Abstract
β-Adrenoceptor (β-AR)-mediated vasodilation, which plays an important physiological role in the regulation of vascular tone, is decreased in two-kidney, one clip (2K-1C) renal hypertension. In this study, downstream pathways related to vascular β-AR activation were evaluated in 2K-1C rats.
Relaxation responses to isoprenaline, forskolin and 8-Br-cAMP were diminished in aortas without endothelium from 2K-1C when compared to those in normotensive two kidney (2K). Basal adenosine-3′,5′-monophosphate (cAMP), as well as isoprenaline-induced increase in cAMP levels, was not different between 2K and 2K-1C aortas.
Contractile responses to caffeine, after depletion and reloading of intracellular Ca2+ stores, were greater in 2K-1C than in 2K. The presence of isoprenaline during the Ca2+-reloading period abolished the differences between groups by increasing caffeine contraction in 2K without changing this response in 2K-1C aortas. Inhibition of the sarcolemmal Ca2+ATPase with thapsigargin markedly attenuated isoprenaline vasodilation in both 2K and 2K-1C and abolished the differences between groups.
Blockade of ATP-sensitive K+ channels (KATP) channels with glibenclamide significantly decreased isoprenaline vasodilation in 2K-1C without affecting this response in 2K. Both vascular gene and protein expression of protein kinase A (PKA), as well as phosphoserine-containing proteins, were increased in 2K-1C vs 2K rats.
In conclusion, decreased isoprenaline vasodilation in 2K-1C hypertensive rats is related to impaired modulation of the sarcolemmal Ca2+ATPase activity. Moreover, KATP channels may play a compensatory role on isoprenaline-induced relaxation in renal hypertension. Both Ca2+ATPase and KATP channel functional alterations, associated with decreased β-AR vasodilation, are paralleled by an upregulation of protein kinase A (PKA) and phosphoserine proteins expression.
Keywords: Renal hypertension, β-adrenoceptor, isoprenaline, cAMP, protein kinase A, Ca2+ATPase, KATP channels
Introduction
Stimulation of β-adrenoceptors (β-AR) on vascular smooth muscle results in relaxation, through activation of membrane-bound adenylate cyclase and augmentation of intracellular adenosine-3′,5′-monophosphate (cAMP) content. Subsequent activation of cAMP-dependent protein kinases (PKA) leads to phosphorylation of critical regulatory proteins, which act to decrease transarcolemmal Ca2+ influx as well as sensitivity of the contractile system to Ca2+ and, most importantly, to increase Ca2+ uptake into the sarcoplasmic reticulum (SR) (Werstiuk & Lee, 2000).
Growing evidence indicates that β-AR activity modulates peripheral vascular tone, thereby controlling the distribution of blood flow to different tissues. Indeed, gene-targeting techniques that selectively inactivate β-AR in mice have demonstrated that these animals exhibit increased blood pressure, decreased vasodilation, as well as increased pressure responses induced by adrenaline infusion (Chruscinski et al., 1999; 2001; Rohrer et al., 1999). Moreover, vascular smooth muscle targeted overexpression of G protein-coupled receptors kinase (GRK), which desensitizes agonist-activated β-AR, induces hypertension and decreases endothelium-independent vasodilation (Eckhart et al., 2002).
Altered vascular tone, a characteristic feature of most forms of experimental and human hypertension, has been associated with impaired endothelium-dependent vasodilation and reduced NO signalling (Puddu et al., 2000; Schiffrin, 2001; Taddei & Salvetti, 2002). However, processes underlying impaired vasodilation in hypertension extend beyond the endothelium and the vascular smooth muscle is also of importance (Touyz, 2002). Accordingly, changes in vascular smooth muscle β-AR signalling may contribute to impaired relaxation in hypertension, shifting the balance between vasoconstrictor and vasodilator mechanisms. In fact, impairment in β-AR-mediated vasodilation has been described in both human and animal forms of hypertension (Tsujimoto et al., 1986; Asano et al., 1988; 1991; Fujimoto et al., 1988; Soltis & Katovich, 1991; Blankesteijn et al., 1996; Cardillo et al., 1997). Interestingly, elevation in GRK levels has also been found in arteries of hypertensive rats (Gross et al., 2000).
We previously demonstrated that aortas from two kidney-one clip (2K-1C) hypertensive rats display decreased endothelium-independent relaxation to isoprenaline, a classical β-AR agonist (Callera & Bendhack, 1999a). However, mechanisms whereby β-AR activation produces decreased vasodilation in 2K-1C hypertension remain unclear. We hypothesized that changes in the adenylate cyclase-cAMP-dependent PKA pathway may represent one of these mechanisms and may contribute to the pathophysiological vascular changes in 2K-1C hypertension. To address this issue, vasodilation induced by agents that stimulate effectors of the β-AR pathway, cyclic nucleotide content and PKA expression were investigated in aortic rings from 2K-1C and normotensive 2K rats.
Methods
Experimental animals
Experimental protocols followed standards and policies of the University of Sao Paulo's Animal Care and Use Committee. Renovascular hypertension was induced in rats as previously described (Callera et al., 2001). Briefly, male Wistar rats (180–200 g) were anaesthetized and after a midline laparotomy, a silver clip with an internal diameter of 0.20 mm was placed around the left renal artery. Normotensive two kidney (2K) rats were submitted to laparotomy only. Animals were maintained in a room with controlled temperature, a 12 h light/dark cycle, and given free access to both food (standard rat chow) and water. Systolic blood pressure (SBP) was measured weekly in nonanaesthetized animals by an indirect tail-cuff method (MLT125R Pulse Transducer/Pressure Cuff coupled to the PowerLab 4/S analogue-to-digital converter, AD Instruments Pty Ltd) and rats were considered to be hypertensive when SBP was higher than 160 mmHg.
