Abstract
We recently described that several 2-(2,5-dimethoxy-4-substituted phenyl)ethylamines (PEAs), including 4-I=2C-I, 4-Br=2C-B, and 4-CH3=2C-D analogs, are partial agonists at 5-HT2C receptors, and show low or even negligible intrinsic efficacy at 5-HT2A receptors. These results raised the proposal that these drugs may act as 5-HT2 antagonists.
To test this hypothesis, Xenopus laevis oocytes were microinjected with the rat clones for 5-HT2A or 5-HT2C receptors. The above-mentioned PEAs and its 4-H analog (2C-H) blocked the 5-HT-induced currents at 5-HT2A, but not at the 5-HT2C receptor, revealing 5-HT2 receptor subtype selectivity. The 5-HT2A receptor antagonism required a 2-min preincubation to attain maximum inhibition.
All PEAs tested shifted the 5-HT concentration–response curves to the right and downward. Their potencies varied with the nature of the C(4) substituent; the relative rank order of their 5-HT2A receptor antagonist potency was 2C-I>2C-B>2C-D>2C-H.
The present results demonstrate that in X. laevis oocytes, a series of 2,5-dimethoxy-4-substituted PEAs blocked the 5-HT2A but not the 5-HT2C receptor-mediated responses. As an alternative hypothesis, we suggest that the psychostimulant activity of the PEAs may not be exclusively associated with partial or full 5-HT2A receptor agonism.
Keywords: Psychotropic phenylethylamines, subtype-selective 5-HT2 receptor antagonists, 5-HT2 receptor antagonists
Introduction
There is abundant evidence in support of the notion that psychostimulants of the indolylalkylamine and phenylethylamine families require the activation of central 5-HT2 receptors (Nichols, 1997). Although radioligand displacement experiments show a reasonable correlation between 5-HT2 receptor affinities in rat brain membranes or cultured cells expressing cloned human 5-HT2 receptors and the human ‘hallucinogenic' potency of many phenylisopropylamines (PIAs, (±)-1-(2,5-dimethoxy-4-substituted phenyl)-2-propylamines), these compounds are not generally subtype selective (Glennon et al., 1992; Nelson et al., 1999). There is a body of evidence, derived from electrophysiological and behavioral assays, that implicates 5-HT2A receptors as a main CNS target for these hallucinogenic and psychotropic drugs (Mckenna & Peroutka, 1989; Pierce & Peroutka, 1989; Krebs-Thomson et al., 1998; Aghajanian & Marek, 1999). 2C-B (2-(4-bromo-2,5-dimethoxyphenyl)-ethylamine) is a popular and recreational psychostimulant phenylethylamine (PEA), known among other street names as ‘nexus' or ‘cyber'. Several related drugs, as [(±)-1- (4-bromo-2,5-dimethoxyphenyl)-2-propylamine] (DOB), with varying substituents at C(4), have similar psychotropic activity probably due to the activation of a common mechanism (Shulgin & Shulgin, 1991; Giroud et al., 1998; de Boer et al., 1999). DOB, a known 5-HT2A/2C agonist, was used as evidence to infer that the corresponding PEA analog 2C-B might also exert its psychotropic actions through the activation of 5-HT2A/2C receptors, either as a full or partial agonist, a feature common to other psychoactive drugs.
Recently, Xenopus laevis oocytes microinjected with clones of the rat 5-HT2A or the 5-HT2C receptors were used to characterize the pharmacology of PIAs and their corresponding PEA pairs (Table 1 and Acuña-Castillo et al., 2002). Although this new experimental model has limitations, in part due to the transient expression of the transfected proteins and likely also to differential engagement of G proteins, the Acuña-Castillo et al. (2002) study demonstrated that the PIAs are generally full 5-HT2C receptor agonists and partial agonists at the 5-HT2A receptor, a finding in accordance with the current literature on the mode of action of these drugs. Furthermore, they reported in the same study that the similarly substituted PEAs are less efficacious agonists, some of them with low or negligible efficacy at the 5-HT2A receptor subtype. Based on the report of Acuña-Castillo et al. (2002), we now suggest that PEAs with null or negligible intrinsic activity might behave as 5-HT2A receptor antagonist in X. laevis oocytes. To test our working hypothesis, 5-HT2A and 5-HT2C receptors were expressed in Xenopus oocytes and evaluated the interaction of a series of PEAs with these receptors. These findings show new evidence in support of the proposal that the hallucinogenic effect of PEAs is not directly related to 5-HT2A agonism, but it might be possibly associated with mechanism(s) or receptor(s) other than the classical partial agonism at the 5-HT2A receptor. This novel concept may enlighten and help to understand the mode of action of PEAs and related psychoactive compounds.
Table 1.
