Abstract
The involvement of peripheral nitric oxide (NO) in febrigenic signaling to the brain has been proposed because peripherally administered NO synthase (NOS) inhibitors attenuate lipopolysaccharide (LPS)-induced fever in rodents. However, how the unstable molecule of NO can reach the brain to trigger fever is unclear. It is also unclear whether NOS inhibitors attenuate fever by blocking febrigenic signaling or, alternatively, by suppressing thermogenesis in brown fat.
Male Wistar rats were chronically implanted with jugular catheters; their colonic and tail skin temperatures (Tc and Tsk) were monitored.
Study 1 was designed to determine whether the relatively stable, physiologically relevant forms of NO, that is, S-nitrosoalbumin (SNA) and S-nitrosoglutathione (SNG), are pyrogenic and whether they enhance LPS fever. At a neutral ambient temperature (Ta) of 31°C, afebrile or LPS (1 μg kg−1, i.v.)-treated rats were infused i.v. with SNA (0.34 or 4.1 μmol kg−1; the controls received NaNO2 and albumin) or SNG (10 or 60 μmol kg−1; the controls received glutathione). Tc of SNA- or SNG-treated rats never exceeded that of the controls.
In Study 2, we tested whether the known fever-attenuating effect of the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) at a subneutral Ta (when fever is brought about by thermogenesis) also occurs at a neutral Ta (when fever is brought about by skin vasoconstriction). At a subneutral Ta of 24°C, L-NAME (2.5 mg kg−1, i.v.) attenuated LPS (10 μg kg−1, i.v.) fever, presumably by inhibiting thermogenesis. At 31°C, L-NAME enhanced LPS fever by augmenting skin vasoconstriction (Tsk fall).
In summary, both SNA and SNG had no pyrogenic effect of their own and failed to enhance LPS fever; peripheral L-NAME attenuated only fever brought about by increased thermogenesis. It is concluded that NO is uninvolved in febrigenic signaling to the brain.
Keywords: Nitrosoalbumin, nitrosoglutathione, nitrosothiols, nitric oxide synthase, L-NAME, thermoregulation, fever, lipopolysaccharide, body temperature, ambient temperature
Introduction
There is little doubt that centrally produced nitric oxide (NO) is involved in fever as an antipyretic agent (Steiner et al., 2002), but the role of peripherally produced NO in the fever response is unclear. It has been hypothesized that peripheral NO is a febrigenic signal to the brain (Steiner & Branco, 2001). The main argument supporting this hypothesis is that peripherally administered inhibitors of NO synthase (NOS) attenuate fevers induced by lipopolysaccharide (LPS) and many other exogenous pyrogens in rats (Scammell et al., 1996; Perotti et al., 1999; Ataoglu et al., 2000; Soszynski, 2001; Kamerman et al., 2002) and guinea-pigs (Roth et al., 1998a; 1999; Kamerman & Fuller, 2000). However, the brief (∼0.1 s) half-life of free NO in vivo (Kelm & Schrader, 1990) and, consequently, its curtailed ability to reach tissue targets (Condorelli & George, 2002) severely limit the mechanisms by which peripheral NO might signal the brain to produce fever.
In the plasma, NO exists in a dynamic balance with low-molecular-mass nitrosothiols (such as S-nitrosoglutathione (SNG)) and high-molecular-mass nitrosothiols (such as S-nitrosoalbumin (SNA)): NO–SNG–SNA (Scharfstein et al., 1994; Gordge et al., 1996; JOURD'HEUIL et al., 2000b; Tsikas et al., 2001). The predominant, most stable species in this equilibrium is SNA: it has a plasma concentration of ∼10 nM (Rassaf et al., 2003) and a half-life of ∼40 min (Stamler et al., 1992). Since they are relatively stable, the nitrosothiols serve as a high-capacity reservoir of NO (Minamiyama et al., 1996; JOURD'HEUIL et al., 2000b). The same nitrosothiols also release NO to its distant targets via tissue-specific reactions with transition metals, enzymes, and reducing agents (Gaston, 1999; Hogg, 2002). The involvement of SNA and SNG in many biological effects of NO, including vasorelaxation and inhibition of platelet aggregation, has been demonstrated (Stamler et al., 1992; Keaney et al., 1993; De Belder et al., 1994; Gordge et al., 1996; Kashiba et al., 1999). It is, therefore, reasonable to hypothesize that, if peripheral NO signals the brain to produce fever, this signaling involves nitrosothiols. Consistent with this hypothesis, the plasma levels of nitrosothiols increase after the peripheral administration of LPS, at least at a high dose (JOURD'HEUIL et al., 2000a). To test whether peripherally administered SNA and SNG are pyrogenic and whether they enhance LPS-induced fever were the first aims of the present work.
Although several studies (Scammell et al., 1996; Roth et al., 1998a; 1999; Perotti et al., 1999; Ataoglu et al., 2000; Kamerman & Fuller, 2000; Soszynski, 2001; Kamerman et al., 2002) attributed the fever-suppressing effect of NOS inhibitors to their action on NO-mediated mechanisms of immune-to-brain febrigenic signaling, an alternative interpretation was not ruled out. Specifically, these studies did not address the possibility that the observed attenuation of fever might have resulted from altered thermogenesis. All referenced studies were conducted in rats and guinea-pigs at an ambient temperature (Ta) of 22–25°C, which is substantially below the thermoneutral zone for these species in most experimental setups (Pace & Rahlman, 1983; Romanovsky et al., 2002). At a subneutral Ta (i.e., in the cold), fever is primarily caused by increased thermogenic activity of the brown adipose tissue (Székely & Szelényi, 1979). As this activity critically depends on the local production of NO (Nagashima et al., 1994; Saha & Kuroshima, 2000), NOS inhibitors might attenuate fever by suppressing brown adipose tissue thermogenesis. To either confirm or rule out this possibility was the second aim of the present work.
To address this second aim, the effect of the widely used NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) on LPS fever was studied both in the cold and at the upper limit of the thermoneutral zone. In the latter case, fever is primarily brought about by tail skin vasoconstriction, and the contribution of thermogenesis for this response is negligible (Székely & Szelényi, 1979). If L-NAME attenuates fever by suppressing thermogenesis, it should fail to affect the febrile response at a neutral Ta. If, however, NO is a febrigenic signal to the brain (as proposed in the earlier studies), L-NAME should attenuate fever regardless of Ta. This approach (i.e., studying an effect on the febrile response at different Ta's to differentiate whether the effect results from altered febrigenic signaling or altered thermoeffector mechanisms) was used successfully in our recent study (Ivanov & Romanovsky, 2002).
Methods
Animals
The studies were conducted in 151 male Wistar rats (B&K Universal, Kent, WA, U.S.A.) weighing 280–320 g at the time of the experiments. Initially, the animals were housed three per standard ‘shoe box'; after surgery, they were caged individually. The cages were kept in a rack equipped with a Smart Bio-Pack ventilation system (model SB4100) and Thermo-Pak temperature control system (model TP2000; Allentown Caging Equipment, Allentown, NJ, U.S.A.); the temperature of the incoming air was maintained at 28°C. Standard rat chow (Teklad Rodent Diet ‘W' 8604; Harlan Teklad, Madison, WI, U.S.A.) and tap water were available ad libitum. The room was on a 12 : 12 h light–dark cycle (lights on at 07:00 h). The cage space was enriched with artificial ‘rat holes' (cylindrical confiners made of stainless steel wire). In addition to spending time in the confiners voluntarily, the rats were systematically habituated to them (seven daily training sessions, 4 h each). The same confiners were used later in the experiments. When well-adapted rats are confined, they exhibit no stress fever (Romanovsky et al., 1998). All experiments were started between 08:00 and 09:00 h. Each rat was used in only one experiment and euthanized with an overdose of sodium pentobarbital (100 mg kg−1, i.v.) at the end of the experiment. The protocols were approved by the Institutional Animal Care and Use Committee of St Joseph's Hospital and Medical Center.