Preparation of isolated aortic rings
Rats were killed by decapitation and the thoracic aortas were isolated. Aortic rings, 4 mm in length, were cut and mounted between two steel hooks in bath chambers (10 ml) for isolated organs containing physiological salt solution (PSS) at 37°C, continuously bubbled with 95% O2 and 5% CO2, pH 7.4. The system was connected to a F-60 force–displacement transducer and the contractile responses were recorded on a polygraph (Narco Biosystems Inc., Houston, TX, U.S.A.). Aortic rings were submitted to a tension of 1.5 g that was adjusted every 15 min during a 60 min equilibration period before the addition of a given drug. At the beginning of the experiments, the aortas were stimulated with 0.1 μM noradrenaline to test their functional integrity. In all experiments, vascular endothelium removal, performed by gently rubbing the lumen side of the aortic rings, was assessed by the absence of relaxation in response to 1 μM acetylcholine in aortas contracted with noradrenaline.
Vascular reactivity studies
Isoprenaline-induced endothelium-independent relaxation was evaluated in aortas without endothelium from both 2K-1C and 2K rats. A complete concentration–response curve for isoprenaline (1 nM to 0.3 μM) was obtained in aortas precontracted with phenylephrine (10–50 nM). Relaxant responses to forskolin (1 nM to 1 μM), an activator of adenylate cyclase (Seamon & Daly, 1981), and to 8-Br cAMP (10 nM to 100 μM), a membrane-permeant cAMP analogue (Rannels & Corbin 1979), were also observed to evaluate downstream mechanisms associated with the β-AR pathway. Phenylephrine concentration was adjusted, so that the magnitude of contraction was the same for 2K (10 nM) and 2K-1C (50 nM) aortas.
The effects of isoprenaline on Ca2+ uptake were evaluated by functional assessment of caffeine-induced Ca2+-release from intracellular stores, as previously described (Tostes et al., 1995). Briefly, aortic rings were contracted with 3 μM phenylephrine in 1.6 mM Ca2+ PSS. To deplete intracellular Ca2+ stores, aortic rings were washed with Ca2+-free PSS containing 1 mM EGTA for 20 min. Intracellular Ca2+ stores were loaded by replacing 1.6 mM Ca2+ PSS for 20 min (loading period). Contractile response to 20 mM caffeine was induced in Ca2+-free PSS. This protocol was repeated in the presence of 0.1 μM isoprenaline, which was added during the loading period.
The contribution of the sarcolemmal Ca2+ATPase to isoprenaline-induced relaxation was assessed in aortas precontracted with phenylephrine and previously incubated for a 30 min-period with 1 μM thapsigargin (TG), a selective inhibitor of the sarcoplasmic reticulum (SR) Ca2+ATPase (Lytton et al., 1991).
Additional experiments were performed to evaluate the contribution of ATP-sensitive K+ channels (KATP) to isoprenaline-induced relaxation. Concentration–effect curves to isoprenaline were performed in aortas precontracted with phenylephrine previously incubated for a 30 min-period with 3 μM glibenclamide (GLI), a blocker of KATP (Marble, 1971).
In order to avoid the possibility of time-dependent changes in vascular responsiveness, a single concentration–effect curve or only one functional protocol to evaluate intracellular Ca2+ release with caffeine was performed in each aorta.
cAMP measurements
Aortic rings without endothelium were preincubated with 1 ml of PSS for 15 min at 37o C. Tissues were then exposed to phenylephrine for 5 min and stimulated with 1 μM isoprenaline for 120 s. All experiments were performed in the presence of the nonspecific phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (100 mM IBMX). Frozen intact aortas were homogenized in 6% trichloroacetic acid and centrifuged at 2000 × g for 15 min. The supernatant was preserved, and the lipid phase was extracted four times with a five-fold excess of water-saturated diethylether and discarded. The water phase was dried at 60°C under nitrogen. cAMP levels were measured by enzyme immunoassay (EIA) using an acetylation procedure with commercially available kits (Amersham cAMP EIA system, RPN 224; Amersham, London, U.K.). The cAMP content was expressed as fentomoles per milligram of protein. Protein content of the samples was measured by the Bradford method (Bradford, 1976).
Aortic mRNA Expression of PKA
Total cellular RNA was isolated from thoracic aorta using TRizol reagent (Life Technology). Digestion of contaminant DNA was performed using DNAse (Promega, Madison, U.S.A.) and RNA was then isolated by phenol–chloroform extraction, precipitated by isopropanol, resuspended in 50 μl DEPC-treated water and quantified using a spectrophotometer (Eppendorf). First-strand complementary DNA (cDNA) was synthesized using 3 μg total RNA in the presence of Superscript II Rnase H-Reverse Transcriptase (Invitrogen Life Technologies, CA, U.S.A.), RNAase inhibitor and 1 μg oligo(dT) primer, at 42°C for 50 min. cDNA was amplified by polymerase chain reaction (PCR) using the following set of primers (5′-3′): PKA sense – ACC CCA TGC TCG TTT CTA TG, antisense – ATG ATC TCT GGG GCC AGG TA; GAPDH, sense – TAT GAT GAC ATC AAG AAG GTG G, antisense – CAC CAC CCT GTT GCT GTA. The best annealing temperature was chosen for each set of primers and experiments were also performed to confirm that the number of cycles used in the PCRs is within the linear amplification range for each gene. The following conditions were used: final volume of 25 μl, 2.5 U Taq Platinum polymerase (Life Technology), an initial denaturing cycle at 94°C for 3 min, and subsequent cycles with denaturation at 94°C for 30 s, annealing at 62°C. Numbers of amplification cycles were: 36 for PKA and 24 for GAPDH, which was used as an internal control for the coamplification. PCR products underwent electrophoresis on 1% agarose gel containing ethidium bromide 0.5 μg/ml and band intensities were measured using a software (Kodak Digital Science, Eastman Kodak Co.). Signals were expressed relatively to the GAPDH intensity in each coamplified sample.
Western blot analysis of immunoreactive PKA and phosphoserine proteins expression
Aortic homogenate proteins (20 μg) were separated by SDS–PAGE (8 and 12% polyacrilamide) and electrophoretically transferred to a nitrocellulose membrane. After blockade of non specific sites with 5% nonfat dry milk, membranes were incubated overnight at 4°C with primary antibodies raised against PKAα (Santa Cruz, Biotechnology, CA, U.S.A.) or phosphoserine-containing proteins (Chemicon International Inc., CA, U.S.A; AB-1603). Membranes were washed with Tris-buffered saline containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated rabbit anti-mouse antibody. A chemiluminescent assay kit was used to detect immunoreactive PKA or phosphoserine proteins and the band intensities were estimated by densitometric analysis.
