Abstract
Cyclooxygenase (COX)-2 expression and activity in response to pro-inflammatory cytokines TNFα and IFNγ was evaluated in the colonic epithelial cell line HT29 and the airway epithelial cell line A549.
TNFα induced concentration- and time-dependent upregulation of COX-2 mRNA, protein and prostaglandin (PG)E2 synthesis.
Co-stimulation of TNFα with IFNγ resulted in reduced COX-2 mRNA and protein expression.
IFNγ had no effect on the stability of TNFα-induced COX-2 mRNA.
TNFα-induced PGE2 biosynthesis was significantly enhanced by the simultaneous addition of IFNγ and was COX-2 dependent.
The combination of IFNγ and TNFα induced the microsomal prostaglandin E synthase (mPGES), comensurate with the enhanced PGE2 synthesis.
These results suggest that, in terms of PGE2 biosynthesis, IFNγ plays a negative regulatory role at the level of COX-2 expression and a positive regulatory role at the level of mPGES expression. This may have important implications for the clinical use of IFNγ in inflammatory diseases.
Keywords: Inflammation, cyclooxygenase, prostaglandin, synthase, cytokine, colon
Introduction
Cyclooxygenase (COX) is the rate-limiting step in the synthesis of the lipid mediators, prostaglandins and thromboxanes, from arachidonic acid. In 1991, two isoforms were found to be responsible for this catalytic activity – the constitutively expressed COX-1 and the inducible COX-2 (Fu et al., 1991; Kujubu et al., 1991). As the site of action of nonsteroidal anti-inflammatory drugs (NSAIDs), the importance of COX activity had been known for some time (Hemler et al., 1976) and the inducible component COX-2 is now recognised as being a pivotal enzyme both in inflammation and carcinogenesis (DuBois et al., 1998). COX-2 is known to be the product of an immediate early gene and can be induced by a number of different stimuli in many contrasting systems including inflammatory cytokines, tumour promoters and growth factors (Herschman, 1996). The COX-derived endoperoxide PGH2 can then be converted to PGE2 by prostaglandin E synthase (PGES). There are two isoforms of this terminal enzyme, a constitutive cytosolic isoform (cPGES) and an inducible microsomal isoform (mPGES). COX-2 and mPGES have been reported to be functionally linked (Murakami et al., 2000), whereas cPGES is thought to be associated with COX-1 (Tanioka et al., 2000).
The pro-inflammatory Th1 cytokine tumour necrosis factor α (TNFα) has been shown to induce COX-2 in many systems (Arias-Negrete et al., 1995; Pang & Knox, 1997; Jobin et al., 1998; Huang et al., 2000). Indeed, using HT-29 intestinal epithelial cells, it has been demonstrated that TNFα induction of COX-2 is completely dependent on the transcription factor nuclear factor κB (NFκB) (Jobin et al., 1998). Increased TNFα levels play a central role in Crohn's disease (CD), as evidenced by the significant therapeutic action of its blockade with monoclonal antibody (Breese et al., 1994; Targan et al., 1997; Van Deventer, 1997).
Interferon-γ (IFNγ) is a cytokine whose role in COX-2 regulation is somewhat less clear. It has been shown to play an important part in the colitis that develops in CD45RBhi CD4+ T-cell restored SCID mice (Powrie et al., 1994). However, it has also been used in therapeutic trials for CD and is an accepted treatment for chronic granulomatous disease (Debinski et al., 1997). This is at odds with reports that show that IFNγ induces COX-2 in human keratinocytes (Arias-Negrete et al., 1995; Matsuura et al., 1999), whereas it inhibits IL-1β-induced transcription of COX-2 in human macrophages (Barrios-Rodiles & Chadee, 1998). In intestinal epithelial cells, we have shown that IFNγ combines with IL-1α to induce nitric oxide synthase (NOS2) expression (Kolios et al., 1995), can act synergistically with TNFα to induce such pro-inflammatory mediators as the chemokine RANTES (regulated upon activation T-cell expressed and secreted), monocyte chemo-attractant protein (MCP)-1 and IL-8 (Kolios et al., 1999) and initiate the apoptotic program (Wright et al., 1999).
In this work, we investigated the possible regulatory role of IFNγ on TNFα-induced COX-2 expression and activity in two unrelated epithelial cell lines, namely the colonic epithelial cell line HT-29 and the lung epithelial cell line A549. This study reveals the disparate effects of IFNγ on TNFα-induced COX-2 transcriptional induction and translation in both cell models when compared to the functional activity in terms of PGE2 biosynthesis.
Methods
Materials
Human recombinant TNFα (specific activity, 6 × 107 U mg−1) was kindly provided by Glaxo (Greenford, U.K.) and human recombinant IFNγ was purchased from Peprotech (London, U.K.). Two cDNAs for COX-2 were used – Oxford Biomedical Research (Oxford, MI, U.S.A.) and Invitrogen Corporation (San Diego, CA, U.S.A.) and shown to be equivalent with regard to specificity. These were labeled with [32P] deoxycytidine 5-α triphosphate (Amersham Life Sciences, U.K.) by random priming incorporation using High Prime (Roche Diagnostics, Lewes, U.K.). Rabbit IgG antibodies to COX-2 and mPGES were purchased from Cayman Chemical (Alexis, U.K.). All other reagents were from Sigma (Poole, U.K.).
