles (≤ 720 minutes). On univariate analyses in Period 1, circadian cycle (1440 minutes) was significantly associated (p < 0.05) only with oxygen administration at data sampling in PR (Table 4), while there were no significant associations in RR or SpO2 (data not shown). In Periods 3 and 4 in PR, gestational age was found to be significantly associated with circadian cycle (p < 0.01) as well as with oxygen administration (p < 0.05). Neither gestational age nor oxygen administration qualified as an independent factor for existence of circadian cycle in multivariate logistic regression models. Clinical parameters were not associated with the existence of significant cycles in amplitude or amplitude index.
Table 4.
Univariate analysis for association of clinical parameters with existence of circadian rhythmicities in PR in Period 1.
| Clinical variables | Cycle 1440 (n = 20) | ≤ 720 (n = 80) | p |
| Gestational age (wks) | 32.7 ± 4.9 | 34.2 ± 4.6 | NS |
| Birth weight (g) | 1930 ± 983 | 2077 ± 900 | NS |
| Apgar Score < 6 (5 min) | 1 (5) | 10 (12.7) | NS |
| Asphyxia | 4 (20) | 17 (21.3) | NS |
| RDS | 4 (20) | 14 (17.3) | NS |
| IUGR | 3 (15) | 6 (7.5) | NS |
| Mean of variables | |||
| Mean PR (/min) | 140.2 ± 8.6 | 135.5 ± 12.8 | NS |
| Mean RR (/min) | 45.7 ± 8.5 | 43.0 ± 8.5 | NS |
| Mean SpO2 (%) | 97.9 ± 1.1 | 97.9 ± 1.3 | NS |
| Treatment of data sampling | |||
| Oxygenation | 18 (90) | 46 (57.5) | 0.02 |
| Intubation | 10 (50) | 25 (31.3) | NS |
| Aminophylline | 1 (5) | 4 (5) | NS |
| Phenobarbital | 0 (0) | 1 (1.3) | NS |
| Midazolam | 3 (15) | 6 (7.5) | NS |
Data are expressed as mean ± SD or n (%). Mann-Whitney U test was performed for continuous variables and Fisher's exact test was performed for categorical variables.
Discussion
Rhythmicity has been previously studied in preterm and term infants for various physiological variables, such as body temperature [24,30], blood pressure [21], heart rate [18], sleep-wake pattern [24], rest-activity pattern [26], melatonin secretion [31], and electroencephalogram [32]. In this study, we have investigated rhythmicity in PR, RR, and SpO2. All of these are important parameters in the regulation of human physiology, and yet little is known about rhythmicity of these variables in neonates. We have shown that most of the analyzed neonates had individual rhythmicity for these parameters with variable cycles after birth, even in extremely immature infants.
Emergence of circadian rhythmicity has been reported to be associated with brain maturation of preterm infants [33,34]. In term neonates, circadian cycles are detected immediately after birth and subsequently disappear and are not detectable until 3 to 4 weeks of postnatal life [22]. It has been suggested that circadian cycles in the early neonatal period are due to maternal influence in utero and that endogenous rhythmicity appears only later [13,17,18]. However, conclusive studies are limited by subject number because of the difficulty in collecting continuous data in NICU. Our sample size of 187 neonates is larger than that of previous studies. As a result, circadian cycles were confirmed in early neonatal period for all parameters either in preterm or term neonates. In PR, comparatively higher percentages of circadian cycles were observed during early neonatal period in preterm neonates and persisted through the later neonatal period, especially in extremely immature infants, while percentages of circadian cycles decreased through the later period in term neonates. These results partially support the previous studies [4,21,23]. The fact that environmental conditions were rhythmic in our study (i.e., presence of a light-dark cycle, of a cycle of NICU staffing, of a cycle of bathing, etc.) prevents us from making inferences about the endogenous or exogenous nature of biological rhythmicity in our subjects.
Although exact factors for the development of rhythmicity are still unclear, it has been suggested that physiological complications may play a role [35]. Among clinical parameters, disease conditions such as respiratory problems or asphyxia, and therapeutic drugs such as phenobarbital or aminophylline, were not associated with emergence of circadian cycles. Only oxygen administration revealed significant association with emergence of circadian cycles in PR within 3 days of birth. Disruption of circadian rhythmicity by reduction of oxygen supply, and restoration by re-oxygenation, has been demonstrated in rats [36,37]. Reduced oxygen activates hypoxia-inducible factor 1(HIF-1) [38], which is involved in oxygen homeostasis. Chilov and colleagues also indicated that oxygen supply modulates the circadian clock at the molecular levels via HIF-1 in the mouse brain [39]. Our observations support these experimental results and suggested that oxygen supply may also influence rhythmicity in humans. Further analyses are required to explore the influencing mechanisms on emergence of rhythmicities in neonates.