Efficacy of PEAs and PIAs at 5-HT2A and 5-HT2C receptors expressed on X. laevis oocytes
| 5-HT2A | 5-HT2C | |
| Imax (%) | Imax (%) | |
| 2C-I | 17±4 | 44±10 |
| 2C-B | 4±2 | 50±11 |
| 2C-D | 6±3 | 48±7 |
| 2C-H | 0 | 76±16 |
| DOI | 46±9 | 90±9 |
| DOB | 57±11 | 58±3 |
The values represent the average ±s.e.m. Intrinsic activity percentage for each drug normalized upon 5-HT maximum response (Acuña-Castillo et al., 2002).
Methods
Oocyte harvesting, microinjection of rat 5-HT2A and 5-HT2C receptor clones, and characterization of 5-HT-induced currents
X. laevis ovary lobes were surgically removed; stage V and VI oocytes were manually defolliculated and further treated with collagenase II as previously described (Acuña-Castillo et al., 2002). Oocytes were then microinjected intracytoplasmatically with 10–20 ng of a cRNA for the rat 5-HT2A or 5-HT2C receptor clones. The oocytes were incubated for 36–48 h at 15°C in standard Barth's solution supplemented with 10 UI/l penicillin–streptomycin, 0.5 mM theophylline and 2 mM pyruvate.
To assess the expression of the 5-HT receptors, oocytes were impaled with two electrodes in a voltage–clamp configuration using an OC-725C oocyte clamp (Warner Instrument Corp.), as detailed previously by Acuña-Castillo et al. (2002); the membrane potential was fixed at −70 mV. To test the expression of the receptors following transfection, the oocytes were perfused for 10-s with a test concentration of either 100 nM 5-HT in the case of the 5-HT2A or 10 nM 5-HT for the 5-HT2C receptor, values that were previously determined to be close to the EC50 for each receptor. These test challenges were applied regularly every 15–20 min, to avoid desensitization of the 5-HT-evoked currents. When an oocyte yielded a significant current, the same concentration of 5-HT was applied several times until a stable response was attained. 5-HT and related analogs were dissolved in perfusion buffer and superfused at a constant flow rate of 2 ml/min. Uninjected oocytes did not respond to 5-HT or to any PEAs tested. A minimum of four oocytes were assessed per protocol; care was taken to examine oocytes harvested from two separate frogs in the performance of concentration–response studies.
Subtype-selective antagonism protocols
After stable 5-H-ev 5-HT-evoked currents were recorded in an oocyte, varying concentrations of each of the four PEAs examined (10−11–10−4 M) were coapplied together with the test concentration of 5-HT (100 or 10 nM for 5-HT2A or 5-HT2C receptors, respectively) for 10 s, to determine whether the different PEAs antagonized the 5-HT induced currents. In all protocols, recovery of the 5-HT-evoked current was mandatory prior to evaluating the effect of an increased concentration of each PEA examined. Recovery was assessed by continual 10-s pulse application of the test concentration of 5-HT until a response in excess of 85% of the initial 5-HT-evoked current was attained. Parallel protocols were performed in oocytes injected with 5-HT2A and 5-HT2C receptors.
Characterization of the nature of the PEA-induced antagonism of the 5-HT2A receptor
Several protocols were designed to detail the mechanism of the 5-HT2A receptor blockade induced by the four PEAs examined. Sets of parallel experiments were performed in oocytes expressing the 5-HT2A receptor subtype.
Onset of the PEA-induced blockade
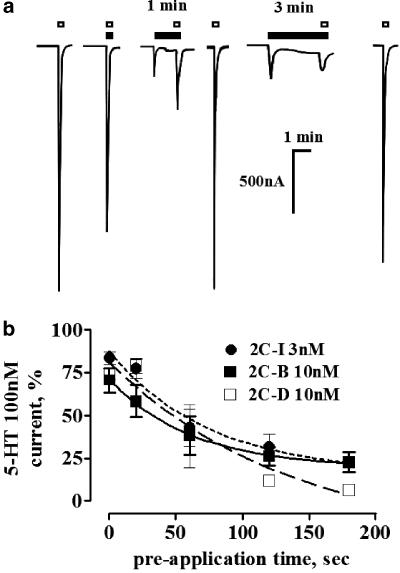
To assess the experimental condition of the PEA application required to demonstrate the blockade of the 5-HT2A receptor, two sets of protocols were conducted. In the first series of experiments, 1–1000 nM PEA was coapplied together with the test concentration of 5-HT. In another set of protocols, the PEAs were preapplied for 20, 60, 120 or 180-s of either 3 nM (2C-I) or 10 nM (2C-B or 2C-D) prior to the coapplication of test 5-HT concentration. The current generated by the test concentration of 5-HT was set as 100% response; the responses recorded in the presence of the PEAs were normalized against this standard. Separate sets of oocytes, harvested from two frogs, were used for each of these determinations. A minimum of four separate oocytes were used for each of these protocols.
