Abstract
Emerging evidence indicates that nucleotide receptors are widely expressed in the nervous system. Here, we present evidence that P2Y and P2X receptors, particularly the P2X7 subtype, are coupled to the phosphoinositide 3-kinase (PI3K)/Akt pathway in astrocytes.
P2Y and P2X receptor agonists ATP, uridine 5′-triphosphate (UTP) and 2′,3′-O-(4-benzoyl)-benzoyl ATP (BzATP) stimulated Akt phosphorylation in primary cultures of rat cortical astrocytes. BzATP induced Akt phosphorylation in a concentration- and time-dependent manner, similar to the effect of BzATP on Akt phosphorylation in 1321N1 astrocytoma cells stably transfected with the rat P2X7 receptor. Activation was maximal at 5 – 10 min and was sustained for 60 min; the EC50 for BzATP was approximately 50 μM. In rat cortical astrocytes, the positive effect of BzATP on Akt phosphorylation was independent of glutamate release.
The effect of BzATP on Akt phosphorylation in rat cortical astrocytes was significantly reduced by the P2X7 receptor antagonist Brilliant Blue G and the P2X receptor antagonist iso-pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulfonic acid, but was unaffected by trinitrophenyl-ATP, oxidized ATP, suramin and reactive blue 2.
Results with specific inhibitors of signal transduction pathways suggest that extracellular and intracellular calcium, PI3K and a Src family kinase are involved in the BzATP-induced Akt phosphorylation pathway.
In conclusion, our data indicate that stimulation of astrocytic P2X7 receptors, as well as other P2 receptors, leads to Akt activation. Thus, signaling by nucleotide receptors in astrocytes may be important in several cellular downstream effects related to the Akt pathway, such as cell cycle and apoptosis regulation, protein synthesis, differentiation and glucose metabolism.
Keywords: Purinergic receptors, astrocytes, BzATP, P2X7, Akt, PI3K, c-Src, calcium
Introduction
Extracellular nucleotides bind to cell surface receptors designated P2 purinergic receptors, which can be divided into two categories: metabotropic, P2Y receptors (G-protein-coupled heptahelical receptors) and ionotropic, P2X receptors (ligand-gated ion channels), as reviewed by Ralevic & Burnstock (1998). The P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13) respond to a variety of naturally occurring agonists, including ATP, uridine 5′-triphosphate (UTP) and their diphosphate analogs, mediating responses through G proteins. A G-protein-coupled receptor that responds to UDP -glucose and other sugar- nucleotides has recently been designated P2Y14 (Abbracchio et al., 2003). In the ionotropic family of purinergic receptors, seven subtypes have been cloned so far (named P2X1–P2X7); they regulate cell function by opening cation channels (Abbracchio & Burnstock, 1994).
Among the P2X receptors, the P2X7 receptor subtype presents some unique features: (1) it requires high concentrations of ATP (in the mM range) to be activated; (2) 2′, 3′-(4-benzoyl)-benzoyl ATP (BzATP) is about 10-fold more potent than ATP; (3) its activation can lead to the formation of reversible plasma membrane pores that allow the entrance of large molecular weight molecules up to 900 Da (Gonzalez et al., 1989; Erb et al., 1990; North, 2002). Indeed, it is also known as the ‘suicidal receptor', as prolonged P2X7 receptor activation is followed by apoptosis-mediated cell death in a variety of cells (Di Virgilio et al., 1998 and references therein). It was recently suggested that this receptor may play a role in cell signaling involving tyrosine phosphorylation and lipid production, as a tyrosine phosphatase and the lipid kinase phosphatidylinositol 4-kinase (PI4K) are part of the P2X7 receptor signaling complex, with nine other proteins (Kim et al., 2001a).
Astrocytes are the major cell type in the mammalian brain, and nucleotides and nucleosides have important roles in the proliferation and differentiation of these cells, as reviewed by Neary et al. (1996). Previous studies revealed that astrocytes express P2Y1, P2Y2 and P2Y4 subtypes as well as P2X1, P2X2, P2X3, P2X4, P2X6 and P2X7 subtypes (King et al., 1996; Lenz et al., 2000; Franke et al., 2001; Kukley et al., 2001; Panenka et al., 2001). Several subtypes of P2Y (Neary & Zhu, 1994; King et al., 1996; Neary et al., 1999) and P2X (Panenka et al., 2001; Gendron et al., 2003a, 2003b) receptors in astrocytes are coupled to extracellular signal-regulated protein kinase (ERK), a key regulator of cellular proliferation and differentiation (Seger & Krebs, 1995). The serine–threonine-specific kinase Akt (protein kinase B) is another signaling protein that has a role in a range of diverse cellular functions that are important physiologically and pathophysiologically, such as cell growth and survival, angiogenesis, glycogen synthesis, protein synthesis and transcription (Brazil & Hemmings, 2001). Extracellular nucleotides can activate Akt via P2Y receptors in renal mesangial cells (Huwiler et al., 2002), but information is lacking as to whether P2X receptors are also coupled to this signaling pathway.
In this study, we show for the first time that P2X receptors, particularly P2X7-like receptors, are involved in Akt activation, and we elucidate steps in the signal transduction pathway leading to Akt phosphorylation by P2X7 receptor stimulation in primary cultures of rat cortical astrocytes.
Methods
Chemicals
All nucleotides, adenosine and signal transduction inhibitors were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). Polyclonal antibodies recognizing total Akt and Akt phosphorylated at specific sites (Ser473 and Thr308) were from Cell Signaling Technology (Beverly, MA, U.S.A.). Peroxidase-conjugated anti-rabbit IgG secondary antibody and nitrocellulose membranes were obtained from Amersham Pharmacia Biotech (Piscataway, NJ, U.S.A.). Enhanced chemiluminescent (ECL) kit (SuperSignal West Femto Maximum Sensitivity Substrate) was purchased from Pierce Biotechnology (Rockford, IL, U.S.A.). Cell culture media were obtained from GIBCO (GIBCO BRL, Carlsbad, CA, U.S.A.). Chemicals and reagents were analytical grade or better.
Cell culture and treatment
Primary astrocytes were obtained from neonatal rat (Fischer) cerebral cortices as described previously (Neary et al., 1994b); the experimental procedure was approved and monitored by the Animal Studies Subcommittee at the Miami VA Medical Center. Cells were seeded at densities of 500,000 cells/35 mm2 plates and cells were not replated before use. At least 99% of the cell population was astrocytes, as determined by staining with cell-specific markers (Neary et al., 1994b). Experiments were conducted with 3- to 4-week-old cultures. Prior to treatment with nucleotides or other agents, cells maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) horse serum were shifted to the quiescent phase by incubation in DMEM containing 0.5% (v/v) horse serum for 48–72 h. Stock solutions of nucleotides, nucleotide analogs, purinergic antagonists and signal transduction inhibitors were divided into single-use aliquots and stored at −80°C.
Expression of recombinant rat P2X7 receptors in 1321N1 cells
Recombinant rat P2X7 receptors were stably expressed in human 1321N1 astrocytoma cells as described previously (Gendron et al., 2003a), using a protocol derived for the expression of the P2Y2 receptor (Parr et al., 1994; Garrad et al., 1998). Briefly, recombinant rat P2X7 receptor cDNA (obtained from GlaxoWellcome, U.K.) incorporated into the pLXSN retroviral vector (P2X7-pLXSN) was transfected into PA317 amphotrophic packaging cells for the production of retroviral vectors. The pLXSN vector was used as a negative control. Then, 1321N1 cells were infected with the retroviral vectors and selected for neomycin resistance with 1 mg ml−1 G418 (GIBCO BRL, Carlsbad, CA, U.S.A.). The 1321N1 cells expressing P2X7-pLXSN were cultured in DMEM (GIBCO BRL, Carlsbad, CA, U.S.A.) containing 5% (v v−1) fetal bovine serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 1 mg ml−1 G418.
