Abstract
The prostanoid receptor(s) on human airways smooth muscle (HASM) cells that mediates the inhibitory effect of PGE2 on interleukin (IL)-1β-induced granulocyte/macrophage colony-stimulating factor (GM-CSF) release has been classified.
IL-1β evoked the release of GM-CSF from HASM cells, which was suppressed by PGE2, 16,16-dimethyl PGE2 (nonselective), misoprostol (EP2/EP3-selective), ONO-AE1-259 and butaprost (both EP2-selective) with pIC50 values of 8.61, 7.13, 5.64, 8.79 and 5.43, respectively. EP-receptor agonists that have selectivity for the EP1- (17-phenyl-ω-trinor PGE2) and EP3-receptor (sulprostone) subtypes as well as cicaprost (IP-selective), PGD2, PGF2α and U-46619 (TP-selective) were poorly active or inactive at concentrations up to 10 μM.
AH 6809, a drug that can be used to selectively block EP2-receptors in HASM cells, antagonised the inhibitory effect of PGE2, 16,16-dimethyl PGE2 and ONO-AE1-259 with apparent pA2 values of 5.85, 6.09 and 6.1 respectively. In contrast, the EP4-receptor antagonists, AH 23848B and L-161,982, failed to displace to the right the concentration–response curves that described the inhibition of GM-CSF release evoked by PGE2 and ONO-AE1-259.
Inhibition of GM-CSF release by PGE2 and 8-Br-cAMP was abolished in cells infected with an adenovirus vector encoding an inhibitor protein of cAMP-dependent protein kinase (PKA) but not by H-89, a purported small molecule inhibitor of PKA.
We conclude that prostanoid receptors of the EP2-subtype mediate the inhibitory effect of PGE2 on GM-CSF release from HASM cells by recruiting a PKA-dependent pathway. In addition, the data illustrate that caution should be exercised when using H-89 in studies designed to assess the role of PKA in biological processes.
Keywords: Gene expression, prostanoid receptors, GM-CSF, ONO-AE1-259, PGE2, human airways smooth muscle, EP2-receptors, PKA inhibitor – cAMP-dependent protein kinase inhibitor (PKIα), PKA inhibitor – H-89, Adenovirus vector – Ad5.CMV.PKIα
Introduction
Colony-stimulating factors (CSFs) such as granulocyte/macrophage colony-stimulating factor (GM-CSF) are responsible for the proliferation and differentiation of cells in the bone marrow (Metcalf, 1985; 1986). These cytokines also modulate the function of mature leukocytes, including eosinophils and neutrophils, promoting their activation and survival (Lopez et al., 1986; Giembycz & Lindsay, 1999). It is believed that by increasing the longevity of proinflammatory cells in tissues, CSFs may perpetuate airway inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD) where eosinophils and neutrophils play a pathogenic role. It is now appreciated that human airways smooth muscle (HASM) cells have a substantial synthetic capacity and can contribute to inflammatory processes through the generation of a plethora of mediators including cytokines, chemokines and bioactive lipids (Johnson & Knox, 1997). Of relevance to the present study is the finding that HASM cells generate GM-CSF (Saunders et al., 1997; Lazzeri et al., 2001) that can be suppressed by endogenously synthesised or exogenously applied prostaglandin (PG) E2 (Clarke et al., 2001; Lazzeri et al., 2001). The possibility exists, therefore, that agonism of specific prostaglandin receptors on HASM cells could provide an effective means of suppressing CSF production in diseases such as asthma and COPD and reduce, respectively, the eosinophil and neutrophil burden in the airways.
Five main classes of G-protein-coupled receptor for the naturally occurring prostanoid agonists have been defined and given the prefix DP, EP, FP, IP and TP (Coleman et al., 1994b; Breyer et al., 2001; Tsuboi et al., 2002) where the first letter refers to the natural ligand most selective for that receptor. Molecular biological techniques subsequently confirmed this pharmacological classification with the cloning and expression of cDNAs for representatives of the five prostanoid receptors in a number of species including humans (Coleman et al., 1994b; Breyer et al., 2001; Tsuboi et al., 2002). The finding that PGE2 potently inhibits the release of GM-CSF from HASM cells in response to IL (interleukin)-1β (Clarke et al., 2001; Lazzeri et al., 2001) suggests that this response is mediated by one or more prostanoid receptors of the EP-subtype. Currently, pharmacological evidence and primary sequence information of partial and full-length cDNA clones indicates the presence of at least four EP-receptor variants denoted EP1, EP2, EP3 and EP4 (Coleman et al., 1994b; Narumiya et al., 1999; Breyer et al., 2001). These receptors can couple to several effector molecules (Breyer et al., 2001), thereby mediating a diverse array of biological responses (Narumiya et al., 1999; Kobayashi & Narumiya, 2002; Tsuboi et al., 2002) that can now be reasonably well defined with agonists and antagonists that can discriminate between EP-receptor subtypes.
The objective of the present study was to characterise the prostanoid receptor(s) through which PGE2 inhibits GM-CSF release from IL-1β-stimulated HASM cells and probe the molecular basis of this effect. To this end, naturally occurring and synthetic prostanoid agonists and antagonists that have selectivity for the EP-receptor subtypes were used. In addition, the role of the cAMP/cAMP-dependent protein kinase (PKA) cascade was assessed using an adenovirus (Ad5) vector encoding the α-isoform of PKA inhibitor protein (PKIα), which is potent, extremely selective for PKA (Olsen & Uhler, 1991; Scarpetta & Uhler, 1993; Collins & Uhler, 1997) and devoid of the problems associated with many small molecule protein kinase inhibitors (Engh et al., 1996; Davies et al., 2000).
Methods
Isolation of HASM cells
Tracheal rings from either lung or heart and lung transplantation donors (7 female, 17 male, age range: 17–57 years; median age 36.5 years) were dissected under sterile conditions in Hanks' balanced salt solution (HBSS; in mM: NaCl 136.8, KCl 5.4, MgSO4 0.8, Na2HPO4·7H2O 0.4, CaCl2·2H2O 1.3, NaHCO3 4.2 and glucose 5.6) supplemented with penicillin (100 U m1−1), streptomycin (100 μg ml−1) and amphotericin B (2.5 μg ml−1). The smooth muscle layer was dissected free of adherent connective tissue and cartilage, and the epithelium was removed using a rounded scalpel blade. The smooth muscle was incubated (30 min; 37°C; 5% CO2/air) in HBSS containing BSA (10 mg ml−1), collagenase (type XI, 1 mg ml−1) and elastase (type I, 3.3 U ml−1). After the removal of any remaining connective tissue, the smooth muscle was chopped finely and incubated for a further 150 min in the enzyme solution described above with the elastase concentration increased to 15 U/ml. Dissociated cells were centrifuged (100 × g, 5 min, 4°C) and resuspended in Dulbecco's-modified Eagle's medium (DMEM) containing heat-inactivated foetal calf serum (FCS; 10% v v−1), sodium pyruvate (1 mM), L-glutamate (2 mM), nonessential amino acids (1 ×) and the antimicrobial agents detailed above.