Surgery
Each rat was subjected to chronic catheterization of the jugular vein. Under ketamine–xylazine–acepromazine (55.6, 5.5, and 1.1 mg kg−1, i.p., respectively) anesthesia and antibiotic (enrofloxacin, 12 mg kg−1, s.c.) protection, the rat was placed on an operating board. A 1-cm longitudinal incision was made on the ventral surface of the neck, 1 cm left of the trachea. The left jugular vein was exposed, freed from its surrounding connective tissue, and ligated. A silicone catheter (ID 0.5 mm, OD 0.9 mm) filled with heparinized (50 U ml−1) pyrogen-free saline was passed into the superior vena cava through the jugular vein and secured in place with ligatures. The 10-cm free end of the silicone catheter was knotted, tunneled under the skin, and exteriorized at the nape. If the rat was designated for an experiment involving blood pressure measurements (Experiment (Exp.) 1), the left carotid artery was also chronically catheterized. The artery was isolated and clamped by a microclip. A PE-50 catheter (ID 0.6 mm, OD 1.0 mm) filled with heparinized saline was inserted into the artery centripetally, the clip was removed, and the catheter was secured in place with ligatures. The free end of the catheter was heat-closed and exteriorized at the nape. The surgical wounds on the ventral and dorsal surfaces of the neck were sutured. The catheters were flushed with heparinized saline every other day.
Instrumentation
All experiments (except for Exp. 1) were performed on Day 5 post-surgery. Each rat was placed in a confiner and equipped with two copper-constantan thermocouples: one for recording the colonic temperature (Tc) and the other for recording the tail skin temperature (Tsk). The colonic thermocouple was inserted 10 cm beyond the anal sphincter and fixed to the base of the tail with an adhesive tape. The skin thermocouple was positioned on the lateral surface of the tail (at the boundary of the proximal and middle thirds) and insulated from the environment with tape. The thermocouples were plugged in to a data logger (Dianachart, Rockaway, NJ, U.S.A.), which was connected to a personal computer. The rat was transferred to a climatic chamber (Forma Scientific, Marietta, OH, U.S.A.) set to Ta of 24.0°C (mild cold exposure) or 31.0°C (the upper limit of the thermoneutral zone; see Romanovsky et al., 2002). According to the manufacturer, the precision of temperature control in this chamber is ±0.1°C, and the uniformity at 30.0°C is ±0.4°C. The jugular catheter was extended with a length of PE-50 tubing filled with saline, and the extension was passed through a wall port and connected to a syringe filled with the drug of interest. This setup permitted i.v. drug administration without disturbing the animal.
The experiment that involved arterial blood pressure recording (Exp. 1) was performed on Day 1 post-surgery at a Ta of 31.0°C. Each rat was prepared as described above, but no thermocouples were attached. The arterial catheter was extended with a length of PE-50 tubing filled with saline. The extension was passed through a wall port and connected to a blood pressure monitor (World Precision Instruments, Sarasota, FL, U.S.A.) via a disposable pressure transducer (Cobe Cardiovascular, Arvada, CO, U.S.A.). The data were registered with the help of a paper chart recorder (Linear Instruments, Delray Beach, FL, U.S.A.).
Drugs
All drugs and reagents were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). A stock suspension of E. coli 0111:B4 LPS (2.5 mg ml−1) in saline was stored at −20°C. At the time of the experiment, the stock was diluted to a final concentration of either 1 μg ml−1 (Study 1) or 10 μg ml−1 (Study 2). The NOS inhibitor L-NAME (2.5, 5, or 10 mg ml−1), its inactive (Rees et al., 1990) enantiomer D-NAME (2.5 or 10 mg ml−1), SNG (5 or 30 μmol ml−1), and reduced glutathione (5 or 30 μmol ml−1) were all dissolved in saline immediately before the experiment.
SNA was synthesized extemporaneously according to Stamler et al. (1992), with minor modifications. Essentially fatty acid-free bovine serum albumin (BSA, 106.4 mg) was dissolved in saline (0.5 ml). NaNO2 (156 μg in 26 μl of saline) was added to achieve a molar NaNO2-to-BSA ratio of 1.4, and the solution was acidified with 0.5 N HCl (1 ml). After a 30-min incubation at room temperature, the solution was neutralized with 0.5 N NaOH (1 ml) and diluted with Tris-buffered saline (10 mM, pH 7.4) to a final volume of 4 ml. If the efficacy of nitrosylation were 100%, the resultant solution would have a final SNA concentration of 400 nmol ml−1. Assuming that the actual yield of SNA in this reaction is 85% (i.e., 85% of BSA molecules are nitrosylated; see Stamler et al., 1992; Seo et al., 1998), the final concentration of SNA was 340 nmol ml−1. Hence, the molar ratio between the added amount of NaNO2 and the synthesized amount of SNA was 1.65, whereas the ratio between the added amount of BSA and the synthesized amount of SNA was 1.18. These molar ratios were used to calculate the amounts of NaNO2 and BSA infused to the control rats in Study 1.
To verify the successful synthesis of SNA, UV-visible scanning was performed with a U-3010 spectrophotometer (Hitachi, Tokyo, Japan). The preparation of SNA, but not that of uncomplexed BSA or NaNO2, presented an absorption maximum at ∼340 nm, which is characteristic for S-nitrosothiols (Stamler et al., 1992; Seo et al., 1998). Assuming a molar absorptivity of 3869 M−1 cm−1 (as reported by Stamler et al. (1992) for a similar preparation of SNA), the value of absorbance measured at 340 nm (1.23, mean of three samples) corresponds to the SNA concentration of 318 nmol ml−1, which differs by only 6% from the concentration calculated based on the product yield in the nitrosylation reaction.
Protocols
Study 1: Thermal effects of SNA and SNG in afebrile and febrile rats. This study consisted of Exps. 1–3, which were performed at a neutral Ta (31°C).
Exp. 1: For both SNA and SNG, infusion rates presumed to be biologically active based on previous reports (Stamler et al., 1992; Keaney et al., 1993; Kashiba et al., 1999) were selected. The effectiveness of the selected infusion rates to cause arterial hypotension under the present experimental conditions was then tested. The rats were infused i.v. with SNA (68 nmol kg−1 min−1, 200 μl kg−1 min−1, 5 min) or SNG (1 μmol kg−1 min−1, 33.3 μl kg−1 min−1, 1 min), and their arterial blood pressure was recorded. For the SNA infusion, the control treatment was an infusion of the reagents employed in SNA synthesis at the corresponding molar ratios (see Drugs): NaNO2 (1.1 μmol kg−1 min−1, 200 μl kg−1 min−1, 0.5 min), followed by BSA (89.1 nmol kg−1 min−1, 200 μl kg−1 min−1, 4.5 min). For the SNG infusion, the control treatment was an infusion of reduced glutathione (1 μmol kg−1 min−1, 33.3 μl kg−1 min−1, 1 min). At the infusion rates used, both SNA and SNG evoked mild hypotensive responses, whereas the control infusions did not affect arterial blood pressure.
Exp. 2 was designed to determine whether SNA and SNG cause fever. In this experiment, the nitrosothiols were infused at the biologically active (mild hypotensive) rates selected in Exp. 1. To increase the chance of finding an effect on Tc, the duration of the infusions was extended substantially. SNA was infused over 60 min, so that the total dose administered was 4.1 μmol kg−1; the controls received NaNO2 (6.8 μmol kg−1) over 6 min, followed by BSA (4.8 μmol kg−1) over 54 min. SNG (or glutathione in the controls) was infused over 60 min, so that the total dose administered was 60 μmol kg−1.
Exp. 3 was designed to determine whether SNA and SNG enhance the small, monophasic fever caused by a low dose of LPS (Romanovsky et al., 1997; Simons et al., 1998). While designing this experiment, it was important to notice that both control groups in Exp. 2 (viz., the group treated with NaNO2 and BSA and that treated with glutathione) exhibited thermal effects (see Results). To eliminate the thermal effects of the control solutions and to prevent their potential interference with the fever response to LPS, the infusion rates were decreased in Exp. 3. Starting 30 min after the injection of LPS (1 μg kg−1, i.v.), the rats were infused with SNA (5.67 nmol kg−1 min−1, 16.8 μl kg−1 min−1) or SNG (167 nmol kg−1 min−1, 33.4 μl kg−1 min−1) for 60 min; the total doses infused were 340 nmol kg−1 and 10 μmol kg−1, respectively. The controls were also injected with LPS and then infused with either NaNO2 (92.4 nmol kg−1 min−1, 16.8 μl kg−1 min−1, 6 min) followed by BSA (7.5 nmol kg−1 min−1, 16.8 μl kg−1 min−1, 54 min), or with glutathione (167 nmol kg−1 min−1, 33.4 μl kg−1 min−1, 60 min).
Study 2: Effect of L-NAME on LPS fever at different Ta's. This study consisted of Exps. 4 and 5.
