Abstract
This study characterises some of the mechanisms and mediators involved in the orofacial nociception triggered by injection of formalin into the upper lip of the rat, by assessing the influence of various treatments on behavioural nociceptive responses (duration of facial rubbing) elicited either by a low subthreshold (i.e. non-nociceptive; 0.63%) or a higher concentration of the algogen (2.5%).
The kininase II inhibitor captopril (5 mg kg−1, s.c.) and prostaglandin(PG) E2 (100 ng lip−1) potentiated both phases of the response to 0.63% formalin, whereas tumour necrosis factor (TNFα; 5 pg lip−1), interleukin(IL)-1β (0.5 pg lip−1), IL-6 (2 ng lip−1) and IL-8 (200 pg lip−1), or the indirectly acting sympathomimetic drug tyramine (200 μg lip−1), each augmented only the second phase of nociception.
Conversely, both phases of nociception induced by 2.5% formalin were inhibited by the bradykinin (BK) B2 receptor antagonist HOE140 (5 μg lip−1) or the selective β1-adrenoceptor antagonist atenolol (100 μg lip−1). However, the BK B1 receptor antagonist des-Arg9-Leu8-BK (1 and 2 μg lip−1), antibody and/or antiserum against each of the cytokines, the adrenergic neurone blocker guanethidine (30 mg kg−1 day−1, s.c., for 3 days) and the cyclooxygenase(COX)-2 inhibitor celecoxib (50 and 200 μg lip−1, s.c.; or 1 and 3 mg kg−1, i.p.) reduced only the second phase of the response. The nonselective COX inhibitor indomethacin and the 5-lipoxygenase activating protein inhibitor MK886 did not change formalin-induced nociception.
Our results indicate that BK, TNF-α, IL-1β, IL-6, IL-8, sympathetic amines and PGs (but not leukotrienes) contribute significantly to formalin-induced orofacial nociception in the rat and the response seems to be more susceptible to inhibition by B2 receptor antagonist and selective COX-2 inhibitor than by B1 receptor antagonist or nonselective COX inhibitor.
Keywords: Formalin, bradykinin, cytokines, prostaglandins, noradrenaline
Introduction
Pain is an essential sensation that usually signals tissue injury inflicted by external or internal damaging events. Sensory information generated by nociceptors located in peripheral tissues is relayed to neurones in the spinal cord which project to the thalamus and cortex to elicit pain (for a review, see Woolf & Salter, 2000). Frequently, tissue damage leads to development of an inflammatory response in which various nociception-producing mediators, such as bradykinin (BK) and substance P come into play, alongside others which promote hyperalgesia (i.e. enhancement of nociceptor sensitivity), including prostaglandins (PG), cytokines and sympathetic amines, to cause inflammatory pain (Coderre et al., 1984; Nakamura & Ferreira, 1987; Duarte et al., 1988; Cunha et al., 1992; Malmberg & Yaksh, 1995; Safieh-Garabedian et al., 2002; for a review, see Calixto et al., 2000).
In 1977, Dubuisson & Dennis introduced the use of formalin to induce nociception in the hind-paw, currently one of the most well established and widely employed experimental models for the study of nociceptive mechanisms. In this model, intraplantar injection of formalin into a hind-paw elicits a biphasic pattern of nociceptive-related behaviours: an early short-lasting neurogenic phase, which has been attributed to a direct activation of C-fibre nociceptors, followed by a second and more sustained inflammatory phase, generally associated with the local release of inflammatory mediators (Franklin & Abbott, 1989; Tjolsen et al., 1992). The possible contribution of BK (Correa & Calixto, 1993), cytokines (Watkins et al., 1994; Wordliczek et al., 2000), sympathetic amines (Fuchs et al., 1999) and arachidonic acid metabolites (Malmberg & Yaksh, 1995) to one or both phases of formalin-induced nociception is firmly established.
Clavelou et al. (1989) adapted the formalin test to assess nociception in the orofacial region of rats. Somatosensory innervation of the face and oral cavity in mammals is provided by ophthalmic, maxillary and mandibular divisions of the trigeminal nerve. The maxillary nerve, among many other structures, supplies the upper lip (for a review, see Lazarov, 2001). There is mounting evidence that, in models of chronic nociception, the anatomical and physiological consequences of nerve injuries of the trigeminal system differ from those seen after peripheral nerve injury (Tal & Devor, 1992; Bongenhielm et al., 1999; for a review, see Fried et al., 2001). Moreover, in humans, craniofacial pain also show some differences from that involving spinal nerves (Matthews, 1989). Nonetheless, possible differences between the roles played by inflammatory mediators following injury of peripheral and trigeminal nerve systems have not yet been objectively investigated. In this regard, the model provided by Clavelou et al. (1989) constitutes a useful tool to evaluate such differences, which have not yet been explored. Therefore, the aim of the present study was to evaluate the participation of well-established mediators such as BK, cytokines, sympathetic amines and arachidonic acid metabolites, in peripheral nociception elicited by formalin in the orofacial region of the rat.