Primary culture of HASM cells
The HASM cell suspension was placed in a tissue culture flask (75 cm2) containing 6 ml supplemented DMEM and allowed to adhere (∼12 h) at 37°C in 5% CO2/air. The culture medium was replaced after 4–5 days (12 ml) and thereafter every 3–4 days. When the cells reached confluence (∼10–14 days) and demonstrated a typical ‘hill and valley' appearance and positive immunostaining for α-actin (routinely >95%, Belvisi et al., 1997), they were seeded onto either 96-well plates (Costar UK Ltd., High Wycombe) at an initial density of 2000 cells per well or six well plates (Costar) at an initial density of 20,000 cell per well for cytokine release and Western blotting experiments, respectively. At subconfluence, the cells were growth arrested by being placed in DMEM containing apotransferin (5 μg ml−1), insulin (1 μM), ascorbate (100 μM) and BSA (0.1% w v−1) for 24 h. The medium was replaced with DMEM containing 3% FCSv v−1 and drugs or appropriate vehicles as described below.
Infection of HASM cells with Ad5.CMV.PKIα
In some experiments, subconfluent, growth-arrested HASM cells were infected (MOI=100) with an E1−/E3− replication-deficient Ad5 vector (Ad5.CMV.PKIα) containing a 251 bp DNA fragment encoding the complete amino-acid sequence of PKIα (Day et al., 1989; Meja et al., 2004), downstream of the constitutively active CMV immediately early promoter (Gomez-Foix et al., 1992; Lum et al., 1999). After 48 h, cells were processed for GM-CSF release and Western blotting as described below. To control for possible biological effects of the virus per se, the vector, Ad5.CMV.Null, expressing no transgene, was used in parallel. Preliminary experiments using immunofluorescence microscopy established that >95% of cells expressed PKIα 48 h after infection. This was determined by enumerating the number of HASM cells expressing the transgene as a percentage of total number of cells that counterstained with the nuclear marker, 4′,6-diamidino-2-phenylindole. Ad5.CMV.PKIα and Ad5.CMV.Null at an MOI of 100 had no effect on HASM cell viability 48 h after infection (data not shown).
Measurement of GM-CSF
HASM cells (naïve and virus-treated) were pretreated for 30 min with indomethacin (10 μM) and, where indicated, receptor antagonist before being exposed for a further 5 min or 30 min to prostanoid agonists or 8-Br-cAMP, respectively. IL-1β (100 pg ml−1) was then added and the cells were incubated at 37°C in a thermostatically controlled incubator under a 5% CO2 atmosphere. At 24 h, the amount of GM-CSF released into the culture supernatant was quantified by a sandwich ELISA (human DuoSet® development system, R&D Systems Europe, Abingdon) according to the manufacturer's instructions. The detection limit of this assay is 7.8 pg ml−1.
Western blot analysis
HASM cells were treated with 3% FCS for 24 h. The medium was removed and the cells washed with HBSS, lysed and proteins extracted in 20 mM Tris HCl – pH 7.4, 100 mM NaCl, 1 mM EDTA, 0.1% (v v−1) Nonidet P-40, 0.05% (w v−1) sodium deoxycholate, 0.025% (w v−1) SDS and 0.1% (v v−1) Triton X-100 supplemented with PMSF (0.1 mg ml−1), leupeptin (10 μg ml−1) and aprotinin (25 μg ml−1). Insoluble protein was removed by centrifugation (10,000 × g; 3 min) and aliquots of the resulting supernatant were diluted 1 : 4 in Laemmeli buffer (62.5 mM Tris-HCl – pH 6.8, 10% (v v−1) glycerol, 1% (w v−1) SDS, 1% (v v−1) β-mercaptoethanol, 0.01% (v v−1) bromophenol blue) and boiled for 5 min. Denatured proteins (25 μg) were separated by SDS–PAGE using a 4–20% gradient gel (BioRad; Hemel Hempstead) and transferred to Hybond enhanced chemiluminescence (ECL) membranes (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire) in Tris buffer (Tris base 50 mM – pH 8.3, glycine 192 mM, 20% v v−1 methanol). The nitrocellulose was incubated overnight in Tris-buffered saline plus Tween-20 (TBS-T) (25 mM Tris-base – pH 7.4, 150 mM NaCl, 0.1% v v−1 Tween 20) containing 5% (w v−1) non-fat dry milk. The filters were incubated for 1 h at room temperature in TBS-T containing 5% non-fat dry milk and an anti-human PKIα, pCREB or β-actin polyclonal antibody (diluted 1 : 500, 1 : 1000 and 1 : 2000, respectively) as indicated. Membranes were washed with TBS-T and incubated with horseradish peroxide-conjugated sheep, anti-rabbit IgG (diluted 1 : 4000) in TBS-T/5% nonfat dry milk for 1 h at room temperature. The nitrocellulose was washed again in TBS-T and developed using ECL™ western blotting detection reagents on Kodak X-OMAT-S film.
Cell viability
At the end of each experiment, cell viability was determined colorimetrically by measuring the reduction of the tetrazolium salt, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), to formazan by mitochondrial dehydrogenases (Mosmann, 1983; Hussain et al., 1993).
Drugs, antibodies and analytical reagents
IL-1β was from R&D systems (Abingdon, Oxon, U.K.), DMEM and HBSS were from Flow Laboratories (Rickmansworth, Hertfordshire, U.K.) and nonessential amino acids were purchased from Life Technologies (Paisley, U.K.). Indomethacin, FCS, MTT, PGD2, PGE2, PGF2α, U-46619 (9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α) and H-89 were from Sigma-Aldrich (Poole, Dorset, U.K.). 16,16-Dimethyl PGE2, 17-phenyl-ω-trinor PGE2, AH 6809 (6-isopropoxy-9-oxoxanthine-2-carboxylic acid), SQ 29,548 (1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenylamino) carbonyl]hydrazino]methyl]-7-oxa bicyclo[2.2.1]hept-2-y1]-5-heptenoic acid), AH 23848B ([1α(Z),2β,5α]-(±)-7-[5-[[(1,1′-biphenyl]-4-ylmethoxy)-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptanoic acid), sulprostone, misoprostol methyl ester and butaprost methyl ester were obtained from Cayman Chemicals (Ann Arbor, MI, U.S.A.). All other synthetic prostanoid reagents were donated by the following: cicaprost (Schering AG, Berlin, Germany); ONO-AE1-259 ((16S)-9-deoxy-9β-chloro-15-deoxy-16-hydroxy-17,17-trimethylene-19,20-didehydro-PGE2 – sodium salt; Ono Pharmaceuticals, Osaka, Japan) and L-161,982 ([4′-[3-butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5-dihydro-[1,2,4]triazol-4-ylmethyl]-biphenyl-2-sulphonic acid (3-methyl-thiophene-2-carbonyl)-amide]; Merck Frosst, Montreal, Canada). Goat anti-human PKIα (code sc#1944) and goat anti-human β-actin (code sc# 1615) were from Autogen Bioclear (Calne, Wiltshire, U.K.). pCREB (code 9191S) was purchased from New England Biolabs (Hitchin, Hertfordshire, U.K.).
