Abstract
The 5-HT receptor subtype that mediates bronchocontraction and the involvement of neuronal and non-neuronal acetylcholine was assessed in murine isolated tracheae.
Atropine (1–10 nM) caused a rightward shift of the methacholine concentration–effect curves (pA2=9.0) but reduced the maximum response to 5-HT, suggesting that 5-HT acts as an indirect agonist. The potency of 5-HT receptor agonists (α-methyl-5-HT≈5-HT>5-carboxamidotryptamine), together with the competitive antagonism of 5-HT by ketanserin (pA2=9.4), suggests the involvement of the 5-HT2A receptor.
While cholinergic twitch responses to electrical field stimulation were abolished by the fast sodium channel inhibitor tetrodotoxin (300 nM), as well as by combined blockade of N-, P- and Q-type voltage-operated calcium channels by ω-conotoxin GVIA (30 nM) and agatoxin IVA (100 nM), responses to 5-HT were unaffected. Similarly, botulinum toxin A (50 nM) inhibited EFS twitch responses, but not contractions to 5-HT.
Choline acetyltransferase immunoreactivity was localised to ganglia and nerve fibres as well as approximately half the epithelial cells in the preparation. Removal of the epithelial layer markedly attenuated the contractile response to 5-HT, but had no effect on contractions to either methacholine or EFS.
These findings suggest that 5-HT, acting at 5-HT2A receptors on mouse tracheal epithelial cells, stimulates these cells to release acetylcholine, which then causes contraction of airway smooth muscle. This phenomenon should be borne in mind in when interpreting studies of murine models of airway disease.
Keywords: Acetylcholine, 5-HT, airway, epithelium
Introduction
5-hydroxytryptamine (5-HT), a product of rodent mast cells and human platelets, causes bronchoconstriction in many mammalian species (Barnes et al., 1998), but not in healthy or asthmatic humans (Cazzola & Matera, 2000). In mice, 5-HT-induced bronchoconstriction is sensitive to atropine (Eum et al., 1999), suggesting that its mechanism of action may depend on cholinergic parasympathetic nerve fibres that innervate the smooth muscle of the airways. Also, since anaphylactic bronchoconstriction in mice is reported to be sensitive to both muscarinic and 5-HT receptor blockade (Eum et al., 1999), the exact mechanism by which 5-HT indirectly contracts airway smooth muscle has bearing on the extensive use of this species in models of airway disease like allergic asthma.
We hypothesised that for 5-HT to cause acetylcholine release both in vivo and in vitro, 5-HT must cause direct release of acetylcholine from postganglionic nerve terminals. One possibility is that 5-HT receptor activation causes depolarisation of airway cholinergic nerve terminals via 5-HT3 receptors (a ligand-gated ion channel), as reported in human and guinea-pig airways (Takahashi et al., 1995). However, we report here that a 5-HT3-selective ligand has no contractile effect in the mouse isolated trachea. Rather, by using a combination of pharmacological and immunohistochemical approaches, we found that 5-HT appears to cause the release of acetylcholine from the epithelium and not from nerves. Therefore, our results suggest that epithelium-derived acetylcholine may be the final mediator of anaphylactic bronchoconstriction in mice and may play a role in altered airway responsiveness in airway disease models.
Methods
In vitro pharmacology
Female BALB/C mice (Charles River, Margate, Kent, U.K.) were killed with an overdose of urethane (20 g kg−1 i.p.) and the entire trachea was removed, dissected free from surrounding connective tissues and cut into two rings. Each ring was mounted on two stainless-steel hooks in a conventional organ bath in a modified Krebs solution (composition (mM): NaCl 118; KCl 5.4; MgSO4 0.57; glucose 11; KH2PO4 1.2; NaHCO3 25; CaCl2 2.5), maintained at 37°C and continuously bubbled with 95% O2 5% CO2. One hook was attached to a Grass FT03 force transducer (Grass Instruments. Quincy, MA, U.S.A.) connected to a MacLab (AD Instruments, Castle Hill, Australia) for isometric tension recording, while the other hook served as an anchor for the preparation. Two platinum electrodes placed on either side of the preparation were used to deliver electrical field stimulation (EFS) to the preparation. After a 15 min equilibration period, a passive tension of 0.5 g was placed on each preparation and after a second 20 min equilibration period the maximum contractile force of the preparation was estimated by applying methacholine (MCh, 100 μM) to the tissue. Once this contraction reached a plateau, the preparation was washed repeatedly and allowed to equilibrate for 30 min and return to original basal tone before experiments were performed. Each preparation acted as its own control and concentration–effect curves were performed by cumulative drug addition. Appropriate vehicle controls were performed to verify that none of the vehicles used had any effect. A control concentration–effect curve for 5-HT or MCh or trains of EFS (single pulses of 0.1 ms, 30 V delivered every 60 s) was established and then repeated 30 min after administration of an inhibitor/antagonist. Data are expressed as the mean±the standard error of the mean of the percentage of the initial maximal contraction to MCh. Nonlinear regression analysis using GraphPad Prism (v 2.01; GraphPad Software, San Diego, CA, U.S.A.) was used to determine pEC50 values. Statistical comparisons of pEC50 and maximal contraction values were performed using a two-tail Student's t-test.
