Abstract
Mefloquine is a chiral neurotoxic antimalarial agent showing stereoselective brain uptake in humans and rats. It is a substrate and an inhibitor of the efflux protein P-glycoprotein.
We investigated the stereoselective uptake and efflux of mefloquine in mice, and the consequences of the combination with an efflux protein inhibitor, elacridar (GF120918) on its brain transport.
Racemic mefloquine (25 mg kg−1) was administered intraperitoneally with or without elacridar (10 mg kg−1). Six to seven mice were killed at each of 11 time-points between 30 min and 168 h after administration. Blood and brain concentrations of mefloquine enantiomers were determined using liquid chromatography.
A three-compartment model with zero-order absorption from the injection site was found to best represent the pharmacokinetics of both enantiomers in blood and brain. (−)Mefloquine had a lower blood and brain apparent volume of distribution and a lower efflux clearance from the brain, resulting in a larger brain/blood ratio compared to (+)mefloquine. Elacridar did not modify blood concentrations or the elimination rate from blood for either enantiomers. However, cerebral AUCinf of both enantiomers were increased, with a stronger effect on (+)mefloquine. The efflux clearance from the brain decreased for both enantiomers, with a larger decrease for (+)mefloquine.
After administration of racemic mefloquine in mice, blood and brain pharmacokinetics are stereoselective, (+)mefloquine being excreted from brain more rapidly than its antipode, showing that mefloquine is a substrate of efflux proteins and that mefloquine enantiomers undergo efflux in a stereoselective manner. Moreover, pretreatment with elacridar reduced the brain efflux clearances with a more pronounced effect on (+)mefloquine.
Keywords: Mefloquine, enantiomer, stereoselectivity, P-glycoprotein, efflux protein, brain uptake, pharmacokinetics, blood–brain barrier, elacridar
Introduction
Mefloquine, α-2-piperidinyl-2,8-bis(trifluoromethyl)-4-quinolinemethanol, an antimalarial agent with two asymmetric carbons, is administered orally as the racemic mixture of the two erythro-enantiomers (+)11R,2′S-mefloquine [(+)mefloquine] and (−)11S,2′R-mefloquine [(−)mefloquine] (Lariam®) in the prevention and treatment of chloroquine-resistant malaria. Serious neuropsychiatric reactions have been reported in approximately 1 : 10,000 healthy subjects receiving prophylaxis and 1 : 300 patients receiving treatment (Weinke et al., 1991; Phillips-Howard & Ter Kuile, 1995). Both enantiomers of mefloquine show similar antimalarial activity against Plasmodium falciparum, the parasite responsible for severe malaria in humans (Basco et al., 1992). The specific neurotoxic effects of separated enantiomers is not known. In case the neurotoxicity is different, it is important to study the cerebral transport of mefloquine enantiomers to determine which enantiomer is less transported into the brain.
Cerebral transport of mefloquine is stereoselective. In two human cases of cerebral malaria treated with oral racemic mefloquine, plasma and brain concentrations of the (−)enantiomer were higher than those of the antipode (Pham et al., 1999). In the rat, after repeated oral administration of the racemic mixture, brain concentrations of (−)mefloquine were always higher than those of the antipode while the contrary was observed in plasma (Baudry et al., 1997). This stereoselectivity observed in the brain uptake indicates that the cerebral transport may be active. An active efflux could be responsible for this active stereoselective transport of mefloquine involving efflux proteins.
The blood–brain barrier (BBB) is composed of capillary endothelial cells with efflux proteins, expressed on the luminal side of the plasma membrane. Among them, the most studied, P-glycoprotein (P-gp) and others, such as MRP and the more recently discovered Breast Cancer Resistance Protein BCRP/MXR/ABCG2 (BCRP) (Litman et al., 2001; Cooray et al., 2002; Sun et al., 2003). All of those efflux proteins are responsible for the extrusion of their substrates from the endothelial cells of the BBB back to the cerebral blood circulation.
Interactions between P-gp and mefloquine have been described. It has been shown that mefloquine was able to inhibit P-gp in different drug-resistant cell lines (Lan et al., 1996; Riffkin et al., 1996; Shao et al., 1997). This inhibitory effect on P-gp was stereoselective when tested on the accumulation of vinblastine in an immortalized rat brain capillary endothelial cell line, GPNT (Pham et al., 2000) and on the retention of cyclosporin A in an immobilized P-gp liquid chromatographic stationary phase (Lu et al., 2001), (+)mefloquine being a more potent inhibitor than its antipode. In GPNT cells, in vitro efflux of racemic mefloquine was decreased by P-gp inhibitors such as verapamil and cyclosporin, indicating that mefloquine could also be a substrate of P-gp (Pham et al., 2000).
No in vivo study has yet demonstrated that mefloquine is a substrate of efflux proteins in the brain.
Many compounds have been shown to reverse this multidrug resistance phenomenon (Sun et al., 2003). Many commercialized drugs such as verapamil and cyclosporin A were discovered to be chemosensitizers capable of modulating the effects of these pumps. More specific inhibitors such as PSC 833 (valspodar), VX-170 (biricodar), LY335979 (zosuquidar) and GF120918 (elacridar) were later developed. Elacridar, (N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolynyl)-ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamine) (also known as GF120918 or GG918) was initially described as a specific P-gp inhibitor. But when BCRP was discovered later on, many articles reported an inhibitory effect of this modulator also on BCRP, proving that elacridar was no longer specific to P-gp. Elacridar is now described as a P-gp and BCRP inhibitor (Maliepaard et al., 2001) without any effect on MRP (Evers et al., 2000). It does not modulate cytochrome metabolism (Cummins et al., 2002).
Knowing that (i) mefloquine is a P-gp substrate; (ii) its cerebral transport is stereoselective; (iii) its neurotoxicity could be concentration-dependent, the objective of this study was to investigate and model the cerebral transport of mefloquine enantiomers in mice after administration of the racemic mixture used in human therapeutics and to determine whether there is a difference in brain transport between the two enantiomers. We have also investigated the consequences of the combination with the efflux protein inhibitor, elacridar on the brain transport of both enantiomers. As a modification of plasma protein binding could influence the brain uptake of mefloquine, the effect of elacridar on mefloquine protein binding was also studied.
