Abstract
ATP-sensitive K+ channels (KATP channels) are composed of pore-forming subunits (Kir6.x) and of regulatory subunits, the sulphonylurea receptors (SURx). Synthetic openers of KATP channels form a chemically heterogeneous class of compounds that are of interest in several therapeutic areas. We have investigated the interaction of a novel dihydropyridine opener, A-312110 ((9R)-9-(4-fluoro-3-iodophenyl)-2,3,5,9-tetrahydro-4H-pyrano[3,4-b]thieno [2,3-e]pyridin-8(7H)-one-1,1-dioxide), with SURs and Kir6/SUR channels in comparison to the cyanoguanidine opener P1075.
In the presence of 1 mM MgATP, A-312110 bound to SUR2A (the SUR in cardiac and skeletal muscle) and to SUR2B (smooth muscle) with Ki values of 14 and 18 nM; the corresponding values for P1075 were 16 and 9 nM, respectively. Decreasing the MgATP concentration reduced the affinity of A312110 binding to SUR2A significantly more than that to SUR2B; for P1075, the converse was true. At SUR1 (pancreatic β-cell), both openers showed little binding up to 100 μM.
In the presence of MgATP, both openers inhibited [3H]glibenclamide binding to the SUR2 subtypes in a biphasic manner. In the absence of MgATP, the high-affinity component of the inhibition curves was absent.
In inside-out patches, the two openers activated the Kir6.2/SUR2A and Kir6.2/SUR2B channels with similar potency (∼50 nM). Both were almost 2 × more efficacious in opening the Kir6.2/SUR2B than the Kir6.2/SUR2A channel.
The results show that the novel dihydropyridine A-312110 is a potent KATP channel opener with binding and channel-opening properties similar to those of P1075.
Keywords: KATP channel openers, dihydropyridine A-312110, cyanoguanidine P1075, binding to SUR and to Kir6.2/SUR, opener binding and MgATP, MgATP shift, opener affinity, opener potency, opener efficacy
Introduction
ATP-sensitive K+ (KATP) channels are composed of pore-forming subunits (Kir6.x) and of sulphonylurea receptors (SURx), which serve as regulatory subunits (Aguilar-Bryan et al., 1995; Sakura et al., 1995). KATP channels have a tetradimeric architecture, (Kir6.x)4(SURx)4 (Clement IV et al., 1997; Shyng & Nichols, 1997). Kir6.x and SURx each are encoded by two genes, giving rise to different subtypes. Additional complexity arises from alternative splicing of the SUR genes (Hambrock et al. (2002b) and references therein). A prominent example for this is given by the SUR2 isoforms, SUR2A and SUR2B, which differ in the use of the last exon (Inagaki et al., 1996; Isomoto et al., 1996). Different Kir 6.x and SURx subtypes combine to form the KATP channels in the various tissues; the resulting channels differ in their pharmacological and biophysical properties (review: Babenko et al., 1998; Gonoi & Seino, 2000; Seino & Miki, 2003).
KATP channels are gated by nucleotides; in particular, Kir6.2 containing KATP channels are closed by ATP and opened by MgADP. Hence, these channels link the metabolic state of the cell to membrane potential and cellular excitability (Ashcroft & Ashcroft, 1990; Seino & Miki, 2003). In the pancreatic β-cell, Kir6.2/SUR1 channels close when plasma glucose increases and thereby couple the early phase of insulin secretion to the plasma glucose level (Cook & Hales, 1984; Seghers et al., 2000; Seino & Miki, 2003). In glucose-responsive neurons in the ventromedial nucleus of the hypothalamus, the channels close when glucose increases (Ashford et al., 1990) and affect glucose homeostasis (Liss & Roeper, 2001). In some vascular beds, Kir6.1/SUR2B channels are activated by cAMP-dependent phosphorylation and contribute to the control vascular tone (Quayle et al., 1997). In the urinary bladder and other smooth muscle, opening of Kir6.x/SUR2B channels exerts a spasmolytic effect (Coghlan et al., 2001). In neurons (mostly Kir6.2/SUR1) and in skeletal and cardiac myocytes (Kir6.2/SUR2A), KATP channels open in response to ischaemia and hypoxia and help to preserve organ function (Fujita & Kurachi, 2000; Seino & Miki, 2003).
The important role of KATP channels in various tissues makes these channels an attractive drug target, and, according to the medical need, either opening or closing of the channel may be required (Lawson, 1996; Coghlan et al., 2001). The best known KATP channel modulators are the antidiabetic sulphonylureas and glinides that promote insulin secretion by binding to SUR1 and closing the channel in the pancreatic β-cell (Sturgess et al., 1985; Proks et al., 2002; Gribble & Reimann, 2003). KATP channel openers (KCOs) also bind to SUR and activate the channel closed by ATP. KCOs generally prefer Kir6.1 SUR2B, thereby inducing vasodilatation and hypotension as the predominant effects (Lawson, 1996; Quast, 1996). They belong to several chemically distinct families including benzopyrans, cyanoguanidines, thioformamides and dihydropyridines (Edwards & Weston, 1990; Coghlan et al., 2001).
Very recently, the synthesis of a dihydropyridine, A-312110, was reported, which has almost no affinity (>100 μM) for L-type Ca2+ channels and which relaxes electrically stimulated bladder strips from guinea-pig with EC50=18.5 nM (Davis-Taber et al., 2003). A-312110 carries an iodine and, after radioiodination, was shown to specifically bind to KATP channels in membranes from guinea-pig heart and bladder, with KD values of 5.8 and 4.9 nM, respectively. The high affinity and high specific activity (2000 Ci mmol−1) make [125I]A-312110 a very valuable addition to the other well-characterised radiolabelled openers, which are both tritiated, for example, the cyanoguanidine [3H] P1075 (Bray & Quast, 1992; Manley et al., 1993) and the benzopyran [3H]217-774 (Manley et al., 2001). It was the aim of this study to compare the binding and the channel-opening properties of A-312110 to those of the standard cyanoguanidine opener P1075 in human embryonic kidney (HEK) 293 cells expressing recombinant SURx and Kir6.2.