Choline acetyltransferase (ChAT) immunohistochemistry
Flat whole-mount preparations were prepared by opening the tracheae longitudinally along the ventral cartilage rings and were pinned to a Syguard lined chamber using fine entomological pins. The preparations were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) and then washed three times (15 min each). A 30 min wash in 80% ethanol, followed by a 30 min wash in DMSO, was used to permeabilise and clear the preparations. After three final washes in PBS, the preparations were blocked for 1 h in PBS containing 1% BSA and then incubated overnight at room temperature in the primary antibody combination (goat anti-human ChAT, diluted 1 : 50; mouse pan anti-human cytokeratin, 1 : 50; both from Sigma, Poole, Dorset, U.K.), diluted in PBS/BSA containing 0.1% Triton X-100 and 0.1% sodium azide. The following day, the preparations were washed three times in PBS (15 min each) and then exposed to a secondary antibody combination (CY3 conjugated donkey anti-goat, diluted 1 : 400; FITC conjugated donkey anti-mouse 1 : 200; both from Jackson Immunoresearch, West Grove, PA, U.S.A.) for 1 h. For nuclear counterstaining, the preparations were then incubated in 7-amino-actinomycin (10 μg ml−1; 10 min; Molecular Probes, Eugene, OR, U.S.A.). Each preparation was then washed in PBS three times (15 min each) before being mounted under a coverslip in buffered glycerol (pH 8.5). Preparations were observed using a BioRad confocal microscope (MRC1024, running Laser Sharp v.3.2 software; BioRad Laboratories, Hemel Hemsptead, Hertfordshire, U.K.) using appropriate filter sets for FITC, CY3 and 7-amino-actinomycin.
Botulinum toxin A (BTXA) treatment
Some preparations were incubated with BTXA (Sigma) to inhibit cholinergic neurotransmission, as previously described in peripheral tissues (Morris et al., 2001). Aliquots of BTXA were stored at −20°C in HEPES buffered saline (composition (mM): NaCl 140; KCl 5; MgCl2 1.0; glucose 10; CaCl2 2.0). Ring preparations of trachea were incubated for 3 h in an equal volume (500 μl) of BTXA and Krebs at 37°C (final BTXA concentration 50 nM) in a tissue culture incubator. Control preparations were incubated in the same volume of Krebs/Tris-buffered saline only. After the incubation period, each preparation was washed several times in buffer and then mounted in organ baths as described above.
Epithelium removal
The epithelium was destroyed by an intratracheal administration of a 1.0 ml solution of 0.1% Triton X-100 in Krebs, as previously described (Cocks et al., 1999) The solution was gently lavaged repeatedly to assist in cell removal for 2 min before the tracheae were dissected from the animals and mounted in organ baths. The entire length of the trachea was used since this procedure reduced the maximum contractile force of the preparations by 50% or more. At the end of the experiment, the preparations were fixed in 4% paraformaldehyde for immunohistochemical (cytokeratin) assessment of epithelial removal (see above). By using whole-mount preparations rather than individual sections, we were able to estimate the epithelial integrity of the entire preparation. Denuded areas were identified as those in which the autofluorescent, fibrous subepithelial connective tissue was clearly visible and cytokeratin-immunoreactive cells were absent.
Materials
5-HT (MCPBG), a-methyl-5-HT, 5-carboxamidotryptamine (5-CT), R-[+]-2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydronaphthalene (8-OHDPAT), 1-(m-chlorophenyl)-bigaunide (m-CPB), ketanserin, atropine, acetyl-β-methylcholine (MCh), ketanserin, BTXA, and agatoxin IVA were purchased from Sigma. ω-Conotoxin was purchased from Tocris (Avonmouth, Bristol, U.K.) and 2-aminoethoxydiphenyl borate (2-APB) from Calbiochem (San Diego, CA, U.S.A.).