Methods
Chemicals
Racemic mefloquine and 14C-mefloquine were kindly provided by Hoffmann La Roche (Basel, Switzerland). The efflux inhibitor, elacridar was graciously supplied by Glaxo Smith Kline (Marly-le-Roi, France).
Influence of elacridar on the plasma protein binding of mefloquine
Mefloquine plasma protein-bound fraction was determined by ultrafiltration using Centrifree® devices (Millipore Corp., Bedford, U.S.A.). Centrifree® devices were filled with samples of 1 ml of mouse plasma spiked with racemic mefloquine at low (714 ng ml−1) and high (5000 ng ml−1) concentrations with or without elacridar (150 ng ml−1). 14C-mefloquine was added to each sample (3 μCi ml−1). The devices were centrifuged (1000 × g, 15 min) and the ultrafiltrate was transferred to Ultima Gold counting fluid (Perkin-Elmer Life and Analytical Sciences, Boston, U.S.A.). Radioactivity was determined by liquid scintillation counting on a Beckman LS 6000 TA counter (Beckman, Galway, Ireland). Percentage of plasma protein binding was calculated as follows:
The statistical significance of differences found between radioactivity levels in samples of mefloquine, alone and with elacridar, was assessed using the nonparametric Wilcoxon test.
Influence of efflux inhibition on the pharmacokinetics of mefloquine enantiomers in whole blood and the brain
Two groups of OF1 (4–5 weeks) female mice (Iffa Credo, L'Arbresle, France) were included in a pharmacokinetic study. All mice received a single intraperitoneal injection of racemic mefloquine (25 mg kg−1 solubilized in PEG600/water (25 : 75, v/v)). Mice from group A received repeated intraperitoneal injections of elacridar (10 mg kg−1 suspended in a PEG600/water mixture (25 : 75, v/v)) while group B received 100 μl of its placebo (elacridar solvent: PEG600/water (25 : 75, v/v)). Injections of elacridar or its placebo were performed 20 min prior to the single mefloquine injection and were repeated twice daily until the mice were killed. Preliminary experiments showed that intraperitoneal injection of a single dose of 10 mg kg−1 of elacridar provided plasma concentrations above the EC50 of the drug (12 ng ml−1) from the first sampling point (10 min) up to at least 7 h after administration with a maximum concentration of 145 ng ml−1 (Imbert et al., 2003). Therefore, injecting elacridar (10 mg kg−1) b.i.d. until killing of mice was adequate to inhibit P-glycoprotein throughout the present experiment.
Mice were killed at the following times after mefloquine treatment: 30 min, 1, 2, 5, 8, 17, 24, 48, 72, 120 and 168 h (six to seven mice per time). Brain and whole blood samples were collected and frozen at −20°C until HPLC analysis. Mefloquine stability under these storage conditions was previously demonstrated.
Experiments were conducted according to the ‘Guidance on the Operation of the Animals (Scientific Procedures) Act 1986'.
Determination of mefloquine enantiomers in whole blood and brain tissue by liquid chromatography
The liquid chromatographic equipment consisted of a WISP 717+ automatic sample injector (Waters, Millipore, Saint Quentin, France), a Shimadzu LC10 AV pump, SPD10 AV spectrophotometric detector and Class VP automated software system (Touzart & Matignon, Les Ulis, France). Mefloquine enantiomers was determined using a sequential achiral–chiral chromatography (Ducharme et al., 1996) with a Lichrospher® 100 RP-18 (5 μm) as achiral column (Lichrocart 125-4 HPLC cartridge) and a Lichrospher® 100 RP-18 (5 μm) (Lichrocart 4-4) guard column (Merck, Darmstadt, Germany) and with a 150 × 4.6 mm Ultron ES-OVM ovomucoid (Agilent Technologies, Massy, France) as chiral column. The mobile phases were composed of: acetonitrile/water (50/50, v/v) modified with orthophosphoric acid (400 μl l−1) and diethylamine (80 μl l−1), for the achiral chromatography and acetonitrile/20 mM pH 5.8 phosphate buffer (20/80, v/v) for the chiral chromatography. Analyses were performed at room temperature, at a flow rate of 1.0 ml min−1 and at 285 and 230 nm for the achiral and chiral chromatographies, respectively.
Mefloquine was extracted from whole blood (200 μl) with 50 μl of internal standard (enpiroline, 10 μg ml−1 in methanol) and 200 μl of methanol. The mixture was vortexed (15 s) and centrifuged (1000 × g, 5 min). The supernatant was evaporated under nitrogen at 60°C. The residue was dissolved in 200 μl of a 1.2 M trisodic phosphate solution and 200 μl of methyl-tert-butyl ether, vortexed and centrifuged (1000 × g, 5 min). The organic supernatant was evaporated under nitrogen at 60°C. The residue was dissolved in mobile phase, and injected into the achiral chromatographic system.
Mefloquine was extracted from brain tissue (200 mg) with 50 μl of internal standard (enpiroline 10 μg ml−1 in methanol) and 3 ml of acetonitrile using an Ultra-Turrax (Jankel & Kunkel) for 15 s and then centrifuged (1000 × g, 10 min). The supernatant was evaporated under nitrogen at 60°C. The residue was dissolved in 500 μl of a 1.2 M trisodic phosphate solution and 3 ml of methyl-tert-butyl ether and shaken for 10 min. The mixture was centrifuged (1000 × g, 10 min) and the supernatant evaporated under nitrogen at 60°C. The residue was dissolved in mobile phase, and injected into the achiral chromatographic system.