Methods
Cell culture and transfection
The mutant SUR2 subtypes SUR2A(Y1206S) and 2B(Y1206S) were constructed from the respective murine SUR2 clones (SUR2A: GenBank D86037, and SUR2B: GenBank D86038; Isomoto et al., 1996), using the QuikChange Site-Directed Mutagenesis System (Stratagene, Amsterdam, The Netherlands) as described (Hambrock et al., 2001). HEK 293 cells were cultured in Minimum Essential Medium containing glutamine and supplemented with 10% foetal bovine serum and 20 μg ml−1 gentamycin (Hambrock et al., 1998). Cells were transfected with the mammalian expression vector pcDNA 3.1 (Invitrogen, Karlsruhe, Germany) containing the coding sequence of rat SUR1 (GenBank X97279) or the murine SUR2 clones mentioned above using lipofectAMINE and OPTIMEM (Invitrogen), and cell lines stably expressing these proteins were generated as described (Hambrock et al., 1998). Cells transiently coexpressing SUR2 and murine Kir6.2 (D50581; Inagaki et al., 1996) were transfected with the plasmids at a molar ratio of 1 : 1. In cotransfections prepared for electrophysiological experiments, the pEGFP-C1 vector (Clontech, Palo Alto, CA, U.S.A.), encoding for green fluorescent protein, was added for identification of transfected cells. At 2–4 days after transfection, cells were used for binding studies and electrophysiological experiments.
Membrane preparation and radioligand-binding competition experiments
For cells stably expressing SUR alone, the antibiotic was withdrawn from the culture medium 3 days prior to membrane preparation. Membranes were prepared as described (Hambrock et al., 1998). Protein concentration was determined according to Lowry et al. (1951) using bovine serum albumin as the standard.
For radioligand-binding competition experiments in the absence/presence of MgATP, membranes were added to an incubation buffer containing (in mM): HEPES 5; NaCl 139; KCl 5; MgCl2 0/2.2; EDTA 1/0 and Na2ATP 0/1, and supplemented with the radioligand ([3H]P1075 ∼3 nM or [3H]GBC ∼2.2 nM) and (unlabelled) A-312110 or P1075. In case of 3 μM MgATP in the incubation medium, MgCl2 was reduced to 1 mM. After equilibrium was reached (25 min for [3H]P1075 and 15 min for [3H]GBC), incubation was stopped by diluting 0.3 ml aliquots (in triplicate) in 8 ml of ice-cold quench solution (50 mM Tris-(hydroxymethyl)-aminomethane, 154 mM NaCl, pH 7.4). The bound and free ligand were separated by rapid filtration over Whatman GF/B filters, washed twice with quench solution and counted for [3H] in the presence of 6 ml of scintillant (Ultima Gold: Packard, Meriden, CT, U.S.A.). Nonspecific binding (BNS) of [3H]P1075/[3H]GBC was determined in presence of 10/100 μM P1075 (Hambrock et al., 2001) and was ∼10/25% of total binding.
Electrophysiological experiments
The patch-clamp technique was used in the inside-out configuration, as described by Hamill et al. (1981). Patch pipettes were drawn from borosilicate glass capillaries (GC 150T, Harvard Apparatus, Edenbridge, U.K.) and heat polished using a horizontal microelectrode puller (Zeitz, Augsburg, Germany). Bath and pipette were filled with a high K+-Ringer solution containing (in mM) KCl 142; NaCl 2.8; MgCl2 1; CaCl2 1; D(+)-glucose 11; HEPES 10; titrated to pH 7.4 with NaOH at 22°C. Filled pipettes had a resistance of 1.0–1.5 MΩ. After excision of the patch, the pipette was moved in front of a pipe with a high K+-Ringer solution containing (in mM) KCl, 143; CaCl2, 1; D(+)-glucose, 11; HEPES, 10; EGTA, 5, titrated to pH 7.2 with NaOH at 22°C. MgCl2 was added such that [Mg2+]free was 0.7 mM. Openers were dissolved as described below and added to the pipe solution. Patches were clamped to −50 mV. Data were recorded with an EPC 9 amplifier (HEKA, Lambrecht, Germany) using the ‘Pulse' software (HEKA). Signals were filtered at 200 Hz using the four-pole Bessel filter of the EPC9 amplifier and sampled with 1 kHz.
Data analysis
Individual binding inhibition curves were analysed using the logarithmic form of the Hill equation
here, A denotes the extent (amplitude) of inhibition, n (=nH) the Hill coefficient and IC50 the midpoint of the curve with pIC50=−log IC50; x is the concentration of the compound under study with px=−log x. If necessary, two-component analysis was used with nH=1 and A2=100−A1.
IC50 values were converted into inhibition constants, Ki, by correcting for the presence of the radioligand, L, according to the Cheng & Prusoff (1973) equation
where KD is the equilibrium dissociation constant of the radioligand. In case of homologous competition experiments, the inhibition constant Ki is identical to the KD value. The correction was always <2.0.
The concentration dependence of channel opening was analysed using the ascending form of the Hill equation and taking all individual data points into account.
Data are shown as means±s.e.m. Fits of the equations to the data were performed according to the method of least squares using the programme SigmaPlot 6.1 (SPSS Science, Chicago, IL, U.S.A.). Individual binding experiments were analysed and the parameters averaged assuming that amplitudes and pIC50 values are normally distributed (Christopoulos, 1998). In the text, KD/Ki values are given, followed by the 95% confidence interval in parentheses. When the decision for the two-sites model was not obvious and the Hill coefficient was between 0.7 and 0.85, the fits to the one- and the two-sites model were compared by Fisher's F-test and the ‘Akaike minimum information criterion', as described in Quast & Mählmann (1982). In calculations involving two mean values with standard errors, propagation of errors was taken into account according to Bevington (1969). Significance of differences between two (normally distributed) parameters was assessed using the two-tailed unpaired Student's t-test.