Akt activation
Quiescent cultures were treated with P2 receptor agonists or antagonists as described in the text. Cells were lysed with Laemmli (1970) sample buffer and protein concentrations were determined by the modified Lowry procedure as described previously (Peterson, 1983) with bovine serum albumin as standard. Lysates containing equal amounts of protein (20–25 μg protein) were subjected to SDS–PAGE (Laemmli, 1970) using 11% (w v−1) acrylamide and transferred to nitrocellulose filters with a Genie electrophoretic blotter (Idea Scientific Inc., Minneapolis, MN, U.S.A.) for 1 h at 12 V in a transfer buffer containing 25 mM Tris, 192 mM glycine and 20% (v v−1) methanol. Membranes were incubated with a blocking solution containing 20 mM Tris, pH 7.7, 137 mM NaCl, 0.1% (v v−1) Tween 20 (TTBS) and 5% (w v−1) nonfat dry milk for 1 h at room temperature, rinsed in TTBS, and then incubated overnight at 4°C with specific antibodies ((rabbit polyclonal anti-phospho-Akt(Ser473), rabbit polyclonal anti-phospho-Akt(Thr308) or rabbit polyclonal anti-total Akt)) diluted 1/1000 in blocking solution. Following three rinses in TTBS, membranes were incubated for 1 h at room temperature with peroxidase-conjugated donkey anti-rabbit IgG diluted 1/2000 in blocking solution. Membranes were washed three times in TTBS, and phospho- and total Akt were detected by ECL with Kodak Biomax film (Eastman Kodak Company, Rochester, NY, U.S.A.). Immunoblot images were scanned and densitometrically analyzed using Molecular Analyst Software (Bio-Rad Laboratories, Hercules, CA, U.S.A.).
RT–PCR analysis
Total RNA was isolated using the TRIzol™ reagent (GIBCO BRL Life Technologies, Rockville, MD, U.S.A.) according to the manufacturer's recommendations. Reverse transcription was carried out using SUPERSCRIPT™ II RNase H− reverse transcriptase (GIBCO-BRL Life Technologies, Rockville, MD, U.S.A.). First-strand complementary DNA was synthesized from 4 μg total RNA in the presence of 0.5 μg oligo(dT)12–18, 1 × First-Strand Buffer, 0.01 M DTT, 0.5 mM dNTP mix and 200 U of SUPERSCRIPT II at 42°C for 50 min. The reaction was stopped by heating at 70°C for 15 min. Polymerase chain reaction was performed using the Eppendorf's MasterTaq™ kit (Brinkmann Instruments, Inc., Westbury, NY, U.S.A.). Oligonucleotide amplification primers were designed from the published rat P2X1–6 (Shibuya et al., 1999) and P2X7 (Humphreys et al., 1998) sequences. P2X1: sense primer, 5′-GAAGTGTGATCTCGACTGGCACGT-3′; antisense primer, 5′-GCGTCAAGTCCGGATCTCGACTAA-3′ (amplifies a 452 bp fragment); P2X2: sense primer, 5′-GAATCAGAGTGCAACCCCAA-3′; antisense primer, 5′-TCACAGGCCATCTACTTGAG-3′ (amplifies a 357 bp fragment); P2X3: sense primer, 5′-TGGCGTTCTGGGTATTAAGATCGG-3′; antisense primer, 5′-CAGTGGCCTGGTCACTGGCGA-3′ (amplifies a 440 bp fragment); P2X4: sense primer, 5′-GAGGCATCATGGGTATCCAGATCAAG-3′; antisense primer, 5′-GAGCGGGGTGGAAATGTAACTTTAG (amplifies a 447 bp fragment); P2X5: sense primer, 5′-GCCGAAAGCTTCACCATTTCCATAA-3′; antisense primer, 5′-CCTACGGCATCCGCTTTGATGTGATAG-3′ (amplifies a 418 bp fragment); P2X6: sense primer, 5′-AAAGACTGGTCAGTGTGTGGCGTTC-3′; antisense primer, -5′-TGCCTGCCCAGTGACAAGAATGTCAA-3 (amplifies a 520 bp fragment); P2X7: sense primer, 5′GGCAGTTCAGGGAGGAATCATGG-3′; antisense primer, 5′-AAAGCGCCAGGTGGCATAGCTC-3′ (amplifies a 939 bp fragment). First-strand cDNA (2 μl) were used in the presence of 1X Taq Master, 1X Taq Buffer, 1.5 mM Mg2+, 0.2 mM dNTP mix, 0.5 μM of each primer and 2.5 U of Taq DNA polymerase in a total volume of 25 μl. PCR was conducted as published previously (Humphreys et al., 1998; Shibuya et al., 1999). Briefly, samples were denatured at 94°C for 5 min. For P2X1–6, 35 cycles of denaturation at 94°C for 30 s, annealing at 57°C (P2X6), 58°C (P2X1, P2X3, P2X4, P2X5) or 61°C (P2X2) for 60 s, and elongation at 72°C for 90 s were conducted. For P2X7, 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 2 min and elongation at 72°C for 2 min were conducted. The final elongation was conducted for 7 min at 72°C. Reactions were also conducted without the reverse transcriptase step as a control for genomic DNA contamination in the RNA sample. Amplification products were resolved by agarose (2%) gel electrophoresis; the predicted size of amplification product was observed. The band was excised and eluted from the gel, purified (Wizard DNA purification kit; Promega), precipitated overnight with ethanol and sequenced.
P2X7 receptor immunoblotting
Nitrocellulose membranes were prepared as described in the Akt activation section and were incubated for 1 h at room temperature with anti-P2X7 antibody (Alomone Labs, Jerusalem, Israel) diluted in TTBS (1/200 dilution). Following three rinses in TTBS, membranes were incubated for 1 h at room temperature with peroxidase-conjugated donkey anti-rabbit IgG diluted 1/2000 in blocking solution. Membranes were washed three times in TTBS, and P2X7 receptors were detected colorimetrically with DAB as substrate for peroxidase. For anti-P2X7 antibody blocking studies, P2X7 receptor antibodies were preincubated with a 50-fold molar excess of peptide antigen in 500 μl TTBS containing 5% (w v−1) BSA for 1 h at room temperature before incubation with filters.
Statistical analysis
The number of experimental replications is given in the figure legends; replicate experiments were conducted with cultures from different seedings. Data were analyzed by Student's t-test for two groups or repeated measures ANOVA followed by post hoc comparisons (Bonferroni test) using an Instat software package (GraphPad Software, San Diego, CA, U.S.A.).
Results
P2X and P2Y purinergic agonists stimulate Akt phosphorylation in astrocytes
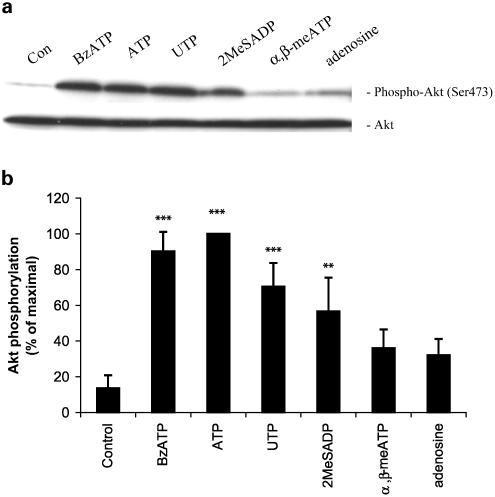
Akt activation is a multistep process involving the phosphorylation of Ser473 and Thr308 residues, and the phosphorylation of these sites closely correlates with the activity of Akt (Chan & Tsichilis, 2001). To address the possibility that purinergic receptor stimulation leads to Akt activation in astrocytes, we tested the ability of several receptor agonists to induce phosphorylation at these sites. As seen in Figure 1a and b, ATP stimulated Ser473 phosphorylation. As ATP can be metabolized to adenosine by ectoNTPDases and ecto-5′-nucleotidases, we tested adenosine to determine whether the ATP effect was mediated by P1/adenosine receptors, since it was shown that the activation of adenosine A1 receptors leads to the activation of Akt in rat hippocampus in vitro and in vivo (Gervitz et al., 2002). However, the effect of adenosine on Akt activation in astrocytes was not statistically significant. Results with an uptake resistant P1 receptor agonist, N6-cyclohexyladenosine, which is a selective adenosine A1 receptor agonist, were similar to adenosine (data not shown), thereby indicating that breakdown of ATP to adenosine is not necessary to activate Akt.
Figure 1.
P2 purinergic receptor stimulation induces Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) Quiescent astrocytes were stimulated for 5 min with BzATP (100 μM), ATP (100 μM), UTP (100 μM), 2MeSADP (10 μM), α,β-meATP (100 μM), adenosine (100 μM) or vehicle. The cell lysates containing 20 μg of protein were subjected to SDS–PAGE (11% acrylamide gel) and Western blot analysis was performed using specific phospho-Ser473 Akt antibody. As a loading control, blots were also probed for total Akt. Data are representative of five independent experiments giving similar results. Bands were visualized by the ECL method. (b) Phospho-Ser473, and total Akt bands of the samples (n=5) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the results were expressed as the percentage of maximal response; values are means±s.e. The effects were statistically significant at **P<0.01 and ***P<0.001.