Methods
Animals
Experiments were conducted on male Wistar rats weighing 180–200 g, housed at 22±1°C on a 12-h by 12-h light/dark cycle (lights on at 07 : 00 h), acclimatised to the laboratory for at least 48 h before use, with free access to food chow and tap water. All experiments and experimental procedures adopted for the in vivo studies were previously approved by the institution's ethics committee for research on laboratory animals and were in accordance with the standards of the European Community Council's directives (86/609/EEC).
Formalin test
The orofacial formalin test was conducted as described by Clavelou et al. (1989), with minor modifications. Briefly, animals were placed individually in an observation chamber (an inverted 30 cm wide 20 cm high glass funnel) for 10 min to minimise any stress-related behavioural changes. The animals were gently held and then received a subcutaneous 50 μl injection of formalin (over a range of concentrations, see below) or saline into the upper lip using a thin needle (12.7 mm × 0.33 mm) and were returned immediately to the observation chamber. The time each animal spent rubbing the injected area with its fore- or hind-paws was recorded cumulatively (using a stopwatch), in consecutive 3-min bins over a period of 30 min, and was considered as an index of nociception. The nociceptive response was clearly biphasic, with an initial component that normally peaked about 3 min after formalin injection (first phase) and subsided transiently over the next 3 min, followed by a second rise in rubbing incidence (second phase) over the remainder of the 30 min observation period (which peaked between 15 and 21 min depending on the concentration of formalin). In view of these characteristics, we considered responses over the first 3-min bin following formalin injection as the first phase of nociception, and those occurring between 12 and 30 min as the second phase. In preliminary experiments, different groups of rats were treated with different concentrations of formalin (0.63, 1.25, 2.5 or 5% v v−1 in saline) to establish the concentration–response relationships for both phases of the nociceptive effect of this algogen. Based on the results from these experiments, we selected the concentrations of 0.63 and 2.5% of formalin for all subsequent experiments, as they were, respectively, the highest subthreshold and lowest maximally effective concentrations to elicit nociceptive behaviour in either phase of the response. The rationale for choosing to use two distinct concentrations of formalin was to optimise visualisation of potentiation of nociception by potentially pronociceptive treatments, by employing the subthreshold 0.63% concentration, whereas responses elicited by the higher concentration (2.5%) would facilitate the detection of putative inhibitory effects of treatments blocking the actions or recruitment of various endogenous pronociceptive mediator candidates.
For the traditional paw test, formalin (only at the maximally effective concentration of 2.5%) or saline (50 μl) was injected subcutaneously into the right hind-paw and the amount of time (s) that animals spent licking the injected paw was recorded cumulatively in 5-min bins over 40 min. The first phase occurred during the first 10 min and the second phase from 20 to 40 min after injection (Dubuisson & Dennis, 1977).
Only a maximum of two animals were observed simultaneously at any one time in either test. All experiments were carried out between 11 : 00 and 19 : 00 h. Each animal was used only once and was immediately killed at the end of the observation period, by cervical dislocation.
Treatment protocols
To assess the possible contribution of endogenous kinins in formalin-induced orofacial nociception, animals were treated systemically, 2 h before local injection of 0.63% formalin or saline, with either captopril (a kininase II inhibitor; 5 mg kg−1, s.c.) or vehicle. In addition, to evaluate the participation of B1 and B2 BK receptors, other animals were treated, 30 min before local 2.5% formalin injection, with either DALBK (a selective kinin B1 receptor antagonist; at 0.5, 1.0 or 2.0 μg lip−1), HOE140 (a selective kinin B2 receptor antagonist; at 1.25, 2.5 or 5.0 μg lip−1) or the same volume of saline. These doses were chosen on the basis of those found to be effective in studies of formalin-induced nociception in the mouse hind-paw by Correa & Calixto (1993).
We next tested if various exogenous cytokines previously shown to cause mechanical hyperalgesia in the rat hind-paw (Cunha et al., 1992) would also potentiate orofacial nociception induced by formalin. To this effect, 2 h before the local injection of 0.63% formalin or saline, the animals received a local injection of TNFα (1.25, 2.5 or 5.0 pg lip−1), IL-1β (0.125, 0.25 or 0.5 pg lip−1), IL-6 (1.0, 1.5 or 2.0 ng lip−1) or IL-8 (100 or 200 pg lip−1) or vehicle. Additionally, other animals were treated locally (i.e. into the upper lip), 1 h before local 2.5% formalin injection, with either TNFα antibody (25 pg lip−1), antiserum against IL-1β, IL-6 or IL-8 (each diluted 1 : 5 in saline), or the same volume of the corresponding vehicle (saline or nonimmune serum diluted 1 : 5, respectively). The doses of cytokines employed were chosen based on their effectiveness in causing mechanical hyperalgesia in the rat hind-paw, whereas those of the corresponding antibody/antiserum have been reported to selectively inhibit the actions of their respective target proteins (Cunha et al., 1992; Cartmell et al., 2000; Lorenzetti et al., 2002).