Data and statistical analyses
Data points, and values in the text and figure legends, represent the mean±s.e.m. of ‘n' independent determinations using tissue from different donors. The concentration–response curves were analysed by least-squares, nonlinear iterative regression with the ‘PRISM' curve fitting program (GraphPad software, San Diego, U.S.A.) and pEC50 and pIC50 values were subsequently interpolated from curves of best-fit. Equieffective molar concentration ratios (e.c.r.) were calculated using the formula: IC50 PGE analogue/IC50 PGE2.
Antagonist affinity was calculated using the equation pKB=log (CR-1) – log [B] (Schild, 1949), where CR is the concentration ratio calculated from the EC50 of agonist in the presence of the antagonist divided by the EC50 of the agonist alone, KB is the equilibrium dissociation constant and [B] is the concentration of antagonist. In the experiments described herein, the term apparent pA2 is substituted for pKB as antagonists were used at one concentration only, which precludes assumptions being made about the nature of the antagonism.
Where appropriate, data were analysed statistically using Student's paired t-test or by one-way ANOVA/Newman–Keuls multiple comparison test. The null hypothesis was rejected when P<0.05.
Results
We have reported previously that IL-1β promotes a time- and concentration-dependent release of GM-CSF from HASM cells with a t1/2 and EC50 of >18 h and 16 pg ml−1, respectively (Clarke et al., 2001). In the experiments described herein, IL-1β was used at 100 pg ml−1 (∼EC90) and GM-CSF was measured in the culture supernatant 24 h after addition of the stimulus. None of the compounds or their vehicles used in these experiments affected cell viability as determined by the reduction of MTT to formazan. None of the vehicles used had any significant effect on GM-CSF release (data not shown).
Effect of naturally occurring prostaglandins, cicaprost and U-46619 on GM-CSF release
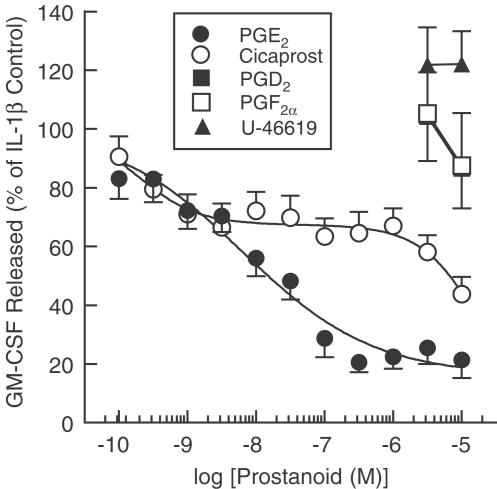
PGD2, PGE2, PGF2α, U-46619 and cicaprost had no effect on GM-CSF release from indomethacin (10 μM; 30 min)-pretreated HASM cells. However, the GM-CSF elaborated in response to IL-1β was suppressed by PGE2 in a concentration-dependent manner with a maximal effect and pIC50 of 79% and 8.61, respectively (Figure 1, Table 1 ). Cicaprost was also active but the concentration–response curves that described the suppression of GM-CSF were complex and better described by a two-site (mean r2=0.956) rather than single sigmoidal function (mean r2=0.765; Figure 1). Analysing the data in this way yielded pIC50 values for the high- and low-affinity components of 9.45, which accounted for 34.1±5.5% of the effect, and 4.69 (estimate), respectively. PGD2, PGF2α and U-46619 had no significant effect on GM-CSF release (Figure 1, Table 1).
Figure 1.
Effect of prostanoids on IL-1β-induced GM-CSF release. Adherent HASM cells were pretreated for 5 min with varying concentrations of PGD2, PGE2, PGF2α, cicaprost and U-46619 before being exposed to IL-1β (100 pg ml−1). Cells were maintained at 37°C in a thermostatically controlled incubator under a 5% CO2 atmosphere and the amount of GM-CSF released into the culture supernatant was quantified at 24 h by a sandwich ELISA. Each data point represents the mean±s.e.m. of four to 13 determinations (see Table 1) using tissue from different donors. Indomethacin (10 μM) was present through the experiment. Note that the data for PGD2 and PGF2α are superimposed. See Methods for further details.
Table 1.
Potency of prostanoid and EP/IP-selective agonists at suppressing GM-CSF generation
| Prostanoid | n | Receptor selectivitya | Inhibition of GM-CSF release pIC50 | e.c.r. PGE2=1 |
|---|---|---|---|---|
| PGE2 | 13 | EP1≈EP2 ≈EP3≈EP4 | 8.61±0.43 (78.6±6.2) | 1 |
| ONO-AE1-259 | 8 | EP2>>>EP1=EP3=EP4 | 8.79±0.26 (8.79±0.3) | 0.7 |
| 16,16-dimethyl PGE2 | 7 | EP2⩾EP3=EP1>EP4 | 7.13±0.37 (83.0±9.2) | 30 |
| Misoprostol | 5 | EP2=EP3>EP1>EP4 | 5.64±0.25 (64.7±6.9) | 935 |
| Butaprost | 4 | EP2>>EP1>EP3>EP4 | 5.43±0.26 (56.1±10.9) | 1518 |
| Cicaprostb | 11 | IP>EP4 | 9.45±0.26 | 0.14 |
| >5 | >4081 | |||
| PGF2α | 4 | FP | >5 (12.5±17.9%) | >4081 |
| PGD2 | 4 | DP | >5 (13.4±13.6%) | >4081 |
| U-46619 | 9 | TP | >5 (−22.1±11.2%) | >4081 |
| 17-phenyl-ω-trinor PGE2 | 4 | EP1>EP3>EP2>EP4 | >5 (9.7±11.8%) | >4081 |
| Sulprostone | 4 | EP3>EP1>>EP2>EP4 | >5 (−22.7±8.6%) | >4081 |
Values in parentheses show the percentage inhibition of GM-CSF release evoked by 10 μM prostanoid agonist. Equieffective concentration ratios >1 and <1 indicate that the agonist in question is less and more potent than PGE2, respectively.
Selectivity derived from studies in isolated cells and tissues.
Concentration–response curve described by a two site sigmoidal function.
Effect of EP-selective prostanoid agonists on GM-CSF release
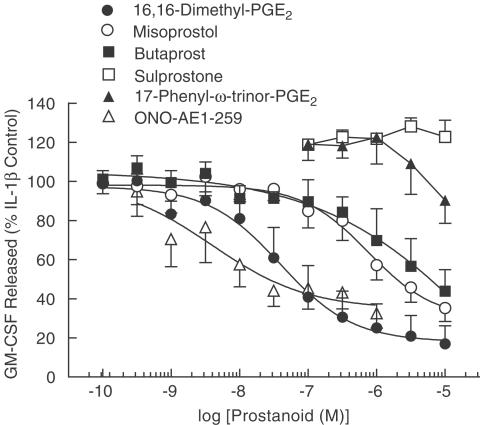
To gain information on the possible role of an EP-receptor subtype in mediating the inhibitory effect of PGE2 on GM-CSF release, a variety of PGE analogues, which differ in their selectivity for the EP1-, EP2-, EP3- and EP4-receptor subtypes, were examined (Figure 2, Table 1). By themselves none of the ligands had any effect on GM-CSF release. However, in IL-1β-stimulated HASM cells, 16,16-dimethyl PGE2, ONO-AE1-259, misoprostol and butaprost suppressed the release of GM-CSF in a concentration-dependent manner, with a maximum inhibition of 83, 68, 65 and 56%, respectively, at the highest concentration (10 μM) of drug examined (Figure 2). The rank order of agonist potency was ONO-AE1-259 >16,16-dimethyl PGE2 > misoprostol > butaprost. 17-Phenyl-ω-trinor PGE2 suppressed GM-CSF output at concentrations above 1 μM whereas sulprostone was inactive at all concentrations examined (Figure 2, Table 1).