To investigate the activation of Akt by P2 receptors, several nucleotide receptor agonists were tested. An agonist of P2X7 and other P2X receptors, BzATP, was effective in activating Akt (Figure 1a and b). Agonists of P2Y receptors, UTP and 2-methylthioadenosine diphosphate (2MeSADP), also significantly increased Ser473 phosphorylation. The same compounds stimulated Thr308 phosphorylation in a similar manner (data not shown). An agonist of P2X1 and P2X3 receptors, α,β-methylene-ATP (α,β-meATP) did not significantly affect Akt phosphorylation.
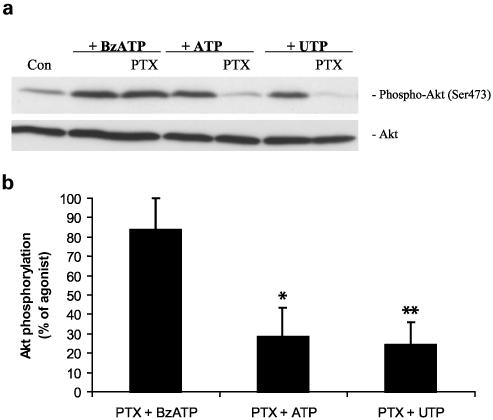
As an alternative approach, we tested the effect of pertussis toxin (PTX), an inactivator of Gi/Go proteins, on the stimulation of Akt phosphorylation by extracellular nucleotides. As shown in Figure 2, PTX was unable to block BzATP-induced Akt phosphorylation but was effective in reducing ATP- or UTP-induced Akt phosphorylation. The results of experiments presented in Figures 1 and 2 indicate that both P2X and P2Y receptors are linked to Akt activation in astrocytes.
Figure 2.
PTX reduces ATP- and UTP-induced Akt phosphorylation but does not affect BzATP-induced Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) Quiescent rat astrocytes were treated with PTX (100 ng/ml) for 24 h before stimulation for 5 min with 100 μM BzATP, ATP or UTP. Cells were harvested and Western blot analyses were performed using specific phospho-Ser473 and total Akt antibodies. Bands were visualized by the ECL method. Data are representative of four independent experiments giving similar results. (b) The phospho-Ser473 and total-Akt bands of the samples (n=4) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the stimulation obtained with BzATP, ATP or UTP was defined as 100%; values are means±s.e. In relation to these controls, fold stimulation for BzATP, ATP and UTP in the presence of PTX were approximately 80, 30 and 30%, respectively. The effect was statistically significant at *P<0.05 and **P<0.01.
P2X nucleotide receptor subtypes are expressed in rat cortical astrocyte cultures
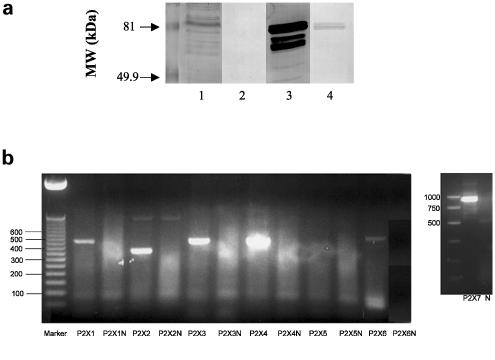
Immunoblot studies with anti-P2X7 receptor antibodies demonstrated the presence of P2X7 receptors in membranes from rat cortical astrocytes (Figure 3a), thereby confirming a previous report (Panenka et al., 2001). A band from astrocyte membranes comigrated with the major P2X7 receptor immunoreactive band from rat brain membranes (Figure 3a, lanes 1 and 3). The additional bands may represent degradation products due to incomplete protease inhibition or lack of specificity of the antibody. To determine antibody specificity, anti-P2X7 receptors antibodies were preincubated with peptide antigen before use. This treatment greatly reduced immunoreactivity in both astrocyte and brain membranes (Figure 3a, lanes 2 and 4). The residual stain in the rat brain membrane sample may represent incomplete antibody neutralization by peptide antigen.
Figure 3.
P2X7 and other P2X purinergic receptor subtypes are expressed in primary cultures of rat cortical astrocytes. In (a), membranes were prepared from rat cortical astrocyte cultures and brain cortical tissue, and immunoblot analysis was conducted as described in Methods with anti-P2X7 receptor antibodies. Lanes 1 and 2, membrane preparation from rat cortical astrocyte cultures; lanes 3 and 4, rat brain membranes; lanes 2 and 4, anti-P2X7 receptor antibodies were incubated with a 50-fold molar excess of peptide antigen for 1 h before use. In (b), total RNAs from cultured rat astrocytes were reverse transcribed and P2X receptor cDNAs were amplified by PCR as described in Methods. Amplification products were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining for P2X1–7. In parallel assays without reverse transcriptase, there were no amplification products (denoted by N), indicating that the bands appearing on the gels were not derived from genomic DNA. P2X1–452 bp; P2X2–357 bp; P2X3–440 bp; P2X4–447 bp; P2X5–418 bp; P2X6–520 bp; P2X7–939 bp.
To investigate the expression of additional P2X nucleotide receptor subtypes in primary cultures of rat cortical astrocytes, we performed RT–PCR analysis with primer pairs designed to amplify astrocyte-derived cDNA encoding specific subtypes, as detailed in Methods. RT–PCR studies yielded amplification products of the expected sizes for six of the seven known P2X receptors (Figure 3b). Thus, rat cortical astrocytes possess P2X1, P2X2, P2X3, P2X4, P2X6 and P2X7 purinergic receptors in addition to the P2Y1, P2Y2 and P2Y4 receptor subtypes previously identified in these cells (Lenz et al., 2000).
Time and concentration dependency of BzATP-stimulated Akt phosphorylation
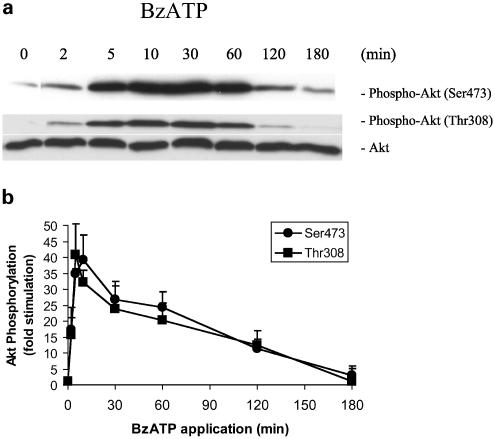
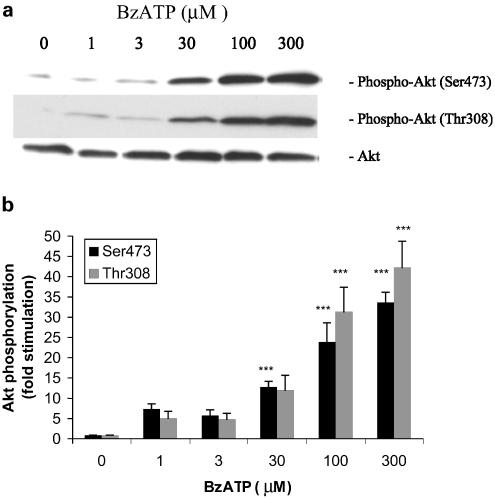
To further explore the activation of Akt by P2X receptors, we performed time-course and dose–response studies with BzATP. Time-course experiments revealed a rapid phosphorylation of Ser473 and Thr308 within 2 min of stimulation with BzATP (Figure 4a and b). Maximal activation occurred within 10 min and persisted for 1 h, remaining above baseline at 2 h poststimulation. Thus, the stimulation of P2X receptors by BzATP induced rapid and sustained Akt activity.
Figure 4.
Time-course of BzATP-stimulated Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) Quiescent rat astrocytes were treated with BzATP (100 μM) for the indicated time periods. Then, cells were harvested and Western blot analyses were performed using specific phospho-Ser473 and phospho-Thr308 Akt antibodies. As a loading control, blots were also probed for total Akt, and bands were visualized by the ECL method. Data are representative of four independent experiments giving similar results. (b) The phospho-Ser473, phospho-Thr308 and total Akt bands of the samples (n=4) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the results were expressed as fold stimulation in relation to the control; values are means±s.e.
As shown in Figure 5a and b, the phosphorylation of Akt by BzATP occurred in a concentration-dependent manner, with the maximal effect at 300 μM with a 5 min BzATP stimulation. The EC50 for BzATP was approximately 50 μM, and the effect was similar at both phosphorylation sites.
Figure 5.