Materials and solutions
[3H]P1075 (specific activity 4.5 TBq (117 Ci) mmol−1) was purchased from Amersham Buchler (Braunschweig, Germany) and [3H]glibenclamide ([3H]GBC, specific activity 1.85 TBq (50 Ci) mmol−1) from Perkin-Elmer Life Sciences (Bad Homburg, Germany). The reagents and media used for cell culture and transfection were from Invitrogen. A-312110 was synthesised as described by Carroll et al. (2001). P1075 was a kind gift from Leo Pharmaceuticals, Ballerup, Denmark. GBC was purchased from Sigma (Deisenhofen, Germany) and Na2ATP was from Roche Diagnostics (Mannheim, Germany). KATP channel modulators were dissolved in dimethyl sulphoxide/ethanol (50%/50%, v v−1) to give stock solutions of 0.1 M. These were further diluted with the same solvent or with incubation buffer to give final solvent concentrations <0.3%. Mg2+-free solutions (no Mg2+ added, contaminating Mg2+⩽10 μM (Forestier & Vivaudou, 1993; EDTA=1 or 5 mM) contained ⩽10 nM [Mg2+]free (Hambrock et al., 1998).
Results
Binding of A-312110 and P1075 to SUR2 subtypes: [3H]P1075 competition experiments
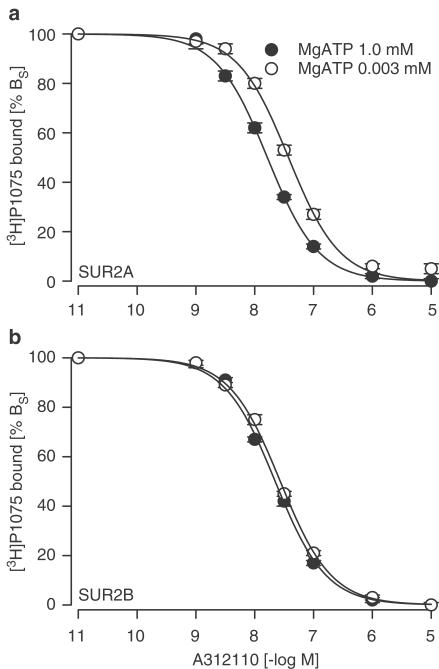
The interaction of A-312110 with SUR2A was first investigated using the tritiated cyanoguanidine opener [3H]P1075. High-affinity binding of openers to SUR requires the presence of MgATP (Schwanstecher et al., 1992; Quast et al., 1993; Dickinson et al., 1997; Hambrock et al., 1998; Schwanstecher et al., 1998) and experiments were first conducted in the presence of 1 mM MgATP, a saturating nucleotide concentration. Figure 1a illustrates the inhibition of [3H]P1075 binding to SUR2A by A-312110. The inhibition curve was regular (nH=1), reached completion and gave a Ki value of 14 nM (Table 1 ). MgATP was then reduced to 3 μM, a manoeuvre that decreased [3H]P1075 binding to 21±1% of that obtained with 1 mM MgATP (see legend to Figure 1). The A-312110 inhibition curve was shifted to the right by a factor f=2.2 (Figure 1a, Table 1). Analogous experiments were performed with P1075 and the results are summarised in Table 1. At 1 mM MgATP, a KD value of 16 nM was determined and this was not affected by the reduction in MgATP (comparison of pKD values by t-test).
Figure 1.
Inhibition of [3H]P1075 binding to SUR2A and 2B by A-312110 at 1 and 0.003 mM MgATP. (a) SUR2A, (b) SUR2B. Data were normalised with respect to % specific binding (% BS) and are means from 4–5 experiments. [3H]P1075 concentration was 3 nM and BS at 1 mM/0.003 mM MgATP was 120±13/25±3 fmol mg–1 for SUR2A and 230±12/60±7 fmol mg−1 for SUR2B, respectively. Individual binding curves were evaluated according to eqn (1), the parameters averaged as described in Methods and the mean values listed in Table 1.
Table 1.
Binding of A-312110 and P1075 to SUR subtypes
| A-312110 | P1075 | |||||||
|---|---|---|---|---|---|---|---|---|
| SUR | Radiolig. | MgATP (μM) | Ki (nM) | A (%)a | f b | KD/Ki (nM) | A (%)a | f b |
| SUR2A | 3H-P1075 | 1000 | 14 (12, 15) | 100 | 2.2 (1.9, 2.6)* | 16 (14, 18) | 100 | 1.3 (1.0, 1.7)c |
| 3 | 30 (28, 33)* | 100 | 20 (16, 25)c | 100 | ||||
| SUR2B | 3H-P1075 | 1000 | 18 (16, 20)* | 100 | 1.3 (1.1, 1.4)* | 9.1 (7.1, 11) | 100 | 2.0 (1.6, 2.5) |
| 3 | 22 (20, 25) | 100 | 18 (16, 20) | 100 | ||||
| SUR2A(YS) | 3H-GBC | 1000 | 8.0 (7.0, 10)* | 69±2 | 140 (100, 190) | 19 (14, 26) | 63±2 | 110 (76, 170) |
| 3000 (1800, 3900) | 31±2 | 1500 (800, 3000) | 37±2 | |||||
| 0 | 1100 (880, 1400)d | 91±6 | 2200 (1700, 2800) | 100 | ||||
| (nH=0.74±0.05)d | (nH=0.89±0.05) | |||||||
| Kir6.2/ | 3H-GBC | 1000 | 30 (24, 37) | 71±6 | 50 (38, 66) | Not done | ||
| SUR2A(YS) | 3500 (1400, 5600) | 29±6 | ||||||
| 0 | 1500 (1300, 1700) | 100 | ||||||
| SUR2B(YS) | 3H-GBC | 1000 | 12 (9, 15)* | 42±5 | 71 (54, 93)* | 5.9 (3.5, 9.8) | 46±4 | 200 (120, 350) |
| 850 (600, 1200) | 58±5 | 710 (490, 1000) | 54±4 | |||||
| 0 | 830 (690, 1000) | 100 | 1200 (1100, 1300) | 100 | ||||
| SUR1 | 3H-GBC | 1000 | Inhibition at 100 μM by 4±3% | Inhibition at 100 μM by 28±6% | ||||
| 0 | Inhibition at 300 μM by 2±2% | Inhibition at 100 μM: 0% | ||||||
Data for A-312110 were obtained as described in Figures 1 and 2; data for P1075 are from Stauss et al. (in preparation). For KD/Ki values, the 95% confidence intervals are given.