Figure 2.
Effect of EP-selective prostanoid agonists on IL-1β-induced GM-CSF release. Adherent HASM cells were pretreated for 5 min with varying concentrations of six synthetic PGE analogues before being exposed to IL-1β (100 pg ml−1). Cells were maintained at 37°C in a thermostatically controlled incubator under a 5% CO2 atmosphere and the amount of GM-CSF released into the culture supernatant was quantified at 24 h by a sandwich ELISA. Each data point represents the mean±s.e.m. of four to 13 determinations (see Table 1) using tissue from different donors. Indomethacin (10 μM) was present through the experiment. See Methods for further details.
Effect of an EP2-receptor antagonist on the inhibition of GM-CSF release evoked by PGE2, 16,16-dimethyl PGE2 and ONO-AE1-259
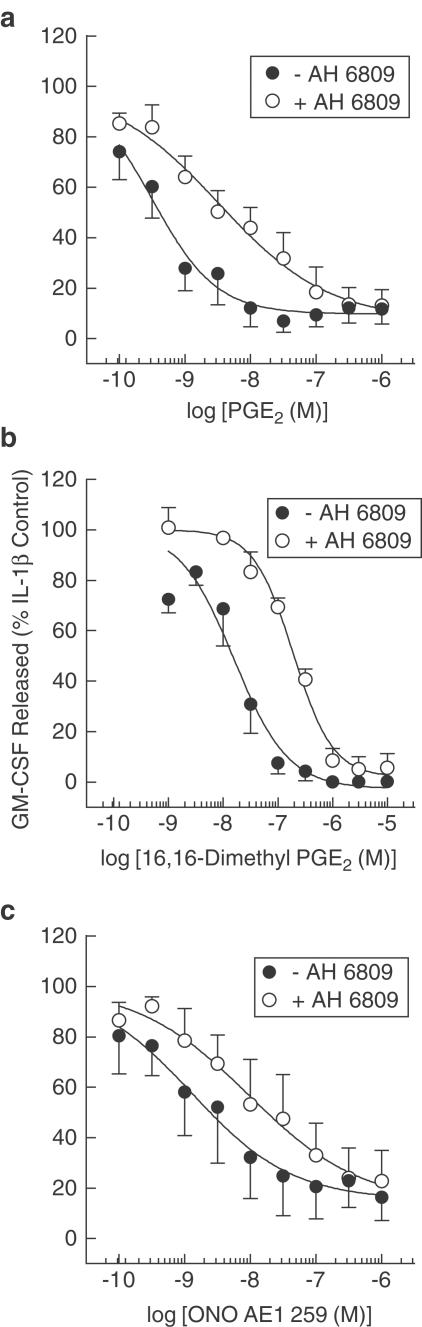
AH 6809 is an antagonist at the human EP1-, EP2- and DP-receptor subtypes (Coleman et al., 1987; Keery & Lumley, 1988). As 17-phenyl-ω-trinor PGE2 (EP1-selective) and PGD2 were inactive in this system (Table 1, Figure 2), AH 6809 was used to determine if EP2 receptors mediated the inhibitory effects of PGE2, 16,16-dimethyl PGE2 and ONO-AE1-259 on GM-CSF release. Pretreatment of HASM cells with AH 6809 (10 μM) produced a rightwards shift of the concentration–response curve that described the inhibition of GM-CSF release by PGE2, 16,16-dimethyl PGE2 and ONO-AE1-259 from which apparent pA2 values of 5.85±0.31, 6.09±0.22 and 6.1±0.7 were derived, respectively (Figure 3). These affinity estimates were not statistically significant from one another (P>0.05; one-way ANOVA).
Figure 3.
Effect of AH 6809 on the inhibition of IL-1β-induced GM-CSF evoked by PGE2, 16,16-dimethyl PGE2 and ONO-AE1-259. Adherent HASM cells were pretreated (30 min) concurrently with indomethacin and AH 6809 (both 10 μM) and then for a further 5 min with PGE2 (a), 16,16-dimethyl (b) or ONO-AE1-259 (c). IL-1β (100 pg ml−1) was then added to the cells and the GM-CSF released into the culture medium was quantified at 24 h by a sandwich ELISA. Each data point represents the mean±s.e.m. of three to five determinations using tissue from different donors.
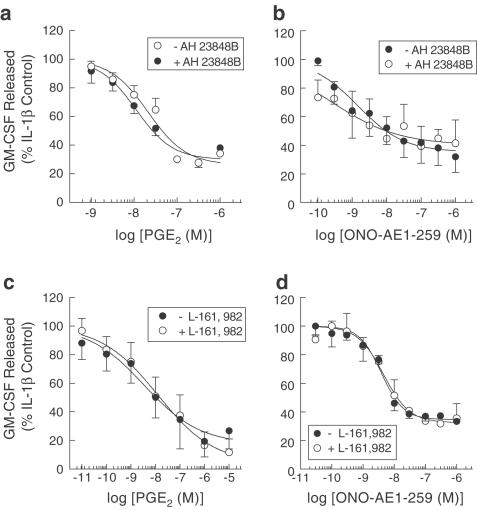
Effect of EP4-receptor antagonists on the inhibition of GM-CSF release evoked by PGE2 and ONO-AE1-259
To evaluate the role of EP4-receptors in mediating suppression of GM-CSF release, the antagonists AH 23848B (Coleman et al., 1994a) and L-161,982 (Machwate et al., 2001) were employed using PGE2 and ONO-AE1-259 as agonists. In the presence of AH 23848B or L-161,982, at concentrations ∼10 and ∼100 times higher than their affinity at EP4-receptors, respectively, the inhibitory effect of PGE2 and ONO-AE1-259 was not significantly altered in terms of IC50 or maximal inhibitory response (Figure 4).
Figure 4.
Effect of AH 23848B and L-161,982 on the inhibition of IL-1β-induced GM-CSF release evoked by PGE2 and ONO-AE1-259. Adherent HASM cells were pretreated (30 min) concurrently with indomethacin (10 μM) and either AH 23848B (30 μM) or L-161,982 (2 μM) and then for a further 5 min with PGE2 (a and b) or ONO-AE1-259 (c and d). IL-1β (100 pg ml−1) was then added to the cells and the GM-CSF released in to the culture medium was quantified at 24 h by a sandwich ELISA. Each data point represents the mean±s.e.m. of three to five determinations using tissue from different donors.