Results
5-HT-induced contractions
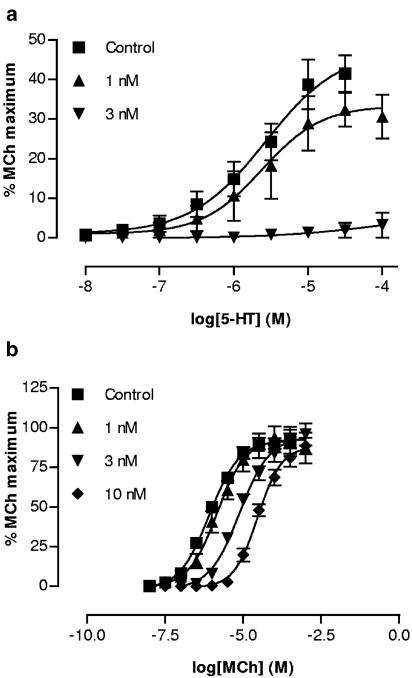
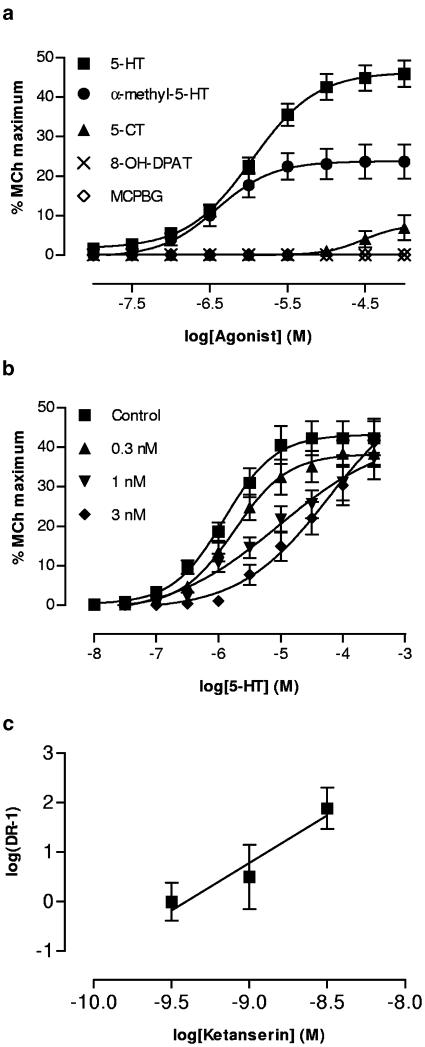
5-HT (30 nM–100 μM) caused concentration-dependent contractions in all preparations studied, with maximum contraction reaching approximately 40% of the maximum response to MCh and a pEC50 of 5.96±0.1 (Figure 1a). Atropine (1–3 nM) reduced the maximum of the 5-HT concentration–effect curve, abolishing contractions at higher concentrations of atropine (Figure 1a). By contrast, atropine caused a dose-dependent parallel rightward shift of the MCh concentration–effect curve with a pA2 of 9.0 (Figure 1b). The 5-HT3 receptor agonist m-PBG had no contractile effect in this preparation (10 nM–300 μM). However, the 5-HT analogue α-methyl-5-HT was slightly more potent than 5-HT (pEC50=6.41±1.8; P=0.023 compared to 5-HT), but elicited a significantly smaller maximum response (Figure 2a). 5-CT also elicited responses in this preparation, but much less potently than 5-HT or α-methyl-5-HT, with a pEC50 of 4.52±0.29. By contrast, 8-OHDPAT was inactive. Ketanserin shifted the 5-HT concentration–effect curve to the right (Figure 2b); the Schild plot (Figure 2c) produced a slope not different from unity and the derived pA2 was 9.4.
Figure 1.
Effect of atropine (1–10 nM) on cumulative concentration–contraction curves for 5-HT (a, n=4) and MCh (b, n=6) in the mouse isolated trachea.
Figure 2.
(a) Cumulative concentration–contraction curves for a variety of 5-HT receptor agonists in the mouse isolated trachea (n=3–6). (b) The effect of ketanserin (0.3–3.0 nM; n=6) on the cumulative concentration–contraction curve for 5-HT. (c) Schild plot of data from panel (b).