Displacement of the 5-HT concentration–response curves by the PEAs; rank order of antagonist potency
In an additional set of experiments, we assessed how PEA applications shifted the 5-HT concentration–response curves. Complete 5-HT concentration–response curves were obtained for single oocytes prior to and following either the coapplication of PEAs with varying concentrations of 5-HT or 2 min after preincubation with each PEA plus a 10-s coapplication of 5-HT plus the PEA. These entire tests were performed in a single oocyte. Results are graphed as 5-HT concentration–response curves. The rank order of PEA antagonism potency was established by estimating the concentration of each PEA required to block 50% of the current generated by the test 5-HT concentration (IC50); this value was interpolated from each PEA antagonism curve. Separate protocols examined the blockade caused by each of the four PEAs studied.
Data quantification and statistical analysis of results
Results are presented as average ±s.e.m. corresponding to experiments performed with four to eight oocytes from at least two separate batches of cells. Based on standard deviation values, parallel experiments revealed that intergroup variability was larger than intra-assay variability for the 5-HT2 receptors. The 5-HT-generated currents were plotted against the PEA concentrations using Graph-Pad Software (Graph-Pad Inc., San Diego, CA, U.S.A.) to obtain the median effective agonist concentration (EC50) or, in the case of the antagonists, their median inhibitory concentration (IC50 and its −log, pIC50 s). Nonparametric analysis, Kruskal–Wallis, Mann–Whitney and Friedman & Quade tests were also used for statistical analysis. In all cases, significance was set at a P-value <0.05.
Drugs and chemicals used
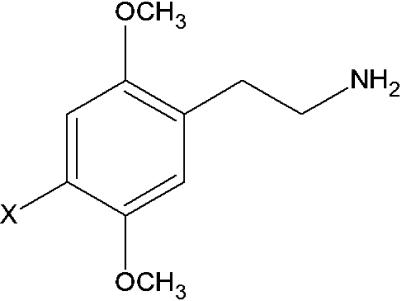
The PEAs 2C-I, 2C-B, 2C-D and 2C-H (structures are schematized in Figure 1) were synthesized as reported by Shulgin & Shulgin (1991) and prepared as hydrochloride salts (with the exception of 2C-B, which was prepared as the hydrobromide salt). These compounds were dissolved in Barth's solution. 5-HT was purchased from RBI (Natick, MA, U.S.A.). The chemicals used to prepare Barth's buffer solution were of analytical grade and were purchased from Merck (Darmstadt, Germany) or Sigma Chemicals (St Louis, MO, U.S.A.).
Figure 1.
Structural formulae of the PEAs 4-(X)-substitutions tested: 2C-I, X=I; 2C-B, X=Br; 2C-D, X=CH3; 2C-H, X=H.
Results
5-HT2A receptor antagonism by 2C-I
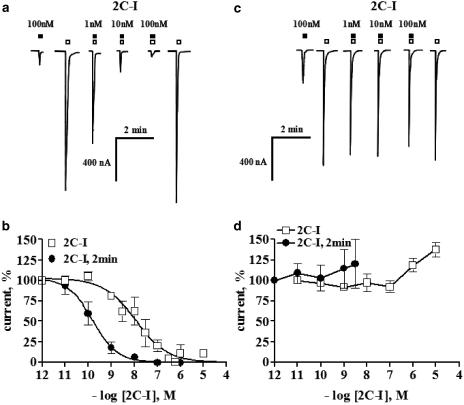
This PEA behaves as a partial agonist with an intrinsic efficacy, relative to 5-HT, of about 15% at 5-HT2A receptors; 100 nM 2C-I elicited a small current (see tracing in Figure 2a), a result consonant with Acuña-Castillo et al. (2002). However, the coapplication of 1 nM 2C-I plus 100 nM 5-HT reduced the current elicited by 5-HT at the 5-HT2A receptor. Increasing concentrations of 2C-I abolished the magnitude of the 5-HT-evoked currents (Figure 2a,b). The antagonism was reversible; the rate of recovery depended on the drug concentration, and higher concentrations resulted in the slowest recoveries; complete recovery was attained at highest 2C-I concentrations after a 45-min washout. The antagonism was time-dependent since a 2-min 2C-I preincubation caused more than a 100-fold increase in the antagonist potency compared with 2C-I coapplications (Figure 2,b).
Figure 2.
Selective 5-HT2A receptor antagonism by 2-CI (2,5-dimethoxy-4-iodo-phenyl)ethylamine. (a) Representative tracing illustrates the blockade of the 100 nM 5-HT-evoked current by coapplication of 1, 10 or 100 nM 2C-I to a single oocyte expressing 5-HT2A receptors. Solid squares represent 2C-I applications, open squares depict 100 nM 5-HT additions. Note that 100 nM 2C-I alone caused a small reproducible current, consistent with its partial agonism. (b) The coapplication of different concentrations of 2C-I plus 100 nM 5-HT blocked the 5-HT-induced currents in a concentration-dependent manner at the 5-HT2A receptor (open squares). A 2-min preapplication of 2C-I increased the magnitude of the blockade about 100-fold (solid circles). (c) Representative polygraph recording shows, in the same oocyte, that 2C-I did not significantly reduce the 10 nM 5-HT-evoked currents at the 5-HT2C receptor. Solid squares represent the 2C-I applications; open squares depict 10 nM 5-HT additions. Consonant with its partial agonist profile, 100 nM 2C-I per se caused a current amounting to 35% of the 10 nM 5-HT response. (d) 2C-I did not block the 10 nM 5-HT-induced currents in an oocyte expressing 5-HT2C receptors when 2C-I was either coapplied or preapplied for 2 min. Coapplications of 2C-I larger than 3 nM were not tested in view of its partial agonism. Symbols indicate the mean average normalized current, and, bars the s.e.m. At least four separate oocytes from two batches of oocytes were analyzed per protocol in all panels.