Effect of 8-Br-cAMP on IL-1β-induced GM-CSF release
Pretreatment of HASM cells with 8-Br-cAMP inhibited the release of GM-CSF from IL-1β (100 pg/ml)-stimulated HASM cells with a pIC50 of 3.76±0.14 (n=3). At the highest concentration of drug tested (1 mM), the elaboration of GM-CSF was suppressed by 96.2±3.1% (n=3).
Role of PKA in the action of PGE2 on GM-CSF release
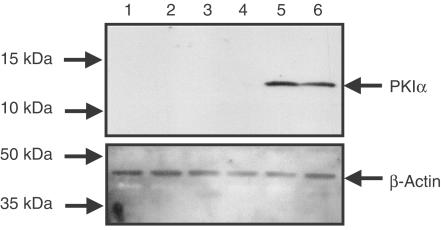
To determine the role of PKA in mediating the effect of PGE2 on GM-CSF release from HASM cells H-89, a purported selective inhibitor of PKA (Chijiwa et al., 1990), and a virus vector, Ad5.CMV.PKIα (Lum et al., 1999; Meja et al., 2004), containing DNA encoding the complete amino-acid sequence of PKIα, an endogenous, potent and highly selective inhibitor of PKA (Glass et al., 1986; Olsen & Uhler, 1991) were employed. In the virus studies, western blotting was used to confirm expression of the PKIα transgene. In uninfected cells, PKIα was not detected in any experiment. However, 48 h after infection with Ad5.CMV.PKIα (MOI=100), a single peptide was labelled by the anti-PKIα antibody that migrated as a 12 kDa band on SDS polyacrylamide gels (Figure 5). In preliminary studies, the efficiency of transgene expression at 48 h was found to be >95% (see Methods for details).
Figure 5.
Expression of PKIα in HASM cells infected with Ad5.CMV.PKIα. Adherent cells were cultured until 50% confluent and then infected with Ad5.CMV.Null, Ad5.CMV.PKIα (MOI=100) or left untreated (naïve) for 48 h at 37°C. Cells were growth arrested in serum-free medium and processed by western blotting for PKIα expression (a) and the house-keeping protein, β-actin (b). Data are representative of three independent determinations using tissue from different donors. See Methods for further details. (Key: lanes 1 & 2, Naïve; lanes 3 & 4, Ad5.CMV.Null; lanes 5 & 6, Ad5.CMV.PKIα).
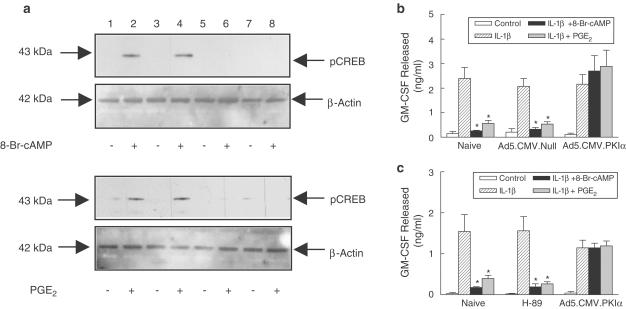
HASM cells exposed to IL-1β elaborated GM-CSF in an amount that was not significantly altered by H-89 (10 μM; 30 min) or following infection with Ad5.CMV.PKIα or Ad5.CMV.Null (MOI=100; 48 h) (Figure 6a,b). PGE2 (10 μM) and 8-Br-cAMP (1 mM), which was used as a positive control, suppressed IL-1β-stimulated GM-CSF release by a mechanism that was prevented in cells expressing the PKIα transgene but not those infected with the empty virus or pretreated with H-89 (Figure 6a,b). Paradoxically, the phosphorylation by 8-Br-cAMP and PGE2 of CREB, a well established substrate for PKA, was abolished in HASM cells infected with Ad5.CMV.PKIα and H-89 confirming that PKA was inhibited by both interventions (Figure 6c).
Figure 6.
Effect of H-89 and PKIα on 8-Br-cAMP- and PGE2-induced GM-CSF release from and CREB phosphorylation in HASM cells. Adherent cells were pretreated with H-89 (10 μM; 30 min) or infected with Ad5.CMV.Null or Ad5.CMV.PKIα (MOI=100; 48 h). 8-Br-cAMP (1 mM) or PGE2 (10 μM) were then added for 30 min. At this point, cells were either processed immediately for CREB phosphorylation by western blotting (a) or exposed to IL-1β (100 pg ml−1) for 24 h to promote GM-CSF release, which was measured by ELISA (b and c). Each bar represents the mean±s.e.m. of four independent determinations using tissue from different donors. Indomethacin (10 μM) was present throughout the experiment. See Methods for further details. *P<0.05, significant inhibition of IL-1β-stimulated GM-CSF release. (Key: lanes 1 & 2, Naïve; lanes 3 & 4, Ad5.CMV.Null; lanes 5 & 6, Ad5.CMV.PKIα, lanes 7 & 8, H-89).
Discussion
We have reported previously that endogenously synthesised and exogenously applied PGE2 can negatively regulate the elaboration of GM-CSF from HASM cells elicited by the proinflammatory cytokine, IL-1β (Clarke et al., 2001; Lazzeri et al., 2001). The experiments described in the present study were designed to further this work by characterising the prostanoid receptor(s) and second messenger pathway through which PGE2 mediates this inhibitory effect. As PGE2 was the most potent prostanoid at suppressing GM-CSF release from HASM cells, we reasoned that this might reflect the activation of an EP-receptor subtype. This conclusion was supported by the additional findings that cicaprost, an IP-receptor agonist, was a weak inhibitor of GM-CSF release and U-46619 (TP-agonist), PGD2 and PGF2α were inactive.
To identify the inhibitory EP-receptor(s) on HASM cells, a pharmacological analysis was performed by examining a panel of agonists and antagonists that have varying degrees of selectivity for the EP-receptor subtypes. The finding that 17-phenyl-ω-trinor PGE2 was greater than three orders of magnitude less active than PGE2 in this system provides persuasive evidence that the elaboration of GM-CSF from HASM cells is not mediated by receptors of the EP1-subtype (Lawrence et al., 1992).