Effect of neuronal voltage-operated calcium channel (VOCC) blockers
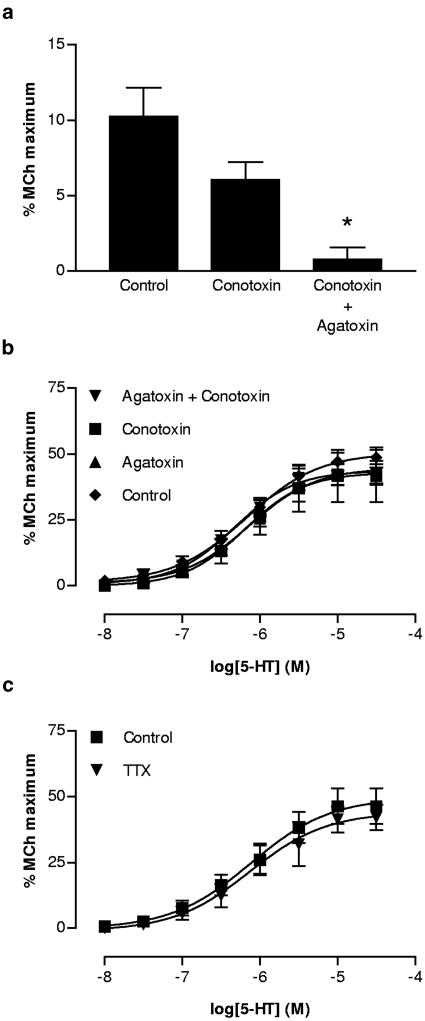
Twitch responses to EFS were not significantly inhibited by ω-conotoxin GVIA (30 nM), but were abolished by the further addition of agatoxin IVa (100 nM; Figure 3a). However, neither of these VOCC blockers modified the response to 5-HT, either alone or in combination (Figure 3b). Similarly, although responses to EFS were abolished by 300 nM tetrodotoxin (data not shown), those to 5-HT were not affected (Figure 3c).
Figure 3.
(a) Effect of ω-conotoxin GVIA (conotoxin; 30 nM) and the combination of conotoxin and agatoxin IVA (agatoxin 100 nM) on cholinergic twitch responses to a single pulse of EFS in the mouse isolated trachea (n=6). The toxins were added sequentially to individual preparations. (b) Lack of effect of conotoxin and agatoxin either alone or in combination on the cumulative concentration–contraction curve for 5-HT (n=3–6). (c) Similar lack of effect of tetrodotoxin (TTX; 300 nM) on the cumulative concentration–contraction curve for 5-HT (n=6).
Effect of IP3 receptor antagonism
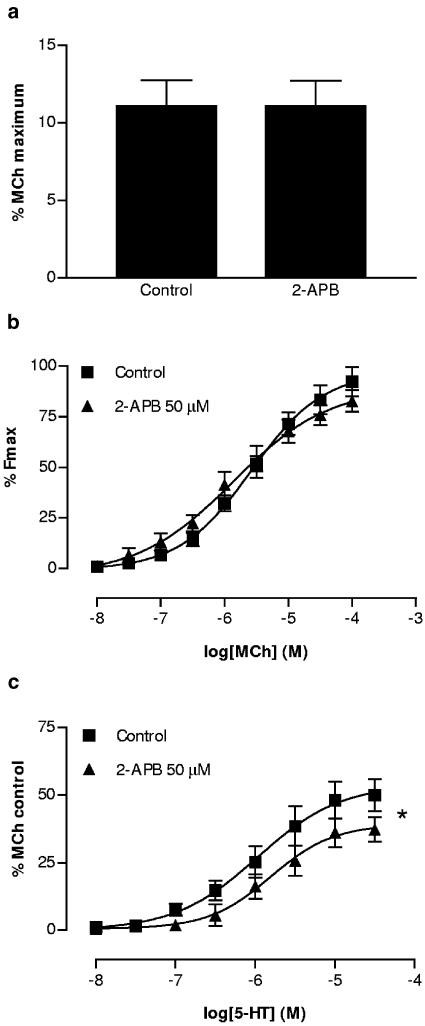
The putative IP3 receptor antagonist 2-APB (50 μM) had no effect on responses to EFS (Figure 4a) or MCh (Figure 4b), yet significantly depressed the maximum response to 5-HT (Figure 4c). The potency of 5-HT was not modified by 2-APB (pEC50 were 5.97±0.22 for 2-APB vs 5.80±18 for control).