For blood and brain, total mefloquine in the mobile phase was collected after the detector and evaporated under nitrogen at 60°C. Then, 500 μl of a 1.2 M trisodic phosphate solution and 500 μl of methyl-tert-butyl ether were added. The mixture was vortexed and centrifuged (1000 × g, 10 min). The organic supernatant was evaporated under nitrogen at 60°C. The residue was dissolved in mobile phase, and injected into the chiral chromatographic system.
HPLC methods were linear over the following ranges: 0–10 and 0–5 μg ml−1 for racemic mefloquine and mefloquine enantiomers, respectively in plasma and brain. Intraday and interday variabilities performed on low-level controls (750 ng ml−1 for racemic mefloquine or 375 ng ml−1 for each enantiomer) or high-level controls (7500 ng ml−1 for racemic mefloquine or 3750 ng ml−1 for each enantiomer) were always under 1.65 and 3.56% in plasma and in brain, respectively. Inaccuracies for these controls were under 1.3 and 3.4% in plasma and in brain, respectively. Limits of quantification were 150ng ml−1 and 150 ng g−1 in plasma and in brain, respectively. Recoveries were 41 and 42% in plasma and brain, respectively.
Statistical analysis
A joint model was developed to describe the pharmacokinetics of the two enantiomers of mefloquine in blood and brain. We used a naive pooling of data (NPD) approach to estimate the parameters of the model, because the data had been obtained by destructive sampling. The parameters θ of the model f(θ,t) were estimated using nonlinear regression weighted by the empirical variance. The estimation was performed using the software R (Ihaka & Gentleman, 1996). We made use of the library nls2 developed by Huet et al. (1996).
To build the pharmacokinetic model, we compared two-, three- and four-compartment models, with different assumptions concerning the physiological structure of the model. Absorption and elimination were assumed to involve only the blood compartment, based on previous knowledge of the absorption and disposition of mefloquine. We also compared different assumptions regarding the absorption model after parenteral administration of mefloquine. For each model tested, the log-likelihood was calculated. Nested models were compared using the log-likelihood ratio test. Non-nested models were compared using the Akaike criterion. We also selected models based on their ability to describe the data, as assessed using diagnostic plots.
Once the structure of the model had been selected, we compared the pharmacokinetic parameters between the two enantiomers and between the two groups. The parameters for (+)mefloquine in the group without elacridar were taken as the reference and denoted θ. We modelled the kth component of the vector of parameters for (−)mefloquine in the same group as θk × αk. The effect of elacridar was also modelled using multiplicative factors. The kth component of the vector of parameters of (+)mefloquine in the group elacridar was modelled as θk × βk. The kth component of the vector of parameters of (−)mefloquine in the group elacridar was modelled as θk × αk × βk × γk, where γk represents the differential effect of elacridar on (−)mefloquine with respect to its antipode.
We used an iterative backward procedure to select which of the αk, βk, γk, could be taken equal to 1. We started from the full model where all the α are different from 1, and we tested which of the α could be fixed to 1 by removing one at a time using log-likelihood ratio tests as above. If for at least one of the nested models, the difference in log-likelihood was nonsignificant, we then removed the α for which the difference in log-likelihood was smallest. The procedure was repeated until none of the αk remaining in the model could be fixed to 1 without a significant loss of log-likelihood. Using the same procedure, we then tested which of the β and γ could be fixed to 1.
Results
The mefloquine plasma protein unbound fractions were 2.70% (±0.43) and 2.82% (±0.58) without and with elacridar, respectively at low mefloquine concentrations and 2.61 (±0.41) and 2.73% (±0.25) without and with elacridar, respectively at high mefloquine concentrations, close to the free fraction values previously reported for rodents (Mu et al., 1975). No difference was observed in free fractions with and without elacridar at low and high mefloquine concentrations.
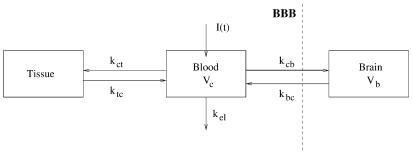
A three-compartment model was found to best represent the pharmacokinetics of both enantiomers and is shown in Figure 1. Let Cc denote the concentration in the blood (central) compartment for a given enantiomer in one of the groups, Cb the concentration in the brain and Qt the amount of the same enantiomer in the tissue compartment. Let kct and ktc denote the rate constants to and from the tissue compartment, and kcb and kbc the rate constants to and from the brain compartment. Let kel denote the elimination rate constant from the central compartment, and Vc and Vb the apparent volumes of distribution, respectively, in the central and brain compartments.
Figure 1.
Three-compartment model used to describe the pharmacokinetics of the two enantiomers of mefloquine in mouse blood and brain.
The equations for the selected model are as follows:
 |
where I(t) is the input of drug into the central compartment. The absorption from the intraperitoneal injection site was best modelled as zero-order absorption, according to graphical diagnostics. Models including first-order absorption and i.v. injection failed to represent adequately the initial concentrations. I(t) was therefore modelled as:
 |
where Tlag is the length of the infusion and D is the dose. Because it represents a breakpoint in the model, Tlag is a difficult parameter to estimate in nonlinear regression. We therefore used a grid-search to find an appropriate value of Tlag.
We fixed Tlag to 0.01 h in the following, a value providing a good fit of the concentrations of mefloquine for both enantiomers in both groups, as assessed by graphical diagnostics. A model where brain efflux was saturable was also tested, but the results (not shown) indicated that even though saturable efflux processes may be involved in the efflux of mefloquine, the approximation of a linear model was acceptable for describing the transport of mefloquine in and out of brain. Finally, models with saturable transport of mefloquine out of tissues were tested but provided a worse fit.
Figures 2 and 3 show the predicted and observed concentration versus time profiles for both enantiomers in blood and brain, respectively. The plots were drawn in logarithmic scale and showed a very good fit for both enantiomers, both in blood and brain. The variability in the measurement was usually small. We assessed the goodness of fit of the models using plots of the residuals versus time, and of the residuals versus predicted values, and these plots confirmed the adequacy of the model (figures not shown). Concentrations of (−)mefloquine are consistently higher than concentrations of (+)mefloquine, in both compartments considered. Comparing the figures on the left side (mefloquine alone) and on the right side (mefloquine coadministered with elacridar), the concentrations of both enantiomers appear unchanged in blood after administration of elacridar, but they are increased in brain.