Cell culture
The human epithelial cell lines HT-29 and A549 were obtained from the European Collection of Animal Cell Cultures. Cells were cultured in humidified incubators at 37°C, 5% CO2 in McCoy's 5A and DMEM (with 2 mM L-glutamine) medium, respectively, supplemented with 10% foetal bovine serum (FBS) and 10 U ml−1 penicillin/streptomycin. The cells were passaged weekly and, for experiments, cells were seeded at 2–3 × 104 cells ml−1 until confluent. Confluent cells were washed and cultured in fresh medium without FBS 24 h before stimulation. Growth-arrested cells were treated with the appropriate concentrations of stimuli in medium without serum and incubated as described above. Cell counting and viability were checked by trypan blue exclusion at the beginning and end of each experiment using representative wells and were always greater than 95%.
Northern analysis for COX-2 mRNA
Total cellular RNA was isolated using RNAsol B. The concentration of RNA was measured by obtaining the absorbance at 260 and 280 nm and 10 μg of RNA was loaded into each well of the agarose gel. Total RNA was separated using formaldehyde, 1% agarose gels and transferred overnight to nylon membrane by capillary blotting. Blots were baked for 20 min at 120°C, pre-hybridised for 1 h and then hybridised with the 32P-labelled COX-2 probe in high SDS hybridisation solution (7% SDS, 0.1 mM EDTA, 0.25 M Na2HPO4 (pH 7.2)) at 63°C overnight as previously described (Soriani et al., 1999). Membranes were washed twice for 5 min with 6 × SSPE, 0.1% SDS at 37°C, then twice for 15 min with 1 × SSPE, 0.1% SDS at 37°C, then two high-stringency washes at 63°C with 2 × SSPE, 0.1% SDS for 5 min. The bound probe was quantified with a phosphorimager (Molecular Dynamics, Sunnyvale, U.S.A.). Equivalent amounts of total RNA load per gel lane were assessed by stripping the membranes and re-probing for β-actin as previously described (Wright et al., 1997), using a digoxigenin-labelled oligonucleotide probe visualised with anti-digoxigenin Fab fragments conjugated to alkaline phosphatase with lumigen PPD as chemiluminescent substrate (all Roche), and also by monitoring 18S and 28S RNA. Membranes were stripped by washing twice in 0.1% SDS at 100oC, followed by cooling to room temperature.
Cell lysis
In all, 107 cells ml−1 were stimulated and incubated at 37°C in McCoy's as indicated. Reactions were terminated by the addition of 1 ml of ice-cold lysis buffer (1% (v v−1) Nonidet P-40, 150 mM NaCl, 50 mM Tris pH 7.5, 5 mM EDTA, 10 mM sodium fluoride, 1 mM phenylmethylsulphonyl fluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 1 μg ml−1 soybean trypsin inhibitor, 1 μg ml−1 pepstatin A, 1 mM sodium orthovanadate and 1 mM sodium molybdate). Lysates were sonicated for 30 s, followed by centrifugation at 14, 000 r.p.m. to remove debris. A Bradford Protein Assay (Bio-Rad) was then performed on the supernatants.
Western analysis for COX-2 and mPGES protein
Equalised aliquots of cell lysate supernatant were boiled in Laemmli buffer and electrophoresed through 7.5% (v v−1) acrylamide gels (with an acrylamide : bis-acrylamide ratio of 37.5 : 1) by SDS–PAGE and the proteins were transferred by electroblotting onto nitrocellulose (BDH, VWR International Ltd, U.K.), as described previously (Wright et al., 1997). The blots were probed with rabbit polyclonal antibodies (1 μg ml−1) and proteins visualised by ECL (Amersham Biosciences U.K. Ltd) with a rabbit anti-goat Ig (0.1 μg ml−1) conjugated with horseradish peroxidase as the secondary antibody.
Prostaglandin E2 ELISA
PGE2 was assayed using a commercially available ELISA from R&D Systems (Abingdon, U.K.). This was carried out as per the manufacturer's guidelines on triplicate cell culture supernatants stimulated for 24 h as described. The results were statistically analysed using a Mann–Whitney U-test with a Bonferroni correction.