Figure 4.
Lack of effect of 2-APB (50 μM) on cholinergic twitch responses to one pulse of EFS (a) In the mouse isolated trachea (n=6). (b) Lack of effect of 2-APB on concentration–contraction curves for MCh. (c) Significant reduction of 5-HT-induced contractions by 2-APB (n=6). * indicates P<0.05, Student's t-test of control vs treated maximal responses.
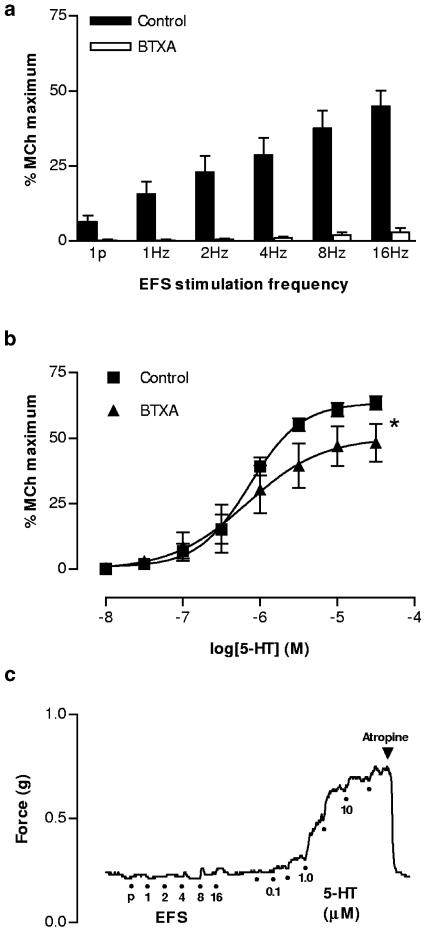
Effect of BTXA
Incubation of tissues with BTXA (50 nM) abolished EFS-induced contractions at low frequencies (1 pulse – 4 Hz) and only very small responses remained at higher stimulation frequencies (Figure 5a). The maximum response to 5-HT was slightly, but significantly reduced in BTXA-treated preparations (Figure 5b), while potency was unaffected (pEC50 6.14±0.28 vs 6.14±0.06, control). Contraction to 5-HT in BTXA-treated preparations was abolished by atropine (Figure 5c).
Figure 5.
Effect of BTXA (50 nM) on contractions to EFS and 5-HT in the mouse isolated trachea. (a) Marked inhibitory effect of BTXA on EFS-induced contractions over a wide range of stimulus frequencies (1p represents a single pulse; n=6). (b) Effect of BTXA incubation on the cumulative concentration–contraction curve for 5-HT (n=6). * indicates that the maximum contraction to 5-HT is significantly reduced by BTXA (P<0.05; paired t-test) compared with buffer-incubated controls. (c) Representative trace of one of the experiments represented in the figures above, demonstrating that in a BTXA-treated preparation responses to EFS are abolished and the remaining response to 5-HT is sensitive to atropine (1 μM).
ChAT immunohistochemistry
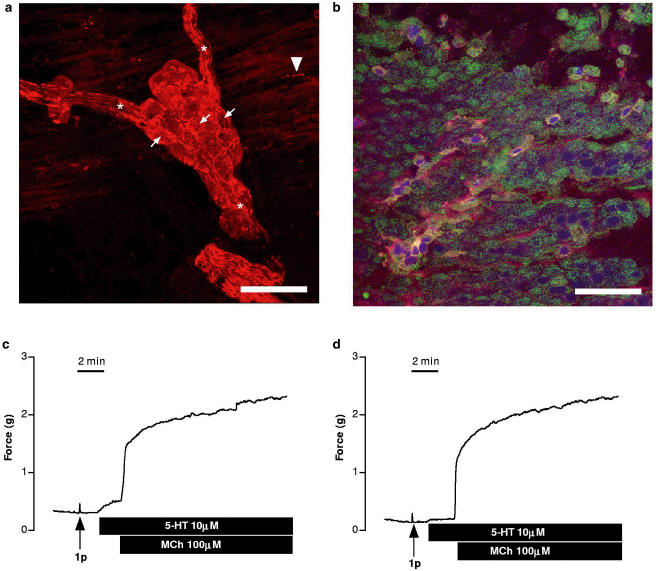
In tracheal whole-mounts, strongly immunofluorescent nerve fibres were observed surrounding less intensely fluorescent nerve cell bodies in local airway ganglia, and fine varicose fibres were observed throughout the trachealis muscle (Figure 6a). Additionally, many of the cytokeratin-positive epithelial cells exhibited punctate intracellular ChAT immunoreactivity (Figure 6b). Nerves and epithelial cells were the only obvious ChAT-immunoreactive cells within the preparation.