Concentration dependency of BzATP-stimulated Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) Quiescent rat astrocytes were treated with BzATP at the indicated concentrations for 5 min. Then, cells were harvested and Western blot analyses were performed using specific phospho-Ser473 and phospho-Thr308 Akt antibodies. As a loading control, blots were also probed for total Akt, and bands were visualized by the ECL method. Data are representative of four independent experiments giving similar results. (b) The phospho-Ser473, phospho-Thr308 and total Akt bands of the samples (n=4) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the results were expressed as fold stimulation in relation to the control; values are means±s.e. The effect was statistically significant at ***P<0.001.
BzATP stimulates Akt phosphorylation via P2X7 receptors
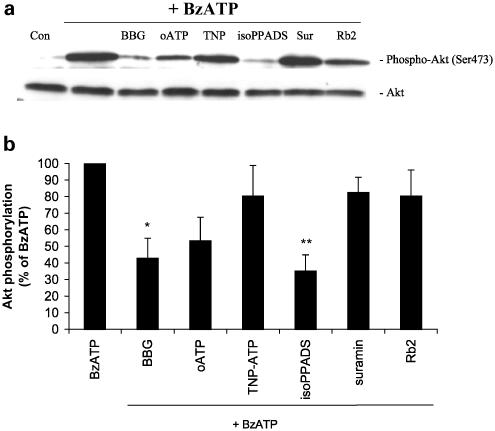
Although the ATP analog BzATP is more potent than ATP at the P2X7 receptor, it acts as a partial agonist at other P2X receptors over the same concentration range (Evans et al., 1995; Surprenant et al., 1996). To determine the P2X receptor subtype at which BzATP was acting to induce Akt phosphorylation in astrocytes, we treated the cultures with Brilliant Blue G (BBG) or oxidized ATP (oATP), antagonists of P2X7 receptors (Murgia et al., 1993; Jiang et al., 2000), prior to stimulation with BzATP (Figure 6a and b). Akt phosphorylation induced by BzATP was significantly decreased by 60% (P<0.05) when BBG was present (Figure 6b). Although the data analysis did not reveal a statistically significant reduction in Akt phosphorylation when cells were treated with oATP, this P2X7 receptor antagonist caused ∼50% inhibition of the effect of BzATP. Akt phosphorylation was not affected by either BBG or oATP alone (data not shown), indicating that the residual phosphorylation was not due to an effect of these antagonists on Akt phosphorylation. iso-pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulfonic acid (Iso-PPADS), an antagonist at the P2X1, P2X2, P2X3 and P2X5 receptor subtypes (Ralevic & Burnstock, 1998; Khakh et al., 2001), effectively reduced BzATP-induced Akt phosphorylation by 65% (P<0.01). On the other hand, 2′ 3′-O-(2, 4, 6-trinitrophenyl) adenosine 5′-triphosphate ((TNP)-ATP), suramin and reactive blue 2 (Rb2), agents that do not antagonize the P2X7 receptor, were not able to inhibit BzATP-induced Akt phosphorylation (Figure 6a and b). Since P2X7 receptors are fully activated by millimolar concentrations of ATP, we treated astrocyte cultures with 1 mM ATP for 5 min in the presence or absence of BBG (1 μM). The activation of Akt by a high concentration of ATP was inhibited about 35% by BBG (data not shown), suggesting that P2X7 receptors contribute about one-third of the activation of Akt at high concentrations of ATP, while the remainder is likely due to the activation by P2Y and perhaps additional P2X receptors.
Figure 6.
P2X7 receptors are involved in BzATP-induced Akt phosphorylation in primary cultures of rat cortical astrocytes. BzATP (100 μM) was applied to the cultures for 5 min in the absence or presence of the indicated purinergic receptor antagonist. Concentrations and preincubation times of compounds were as follows: BBG 1 μM, 15 min; oATP 500 μM, 2 h; TNP-ATP 1 μM, 5 min; iso-PPADS 50 μM, 15 min; suramin 100 μM, 15 min; Rb2 50 μM, 15 min. Control cells were treated with vehicle alone. Cell lysates containing 20 μg of protein were subjected to SDS–PAGE and Western blot analysis was performed using specific phospho-Ser473 Akt antibody and total Akt antibody as loading control. Bands were visualized by the ECL method according to the manufacturer's recommendation. (a) Data are representative of five independent experiments giving similar results. (b) The phospho-Ser473 and total Akt bands of the samples (n=5) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the level of Akt phosphorylation with BzATP (40-fold stimulation) was defined as 100%; values are means±s.e. The effect was statistically significant at *P<0.05 and **P<0.01.
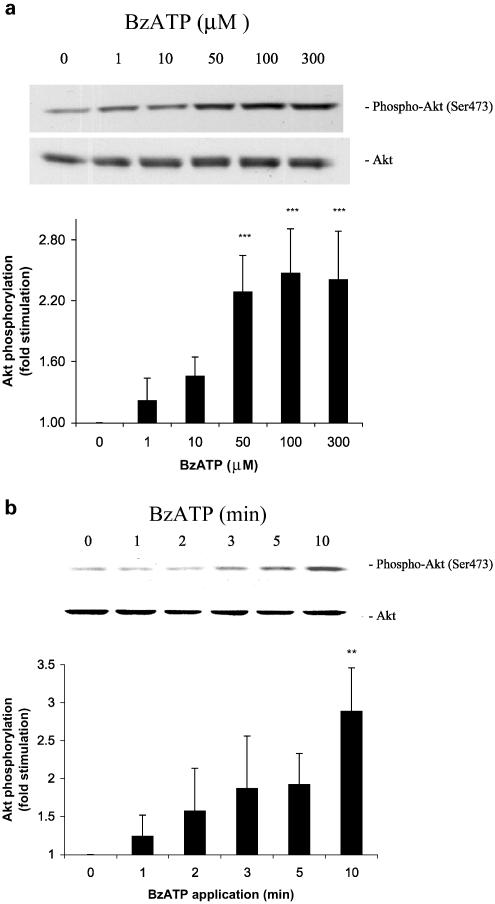
To confirm that P2X7 receptors can couple to Akt activation, recombinant rat P2X7 receptors were stably expressed in 1321N1 astrocytoma cells that lack or express very low levels of P2 receptor activities (Parr et al., 1994). The activation of P2X7 receptors by BzATP in the 1321N1-P2X7 cell transfectants showed that Akt phosphorylation occurred with a similar time dependence (Figure 7a) and dose–response (Figure 7b) to the effects of BzATP on Akt activation in astrocytes (Figures 4 and 5). We also conducted experiments in P2X7-expressing 1321N1 cells to determine the effect of inhibitors on BzATP-induced Akt phosphorylation in cells that do not express other P2 receptors. We found that 94% of BzATP-induced Akt phosphorylation was inhibited by 1 μM BBG (n=3; data not shown). In control experiments, treatment of cells expressing the vector alone (without P2X7 receptor cDNA) or non transfected 1321N1 cells with BzATP or treatment of 1321N1-P2X7 cells with vehicle did not significantly enhance Akt phosphorylation (data not shown). These data demonstrate that recombinant P2X7 receptors expressed in 1321N1 cells are coupled to Akt phosphorylation and support the conclusion that activation of P2X7 receptors by BzATP is responsible for Akt phosphorylation in rat cortical astrocytes.
Figure 7.
BzATP induces Akt phosphorylation in 1321N1 astrocytoma cells expressing the rat P2X7 receptor. Human 1321N1 cells stably expressing the P2X7 receptor or pLXSN-transfected negative controls were incubated with (a) the indicated concentration of BzATP or (b) 100 μM BzATP for (a) 7.5 min or (b) the indicated time. Then, cells were harvested, Western blot analyses were performed using specific anti-phospho-Ser473 and anti-total Akt antibodies, and bands were visualized by the ECL method. Data are representative of (a) six and (b) three independent experiments giving similar results. The phospho-Ser473 and total Akt bands of the samples were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the results were expressed as fold increase in relation to the control; values are means±s.e. The effect was statistically significant at **P<0.01 and ***P<0.001.