Other experiments were undertaken to address the role of sympathetic mechanisms in orofacial nociception evoked by formalin. In the first of these, animals were treated daily with either saline or guanethidine (a postganglionic sympathetic neuron blocker; at 30 mg kg−1 day−1, s.c.) for 3 days. On the fourth day, these animals were given an injection of either saline or tyramine (a releaser of noradrenaline from postganglionic sympathetic neurones; at 200 μg lip−1), which was followed 2 h later by a similar injection of either formalin (at either 0.63 or 2.5%) or saline. Other animals were treated locally, 30 min prior to local 2.5% formalin injection, with a single injection of either atenolol (a selective β1 adrenoceptor antagonist; at 6.25, 25 or 100 μg lip−1) or saline. The drug dosages and treatment protocols employed in these sets of experiments were chosen on the basis of their efficacies in a previous study conducted using a model of mechanical hyperalgesia (Nakamura & Ferreira, 1987).
A final set of experiments was carried out to examine the contribution of arachidonic acid-derived products of both the cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) pathways to formalin-induced orofacial nociception. Some rats were given a local injection of either PGE2 (100 ng lip−1) or vehicle 3 h before injecting 0.63% formalin or saline into the upper lip. Other groups of animals received a local 2.5% formalin injection into the lip 30 min after treatment with one of the COX inhibitors indomethacin (2 or 4 mg kg−1, i.p.; or 100 or 300 μg lip−1), meloxicam (0.31, 1.25 or 5 mg kg−1, i.p.) or celecoxib (0.3, 1.0 or 3.0 mg kg−1, i.p.; or 12.5, 50 or 200 μg lip−1), or 1 h after treatment with the inhibitor of 5-LOX activating protein MK 886 (0.3, 1.0 and 3.0 mg kg−1, i.p), or an equal volume of the corresponding vehicle; Tris buffer at 0.2 M and pH 8.2, in the case of indomethacin; carboxymethylcellulose solution at 0.05% in the case of the COX inhibitors meloxicam and celecoxib; carboxymethylcellulose solution at 0.1% in the case of MK 886. We also tested the influence of indomethacin (2 or 4 mg kg−1, i.p.) or celecoxib (3.0 mg kg−1, i.p.; or 200 μg paw−1) on both phases of nociception induced, 30 min later, by injection of 2.5% formalin into the right hind-paw, to enable a comparison between both models of formalin-induced nociception. The doses of PGE2, indomethacin, meloxicam, celecoxib and MK 886 were selected on the basis of their previously reported efficacies in causing (PGE2; Nakamura & Ferreira, 1987) or blocking (all others; Yamamoto & Nozaki-Taguchi, 2002; Bianchi & Panerai, 2002; Tonussi & Ferreira, 1999) inflammatory mechanical and thermal hyperalgesia.
Materials
The formalin used in these experiments was 37% formaldehyde from Merck S/A, Rio de Janeiro, Brazil. The sources of other drugs are as follows: indomethacin (a gift from Merck, Sharp & Dohme, São Paulo, Brazil), celecoxib (Searle-Pfizer, Caguas, Puerto Rico), meloxicam (Boehringer Ingelheim, Buenos Aires, Argentina), PGE2, atenolol, tyramine, guanethidine monosulphate, captopril (all from Sigma Chemical Company, St Louis, U.S.A.); HOE 140 (D-Arg-Arg-Pro-Hyp-Gly-3-[2-thienyl]-Ala-Ser-D1,2,3,4 tetrahydro-3-isoquinoline carbonyl-L-[2α,3β,7αβ-octahydro-1H-indole-2-carbonyl-Arg), Hoechst AG, Frankfurt, Germany; DALBK (des-Arg9-Leu8-BK), Peninsula Laboratories, Belmont, U.S.A.); MK 886 (3-[1-{p-chlorobenzyl}-5-{isopropyl}-3-tert-butylthioindol-2-yl]-2,2-dimethylpropanoic acid), BIOMOL Research Laboratories, Milan, Italy. The cytokines, recombinant murine TNFα, recombinant rat IL-1β and IL-6, and recombinant human IL-8 and goat anti-mouse TNFα antibody were from R&D Systems Inc. (Minneapolis, U.S.A.). Sheep anti-rat IL-1β, sheep anti-human IL-8 and sheep anti-rat IL-6 antisera were from the National Institute for Biological Standards and Control (NIBSC, Hertfordshire, England) and were generous gifts from Dr Stephen Poole.
Statistical analysis
All results are presented as the mean±s.e.m. of 6–10 observations. Statistical comparison of the data was performed by analysis of variance (ANOVA) followed by the Bonferroni test. The P-values equal to or smaller than 0.05 were considered significant.