Amplitude (extent) of inhibition in % specific binding (% BS); in case of biphasic competition curves, A2=100−A1.
f denotes the MgATP shift calculated as f=10 exp[pKi (1 mM MgATP)−pKi (3 μM MgATP)]. In case of a biphasic curve at 1 mM MgATP, the pKi of the high-affinity component was used. f values are log normally distributed and were compared on the log scale.
KD value not different from that at 1 mM MgATP; f value not different from 1.0.
The fit to the two-sites model with nH=1 gave Ki values of 360 (190, 680) and 6200 (3400, 11,000) nM and amplitudes of 58±5 and 42±5% for the high- and low-affinity component, respectively. The two analyses were statistically equivalent as assessed by the F-test and the Akaike criterion.
Indicates a significant difference (P<0.05) between the respective parameters for A-312110 and P1075.
The interaction of A-312110 with SUR2B is shown in Figure 1b and Table 1. The Ki values obtained in the presence of 1 and 0.003 mM MgATP differed slightly from those determined for SUR2A. Reduction of MgATP reduced again the specific binding of the radioligand and induced a small but still significant rightward shift of the inhibition curve (f=1.3). This shift was significantly smaller than that observed for binding to SUR2A (f=2.2; P<0.05; comparison of log f values), whereas the opposite was true for P1075 (Table 1).
Binding of A-312110 and P1075 to SUR subtypes: [3H]GBC inhibition experiments
The interaction of openers with SUR can also be studied using [3H]GBC as the radioligand; this gives additional information since openers interfere with GBC binding by allosteric inhibition (Bray & Quast, 1992; Schwanstecher et al., 1992; Hambrock et al., 2002a). The low affinity of the SUR2 subtypes for GBC (KD∼25–30 nM; Hambrock et al., 2001; Stauß et al., unpublished results) is, however, insufficient for high-precision binding studies with this lipophilic radioligand. We have therefore prepared SUR2 mutants in which Tyr in position 1206 is replaced by Ser, which is the corresponding amino acid in SUR1. This mutation increases the affinity of the SUR2 subtypes for GBC by ∼25 × (Toman et al., 2000) or ∼10 × (Hambrock et al., 2001; Stauß et al., unpublished results), while decreasing the opener affinity by less than 2 × (Hambrock et al., 2001; Russ et al., 2003; Stauß et al., unpublished results).
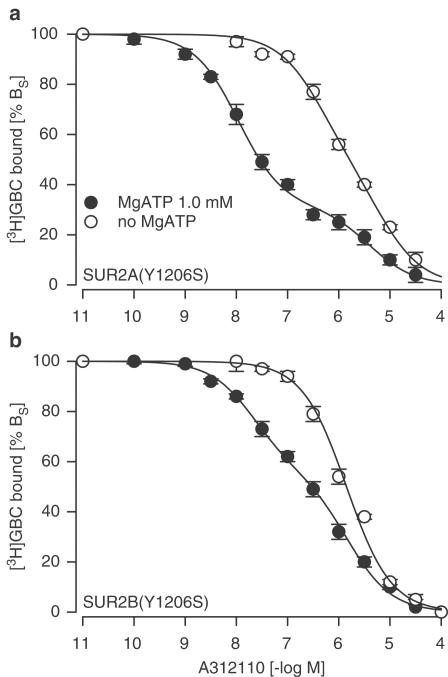
Figure 2a illustrates the inhibition of [3H]GBC binding to SUR2A(YS) by A-312110. In the presence of 1 mM MgATP, the inhibition curve was strongly biphasic. The high-affinity component comprised 69% of the total inhibition with a Ki value of 8 nM; the low-affinity component (31%) had a Ki value of 3000 nM (Table 1). In the absence of MgATP (no ATP, presence of 1 mM EDTA), the inhibition curve was shifted rightwards and looked essentially homogeneous with Ki=1.1 μM; however, the Hill coefficient of 0.74±0.05 (<1.0;P<0.001) suggested slight heterogeneity of the binding sites. The fit to a two-sites model gave two components with Ki values of 360 and 6200 nM, respectively, and was statistically equivalent to the Hill fit (see Table 1 for details). Inspection of Table 1 shows that similar results were obtained with P1075: In the presence of 1 mM MgATP, the inhibition curve was strongly biphasic; in its absence, the curve was essentially monophasic (nH=0.89±0.05<1.0; P<0.05) and could not be broken down into two components.
Figure 2.
Inhibition of [3H]GBC binding to SUR2(YS) by A-312110 in the presence of 1 and 0 mM MgATP. (a) SUR2A(YS), (b) SUR2B(YS). Data are means from four experiments and are presented as % BS. At the [3H]GBC concentration of 2.2 nM, BS in the absence of MgATP was 204±8 and 370±15 fmol mg–1 for mutant SUR2A and SUR2B, respectively; MgATP (1 mM) reduced BS to 67±4/57±3 for mutant SUR2A/2B, respectively, without affecting KD (Hambrock et al., 2002a). For parameters, see Table 1.