Figure 6.
(a) Confocal micrograph showing the immunohistochemical localisation of ChAT in parasympathetic ganglia located on the dorsal surface of the mouse trachea. This is a montage of 16 1-μm z-sections through the ganglion. Preganglionic cholinergic nerve fibres converging on the ganglia in nerve trunks (asterisks) and surrounding the more weakly stained postganglionic neurons (small arrows). A fine varicose fibre (arrowhead) is apparent in the outermost layer of the smooth muscle, which is included in the lowest section of the stack. Scale bar=40 μm. (b) Confocal micrograph of a series of z-sections through the entire epithelium, illustrating the distribution of ChAT (red) and cytokeratin (green). Nuclei (blue) have been stained with 7-amino-actinomycin. Cytokeratin is present in virtually all cells, while a subpopulation of cells contains intracellular ChAT immunoreactivity. Scale bar=40 μm. (c) Representative trace showing one of six experiments in which only partial removal of the epithelium was achieved such that 5-HT-induced contractions were attenuated by more than 50% although the preparations still responded to MCh and a single pulse (1p) of EFS. (d) Representative trace of an identical experiment run in parallel with the trace in panel (c). This preparation, and three others, similarly maintained responsiveness to MCh and EFS, but only responded feebly, if at all, to 5-HT and were subsequently found to have virtually no epithelial cells present.
Effect of epithelium removal
The Triton X-100 lavage procedure, designed to selectively remove the airway epithelium, resulted in damage to the epithelial layer of all preparations, but was variably effective in actually removing all cells. However, the preparations were readily categorised into two groups. Firstly, in six preparations, patches of cells remained (representing approximately 30% of the surface area in total) and in these preparations 5-HT consistently caused contractions less than 15% of the maximum response to MCh (Figure 6c). By contrast, in a further four preparations, the epithelium had been reduced to only a few scattered cells and the response to 5-HT was essentially abolished (Figure 6d). Relative to the maximal response to MCh, responses to EFS were unchanged by either partial or near-complete removal of the epithelium (Figure 6c, d; cf. Figure 5a).
Discussion
The present studies provide structural and pharmacological evidence that the cholinergic contractile response to 5-HT in the mouse isolated trachea depends on a non-neuronal source of acetylcholine, most likely the airway epithelium.
In agreement with previous studies (Eum et al., 1999), the contractile response to 5-HT observed here was sensitive to the nonselective muscarinic antagonist atropine. Furthermore, rather than causing a rightward shift of the curve with no change in the maximal response (as was the case for MCh), atropine both right-shifted and reduced the maximum of the 5-HT concentration–contraction curve, suggestive of indirect agonism (Black et al., 1985). This supports the earlier suggestion (Eum et al., 1999) that 5-HT does not act directly on receptors located on smooth muscle cells, but induces acetylcholine release that causes contraction.
Initially, we suspected that 5-HT might depolarise nerve terminals by acting at ligand-gated ion channel 5-HT3 receptors that have been reported in other species (Takahashi et al., 1995). However, the 5-HT3 selective agonist m-CPB (Hoyer et al., 1994; Uphouse, 1997) did not cause contraction in the mouse isolated trachea. Using several previously characterised 5-HT analogues, we pharmacologically defined the receptor mediating the response to 5-HT. The rank order potency of 5-HT≈α-methyl-5-HT>5-CT is reminiscent of 5-HT2A receptors (Hoyer et al., 1994). However, there are subtle differences between our analysis and a previous characterisation of 5-HT2A receptors in the mouse isolated aorta (McKune & Watts, 2001). Thus, 5-CT did not appear to be a full agonist and was slightly more potent than 5-HT, while 8-OH-DPAT was inactive in this preparation. By contrast, McKune & Watts (2001) found that 5-CT was less potent but equally efficacious as 5-HT, and that high concentrations of 8-OHDPAT were able to contract the mouse isolated aorta. Whether these differences in 5-HT2A receptor pharmacology are due to post-translational modifications or splice variation is uncertain. Nevertheless, the pA2 (9.4) that we obtained for the known 5-HT2A antagonist ketanserin is in good agreement with previous reports of the pharmacology of this 5-HT receptor subtype in mice (9.72; McKune & Watts, 2001).