Results
IFNγ inhibits the induction of COX-2 mRNA by TNFα
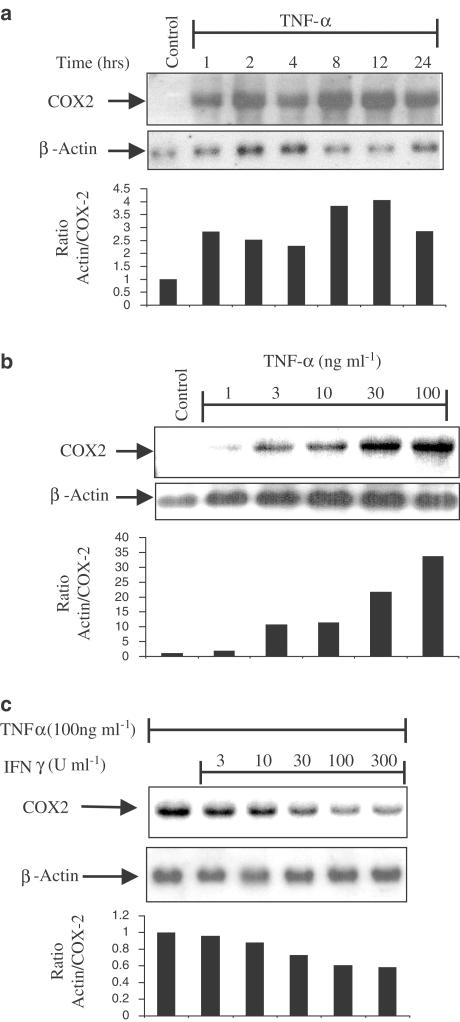
The ability of the pro-inflammatory cytokine TNFα to induce COX-2 mRNA was investigated. Confluent monolayers of HT-29 cells were initially stimulated with TNFα (100 ng ml−1) and harvested at time points over the ensuing 24 h. COX-2 mRNA was detected by Northern blot analysis. A biphasic response to TNFα-induced COX-2 was seen in which an initial mRNA peak was reached at 2 h, followed by a second wave of mRNA expression after 4 h, which was reduced by 24 h (Figure 1a). To investigate in what way this induction of COX-2 was dependent on concentration of the stimulating cytokine, HT-29 cells were stimulated with increasing concentrations of TNFα (1–100 ng ml−1) (Figure 1b) for 2 h before isolating the mRNA for Northern analysis for COX-2 mRNA expression. This revealed a concentration-dependent increase in COX-2 mRNA.
Figure 1.
Cytokine regulation of COX-2 expression. Northern analyses of mRNA isolated from HT-29 cells exposed for various time points up to 24 h with TNFα (100 ng ml−1) (a); exposed for 2 h to increasing concentrations of TNFα (1–100 ng ml−1) (b) and TNFα (100 ng ml−1) in the presence of increasing concentrations of IFNγ (3–300 U ml−1) and probed for COX-2 (upper panels) (c). Membranes were stripped and reprobed for β-actin to demonstrate equal loading (lower panels). Histobars represent the ratio of COX-2 mRNA against β-actin mRNA from each experiment, representative of at least three others, with negative or positive controls as a ratio of 1. Blots are from single experiments, but are representative of at least three others.
Having demonstrated the kinetics and concentration-dependent characteristics of COX-2 induction by TNFα, we next investigated the possibility of a regulatory role of IFNγ on this stimulation. HT-29 cells were simultaneously stimulated with TNFα (Figure 1c) in the presence of increasing concentrations of IFNγ (3–300 U ml−1). IFNγ inhibits the stimulation of COX-2 mRNA by TNFα and this inhibition is concentration-dependent.
IFNγ does not alter the stability of TNFα-stimulated COX-2 mRNA
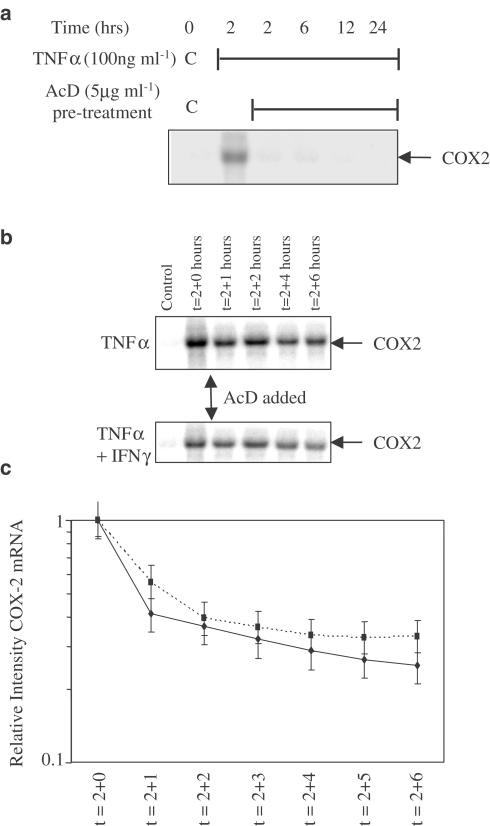
In order to further characterise the mechanism of the inhibitory action of IFNγ on TNFα-stimulated COX-2 mRNA, we proceeded to investigate whether IFNγ altered COX-2 mRNA stability. To do this, we initially ensured that actinomycin D (AcD) inhibited TNFα-stimulated COX-2 mRNA expression. HT-29 cells were treated with AcD (5 μg ml−1) for 1 h prior to the addition of TNFα, and mRNA isolated over the next 24 h shows complete inhibition of COX-2 transcription (Figure 2a). HT-29 cells were then stimulated for 2 h by TNFα in the presence or absence of IFNγ before the addition of AcD to the media. The subsequent inhibition of COX-2 transcription demonstrates that the half-life of TNFα-stimulated COX-2 mRNA is not significantly altered by IFNγ (Figure 2b, c).
Figure 2.