Solutions and drugs
Composition of PSS was the following (mM): 130.0. NaCl, 1.6 CaCl2, 4.7 KCl, 1.17 MgSO4, 1.18 KH2PO4, 14.9 NaHCO3, 0.026 EDTA, 5.5 dextrose. In some experiments, we used Ca2+-free solution with the same PSS composition except for containing 1.0 mM ethylene glycol bis-(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) and no CaCl2. Noradrenaline, phenylephrine and isoprenaline were obtained from Sigma Chemical Co. (St Louis, MO- U.S.A.); acetylcholine, 8-Br cAMP, forskolin, TG and GLI, were from Research Biochemicals International (Natick, MA, U.S.A.).
Data analysis
Results are expressed as means±s.e.m. and n indicates the number of animals. Responses to relaxant agents are expressed as a percentage of the preceding contraction induced by phenylephrine. Caffeine responses were expressed as g of contraction. Statistical analysis was performed by using ANOVA two-way followed by the Bonferroni post hoc test, when appropriate. Single comparisons were made using Student's unpaired t-test. The P-value <0.05 was taken as significant.
Results
At 6 weeks after surgery, SBP was significantly increased in 2K-1C (203±4 mmHg, n=28, P<0.001) vs 2K rats (116±3 mmHg, n=24).
Relaxant response of aortic rings
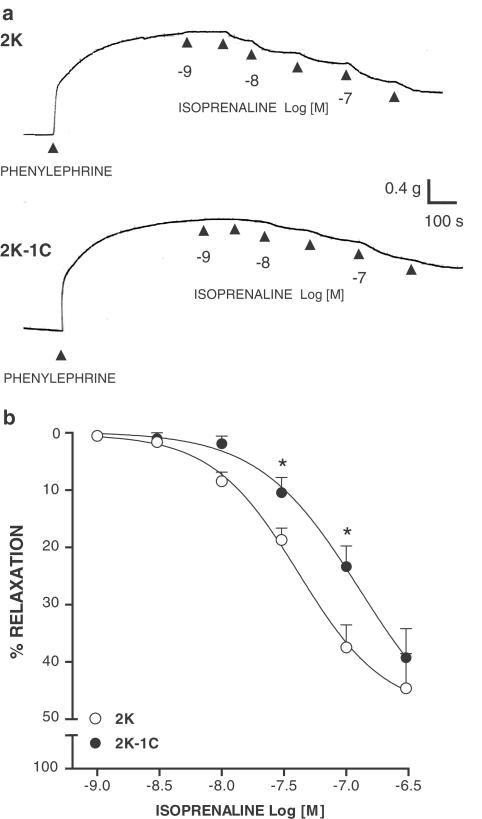
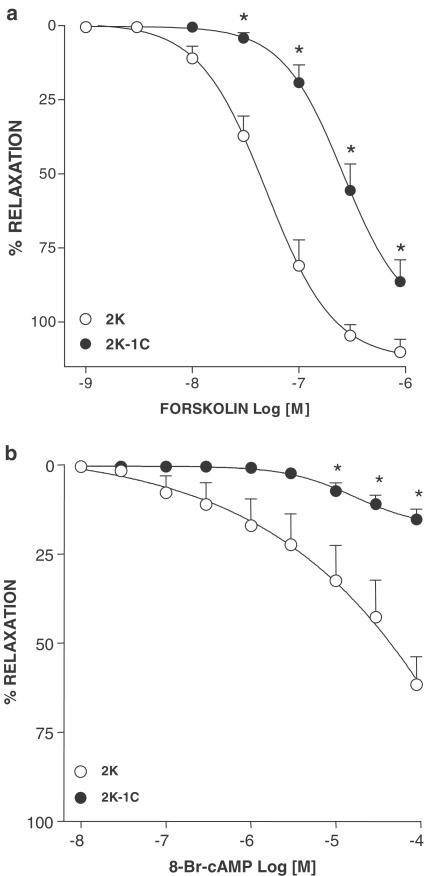
Isoprenaline induced a concentration-dependent relaxation in endothelium-denuded aortas contracted with phenylephrine (Figure 1a, b). Relaxation to isoprenaline was reduced in aortas from 2K-1C (P<0.01; n=7) compared with those from 2K rats (n=7). Relaxation to forskolin (Figure 2a) was decreased in aortas from 2K-1C (P<0.001; n=7) compared with 2K (n=8). Similar results were obtained using 8-Br cAMP (Figure 2b): a decreased relaxation in 2K-1C aortas (P<0.001; n=9) compared with 2K aortas (n=8).
Figure 1.
(a) Representative traces showing the relaxant response to isoprenaline in aortas without endothelium precontracted with phenylephrine from 2K and 2K-1C rats. (b) Concentration-effect curves to isoprenaline. Results are presented as mean±s.e.m. * (P<0.05) vs 2K.
Figure 2.
Concentration–effect curves to Forskolin (a) and 8-Br-cAMP (b) in aortas without endothelium precontracted with phenylephrine from 2K and 2K-1C rats. Results are presented as mean±s.e.m. * (P<0.05) vs 2K.
Effects of isoprenaline on cAMP levels
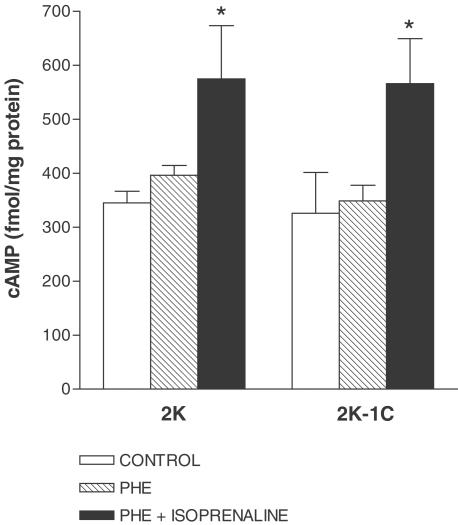
As shown in Figure 3, basal cAMP levels in aortas from 2K-1C (325±75 fmol (mg protein)−1; n=4) did not differ from those in 2K (345±21 fmol (mg protein)−1; n=7). Phenylephrine did not alter basal levels of cAMP in 2K-1C (348±28 fmol (mg protein)−1; n=5) or 2K aortas (396±18 fmol (mg protein)−1; n=4). The increase in cAMP levels induced by isoprenaline was similar between 2K (575±98 fmol (mg protein)−1; n=7) and 2K-1C (566±83 fmol mg (protein)−1; n=5) aortas.