Exp. 4: Peripheral administration of L-NAME causes dose-dependent hypothermia in rats at subneutral Ta's (De Luca et al., 1995; Scammell et al., 1996; Branco et al., 1997; Steiner et al., 1998), an effect that would potentially interfere with the fever response to LPS. To avoid such an effect, the highest dose of L-NAME that is thermally ineffective in afebrile rats at both subneutral and neutral Ta's was found in Exp. 4. At a subneutral Ta (24°C), the rats were injected with L-NAME (2.5, 5, or 10 mg kg−1, i.v.) or D-NAME (10 mg kg−1) in a volume of 1 ml kg−1. Only the lowest dose caused no hypothermia (see Results). The thermal ineffectiveness of this dose was then verified in afebrile rats at a neutral Ta (31°C). Importantly, this dose of L-NAME is adequate to study the physiological roles of peripherally produced NO: it markedly suppresses NOS activity in peripheral tissues (Rees et al., 1990), but is expected to have little effect on brain NOS (Iadecola et al., 1994). This dose was selected for Exp. 5.
Exp. 5 was designed to determine the effect of L-NAME on LPS-induced fever at both subneutral and neutral Ta's. To this end, L-NAME or D-NAME (2.5 mg kg−1, i.v.) was injected 30 min before LPS (10 μg kg−1, i.v.) at either 24 or 31°C. The 10 μg kg−1 dose of LPS causes a long-lasting, polyphasic fever of a relatively high magnitude (Romanovsky et al., 1997; 1998). This dose of LPS was chosen because the expected effect of L-NAME was a reduction in the fever height. The duration of the febrile response to this dose of LPS was not a problem because the NOS-inhibiting effect of a single injection of L-NAME lasts a few hours to a few days (Rees et al., 1990; Iadecola et al., 1994).
Data processing and analysis
The absolute value of Tc, rather than the change in Tc, was used to evaluate deep body temperature responses (for justification, see Romanovsky et al., 2003). The heat loss index (HLI) was used to evaluate the thermoeffector responses of tail skin vasculature. As justified elsewhere (Romanovsky et al., 2002), the HLI was calculated according to the formula: HLI=(Tsk–Ta)/(Tc–Ta). The theoretical limits of the HLI are 0 (maximal skin vasoconstriction) and 1 (maximal vasodilation). In practice, however, the upper limit depends on the position of the tail skin thermocouple. When Tsk is measured at the boundary of the proximal and middle thirds of the tail, as in the present work, the HLI rarely exceeds 0.6 (Romanovsky et al., 2002). The mean arterial pressure (MAP) was calculated according to the formula: MAP=APmin+(APmax–APmin)/3 (Sindrup et al., 1991), where APmin and APmax are the minimal and maximal values of arterial pressure, respectively. The Tc, HLI, and MAP responses were compared across treatments and time points by two-way ANOVA for repeated measures using Statistica AX'99 (StatSoft, Tulsa, OK, U.S.A.). The data are reported as means±s.e.
Results
Study 1: Thermal effects of SNA and SNG in afebrile and febrile rats
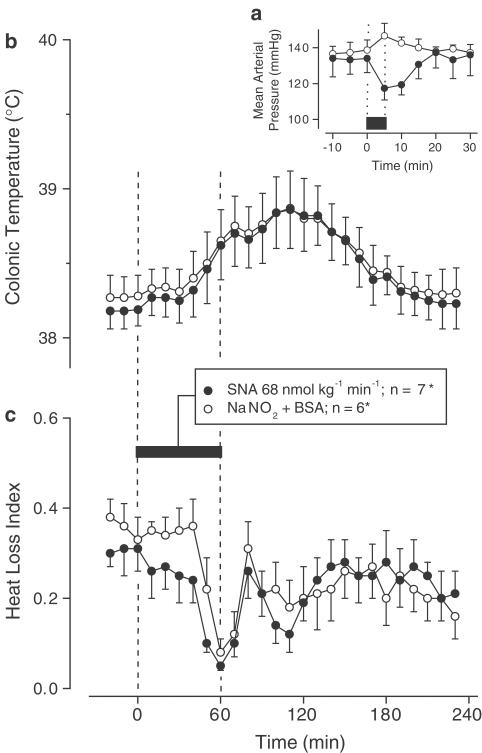
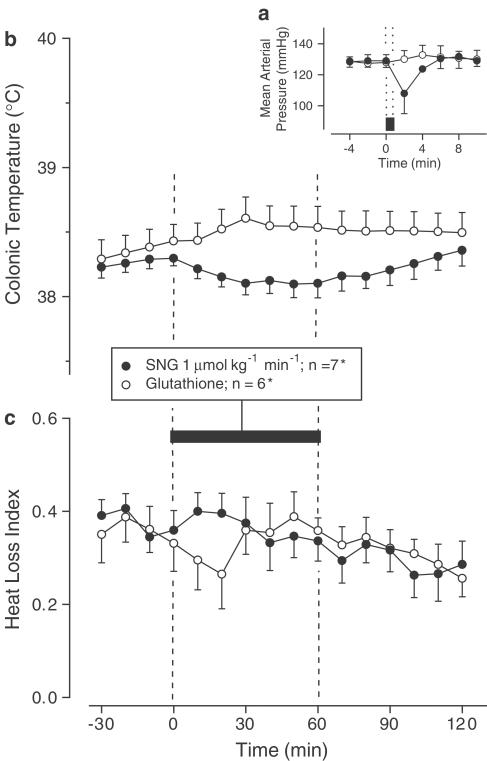
Exp. 1: When infused i.v. at the rate of 68 nmol kg−1 min−1, SNA produced mild arterial hypotension (MAP decrease of ∼20 mmHg; P=3.4 × 10−2), whereas the control infusion (NaNO2+BSA) had no effect on the MAP (Figure 1a). The i.v. infusion of SNG (but not of glutathione) at the rate of 1 μmol kg−1 min−1 caused a similar decrease in the MAP (P=3.9 × 10−2; Figure 2a). The biologically active (mild hypotensive) infusion rates of SNA and SNG used in this experiment and the corresponding rates of the control solutions were used in Exp. 2.
Figure 1.
Effects of SNA (infusion rate indicated) on the MAP (a), colonic temperature (b), and HLI (c) of afebrile rats at a neutral ambient temperature of 31°C. The controls received the corresponding amounts of NaNO2 and BSA; see Methods for details. The black bars indicate the duration of infusions. *In panel (a), n=3 for each group.
Figure 2.
Effects of SNG (infusion rate indicated) on the MAP (a), colonic temperature (b), and HLI (c) of afebrile rats at 31°C. The controls received reduced glutathione. The black bars indicate the duration of infusions. *In panel (a), n=3 for each group.
Exp. 2: When NaNO2 and BSA were administered sequentially (as a control to the SNA infusion), they evoked a biphasic rise in Tc (Figure 1b) accompanied by tail skin vasoconstriction (biphasic decrease in the HLI; Figure 1c). Experiments with an i.v. infusion of BSA alone (data not shown) suggest that these changes are likely caused by the BSA preparation. When SNA was infused for 60 min, the Tc (Figure 1b) and HLI (Figure 1c) responses were identical to those of the controls. The infusion of glutathione (control to SNG) tended to produce a small monophasic increase in Tc (Figure 2b) and a monophasic decrease in the HLI (Figure 2c), whereas SNG tended to reduce Tc (Figure 2b) and did not cause skin vasoconstriction (Figure 2c). Although not significant by themselves, the changes in Tc of the SNG- and glutathione-treated rats differed significantly from each other (P=3.4 × 10−2).