Results
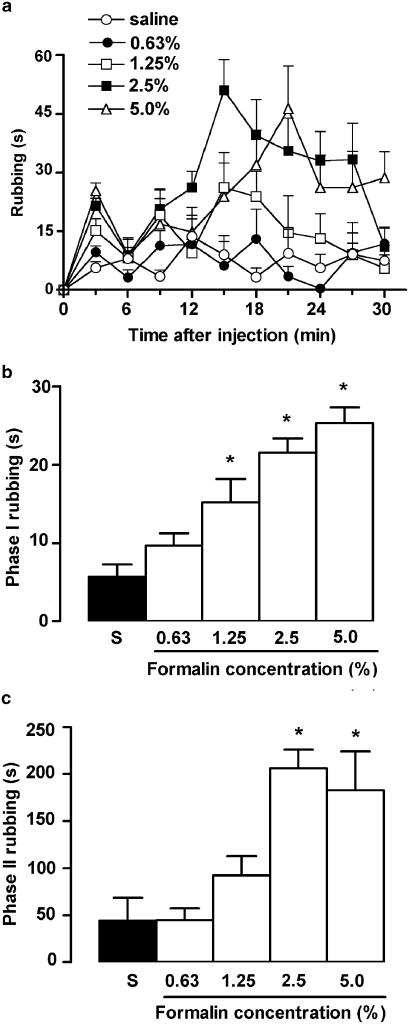
Injection of formalin into the upper lip caused a concentration-dependent and biphasic behavioural nociceptive response characterised by rubbing of the injected area (Figure 1a). The incidence of rubbing behaviour during the first – neurogenic – phase (first 3 min) was significantly increased over that of control saline-injected rats by 1.25, 2.5 or 5% formalin (Figure 1b), whereas only 2.5 and 5% formalin effectively increased nociceptive behaviour during the second – inflammatory – phase of the response (i.e. 12 to 30 min after injection; Figure 1c). Indeed, the second-phase responses elicited by both concentrations were similar and, thus, apparently maximal. Based on these initial results, we selected only two concentrations of formalin for use throughout the remainder of the study: (a) 0.63%, as it was clearly subthreshold for eliciting either phase of nociceptive response per se, and could thus be amenable to potentiation by various treatments; and (b) 2.5%, as this concentration caused very robust nociceptive behaviour in each of the two phases of the response, which renders it susceptible to clear-cut inhibition by treatments with potential blockers of inflammatory nociception. None of the vehicles used in these various treatments induced statistically significant nociceptive responses per se, and these data have been omitted from the subsequent figures, for sake of clarity.
Figure 1.
Nociceptive responses induced by formalin administration into the upper lip of rats, evaluated as the time the animals spent rubbing the injected site, in seconds. (a) Time course of rubbing responses displayed throughout consecutive 3-min bins during the first 30 min after injection of formalin (50 μl at 0.63, 1.25, 2.5, 5.0%) or saline. (b, c) Total rubbing time induced by formalin (open bars) and saline (S, closed bars) during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following injection, respectively. Each value represents the mean±s.e.m. recorded from 10 animals. Asterisks denote P<0.05 relative to the corresponding saline-injected control value (ANOVA followed by Bonferroni's test).
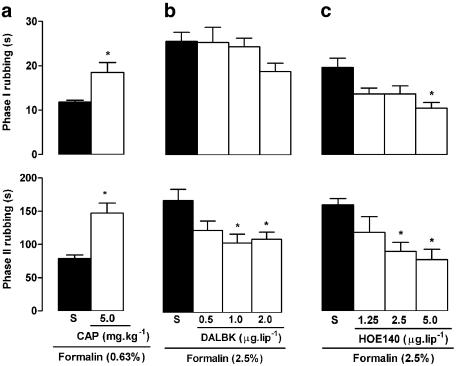
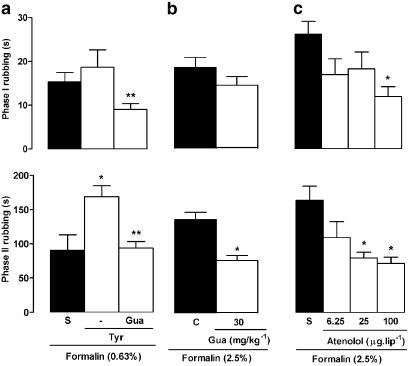
Pretreatment with captopril (5 mg kg−1, 2 h before formalin), a kininase II inhibitor, increased both phases of the response to 0.63% formalin (Figure 2a), without affecting control rubbing responses induced by saline (results not shown). Local administration of the B1 receptor antagonist DALBK (0.5, 1.0 and 2.0 μg lip−1, 30 min before formalin) reduced only the second phase of nociception triggered by 2.5% formalin (Figure 2b), while the B2 receptor antagonist HOE 140 (1.25, 2.5 and 5.0 μg lip−1) reduced both phases of this response (Figure 2c).