EP2- and EP4-receptors can couple to adenylyl cyclase via Gs and enhance cAMP biosynthesis (Coleman et al., 1994a; Breyer et al., 2001). As 8-Br-cAMP was found to suppress GM-CSF release from HASM cells, it was considered likely that the EP2- and/or EP4-receptor might mediate the inhibitory effect of PGE2 and related ligands in this system. Although agonism of certain splice variants of the human EP3-receptor (e.g. EP3B, EP3C) can also couple positively to adenylyl cyclase (Namba et al., 1993), the failure of sulprostone to suppress GM-CSF release suggests that the EP3-subtype does not regulate the expression of the CSF2 gene in HASM cells. As illustrated in Figure 3, PGE2, 16,16-dimethyl PGE2, butaprost, misoprostol and ONO-AE1-259 effectively reduced the elaboration of GM-CSF. Evidence that these ligands were acting through EP2-receptors was derived primarily from two pieces of data. First, the agonist activity of butaprost and ONO-AE1-259 reported in the present study is considered diagnostic of EP2-receptors (Boie et al., 1997; Kiriyama et al., 1997; Suzawa et al., 2000). Second, AH 6809 antagonised the inhibitory effect of PGE2 and related ligands with an affinity consistent with EP2-receptor-mediated events in other tissues including human myometrium (pA2=5.85, (Hillock & Crankshaw, 1999) and the human recombinant EP2-subtype expressed in Chinese hamster ovary (CHO) cells (pA2=6.5 (Woodward et al., 1995). It is noteworthy that AH 6809 is also an antagonist at the EP1- and DP-receptor (Coleman et al., 1987; Keery & Lumley, 1988; Woodward et al., 1995). However, as neither 17-phenyl-ω-trinor PGE2 nor PGD2 inhibited GM-CSF release, AH 6809 was used as a selective antagonist at the human EP2-receptor. Despite antagonist affinity estimates implicating EP2-receptors in mediating the inhibition of GM-CSF release, several observations were not entirely consistent with this conclusion. For example, a prediction based on data obtained with established EP2-receptor-containing tissues such as the rabbit ear artery and cat trachea is that butaprost should be 10- to 100-times less potent than PGE2 (Gardiner, 1986; Coleman et al., 1988; Nials et al., 1993; Lydford et al., 1996). However, in HASM cells, butaprost was considerably weaker than predicted with an e.c.r. of 1518 relative to PGE2. Similarly, while misoprostol effectively reduced GM-CSF release, it was 935-times less potent than PGE2 unlike its activity on the rabbit ear artery and the cat trachea where it is equieffective with the natural ligand (Coleman et al., 1988; Lydford et al., 1996). Consequently, the rank order of agonist potency for the suppression of GM-CSF output from HASM cells was markedly different from other EP2-receptor-containing tissues. However, rather than invoke the involvement of multiple or a novel EP-receptor subtype(s), we suggest that a likely cause of these discrepancies is our use of the methyl esters of butaprost and misoprostol, which are considerably less potent (typically 10–300-times) than their respective free acids (Tsai et al., 1991; Abramovitz et al., 2000; Tani et al., 2001). For example, butaprost free acid has an affinity (Ki∼80 nM) for the human and murine recombinant EP2-receptor subtype that is 39- and 32-fold higher than butaprost methyl ester (Ki∼2.9 μM) respectively (Abramovitz et al., 2000; Tani et al., 2001). A more dramatic difference is seen with misoprostol where the free acid is >300-times more potent (Ki=34 nM) than the methyl ester (Ki=10.3 μM) (Abramovitz et al., 2000). If this interpretation is correct, then the present study highlights a fundamental difference between HASM cells and other EP2-receptor-expressing preparations where the methyl esters of misoprostol and butaprost have the expected e.c.r. relative to PGE2. Mechanistically, this would imply that unlike many tissues HASM cells lack appreciable esterase activity and cannot convert esterified compounds such as misoprostol and butaprost to their free acids. Thus, these apparent anomalies not withstanding, the data obtained in the present study provide good evidence that PGE2 suppresses GM-CSF release by interacting with prostanoid receptors of the EP2-subtype. This conclusion is consistent with the expression by cultured HASM cells of mRNA and protein for the EP2-receptor subtype (Clarke, D.L., Belvisi, M.G., Smith, S.J., Yacoub, M.H., Meja, K.K., Newton, R., Slater, D.M., Giembycz, M.A. – manuscript under review).
In other human tissues including synovial fibroblasts (Inoue et al., 2002), monocyte-derived macrophages (Takayama et al., 2002) and peripheral blood mononuclear cells (Takahashi et al., 2002), PGE2 has been shown to regulate cytokine, chemokine and adhesion molecule expression by acting through EP4-receptors. The failure of two EP4-receptor antagonists, AH 23848B and L-161,982 (Coleman et al., 1994a; Machwate et al., 2001), to displace to the right the concentration-response curves that described the inhibition of GM-CSF release by PGE2 and ONO-AE1-259 at a concentration ∼10 and 100 times greater than their affinity at EP4-receptors, respectively, indicates that this is not the case in HASM cells. This conclusion is further supported by the finding that PGE2 did not suppress the elaboration of GM-CSF in the subnanomolar concentration range, which is typical of many other EP4-receptor-expressing preparations (Coleman et al., 1994b).
Agonism of EP2-receptors evokes responses that are thought to rely exclusively on the activation of the cAMP/PKA cascade (Tsuboi et al., 2002). However, in many biological systems, compelling evidence implicating this signalling pathway is difficult to gain as many compounds marketed as PKA inhibitors, such as H-89, are isoquinolinesulphonyl derivatives, which are remarkably nonselective (Davies et al., 2000), presumably because they block a conserved ATP-binding site found among many protein kinases (Engh et al., 1996). The reported limitations in H-89 prompted us to adopt an additional approach to establish the role of PKA in the inhibition, by PGE2 and 8-Br-cAMP, of IL-1β-induced GM-CSF release. Thus, HASM cells were infected with an adenovirus vector encoding the complete amino-acid sequence of PKIα, one of three endogenous, potent (Ki∼50–100 pM) and highly selective inhibitors of PKA (Olsen & Uhler, 1991; Scarpetta & Uhler, 1993; Collins & Uhler, 1997; Meja et al., 2004). Indeed, PKIα, at a concentration ∼106 times higher than its affinity for PKA, does not inhibit the highly homologous cGMP-dependent protein kinase (Glass et al., 1986) to which it is most closely related (Takio et al., 1984). As shown in Figure 6, CREB phosphorylation and the inhibition of GM-CSF release evoked by PGE2 and 8-Br-cAMP was abolished in HASM cells expressing PKIα, indicating that the CSF2 gene is negatively regulated, directly or indirectly, by PKA. H-89 also prevented PGE2- and 8-Br-cAMP-induced CREB phosphorylation under identical experimental conditions but, in contrast to PKIα-expressing cells, failed to block the reduction in GM-CSF output. These results agree with data published by Takayama et al. (2002), who found that H-89 abolished CREB phosphorylation in human monocyte-derived macrophages evoked by PGE2 and 8-Br-cAMP but not the inhibition of MIP-1β release. Similarly, the ability of 8-Br-cAMP and β2-adrenoceptor agonists to suppress the release of eotaxin from TNFα-stimulated HASM cells was not prevented by H-89 (Pang & Knox, 2001). Taken together, these results imply that although H-89 abolishes cAMP-dependent CREB phosphorylation, other signalling intermediates are affected that oppose the functional consequences associated with the inhibition of PKA. The identity of these additional intracellular targets in HASM cells is unknown and was not a subject of this investigation. However, isoquinoline sulphonamides can bind many proteins with high affinity including certain carboxy-terminal domain kinases, which have a profound influence on gene transcription (Dubois et al., 1994). Based on these data, we suggest that caution should be exercised when using H-89 in studies designed to assess the role of PKA in biological responses.