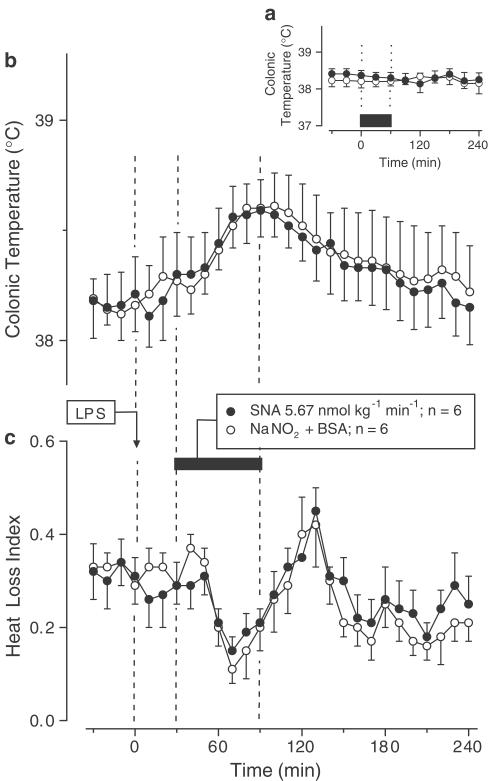
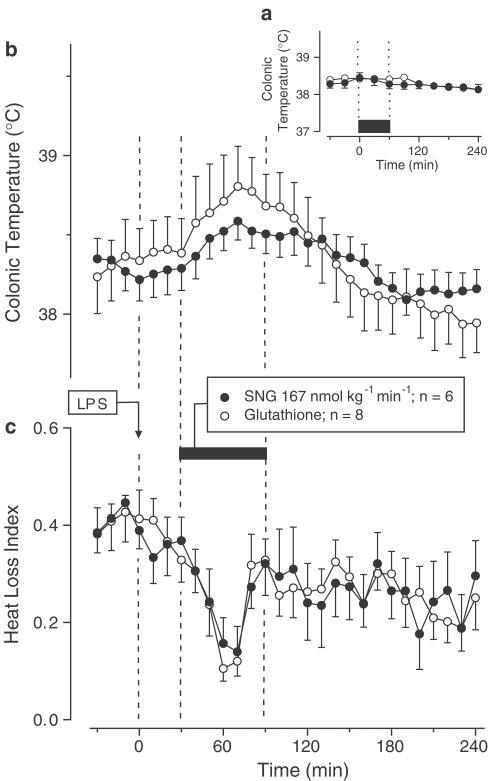
Exp. 3: To avoid potential interference of the thermal effects of the control solutions with LPS fever (subject of study in Exp. 3), SNA, SNG, and their control solutions were infused at lower rates: 5.67 nmol kg−1 min−1 for SNA and 167 nmol kg−1 min−1 for SNG. At these infusion rates, the nitrosothiols and their respective control solutions produced no change in Tc of the afebrile rats (Figures 3a and 4a). When SNA or SNG was infused during the rising phase of LPS (1 μg kg−1, i.v.)-induced monophasic fever, they affected neither the febrile Tc rise (Figures 3b and 4b) nor the associated decrease in the HLI (Figures 3c and 4c).
Figure 3.
Effects of SNA (infusion rate indicated) on the colonic temperature of afebrile rats (a) and on the colonic temperature (b) and HLI (c) of LPS (1 μg kg−1, i.v.)-treated rats at 31°C. The controls were infused with the corresponding amounts of NaNO2 and BSA. The black bars indicate the duration of infusions.
Figure 4.
Effects of SNG (infusion rate indicated) on the colonic temperature of afebrile rats (a) and on the colonic temperature (b) and HLI (c) of LPS (1 μg kg−1, i.v.)-treated rats at 31°C. The controls received reduced glutathione. The black bars indicate the duration of infusions.
Study 2: Effect of L-NAME on LPS fever at different Ta's
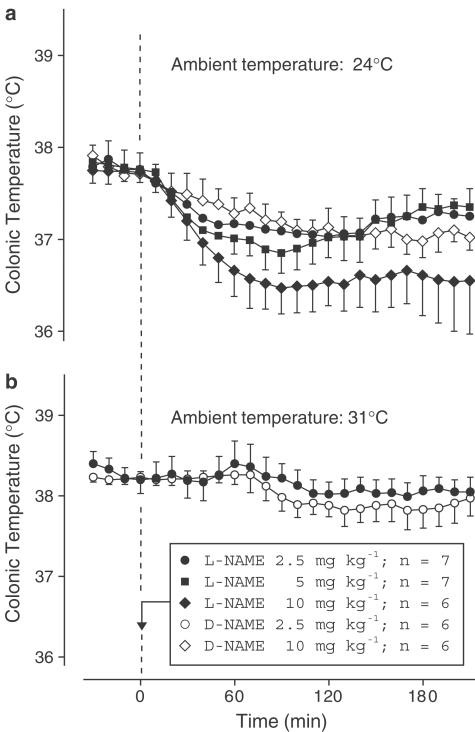
Exp. 4: At a subneutral Ta of 24°C, the D-NAME-treated rats presented a slight (∼0.5°C) decrease in Tc over the course of the experiment (Figure 5a). Such a decrease often occurs in untreated or saline-treated rats during the light phase of the day at a subneutral and even neutral Ta (Honma & Hiroshige, 1978; Romanovsky et al., 1996; Simons et al., 1998; Gautier & Murariu, 1999; Ivanov & Romanovsky, 2002; Ivanov et al., 2003a), presumably reflecting the circadian rhythm of Tc (Honma & Hiroshige, 1978; Scales & Kluger, 1987). Compared to D-NAME, L-NAME evoked a larger decrease in Tc (Figure 5a). This decrease was dose-dependent: whereas Tc of the D-NAME-treated controls was 37.2±0.1°C at 90 min postinjection, Tc of the L-NAME-treated rats was 37.1±0.2°C (2.5 mg kg−1), 36.9±0.2°C (5 mg kg−1), or 36.5±0.3°C (10 mg kg−1). The thermal responses to the two higher doses of L-NAME were significantly different from that of the control group (P=3.7 × 10−2 and P=1.3 × 10−2, respectively), but the response to the lowest dose (2.5 mg kg−1) was not. This lowest dose was also thermally ineffective at a neutral Ta of 31°C (Figure 5b). At neither 24°C nor 31°C did this dose interfere with the basal functioning of skin vessels, as evidenced by the absence of any changes in the HLI (data not shown). This thermally ineffective dose of L-NAME was used in Exp. 5.
Figure 5.
Effect of intravenous injection of the NOS inhibitor L-NAME (doses indicated) or its inactive enantiomer D-NAME (doses indicated) on the colonic temperature of rats at an ambient temperature of 24°C (a) or 31°C (b).
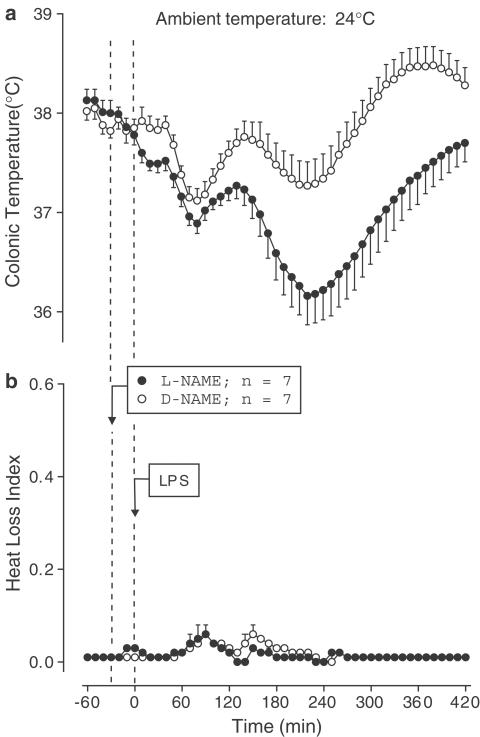
Exp. 5: The effect of L-NAME on LPS (10 μg kg−1, i.v.)-induced fever was studied at both subneutral (24°C) and neutral (31°C) Ta's. At 24°C, the D-NAME-treated controls responded to LPS with complex changes in Tc: the initial drop (nadir at ∼80 min post-LPS) was followed by two Tc rises (peaks at ∼140 and 370 min) (Figure 6a). During the second rise, Tc reached a febrile level (∼0.5°C above the baseline). Pretreatment with L-NAME had little effect on the initial hypothermia, but it markedly attenuated the first (P=2.9 × 10−2) and second (P=1.0 × 10−2) Tc rises (Figure 6a). In both the D-NAME- and L-NAME-treated rats, the HLI remained at a near-zero level throughout the experiment, indicating maximal tail skin vasoconstriction (Figure 6b).
Figure 6.
Effect of pretreatment with L-NAME (2.5 mg kg−1, i.v.) or D-NAME on the colonic temperature (a) and HLI (b) of LPS (10 μg kg−1, i.v.)-treated rats exposed to an ambient temperature of 24°C.