IFNγ does not affect the stability of COX-2 mRNA. (a) Northern analysis of mRNA isolated from HT-29 cells exposed to TNFα (100 ng ml−1) for a 24-h time course, having been exposed to 1 h pretreatment with the transcription inhibitor AcD (5 μg ml−1). Northern analyses of mRNA isolated from HT-29 cells exposed to TNFα (100 ng ml−1) (b, upper panel) or TNFα and IFNγ (300 U ml−1) (b, lower panel) over an 8-h time course in the presence of AcD (5 μg ml−1) added after 2 h to prevent new RNA synthesis. (c) Logarithmic line plot of (intensity) of COX-2 mRNA, as measured by Northern analyses in (b). Units are arbitrary where maximum intensity is 1 vs time in hours. The decay in the mRNA signal was plotted to allow calculation of the half life – time at which intensity is 0.5. The blots shown are representative of three experiments. The membranes were photographed to show the 18S and 28S bands to ensure equal loading (not shown for clarity).
IFNγ downregulates TNFα-induced COX-2 protein
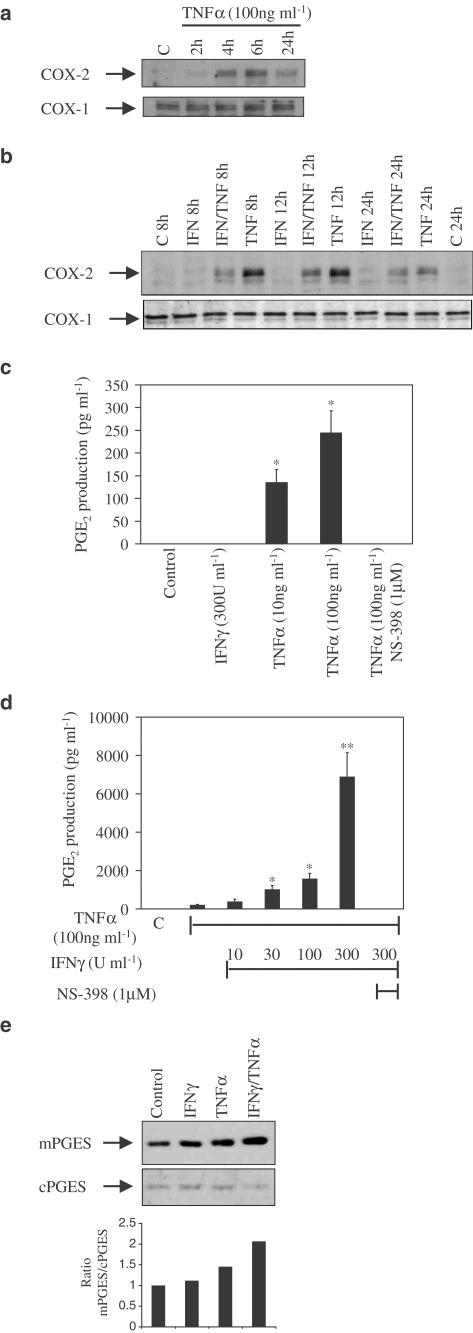
We next investigated whether the inhibitory action of IFNγ on cytokine-stimulated COX-2 mRNA was also seen at the level of COX-2 protein. As with COX-2 mRNA, TNFα causes a time-dependent increase in COX-2 protein, initially visible at 2 h and maximum between 6 and 8 h, while having no effect on the constitutive COX-1 (Figure 3a). The additional presence of IFNγ (300 U ml−1) reduces COX-2 protein expression over 24 h compared to TNFα alone (Figure 3b), which correlates with the transcriptional data.
Figure 3.
Cytokine regulation of COX-2 protein and activity. (a) Western analyses of protein isolated from HT-29 cells stimulated with TNFα (100 ng ml−1) over a 24 h time course and probed with specific antibodies for COX-2 (upper panel) and COX-1 (lower panel). (b) Western analysis of protein isolated from HT-29 cells exposed for up to 24 h to either TNFα (100 ng ml−1) or IFNγ (300 U ml−1) alone and in combination. Lane C represents an unstimulated control sample. Blots are from single experiments, but are representative of at least three others. (c) An ELISA for PGE2 (pg ml−1) using supernatants from HT-29 cells stimulated with either TNFα (10 and 100 ng ml−1) alone for 24 h or (d) in the presence of increasing concentrations of IFNγ (10–300 U ml−1) and then the addition of the specific COX-2 inhibitor NS-398. Lane C represents an unstimulated control sample. Data are the mean±s.e.m. of three separate experiments. *P⩽0.05; **P⩽0.001. (e) Cytokine induction of mPGES. Western analysis of HT-29 cells exposed to cytokine alone (concentrations as previous experiments) or in combination for 24 h (as indicated). Membranes were probed for mPGES and cPGES. Lane C represents an unstimulated control sample. Blots are representative of three separate experiments.