Cicaprost (Sturzebecher et al., 1986) also suppressed GM-CSF release from HASM cells although the concentration–response relationship was complex and better accommodated by a two-site sigmoidal function. The IC50 of cicaprost for the high-affinity component, which accounted for ∼34% of the response, was comparable to that found on well characterised IP-receptor containing tissues including human platelets and vascular smooth muscle, suggesting that the suppression of GM-CSF output could also be mediated through this receptor subtype (Sturzebecher et al., 1986; Stanford et al., 2000). Although IP-receptor antagonists are currently unavailable, the knowledge that HASM cells express IP-receptors (Clarke, D.L., Belvisi, M.G., Smith, S.J., Yacoub, M.H., Meja, K.K., Newton, R., Slater, D.M., Giembycz, M.A. – manuscript under review) and that cicaprost is a potent and selective IP-receptor agonist (Abramovitz et al., 2000; Dong et al., 1986) with weak activity at the human EP2-receptor subtype (Breyer et al., 2001) supports this conclusion. Moreover, a role for EP4-receptors can be excluded on the basis that although cicaprost binds with nanomolar affinity to this subtype (Abramovitz et al., 2000), neither AH 23848B nor L-161,982 antagonised the inhibitory effect of PGE2, ONO-AE1-259 and 16,16-dimethyl-PGE2 on GM-CSF release. At high concentrations (>1 μM) of cicaprost, the second component of the response curve became evident (IC50 ∼20 μM), which, based on the aforementioned discussion, may involve the EP2-subtype.
In conclusion, PGE2 inhibited GM-CSF release from IL-1β-stimulated HASM cells by acting through prostanoid receptors of the EP2-subtype and this effect was mediated by PKA. Evidence is also provided that agonism of IP-receptors may also negatively regulate the expression of the CSF2 gene. Finally, H-89 did not reproduce the effect of PKIα, possibly due to its ability to inhibit other protein kinases (Davies et al., 2000), and should not be considered a selective inhibitor of PKA.
Acknowledgments
This work was supported by an MRC/Aventis collaborative Studentship to D.L.C. M.G.B. is funded by the Harefield Research Foundation. M.A.G. is an Alberta Heritage Senior Medical Scholar and is funded by the Canadian Institutes of Health Research (#73-1279). We thank Ono Pharmaceuticals (Osaka, Japan), Schering AG (Berlin, Germany) and Merck Frosst (Montreal, Canada) for the generous gifts of ONO-AE1-259, cicaprost and L-161,982, respectively.
Abbreviations
- CTD
carboxy-terminal domain
- CHO
Chinese hamster ovary
- COPD
chronic obstructive pulmonary disease
- CSF
colony-stimulating factor
- DMEM
Dulbecco's modified Eagle's medium
- HBSS
Hanks' balanced salt solution
- IL
interleukin
References
- ABRAMOVITZ M., ADAM M., BOIE Y., CARRIERE M., DENIS D., GODBOUT C., LAMONTAGNE S., ROCHETTE C., SAWYER N., TREMBLAY N.M., BELLEY M., GALLANT M., DUFRESNE C., GAREAU Y., RUEL R., JUTEAU H., LABELLE M., OUIMET N., METTERS K.M. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M.A., HADDAD E., HIRST S.J., YACOUB M.H., BARNES P.J., MITCHELL J.A. Induction of cyclo-oxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br. J. Pharmacol. 1997;120:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOIE Y., STOCCO R., SAWYER N., SLIPETZ D.M., UNGRIN M.D., NEUSCHAFER-RUBE F., PUSCHEL G.P., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- BREYER R.M., BAGDASSARIAN C.K., MYERS S.A., BREYER M.D. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- CLARKE D.L., PATEL H.J., MITCHELL J.A., YACOUB M.H., GIEMBYCZ M.A., BELVISI M.G. Regulation of the release of colony-stimulating factors from human airway smooth muscle cells by prostaglandin E2. Am. J. Respir. Crit Care Med. 2001;163:A515. [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLETT A., SHELDRICK R.L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994a;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., HUMPHREY P.P.A., SHELDRICK R.L., WHITE B.P. Gastric antisecretory prostanoids: actions at different prostanoid receptors. Br. J. Pharmacol. 1988;95:724P. [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive EP-receptors. Adv. Prostaglandin Thromb. Leukoc. Res. 1987;17A:467–470. [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994b;46:205–229. [PubMed] [Google Scholar]
- COLLINS S.P., UHLER M.D. Characterization of PKIγ, a novel isoform of the protein kinase inhibitor of cAMP-dependent protein kinase. J. Biol. Chem. 1997;272:18169–18178. doi: 10.1074/jbc.272.29.18169. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY R.N., WALDER J.A., MAURER R.A. A protein kinase inhibitor gene reduces both basal and multi-hormone-stimulated prolactin gene transcription. J. Biol. Chem. 1989;264:431–436. [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L., WILSON N.H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br. J. Pharmacol. 1986;87:97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS M.F., NGUYEN V.T., BELLIER S., BENSAUDE O. Inhibitors of transcription such as 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole and isoquinoline sulfonamide derivatives (H-8 and H-7) promote dephosphorylation of the carboxyl-terminal domain of RNA polymerase II largest subunit. J. Biol. Chem. 1994;269:13331–13336. [PubMed] [Google Scholar]
- ENGH R.A., GIROD A., KINZEL V., HUBER R., BOSSEMEYER D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J. Biol. Chem. 1996;271:26157–26164. doi: 10.1074/jbc.271.42.26157. [DOI] [PubMed] [Google Scholar]
- GARDINER P.J. Characterization of prostanoid relaxant/inhibitory receptors (ψ) using a highly selective agonist, TR4979. Br. J. Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., LINDSAY M.A. Pharmacology of the eosinophil. Pharmacol. Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- GLASS D.B., CHENG H.C., KEMP B.E., WALSH D.A. Differential and common recognition of the catalytic sites of the cGMP-dependent and cAMP-dependent protein kinases by inhibitory peptides derived from the heat-stable inhibitor protein. J. Biol. Chem. 1986;261:12166–12171. [PubMed] [Google Scholar]
- GOMEZ-FOIX A.M., COATS W.S., BAQUE S., ALAM T., GERARD R.D., NEWGARD C.B. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J. Biol. Chem. 1992;267:25129–25134. [PubMed] [Google Scholar]
- HILLOCK C.J., CRANKSHAW D.J. Inhibitory prostanoid EP receptors in human non-pregnant myometrium. Eur. J. Pharmacol. 1999;378:99–108. doi: 10.1016/s0014-2999(99)00442-2. [DOI] [PubMed] [Google Scholar]