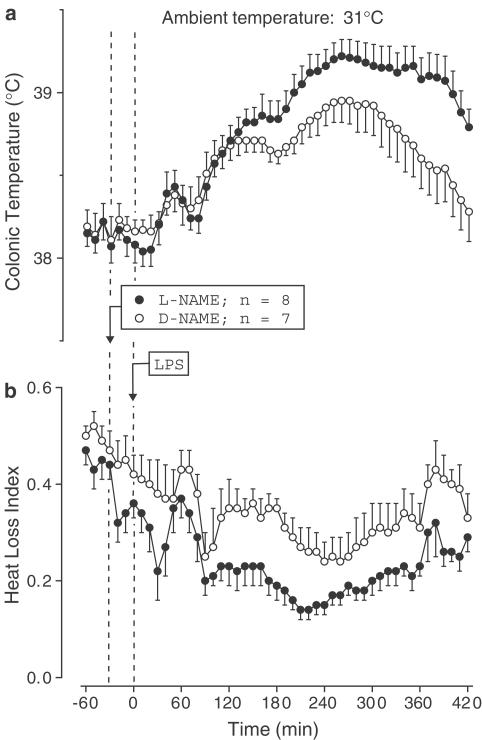
At 31°C, the D-NAME-treated controls responded to LPS with a typical polyphasic fever: three febrile phases peaked at ∼60, 150, and 280 min post-LPS (Figure 7a), and they were preceded by three waves of skin vasoconstriction (decrease in the HLI; Figure 7b). In contrast to its fever-reducing effect at 24°C, L-NAME significantly enhanced the second (P=2.8 × 10−2) and third (P=1.2 × 10−2) febrile phases (Figure 7a). In contrast to its lack of effect on the HLI at 24°C, L-NAME tended to enhance the LPS-induced skin vasoconstriction at the first and second febrile phases and significantly (P=1.4 × 10−2) enhanced it at the third phase (Figure 7b).
Figure 7.
Effect of L-NAME (2.5 mg kg−1, i.v.) or D-NAME on the colonic temperature (a) and HLI (b) of LPS (10 mg kg−1, i.v.)-treated rats exposed to an ambient temperature of 31°C.
Discussion
Several short-lived and/or poorly water-soluble mediators of fever (e.g., prostaglandin (PG) E2 and platelet-activating factor (PAF)) are solubilized, stabilized, and transported in the bloodstream by carrier molecules, primarily serum albumin (Clay et al., 1990; Peters, 1996). Consequently, their predominant, albumin-bound form is physiologically the most relevant (Peters, 1996). Experiments in which these mediators are administered in the albumin-bound form are more likely to reveal their intrinsic physiological actions. The use of the albumin-bound form was crucial to show that i.v. PGE2 causes fever, thus providing an important argument in favor of the febrigenic role of blood-borne PGE2 (Romanovsky et al., 1999). The albumin-bound form was also important for the discovery of the pyrogenic action of PAF (Ivanov et al., 2003b). Since NO is also a short-lived molecule that circulates primarily in its more stable, bound form (either to albumin (SNA) or to low-molecular-mass molecules such as glutathione (SNG)), we investigated whether SNA and SNG could have pyrogenic effects of their own and/or enhance LPS-induced fever.
Even at biologically active (mild hypotensive) doses, neither SNA nor SNG increased Tc of afebrile rats at a neutral Ta to above the levels seen in the controls. SNG even tended to decrease Tc, an action that agrees with the ability of NO donors to elicit hypothermia in rabbits in a thermoneutral environment (Mathai et al., 1997). When SNA and SNG were infused during LPS-induced fever, they also failed to exaggerate this response. (The infusion rates for the fever experiment were selected as the maximal rates at which the control solutions were thermally ineffective in afebrile rats.) SNA and SNG also failed to affect the HLI of either afebrile or febrile rats. Although the latter observation suggests that the tail skin vasculature in the rat is insensitive to circulating nitrosothiols, it does not rule out the possibility that locally produced, free NO may still play a role in thermoregulatory cutaneous vasodilation. Indeed, such vasodilation in the human forearm can be blocked by dialysis of a NOS inhibitor (Minson et al., 2001).
Although the low responsiveness of skin blood vessels to nitrosothiols in vivo (De Belder et al., 1994; present results) seemingly contradicts the high responsiveness of other vessels (e.g. the aorta) to nitrosothiols in vitro (Stamler et al., 1992; Seo et al., 1998; Jia & Stamler, 1999), the sensitivity to the vasorelaxant action of nitrosothiols is known to vary widely among vascular beds (Jia & Stamler, 1999; Gluckman et al., 2002). In contrast to the spontaneous release of NO by many synthetic donors, the release of NO from nitrosothiols depends on the presence of transition metals, enzymes, and reducing agents, many of which are distributed throughout the body in a tissue-specific manner (Gaston, 1999; Hogg, 2002). For example, SNG readily releases NO in platelets due to the presence of a copper-dependent enzyme in these postcellular structures (Gordge et al., 1996). The effects of nitrosothiols are also likely to be less tissue-specific in vitro than in vivo. In vitro, nitrosothiols promptly release NO by reacting with transition metal ions that exist as unavoidable contaminants of all buffers. In vivo, transition metal ions are actively sequestered; they are present at much lower concentrations and distributed throughout the body in a tissue-specific manner (Hogg, 2002).
Since both SNA and SNG lacked a febrigenic effect of their own and failed to enhance fever in the present work, they are likely uninvolved in febrigenic signaling to the brain. However, a few speculative scenarios are still compatible with an involvement of free NO, produced outside or at the blood–brain barrier, in febrigenic signaling. One scenario is that NO may be produced in the liver, in the vicinity of sensory endings of the nodose ganglion neurons. These neurons, which are stimulated by NO (Lawrence et al., 1997), convey a centripetal febrigenic message (Simons et al., 1998), which is likely to be important for triggering monophasic fever and, possibly, the first, early phase of polyphasic fever (Romanovsky et al., 2000). Another unstable mediator, PGE2, which is synthesized in the liver already at the onset of the first febrile phase (Ivanov et al., 2002), can also activate hepatic vagal sensory fibers (Niijima, 1996; Ek et al., 1998).
A second scenario emphasizes the hypothalamic microvasculature, which produces febrigenic PGE2 (Matsumura et al., 1998; Yamagata et al., 2001) during the second and third febrile phases (Ivanov et al., 2002). By analogy, it is conceivable that circulating LPS and cytokines can induce NO synthesis in hypothalamic capillaries. After being released from their antiluminal surface, NO (or NO-derived species) may trigger fever by increasing the permeability of the blood–brain barrier (Mayhan, 1999) to circulating pyrogenic molecules or by activating an important PGE2-synthesizing enzyme, cyclooxygenase-2 (Salvemini et al., 1993; Goodwin et al., 1999).
Yet, a completely different scenario is possible: NO may be uninvolved in febrigenic signaling. Although this less intriguing scenario has received little attention in the literature, it is consistent with the following data. First, when L-NAME attenuates the febrile response to peripheral pyrogens in a cool environment, it decreases neither the blood levels of propyretic cytokines interleukin (IL)-6 and tumor necrosis factor-α (Roth et al., 1998a) nor the brain level of PGE2 (Redford et al., 1995). Second, fevers caused by central administration of IL-1β (Monroy et al., 2001) or by psychological stressors (De Paula et al., 2000; Soszynski, 2001) in a cool environment are also sensitive to peripheral administration of NOS inhibitors – even though they are initiated within the brain and do not require transduction of a febrigenic signal from the periphery. Third, systemic production of NO may not even be enhanced during fever. Only high doses of LPS (those that cause shock and hypothermia) induce overexpression of the inducible NOS isoform (Titheradge, 1999), leading to a massive production of NO and to a surge of plasma SNA (JOURD'HEUIL et al., 2000a). Lower, pyrogenic doses suppress NO synthesis, possibly by inhibiting constitutive NOS isoforms (Riedel, 1997; Steiner et al., 2002).