Figure 3.
Effect of isoprenaline on cAMP levels in aortas from 2K and 2K-1C. cAMP content was evaluated in the absence (control) or in the presence of 0.3 μM phenylephrine (PHE) and following the addition of 1 μM isoprenaline. Results are presented as mean±s.e.m. and are expressed as fentomoles per milligram of protein. * (P<0.05) vs control.
Effects of isoprenaline on caffeine-induced Ca2+ release
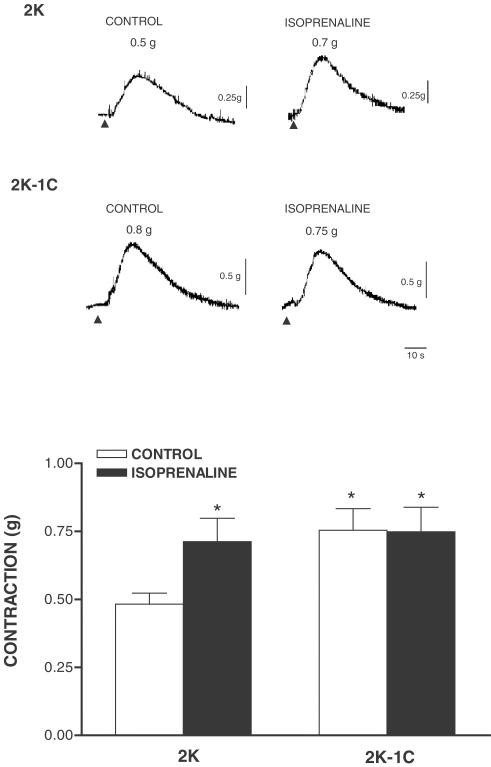
Figure 4 shows that contractile response to caffeine after depletion and reloading of intracellular Ca2+ stores is greater in 2K-1C (0.75±0.08 g; P<0.01, n=9) compared to 2K (0.48±0.04 g; n=8) aortas. When extracellular Ca2+ was restored (Ca2+-loading period) in the presence of isoprenaline, tonic contraction to caffeine was increased in 2K aortas (0.71±0.08 g; P<0.01, n=8), but did not change in 2K-1C aortas (0.74±0.09 g; n=8) abolishing the difference between groups.
Figure 4.
Top: Representative traces of the effect of isoprenaline on caffeine-induced contraction. Aortic rings from 2K and 2K-1C rats were stimulated with PHE in 1.6 mM Ca2+ Krebs solution. When a stable contraction was reached, aortas were washed in Ca2+-free Krebs solution containing 1 mM EGTA for 20 min. Aortic rings were further equilibrated in 1.6 mM Ca2+ solution for 20 min in the absence (control) or in the presence of 1 μM isoprenaline and were stimulated with 20 mM caffeine in Ca2+-free Krebs solution. Bottom: bar graphs representing the effect of isoprenaline on caffeine-induced contraction. Results are presented as mean±s.e.m. * (P<0.05) vs 2K control.
Effects of TG and GLI on isoprenaline-induced relaxation
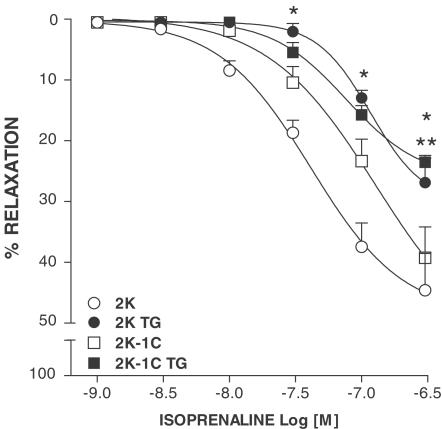
As shown in Figure 5, the inhibition of the sarcolemmal Ca2+ATPase with TG markedly attenuated isoprenaline-induced relaxation in 2K (P<0.001; n=6) and 2K-1C (P<0.001; n=6) aortas, and abolished the differences in isoprenaline vasodilation between groups.
Figure 5.
Effects of 0.1 μM TG on concentration–response curves to isoprenaline in phenylephrine-contracted aortas from 2K and 2K-1C rats. Results are presented as mean±s.e.m. * (P<0.05) 2K vs 2K TG; ** (P<0.05) 2K-1C vs 2K-1C TG.
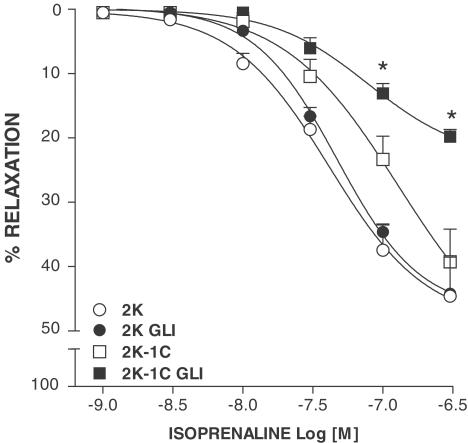
The blockade of KATP channels with GLI significantly decreased isoprenaline-induced maximum vasodilation in 2K-1C (P<0.001; n=6) without affecting isoprenaline response in 2K aortas (n=6), as shown in Figure 6.
Figure 6.
Effects of 0.1 μM glibenclamide (GLI) on concentration–response curves to isoprenaline in phenylephrine-contracted aortas from 2K and 2K-1C. Symbols and bars indicate means±s.e.m. * (P<0.05) 2K-1C vs 2K-1C GLI.