Figure 2.
Concentrations versus time in blood for both enantiomers of mefloquine (MQ) after a single intraperitoneal administration of 25 mg kg−1 of racemic mefloquine. The plots are separated by group (left, mefloquine alone; right, mefloquine coadministered with the inhibitor). In each plot, the predicted and observed concentrations are shown for (+)MQ (observations: triangles, predictions: full line) and (−)MQ (observations: x; predictions: dotted line).
Figure 3.
Concentrations versus time in brain for both enantiomers of mefloquine (MQ) after a single intraperitoneal administration of 25 mg kg−1 of racemic mefloquine. The plots are separated by group (left, mefloquine alone; right, mefloquine coadministered with the inhibitor). In each plot, the predicted and observed concentrations are shown for (+)MQ (observations: triangles, predictions: full line) and (−)MQ (observations: x; predictions: dotted line).
The concentrations in the blood compartment were reported as μg ml−1, so Vc was an apparent volume (and could also be denoted Vc/F) estimated as ml, but concentrations in the brain compartment were reported as μg g−1 of brain, so that the volume of distribution in the brain was expressed in g of brain, as usual in studies of the BBB. From this model, we also derived the clearance parameters and areas under the concentration curve (AUC): elimination clearance from blood was obtained as CLel=kelVc, clearance from the brain as Cbc=kbcVb, and clearance from blood to brain as Ccb=kcbVc. Blood AUC was calculated as AUCc=Dose/CLel, and brain AUC was AUCb=kcb/kel Dose/CLbc.
Based on the model, we compared the pharmacokinetic parameters of the two enantiomers, estimated in the two groups. The results are shown in Table 1, where the values for the (+) enantiomer in the group receiving mefloquine alone were taken as the reference, and the values of α, β and γ that differ from 1 are given. The standard errors of estimation for each value are given in brackets. They were small (less than 15% for most parameters except kct for which it was 26% and for ktc which was poorly estimated), denoting a good estimation for most parameters. The information from this table can also be summarized by calculating the parameter values for each enantiomer in each group. For instance, the value of kbc is 0.107 h−1 for (+)mefloquine and 0.107 × 0.80=0.086 h−1 for (−)mefloquine in the group with mefloquine alone, and respectively 0.107 × 1.62=0.173 h−1 and 0.107 × 0.80 × 1.62=0.139 h−1 in the group with elacridar.
Table 1.
Estimated parameters (and standard errors of estimation in brackets)
| Mefloquine alone | Pretreatment with elacridar | |||
|---|---|---|---|---|
| Parameters | Value for (+)MQ | α | β | γ |
| Reference values for (+)MQ | Changes for (−)MQ compared to the reference | Changes for (+)MQ compared to the reference | Changes for (−)MQ compared to the reference | |
| kel (h−1) | 0.047 (0.001) | 1 | 1 | 1 |
| kcb (h−1) | 0.127 (0.016) | 1 | 1 | 1 |
| kbc (h−1) | 0.107 (0.004) | 0.80 (0.03) | 1.62 (0.08) | 1 |
| kct (h−1) | 0.031 (0.008) | 1 | 1 | 1 |
| ktc (h−1) | 0.256 (0.234) | 1 | 0.17 (0.17) | 1 |
| Vc (ml) | 122 (3) | 0.53 (0.01) | 1 | 1 |
| Vb (g) | 76 (9) | 0.44 (0.01) | 0.25 (0.01) | 1.59 (0.05) |
The values for (+)mefloquine [(+)MQ] in the group given mefloquine without the P-gp inhibitor are taken as reference. Then, significant changes (α, β, γ) in the values of these reference parameters are given
Using the parameterization explained in Methods, we found that most of the α were not significantly different from 1. Table 2 shows the derived parameters for the two enantiomers, with or without elacridar. In this table, many parameters do not change after administration of elacridar. Indeed, in the final model, because the elimination rate constant kel and the volume of distribution in blood Vc did not change when the animals were pretreated with elacridar, the clearance from blood CLel=Vc × kel was also unchanged. On the other hand, the efflux clearance from brain CLbc=Vb × kbc was different both between enantiomers and between groups because of differences in Vb and kbc.
Table 2.
Derived parameters of (+)mefloquine [(+)MQ] and (−)mefloquine [(−)MQ] estimated for each of the two groups with or without elacridar
| Mefloquine alone | Pretreatment with elacridar | |||
|---|---|---|---|---|
| Parameters | (+)MQ | (−)MQ | (+)MQ | (−)MQ |
| CLcb (ml h−1) | 15.5 | 8.2 | 15.5 | 8.2 |
| CLbc (g h−1) | 8.2 | 2.9 | 3.3 | 1.9 |
| CLel (ml h−1) | 5.7 | 3.6 | 5.7 | 3.6 |
| Vc (ml) | 122 | 65 | 122 | 65 |
| Vb (g) | 77 | 34 | 19 | 13 |
| AUCc (μg ml−1 h) | 66 | 104 | 66 | 104 |
| AUCb (μg g−1 h) | 124 | 349 | 307 | 533 |
The only differences between the two enantiomers seen in Table 1 were that the volumes of distribution were lower for (−)mefloquine, by 53% for Vc (P<0.001) and by 44% for Vb (P<0.001), and that the (−)mefloquine enantiomer had a lower rate constant of transfer from brain to blood, kbc (P<0.001). Several parameters also remained unchanged for both enantiomers after the administration of elacridar: the elimination rate constant from the blood, as well as the transfer rate constants from the blood to the brain and the tissues, were unchanged. The transfer rate constant from the tissues to the blood ktc was reduced by 83% in the presence of elacridar for both enantiomers (P=0.03), but the estimate of the parameter and the associated β had a large standard error of estimation indicating that these parameters were estimated with a poor precision. The volumes of distribution in brain were lower by 75% for (+)mefloquine (P<0.001) and 60% for its antipode (P<0.001) in the presence of elacridar when compared to the values of the parameters in the group mefloquine alone. The volumes of distribution in blood were unchanged for both enantiomers. These findings are in accordance with Figures 2 and 3, where blood concentrations were different between the two enantiomers, but unchanged in the presence of the inhibitor while brain concentrations increased. Unexpectedly, the transfer rate constant from the brain to the blood kbc was increased in the presence of elacridar (P<0.001). However, because the apparent volumes of distribution in the brain decreased, the efflux clearances of both enantiomers decreased in the presence of the inhibitor as shown in Table 2. For (+)mefloquine, the efflux clearance out of the brain CLbc decreased by 60%, and for (−)mefloquine, the efflux clearance which was a third of that of (+)mefloquine decreased by 35% in the presence of elacridar.