In the present work, L-NAME attenuated LPS fever in a cool environment but did not attenuate (and rather enhanced) it at a neutral Ta. These results are consistent with multiple studies showing that NOS inhibitors suppress fever in rats (Reimers et al., 1994; Scammell et al., 1996; Roth et al., 1998b; Perotti et al., 1999; Ataoglu et al., 2000; Soszynski, 2001; Kamerman et al., 2002) and guinea-pigs (Roth et al., 1998a; 1999; Kamerman & Fuller, 2000) at 22–25°C, that is, at their subneutral Ta's (Pace & Rahlman, 1983; Romanovsky et al., 2002). Our finding also agrees with studies showing that NOS inhibitors do not suppress fever in rabbits (Kapás et al., 1994; Gagalo et al., 1996; Riedel, 1997), cats (Redford et al., 1995), and pigs (Parrott et al., 1998) at 20–26°, that is, at their neutral Ta's (Gonzalez et al., 1971; Gautier et al., 1989; Noblet et al., 1997). Together with our data, these studies suggest that NO affects fever by acting on a process or mechanism that strongly depends on Ta. Febrigenic signaling is unlikely to be a subject of such dependence.
What strongly depends on Ta is the set of thermoeffectors recruited for the development of fever. In the cold, fever is brought about largely by thermogenesis; at thermoneutrality, its major effector mechanism is skin vasoconstriction (Crawshaw & Stitt, 1975; Székely & Szelényi, 1979). Therefore, it can be inferred that NOS inhibitors attenuate fever by affecting thermoregulatory effector mechanisms rather than by blocking febrigenic signaling to the brain. Indeed, both activation of thermogenesis in the brown adipose tissue (Nagashima et al., 1994; Saha & Kuroshima, 2000; Kikuchi-Utsumi et al., 2002) and dilation of skin vasculature (Minson et al., 2001) are thought to depend critically on locally produced NO. In the present work, the fever-attenuating effect of L-NAME at a subneutral Ta was not associated with changes in the HLI (the tail skin vessels remained constricted); the effect of L-NAME likely resulted from inhibition of thermogenesis. In contrast, the fever-enhancing effect of L-NAME at a neutral Ta probably reflects enhanced vasoconstriction in the tail because L-NAME augmented the LPS-induced decreases in the HLI. These results are in agreement with a recent report (Kamerman et al., 2003) showing that the thermal effect of peripheral L-NAME in afebrile rats also depends on Ta: L-NAME inhibits thermogenesis and causes hypothermia at a subneutral, but not at a neutral Ta.
Conclusions
The major findings of the present work can be summarized as follows. Neither SNA nor SNG (physiologically relevant forms of NO) have a febrigenic action of their own or enhance LPS fever. Intravenous L-NAME (a NOS inhibitor) attenuates LPS-induced fever in rats only at a subneutral Ta, that is, when this response is predominantly brought about by nonshivering thermogenesis. Intravenous L-NAME does not attenuate LPS fever when it is brought about by skin vasoconstriction (at or above thermoneutrality). Rather, L-NAME enhances the febrile response to LPS at thermoneutrality by enhancing skin vasoconstriction. Together, these results suggest that peripheral NO is uninvolved in febrigenic signaling to the brain.
Perspectives
While this report was in preparation, Kozak & Kozak (2003) used selective genetic deletion of individual isoforms of NOS and chronic oral administration of a NOS inhibitor to study LPS- and turpentine oil-induced fevers in mice. The authors' conclusion that the inducible and neuronal isoforms of NOS (but not the endothelial isoform) are involved in febrigenic signaling contradicts the main conclusion of the present work. Such a contradiction can be attributed to at least two methodological factors. First, due to compensatory mechanisms of genetic ablation in knockout and mutant animals, experiments in such animals (Ivanov et al., 2003a), and specifically in NOS knockout mice (Szabó, 1998), often lead to conclusions later proved erroneous by further pharmacological studies. Second, when administered chronically (as done by Kozak & Kozak, 2003), NOS inhibitors often produce qualitatively different results than when administered acutely (Bryant et al., 1995). Although the main conclusion of our present work differs from that of Kozak & Kozak (2003), we agree with their final remark that the complex relationships between the NOS system and the febrile response are far from being understood and that the role of NO in fevers caused by various pyrogenic insults merits further investigation.
Acknowledgments
We thank Drs R.J. Lukas and Y.-P. Kuo for their help with spectrophotometric analyses of SNA; Drs. A.D. Craig, Jr. and W.A. Eckert, III, for their help with arterial blood pressure measurements; M. Milovancevic, S. Patel, and C. Thai for technical assistance; and Drs. S.A. Kick and V.F. Turek for editing the manuscript. The work was funded in part by a National Institute of Neurological Disorders and Stroke grant NS-41233 (A.A. Romanovsky). A.I. Ivanov's present address: Department of Pathology and Laboratory Medicine, Emory University, Atlanta, GA 30322, U.S.A.
Abbreviations
- BSA
bovine serum albumin
- D-NAME
Nω-nitro-D-arginine methyl ester
- Exp(s)
experiment(s)
- HLI
heat loss index
- IL
interleukin
- L-NAME
Nω-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAF
platelet-activating factor
- PG
prostaglandin
- SNA
S-nitrosoalbumin
- SNG
S-nitrosoglutathione
- Ta
ambient temperature
- Tc
colonic temperature
- Tsk
tail skin temperature
References
- ATAOGLU H., DOGAN M.D., MUSTAFA F., AKARSU E.S. Candida albicans and Saccharomyces cerevisiae cell wall mannans produce fever in rats: role of nitric oxide and cytokines. Life Sci. 2000;67:2247–2256. doi: 10.1016/s0024-3205(00)00804-3. [DOI] [PubMed] [Google Scholar]
- BRANCO L.G.S., CARNIO E.C., BARROS R.C.H. Role of nitric oxide pathway in hypoxia-induced hypothermia of rats. Am. J. Physiol. 1997;273:R967–R971. doi: 10.1152/ajpregu.1997.273.3.R967. [DOI] [PubMed] [Google Scholar]
- BRYANT C.E., ALLCOCK G.H., WARNER T.D. Comparison of effects of chronic and acute administration of NG-nitro-L-arginine methyl ester to the rat on inhibition of nitric oxide-mediated responses. Br. J. Pharmacol. 1995;114:1673–1679. doi: 10.1111/j.1476-5381.1995.tb14956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAY K.L., JOHNSON C., HENSON P. Binding of platelet activating factor to albumin. Biochim. Biophys. Acta. 1990;1046:309–314. doi: 10.1016/0005-2760(90)90246-t. [DOI] [PubMed] [Google Scholar]
- CONDORELLI P., GEORGE S.C. Free nitric oxide diffusion in the bronchial microcirculation. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H2660–H2670. doi: 10.1152/ajpheart.00003.2002. [DOI] [PubMed] [Google Scholar]
- CRAWSHAW L.I., STITT J.T. Behavioral and autonomic induction of prostaglandin E1 fever in squirrel monkeys. J. Physiol. 1975;244:197–206. doi: 10.1113/jphysiol.1975.sp010791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BELDER A.J., MACALLISTER R., RADOMSKI M.W., MONCADA S., VALLANCE P.J. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc. Res. 1994;28:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- DE LUCA B., MONDA M., SULLO A. Changes in eating behavior and thermogenic activity following inhibition of nitric oxide formation. Am. J. Physiol. 1995;268:R1533–R1538. doi: 10.1152/ajpregu.1995.268.6.R1533. [DOI] [PubMed] [Google Scholar]
- DE PAULA D., STEINER A.A., BRANCO L.G.S. The nitric oxide pathway is an important modulator of stress-induced fever in rats. Physiol. Behav. 2000;70:505–511. doi: 10.1016/s0031-9384(00)00295-x. [DOI] [PubMed] [Google Scholar]