It was also of interest to examine whether coexpression of SUR2A(YS) with Kir6.2 affected the allosteric interactions between [3H]GBC and A-312110 binding. In the presence of MgATP, the A-312110 inhibition curve was again biphasic (Table 1). Coexpression shifted the high-affinity component from 8 to 30 nM, whereas the low-affinity component and the ratio of the amplitudes remained unchanged. In the absence of MgATP, the A-312110 inhibition curve was now strictly monophasic (nH=1.0 compared to 0.74 with SUR2A(YS) expressed alone) with a KD of 1.5 μM. This value was similar to that found when the inhibition curve for SUR2A(YS) alone was analysed with a one-component Hill model (Table 1). Hence, both in the presence and absence of MgATP, coexpression with Kir6.2 reduced the heterogeneity in the interaction of A-312110 with SUR2A(YS), apparent in the [3H]GBC-binding studies.
The analogous experiments using SUR2B(YS) are shown in Figure 2b and the results are summarised in Table 1. As with SUR2A(YS), the A-312110 and P1075 inhibition curves were biphasic in the presence of 1 mM MgATP; in the absence of MgATP, they were strictly monophasic. The respective Ki values were similar to those determined with SUR2A(YS) (Table 1). A clear difference between SUR2 subtypes was the amplitude ratio of the biphasic inhibition curves: for both openers, the high-affinity component was significantly smaller for SUR2B(YS) than for SUR2A(YS).
In [3H]GBC-binding experiments using SUR1 and in the presence of MgATP, A-312110 did not show a significant interaction with SUR1; P1075 was very week.
Electrophysiological experiments
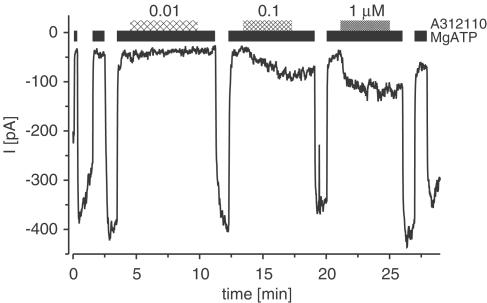
The channel-opening ability of the KCOs was examined in inside-out patches from HEK cells expressing Kir6.2/SUR2x channels. Experiments were performed in the presence of 1 mM MgATP to keep the channel closed. Figure 3 shows a recording of A-312110 opening the Kir6.2/SUR2A channel. In this experiment, A-312110 was ineffective at 0.01 μM, but elicited currents at 0.1 and 1 μM. The currents faded slowly upon washout of the drug; in the absence of MgATP, fading was accelerated (cf. Gribble et al., 2000; Reimann et al., 2000).
Figure 3.
Recording from an inside-out patch showing activation of the Kir6.2/SUR2A channel by A-312110. After excision of the patch into nucleotide-free solution, a current was present which was essentially abolished by superfusion with MgATP (1 mM). This showed that the current was indeed flowing through KATP channels. A-312110 was applied in the presence of MgATP as indicated by the hatched bars. Holding potential was −50 mV; experiments were performed in symmetrical high K+ buffer at 22°C.
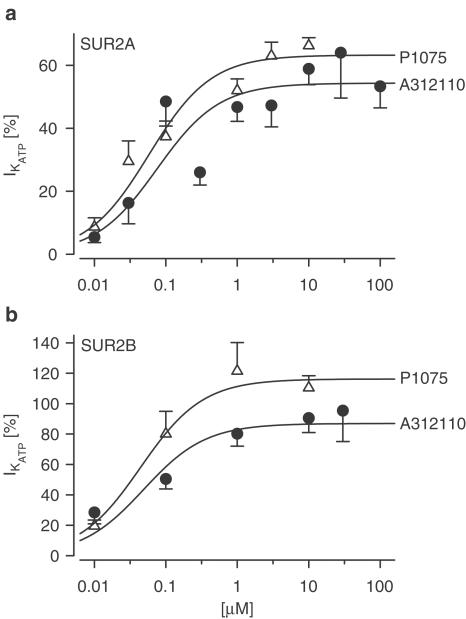
In the course of these experiments, it was observed that MgATP (1 mM) inhibited Kir6.2/SUR2A channels more strongly thanKir6.2/SUR2B channels (see the legend of Figure 4 for details). Figure 4 illustrates the concentration-dependent activation of the Kir6.2/SUR2x channels by the openers; the parameters of the activation curves are listed in Table 2 . A-312110 and P1075 were of similar potency (∼50 nM) in activating the two channels. The maximum effects (amplitudes) obtained from the Hill fit showed that at both channels P1075 was ∼20–30% more efficacious than A312110 (Table 2); however, if only the data in the saturation region were considered (Figure 4), this difference was not maintained. It is also seen that both openers were ∼75% more efficacious in activating the Kir6.2/SUR2B than the Kir6.2/SUR2A channel (efficacy ratios of 170±21 and 184±12% for A-312110 and P1075, respectively; propagation of errors taken into account).
Figure 4.
Concentration-dependent activation of Kir6.2/SUR2x channels by A-312110 and P1075. (a) Kir6.2/SUR2A, (b) Kir6.2/SUR2B channels. Data were obtained from experiments as shown in Figure 3. The KCO-induced current (IKCO) was normalised with respect to the peak current in the absence of MgATP before run-down, since run-down is reversed by MgATP (Ribalet et al., 2000). Concentration dependencies were analysed using the logarithmic form of the Hill equation with nH=1; n=3−12 per data point. Control currents in the presence of MgATP (1 mM) and absence of opener were 3.5 (0, 29)% of the current in the absence of ATP for Kir6.2/SUR2A (n=174) and 6.5 (0, 33) for Kir6.2/SUR2B (n=74; median and 95% confidence interval; P=0.027 by Mann–Whitney rank sum test).
Table 2.
Activation of KATP channels by A-312110 and P1075
| A-312110 | P1075 | |||
|---|---|---|---|---|
| Channel | EC50 (nM) | A (%) | EC50 (nM) | A (%) |
| Kir6.2/SUR2A | 58 (25,137) | 51±5 | 58 (35,96) | 63±2a |
| Kir6.2/SUR2B | 49 (19,123) | 87±7b | 44 (17,116) | 116±9a,b |
Parameters are from fits of the logistic equation with nH=1 to the individual data points obtained as shown in Figure 3; mean values are presented in Figure 4. A=amplitude.