P2X7 receptor-mediated activation of Akt is independent of glutamate release
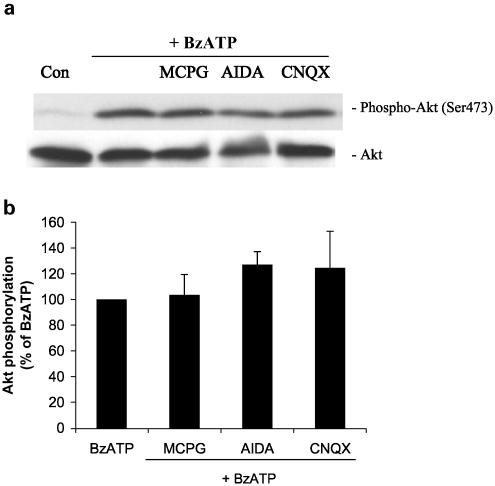
It was reported by Duan et al. (2003) that stimulation of P2X7 receptors induced a strong release of glutamate from mouse cortical astrocytes, and that Akt activity is coupled to the activation of ionotropic and metabotropic glutamate receptors (Perkinton et al., 1999; D'Onofrio et al., 2001; Iacovelli et al., 2003). To determine whether BzATP-induced glutamate release leads to Akt phosphorylation, astrocytes were treated with different glutamatergic antagonists prior to BzATP (Figure 8). BzATP-induced phosphorylation of Akt was not modified when cells were treated with α-methyl-4-carboxyphenylglycine (MCPG) (nonselective groups I and II metabotropic glutamate receptor antagonist), (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA) (selective antagonist of mGlu1a) or 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) (ionotropic glutamate receptor antagonist). In addition, glutamate (100 μM or 1 mM) did not induce significant Akt phosphorylation (data not shown). These results indicate that the P2X7 receptor-mediated activation of Akt is not due to glutamate release.
Figure 8.
BzATP-induced Akt phosphorylation is independent of glutamate release in primary cultures of rat cortical astrocytes. (a) Quiescent rat astrocytes were pre-treated with MCPG (100 μM), AIDA (100 μM) or CNQX (100 μM) for 30 min before stimulation for 5 min with 100 μM BzATP. Then, cells were harvested, Western blot analyses were performed using specific phospho-Ser473 and total Akt antibodies and bands were visualized by the ECL method. Data are representative of three independent experiments giving similar results. (b) The phospho-Ser473 and total Akt bands of the samples (n=3) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the stimulation obtained with BzATP (40-fold stimulation vs untreated control) was defined as 100%; values are means±s.e.
Involvement of calcium, PI3K and a Src family kinase in Akt phosphorylation mediated by P2X7 receptors
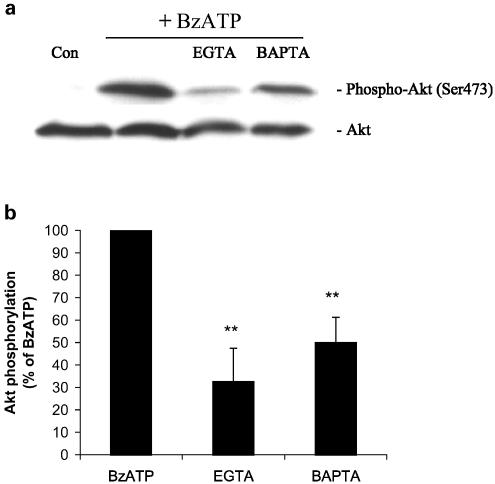
To evaluate the pathway upstream of Akt phosphorylation activated by P2X7 receptors, we treated the cells with several signal transduction inhibitors prior to the addition of BzATP. P2X7 receptors are ligand-gated ion channels that allow the entrance of cations such as Ca2+, Na+ and K+ when activated. Introduction of the Ca2+ chelator BAPTA into rat astrocytes by a 30-min pretreatment with 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetrakis (BAPTA-AM) inhibited BzATP-induced Akt phosphorylation by 50% (P<0.01) (Figure 9a and b), suggesting that Akt activation was linked to an increase in the concentration of intracellular Ca2+. Chelation of extracellular Ca2+ by the addition of ethylene glycol-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA) reduced the BzATP-induced Akt phosphorylation by 70% (P<0.01). These findings suggest a role for intra- and extracellular calcium in Akt activation by P2X7 receptors.
Figure 9.
Ca2+ chelators reduce BzATP-induced Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) BzATP (100 μM) was applied to the quiescent cultures for 5 min in the absence or presence of 5 mM EGTA (5 min preincubation) or 30 μM BAPTA-AM (30 min preincubation). Then, cells were harvested, Western blot analyses were performed using specific phospho-Ser473 and total Akt antibodies and bands were visualized by the ECL method. Data are representative of four independent experiments giving similar results. (b) The phospho-Ser473 and total Akt bands of the samples (n=4) were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the stimulation obtained with BzATP (40-fold stimulation vs untreated control) was defined as 100%; values are means±s.e. The effect was statistically significant at **P<0.01.
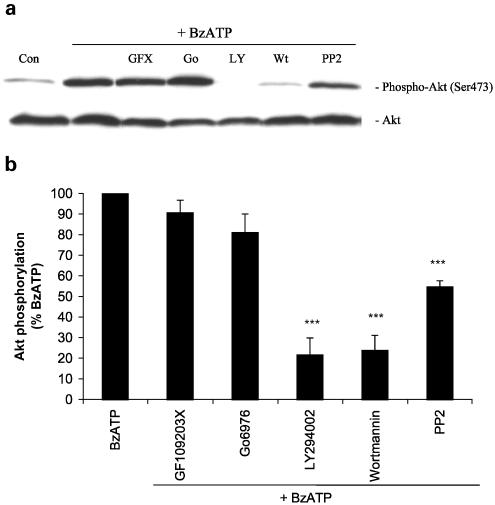
Increases in the intracellular calcium concentration can activate some isoforms of protein kinase C (PKC), and PKC has been shown to lie upstream of Akt in some cells (Gliki et al., 2002; Bauer et al., 2003). Therefore, we tested two PKC inhibitors: GF 109203X, an inhibitor of Ca2+-dependent and -independent PKCs, and Gö6976, an inhibitor selective for Ca2+-dependent PKCs (Martiny-Baron et al., 1993). Pretreatment of astrocytes with these inhibitors had no effect on BzATP-induced Akt phosphorylation, as shown in Figure 10a and b. PI3K inhibitors LY294002 and wortmannin and the Src family kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d]pyrimidine (PP2) were effective in reducing BzATP-induced phosphorylation of Akt by 80, 80 and 45%, respectively (Figure 10b). These results suggest that calcium, c-Src (or a related tyrosine kinase) and PI3K are components of the Akt signaling pathway coupled to P2X7 receptors in astrocytes.
Figure 10.
Involvement of PI3K and c-Src in BzATP-induced Akt phosphorylation in primary cultures of rat cortical astrocytes. (a) BzATP (100 μM) was applied to quiescent cultures for 5 min in the absence or presence of the indicated inhibitor. Concentrations and preincubation times of these compounds were as follows: GF109203X 5 μM, 20 min; Gö 6976 25 μM, 20 min; LY294002 50 μM, 30 min; wortmannin 100 nM, 30 min; PP2 5 μM, 30 min. Then, cells were harvested, Western blot analyses were performed using specific phospho-Ser473 and total Akt antibodies and bands were visualized by the ECL method. Data are representative of four independent experiments giving similar results. Control cells were treated with vehicle alone (0.5% DMSO). (b) The phospho-Ser473 and total Akt bands of the samples were densitometrically evaluated. The phospho-Akt/total Akt ratio was determined, and the stimulation obtained with BzATP (40-fold stimulation) was defined as 100%; values are means±s.e. The effect was statistically significant at ***P<0.001.
Discussion
Nucleotides and nucleosides exert diverse and complex trophic effects in the central nervous system, as multiple nucleotide receptor subtypes exist on glial and neuronal cells (Neary et al., 1996). In astrocytes, P2Y receptor activation is associated with increased stellation, GFAP content and DNA synthesis, and the activation of a calcium-independent PKC and the ERK pathway (Neary & Zhu, 1994; Neary et al., 1994a, 1994b, 1999; Brambilla et al., 2002, 2003). More recently, studies have focused on signaling mechanisms initiated by P2X7 receptor activation in glial cells. In astrocytes, stimulation of the P2X7 receptor leads to the activation of the MAPKs ERK1, ERK2 and p38, resulting in an increase in monocyte chemoattractant protein-1 expression (Panenka et al., 2001). In 1321N1 human astrocytoma cells, transfected rat P2X7 receptors are coupled to ERK activation (Gendron et al., 2003a), while in RBA-2 cells, a permanent type-2 astrocyte cell line, stimulation of P2X7 receptors is closely associated with the activation of PKC and phospholipase D (Sun et al., 1999). Although there is evidence of a link between P2X7 receptor activation and the mitogen-activated protein kinase (MAPK) and PKC pathways, there is no description thus far of P2X7-mediated activation of Akt.