Brain uptake of mefloquine enantiomers, represented as the brain–blood concentration ratios with and without pretreatment with elacridar is represented in Figure 4. Without pretreatment with elacridar, (+)mefloquine showed a lower brain–blood ratio when compared to its antipode, with the ratio between the volumes of distribution of brain and blood also lower for (−)mefloquine (0.51) than for (+)mefloquine (0.62). However, when we tested a model where α × γ =1 (α × β × γ=β) for this parameter using a log-likelihood ratio test, this difference in ratios was not statistically significant. Figure 4 also shows that enantiomeric ratios are inverted during treatment with elacridar, when compared without elacridar. When comparing the AUC over the whole period of time, the brain–blood ratios for both enantiomers are increased in the presence of elacridar [1.83 for (+)mefloquine and 1.73 for (−)mefloquine in the presence of elacridar, versus respectively 0.74 and 1.11 in the absence of the inhibitor], indicating increased relative uptake to the brain. This increase is stereoselective: the ratio of the brain–blood AUC ratio of (+)mefloquine to the brain–blood AUC ratio of its antipode is 0.67 in the absence of elacridar (confidence interval: [0.62, 0.72]) while it is 1.07 (confidence interval: [1.00, 1.13]) in the presence of the inhibitor, and the difference is statistically significant. Moreover, 1 is on the boundary of the second confidence interval which suggests that the stereoselectivity observed in mefloquine brain uptake without elacridar is in fact suppressed with the pretreatment with elacridar.
Figure 4.
Mean brain/whole blood concentration ratios (s.d.) for racemic mefloquine (Racemic) (circle, full line) and the enantiomers of mefloquine (+)MQ (triangle, dotted line) and (−)MQ (x, dotted line) after a single intraperitoneal administration of 25 mg kg−1 of racemic mefloquine. The plots are separated by group (left, mefloquine alone; right, mefloquine coadministered with the inhibitor).
Discussion
Modelling the pharmacokinetics of mefloquine in blood and brain
In the present study, we jointly modelled the pharmacokinetics of the two enantiomers of mefloquine in mice, in the presence or absence of elacridar, an inhibitor of P-glycoprotein. Data from blood and brain were obtained in the same animals. A compartmental model was developed and the parameters obtained through nonlinear regression.
The pharmacokinetics of mefloquine have been modelled in man before, and a two-compartment model was mostly used to describe the time-course of the concentrations after oral administration (Hellgren et al., 1997; Svensson et al., 2002). In our study, brain concentrations were also available and we were able to develop a three-compartment model, with a blood compartment, a brain compartment, and a tissue compartment to aggregate the remaining distribution compartments of mefloquine. More complex models reflecting saturable efflux processes were also tested, but either we could not estimate the associated parameters because of identifiability problems, or these models did not provide a significant improvement over first- or zero-order processes.
Modelling the pharmacokinetics of mefloquine therefore allowed us to describe the system in a satisfactory manner and to test for changes in the parameters when adding the P-gp inhibitor, elacridar. We could also jointly fit the brain and blood concentrations. As such, modelling provides a more insightful analysis than noncompartmental approaches.
Stereoselectivity in blood pharmacokinetics
In our study, pharmacokinetics of mefloquine were stereoselective in mice after intraperitoneal administration of racemic mefloquine with higher whole blood concentrations and mean AUC value for (−)mefloquine when compared to its antipode. In the group where mefloquine is administered alone, we found apparent volumes of distribution for (−)mefloquine lower by about 50% both in blood and brain, resulting in higher observed concentrations. This could be the result of a higher bioavailability for (−)mefloquine, since in the present study we do not have intravenous data to assess the absolute bioavailability. kel was the same for both enantiomers, with an estimated value of 0.047 h−1 yielding a half-life of 14.7 h. Using noncompartmental methods, Chung et al. (1982) reported an elimination half-life of 16.1 h in mice, very close to the value we obtain through modelling. As a result of the lower volume of distribution for (−)mefloquine, this enantiomer has a lower systemic clearance compared to its antipode, similar to what has been reported in man (Hellgren et al., 1997).
The higher AUC value in whole blood for (−)mefloquine was also observed in plasma samples after oral administration in adult Caucasian healthy subjects (Gimenez et al., 1994), adult Thai healthy subjects (Martin et al., 1994) and Thai malaria children (Bourahla et al., 1996). In contrast, after repeated oral administration of the racemic mixture in rats, plasma concentrations of (+)mefloquine were higher than those of its antipode (Baudry et al., 1997), opposite to those obtained after oral administration in humans and intraperitoneal injection in mice.