Significantly different from the value with A-312110 (P<0.05).
Significantly different from the value with Kir6.2/SUR2A (P<0.01).
Discussion
Affinity to SUR subtypes
At saturating MgATP, the [3H]P1075–A-312110 inhibition curves for SUR2A and 2B were monophasic and gave the same Ki value (∼15 nM); hence, in the binding step, the new opener showed no selectivity for one of the SUR2 subtypes. This conclusion agrees with Davis-Taber et al. (2003), who measured [125I]A-312110 binding in membranes from guinea-pig heart (Kir6.2/SUR2A) and bladder (Kir6.x/SUR2B) and obtained KD values of 5.8 and 4.9 nM, respectively. The three-fold difference in the KD (Ki) values from the two studies probably reflects differences in experimental conditions (e.g. presence or absence of an ATP-regenerating system and/or native vs recombinant system, etc.). In contrast to A-312110, P1075 showed a slight (∼two-fold) selectivity for SUR2B over SUR2A. This agrees with results obtained in the recombinant system (Schwanstecher et al., 1998; Hambrock et al., 1999) and in membranes from native tissues (Davis-Taber et al., 2003).
With [3H]GBC as the radioligand and in the presence of MgATP, the inhibition curves of the two openers to the SUR2(YS) subtypes were biphasic. The Ki values of the high-affinity components were close to the Ki values obtained with [3H]P1075, and thus reflected the true affinity of the openers for the SUR2 subtypes in the presence of MgATP. The Ki values of the low-affinity components agreed well with those using [3H]GBC in the absence of MgATP. Comparing the inhibition of [3H]GBC binding to SUR2B(YS) by a large number of KCOs, we recently found that cyanoguanidines, benzopyrans and aprikalim showed this biphasic behaviour and these openers were classified as ‘typical' KCOs; in contrast, nicorandil, minoxidil sulphate and diazoxide were monophasic (Russ et al., 2003). This study shows that the dihydropyridine A-312110 also belongs to the group of ‘typical' KCOs and that the biphasic [3H]GBC-opener inhibition curves, first observed with SUR2B(YS) (Hambrock et al., 2001; Hambrock et al., 2002a; Russ et al., 2003), occur also with SUR2A(YS).
MgATP dependence
Turning first to the [3H]P1075 experiments, we recall that high-affinity opener binding to SUR requires the presence of MgATP. Binding of MgATP converts SUR into a high-affinity state for openers (Schwanstecher et al., 1998), in which the SUR-opener complex is stabilised by a decreased dissociation rate (Gribble et al., 2000; Reimann et al., 2000). SUR exhibits ATPase activity and the two nucleotide-binding domains of SUR are linked by positive cooperativity and act in concert (Ueda et al., 1999; Bienengraeber et al., 2000). Reducing MgATP from 1 mM to 3 μM decreased the binding of [3H]P1075 (3 nM) to SUR2A and 2B by 75–80%. This loss reflects conversion of SUR to the low-affinity state for openers due to the lack of MgATP. The fraction of SUR which can still be labelled by [3H]P1075 is in a state different from that at high MgATP, probably due to differences in occupation of the nucleotide-binding domains by MgATP and its hydrolysis products. Considering SUR2B, we have found that in this low MgATP state the affinity of SUR for openers is generally reduced and that for blockers like GBC and phloxin B is increased (Hambrock et al., 2000). Here we found that for SUR2B reduction of MgATP gave a smaller shift for A-312110 than for P1075 (1.3 vs 2.0), whereas for SUR2A the opposite was true (2.2 vs 1.3).
Use of [3H]GBC as the radioligand offers the advantage that opener binding can be monitored in the presence and the complete absence of MgATP; the Ki values under the two conditions reflect the opener affinity of SUR in these two states. For SUR2B(YS), we found for A-312110 a shift of 70 which is significantly lower than for P1075 (200; this study) and that for the other ‘typical openers' (∼200; Russ et al., 2003). For SUR2A(YS), the absence of MgATP increased the Ki values of A-312110 and P075 to a similar degree (140 vs 110, respectively). Taken together, the results show that reduction of MgATP decreased the affinity of SUR2B for A-312110 significantly less than for P1075 (and other typical KCOs); for SUR2A, the [3H]P1075 experiments showed the converse.
Channel opening
The two openers activated the Kir6.2/SUR2A and /SUR2B channels with the same potency (EC50 values ∼50 nM at 22°C). This is lower than the KD/Ki values for binding to SUR2 (without coexpression of Kir6.2) determined here (∼10–20 nM) and the KD values for binding to native channels in membranes from smooth muscle and heart (5–6 nM; Bray & Quast, 1992; Löffler-Walz & Quast, 1998; Davis-Taber et al., 2003; all values at 37°C). This discrepancy has generally been observed with KCOs (see, for example, Schwanstecher et al., 1998) and is aggravated if both binding and channel opening are assessed at the same temperature (37°C; Quast et al., 1993). It probably reflects the complex signal transduction within the channel in which KCO binding affects the ATPase activity of SUR, thereby determining the channel activity (Bienengraeber et al., 2000; Zingman et al., 2001). Regarding the efficacies in channel opening, the two openers were similar as well. Interestingly, both openers exhibited an almost two times higher efficacy in activating the Kir6.2/SUR2B than the SUR2A channel, and this may be related to the stronger inhibition of the Kir6.2/SUR2A channels by MgATP (see also Reimann et al., 2000).
In conclusion, we have characterised here the binding and channel-opening properties of a novel dihydropyridine KCO, A-312110, in comparison to the standard opener P1075, in recombinant systems. The results show that A-312110 is a potent KCO with properties similar to those of P1075. The new opener, which is available in radioiodinated form (Davis-Taber et al., 2003), makes a welcome addition to the chemically diverse group of KATP channel openers.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Qu 100/3-1, U.Q.), the Federal Ministry of Education, Science, Research and Technology (Fö 01KS9602) and the Interdisciplinary Center of Clinical Research (IZKF), Tübingen. We thank Drs Y. Kurachi and Y. Horio (Osaka) for the generous gift of the murine clones of SUR2A, 2B and Kir6.2, and Dr C. Derst, Marburg for the rat clone of SUR1.