Akt is a 57 kDa Ser/Thr kinase involved in a variety of biological effects, such as cell survival, cell cycle and apoptosis regulation, protein synthesis, differentiation and glucose metabolism (Downward, 1998; Cantrell, 2001). Considering the numerous cellular events mediated by Akt, and the important effects of purinergic receptor agonists in the central nervous system, we studied the effects of several ATP analogs on Akt phosphorylation, with a particular emphasis on the activation of Akt by P2X7 receptors and the signaling mechanisms involved in this pathway. Using primary cultures of rat cortical astrocytes, we determined that among the ATP analogs tested, ATP, BzATP, UTP and 2MeSADP were able to induce Akt phosphorylation. Extracellular ATP can be hydrolyzed to ADP and AMP by ecto-NTPDases (i.e. ‘ecto-nucleoside triphosphate diphosphohydrolases') and finally to adenosine by ecto-5′-nucleotidases (Zimmermann, 2001). Although Akt activity was linked to adenosine A3 receptors in Chinese hamster ovary cells (Schulte & Fredholm, 2002) and to adenosine A1 receptors in rat hippocampus (Gervitz et al., 2002), adenosine or N6-cyclohexyladenosine treatment in rat cortical astrocyte cultures did not significantly induce Akt phosphorylation, indicating that degradation of ATP to adenosine is not needed for Akt activation. Our results with PTX indicate that Gi or Go protein is involved in ATP and UTP signaling to the Akt pathway. This is in line with a report from Huwiler et al. (2002), who showed that ATP and UTP activation of Akt was dependent on Gi/Go proteins in renal mesangial cells. A P2Y2 receptor subtype that is activated equipotently by ATP and UTP has been shown to couple to Go proteins through interactions with αvβ3 integrins, although this receptor also mediates signal transduction by coupling to Gq (Erb et al., 2001). In contrast, PTX did not reduce BzATP-induced Akt phosphorylation, indicating that the effect of BzATP is mediated by P2X receptors.
As pointed out by North (2002), the fact that BzATP is more potent than ATP at the P2X7 receptor has led to the widespread use of BzATP as an agonist at P2X7 receptors and to the belief that BzATP is selective for P2X7 receptors. However, BzATP is an effective agonist at similar or lower concentrations at other P2X receptors (Evans et al., 1995; Bianchi et al., 1999) and can also act at the P2Y11 receptor subtype (Communi et al., 1999). Although rat cortical astrocyte cultures do not express functional P2Y11 receptors (Lenz et al., 2000), they do express several P2X receptor subtypes, including the P2X7 receptor (Figure 3). To evaluate which P2X receptors are linked to Akt phosphorylation, we used a series of P2 receptor antagonists (Figure 6). BBG, an antagonist of P2X7 receptors, significantly reduced BzATP-induced Akt phosphorylation. In addition, iso-PPADS, an antagonist of P2X receptors other than P2X7 receptors (Ralevic & Burnstock, 1998; Khakh et al., 2001), significantly inhibited BzATP-induced Akt phosphorylation. These results suggest that P2X7 and perhaps other P2X receptors are coupled to Akt. However, oxidized ATP did not significantly reduce BzATP-induced Akt phosphorylation. It should be noted that Hibell et al. (2001) emphasized the need for caution when using oATP to define P2X7 receptor-mediated effects, as oATP potency can be affected by temperature, NaCl presence in the media and the nature of the P2X7 receptor ortholog studied. Additional caution in the interpretation of oATP results was indicated by more recent reports that oATP can act independently of P2 receptors (Di Virgilio, 2003; Beigi et al., 2003). Supporting the notion that P2X7 receptors are linked to Akt in astrocytes is the observation that millimolar ATP also stimulated Akt phosphorylation. P2X2 receptors may not be involved in Akt phosphorylation because, although it was described that BBG can antagonize P2X2 receptors expressed in Xenopus oocytes (King et al., 1997), our results indicate that the P2X2 receptor antagonists suramin and reactive blue 2 (King et al., 1997; Bianchi et al., 1999) did not modify Akt phosphorylation induced by BzATP. While α,β-meATP, an agonist at P2X1, P2X3 and P2X4 receptor subtypes (Valera et al., 1994; Bo et al., 1995; Lewis et al., 1995), induced a weak increase in Akt phosphorylation, this effect was not significant (Figure 1). Consistent with this result, TNP-ATP, a potent antagonist of P2X1, P2X3 and P2X2/3 receptors (Virginio et al., 1998), was not able to reduce Akt phosphorylation induced by BzATP. However, the use of TNP-ATP in multicellular preparations may be limited by its susceptibility to enzymatic breakdown (Lewis et al., 1998). Hence, our data suggest that the effect of BzATP on Akt phosphorylation in primary cultures of rat cortical astrocytes is mediated by P2X7 receptors, although other P2X receptor subtypes may also contribute to the response. Since the paucity of selective P2 receptor antagonists can make the identification of specific P2 receptor subtypes involved in an effect particularly difficult in cells expressing multiple P2 receptor subtypes, we determined that expression of the rat P2X7 receptor in 1321N1 astrocytoma cells lacking endogenous P2X receptor activities leads to the appearance of BzATP-induced Akt phosphorylation in a time- and dose-dependent manner (Figure 7). These results clearly show that activation of the P2X7 receptor subtype can induce Akt phosphorylation. As the P2X7 antagonist BBG blocked BzATP-induced Akt phosphorylation in P2X7 receptor-expressing 1321N1 cells by 94% compared to 60% inhibition in primary cultures of rat cortical astrocytes, BzATP may activate additional P2X receptors in these cells.
After confirming that P2X7 receptors are linked to Akt activation, we ascertained whether this effect could be mediated by glutamate, as it was recently reported that P2X7 receptor activation in astrocytes causes substantial glutamate efflux (Duan et al., 2003). Primary cultures of rat astrocytes express ionotropic (iGluR1, iGluR4) and metabotropic (mGluR1, mGluR5) glutamatergic receptors (Gebremedhin et al., 2003), and the PI3K/Akt pathway is linked to AMPA-selective and metabotropic glutamate receptors (Perkinton et al., 1999; D'Onofrio et al., 2001; Iacovelli et al., 2003). To test the hypothesis that BzATP could induce Akt phosphorylation through glutamate release, we preincubated primary rat cortical astrocyte cultures with different glutamatergic antagonists. However, none of the antagonists tested were able to reduce BzATP-induced Akt phosphorylation. Moreover, glutamate, in a range of 100 μM–1 mM, only weakly stimulated Akt phosphorylation. These results indicate that glutamate does not mediate the activation of Akt by P2X7 receptors.
Structural and signaling proteins form part of the P2X7 receptor complex and are key molecular elements in downstream signaling from P2X7 receptors to the cytoskeleton (Kim et al., 2001a). The presence of the lipid kinase phosphatidylinositol 4-kinase (PI4K) in the P2X7 receptor complex suggests that this ion channel may play a role in cell signaling involving lipid production, since PI4Ks convert phosphatidylinositol (PtdIns) to PtdIns-4-P (Fruman et al., 1998). The PtdIns-4-P thus generated by PI4K can serve as a substrate for PI3K, which in turn produces the phosphatidylinositols that are involved in Akt activation. Indeed, the present study provides the evidence that the P2X7 receptor can mediate Akt activation through a cellular pathway that is dependent on PI3K, because preincubation of the cells with the PI3K inhibitors LY294002 and wortmannin reduced Akt phosphorylation by 80% (Figure 10b). The phosphorylation of Akt induced by BzATP may also be dependent on a tyrosine kinase such as c-Src, as preincubation of the cells with PP2, an inhibitor of Src-family tyrosine kinases (Hanke et al., 1996), reduced Akt phosphorylation by 45% (Figure 10b). An alternative explanation for the inhibitory effect of c-Src and PI3K inhibitors on BzATP-induced Akt phosphorylation is that these enzymes maintain P2X7 receptors in an activated state (e.g. due to receptor phosphorylation). However, although the resting P2X7 receptor is phosphorylated on tyrosine (Kim et al., 2001a), and although several ion channels (maxi-K channels and NMDA receptors for example) can be tyrosine phosphorylated by Src family kinases, c-Src did not significantly alter the kinetics or current run-down stimulated by BzATP in HEK293 cells stably expressing rat P2X7 receptors (Kim et al., 2001a). Nonetheless, further studies on the effect of pathway inhibitors on P2X7 channel activity and on the activation of Src family kinases and PI3K by P2X7 receptor stimulation are needed to confirm that P2X7 receptors signal to Akt via these enzymes.