Several points may explain these differences of stereoselectivity. (i) It could be a species-dependent stereoselective pharmacokinetics. This phenomenon has been often described between human and rodent pharmacokinetics but is less common within rodents between rats and mice. Species-dependent stereoselective affinities have been described for transporters (Donly et al., 2000) but this phenomenon has not been described in particular for efflux proteins (ii) This difference of stereoselectivity between rats and mice could also be explained by the difference of route of administration (oral in rats (Baudry et al., 1997) versus intraperitoneal in mice in our study). Stereoselectivity may occur during the absorption through the intestinal membrane or through the peritoneum. It also may be due to a stereoselective first-pass effect. (iii) Chiral inversion could also appear through the intestinal barrier as described for the anticonvulsant stiripentol (Tang et al., 1994). However, this stereoconversion may be ruled out for mefloquine as, after oral administration of one of the separated enantiomer, no antipode was detected in plasma and brain (Baudry et al., 1997). (iv) The difference in biological samples where mefloquine was determined (plasma for rats and whole blood for mice) could also explain the difference of stereoselectivity. However, this explanation can also be ruled out, no stereoselective difference in mefloquine accumulation having been described in erythrocytes (Vidrequin et al., 1996).
Stereoselectivity in brain uptake
Mefloquine is a lipophilic compound, highly distributed in tissues, such as brain parenchyma. Cerebral uptake of its enantiomers was studied in rats after repeated oral administration of the racemic mixture (Baudry et al., 1997). It showed that (−)mefloquine was more concentrated in the brain parenchyma than its antipode whereas the contrary was observed in plasma. This stereoselective brain uptake strongly suggests that an active transport of mefloquine occurs during the brain uptake of this antimalarial agent. In the present study performed on mice, as in a previous study in humans (Pham et al., 1999), concentrations of (−)mefloquine were higher in brain as also observed in blood.
Stereoselective modulation of brain efflux by elacridar
The increase in mefloquine brain uptake that we observed following a pretreatment with elacridar can be due to a modulation of efflux proteins by elacridar inhibiting the efflux of mefloquine during its transfer through the BBB. Mefloquine is highly bound to plasma proteins (Mu et al., 1975) and elacridar could also have displaced it from its plasma protein binding, increasing its free fraction in blood and therefore the fraction crossing the BBB. The fact that we did not observed any modification of protein binding by elacridar using ultrafiltration and that the stereoselectivity observed in brain–blood ratios between the two enantiomers was suppressed after pretreatment with elacridar (Figure 4) strongly suggests that this stereoselectivity was due to efflux proteins.
The present study demonstrated that, in vivo, mefloquine enantiomers undergo efflux in a stereoselective manner. It is interesting to note that while efflux proteins show a vast diversity in the structure of their substrates and therefore are very unselective regarding their substrates, these efflux proteins can also be able to differentiate very close structures as those of two enantiomers of a chiral compound.
Information is available regarding the interaction between mefloquine and P-gp but nothing is known about the interaction between mefloquine and either MRP or BCRP. Elacridar is an inhibitor of both P-gp and BCRP and the fact that the brain uptake of mefloquine was increased by a pretreatment with this modulator indicates that mefloquine could be a substrate of P-gp, or BCRP, or both. Pham et al. (2000) used verapamil, cyclosporin A and chlorpromazine as chemosensitizers to investigate the intracellular uptake of mefloquine in the rat brain capillary endothelial cell line, GPNT (Pham et al., 2000). Verapamil was described as an inhibitor of P-gp but not of BCRP while cyclosporin A is a modulator of both P-gp and BCRP (Ozvegy et al., 2001). No information is available regarding the interaction of chlorpromazine and BCRP. To confirm the substrate characteristics of mefloquine with respect to either P-gp or BCRP, the pharmacokinetics and brain uptake of mefloquine enantiomers should be investigated in knockout mdr1a(−/−)1b(−/−) and bcrp1(−/−) and their respective wild-type species (Schinkel et al., 1996; Jonker et al., 2002).
In the present study, coadministration of elacridar with racemic mefloquine increased AUC values in the brain with a stronger effect on (+)mefloquine compared to its antipode and did not modify mefloquine concentrations in whole blood. The volumes of distribution in brain of the two enantiomers both decreased in the presence of elacridar, an inhibitor of P-gp, but there was no change in the apparent volume of distribution in blood Vc, suggesting that systemic bioavailability was unchanged. The excretion rate of mefloquine from tissue to blood, ktc, decreased in the presence of elacridar. Because elacridar is a P-gp inhibitor and P-gp is distributed throughout the body, this finding could be explained by the inhibition of P-gp in the tissues compartment. There was no change in the systemic elimination rate constant kel when the inhibitor was given. The apparent clearance out of the brain CLbc and therefore the efflux out of brain also decreased in the presence of the inhibitor, by 40% for (+)mefloquine and by 65% for (−)mefloquine, as shown in Table 2. This is due to a large decrease in Vb [75% for (+)mefloquine and 60% for (−)mefloquine].
In terms of stereoselectivity of modulators of efflux proteins, similar efficacy in modulating drug transport has been described for enantiomers of calcium antagonists whereas stereoselectivity in reversal of multidrug resistance has been reported for butaclamol (Szabo & Molnar, 1998). As for stereoselectivity of substrates of efflux proteins, no stereoselectivity of P-gp-mediated transport has been reported for the enantiomers of verapamil (Sandstrom et al., 1998), citalopram (Rochat et al., 1999) and daunorubicin (Loetchutinat et al., 2001). Stereoselective P-gp transport was described in vitro for diastereoisomers of d4T analogs (Siccardi et al., 2003) and the diastereoisomers quinine and quinidine (Hooiveld et al., 2002). Our study is the first in vivo evidence for enantioselectivity in cerebral efflux transport.
Mefloquine is widely used in the treatment of chloroquine-resistant malaria and is often prescribed in combination with other antimalarial agents. This antimalarial agent is often responsible for potentially serious neuropsychiatric reactions. In cerebral malaria, the parasite does not cross the BBB, stays in the cerebral capillary circulation and is responsible for immune and inflammatory reactions. Red blood cells parasitized with Plasmodium falciparum adhere to non parasitized erythrocytes and obstruct brain microcirculation. For all these reasons (no transport into the brain of the parasite and neurotoxicity of mefloquine), it is better to avoid mefloquine transport to the brain. For a similar level of blood concentrations of enantiomers, (+)mefloquine seems to give lower brain concentrations than its antipode and could be, therefore, more adequate to reduce neurotoxicity. However, in case of combination with a drug capable of inhibiting efflux proteins, such as elacridar, this advantage disappears.