Abbreviations
- A-312110
(9R)-9-(4-fluoro-3-iodophenyl)-2,3,5,9-tetrahydro-4H-pyrano[3,4-b]thieno [2,3-e]pyridin-8(7H)-one-1,1-dioxide
- GBC
glibenclamide
- HEK cells
human embryonic kidney 293 cells
- KATP channel
ATP-sensitive K+ channel
- KCO
KATP channel opener
- Kir
inwardly rectifying K+ channel
- P1075
N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine
- SUR
sulphonylurea receptor
- SUR2(YS)
SUR2(Y1236S)
References
- AGUILAR-BRYAN L., NICHOLS C.G., WECHSLER S.W., CLEMENT J.P., IV, BOYD A.E., III, GONZÁLES G., HERRERA-SOZA H., NGUY K., BRYAN J., NELSON D.A. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- ASHCROFT S.J.H., ASHCROFT F.M. Properties and functions of ATP-sensitive K-channels. Cell. Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- ASHFORD M.L., BODEN P.R., TREHERNE J.M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Arch. Eur. J. Physiol. 1990;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- BABENKO A.P., AGUILAR-BRYAN L., BRYAN J. A view of SUR/KIR6.X, KATP channels. Annu. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- BEVINGTON P.R. Data Reduction and Error Analysis for the Physical Sciences 1969New York: McGraw-Hill; 55, 92–65.and [Google Scholar]
- BIENENGRAEBER M., ALEKSEEV A.E., ABRAHAM M.R., CARRASCO A.J., MOREAU C., VIVAUDOU M., DZEJA P.P., TERZIC A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- BRAY K.M., QUAST U. A specific binding site for K+ channel openers in rat aorta. J. Biol. Chem. 1992;267:11689–11692. [PubMed] [Google Scholar]
- CARROLL W.A., AGRIOS K.A., ALTENBACH R.J., DRIZIN I., KORT M.E.Inventors, Abbot Laboratories, assignee. Preparation of 4-arylbisannelated-1, 4-dihydropyridines as potassium channel openers 2001. U.S. Patent 6,191,140B1.2001 February 20
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS A. Assessing the distribution of parameters in models of ligand–receptor interaction: to log or not to log. Trends Pharmacol. Sci. 1998;19:351–357. doi: 10.1016/s0165-6147(98)01240-1. [DOI] [PubMed] [Google Scholar]
- CLEMENT J.P., IV, KUNJILWAR K., GONZALEZ G., SCHWANSTECHER M., PANTEN U., AGUILAR-BRYAN L., BRYAN J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- COGHLAN M.J., CARROLL W.A., GOPALAKRISHNAN M. Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J. Med. Chem. 2001;44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- COOK D.L., HALES C.N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- DAVIS-TABER R., MOLINARI E.J., ALTENBACH R.J., WHITEAKER K.L., SHIEH C.-C., ROTERT G., BUCKNER S.A., MALYSZ J., MILICIC I., MCDERMOTT J.S., GINTANT G.A., COGHLAN M.J., CARROLL W.A., SCOTT V.E., GOPALAKRISHNAN M. 125I]A-312110, a novel high-affinity 1,4-dihydropyridine ATP-sensitive K+ channel opener: characterization and pharmacology of binding. Mol. Pharmacol. 2003;64:143–153. doi: 10.1124/mol.64.1.143. [DOI] [PubMed] [Google Scholar]
- DICKINSON K.E.J., BRYSON C.C., COHEN R.B., ROGERS L., GREEN D.W., ATWAL K.S. Nucleotide regulation and characteristics of potassium channel opener binding to skeletal muscle membranes. Mol. Pharmacol. 1997;52:473–481. doi: 10.1124/mol.52.3.473. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. Structure activity relationships of K+ channel openers. Trends Pharmacol. Sci. 1990;11:417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- FORESTIER C., VIVAUDOU M. Modulation by Mg2+ and ADP of ATP-sensitive potassium channels in frog skeletal muscle. J. Membr. Biol. 1993;132:87–94. doi: 10.1007/BF00233054. [DOI] [PubMed] [Google Scholar]
- FUJITA A., KURACHI Y. Molecular aspects of ATP-sensitive K+ channels in the cardiovascular system and K+ channel openers. Pharmacol. Ther. 2000;85:39–53. doi: 10.1016/s0163-7258(99)00050-9. [DOI] [PubMed] [Google Scholar]
- GONOI T., SEINO S.Structure and function of ATP-sensitive K+ channels Pharmacology of Ionic Channel Function: Activators and Inhibitors 2000New York: Springer Verlag; 271–295.ed. Endo, M., Kurachi, Y. & Mishina, M. pp [Google Scholar]
- GRIBBLE F.M., REIMANN F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- GRIBBLE F.M., REIMANN F., ASHFIELD R., ASHCROFT F.M. Nucleotide modulation of pinacidil stimulation of the cloned KATP channel Kir6.2/SUR2A. Mol. Pharmacol. 2000;57:1256–1261. [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., KLOOR D., DELABAR U., HORIO Y., KURACHI Y., QUAST U. ATP-sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol. Pharmacol. 1999;55:832–840. [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., KURACHI Y., QUAST U. Mg2+ and ATP dependence of KATP channel modulator binding to the recombinant sulphonylurea receptor, SUR2B. Br. J. Pharmacol. 1998;125:577–583. doi: 10.1038/sj.bjp.0702109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., QUAST U. Glibenclamide binding to sulphonylurea receptor subtypes: dependence on adenine nucleotides. Br. J. Pharmacol. 2002a;136:995–1004. doi: 10.1038/sj.bjp.0704801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., RUSS U., QUAST U.Allosteric coupling between the binding sites for sulphonylureas and ATP on the sulphonylurea receptor SUR2B Naunyn-Schmiedeberg's Arch. Pharmacol. (Suppl.) 2000361R76(abstract) [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., RUSS U., LANGE U., QUAST U. Characterization of a mutant sulfonylurea receptor SUR2B with high affinity for sulfonylureas and openers: differences in the coupling to Kir6.x subtypes. Mol. Pharmacol. 2001;60:190–199. doi: 10.1124/mol.60.1.190. [DOI] [PubMed] [Google Scholar]