IFNγ upregulates TNFα-induced COX-2-dependent PGE2 production
Considering the inhibitory action of IFNγ on COX-2 at the mRNA and protein level, we assessed the regulatory action of IFNγ on the functional activity of COX-2. For a marker of COX-2 functional activity we assayed PGE2, a major prostaglandin product of intestinal COX-2. HT-29 cells were stimulated with IFNγ and increasing doses of TNFα for 24 h and supernatants collected for measurement of PGE2. Unstimulated cells produce no demonstrable PGE2 and stimulation with IFNγ alone has no effect. Increasing concentrations of TNFα cause a small but significant rise in PGE2 levels (Figure 3c). However, when TNFα stimulation of HT-29 cells is combined with increasing concentrations of IFNγ, there is a highly significant 30-fold synergistic increase in production of PGE2 (Figure 3d). Pretreatment with NS-398, a specific inhibitor of COX-2 activity, completely abrogated cytokine-induced PGE2 biosynthesis (Figure 3c, d).
IFNγ enhances TNFα-induced expression of microsomal PGE synthase
The data regarding IFNγ regulation of TNFα-induced COX-2 versus PGE2 synthesis was both surprising and conflicting. We therefore decided to explore the possible regulation of PGE2 synthesis at sites distal to COX-2. In order to determine whether the terminal enzyme mPGES was induced by the pro-inflammatory cytokines used in our model, stimulations of TNFα alone and in combination with IFNγ were carried out for 24 h and compared in terms of mPGES expression at the protein level. Interestingly, mPGES was found to have some basal, constitutive presence in these cells. This level was unchanged by IFNγ. TNFα alone showed some moderate induction of mPGES, whereas the combinations of TNFα with IFNγ significantly enhanced mPGES induction, which correlates with the PGE2 data at 24 h (Figure 3e).
Regulation of TNFα-induced mPGES by IFNγ is common to A549 epithelial cells
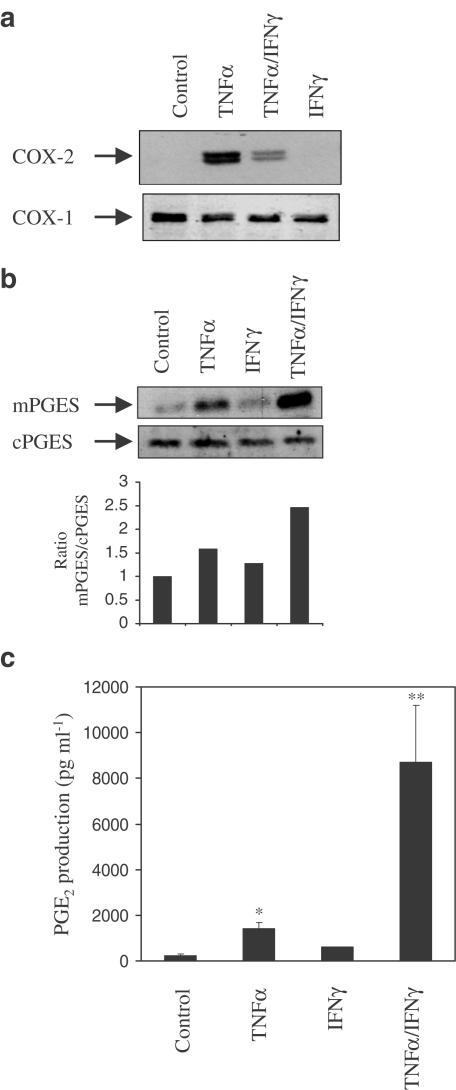
The investigation of intestinal epithelial cell biology is limited by the inability to culture and passage freshly isolated primary intestinal epithelial cells (IECs) (Evans et al., 1994). In order to verify that this regulation of mPGES was not a peculiarity of HT-29 epithelial cells, we initially repeated key experiments in two other human colonic epithelial cell lines, namely Caco-2 and DLD. TNFα did not induce COX-2 in Caco-2 cells and DLD cells exhibited constitutive COX-2 protein expression, which could not be further induced by TNFα (data not shown). We then chose to use the lung epithelial cell line A549, which had already been shown to express both COX-2 and mPGES in response to TNFα (Thoren & Jakobsson, 2000). Thus, TNFα-induced COX-2 protein expression at 24 h was significantly reduced by co-stimulation with IFNγ (Figure 4a). The coordinate expression of mPGES by TNFα is enhanced by the addition of IFNγ (Figure 4b), which supports the HT-29 data. In addition, PGE2 production in response to both cytokines is 10-fold greater than TNFα alone (Figure 4c).
Figure 4.
Cytokine regulation of PGE2 production in lung epithelial A549 cells. Western analyses of protein isolated from A549 cells stimulated with either vehicle or TNFα (100 ng ml−1) alone or in combination with IFNγ (300 U ml−1) after 24 h and probed with specific antibodies for COX-2 (a) and mPGES (b). Blots are from single experiments, but are representative of at least three others. (c) An ELISA for PGE2 (pg ml−1) using supernatants from A549 cells stimulated with vehicle or TNFα (100 ng ml−1) for 24 h either alone or in the presence of IFNγ (10–300 U ml−1). Data are the mean±s.e.m. of three separate experiments. *P≤0.05; **P≤0.001.