- EK M., KUROSAWA M., LUNDEBERG T., ERICSSON A. Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGALO I.T., HAC E.E., KOROLKIEWICZ K.Z., MATUSZEK M.T., SZREDER Z. Do sodium nitroprusside and L-NAME affect pyrogen fever in rabbits. Acta Physiol. Hung. 1996;84:289–290. [PubMed] [Google Scholar]
- GASTON B. Nitric oxide and thiol groups. Biochim. Biophys. Acta. 1999;1411:323–333. doi: 10.1016/s0005-2728(99)00023-7. [DOI] [PubMed] [Google Scholar]
- GAUTIER H., BONORA M., REMMERS J.E. Effects of hypoxia on metabolic rate of conscious adult cats during cold exposure. J. Appl. Physiol. 1989;67:32–38. doi: 10.1152/jappl.1989.67.1.32. [DOI] [PubMed] [Google Scholar]
- GAUTIER H., MURARIU C. Role of nitric oxide in hypoxic hypometabolism in rats. J. Appl. Physiol. 1999;87:104–110. doi: 10.1152/jappl.1999.87.1.104. [DOI] [PubMed] [Google Scholar]
- GLUCKMAN T.L., GROSSMAN J.E., FOLTS J.D., KRUSE-ELLIOTT K.T. Modulation of endotoxin-induced cardiopulmonary dysfunction by S-nitrosoalbumin. J. Endotoxin Res. 2002;8:17–26. [PubMed] [Google Scholar]
- GONZALEZ R.R., KLUGER M.J., HARDY J.D. Partitional calorimetry of the New Zealand white rabbit at temperatures 5–35°C. J. Appl. Physiol. 1971;31:728–734. doi: 10.1152/jappl.1971.31.5.728. [DOI] [PubMed] [Google Scholar]
- GOODWIN D.C., LANDINO L.M., MARNETT L.J. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxidase synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- GORDGE M.P., HOTHERSALL J.S., NEILD G.H., DUTRA N. Role of copper (I)-dependent enzyme in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1996;119:533–538. doi: 10.1111/j.1476-5381.1996.tb15704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGG N. The biochemistry and physiology of S-nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- HONMA K.-I., HIROSHIGE T. Internal synchronization among several circadian rhythms in rats under constant light. Am. J. Physiol. 1978;235:R243–R249. doi: 10.1152/ajpregu.1978.235.5.R243. [DOI] [PubMed] [Google Scholar]
- IADECOLA C., XU X., ZHANG F., HU J., EL-FAKAHANY E.E. Prolonged inhibition of brain nitric oxide synthase by short-term systemic administration of nitro-L-arginine methyl ester. Neurochem. Res. 1994;4:501–505. doi: 10.1007/BF00967330. [DOI] [PubMed] [Google Scholar]
- IVANOV A.I., KULCHITSKY V.A., ROMANOVSKY A.A. Role for the cholecystokinin-A receptor in fever: a study of a mutant rat strain and a pharmacological analysis. J. Physiol. 2003a;547:941–949. doi: 10.1113/jphysiol.2002.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOV A.I., PATEL S., KULCHITSKY V.A., ROMANOVSKY A.A. Platelet-activating factor: a previously unrecognized mediator of fever. J. Physiol. 2003b;553:221–228. doi: 10.1113/jphysiol.2003.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOV A.I., PERO R.S., SHECK A.C., ROMANOVSKY A.A. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1104–R1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- IVANOV A.I., ROMANOVSKY A.A. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R311–R316. doi: 10.1152/ajpregu.00376.2001. [DOI] [PubMed] [Google Scholar]
- JIA L, STAMLER J.S. Dual actions of S-nitrosylated derivative of vasoactive intestinal peptide as a vasoactive intestinal peptide-like mediator and a nitric oxide carrier. Eur. J. Pharmacol. 1999;366:79–86. doi: 10.1016/s0014-2999(98)00921-2. [DOI] [PubMed] [Google Scholar]
- JOURD'HEUIL D., GRAY L., GRISHAM M.B. S-nitrosothiol formation in blood of lipopolysaccharide-treated rats. Biochem. Biophys. Res. Commun. 2000a;273:22–26. doi: 10.1006/bbrc.2000.2892. [DOI] [PubMed] [Google Scholar]
- JOURD'HEUIL D., HALLEN K., FEELISCH M., GRISHAM M.B. Dynamic state of S-nitrosothiols in human plasma and whole blood. Free Radic. Biol. Med. 2000b;28:409–417. doi: 10.1016/s0891-5849(99)00257-9. [DOI] [PubMed] [Google Scholar]
- KAMERMAN P., FULLER A. Effects of nitric oxide synthase inhibitors on the febrile response to lipopolysaccharide and muramyl dipeptide in guinea pigs. Life Sci. 2000;67:2639–2645. doi: 10.1016/s0024-3205(00)00847-x. [DOI] [PubMed] [Google Scholar]
- KAMERMAN P., LABURN H.P., MITCHELL D. Inhibitors of nitric oxide synthase block cold-induced thermogenesis in rats. Can. J. Physiol. Pharmacol. 2003;81:834–838. doi: 10.1139/y03-069. [DOI] [PubMed] [Google Scholar]
- KAMERMAN P., MITCHELL D., LABURN H.P. Effects of nitric oxide synthase inhibitors on the febrile response to muramyl dipeptide and lipopolysaccharide in rats. J. Comp. Physiol. B. 2002;172:441–446. doi: 10.1007/s00360-002-0273-0. [DOI] [PubMed] [Google Scholar]
- KAPÁS L., SHIBATA M., KIMURA M., KRUEGER J.M. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am. J. Physiol. 1994;266:R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- KASHIBA M., KASAHARA E., CHIEN C.C., INOUE M. Fates and vascular action of S-nitrosoglutathione and related compounds in the circulation. Arch. Biochem. Biophys. 1999;363:213–218. doi: 10.1006/abbi.1998.1055. [DOI] [PubMed] [Google Scholar]
- KEANEY J.F., SIMON D.I., STAMLER J.S., JARAKI O., SCHARFSTEIN J., VITA J.A., LOSCALZO J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J. Clin. Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELM M., SCHRADER J. Control of coronary vascular tone by nitric oxide. Circ. Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- KIKUCHI-UTSUMI K., GAO B., OHINATA H., HASHIMOTO M., YAMAMOTO N., KUROSHIMA A. Enhanced gene expression of endothelial nitric oxide synthase in brown adipose tissue during cold exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R623–R626. doi: 10.1152/ajpregu.00310.2001. [DOI] [PubMed] [Google Scholar]
- KOZAK W., KOZAK A. Differential role of nitric oxide synthase isoforms in fever of different etiologies: studies using NOS gene-deficient mice. J. Appl. Physiol. 2003;94:2534–2544. doi: 10.1152/japplphysiol.01042.2002. [DOI] [PubMed] [Google Scholar]
- LAWRENCE A.J., KRSTEW E., JARROTT B. Complex interactions between nitric oxide and adenosine receptors in the rat isolated nodose ganglion. Eur. J. Pharmacol. 1997;328:83–88. doi: 10.1016/s0014-2999(97)83032-4. [DOI] [PubMed] [Google Scholar]
- MATHAI M.L., HJELMQVIST H., HEIL R., GERSTBERGER R. Nitric oxide increases cutaneous and respiratory heat dissipation in conscious rabbits. Am. J. Physiol. 1997;272:R1691–R1697. doi: 10.1152/ajpregu.1997.272.6.R1691. [DOI] [PubMed] [Google Scholar]
- MATSUMURA K., CAO C., OZAKI M., MORII H., NAKADATE K., WATANABE Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J. Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYHAN W.G. VEGF increases the permeability of the blood–brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am. J. Physiol. 1999;276:C1148–C1153. doi: 10.1152/ajpcell.1999.276.5.C1148. [DOI] [PubMed] [Google Scholar]
- MINAMIYAMA Y., TAKEMURA S., INOUE M. Albumin is an important vascular tonus regulator as a reservoir of nitric oxide. Biochem. Biophys. Res. Commun. 1996;225:112–115. doi: 10.1006/bbrc.1996.1138. [DOI] [PubMed] [Google Scholar]
- MINSON C.T., BERRY L.T., JOYNER M.J. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- MONROY M., KULUZ J.W., HE D., DIETRICH W.D., SCHLEIEN C.L. Role of nitric oxide in the cerebrovascular and thermoregulatory response to interleukin-1β. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1448–H1453. doi: 10.1152/ajpheart.2001.280.4.H1448. [DOI] [PubMed] [Google Scholar]
- NAGASHIMA T., OHINATA H., KUROSHIMA A. Involvement of nitric oxide in noradrenaline-induced increase in blood flow through brown adipose tissue. Life Sci. 1994;54:17–25. doi: 10.1016/0024-3205(94)00573-7. [DOI] [PubMed] [Google Scholar]