Acknowledgments
Hélène Chacun for helping us to conduct investigations with radiolabelled compounds
Abbreviations
- AUC
area under the curve
- BBB
blood–brain barrier
- P-gp
P-glycoprotein
References
- BASCO L.K., GILLOTIN C., GIMENEZ F., FARINOTTI R., LEBRAS J. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br. J. Clin. Pharmacol. 1992;33:517–520. doi: 10.1111/j.1365-2125.1992.tb04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUDRY S., PHAM Y.T., BAUNE B., VIDREQUIN S., CREVOISIER C., GIMENEZ F., FARINOTTI R. Stereoselective passage of mefloquine through the blood–brain barrier in the rat. J. Pharm. Pharmacol. 1997;49:1086–1090. doi: 10.1111/j.2042-7158.1997.tb06047.x. [DOI] [PubMed] [Google Scholar]
- BOURAHLA A., MARTIN C., GIMENEZ F., SINGHASIVANON V., ATTANATH P., SABCHEARON A., CHONGSUPHAJAISIDDHI T., FARINOTTI R. Stereoselective pharmacokinetics of mefloquine in young children. Eur. J. Clin. Pharmacol. 1996;50:241–244. doi: 10.1007/s002280050100. [DOI] [PubMed] [Google Scholar]
- CHUNG H., JIMMERSON V.R., ROZMAN R.S., SANDERS J.E. Disposition of the diastereoisomer of mefloquine in mice. Pharmacology. 1982;24:267–274. doi: 10.1159/000137606. [DOI] [PubMed] [Google Scholar]
- COORAY H.C., BLACKMORE C.G., MASKELL L., BARRAND M.A. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- CUMMINS C.L., JACOBSEN W., BENET L.Z. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002;300:1036–1045. doi: 10.1124/jpet.300.3.1036. [DOI] [PubMed] [Google Scholar]
- DONLY C., JEVNIKAR J., MCLEAN H., CAVENEY S. Substrate-stereoselectivity of a high-affinity glutamate transporter cloned from the CNS of the cockroach Diploptera punctata. Insect Biochem. Mol. Biol. 2000;30:369–376. doi: 10.1016/s0965-1748(00)00004-7. [DOI] [PubMed] [Google Scholar]
- DUCHARME J., FERNANDEZ C., GIMENEZ F., FARINOTTI R. Critical issues in chiral drug analysis in biological fluids by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1996;686:65–75. doi: 10.1016/s0378-4347(96)00274-5. [DOI] [PubMed] [Google Scholar]
- EVERS R., KOOL M., SMITH A.J., VAN DEEMTER L., DE HAAS M., BORST P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br. J. Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ F., PENNIE R.A., KOREN G., CREVOISIER C., WAINER I.W., FARINOTTI R. Stereoselective pharmacokinetics of mefloquine in healthy Caucasians after multiple doses. J. Pharm. Sci. 1994;83:824–827. doi: 10.1002/jps.2600830613. [DOI] [PubMed] [Google Scholar]
- HELLGREN U., BERGGREN-PALME I., BERGQVIST Y., JERLING M. Enantioselective pharmacokinetics of mefloquine during long-term intake of the prophylactic dose. Br. J. Clin. Pharmacol. 1997;44:119–124. doi: 10.1046/j.1365-2125.1997.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOIVELD G.J., HEEGSMA J., VAN MONTFOORT J.E., JANSEN P.L., MEIJER D.K., MULLER M. Stereoselective transport of hydrophilic quaternary drugs by human MDR1 and rat Mdr1b P-glycoproteins. Br. J. Pharmacol. 2002;135:1685–1694. doi: 10.1038/sj.bjp.0704620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUET S., BOUVIER A., GRUET M., JOLIVET E. Statistical Tools for Nonlinear Regression. New York: Springer-Verlag; 1996. [Google Scholar]
- IHAKA R., GENTLEMAN R. A language for data analysis and graphics. J. Comput. Graph Stat. 1996;5:299–314. [Google Scholar]
- IMBERT F., JARDIN M., FERNANDEZ C., GANTIER J.C., DROMER F., BARON G., MENTRE F., VAN BEIJSTERVELDT L., SINGLAS E., GIMENEZ F. Effect of efflux inhibition on brain uptake of itraconazole in mice infected with Cryptococcus neoformans. Drug Metab. Dispos. 2003;31:319–325. doi: 10.1124/dmd.31.3.319. [DOI] [PubMed] [Google Scholar]
- JONKER J.W., BUITELAAR M., WAGENAAR E., VAN DER VALK M.A., SCHEFFER G.L., SCHEPER R.J., PLOSCH T., KUIPERS F., ELFERINK R.P., ROSING H., BEIJNEN J.H., SCHINKEL A.H. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAN L.B., AYESH S., LYUBIMOV E., PASHINSKY I., STEIN W.D. Kinetic parameters for reversal of the multidrug pump as measured for drug accumulation and cell killing. Cancer Chemother. Pharmacol. 1996;38:181–190. doi: 10.1007/s002800050468. [DOI] [PubMed] [Google Scholar]
- LITMAN T., DRULEY T.E., STEIN W.D., BATES S.E. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol. Life Sci. 2001;58:931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOETCHUTINAT C., HEYWANG C., PRIEBE W., GARNIER-SUILLEROT A. The absence of stereoselective P-glycoprotein- and multidrug resistance-associated protein-mediated transport of daunorubicin. Biochem. Pharmacol. 2001;62:561–567. doi: 10.1016/s0006-2952(01)00703-1. [DOI] [PubMed] [Google Scholar]