Vascular gene and protein expression of PKA and phosphoserine-containing proteins
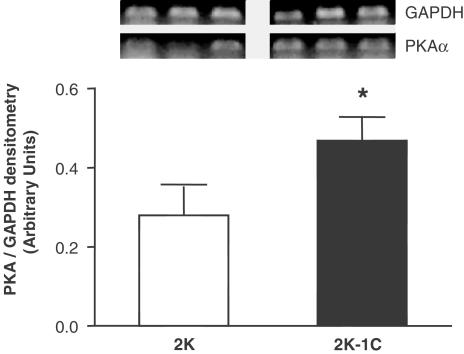
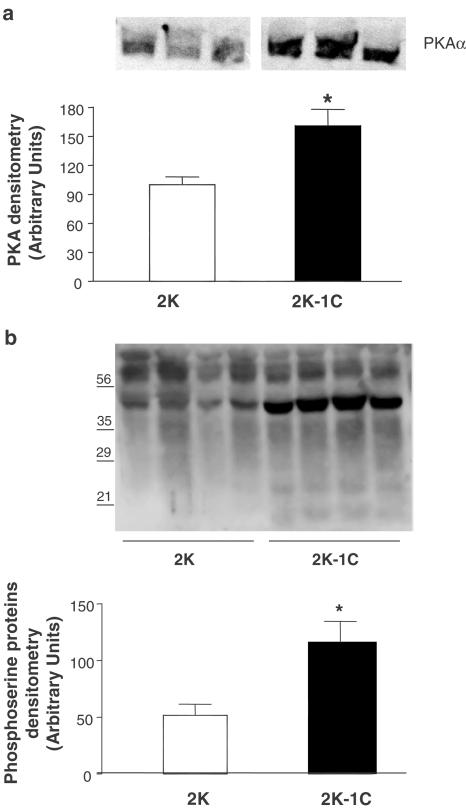
Figure 7 shows PKA gene expression in aortas from 2K-1C and 2K rats. Increased vascular PKA mRNA expression was observed in 2K-1C (0.5±0.1; n=6, P<0.05) vs 2K (0.3±0.1, n=6). Analysis of Western blots (n=4) showed that both PKA protein expression (Figure 8a) and the content of phosphoserine proteins (Figure 8b) were increased in aortas from 2K-1C (P<0.05) compared to 2K.
Figure 7.
Top panel: Representative photographies of RT–PCR products of 3 μg total RNA extracted from aortas of 2K and 2K-1C. Bottom panel: Graph showing the relative optical density values for PKA in the 2K and 2K-1C groups. Values were normalized by the corresponding RT–PCR products for GAPDH. Results are mean±s.e.m.* (P<0.05) vs 2K.
Figure 8.
Representative photographies of Western Blot (top panel) and densitometric group data (bottom panel) evaluating expression of PKA protein (a) and phosphoserine-containing proteins (b) in aortas from 2K and 2K-1C rats. Results are mean±s.e.m. * (P<0.05) vs 2K.
Discussion
Earlier reports have shown that two-kidney, one clip (2K-1C) renal hypertension is associated with changes in vascular reactivity (Cauvin & Pegram, 1983; Dohi et al., 1991; Van De Voorde et al., 1992; Bennett et al., 1993; Callera et al., 2000). Considering that reduced vasodilation in renal hypertension may also be related to an abnormal function of vascular smooth muscle cells and that β-AR-mediated vasodilation, which is impaired in aortas from 2K-1C rats (Callera & Bendhack, 1999a), is thought to play an important physiological role in the regulation of vascular tone, the present study was carried out to further characterize changes in downstream signalling pathways linked to β-AR-induced relaxation in aortas from 2K-1C rats.
Since isoprenaline mediates vasodilation through activation of adenylate cyclase and augmentation of intracellular cAMP levels (Itoh et al., 1982), changes in the adenylate cyclase-cAMP pathway could explain the blunted β-AR vasodilation in 2K-1C hypertensive rats. We initially tested this hypothesis evaluating the relaxant effects of forskolin, which directly stimulates adenylate cyclase, and by measuring cAMP levels. Forskolin-induced relaxation was decreased in 2K-1C aortas. However, isoprenaline-induced augmentation of cAMP was not altered in 2K-1C aortas, suggesting that mechanisms downstream to the activation of adenylate cyclase and generation of cAMP contribute to the decreased β-AR vasodilation in 2K-1C hypertensive rats. To test for changes in cAMP-activated mechanisms in 2K-1C aortas, we evaluated the effects of 8-Br cAMP, a membrane-permeant cAMP analogue. Relaxation induced by 8-Br cAMP was decreased in 2K-1C aortas, a finding that strongly supports our suggestion that mechanisms downstream to cAMP generation are involved in the decreased β-AR-induced vasodilation in renal hypertension.
The overall physiological effect due to activation of vascular smooth muscle β-AR is a decrease in intracellular Ca2+ levels leading to vasodilation (Werstiuk & Lee, 2000). It has been assumed that augmentation of cAMP activates cAMP-dependent PKA, which subsequently phosphorylates various critical regulatory target proteins (Murray, 1990; Haynes et al., 1992, Eckly Michel et al., 1997). Activation of the SR Ca2+ATPase, which is probably mediated through the phosphorylation of phospholamban, and increased SR Ca2+ uptake are key mechanisms involved in the vascular actions of agents that stimulate the cAMP/PKA pathway, such as β-AR agonists (Watras, 1988; Mundiña-Weilenmann et al., 2000).
Therefore, we further investigated mechanisms involved in the decreased β-AR relaxation in 2K-1C aortas, by evaluating the functional activation of the SR Ca2+ATPase. The magnitude of the contractile response to caffeine, obtained after depletion and reloading of intracellular Ca2+ stores, was used to evaluate the effects of isoprenaline on intracellular Ca2+ recycling. As we previously demonstrated, caffeine-induced contraction is increased in 2K-1C aortas, reflecting a greater storage of Ca2+ into the SR and no changes in the SR Ca2+ATPase functional activity in 2K-1C vessels (Callera & Bendhack, 1999b). However, isoprenaline increased the contraction to caffeine in 2K aortas without affecting caffeine responses in 2K-1C, indicating that isoprenaline is able to stimulate SR Ca2+ uptake during the reloading period only in aortas from normotensive rats. Therefore, it is possible that cAMP-induced activation of the SR Ca2+ATPase is abnormal/decreased in 2K-1C aortas. The use of TG, a potent inhibitor of the SR Ca2+ATPase pump that prevents the ability of SR to take up Ca2+ (Lytton et al., 1991), provided further support for the suggestion that isoprenaline produces its effects by activating Ca2+ uptake into the SR. TG not only decreased the relaxation to isoprenaline but also abolished the differences in isoprenaline relaxation between 2K-1C and 2K aortas, indicating that a decreased cAMP/PKA-induced activation of SR Ca2+ uptake contributes to the decreased isoprenaline-mediated vasodilation in 2K-1C. However, one should also consider that the SR Ca2+ buffering ability in 2K-1C aortas may be already at its maximum and, consequently, isoprenaline may not be able to further stimulate Ca2+ uptake.