- NIIJIMA A. The afferent discharges from sensors for interleukin-1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- NOBLET J., DOURMAD J.Y., ETIENNE M., DIVIDICH J. Energy metabolism in pregnant sows and newborn pigs. J. Anim. Sci. 1997;75:2708–2714. doi: 10.2527/1997.75102708x. [DOI] [PubMed] [Google Scholar]
- PACE N., RAHLMAN D.F. Thermoneutral zone and scaling of metabolic rate on body mass of small mammals. Physiologist. 1983;26:S51–S52. [PubMed] [Google Scholar]
- PARROTT R.F., VELLUCCI S.V., LLOYD D.M. Effects of intravenous nitric oxide inhibitors on endotoxin-induced fever in prepubertal pigs. Gen. Pharmacol. 1998;31:371–376. doi: 10.1016/s0306-3623(98)00036-6. [DOI] [PubMed] [Google Scholar]
- PEROTTI C.A., NOGUEIRA M.S., ANTUNES-RODRIGUES J., CARNIO E.C. Effects of a neuronal nitric oxide synthase inhibitor on lipopolysaccharide-induced fever. Braz. J. Med. Biol. Res. 1999;32:1381–1387. doi: 10.1590/s0100-879x1999001100008. [DOI] [PubMed] [Google Scholar]
- PETERS T., Jr . All about Albumin: Biochemistry, Genetics and Medical Applications. San Diego, CA: Academic Press; 1996. [Google Scholar]
- RASSAF T., BRYAN N.S., MALONEY R.E., SPECIAN V., KELM M., KALYANARAMAN B., RODRIGUEZ J., FEELISCH M. NO adducts in mammalian red blood cells: too much or too little. Nat. Med. 2003;9:481–482. doi: 10.1038/nm0503-481. [DOI] [PubMed] [Google Scholar]
- REDFORD J., BISHAI I., COCEANI F. Pyrogen–prostaglandin coupling in the pathogenesis of fever: evidence against a role for nitric oxide. Can. J. Physiol. Pharmacol. 1995;73:1466–1474. doi: 10.1139/y95-204. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIMERS J.L., BJERRE U., MANDRUP-POULSEN T., NERUP J. Interleukin-1β induces diabetes and fever in normal rats by nitric oxide via induction of different nitric oxide synthases. Cytokine. 1994;6:512–520. doi: 10.1016/1043-4666(94)90079-5. [DOI] [PubMed] [Google Scholar]
- RIEDEL W. Antipyretic role of nitric oxide during endotoxin-induced fever in rabbits. Int. J. Tissue Reac. 1997;xix:171–178. [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., KARMAN E.K. Blood-borne, albumin-bound prostaglandin E2 may be involved in fever. Am. J. Physiol. 1999;276:R1840–R1844. doi: 10.1152/ajpregu.1999.276.6.R1840. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., SCHIMANSKY Y.P. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., SZÉKELY M. Neural route of pyrogen signaling to the brain. Clin. Infect. Dis. 2000;31:S162–S167. doi: 10.1086/317515. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SHIDO O., SAKURADA S., SUGIMOTO N., NAGASAKA T. Endotoxin shock: thermoregulatory mechanisms. Am. J. Physiol. 1996;270:R693–R703. doi: 10.1152/ajpregu.1996.270.4.R693. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SIMONS C.T., KULCHITSKY V.A. Biphasic fevers often consist of more than two phases. Am. J. Physiol. 1998;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SIMONS C.T., SZÉKELY M., KULCHITSKY V.A. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SUGIMOTO N., SIMONS C.T., HUNTER W.S. The organum vasculosum laminae terminalis (OVLT) in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R420–R428. doi: 10.1152/ajpregu.00757.2002. [DOI] [PubMed] [Google Scholar]
- ROTH J., STORR B., GOLDBACH J.-M., VOIGT K., ZEISBERGER E. Dose-dependent attenuation of lipopolysaccharide-fever by inhibitors of inducible nitric oxide-synthase in guinea pigs. Eur. J. Pharmacol. 1999;383:177–187. doi: 10.1016/s0014-2999(99)00627-5. [DOI] [PubMed] [Google Scholar]
- ROTH J., STORR B., VOIGT K., ZEISBERGER E. Inhibition of nitric oxide synthase attenuates lipopolysaccharide-induced fever without reduction of circulating cytokines in guinea pigs. Pflugers Arch. 1998a;436:858–862. doi: 10.1007/s004240050715. [DOI] [PubMed] [Google Scholar]
- ROTH J., STORR B., VOIGT K., ZEISBERGER E. Inhibition of nitric oxide synthase results in a suppression of interleukin-1β-induced fever in rats. Life Sci. 1998b;62:345–350. doi: 10.1016/s0024-3205(98)00179-9. [DOI] [PubMed] [Google Scholar]
- SAHA S.K., KUROSHIMA A. Nitric oxide and thermogenic function of brown adipose tissue in rats. Jpn. J. Physiol. 2000;50:337–342. doi: 10.2170/jjphysiol.50.337. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SIEBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCALES W.E., KLUGER M.J. Effect of antipyretic drugs on circadian rhythm in body temperature of rats. Am. J. Physiol. 1987;253:R306–R313. doi: 10.1152/ajpregu.1987.253.2.R306. [DOI] [PubMed] [Google Scholar]
- SCAMMELL T.E., ELMQUIST J.K., SAPER C.B. Inhibition of nitric oxide synthase produces hypothermia and depresses lipopolysaccharide fever. Am. J. Physiol. 1996;271:R333–R338. doi: 10.1152/ajpregu.1996.271.2.R333. [DOI] [PubMed] [Google Scholar]
- SCHARFSTEIN J.S., KEANEY J.F., JR, SLIVKA A., WELCH G.N., VITA J.A., STAMLER J.S., LOSCALZO J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J. Clin. Invest. 1994;94:1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEO S., LANDRY D.W., OLIVER J.A. Renin inhibits the vasorelaxation induced by nitroso albumin. Biochem. Biophys. Res. Commun. 1998;250:94–98. doi: 10.1006/bbrc.1998.9281. [DOI] [PubMed] [Google Scholar]
- SIMONS C.T., KULCHITSKY V.A., SUGIMOTO N., HOMER L.D., SZÉKELY M., ROMANOVSKY A.A. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis. Am. J. Physiol. 1998;275:R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- SINDRUP J.H., KASTRUP J., CHRISTENSEN H., JØRGENSEN B. Nocturnal variations in peripheral blood flow, systemic blood pressure, and heart rate in humans. Am. J. Physiol. 1991;261:H982–H988. doi: 10.1152/ajpheart.1991.261.4.H982. [DOI] [PubMed] [Google Scholar]
- SOSZYNSKI D. The inhibition of nitric oxide synthase suppresses LPS- and psychological-stress-induced fever in rats. Physiol. Behav. 2001;72:65–72. doi: 10.1016/s0031-9384(00)00375-9. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SIMUN D.I., OSBORNE J., MULLINS M.E., JARAKI O., MICHEL T., SINGEL D., LOSCALZO J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. U.S.A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINER A.A., ANTUNES-RODRIGUES J., MCCANN S.M., BRANCO L.G.S. Antipyretic role of the NO-cGMP pathway in the anteroventral preoptic region of the rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R584–R593. doi: 10.1152/ajpregu.00391.2001. [DOI] [PubMed] [Google Scholar]
- STEINER A.A., BRANCO L.G.S. Nitric oxide in the regulation of body temperature and fever. J. Therm. Biol. 2001;26:325–330. [Google Scholar]
- STEINER A.A., CARNIO E.C., ANTUNES-RODRIGUES J., BRANCO L.G.S. Role of nitric oxide in systemic vasopressin-induced hypothermia. Am. J. Physiol. 1998;275:R937–R941. doi: 10.1152/ajpregu.1998.275.4.R937. [DOI] [PubMed] [Google Scholar]
- SZABÓ C. Role of nitric oxide in endotoxin shock: an overview of recent advances. Ann. N. Y. Acad. Sci. 1998;851:422–425. doi: 10.1111/j.1749-6632.1998.tb09019.x. [DOI] [PubMed] [Google Scholar]
- SZÉKELY M., SZELÉNYI Z. Endotoxin fever in the rat. Acta Physiol. Acad. Sci. Hung. 1979;53:265–277. [PubMed] [Google Scholar]
- TITHERADGE M.A. Nitric oxide in septic shock. Biochim. Biophys. Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- TSIKAS D., SANDMANN J., LUESSEN P., SAVVA A., ROSSA S., STICHTENOTH D.O., FRÖLICH J.C. S-transnitrosylation of albumin in human plasma and blood in vitro and in vivo in the rat. Biochim. Biophys. Acta. 2001;1546:422–434. doi: 10.1016/s0167-4838(01)00166-2. [DOI] [PubMed] [Google Scholar]
- YAMAGATA K., MATSUMURA K., INOUE W., SHIRAKI T., SUZUKI K., YASUDA S., SUGIURA H., CAO C., WATANABE Y., KOBAYASHI S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J. Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]