Figure 2.
Influence of captopril and BK B1 and B2 receptor antagonist treatment on nociceptive behaviour induced by formalin injection into the upper lip of rats. Upper and lower panels show total rubbing time induced by formalin during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following injection, respectively. Rats received (a) the kininase II inhibitor captopril (CAP; 5 mg kg−1, s.c., 2 h before), (b) the B1 receptor antagonist Des-Arg9-Leu8-BK (DALBK; 0.5, 1.0 or 2.0 μg lip−1, 30 min before) or (c) the B2 receptor antagonist HOE 140 (1.25, 2.5 or 5.0 μg lip−1, 30 min before) or vehicle alone (saline, S; closed bars). Note that all animals received 50 μl of formalin, either at 0.63% in (a) or at 2.5% in (b) and (c). Each value represents the mean±s.e.m. recorded from six animals. Asterisks denote P<0.05 relative to the corresponding saline-injected control value (ANOVA followed by Bonferroni's test).
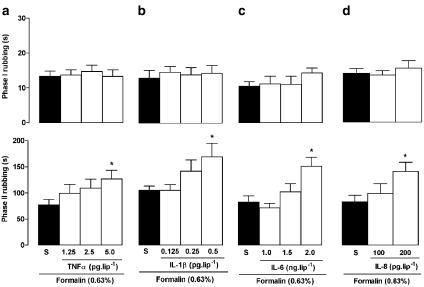
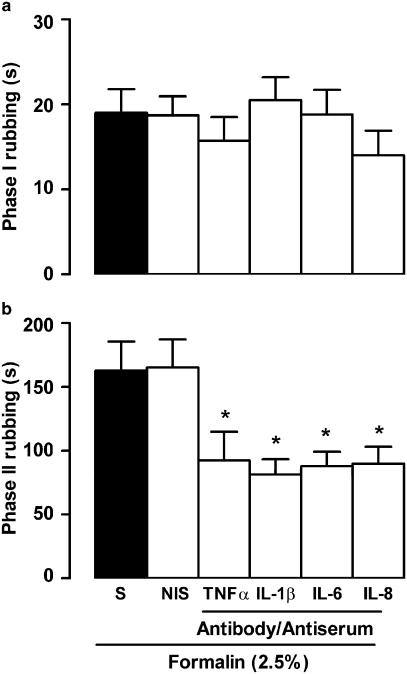
As shown in Figure 3, the first phase of the nociceptive response to 0.63% formalin was unaffected by prior treatment (2 h before) with the cytokines TNFα (1.25, 2.5 and 5.0 pg lip−1), IL-1β (0.125, 0.25 and 0.5 pg lip−1), IL-6 (1.0, 1.5 and 2.0 ng lip−1) or IL-8 (100 and 200 pg lip−1), but all these cytokines increased the second phase of the response, at the highest doses tested. On the other hand, when rats were treated, 1 h before 2.5% formalin, with an antibody against TNFα (25 pg lip−1) or with antiserum against IL-1β, IL-6 or IL-8 (diluted 1 : 5 in saline), nociceptive responses during the first phase were unaltered, but those comprising the second phase were significantly reduced (Figure 4). None of the cytokines or antisera/antibody treatments affected rubbing responses elicited by saline (data not shown).
Figure 3.
Influence of prior local treatment with various cytokines on nociceptive behaviour induced by formalin injection into the upper lip of rats. Upper and lower panels show total rubbing time induced by 50 μl of 0.63% formalin during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following its injection, respectively. Rats received (a) TNFα, (b) IL-1β, (c) IL-6 or (d) IL-8 or vehicle alone (saline, S; closed bars), 2 h before formalin. Note that all animals received formalin injection. Each value represents the mean±s.e.m. recorded from six animals. Asterisks denote P<0.05 relative to the corresponding saline-injected control value (ANOVA followed by Bonferroni's test).
Figure 4.
Influence of prior local treatment with antibody/antiserum against various cytokines on nociceptive behaviour induced by formalin injection into the upper lip of rats. (a, b) Total rubbing time induced by 50 μl of 2.5% formalin during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following its injection, respectively. Rats received either antibody against TNFα, or antisera against IL-1β, IL-6 or IL-8 (each diluted 1 : 5 in saline), and control animals were given the corresponding vehicle (nonimmune serum, NIS or saline, S; closed bars), 1 h beforehand. Note that all animals received formalin injection. Each value represents the mean±s.e.m. recorded from six animals. Asterisks denote P<0.05 relative to the corresponding NIS- or saline-injected control value (ANOVA followed by Bonferroni's test).