Release of autonomic neurotransmitters requires calcium entry via neuronal voltage-gated calcium channels (Waterman, 2000). Usually, ω-conotoxin GVIA-sensitive N-type VOCCs are required for acetylcholine release, while agatoxin IVA-sensitive P- and Q-type VOCCs are required for cotransmitter release following stronger stimulation (Waterman, 2000). In the mouse trachea, N-type VOCCs appeared to contribute only partly, if at all, to acetylcholine release following a single pulse, while responses were abolished by inhibitors of all the three VOCC types. This is not unprecedented since ω-conotoxin fails to affect cholinergic neurotransmission in the guinea-pig heart (Hong & Chang, 1995) and other tissues (Waterman, 2000). More importantly, while blockade of voltage-gated calcium channels was able to inhibit cholinergic EFS-induced responses, the response to 5-HT was unaffected, implying that this response was unlikely due to classic, VOCC-dependent release of acetylcholine from nerves. Thus, we considered two other possibilities to explain the atropine-sensitive contraction to 5-HT. First, that acetylcholine release from nerve terminals does not require VOCCs following 5-HT receptor occupation and, second, that 5-HT is able to induce release of acetylcholine from a non-neuronal source within the trachea. Both of these possibilities are plausible, since ChAT has previously been reported in non-neuronal airway tissues (Wessler & Kirkpatrick, 2001) and intracellular calcium stores have been demonstrated in autonomic neurons (Brain et al., 2001).
We initially compared the mechanisms of calcium mobilisation underlying responses to cholinergic nerve stimulation and those to 5-HT and MCh in order to determine if and how these responses differ. Neither EFS- nor MCh-induced contractions were affected by the IP3 antagonist 2-APB (Ascher-Landsberg et al., 1999), although the maximum response to 5-HT was significantly reduced in the presence of this drug. This small inhibitory effect of 2-APB on 5-HT-induced contractions suggests that these contractions may rely to a minor extent on an intracellular calcium pool. While 2-APB exhibits a number of other activities relevant to calcium mobilisation (Bootman et al., 2002; Harks et al., 2003), the specific mechanism of action is not central to our findings since this compound pharmacologically distinguishes responses to MCh, EFS from those to 5-HT, suggesting a difference between cholinergic transmission and release of acetylcholine by 5-HT.
To further rule out the possibility that 5-HT released acetylcholine from cholinergic nerves within the trachea, we incubated preparations with BTXA, a clostridial metalloprotease that cleaves SNARE proteins essential for neuronal vesicle exocytosis (Humeau et al., 2000) and as such inhibits responses to cholinergic nerve stimulation. This treatment abolished responses to EFS but had only a small effect on responses to 5-HT, which is suggestive of a non-neuronal source of acetylcholine. The small inhibitory effect of botulinum toxin on the maximum response to 5-HT may reflect an action on other SNARE proteins that could be involved in release of acetylcholine from non-neuronal sources. Indeed, clostridial neurotoxins are known to inhibit secretion from a variety of non-neuronal cells, including secretory epithelia such as pancreatic acinar cells (Humeau et al., 2000). Since previous reports have indicated several possible non-neuronal sources of acetylcholine in the airways (Wessler & Kirkpatrick, 2001), we performed choline ChAT immunohistochemistry to identify candidate cellular sources of acetylcholine in the mouse trachea. As previously reported in the airways of other species (Wessler & Kirkpatrick, 2001), the epithelium was found to contain high levels of choline-acetyltransferase. Most convincingly, when the epithelial layer of the preparations had been successfully removed, responses to 5-HT were essentially abolished while those to EFS and MCh were maintained. These observations, coupled with the differences in pharmacology between EFS-induced and 5-HT-induced contractions, strongly suggest that the acetylcholine liberated by 5-HT in the mouse isolated trachea is from the epithelium.
In conclusion, our results suggest that 5-HT indirectly contracts mouse isolated airways via 5-HT2A-dependent release of acetylcholine from the epithelium. Although non-neuronal acetylcholine has been demonstrated in the airways (Wessler & Kirkpatrick), this is the first report that such stores are functional in whole tissues. 5-HT does not constrict human airways in either healthy volunteers or in subjects with asthma (Cushley et al., 1986; Barnes et al., 1998; Cazzola & Matera, 2000). However, our findings point to the potential for epithelium-derived acetylcholine to contribute to airway responses to other inflammatory mediators that may contribute to excessive tone in airway diseases in mice and humans.