In contrast to the activity of this PEA at the 5-HT2A receptor, 2C-I averaged 35% of intrinsic activity at the 5-HT2C receptor and did not alter significantly the currents evoked by 5-HT (see tracings in Figure 2c) on this receptor, revealing 5-HT2 receptor subtype selectivity.
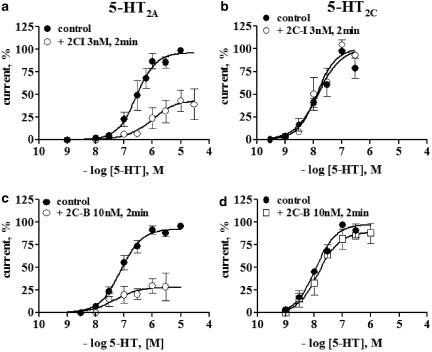
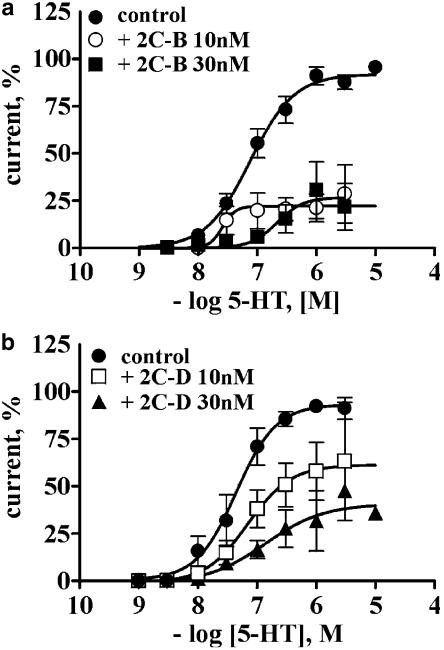
Consistent with the apparent selectivity of PEAs for the 5-HT2A receptor, within the 5-HT2 receptor family, 3 nM 2C-I did not modify the 5-HT concentration–response curve at the 5-5-HT2C receptor, even after 2 min of 2-CI application (Figure 3a,b). Qualitative similar results were also obtained when 30–100 nM 2C-I was co-applied with 5-HT (data not shown). The finding that the preincubation of 3 nM 2C-I for 60–180 s significantly augmented the magnitude of the 5-HT2A receptor blockade, as compared to the coapplication protocol, shows that the maximum inhibition is reached in about 3 min (Figure 4b).
Figure 3.
2C-I and 2C-B block selectively the 5-HT2A but not the 5-HT2C receptor. 5-HT concentration–response curves were performed in separate batches of oocytes injected either with the 5-HT2A or the 5-HT2C receptor subtypes. A 2-min preapplication of 3 nM 2C-I caused a downward displacement of the 5-HT concentration-response curve of the 5-HT2A receptor (a), without altering the activity of the 5-HT2C receptor (b). Likewise, 10 nM 2C-B only blocked, the 5-HT-induced currents elicited by the 5-HT2A (c) but not the 5-HT2C receptor (d). Symbols indicate the mean average normalized current, and bars the s.e.m. At least four separate oocytes from two batches of oocytes were analyzed per protocol.
Figure 4.
Time-dependent PEA antagonism at the 5-HT2A receptor. (a) Typical tracings of the currents evoked by 100 nM 5-HT (open squares) in an oocyte expressing the 5-HT2A receptor. Coapplication or the preapplication of 10 nM 2C-B (solid squares) reduced the magnitude of the 5-HT-evoked currents in a time-dependent manner. The sole application of 10 nM 2C-B caused a transient partial agonist response amounting to 10% of the standard 5-HT challenge. (b) Quantification of the time dependence of the 100 nM 5-HT challenge blockade elicited by 2C-I, 2C-B and 2C-D. Symbols indicate the mean average of the normalized currents, and bars the s.e.m. At least four separate oocytes were assessed per each PEA curve. In each curve, the data derived for the 60, 120 or 180-s preapplication were significantly different from that attained by the coapplication of each PEA plus 100 nM 5-HT (P<0.05, the asterisks were avoided for the sake of clarity).