Treatment with the indirectly acting sympathomimetic drug tyramine (200 μg lip−1, 2 h before formalin) failed to elicit nociceptive responses per se, but potentiated the second (but not the first) phase of nociception triggered by 0.63% formalin (Figure 5a). Prior treatment with the sympathetic postganglionic neurone blocker guanethidine (30 mg kg−1 day−1), for 3 days, abolished this pronociceptive effect of tyramine and also depressed the magnitude of second-phase responsiveness to 2.5% formalin (Figure 5a and b). Likewise, rats treated with the selective β1 adrenoceptor antagonist atenolol (25 μg lip−1) displayed less nociceptive behaviour during the second phase only, but a higher dose (100 μg lip−1) reduced both phases significantly (Figure 5c).
Figure 5.
Influence of sympathetic activation or blockade on nociceptive behaviour induced by formalin injection into the upper lip of rats. Upper and lower panels show total rubbing time induced by formalin during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following its injection, respectively. Rats received either (a) the noradrenaline releasing drug tyramine (Tyr, 200 μg lip−1, 2 h beforehand), (b) daily injections of the sympathetic postganglionic neurone-blocking agent guanethidine (Gua, 30 mg kg−1 day−1, s.c.) for 3 days or (c) the β1-adrenoceptor antagonist atenolol (6.25, 25 and 100 μg lip−1, 30 min before), or vehicle alone (saline, S; closed bars). Note that all animals received a 50 μl injection of formalin, either at 0.63% in (a) or at 2.5% in (b) and (c), and also that a group of Tyr-treated rats in (b) was treated first with Gua. Each value represents the mean±s.e.m. recorded from six to 10 animals. Asterisks denote P<0.05 relative to the corresponding saline-injected control value (ANOVA followed by Bonferroni's test).
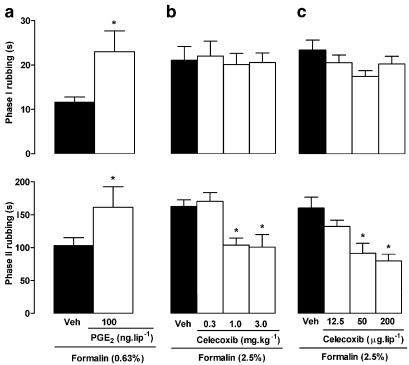
The local injection of PGE2 (100 ng lip−1, 3 h before formalin) potentiated both phases of the nociception induced by 0.63% formalin (Figure 6a), but did not elicit nociceptive behaviour per se (data not shown). Conversely, the selective COX-2 inhibitor celecoxib (given i.p. at 0.3, 1 and 3 mg kg−1 or locally at 12.5, 50 and 200 μg lip−1, 30 min before 2.5% formalin) reduced the incidence of rubbing during the second phase of the response (Figures 6b and c, respectively). In addition, meloxicam (at 1.25 and 5 mg kg−1, i.p.) also reduced significantly the second phase of the nociception induced by 2.5% formalin (87.6±12.4 and 80.2±12.7 s, respectively, compared to 178.9±11.6 for vehicle plus formalin-treated animals). In sharp contrast, the nonselective COX inhibitor indomethacin (2 and 4 mg kg−1, i.p.; or 100 and 300 μg lip−1) did not modify the nociceptive response to 2.5% formalin in the lip (data not shown). However, when 2.5% formalin was injected into the hind-paw, the second phase of the nociceptive behaviour was effectively reduced by systemic treatment with indomethacin, as occurred following local (but not systemic) treatment with celecoxib (200 μg paw−1; Table 1).
Figure 6.
Influence of PGE2 and selective COX-2 inhibition with celecoxib on nociceptive behaviour induced by formalin injection into the upper lip of rats. Upper and lower panels show total rubbing time induced by formalin during the first 3 min (0–3 min; phase I) and between 12 and 30 min (phase II) following injection, respectively. Rats received either (a) PGE2 (100 ng lip−1, 3 h before), (b) systemic celecoxib (0.3, 1 or 3 mg kg−1, i.p., 30 min before) or (c) local celecoxib (12.5, 50 or 200 μg kg−1, 30 min before), or the corresponding vehicle (Veh; closed bars). Note that all animals received a 50 μl injection of formalin, either at 0.63% in (a) or at 2.5% in (b) and (c). Each value represents the mean±s.e.m. recorded from six animals. Asterisks denote P<0.05 relative to the corresponding saline-injected control value (ANOVA followed by Bonferroni's test).
Table 1.
Influence of indomethacin and celecoxib on nociceptive responses induced in rats by i.p. injection of 2.5% formalin into the hind-paw
| Drug treatment | Nociceptive responses (paw licking and lifting, in s) | % inhibition of phase II responses | |
|---|---|---|---|
| Phase I | Phase II | ||
| Intraperitoneal route | |||
| Control | 129.4±14.9 | 917.0±15.5 | – |
| Indomethacin 2 mg kg−1 | 115.1±19.2 | 522.7±42.6* | 43* |
| Indomethacin 4 mg kg−1 | 121.0±10.2 | 504.3±32.2* | 45* |
| Control | 134.8±3.0 | 812.7±34.1 | – |
| Celecoxib 3 mg kg−1 | 121.0±1.9 | 809.7±22.7 | 0.4 |
| Intraplantar route | |||
| Control | 130.3±2.8 | 822.2±15.5 | – |
| Celecoxib 200 μg paw−1 | 118.8±5.2 | 664.2±14.3* | 19* |
Values correspond to mean±s.e.m. of six to 10 animals.