To address the possibility that a decrease in cAMP/PKA-induced activation of regulatory proteins, such as the SR Ca2+ ATPase, may contribute to the decreased isoprenaline-mediated relaxation in 2K-1C aortas, we evaluated both the expression of PKA and the content of phosphoserine proteins in aortas from these animals. Our finding of increased vascular PKA expression and also of increased phosphoserine-containing proteins in 2K-1C compared to 2K was surprising in view of the decreased β-AR-induced vasodilation in 2K-1C (Callera & Bendhack, 1999a), and discharges the possibility of decreased cAMP/PKA signalling. It is possible that vascular upregulation of PKA expression or activity may be a consequence of the increased intracellular Ca2+ concentration in aortas from 2K-1C (Callera et al., 2001). However, one should also consider the possibility that a primary change in PKA expression or activity may be involved in the greater intracellular SR Ca2+ accumulation observed in aortas from 2K-1C rats (Callera & Bendhack, 1999b). Mechanisms whereby PKA stimulates Ca2+-ATPase activity in 2K-1C aortas, such as by phospholamban phosphorylation, remains to be determined.
Still regarding the vascular actions of agents that activate the cAMP pathway, it is known that a significant component of the relaxant responses to these agents involve membrane hyperpolarization by the opening of K+ channels (Allen et al., 1985; Ousterhout & Sperelakis, 1987). In view of the importance of membrane potential as a determinant of smooth muscle tone, β-AR–mediated hyperpolarization may play an important role in the control of vascular tone. In fact, decreased isoprenaline-induced hyperpolarization has been described in hypertension (Stekiel et al., 1993; Goto et al., 2001; Fujii et al., 1999). Although controversy exists regarding the type of K+ channels involved in β-AR-stimulated vasodilation (Satake et al., 1996; Huang & Kwok, 1998; Sadoshima et al., 1988; White et al., 2001), several investigators have linked β-AR responses to activation of (KATP) channels (Miyoshi & Nakaya, 1993; Nakashima & Vanhoutte, 1995; Randall & McCulloch, 1995; Sheridan et al., 1997). Considering that a defective relaxant action of KATP channels activators has been described in different vascular beds from hypertensive animals (Kitazono et al., 1993; Ohya et al., 1996; Takaba et al., 1996), we tested the effects of GLI on isoprenaline-induced relaxation in 2K-1C and 2K aortas. Interestingly, the blockade of KATP with GLI had no effects in the vasodilation to isoprenaline in 2K, but blunted the response to isoprenaline in 2K-1C aortas. Consistent with this finding, no effects of glibenclamide in aortas from normotensive rats have been observed (Satake et al., 1996). Since a significant component of the β-AR vasodilation involves the opening of KATP channels only in 2K-1C aortas, we suggest that mechanisms modulating KATP activity, possibly PKA-regulated channel activity, are altered in vessels from renal hypertensive rats.
In conclusion, since functional β-AR play such a prominent and multifaceted role in vascular smooth muscle cells relaxation, it is important to understand how their activation contributes to altered vascular function in hypertension. Accordingly, the present study demonstrates for the fist time that decreased isoprenaline-induced vasodilation in 2K-1C hypertensive rats is related to impaired β-AR-mediated modulation of the SR Ca2+ATPase activity. Moreover, KATP channels seem to play a compensatory role in isoprenaline-induced relaxation in renal hypertension. Both Ca2+ATPase and KATP channel functional alterations, which are associated with decreased β-AR vasodilation, are paralleled by an upregulation of PKA and phosphoserine proteins expression.
Acknowledgments
We thank Miriam CC Melo, Luciene Ribeiro and Marta Rodrigues for excellent technical assistance and the financial support of FAPESP, PRONEX and CNPq, Brazil.
Abbreviations
- 2K
normotensive rats
- 2K-1C
two kidney-one clip hypertensive rats
- β-AR
β-adrenoceptor
- cAMP
adenosine-3′,5′-monophosphate
- GLI
glibenclamide
- GRK
G protein-coupled receptors kinase
- KATP
ATP-sensitive K+ channels
- PKA
protein kinase A
- PSS
physiological salt solution
- SBP
Systolic blood pressure
- SR
sarcoplasmic reticulum
- TG
thapsigargin
References
- ALLEN S.L., BEECH D.J., FOSTER R.W., MORGAN G.P., SMALL R.C. Electrophysiological and other aspects of the relaxant action of isoprenaline in guinea-pig isolated trachealis. Br. J. Pharmacol. 1985;86:843–854. doi: 10.1111/j.1476-5381.1985.tb11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANO M., MASUZAWA K., MATSUDA T. Evidence for reduced beta-adrenoceptor coupling to adenylate cyclase in femoral arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 1988;94:73–86. doi: 10.1111/j.1476-5381.1988.tb11501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANO M., MASUZAWA K., MATSUDA T., ASANO T. Decreased responsiveness to β-adrenoceptor agonists in arterial strips from spontaneously hypertensive rats is not associated with alterations in β-adrenoceptors. J. Hypertens. 1991;9:607–613. doi: 10.1097/00004872-199107000-00005. [DOI] [PubMed] [Google Scholar]
- BENNETT M.A., WATTP A.C., THURSTON H. Impaired endothelium-dependent relaxation in two-kidney, one clip Goldblatt hypertension: effect of vasoconstrictor prostanoids. J. Hypertens. 1993;11 Suppl. 5:S134–S135. [PubMed] [Google Scholar]