Discussion
The work presented here demonstrates the complexity involved in regulating prostaglandin biosynthesis. In colon epithelial cells, COX-2 can be induced by TNFα in a time- and concentration-dependent manner. The biphasic nature of this induction is likely to be related to the temporal phases of inflammation, in that the acute phase is pro-inflammatory and the later phase is anti-inflammatory (Gilroy et al., 1999). The regulatory action of the Th1 cytokine IFNγ on epithelial COX-2 expression has disparate effects at the mRNA and protein level, compared with its effects on one of the major COX-2 products, namely PGE2. In combination with TNFα, IFNγ inhibits transcription of the COX-2 gene, leading to less translation into COX-2 protein when compared to TNFα alone. In contrast, the combination of cytokines amplifies the otherwise moderate PGE2 production observed in the presence of TNFα. This substantial increase appears to be due to the concurrent induction of the mPGES. mPGES expression and PGE2 production were similarly regulated by TNFα and IFNγ. This is the first indication that IFNγ differentially regulates the PGE2 biosynthetic pathway by reducing COX-2 expression, but increases PGE2 synthase levels in order to drive the reaction towards specific PGE2 synthesis, and may be a common point of regulation in epithelial cells.
There are a number of ways in which to regulate COX-2 expression and activity. There is evidence that COX-2 is regulated at the transcriptional level to alter mRNA synthesis (Reddy et al., 2000), post-transcriptional alteration of mRNA stability (Dixon et al., 2000; Huang et al., 2000), changing the translational rate (Dixon et al., 2000), feedback mechanisms by signalling intermediates (Weaver et al., 2001), as well as the demonstration of variation between different intestinal cell types of COX-2 protein stability (Shao et al., 2000). Finally, the activity of the protein can be altered as evidenced by the effects of specific COX-2 inhibitors. Conclusions cannot be drawn about the regulatory action of a compound or molecule of interest until each level of expression and activity is investigated. In this study, it may be assumed that if the total amount of mRNA is decreased by the action of IFNγ, and that there is no effect on the stability of the mRNA, then the IFNγ must be decreasing the transcription. In addition, this decreased transcription is reflected in decreased COX-2 protein, the stability of which is also unaffected by IFNγ (data not shown). However, the overall effect of IFNγ, when considering the functional output of COX-2 activity, is a synergistic action with TNFα in the synthesis of PGE2, as has been previously described in this system (Arias-Negrete et al., 1995; Warhurst et al., 1998). This disparity implies that IFNγ must act at a point downstream of COX-2 activity.
The recent identification of an inducible microsomal PGE synthase that converts the endoperoxide PGH2 to PGE2 (Jakobsson et al, 1999) has highlighted the multiple points of regulation in PGE2 biosynthesis. Although COX-2 and mPGES are thought to be functionally linked (Murakami et al., 2000), there appears to be temporal differences in their expression in colorectal cancer cells (Yoshimatsu et al., 2001). This latter study demonstrated that TNFα induced COX-2 within 3 h and mPGES after 24 h, an event that was similarly observed following IL-1β treatment of synoviocytes (Stichtenoth et al., 2001). Other groups have seen coordinate induction of these two enzymes by TNFα and IL-1β in A549 lung epithelial cells (Thoren & Jakobsson, 2000) and orbital fibroblasts (Han et al., 2002). In both A549 and HT-29 epithelial cells, TNFα-induced COX-2 expression peaks at 6–8 h, although detectable PGE2 levels only occur at 24 h. The coordinate induction of mPGES at 24 h by IFNγ goes some way in explaining the highly significant rise in PGE2 at this time point. TNFα may induce COX-2 expression and activity, but the prostaglandin profile arising from this must be further defined by specific prostaglandin synthases. Also, it cannot be ruled out that IFNγ may act even further downstream and inhibit enzymes involved in the catabolic metabolism of PGE2, such as PG-15-dehydrogenase.
The physiological significance of this data is unclear. The role of COX-2 in inflammatory conditions is still debated. Acute production of COX-2 is likely to be pro-inflammatory and so the synergistic effect of TNFα and IFNγ, both classically regarded as promoting inflammation, would seem unsurprising. However, altered regulation of COX-2 over time, resulting in a different prostaglandin profile, may mediate the hypothesised beneficial actions of the enzyme implied in clinical studies of its pharmacological inhibition (Reuter et al., 1996) and its loss in knockout mice (Morteau et al., 2000). That is to say that downregulation of COX-2 may not only reduce the production of PGE2, but also reduce or halt the production of other prostaglandins that may be involved in resolution and restitution of inflammatory responses. However, the therapeutic use of IFNγ in this context needs careful evaluation. While IFNγ may partially inhibit TNFα-induced COX-2, it is important to note that it does not completely abrogate expression. Thus, one might imagine that other products of COX-2 activity can still be formed, although perhaps to a lesser degree. The partial inhibition of COX-2 could be regarded as a beneficial outcome, in terms of inflammation; however, one deleterious side effect might be that the IFNγ-induced modulation of mPGES ensures the channelling away from ‘resolution' type COX-2 products toward ‘inflammatory' type PGE2 synthesis.