P<0.05 relative to the respective control group (vehicle+2.5% formalin-treated animals).
The 5-LOX-activating protein inhibitor MK886 (given i.p. at 0.3, 1 and 3 mg kg−1, 1 h before 2.5% formalin) did not modify the incidence of rubbing during both phases of the response (phase I: 15.7±2.5, 16.8±2.5 and 19.7±2.7 s, respectively, compared to 18.5±2.6 s for vehicle plus formalin-treated animals; phase II: 141.5±15.3, 140.7±22.6 and 124.5±24.3 s, respectively, compared to 156.3±22.1 s for vehicle plus formalin-treated animals).
Discussion
The neurochemical and neurophysiological changes induced by injury to nerves of the trigeminal system are clearly distinct from those seen following lesions of other peripheral nerves (for a review, see Fried et al., 2001). For example, the onset of spontaneous nociception in the former case is more delayed (but can be sustained for very long periods) and is not associated with sprouting of ganglionic sympathetic terminals (Tal & Devor, 1992; Bongenhielm et al., 1999). Nonetheless, present knowledge about the specific mechanisms underlying trigeminal nociception, whether neuropathic or inflammatory in origin, is still remarkably sparse. In this regard, the current study provides pharmacological insight into some of the mechanisms involved in nociception induced by formalin in the upper lip of the rat, which is innervated by the trigeminal system. The data provided in this paper reveal significant contributions of BK, several cytokines, sympathetic mechanisms and eicosanoids to nociceptive behaviour associated with this model of orofacial nociception.
It is well established that BK, one of the most potent endogenous algogens known, participates in nociception in various models including that induced by formalin in the hind-paw of mice and rats (Chau et al., 1991; Chapman & Dickenson, 1992; Correa & Calixto, 1993; for a review, see Calixto et al., 2000). This also seems to hold true regarding formalin-induced orofacial nociception, as captopril, which blocks BK degradation by kininase II, augmented both phases of the response to the subthreshold concentration of the algogen, without causing nociception per se. Moreover, we also observed that prior local treatment with selective antagonists of B1 (DALBK) and B2 (HOE 140) receptors attenuated the second phase and both phases of the response to 2.5% formalin, respectively. Thus, the roles of B1 and B2 receptors in mediating the endogenous BK-dependent components of orofacial nociception triggered by formalin show some similarity to those seen following injection into the rat hind-paw (Chapman & Dickenson, 1992; Rupniak et al., 1997), although other studies found that DALBK also blocks the first (neurogenic) phase of the response to intraplantar formalin injection in the rat (Sufka & Roach, 1996) and mouse (Correa & Calixto, 1993). However, the effects of HOE 140 in the paw seemed to be modest (maximal inhibition of about 40%, Correa & Calixto, 1993), while in the orofacial model similar doses produced a more intense effect (maximal inhibition of about 52%). B1 receptors are rarely expressed constitutively, but are frequently upregulated following trauma or by inflammatory stimuli (Schanstra et al., 1998). Thus, it is unclear if the B1 receptors only contribute to the second phase of orofacial nociception because they are expressed solely during this latter stage, or if they are present from the start, but are coupled to signalling mechanisms specific for the inflammatory phase of the response.
BK can activate Aδ and C sensory neurones directly, but in addition also releases many inflammatory mediators that contribute to amplification of the nociceptive response, such as cytokines, PGs and neuropeptides (for a review, see Calixto et al., 2000). It is well known that intraplantar injections of TNFα, IL-1β, IL-6 and chemokines induce hyperalgesia and nociception in the rat hind-paw (Cunha et al., 1992; Perkins & Kelly, 1994; Woolf et al., 1997). There is also limited evidence that their endogenous production contributes effectively to formalin-induced nociception, largely through actions at spinal levels of the CNS (Watkins et al., 1997). The current study reveals that local injections of TNFα, IL-1β, IL-6 and IL-8 into the lip, at doses which evoke marked mechanical hyperalgesia in the rat hind-paw (Cunha et al., 1992), are each capable of increasing the second phase of formalin-induced orofacial nociception. In addition, as the various cytokines failed to cause nociceptive responses per se, their influence was truly hyperalgesic, at least at the doses we tested. The fact that local treatment with specific antibody/antisera against these cytokines reduced the second phase of the response to 2.5% formalin would appear to confirm that peripherally produced TNFα, IL-1β, IL-6 and IL-8 are involved in this process. Cytokines seem to act through the induction of other cytokines, as well as chemokines (Dinarello et al., 1987, De Leo & Yezierski, 2001; Boddeke, 2001) and PG (Follenfant et al., 1989; Crestani et al., 1991; Watkins et al., 1994). On the other hand, although IL-8 causes hind-paw hyperalgesia sensitive to blockade by β-adrenoceptor antagonists in the rat (Cunha et al., 1991), this species does not express this chemokine. Instead, the rat produces CINC-1, which belongs to the same chemokine family (CXC), acts on the same CXCR2 receptor and shares more than 80% homology with IL-8 (Shibata et al., 2000).