- BLANKESTEIJN W.M., RAAT N.J.H., WILLEMS H.G.M., THIEN T. β-Adrenergic relaxation in mesenteric resistance arteries of spontaneously hypertensive and wistar–kyoto rats: the role of precontractiom and intracellular Ca2+ J. Cardiovasc. Pharmacol. 1996;27:27–32. doi: 10.1097/00005344-199601000-00005. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CALLERA G.E., BENDHACK L.M. Role of endothelium on decreased relaxation to isoprenaline in renal hypertensive rats. Hypertension. 1999a;33:169P. [Google Scholar]
- CALLERA G.E., BENDHACK L.M. Contribution of sarcoplasmic reticulum uptake and L-type calcium channels to altered vascular responsiveness in the aorta of renal hypertensive rats. Gen. Pharmacol. 1999b;33:457–466. doi: 10.1016/s0306-3623(99)00042-7. [DOI] [PubMed] [Google Scholar]
- CALLERA G.E, VARANDA W.A., BENDHACK L.M. Impaired relaxation to Ach in 2K-1C hypertensive rat aortas involves changes in membrane hyperpolarization instead of an abnormal contribution of endothelial factors. Gen. Pharmacol. 2000;34:379–389. doi: 10.1016/s0306-3623(01)00075-1. [DOI] [PubMed] [Google Scholar]
- CALLERA G.E, VARANDA W.A., BENDHACK L.M. Ca2+ influx is increased in 2-kidney, 1-clip hypertensive rat aorta. Hypertension. 2001;38:592–596. doi: 10.1161/hy09t1.096248. [DOI] [PubMed] [Google Scholar]
- CARDILLO C., KILCOYNE C.M., QUYYUMI A.A., CANNON R.O., PANZA J.A. Decreased vasodilation response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–921. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- CAUVIN C., PEGRAM B. Decreased relaxation of isolated mesenteric resistance vessels from two kidney, one-clip Goldblatt hypertensive rats. Clin. Exp. Hypertens. 1983;5:383–400. doi: 10.3109/10641968309069496. [DOI] [PubMed] [Google Scholar]
- CHRUSCINSKI A., BREDE M.E., MEINEL L., LOHSE M.J., KOBILKA B.K., HEIN L. Differential distribution of β-adrenergic receptor subtypes in blood vessels of knockout mice lacking β1- or β2-adrenergic receptors. Mol. Pharmacol. 2001;60:955–962. doi: 10.1124/mol.60.5.955. [DOI] [PubMed] [Google Scholar]
- CHRUSCINSKI A.J., ROHRER D.K., SCHAUBLE E., DESAI K.H., BERNSTEIN D., KOBILKA B.K. Targeted disruption of the β2 adrenergic receptor gene. J. Biol. Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- DOHI Y., CRISCIONE L., LÜSCHER T.F. Renovascular hypertension impairs formation of endothelium-derived relaxing factors and sensitivity to endothelin-1 in resistance arteries. Br. J. Pharmacol. 1991;104:349–354. doi: 10.1111/j.1476-5381.1991.tb12434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKHART A.D., OZAKI T., TEVAEARAI H., ROCKMAN H.A., KOCH W.J. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signalling and increases resting blood pressure. Mol. Pharmacol. 2002;61:749–758. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- ECKLY-MICHEL A., MARTIN V., LUGNIER C. Involvement of cyclic nucleotide-dependent protein kinases incyclic AMP-mediated vasorelaxation. Br. J. Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., ONAKA U., GOTO K., ABE I., FUJISHIMA M. Impaired isoproterenol-induced hyperpolarization in isolated mesenteric arteries of aged rats. Hypertension. 1999;34:222–228. doi: 10.1161/01.hyp.34.2.222. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., DOHI Y., AOKI K., MATSUDA T. Altered vascular beta adrenoceptor-mediated relaxation in deoxycorticosterone-salt hypertensive rats. J. Pharmacol. Exp. Ther. 1988;244:716–723. [PubMed] [Google Scholar]
- GOTO K., FUJII K., ABE I. Impaired β-adrenergic hyperpolarization in arteries from prehypertensive spontaneously hypertensive rats. Hypertension. 2001;37:609–613. doi: 10.1161/01.hyp.37.2.609. [DOI] [PubMed] [Google Scholar]
- GROSS R., CHORAZYCZEWSKI J., MEEK M.D., BENOVIC J.L., FERGUSON S.S., FELDMAN R.D. G-protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension. 2000;35:38–42. doi: 10.1161/01.hyp.35.1.38. [DOI] [PubMed] [Google Scholar]
- HAYNES J., ROBINSON J., SAUNDERS L., TAYLOR A.E., STRADA S.J. Role of cAMP-dependent protein kinase in cAMP-mediated vasodilation. Am. J. Physiol. 1992;262:H511–H516. doi: 10.1152/ajpheart.1992.262.2.H511. [DOI] [PubMed] [Google Scholar]
- HUANG Y., KWOK K.H. Beta-adrenoceptor-mediated relaxation inhibited by tetrapentylammonium ions in rat mesenteric artery. Life Sci. 1998;62:19–25. doi: 10.1016/s0024-3205(97)01065-5. [DOI] [PubMed] [Google Scholar]
- ITOH T., IZUMI H., KURIYAMA H. Mechanisms of relaxation induced by activation of beta-adrenoceptors in smooth muscle cells of the guinea-pig mesenteric artery. J. Physiol. 1982;326:475–493. doi: 10.1113/jphysiol.1982.sp014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITAZONO T., HESITAD D.D., FARACI F.M. ATP-sensitive potassium channels in the basilar artery during chronic hypertension. Hypertension. 1993;22:677–681. doi: 10.1161/01.hyp.22.5.677. [DOI] [PubMed] [Google Scholar]
- LYTTON J., WESTLIN M., HANLEY M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- MARBLE A. Glibenclamide, a new sulphonylurea: whither oral hypoglycaemic agents. Drugs. 1971;1:109–115. doi: 10.2165/00003495-197101020-00001. [DOI] [PubMed] [Google Scholar]
- MIYOSHI H., NAKAYA Y. Activation of ATP-sensitive K1-channels by cyclic AMP-dependent protein kinase in cultured smooth muscle cells of porcine coronary artery. Biochem. Biophys. Res. Commun. 1993;193:240–247. doi: 10.1006/bbrc.1993.1615. [DOI] [PubMed] [Google Scholar]