5-HT2A receptor antagonism by 2C-B
The replacement of the iodine atom by a bromine (2C-B) also resulted in a 5-HT2A receptor antagonist, with reversible and time-dependent properties much like those described for 2C-I. The potency of 2C-B as a 5-HT2A antagonist is about 30-fold lower than that of 2C-I (Table 2 ). Similar to 2C-I, a 2-min preapplication of 10 nM 2C-B elicited a downward displacement of the 5-HT concentration–response curve at the 5-HT2A receptor (maximal current reached 22%) but not at the 5-HT2C receptor (Figure 3c,d and 5a). The time dependence of the antagonism is similar to that described for 2C-I (Figure 4b). The reversibility of the antagonism is illustrated in a representative tracing shown in Figure 4a.
Table 2.
Potency of PEAs as antagonist at the 5-HT2A receptor expressed in X. laevis oocytes
| 5-HT2A | ||
| pIC50 | Inhibition (%) | |
| 2C-I | 9.82±0.17 | 100 |
| 2C-B | 8.28±0.12 | 100 |
| 2C-D | 7.42±0.16 | 100 |
| 2C-H | 4.75±2.30 | 100 |
The values represent the average ±s.e.m. pIC50: −log IC50. Percentage of inhibition of a response elicited by a standard 5-HT concentration (10 nM for 5-HT2A receptor).
Figure 5.
2C-B and 2C-D antagonize the 5-HT2A receptor subtype. (a) A 2-min preapplication of 10 or 30 nM 2C-B elicited a downward displacement of the 5-HT concentration–response curve. (b) Concentration-dependent blockade of the 5-HT currents elicited by 2-min incubation with either 10 or 30 nM 2C-D. Symbols indicate the average normalized current, and bars the s.e.m. four to six oocytes from two batches of oocytes were analyzed per curve.
2C-D and 2C-H are also selective 5-HT2A receptor antagonists
Replacement of the halogen atom at C(4) by a methyl group or a H atom also resulted in compounds that reversibly antagonized the 5-HT-evoked currents at the 5-HT2A receptor in a concentration- and time-dependent manner. A 2-min application of 10 or 30 nM 2C-D shifted to the right and downwards the 5-HT concentration–response curves at the 5-HT2A receptor, reducing the maximal 5-HT response to 61.3±11.3 and 40.6±13.4%, respectively (Figure 5b). These PEAs did not modify the current evoked by 5-HT at the 5-HT2C receptor (data not shown). As in the case of the other PEAs, the blockade was reversible and required a 2-min preincubation to attain full blockade. The time course of the blockade was essentially indistinguishable from that of the halogen-substituted PEAs (Figure 4b).
Relative 5-HT2A receptor antagonist potency
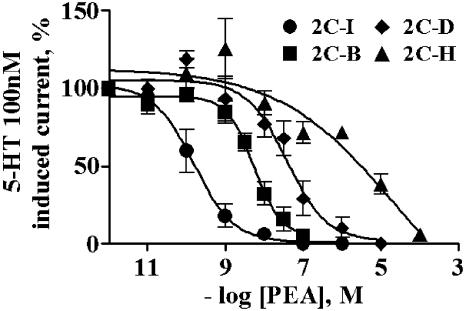
Increasing the concentration of the PEAs reduced proportionally the magnitude of a challenge 5-HT-evoked current at the 5-HT2A receptor. 2C-I was the most potent while 2C-H was the least active; the PEA concentration–response curves were parallel (Figure 6).
Figure 6.
Potency of several PEAs as 5-HT2A receptor antagonists. The four 4-substituted PEAs antagonized with varying potencies the 100 nM 5-HT-evoked currents in oocytes microinjected with the 5-HT2A receptor. In these protocols, the PEAs were preapplied for 2 min prior to challenge with 100 nM 5-HT. The IC50 of each PEA was interpolated from these curves, and is listed in Table 2 as the pIC50 (−log of the IC50). Symbols represent the mean values, and bars the SEM. four to six oocytes from two batches of oocytes were analyzed per curve.
Discussion
The present results demonstrate that in X. laevis oocytes, 2C-B, and a series of C(4)-substituted analogs, are 5-HT2A receptor antagonists; they appear to be selective within the 5-HT2 receptor family since no inhibition of the 5-HT-evoked currents was detected at the 5-HT2C receptor. We are fully aware that the present data do not allow us to deduce the nature of the psychostimulant mechanisms of these PEAs; however, the present results demonstrate novel pharmacological properties that might be of interest in the understanding of their psychostimulant properties. Parallel behavioral studies are required to complement the present investigation. Considering that 2C-B, 2C-I and 2C-D are recognized to be hallucinogenic in humans (Shulgin & Shulgin, 1991), the present results cast doubts on the generally accepted notion that the phenylalkylamine hallucinogens act only as full or partial 5-HT2A agonists.