- LU L., LEONESSA F., BAYNHAM M.T., CLARKE R., GIMENEZ F., PHAM Y.T., ROUX F., WAINER I.W. The enantioselective binding of mefloquine enantiomers to P-glycoprotein determined using an immobilized P-glycoprotein liquid chromatographic stationary phase. Pharm. Res. 2001;18:1327–1330. doi: 10.1023/a:1013098213770. [DOI] [PubMed] [Google Scholar]
- MALIEPAARD M., SCHEFFER G.L., FANEYTE I.F., VAN GASTELEN M.A., PIJNENBORG A.C., SCHINKEL A.H., VAN DE VIJVER M.J., SCHEPER R.J., SCHELLENS J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- MARTIN C., GIMENEZ F., BANGCHANG K.N., KARBWANG J., WAINER I.W., FARINOTTI R. Whole blood concentrations of mefloquine enantiomers in healthy Thai volunteers. Eur. J. Clin. Pharmacol. 1994;47:85–87. doi: 10.1007/BF00193485. [DOI] [PubMed] [Google Scholar]
- MU J.Y., ISRAILI Z.H., DAYTON P.G. Studies of the disposition and metabolism of mefloquine HCl (WR 142,490), a quinolinemethanol antimalarial, in the rat. Limited studies with an analog, WR 30,090. Drug Metab. Dispos. 1975;3:198–210. [PubMed] [Google Scholar]
- OZVEGY C., LITMAN T., SZAKACS G., NAGY Z., BATES S., VARADI A., SARKADI B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem. Biophys. Res. Commun. 2001;285:111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- PHAM Y.T., NOSTEN F., FARINOTTI R., WHITE N.J., GIMENEZ F. Cerebral uptake of mefloquine enantiomers in fatal cerebral malaria. Int. J. Clin. Pharmacol. Ther. 1999;37:58–61. [PubMed] [Google Scholar]
- PHAM Y.T., REGINA A., FARINOTTI R., COURAUD P., WAINER I.W., ROUX F., GIMENEZ F. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochim. Biophys. Acta. 2000;1524:212–219. doi: 10.1016/s0304-4165(00)00160-4. [DOI] [PubMed] [Google Scholar]
- PHILLIPS-HOWARD P.A., TER KUILE F.O. CNS adverse events associated with antimalarial agents. Fact or fiction. Drug Saf. 1995;12:370–383. doi: 10.2165/00002018-199512060-00003. [DOI] [PubMed] [Google Scholar]
- RIFFKIN C.D., CHUNG R., WALL D.M., ZALCBERG J.R., COWMAN A.F., FOLEY M., TILLEY L. Modulation of the function of human MDR1 P-glycoprotein by the antimalarial drug mefloquine. Biochem. Pharmacol. 1996;52:1545–1552. doi: 10.1016/s0006-2952(96)00556-4. [DOI] [PubMed] [Google Scholar]
- ROCHAT B., BAUMANN P., AUDUS K.L. Transport mechanisms for the antidepressant citalopram in brain microvessel endothelium. Brain Res. 1999;831:229–236. doi: 10.1016/s0006-8993(99)01461-4. [DOI] [PubMed] [Google Scholar]
- SANDSTROM R., KARLSSON A., LENNERNAS H. The absence of stereoselective P-glycoprotein-mediated transport of R/S-verapamil across the rat jejunum. J. Pharm. Pharmacol. 1998;50:729–735. doi: 10.1111/j.2042-7158.1998.tb07133.x. [DOI] [PubMed] [Google Scholar]
- SCHINKEL A.H., WAGENAAR E., MOL C.A., VAN DEEMTER L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAO Y.M., AYESH S., STEIN W.D. Mutually co-operative interactions between modulators of P-glycoprotein. Biochim. Biophys. Acta. 1997;1360:30–38. doi: 10.1016/s0925-4439(96)00065-8. [DOI] [PubMed] [Google Scholar]
- SICCARDI D., KANDALAFT L.E., GUMBLETON M., MCGUIGAN C. Stereoselective and concentration-dependent polarized epithelial permeability of a series of phosphoramidate triester prodrugs of d4T: an in vitro study in Caco-2 and Madin–Darby canine kidney cell monolayers. J. Pharmacol. Exp. Ther. 2003;307:1112–1119. doi: 10.1124/jpet.103.056135. [DOI] [PubMed] [Google Scholar]
- SUN H., DAI H., SHAIK N., ELMQUIST W.F. Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- SVENSSON U.S., ALIN H., KARLSSON M.O., BERGQVIST Y., ASHTON M. Population pharmacokinetic and pharmacodynamic modelling of artemisinin and mefloquine enantiomers in patients with falciparum malaria. Eur. J. Clin. Pharmacol. 2002;58:339–351. doi: 10.1007/s00228-002-0485-y. [DOI] [PubMed] [Google Scholar]
- SZABO D., MOLNAR J. The role of stereoselectivity of chemosensitizers in the reversal of multidrug resistance of mouse lymphoma cells. Anticancer Res. 1998;18:3039–3044. [PubMed] [Google Scholar]
- TANG C., ZHANG K., LEPAGE F., LEVY R.H., BAILLIE T.A. Metabolic chiral inversion of stiripentol in the rat. II. Influence of route of administration. Drug Metab. Dispos. 1994;22:554–560. [PubMed] [Google Scholar]
- VIDREQUIN S., GIMENEZ F., BASCO L.K., MARTIN C., LEBRAS J., FARINOTTI R. Uptake of mefloquine enantiomers into uninfected and malaria-infected erythrocytes. Drug Metab. Dispos. 1996;24:689–691. [PubMed] [Google Scholar]
- WEINKE T., TRAUTMANN M., HELD T., WEBER G., EICHENLAUB D., FLEISCHER K., KERN W., POHLE H.D. Neuropsychiatric side effects after the use of mefloquine. Am. J. Trop Med. Hyg. 1991;45:86–91. doi: 10.4269/ajtmh.1991.45.86. [DOI] [PubMed] [Google Scholar]