The increased amounts of PGE2 observed in ulcerative colitis and CD (McCartney et al., 1999) may contribute towards the predisposition of these conditions to colon cancer. Overexpression of mPGES is evident in colorectal adenomas and cancer (Yoshimatsu et al., 2001) and it would be important to ascertain whether mPGES is upregulated in IBD, because enhanced expression of both COX-2 and mPGES could explain the increased amounts of PGE2 present in these conditions. Since PGE2 overproduction is thought to be tumorigenic (Dannenberg & Zakim, 1999; Sheng et al., 2001), inhibiting its production through the specific inhibition of mPGES could maintain the beneficial role perceived for COX-2 in IBD and reduce the risk of developing colon cancer. Manipulating the prostaglandin profile in this way may provide a novel therapeutic target for IBD.
Acknowledgments
This work was supported by funds from the Wellcome Trust (to S.G.W. and K.L.W.), National Association for Colitis and Crohn's Disease (to D.A.F.R.) and Digestive Disorders Foundation (to S.A.W.).
Abbreviations
- AcD
actinomycin D
- CD
Crohn's disease
- COX
cyclooxygenase
- FBS
foetal bovine serum
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- MCP
monocyte chemo-attractant protein
- mPGES
microsomal prostaglandin E synthase
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor κB
- NOS
nitric oxide synthase
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- RANTES
regulated upon activation T-cell expressed and secreted
- SCID
severe combined immune deficiency
- TNF
tumour necrosis factor
- UC
ulcerative colitis
References
- ARIAS-NEGRETE S., KELLER K., CHADEE K. Pro-inflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem. Biophys. Res. Commun. 1995;208:582–589. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- BARRIOS-RODILES M., CHADEE K. Novel regulation of cyclooxygenase-2 expression and prostaglandin E2 production by IFN-γ in human macrophages. J. Immunol. 1998;161:2441–2448. [PubMed] [Google Scholar]
- BREESE E.J., MICHIE C.A., NICHOLLS S.W., MURCH S.H., WILLIAMS C.B., DOMIZIO P., WALKERSMITH J.A., MACDONALD T.T. Tumor necrosis factor-α producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- DANNENBERG A.J., ZAKIM D. Chemoprevention of colorectal cancer through inhibition of cyclooxygenase-2. Semin. Oncol. 1999;26:499–504. [PubMed] [Google Scholar]
- DEBINSKI H., FORBES A., KAMM M.A. Low dose interferon-γ for refractory Crohn's disease. Italian J. Gastroenterol. 1997;29:403–406. [PubMed] [Google Scholar]
- DIXON D.A., KAPLAN C.D., MCINTYRE T.M., ZIMMERMAN G.A., PRESCOTT S.M. Post-transcriptional control of cyclooxygenase-2 gene expression. J. Biol. Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- DUBOIS R.N., ABRAMSON S.B., CROFFORD L., GUPTA R.A., SIMON L.S., VAN DE PUTTE L.B.A., LIPSKY P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- EVANS G.S., FLINT N., POTTEN C.S. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu. Rev. Physiol. 1994;56:399–417. doi: 10.1146/annurev.ph.56.030194.002151. [DOI] [PubMed] [Google Scholar]
- FU J.Y., MASFERRER J.L., SEIBERT K., RAZ A., NEEDLEMAN P. The induction and suppression of prostaglandin H2 synthase (cylooxygenase) in human monocytes. J. Biol. Chem. 1991;265:16737–16740. [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., WILLIS D., CHIVERS J., PAUL-CLARK M.J., WILLOUGHBY D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- HAN R., TSUI S., SMITH T.J. Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J. Biol. Chem. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- HEMLER M., LANDS W.E.M., SMITH W.L. Purification of the cyclo-oxygenase that forms prostaglandins. Demonstration of the two forms of iron in the holoenzyme. J. Biol. Chem. 1976;251:5575–5579. [PubMed] [Google Scholar]
- HERSCHMAN H.R. Prostaglandin synthase 2. Biochim. Biophys. Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- HUANG Z.F., MASSEY J.B., VIA D.P. Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability by interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α) in human in vitro differentiated macrophages. Biochem. Pharmacol. 2000;59:187–194. doi: 10.1016/s0006-2952(99)00312-3. [DOI] [PubMed] [Google Scholar]