There is much evidence that links hallucinogenic drug activity with their affinity at 5-HT2A receptors, or with their efficacy to alter membrane inositide turnover (Glennon et al., 1992; Nichols et al., 1994; Chambers et al., 2001). This notion appears well accepted and taken as a ‘dogma' to understand the effects of psychotropic drugs, and predicts that most hallucinogenic compounds act mainly as full or partial 5-HT2A agonists. Notwithstanding, there is a growing body of evidence that does not support this assumption. Pierce & Peroutka (1990) described that D-lysergic acid diethylamide (LSD), a prototypical indoleamine hallucinogenic, acts as a potent 5-HT2 antagonist. Recently, Rabin et al. (2002), showed that in PC12 cells expressing the 5-HT2A receptor both hallucinogenic and nonhallucinogenic drugs, belonging to the indoleamine or phenylethylamine families, stimulate phosphoinositide hydrolysis, one of the intracellular signaling messengers characteristic of the 5-HT2 family of receptors. Comparison of these results with previous drug discrimination studies suggests an apparent lack of correlation between in vivo hallucinogenic drug action and efficacy in stimulating phosphoinositide hydrolysis. The involvement of phospholipase A2 has not been definitively excluded (Rabin et al., 2002). Altogether, the latter findings may be interpreted as evidence against the notion that 5-HT2A activation is the sole critical receptor and signaling mechanism involved in the stimulus effects of hallucinogens.
Only within the past decade, fairly bulky ligands such as compounds RS 102221, SB 242084, MDL 100907 and 4F 4PP can discriminate among 5-HT2 receptor subtypes (Herndon et al., 1992; Sorensen et al., 1993; Kennett et al., 1997; Bonhaus et al, 1997; Acuña-Castillo et al., 2002), and have become useful tools to explore the physiology of the 5-HT2 receptors in vivo and in vitro. Inasmuch as we are aware, this is the first report of a series of compounds that antagonize the 5-HT2A receptor and which appear to be selective within the 5-HT2 receptor subtypes.
Although we recognize that the limited number of PEAs examined precludes a confirmatory conclusion, a parsimonious interpretation, based on the relative rank order of potencies of PEAs as antagonist at the 5-HT2A receptor, strongly suggests that this receptor may have a hydrophobic pocket to accommodate a C(4) substituent, leading to distinct receptor antagonism affinity. We propose that iodine fits optimally into this hypothetical pocket, resulting in the most potent antagonist. Based on the present findings, we suggest that the 5-HT2C receptor, which bears evident structural similarities with the 5-HT2A receptor, may not have a similar pocket or the site may be more hydrophilic, since 2C-I, 2C-B and 2C-D, which have hydrophobic C(4) substituents, do not possess an antagonist profile in its interaction at this receptor. In contrast, the putative antagonist site on the 5-HT2C receptor may accommodate a hydrophilic and possibly hydrated nitro group that leads to antagonism as described by Acuña-Castillo et al. (2002). The lack of PEA antagonism at the 5-HT2C receptor might also be related to their greater intrinsic activity in this receptor, which in the case of 2C-I averaged 35%, a condition favoring partial agonism rather than 5-HT2C receptor antagonism.
In addition to the hypothesis that the PEAs fit into a pocket proper of the 5-HT2A receptor, which might help account for their exclusive 5-HT2A receptor antagonism, we have not overlooked alternative explanations to explain the PEAs' behavior. One possibility refers to functional receptor coupling selectivity, also known as differential engagement of G proteins, which may influence the interpretation of the present results (see Kurrash-Orbaugh et al., 2003), pointing out that G proteins may couple within the same cell to various effectors. A substantial spare receptor reserve could also hinder the real-time kinetics of the receptor transduction mechanism. Additionally, the present results and the experimental protocol used cannot discard that PEAs interact with other relevant brain receptors, which might be involved in hallucinogenesis. In support of this notion, multiple evidences support the view that PEAs may modulate indirectly the NMDA (Blank et al., 1996; Arvanov et al., 1999) or metabotropic glutamate receptors (Gewirtz and Marek, 2000). It is also well known that PEAs and related compounds facilitate the release of dopamine (Benloucif et al., 1993), a preponderant transmitter involved in the brain circuitry related to psychostimulant drug responses and their addictive mechanisms. In addition, although we did not address experimentally the putative interaction of PEAs with dopaminergic receptors, it is entirely possible that these receptors are also targets of their action. At present, we cannot fully discard that 5-HT2C receptor itself may play a role in the psychostimulant action of the PEAs, since most of these compounds are partial agonists at this receptor. The 5-HT2B receptor has not been established relevant to psychostimulation; furthermore, its expression is restricted to brain sites not linked to the action of these compounds (Kursar et al., 1994; Pompeiano et al., 1994; Bonhaus et al., 1995). Although some phenylisopropylamine derivatives, structurally related to the PEAs, have 30-40 times lower affinity at 5-HT2B than at 5-HT2A receptors (Nelson et al., 1999), we cannot exclude their participation in the hallucinogenic response of the PEAs. However, based on the classical psychopharmacological association of the PIAs with 5-HT2A/2C receptors (Fiorella et al., 1995) and their lower affinity at the 5-HT2B receptor, we favor the action of the PEAs at the other 5-HT2 receptor subtypes.