Ca2+ influx is also involved in P2X7 receptor-mediated Akt activation, as the chelation of extracellular and intracellular Ca2+ with EGTA and BAPTA reduced Akt phosphorylation by 70 and 50%, respectively. Increase in the intracellular Ca2+ concentration can activate PKCs, which are upstream of Akt in some cells and either activate or inactivate Akt depending on the cell type and PKC isoform involved (Gliki et al., 2002; Bauer et al., 2003; Motley et al., 2003; Tanaka et al., 2003). However, in primary cultures of rat cortical astrocytes, neither of the PKC inhibitors tested reduced the level of BzATP-induced Akt phosphorylation. The elevation in the intracellular Ca2+ concentration promoted by the P2X7 receptor is probably an important feature in the activation of the Akt pathway. The calcium messenger system is an upstream activator of c-Src in several cell types, including endothelial (Okuda et al., 1999) and skeletal muscle cells (Buitrago et al., 2001), and we found that c-Src or a related tyrosine kinase is involved in signaling to Akt (Figure 10). Gendron et al. (2003a) demonstrated that P2X7 receptors can activate the proline rich/Ca2+-activated tyrosine kinase Pyk2, which can then form a complex with Src and initiate signaling complex formation. The calcium entry promoted by P2X7 receptor activation and subsequent Pyk2 phosphorylation could lead to the formation of Pyk2/Src/Shc and Pyk2/Src/Grb2 complexes, and ultimately, the interaction of Shc and Grb2 would recruit the guanine nucleotide exchange factor Sos, leading to Ras activation, which has PI3K as one of its downstream effectors (Kodaki et al., 1994). Alternatively, Src may associate directly with PI3K leading to its activation, or directly phosphorylate Akt, as previously shown by Chen et al. (2001). The possibility remains that Ca2+ can directly trigger Akt phosphorylation through Ca2+/calmodulin-dependent protein kinase kinase, as reported by Yano et al. (1998).
Sustained activation of P2X7 receptors is toxic to several cell types and can cause membrane disruption in HEK cells expressing rat P2X7 receptors (Virginio et al., 1999) and apoptosis and necrosis in rat glomerular mesangial cells (Schulze-Lohoff et al., 1998). However, stimulation of P2X7 receptors in primary cultures of mouse cortical astrocytes did not cause cell lysis (Duan et al., 2003), as evaluated by lactic dehydrogenase release. In rat cortical astrocyte cultures, a 24 h treatment with BzATP was not toxic to the cells, as evaluated by trypan blue exclusion and ‘live–dead' assays (Y.F. Shi, B. Kucher and J.T. Neary, unpublished observations). It was speculated that astrocyte resistance to P2X7 receptor-induced cell lysis is due to the density of receptor expression or to the fact that glial P2X7 receptors exist in monomeric form, whereas in other cell types they form multimeric complexes (Kim et al., 2001b). Collectively, these findings lead us to speculate that astrocytes are resistant to P2X7 receptor-mediated cell lysis because their activation increases Akt activity, which has an important role in cell survival mediated by phosphorylation of a variety of different targets, such as BAD and caspase-9 (Cross et al., 2000). However, the exact physiological conditions under which astrocyte P2X7 receptors can be activated are not clear. As P2X7 receptors can be activated by high concentrations of ATP, it is hypothesized that they can be stimulated in neurological diseases or after injury, situations where high concentrations of ATP can be released from cells into the interstitial milieu.
In conclusion, our data indicate that stimulation of astrocytic P2X7 receptors, as well as other P2 receptors, leads to Akt activation by a pathway that is dependent on extracellular and intracellular calcium, a c-Src-related tyrosine kinase and PI3K. These results suggest that nucleotide receptors in astrocytes may be important in several downstream effects related to the Akt pathway, such as cell cycle and apoptosis regulation, protein synthesis, differentiation and glucose metabolism.
Acknowledgments
This work was supported by Department of Veterans Affairs (J.T.N.), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq)/Brazil (M.C.J.-S.) and the NIH (PO1 AG18357 to G.A.W. and P20 RR15565 to F.A.G. and G.A.W.). We are grateful to You-Fang Shi for preparation of astrocyte cultures.
Abbreviations
- α,β-meATP
α,β-methylene-ATP
- 2MeSADP
2-methylthioadenosine diphosphate
- AIDA
(Rs)-1-aminoindan-1,5-dicarboxylic acid
- BAPTA-AM
1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethylester)
- BBG
Brilliant Blue G
- BzATP
2′,3′-O-(4-benzoyl)-benzoyl ATP
- CNQX
6-Cyano-7-nitroquinoxaline-2,3-dione
- DMEM
Dulbecco's modified Eagle's medium
- D-PBS
Dulbecco's phosphate-buffered saline
- ECL
enhanced chemiluminescence
- EGTA
ethylene glycol-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid
- ERK
extracellular signal-regulated protein kinase
- iso-PPADS
iso-pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulfonic acid
- MAPK
mitogen-activated protein kinase
- MCPG
α-methyl-4-carboxyphenylglycine
- oATP
periodate-oxidized ATP
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- SDS–PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- PI3K
phosphoinositide 3-kinase
- PtdIns
phosphatidylinositol
- PKC
protein kinase C
- Rb2
reactive Blue 2
- TNP-ATP
2′,3′-O-(2, 4, 6,-trinitrophenyl) adenosine 5′–triphosphate
- UTP
uridine 5′-triphosphate
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., BURNSTOCK G. Purinoreceptors: are there families of P2X and P2Y purinoreceptors. Pharmac. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- BAUER B., JENNY M., FRESSER F., ÜBERALL F., BAIER G. Akt1/PKBα is recruited to lipid rafts and activated downstream of PKC isotypes in CD3-induced T cell signalling. FEBS Lett. 2003;541:155–162. doi: 10.1016/s0014-5793(03)00287-4. [DOI] [PubMed] [Google Scholar]
- BEIGI R.D., KERTESY S.B., AQUILINA G., DUBYAK G.R. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br. J. Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHI B.R., LYNCH K.J., TOUMA E., NIFORATOS W., BURGARD E.C., ALEXANDER K.M., PARK H.S., YU H., METZGER R., KOWALUK E., JARVIS M.F., VAN BIESEN T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur. J. Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoreceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA R., NEARY J.T., CATTABENI F., COTTINI L., D'IPPOLITO G., SCHILLER P.R., ABBRACCHIO M.P. Induction of COX-2 and reactive gliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signalling. J. Neurochem. 2002;83:1285–1296. doi: 10.1046/j.1471-4159.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA R., NEARY J.T., FUMAGALLI M., COTTINI L., CATTABENI F., SCHILLER P.R., ABBRACCHIO M.P. P2Y receptors in brain astroglial cells: identification of a gliotic P2Y receptor coupled to activation of a calcium-independent Ras/ERK1/2 pathway. Drug Dev. Res. 2003;59:161–170. [Google Scholar]
- BRAZIL D.P., HEMMINGS B.A. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- BUITRAGO C., BOLAND R., BOLAND A.R. The tyrosine kinase c-Src is required for 1,25-(OH)2-vitamin D3 signalling to the nucleus in muscle cells. Biochim. Biophys. Acta. 2001;1541:179–187. doi: 10.1016/s0167-4889(01)00142-2. [DOI] [PubMed] [Google Scholar]
- CANTRELL D.A. Phosphoinositide 3-kinase signaling pathways. J. Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- CHAN T.O., TSICHILIS P.N. PDK2: a complex tail in one Akt. Science's STKE. 2001;66:1–5. doi: 10.1126/stke.2001.66.pe1. [DOI] [PubMed] [Google Scholar]
- CHEN R., KIM O., YANG J., SATO K., EISENMANN K.M., MCCARTHY J., CHEN H., QIU Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., ROBAYE B., BOEYNAEMS J. Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS T.G., SCHEEL-TOELLNER D., HENRIQUEZ N.V., DEACON E., SALMON M., LORD J.M. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F. Novel data point to a broader mechanism of action of oxidized ATP: the P2X7 receptor is not the only target. Br. J. Pharmacol. 2003;140:441–443. doi: 10.1038/sj.bjp.0705469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FALZONI S., FERRARI D., SANZ J.M., VENKETARAMAN V., BARICORDI O.R. Cytolytic P2X receptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- D'ONOFRIO M., CUOMO L., BATTAGLIA G., NGOMBA R.T., STORTO M., KINGSTON A.E., ORZI F., DE BLASI A., DI IORIO P., NICOLETTI F., BRUNO V. Neuroprotection mediated by glial group II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J. Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- DOWNWARD J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- DUAN S., ANDERSON C.M., KEUNG E.C., CHEN Y., SWANSON R.A. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERB L., LIU J., OCKERHAUSEN J., KONG Q.M., GARRAD R.C., GRIFFIN K., NEAL C., KRUGH B., SANTIAGO-PEREZ L.I., GONZALEZ F.A., GRESHAM H.D., TURNER J.T., WEISMAN G.A. An RGD sequence in the P2Y2 receptor interacts with αvβ3 integrins and is required for Go-mediated signal transduction. J. Cell Biol. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERB L., LUSTIG K.D., AHMED A.H., GONZALEZ F.A., WEISMAN G.A. Covalent incorporation of 3′-O-(4-benzoyl)benzoyl-ATP into a P2 purinoreceptor in transformed mouse fibroblasts. J. Biol. Chem. 1990;265:7424–7431. [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X-purinoreceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- FRANKE H., GROSCHE J., SCHÄDLICH H., KRÜGEL U., ALLGAIER C., ILLES P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001;108:421–429. doi: 10.1016/s0306-4522(01)00416-x. [DOI] [PubMed] [Google Scholar]