Acknowledgments
These studies were funded by the Wellcome Trust and the NHMRC Australia.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- BTXA
botulinum toxin A
- ChAT
choline acetyltransferase
- 5-CT
5-carboxamidotryptamine
- EFS
electrical field stimulation
- 5-HT
5-hydroxytryptamine
- MCh
methacholine
- m-CPB
1-(m-chlorophenyl)-bigaunide
- 8-OHDPAT
R-[+]-2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydronaphthalene
- VOCC
voltage-operated calcium channel
References
- ASCHER-LANDSBERG J., SAUNDERS T., ELOVITZ M., PHILLIPPE M. The effects of 2-aminoethoxydiphenyl borate, a novel inositol 1,4,5-triphosphate receptor modulator on myometrial contractions. Biochem. Biophys. Res. Commun. 1999;264:979–982. doi: 10.1006/bbrc.1999.1602. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., CHUNG K.F., PAGE C.P. Inflammatory mediators of asthma: an update. Pharmacol. Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Pharmacological analysis of the pentagastrin–tiotidine interaction in the mouse isolated stomach. Br. J. Pharmacol. 1985;86:589–599. doi: 10.1111/j.1476-5381.1985.tb08935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTMAN M.D., COLLINS T.J., MACKENZIE L., RODERICK H.L., BERRIDGE M.J., PEPPIATT C.M. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- BRAIN K.L., TROUT S.J., JACKSON V.M., DASS N., CUNNANE T.C. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]
- CAZZOLA M., MATERA M.G. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol. Sci. 2000;21:13–16. doi: 10.1016/s0165-6147(99)01408-x. [DOI] [PubMed] [Google Scholar]
- CUSHLEY M.J., WEE L.H., HOLGATE S.T. The effect of inhaled 5-hydroxytryptamine (5-HT, serotonin) on airway calibre in man. Br. J. Clin. Pharmacol. 1986;22:487–490. doi: 10.1111/j.1365-2125.1986.tb02923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- EUM S.-Y., NOREL X., LEFORT J., LABAT C., VARGAFTIG B.B., BRINK C. Anaphylactic bronchoconstriction in BP2 mice: interactions between serotonin and acetylcholine. Br. J. Pharmacol. 1999;126:312–316. doi: 10.1038/sj.bjp.0702304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKS E.G.A., CAMINA J.P., PETERS P.H.J., YPEY D.L., SCHEENEN W.J.J.M., VAN ZOELEN E.J.J., THEUVENET A.P.R. Besides affecting intracellular calcium signalling, 2-APB reversibly blocks gap junctional coupling in confluent monolayers, thereby allowing the measurement of single-cell membrane currents in undissociated cells. FASEB J. 2003;17:941–943. doi: 10.1096/fj.02-0786fje. [DOI] [PubMed] [Google Scholar]
- HONG S.J., CHANG C.C. Calcium channel subtypes for the sympathetic and parasympathetic nerves of guinea-pig atria. Br. J. Pharmacol. 1995;116:1577–1582. doi: 10.1111/j.1476-5381.1995.tb16375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUMEAU Y., DOUSSAU F., GRANT N.J., POULAIN B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. doi: 10.1016/s0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- MCKUNE C.M., WATTS S.W. Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling. J. Pharmacol. Exp. Ther. 2001;297:88–95. [PubMed] [Google Scholar]
- MORRIS J.L., JOBLING P., GIBBINS I.L. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2124–H2132. doi: 10.1152/ajpheart.2001.281.5.H2124. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., WARD J.K., TADJKARIMI S., YACOUB M.D., BARNES P.J., BELVISI M.G. 5-hydroxytryptamine facilitates cholinergic bronchoconstriction in human and guinea-pig airways. Am. J. Respir. Crit. Care Med. 1995;152:377–380. doi: 10.1164/ajrccm.152.1.7599849. [DOI] [PubMed] [Google Scholar]
- UPHOUSE L. Multiple serotonin receptors: too many, not enough, or just the right number. Neurosci. Biobehav. Rev. 1997;21:679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- WATERMAN S.A. Voltage-gated calcium channels in autonomic neuroeffector transmission. Prog. Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- WESSLER I.K., KIRKPATRICK C.J. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulmon. Pharmacol. Ther. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]