We consistently observed that the PEAs require at least 2-min equilibrium to attain the maximal inhibition. Several possible explanations may be adduced to explain this finding, which is likely not related to the rate of drug diffusion considering the relative low molecular weight of these drugs, and the fact that 5-HT evoked within 10 s its electrophysiological response. We may assume that the kinetics of formation of the PEA-receptor complex is slow because either the rate of receptor association or the rate of their access to the binding site is slower than for 5-HT in their access from extracellular space. With regard to their rather slow on-rate of action, we are aware that bulky C(4) substituents may slow the rates of on and off binding, accounting for the slow onset of the blockade described for these PEAs. However, the present data report that even a hydrogen at C(4) has a slow onset of antagonism and acts as an antagonist, albeit of low potency. Therefore, we must consider the molecule as a whole rather than looking exclusively at the role of the C(4) substituent on the action of this series of compounds. In this regard, Nelson (1991) discussed the influence of bulky lipophilic substituents as determinants of on and off rates of agonist–antagonist binding. The present technique does not allow us to derive firm conclusions except to describe this common property.
Based on the present findings, we foresee that one of the PIA enantiomers, the one with less intrinsic activity, may mimic the action of its corresponding PEA, and therefore behave as a 5-HT2A receptor antagonist. Using X. laevis oocytes transfected with 5-HT2 receptors, we observed that (R)-DOI is a full 5-HT2C receptor agonist that behaves as a partial 5-HT2A receptor agonist. In contrast, (S)-DOI has negligible efficacy at the 5-HT2A receptor. Consistent with the present findings, (S)-DOI reduces 5-HT or even (R)-DOI-evoked currents, evidencing an antagonistic profile at the 5-HT2C receptor (Villalobos et al., unpublished observations).
The present findings highlight the subtle pharmacological differences in the interaction of ligands acting differentially at the 5-HT2A and 5-HT2C receptors, and the intricacies of drug action as evidenced by their remarkable selectivity of action. The profile of 2C-B and related PEAs as 5-HT2A antagonists might be considered a turning point in the future development of competitive more potent and selective 5-HT2A receptor antagonists. It is our hope that these compounds will allow a more comprehensive understanding of the physiology of the 5-HT2 receptors and their involvement in psychotropic and hallucinogenic responses.
In summary, PEAs such as 2C-B and congeners behaved as potent and reversible 5-HT2A receptor antagonists in X. laevis oocytes; they appear to be selective within the 5-HT2 receptor family. Based on the present work, we raise the working hypothesis that the psychostimulant effect of 2C-B and related PEAs might not necessarily the linked to 5-HT2A receptor activation, but like LSD, might act through other mechanism(s) or other receptors than the 5-HT2A subtype. The present results maintain open the controversy about 5-HT2 receptors and the mechanisms of hallucinogenesis. Further pharmacological, behavioral and molecular evidences must be sought for a full understanding of the mechanism(s) of their psychostimulant properties.
Acknowledgments
We appreciate and thank the editorial assistance of Professsors G. Owen and R. Andrade for critically reading the manuscript. This research was partially funded by the Center for Cell Regulation and Pathology (FONDAP, Grant 1398001). The Millennium Institutes for Fundamental and Applied Biology (MIFAB) and for Advanced Studies in Cell Biology and Biotechnology (CBB) also contributed to the funding of this research. Grant DICYT 020091SB provided additional funds.
Abbreviations
- 2C-B
4-bromo-2,5-dimethoxyphenethylamine
- 2C-D
4-methyl-2,5-dimethoxyphenethylamine
- 2C-H
2,5-dimethoxyphenethylamine
- 2C-I
4-iodo-2,5-dimethoxyphenethylamine
- 2C-N
4-nitro-2,5-dimethoxyphenethylamine
- 4F 4PP
4-(4-fluorobenzoyl)-1-(4–phenylbutyl) piperidine
- DOB
(±)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane
- DOI
(±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- PEA
phenylethylamine
- PIA
phenylisopropylamine
- RS 102221
8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro [4,5]decane-2,4-dione
References
- ACUÑA-CASTILLO C., VILLALOBOS C., MOYA P.R., SÁEZ P., CASSELS B.K., HUIDOBRO-TORO J.P. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGHAJANIAN G.K., MAREK G.J. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- ARVANOV V.L., LIANG X., RUSSO A., WANG R.Y. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefontal cortex. Eur. J. Neurosci. 1999;11:3064–3072. doi: 10.1046/j.1460-9568.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- BENLOUCIF S., KEEGAN M., GALLOWAY M. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J. Pharmacol. Exp. Ther. 1993;265:373–377. [PubMed] [Google Scholar]