- MUNDIÑA-WEILENMANN C., VITTONE L., RINALDI G., SAID M., CINGOLANI G.C., MATTIAZZI A. Endoplasmic reticulum contribution to the relaxant effect of cGMP- and cAMP-elevating agents in feline aorta. Am. J. Physiol. 2000;278:H1856–H1865. doi: 10.1152/ajpheart.2000.278.6.H1856. [DOI] [PubMed] [Google Scholar]
- MURRAY K.J. Cyclic AMP and mechanisms of vasodilation. Pharmacol. Ther. 1990;47:329–345. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA M., VANHOUTTE P.M. Isoproterenol causes hyperpolarization through opening of ATP-sensitive potassium channels in vascular smooth muscle of the canine saphenous vein. J. Pharmacol. Exp. Ther. 1995;272:379–384. [PubMed] [Google Scholar]
- OHYA Y., SETOGUCHI M., FUJII K., NAGAO T., ABE I., FUJISHIMA M. Impaired action of levcromakalim on ATP-sensitive K+ channels in mesenteric artery cells from spontaneously hypertensive rats. Hypertension. 1996;27:1234–1239. doi: 10.1161/01.hyp.27.6.1234. [DOI] [PubMed] [Google Scholar]
- OUSTERHOUT J.M., SPERELAKIS N. Cyclic nucleotides depress action potentials in cultured aortic smooth muscle cells. Eur. J. Pharmacol. 1987;144:7–14. doi: 10.1016/0014-2999(87)90003-3. [DOI] [PubMed] [Google Scholar]
- PUDDU P., PUDDU G.M., ZACA F., MUSCARI A. Endothelial dysfunction in hypertension. Acta Cardiol. 2000;55:221–232. doi: 10.2143/AC.55.4.2005744. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., MCCULLOCH A.I. The involvement of ATP-sensitive potassium channels in beta-adrenoceptor-mediated vasorelaxation in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1995;115:607–612. doi: 10.1111/j.1476-5381.1995.tb14975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANNELS S.R., CORBIN J.D. Characterization of small cAMP-binding fragments of cAMP-dependent protein kinases. J. Biol. Chem. 1979;254:8605–8610. [PubMed] [Google Scholar]
- ROHRER D.K., CHRUSCINSKI A., SCHAUBLE E.H., BERNSTEIN D., KOBILKA B.K. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J. Biol. Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J., AKAIKE N., KANAIDE H., NAKAMURA M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aorta. Am. J. Physiol. 1988;255:H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- SATAKE N., SHIBATA M., SHIBATA S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br. J. Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFRIN E.L. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J. Cardiovasc. Pharmacol. 2001;38 Suppl. 2:S3–S6. doi: 10.1097/00005344-200111002-00002. [DOI] [PubMed] [Google Scholar]
- SEAMON K.B., DALY J.W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J. Cyclic Nucleotide Res. 1981;7:201–224. [PubMed] [Google Scholar]
- SHERIDAN B.C., MCINTYRE R.C., MELDRUM D.R., FULLERTON D.A. KATP channels contribute to β- and adenosine receptor-mediated pulmonary vasorelaxation. Am. J. Physiol. 1997;273:L950–L956. doi: 10.1152/ajplung.1997.273.5.L950. [DOI] [PubMed] [Google Scholar]
- SOLTIS E.E., KATOVICH M.J. Reduction in aortic smooth muscle beta-adrenergic responsiveness results in enhanced norepinephrine responsiveness in the Dahl salt-sensitive rat. Clin. Exp. Hypertens. 1991;13:117–132. doi: 10.3109/10641969109082618. [DOI] [PubMed] [Google Scholar]
- STEKIEL W.J., CONTNEY S.J., RUSCH N.J. Altered ß-receptor control of in situ membrane potential in hypertensive rats. Hypertension. 1993;21:1005–1009. doi: 10.1161/01.hyp.21.6.1005. [DOI] [PubMed] [Google Scholar]
- TADDEI S., SALVETTI A. Endothelial dysfunction in essential hypertension: Clinical implications. J. Hypertens. 2002;20:1671–1674. doi: 10.1097/00004872-200209000-00001. [DOI] [PubMed] [Google Scholar]
- TAKABA H., NAGAO T., IBAYASHI S., KITAZONO T., FUJII K., FUJISHIMA M. Altered cerebrovascular response to a potassium channel opener in hypertensive rats. Hypertension. 1996;28:143–146. doi: 10.1161/01.hyp.28.1.143. [DOI] [PubMed] [Google Scholar]
- TOSTES R.C.A., TRAUB O., BENDHACK L.M., WEBB R.C. Sarcoplasmic reticulum Ca2+ uptake in aorta from deoxycorticosterone acetate hypertensive rats: functional assessment with cyclopiazonic acid. Can. J. Physiol. Pharmacol. 1995;73:1536–1545. doi: 10.1139/y95-212. [DOI] [PubMed] [Google Scholar]
- TOUYZ R.M. Impaired vasorelaxation in hypertension: beyond the endothelium. J. Hypertens. 2002;20:371–373. doi: 10.1097/00004872-200203000-00007. [DOI] [PubMed] [Google Scholar]
- TSUJIMOTO G., LEE C.H., HOFFMAN B.B. Age-related decrease in beta adrenergic receptor-mediated vascular smooth muscle relaxation. J. Pharmacol. Exp. Ther. 1986;239:411–415. [PubMed] [Google Scholar]
- VAN DE VOORDE J., VANHEEL B., LEUSEN I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ. Res. 1992;70:1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- WATRAS J. Regulation of calcium uptake in bovine aortic sarcoplasmic reticulum by cyclic AMP-dependent protein kinase. J. Mol. Cell Cardiol. 1988;20:711–723. doi: 10.1016/s0022-2828(88)80016-6. [DOI] [PubMed] [Google Scholar]
- WERSTIUK E.S., LEE R.M.K.W. Vascular β-adrenoceptor function in hypertension and in aging. Can. J. Physiol. 2000;78:433–452. [PubMed] [Google Scholar]
- WHITE R., BOTTRILL F.E., SIAU D., HILEY C.R. Protein kinase A-dependent and -independent effects of isoproterenol in rat isolated mesenteric artery: interactions with levcromakalim. J. Pharmacol. Exp. Ther. 2001;298:917–924. [PubMed] [Google Scholar]