- HAMBROCK A., PREISIG-MÜLLER R., RUSS U., PIEHL A., HANLEY P.J., RAY J., DAUT J., QUAST U., DERST C. Four novel splice variants of sulfonylurea receptor 1. Am. J. Physiol. (Cell Physiol.) 2002b;283:C587–C598. doi: 10.1152/ajpcell.00083.2002. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. – Eur. J. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, WANG C.Z., AGUILAR-BRYAN L., BRYAN J., SEINO S. A family of suphonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KONDO C., YAMADA M., MATSUMOTO S., HIGASHIGUCHI O., HORIO Y., MATSUZAWA Y., KURACHI Y. A novel sulfonylurea receptor forms with BIR (KIR6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- LAWSON K. Potassium channel activation: a potential therapeutic approach. Pharmacol. Ther. 1996;70:39–63. doi: 10.1016/0163-7258(96)00003-4. [DOI] [PubMed] [Google Scholar]
- LISS B., ROEPER J. Molecular physiology of neuronal K-ATP channels. Mol. Membr. Biol. 2001;18:117–127. [PubMed] [Google Scholar]
- LÖFFLER-WALZ C., QUAST U. Binding of KATP channel modulators in rat cardiac membranes. Br. J. Pharmacol. 1998;123:1395–1402. doi: 10.1038/sj.bjp.0701756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MANLEY P.W., LÖFFLER-WALZ C., RUSS U., HAMBROCK A., MOENIUS T., QUAST U. Synthesis and characterization of a novel tritiated KATP channel opener with a benzopyran structure. Br. J. Pharmacol. 2001;133:275–285. doi: 10.1038/sj.bjp.0704071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANLEY P.W., QUAST U., ANDRES H., BRAY K.M. Synthesis of and radioligand binding studies with a tritiated pinacidil analogue: receptor interactions of structurally different classes of potassium channel openers and blockers. J. Med. Chem. 1993;36:2004–2010. doi: 10.1021/jm00066a009. [DOI] [PubMed] [Google Scholar]
- PROKS P., REIMANN F., GREEN N., GRIBBLE F., ASHCROFT F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51 Suppl 3:S368–S376. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- QUAST U.Effects of potassium channel activators in isolated blood vessels Potassium Channels and their Modulators: From Synthesis to Clinical Experience 1996London: Taylor & Francis; 173–195.ed. Evans, J.M., Hamilton, T.C., Longman, S.D. & Stemp, G. pp [Google Scholar]
- QUAST U., BRAY K.M., ANDRES H., MANLEY P.W., BAUMLIN Y., DOSOGNE J. Binding of the K+ channel opener [3H]P1075 in rat isolated aorta: relationship to functional effects of openers and blockers. Mol. Pharmacol. 1993;43:474–481. [PubMed] [Google Scholar]
- QUAST U., MÄHLMANN H. Interaction of [3H]flunitrazepam with the benzodiazepine receptor: evidence for a ligand-induced conformation change. Biochem. Pharmacol. 1982;31:2761–2768. doi: 10.1016/0006-2952(82)90130-7. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- REIMANN F., GRIBBLE F.M., ASHCROFT F.M. Differential response of KATP channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol. Pharmacol. 2000;58:1318–1325. doi: 10.1124/mol.58.6.1318. [DOI] [PubMed] [Google Scholar]
- RIBALET B., JOHN S.A., WEISS J.N. Regulation of cloned ATP-sensitive K channels by phosphorylation, MgADP, and phosphatidylinositol bisphosphate (PIP2) – A study of channel rundown and reactivation. J. Gen. Physiol. 2000;116:391–409. doi: 10.1085/jgp.116.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSS U., LANGE U., LÖFFLER-WALZ C., HAMBROCK A., QUAST U. Binding and effect of KATP channel openers in the absence of Mg2+ Br. J. Pharmacol. 2003;139:368–380. doi: 10.1038/sj.bjp.0705238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURA H., ÄMMÄLÄ C., SMITH P.A., GRIBBLE F.M., ASHCROFT F.M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic ß-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- SCHWANSTECHER M., BRANDT C., BEHRENDS S., SCHAUPP U., PANTEN U. Effect of MgATP on pinacidil-induced displacement of glibenclamide from the sulphonylurea receptor in a pancreatic ß-cell line and rat cerebral cortex. Br. J. Pharmacol. 1992;106:295–301. doi: 10.1111/j.1476-5381.1992.tb14331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DÖRSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C., BRYAN J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGHERS V., NAKAZAKI M., DEMAYO F., AGUILAR-BRYAN L., BRYAN J. Sur1 knockout mice – a model for KATP channel-independent regulation of insulin secretion. J. Biol. Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- SEINO S., MIKI T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- SHYNG S.-L., NICHOLS C.G. Octameric stoichiometry of the KATP channel complex. J. Gen. Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURGESS N.C., ASHFORD M.L., COOK D.L., HALES C.N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985;2:474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- TOMAN A., UHDE I., SCHWANSTECHER M.A single residue in SURs is essential for sulphonylurea binding Naunyn-Schmiedeberg's Arch.Pharmacol. (Suppl.) 2000361R75(abstract) [Google Scholar]
- UEDA K., KOMINE J., MATSUO M., SEINO S., AMACHI T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINGMAN L.V., ALEKSEEV A.E., BIENENGRAEBER M., HODGSON D., KARGER A.B., DZEJA P.P., TERZIC A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]