Conclusion
Preterm neonates are at great risk of life-threatening events such as infection, respiratory distress or circulatory failure. As shown in this study, co-existence of circadian cycles with low amplitude in preterm neonates may complementarily support immature homeostasis and function against unstable physiological condition. Our results should aid further research on physiological rhythmicity in neonates.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
EB and MB participated in all data collection, in the analysis and discussion of the results, and in the writing of the manuscript. MO participated in clinical data collection and advised on clinical implications of physiological rhythmicity. HY established the NICU local area network system (NICU LAN system) for physiological data recording and advised on clinical implications of physiological rhythmicity. MK provided advice on neonatal physiology and physiological rhythmicity. YK organized the study group, obtained grant support, and supervised the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful to Rebecca M. Warner for her invaluable advice and cooperation.
Contributor Information
Esmot ara Begum, Email: esmotara@hotmail.com.
Motoki Bonno, Email: bonnomo@hotmail.com.
Makoto Obata, Email: m.obata@zb.ztv.ne.jp.
Hatsumi Yamamoto, Email: hatsumi@alles.or.jp.
Masatoshi Kawai, Email: m_kawai@r2.dion.ne.jp.
Yoshihiro Komada, Email: komada@clin.medic.mie-u.ac.jp.
References
- Hammarlund K, Stromberg B, Sedin G. Heat loss from the skin of preterm and fullterm newborn infants during the first weeks after birth. Biol Neonate. 1986;50:1–10. doi: 10.1159/000242554. [DOI] [PubMed] [Google Scholar]
- Bauer K, Versmold H. Postnatal weight loss in preterm neonates less than 1,500 g is due to isotonic dehydration of the extracellular volume. Acta Paediatr Scand Suppl. 1989;360:37–42. doi: 10.1111/j.1651-2227.1989.tb11280.x. [DOI] [PubMed] [Google Scholar]
- Di Fiore JM, Arko MK, Miller MJ, Krauss A, Betkerur A, Zadell A, Kenney SR, Martin RJ. Cardiorespiratory events in preterm infants referred for apnea monitoring studies. Pediatrics. 2001;108:1304–1308. doi: 10.1542/peds.108.6.1304. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Kok JH. Circadian rhythms in early human development. Early Hum Dev. 1991;26:121–128. doi: 10.1016/0378-3782(91)90016-V. [DOI] [PubMed] [Google Scholar]
- Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics. 2003;112:373–381. doi: 10.1542/peds.112.2.373. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Kok JH, Boer K, Wolf H. Perinatal development of human circadian rhythms: role of the foetal biological clock. Neurosci Biobehav Rev. 1992;16:371–378. doi: 10.1016/S0149-7634(05)80207-6. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci. 2004;24:2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mayes L, Jacobs H, Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113:833–839. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- Glass L. Synchronization and rhthmic process in physiology. Nature. 2001;410:277–284. doi: 10.1038/35065745. [DOI] [PubMed] [Google Scholar]
- Lewy H, Naor Z, Ashkenazi IE. From ultradian to infradian rhythms: LH release patterns in vitro. Chronobiol Int. 1999;16:441–450. doi: 10.3109/07420529908998719. [DOI] [PubMed] [Google Scholar]
- Lunshof S, Boer K, Wolf H, van Hoffen G, Bayram N, Mirmiran M. Fetal and maternal diurnal rhythms during the third trimester of normal pregnancy: outcomes of computerized analysis of continuous twenty-four-hour fetal heart rate recordings. Am J Obstet Gynecol. 1998;178:247–254. doi: 10.1016/S0002-9378(98)80008-2. [DOI] [PubMed] [Google Scholar]
- Visser GH, Goodman JD, Levine DH, Dawes GS. Diurnal and other cyclic variations in human fetal heart rate near term. Am J Obstet Gynecol. 1982;142:535–544. doi: 10.1016/0002-9378(82)90757-8. [DOI] [PubMed] [Google Scholar]
- Patrick J. Influence of maternal heart rate and gross fetal movements on the daily pattern of pattern of fetal heart rate near term. Am J Obstet Gynecol. 1982:533–538. doi: 10.1016/0002-9378(82)90222-8. [DOI] [PubMed] [Google Scholar]
- Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am J Obstet Gynecol. 1982;142:363–371. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- Seron-Ferre M, Ducsay CA, Valenzuela GJ. Circadian rhythms during pregnancy. Endocr Rev. 1993;14:594–609. doi: 10.1210/er.14.5.594. [DOI] [PubMed] [Google Scholar]
- D'Souza SW, Tenreiro S, Minors D, Chiswick ML, Sims DG, Waterhouse J. Skin temperature and heart rate rhythms in infants of extreme prematurity. Arch Dis Child. 1992;67:784–788. doi: 10.1136/adc.67.7_spec_no.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proc Natl Acad Sci USA. 1999;96:2426–2429. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou G, Greenough A, Kavvadia V, Mantagos S. Blood pressure rhythms during the perinatal period in very immature, extremely low birthweight neonates. Early Hum Dev. 1999;56:49–56. doi: 10.1016/S0378-3782(99)00034-1. [DOI] [PubMed] [Google Scholar]
- Ardura J, Andres J, Aldana J, Revilla MA, Aragon MP. Heart rate biorhythm changes during the first three months of life. Biol Neonate. 1997;72:94–101. doi: 10.1159/000244471. [DOI] [PubMed] [Google Scholar]
- Updike PA, Accurso FJ, Jones RH. Physiologic circadian rhythmicity in preterm infants. Nurs Res. 1985;34:160–163. [PubMed] [Google Scholar]
- Bueno C, Diambra L, Menna-Barreto L. Sleep-Wake and Temperature Rhythms in Preterm Babies Maintained in a Neonatal Care Unit. Sleep Research Online. 2001;4:77–82. [Google Scholar]
- Schimmel M, Waterhouse J, Marques MD, Weinert D. Circadian and Ultradian Rhythmicities in very premature neonates Maintained in Incubators. Biol Rhythm Res. 2002;33:83–112. doi: 10.1076/brhm.33.1.83.1321. [DOI] [Google Scholar]
- Korte J, Wulff K, Oppe C, Siegmund R. Ultradian and circadian activity-rest rhythms of preterm neonates compared to full-term neonates using actigraphic monitoring. Chronobiol Int. 2001;18:697–708. doi: 10.1081/CBI-100106082. [DOI] [PubMed] [Google Scholar]
- Weinert D, Sitka U, Minors DS, Waterhouse JM. The development of circadian rhythmicity in neonates. Early Hum Dev. 1994;36:117–126. doi: 10.1016/0378-3782(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Warner RM. Spectral Analysis of Time-series Data. The Goldford Press, New York, London; 1998. [Google Scholar]
- Russell RJ. Significance table for the result of fast fourier transformations. British Journal of Mathematical and Statistical Psychology. 1985;38:116–119. [Google Scholar]
- Thomas KA. The emergence of body temperature biorhythm in preterm infants. Nurs Res. 1991;40:98–102. [PubMed] [Google Scholar]
- Ardura J, Gutierrez R, Andres J, Agapito T. Emergence and evolution of the circadian rhythm of melatonin in children. Horm Res. 2003;59:66–72. doi: 10.1159/000068571. [DOI] [PubMed] [Google Scholar]
- Wakayama K, Ogawa T, Goto K, Sonoda H. Development of ultradian rhythm of EEG activities in premature babies. early Human Development. 1993;32:11–30. doi: 10.1016/0378-3782(93)90089-D. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Bernardo L, Jenkins SL, Ma XH, Brenna JT, Nathanielsz PW. Growth, neurobehavioral and circadian rhythm development in newborn baboons. Pediatr Res. 2001;49:673–677. doi: 10.1203/00006450-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Baldwin RB, Boeddiker M, Ariagno RL. Development of circadian rhythms in premature infants. Sleep Research Online. 1999;2 [Google Scholar]
- Thomas KA. Biological rhythm development in preterm infants: does health status influence body temperature circadian rhythm? Res Nurs Health. 2001;24:170–180. doi: 10.1002/nur.1020. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Seifert EL. Hypoxic depression of circadian rhythms in adult rats. J Appl Physiol. 2000;88:365–368. doi: 10.1152/jappl.2000.88.2.365. [DOI] [PubMed] [Google Scholar]
- Bishop B, Silva G, Krasney J, Salloum A, Roberts A, Nakano H, Shucard D, Rifkin D, Farkas G. Circadian rhythms of body temperature and activity levels during 63 h of hypoxia in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1378–R1385. doi: 10.1152/ajpregu.2000.279.4.R1378. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. Faseb J. 2001;15:2613–2622. doi: 10.1096/fj.01-0092com. [DOI] [PubMed] [Google Scholar]