- BLANK T., ZWART R., NIJHOLT I., SPIESS J. Serotonin 5-HT2 receptor activation potentiates N-methyl-D-aspartate receptor-mediated ion currents by a protein kinase C-dependent mechanism. J. Neurosci. Res. 1996;45:153–160. doi: 10.1002/(SICI)1097-4547(19960715)45:2<153::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- de BOER D., GIJZELS M.J., Bosman IJ., Maes R.A. More data about the new psychoactive drug 2C-B. J. Anal. Toxicol. 1999;23:227–228. doi: 10.1093/jat/23.3.227. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., WEINHART K.K., TAYLOR M., DESOUZA A., MCNEELEY P.M., SZCZEPANSKI K., FONTANA D.J., TRINH J., ROCHA C.L., DAWSON M.W., FLIPPIN L.A., EGLEN R.M. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., BACH C., DeSOUZA A., SALAZAR F.H., MATSUOKA B.D., ZUPPAN P., CHAN H.W., EGLEN R.M. The pharmacology and distribution of human 5-hydroxytryptamine 2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS J., KURRASH-ORBAUGH D., PARKER M., NICHOLS D. Enantiospecific synthesis and pharmacological evaluation of a series of super-potent conformationally restricted 5-HT2A/2C receptor agonists. J. Med. Chem. 2001;44:1003–1010. doi: 10.1021/jm000491y. [DOI] [PubMed] [Google Scholar]
- FIORELLA D., RABIN R.A., WINTER J.C. The role of 5-HT2A and the 5-HT2C receptors in the stimulus effects of hallucinogenic drugs: I. Antagonist correlation analysis. Psychopharmacology. 1995;12:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- GEWIRTZ J.C., MAREK G.J. Behavioral evidence for interaction between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- GIROUD C., AUGSBURGER M., RIVIER L., MANGIN P., SADEGHIPOUR F., VARESIO E., VEUTHEY J.L., KAMALAPRIJA P. 2C-B: a new psychoactive phenylethylamine recently discovered in Ecstasy tablets sold on the Swiss black market. J. Anal. Toxicol. 1998;22:345–354. doi: 10.1093/jat/22.5.345. [DOI] [PubMed] [Google Scholar]
- GLENNON R.A., RAGHUPATHI R., BARTYZEL P., TEITLER M., LEONHARDT S. Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J. Med. Chem. 1992;35:734–740. doi: 10.1021/jm00082a014. [DOI] [PubMed] [Google Scholar]
- HERNDON J., ISMAIEL A., INGHER S., TEITLER M., GLENNON A. Ketanserin analogues: structure-affinity relationships for 5-HT2 and 5-HT1C serotonin receptor binding. J. Med. Chem. 1992;35:4903–4910. doi: 10.1021/jm00104a017. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., TRAIL B., RILEY G., HOLLAND V., AVENELL K.Y., STEAN T., UPTON N., BROMIDGE S., FORBES I.T., BROWN A.M., MIDDLEMISS D.N., BLACKBURN T.P. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- KREBS-THOMSON K., PAULUS M.P., GEYER M.A. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- KURRASH-ORBAUGH D., WATTS V., BARKER E., NICHOLS D. Serotonin 5-hydroxytryptamine2a receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- KURSAR J.D., NELSON D.L., WAINSCOTT D.B., BAEZ M. Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol. Pharmacol. 1994;46:227–234. [PubMed] [Google Scholar]
- McKENNA D.J., PEROUTKA S.J. Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(-)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J. Neurosci. 1989;9:3482–3490. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON D.L. Structure–activity relationships at 5-HT1A receptors: binding profiles and intrinsic activity. Pharmacol. Biochem. Behav. 1991;40:1041–1051. doi: 10.1016/0091-3057(91)90124-k. [DOI] [PubMed] [Google Scholar]
- NELSON D.L., LUCAITES V.L., WAINSCOTT D.B., GLENNON R.A. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn–Schmiedeberg's Arch. Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- NICHOLS D.E.Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens Handbook of Experimental Pharmacology 1997129Berlin, Heidelberg: Springer; 563–585.ed. BAUMGARTEN , H.G. & G. M. Chapter 21 pp [Google Scholar]
- NICHOLS D.E., FRESCAS S., MARONA-LEWITKA D., HUANG X., ROTH B., GUDELSKY G., NASH F. 1-(2,5-Dimethoxy-4-(trifluoromethy1)phenyl)-2-aminopropane: a potent serotonin 5-HT2A/2C agonist. J. Med. Chem. 1994;37:4346–4351. doi: 10.1021/jm00051a011. [DOI] [PubMed] [Google Scholar]
- PIERCE P.A., PEROUTKA S.J. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology (Berl) 1989;97:118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- PIERCE P.A., PEROUTKA S.J. Antagonist properties of D-LSD at 5-hydroxytryptamine2 receptors. Neuropsychopharmacology. 1990;3:503–508. [PubMed] [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- RABIN R., MEREDITH R., MIREILLE D., WINTER J.C. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol. Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- SHULGIN A.T., SHULGIN A. Berkeley, CA: Transform Press; 1991. PIHKAL—A Chemical Love Story. [Google Scholar]
- SORENSEN S.M., KEHNE J.H., FADAYEL G.M., HUMPHREYS T.M., KETTELER H.J., SULLIVAN C.K., TAYLOR V.L., SCHMIDT C.J. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J. Pharmacol. Exp. Ther. 1993;266:684–691. [PubMed] [Google Scholar]