- HUSSAIN R.F., NOURI A.M., OLIVER R.T. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods. 1993;160:89–96. doi: 10.1016/0022-1759(93)90012-v. [DOI] [PubMed] [Google Scholar]
- INOUE H., TAKAMORI M., SHIMOYAMA Y., ISHIBASHI H., YAMAMOTO S., KOSHIHARA Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br. J. Pharmacol. 2002;136:287–295. doi: 10.1038/sj.bjp.0704705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S.R., KNOX A.J. Synthetic functions of airway smooth muscle in asthma. Trends Pharmacol. Sci. 1997;18:288–292. doi: 10.1016/s0165-6147(97)01092-4. [DOI] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T., NARUMIYA S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68-69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZERI N., BELVISI M.G., PATEL H.J., YACOUB M.H., FAN C.K., MITCHELL J.A. Effects of prostaglandin E2 and cAMP elevating drugs on GM-CSF release by cultured human airway smooth muscle cells. Relevance to asthma therapy. Am. J. Respir. Cell Mol. Biol. 2001;24:44–48. doi: 10.1165/ajrcmb.24.1.4027. [DOI] [PubMed] [Google Scholar]
- LOPEZ A.F., WILLIAMSON D.J., GAMBLE J.R., BEGLEY C.G., HARLAN J.M., KLEBANOFF S.J., WALTERSDORPH A., WONG G., CLARK S.C., VADAS M.A. Recombinant human granulocyte–macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J. Clin. Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUM H., JAFFE H.A., SCHULZ I.T., MASOOD A., RAYCHAUDHURY A., GREEN R.D. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am. J. Physiol. 1999;277:C580–C588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- LYDFORD S.J., MCKECHNIE K.C., DOUGALL I.G. Pharmacological studies on prostanoid receptors in the rabbit isolated saphenous vein: a comparison with the rabbit isolated ear artery. Br. J. Pharmacol. 1996;117:13–20. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHWATE M., HARADA S., LEU C.T., SEEDOR G., LABELLE M., GALLANT M., HUTCHINS S., LACHANCE N., SAWYER N., SLIPETZ D., METTERS K.M., RODAN S.B., YOUNG R., RODAN G.A. Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol. Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- MEJA K.K., CATLEY M.C., CAMBRIDGE L.M., BARNES P.J., LUM H., NEWTON R., GIEMBYCZ M.A.Adenovirus-mediated delivery and expression of a cAMP-dependent protein kinase inhibitor gene to BEAS-2B epithelial cells abolishes the anti-inflammatory effects of rolipram, salbutamol and prostaglandin E2: a comparison with H-89 J.Pharmacol.Exp.Ther. 2004309(in press) [DOI] [PubMed] [Google Scholar]
- METCALF D. The granulocyte–macrophage colony stimulating factors. Cell. 1985;43:5–6. doi: 10.1016/0092-8674(85)90004-2. [DOI] [PubMed] [Google Scholar]
- METCALF D. The molecular biology and functions of the granulocyte–macrophage colony-stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NAMBA T., SUGIMOTO Y., NEGISHI M., IRIE A., USHIKUBI F., KAKIZUKA A., ITO S., ICHIKAWA A., NARUMIYA S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S., SUGIMOTO Y., USHIKUBI F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- NIALS A.T., VARDEY C.J.D.L.M., THOMAS M., SPARROW S.J., SHERHERD G.D., COLEMAN R.A. AH 13205, a selective prostanoid EP2-agonist. Cardiovasc. Drug Rev. 1993;11:165–179. [Google Scholar]
- OLSEN S.R., UHLER M.D. Isolation and characterization of cDNA clones for an inhibitor protein of cAMP-dependent protein kinase. J. Biol. Chem. 1991;266:11158–11162. [PubMed] [Google Scholar]
- PANG L., KNOX A.J. Regulation of TNFα-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J. 2001;15:261–269. doi: 10.1096/fj.00-0103com. [DOI] [PubMed] [Google Scholar]
- SAUNDERS M.A., MITCHELL J.A., SELDON P.M., YACOUB M.H., BARNES P.J., GIEMBYCZ M.A., BELVISI M.G. Release of granulocyte-macrophage colony stimulating factor by human cultured airway smooth muscle cells: suppression by dexamethasone. Br. J. Pharmacol. 1997;120:545–546. doi: 10.1038/sj.bjp.0700998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCARPETTA M.A., UHLER M.D. Evidence for two additional isoforms of the endogenous protein kinase inhibitor of cAMP-dependent protein kinase in mouse. J. Biol. Chem. 1993;268:10927–10931. [PubMed] [Google Scholar]
- SCHILD H.O. pAx and competitive drug antagonism. Br. J. Pharmacol. Chemother. 1949;4:277–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANFORD S.J., PEPPER J.R., MITCHELL J.A. Release of GM-CSF and G-CSF by human arterial and venous smooth muscle cells: differential regulation by COX-2. Br. J. Pharmacol. 2000;129:835–838. doi: 10.1038/sj.bjp.0703151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURZEBECHER S., HABEREY M., MULLER B., SCHILLINGER E., SCHRODER G., SKUBALLA W., STOCK G., VORBRUGGEN H., WITT W. Pharmacological profile of a novel carbacyclin derivative with high metabolic stability and oral activity in the rat. Prostaglandins. 1986;31:95–109. doi: 10.1016/0090-6980(86)90228-5. [DOI] [PubMed] [Google Scholar]
- SUZAWA T., MIYAURA C., INADA M., MARUYAMA T., SUGIMOTO Y., USHIKUBI F., ICHIKAWA A., NARUMIYA S., SUDA T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3 and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI H.K., IWAGAKI H., YOSHINO T., MORI S., MORICHIKA T., ITOH H., YOKOYAMA M., KUBO S., KONDO E., AKAGI T., TANAKA N., NISHIBORI M. Prostaglandin E2 inhibits IL-18-induced ICAM-1 and B7.2 expression through EP2/EP4 receptors in peripheral blood mononuclear cells. J. Immunol. 2002;168:4446–4454. doi: 10.4049/jimmunol.168.9.4446. [DOI] [PubMed] [Google Scholar]
- TAKAYAMA K., GARCIA-CARDENA G., SUKHOVA G.K., COMANDER J., GIMBRONE M.A., JR, LIBBY P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- TAKIO K., WADE R.D., SMITH S.B., KREBS E.G., WALSH K.A., TITANI K. Guanosine cyclic 3′,5′-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984;23:4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- TANI K., NAGANAWA A., ISHIDA A., EGASHIRA H., SAGAWA K., HARADA H., OGAWA M., MARUYAMA T., OHUCHIDA S., NAKAI H., KONDO K., TODA M. Design and synthesis of a highly selective EP2-receptor agonist. Bioorg. Med. Chem. Lett. 2001;11:2025–2028. doi: 10.1016/s0960-894x(01)00359-6. [DOI] [PubMed] [Google Scholar]
- TSAI B.S., KESSLER L.K., STOLZENBACH J., SCHOENHARD G., BAUER R.F. Expression of gastric antisecretory and prostaglandin E receptor binding activity of misoprostol by misoprostol free acid. Dig. Dis. Sci. 1991;36:588–593. doi: 10.1007/BF01297024. [DOI] [PubMed] [Google Scholar]
- TSUBOI K., SUGIMOTO Y., ICHIKAWA A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68-69:535–556. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPERL D.J., BURKEY T.H., REGAN J.W. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]