- FRUMAN D.A., MEYERS R.E., CANTLEY L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- GARRAD R.C., OTERO M.A., ERB L., THEISS P.M., CLARKE L.L., GONZALEZ F.A., TURNER J.T., WEISMAN G.A. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor: consequences of truncation of the C-terminus. J. Biol. Chem. 1998;273:29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- GEBREMEDHIN D., YAMAURA K., ZHANG C., BYLUND J., KOEHLER R.C., HARDER D.R. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J. Neurosci. 2003;23:1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENDRON F., NEARY J.T., THEISS P.M., SUN G.Y., GONZALEZ F.A., WEISMAN G.A. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am. J. Cell Physiol. 2003a;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- GENDRON F., NEWBOLD N.L., VIVAS-MEJIA P.E., WANG M., NEARY J.T., SUN G.Y., GONZALEZ F.A., WEISMAN G.A. Signal transduction pathways for P2Y2 and P2X7 nucleotide receptors that mediate neuroinflammatory responses in astrocytes and microglial cells. Biomedical Res. 2003b;14:47–61. [Google Scholar]
- GERVITZ L.M., NALBANT D., WILLIAMS S.C., FOWLER J.C. Adenosine-mediated activation of Akt/protein kinase B in the rat hippocampus in vitro and in vivo. Neurosci. Lett. 2002;328:175–179. doi: 10.1016/s0304-3940(02)00495-0. [DOI] [PubMed] [Google Scholar]
- GLIKI G., WHEELER-JONES C., ZACHARY I. Vascular endothelial growth factor induces protein kinase C (PKC)-dependent Akt/PKB activation and phosphatidylinositol-3-kinase-mediated PKCδ phosphorylation: role of PKC in angiogenesis. Cell Biol. Int. 2002;26:751–759. doi: 10.1016/s1065-6995(02)90926-1. [DOI] [PubMed] [Google Scholar]
- GONZALEZ F.A., AHMED A.H., LUSTIG K.D., ERB L., WEISMAN G.A. Permeabilization of transformed mouse fibroblasts by 3′-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate and the desensitization of the process. J. Cell. Physiol. 1989;139:109–115. doi: 10.1002/jcp.1041390116. [DOI] [PubMed] [Google Scholar]
- HANKE J.H., GARDNER J.P., DOW R.L., CHANGELIAN P.S., BRISSETTE W.H., WERINGER E.J., POLLOCK B.A., CONNELLY P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- HIBELL A.D., THOMPSON K.M., XING M., HUMPHREY P.P.A., MICHEL A.D. Complexities of measuring antagonist potency at P2X7 receptor orthologs. J. Pharmacol. Exp. Ther. 2001;296:947–957. [PubMed] [Google Scholar]
- HUMPHREYS B.D., VIRGINIO C., SURPRENANT A., RICE J., DUBYAK G.R. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol. Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- HUWILER A., RÖLZ W., DORSCH S., REN S., PFEILSCHIFTER J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y2 purinoreceptor in renal mesangial cells. Br. J. Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IACOVELLI L., BRUNO V., SALVATORE L., MELCHIORRI D., GRADINI R., CARICASOLE A., BARLETTA E., DE BLASI A., NICOLETTI F. Native group III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol 3-kinase pathways. J. Neurochem. 2003;82:216–223. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- JIANG L.H., MACKENZIE A.B., NORTH A.L., SURPRENANT A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol. Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- KHAKH B., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SÉGUÉLA P., VOIGT M., HUMPHREY P.P.A. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KIM M., JIANG L., WILSON H.L., NORTH R.A., SURPRENANT A. Proteomic and functional evidence for a P2X7 receptor signaling complex. EMBO J. 2001a;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM M., SPELTA V., SIM J., NORTH R.A., SURPRENANT A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J. Biol. Chem. 2001b;276:23262–23267. doi: 10.1074/jbc.M102253200. [DOI] [PubMed] [Google Scholar]
- KING B.F., NEARY J.T., ZHU Q., WANG S., NORENBERG M.D., BURNSTOCK G. P2 purinoreceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- KING B.F., WILDMAN S.S., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br. J. Pharmacol. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KODAKI T., WOSCHOLSKI R., HALLBERG B., RODRIGUEZ-VICIANA P., DOWNWARD J., PARKER P.J. The activation of phosphatidylinositol 3-kinase by Ras. Curr. Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- KUKLEY M., BARDEN J.A., STEINHAUSER C., JABS R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LENZ G., GOTTFRIED C., LUO Z., AVRUCH J., RODNIGHT R., NIE W., KANG Y., NEARY J.T. P2Y purinoreceptor subtypes recruit different Mek activators in astrocytes. Br. J. Pharmacol. 2000;129:927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LEWIS C., SURPRENANT A., EVANS R.J. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5-triphosphate (TNP-ATP) – a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARME D., SCHACHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MOTLEY E.D., EGUCHI K., GARDNER C., HICKS A.L., REYNOLDS C.M., FRANK G.D., MIFUNE M., OHBA M., EGUCHI S. Insulin-induced Akt activation is inhibited by angiotensin II in the vasculature through protein kinase C-α. Hypertension. 2003;41:775–780. doi: 10.1161/01.HYP.0000051891.90321.12. [DOI] [PubMed] [Google Scholar]
- MURGIA M., HANAU S., PIZZO P., RIPPA M., DI VIRGILIO F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- NEARY J.T., BAKER L., JORGENSEN S.L., NORENBERG M.D. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994a;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., KANG Y., BU Y., YU B., AKONG K., PETERS C.M. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEARY J.T., RATHBONE M.P., CATTABENI F., ABBRACCHIO M.P., BURNSTOCK G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., WHITTEMORE S.R., ZHU Q., NORENBERG M.D. Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factor and extracellular ATP. J. Neurochem. 1994b;63:490–494. doi: 10.1046/j.1471-4159.1994.63020490.x. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., ZHU Q. Signaling by ATP receptors in astrocytes. NeuroReport. 1994;5:1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- OKUDA M., TAKAHASHI M., SUERO J., MURRY C.E., TRAUB O., KAWAKATSU H., BERK B.C. Shear stress stimulation of p130Cas tyrosine phosphorylation requires calcium-dependent c-Src activation. J. Biol. Chem. 1999;274:26803–26809. doi: 10.1074/jbc.274.38.26803. [DOI] [PubMed] [Google Scholar]
- PANENKA W., JIJON H., HERX L.M., ARMSTRONG J.N., FEIGHAN D., WEI T., YONG V.W., RANSOHOFF R.M., MACVICAR B.A. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J. Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BURCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor: a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINTON M.S., SIHRA T.S., WILLIAMS R.J. Ca2+-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J. Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON G.L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SCHULTE G., FREDHOLM B.B. Signaling pathway through the human adenosine A3 receptor expressed in Chinese hamster ovary cells to the extracellular-signal regulated kinase 1/2. Mol. Pharmacol. 2002;62:1137–1146. doi: 10.1124/mol.62.5.1137. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., HUGO C., ROST S., ARNOLD S., GRUBER A., BRÜNE B., STERZEL R.B. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 1998;275:F962–971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- SEGER R., KREBS E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- SHIBUYA I., TANAKA K., HATTORI Y., UEZONO Y., HARAYAMA N., NOGUCHI J., UETA Y., IZUMI F., YAMASHITA H. Evidence that multiple P2X purinoreceptors are functionally expressed in the rat supraoptic neurons. J. Physiol. 1999;514:351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN S.H., LIN L., HUNG A.C., KUO J. ATP-stimulated Ca2+ influx and phospholipase D activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J. Neurochem. 1999;73:334–343. doi: 10.1046/j.1471-4159.1999.0730334.x. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., GAVRIELIDES M.V., MITSUUCHI Y., FUJII T., KAZANIETZ M.G. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through the activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VIRGINIO C., MACKENZIE A., NORTH R.A., SURPRENANT A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J. Physiol. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G., SURPRENANT A., NORTH A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- YANO S., TOKUMITSU H., SODERLING T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev. Res. 2001;52:44–56. [Google Scholar]