- JAKOBSSON P.-J., THOREN S., MORGENSTERN R., SAMUELSSON B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOBIN C., MORTEAU O., HAN D.S., SARTOR R.B. Specific NF-κB blockade selectively inhibits tumour necrosis factor-α-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95:537–543. doi: 10.1046/j.1365-2567.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLIOS G., BROWN Z., ROBSON R.L., ROBERTSON D.A.F., WESTWICK J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br. J. Pharmacol. 1995;116:2866–2872. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLIOS G., WRIGHT K.L., JORDAN N.J., LEITHEAD J.B., ROBERTSON D.A.F., WESTWICK J. C–X–C and C–C chemokine expression and secretion by the human colonic epithelial cell line, HT-29: differential effect of T lymphocyte- derived cytokines. Eur. J. Immunol. 1999;29:530–536. doi: 10.1002/(SICI)1521-4141(199902)29:02<530::AID-IMMU530>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- KUJUBU D.A., FLETCHER B.S., VARNUM B.C., LIM R.W., HERSCHMAN H.R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- MATSUURA H., SAKAUE M., SUBBARAMAIAH K., KAMITANI H., ELING T.E., DANNENBERG A.J., TANABE T., INOUE H., ARATA J., JETTEN A.M. Regulation of cyclooxygenase-2 by interferon-γ and transforming growth factor-α in normal human epidermal keratinocytes and squamous carcinoma cells. J. Biol. Chem. 1999;274:19148–29138. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- MCCARTNEY S.A., MITCHELL J.A., FAIRCLOUGH P.D., FARTHING M.J.G., WARNER T.D. Selective COX-2 inhibitors and human inflammatory bowel disease. Aliment. Pharmacol. Ther. 1999;13:1115–1117. doi: 10.1046/j.1365-2036.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- MORTEAU O., MORHAM S.G., SELLON R., DIELEMAN L.A., LANGENBACH R., SMITHIES O., SARTOR R.B. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAKAMI M., NARABA H., TANIOKA T., SEMMYO N., NAKATANI Y., KOJIMA F., IKEDA T., FUEKI M., UENO A., OH-ISHI S., KUDO I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- PANG L., KNOX A.J. Effect of interleukin-1β, tumour necrosis factor-α and interferon-γ on the induction of cyclooxygenase-2 in cultured human airway smooth muscle cells. Br. J. Pharmacol. 1997;121:579–587. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWRIE F., LEACH M.W., MAUZE S., MENON S., BARCOMB CADDLE L., COFFMAN R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- REDDY S.T., WADLEIGH D.J., HERSCHMAN H.R. Transcriptional regulation of the cyclooxygenase-2 gene in activated mast cells. J. Biol. Chem. 2000;275:3107–3113. doi: 10.1074/jbc.275.5.3107. [DOI] [PubMed] [Google Scholar]
- REUTER B.K., ASFAHA S., BURET A., SHARKEY K.A., WALLACE J.L. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J. Clin. Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAO J., SHENG H., INOUE H., MORROW J.D., DUBOIS R.N. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J. Biol. Chem. 2000;275:33951–33956. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- SHENG H., SHAO J., WASHINGTON M.K., DUBOIS R.N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- SORIANI M., LUSCHER P., TYRRELL R.M. Direct and indirect modulation of ornithine decarboxylase and cyclooxygenase by UVB radiation in human skin cells. Carcinogenesis. 1999;20:727–732. doi: 10.1093/carcin/20.4.727. [DOI] [PubMed] [Google Scholar]
- STICHTENOTH D.O., THOREN S., BIAN H., PETERS-GOLDEN M., JAKOBSSON P.-J., CROFFORD L.J. Microsomal prostaglandin E synthase is regulated by pro-inflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J. Immunol. 2001;167:469–474. doi: 10.4049/jimmunol.167.1.469. [DOI] [PubMed] [Google Scholar]
- TANIOKA T., NAKATANI Y., SEMMYO N., MURAKAMI M., KUDO I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- TARGAN S.R., HANAUER S.B., VAN DEVENTER S.J., MAYER L., PRESENT D.H., BRAAKMAN T., DEWOODY K.L., SCHAIBLE T.F., RUTGEERTS P.J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor-α for Crohn's disease. Crohn's Disease cA 2 study Group. N. Engl. J. Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- THOREN S., JAKOBSSON P.-J. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Inhibition by NS-398 and leukotriene C4. Eur. J. Biochem. 2000;267:6428–6434. doi: 10.1046/j.1432-1327.2000.01735.x. [DOI] [PubMed] [Google Scholar]
- VAN DEVENTER S.J. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARHURST A.C., HOPKINS S.J., WARHURST G. Interferon-γ induces differential upregulation of α and β chemokine secretion in colonic epithelial cell lines. Gut. 1998;42:208–213. doi: 10.1136/gut.42.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER S.A., RUSSO M.P., WRIGHT K.L., KOLIOS G., JOBIN C., ROBERTSON D.A.F., WARD S.G. Regulatory role of phosphatidylinositol 3-kinase on TNF-α-induced cyclooxygenase-2 expression in colonic epithelial cells. Gastroenterology. 2001;120:1117–1127. doi: 10.1053/gast.2001.23257. [DOI] [PubMed] [Google Scholar]
- WRIGHT K., WARD S.G., KOLIOS G., WESTWICK J. Activation of phosphatidylinositol 3-kinase by interleukin-13. An inhibitory signal for inducible nitric-oxide synthase expression in epithelial cell line HT-29. J. Biol. Chem. 1997;272:12626–12633. doi: 10.1074/jbc.272.19.12626. [DOI] [PubMed] [Google Scholar]
- WRIGHT K.L., KOLIOS G., WESTWICK J., WARD S.G. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. J. Biol. Chem. 1999;274:17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- YOSHIMATSU K., GOLIJANIN D., PATY P.B., SOSLOW R.A., JAKOBSSON P.-J., DELELLIS R.A., SUBBARAMAIAH K., DANNENBERG A.J. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin. Cancer Res. 2001;7:3971–3976. [PubMed] [Google Scholar]