The sympathetic nervous system exerts an important facilitatory role in the generation and processing of nociceptive signals, especially (but not exclusively) in inflammatory states (Coderre et al., 1984; Nakamura & Ferreira, 1987; Duarte et al., 1988). In this regard, phentolamine and propranolol (α- and β-adrenoceptor antagonists, respectively), guanethidine (a sympathetic postganglionic neurone-blocking agent) and surgical sympathectomy all block nociception induced by endotoxin (Safieh-Garabedian et al., 2002) or formalin in the rat hind-paw (Fuchs et al., 1999). The sympathetic nervous system also seems to be implicated in orofacial nociception caused by formalin, as it was susceptible to inhibition by the selective β1 adrenoceptor antagonist atenolol or guanethidine (both phases), and was amplified in a guanethidine-sensitive fashion by tyramine (second phase only), a releaser of noradrenaline from catecholaminergic nerve terminals. The reduction of the first phase by atenolol and guanethidine may be related to a reduction in the nociceptor activation threshold (Coderre et al., 1984). At least for atenolol, this may be due to a local anaesthetic activity observed at higher doses for several β blockers (Haeusler, 1990).
Arachidonic acid metabolites of the COX pathways (i.e. PGs) are critically involved in the hyperalgesic effects of BK, TNFα, IL-1β and IL-6 in the rat paw (Cunha et al., 1992). In addition, the second (inflammatory) phase of nociception triggered by formalin in the paw is markedly reduced by nonsteroidal anti-inflammatory drugs, such as the nonselective COX inhibitor indomethacin (Malmberg & Yaksh, 1995 and present study). Local administration of PGE2 potentiated both phases of orofacial nociception induced by a subthreshold concentration of formalin. Surprisingly, however, we observed that indomethacin, at local or systemic doses that effectively inhibited nociceptive behaviour induced by formalin in the paw, did not modify the orofacial nociception triggered by this algogen. These results could suggest that sensitisation of sensory fibres of the trigeminal system is less dependent on local PG generation than that seen in hind-paw nociceptors, even though they are responsive to exogenous PGs. Alternatively, nociceptive responses elicited by stimulation of orofacial nociceptive fibres might simply be less susceptible to inhibitors of COX than those evoked from the hind-paw. Indeed, we found that the second phase of formalin-induced orofacial nociception was reduced substantially by local or systemic treatment with celecoxib, a selective COX-2 inhibitor (Riendeau et al., 2001). We also obtained similar findings using meloxicam, which displays less selectivity than celecoxib as an inhibitor of the COX-2 isoform (Riendeau et al., 2001).
Data on the efficacy of celecoxib and other selective COX-2 inhibitors against formalin-induced nociception in the hind-paw is controversial. Some studies found that such substances were ineffective when given by i.p. or intrathecal routes (Dirig et al., 1997; Euchenhofer et al., 1998), while others detected significant intrathecal antinociceptive effects (Yamamoto & Nozaki-Taguchi, 1996; Torres-López et al., 2002). However, the onset of intrathecal celecoxib-induced antinociception seen in the latter studies was much faster than the time required (2–6 h) for upregulation of COX-2 mRNA expression in the spinal cord (Seibert et al., 1994; Beiche et al., 1996). If the local effect of celecoxib against formalin-induced orofacial nociception truly reflects inhibition of COX-2 activity, it seems that PG generated locally is more important than that formed in the spinal cord, at least in the trigeminal region. However, we cannot discard the participation of centrally induced COX-2. Recently, Choi et al. (2003) showed that COX-2 induced in the central nervous system plays an important role in IL-1β-induced orofacial hyperalgesia. Alternatively, additional mechanisms other than COX-2 inhibition could be contributing to the local effect of this drug (Iñiguez et al., 1999; Cuzzocrea et al., 2002). If the orofacial formalin test could be related to some human pathological conditions, then the clinical efficacy of some known nonselective nonsteroidal anti-inflammatory drugs may be limited, while selective COX-2 inhibitors such as celecoxib may be more valuable. Indeed, some clinical studies have been carried out and celecoxib and rofecoxib, another selective COX-2 inhibitor, have provided similar or greater overall analgesic efficacy than nonselective COX inhibitors (Morrison et al., 1999; Chang et al., 2002; Khan et al., 2002). In addition, although the injection of formalin in the lip constitutes a model for acute orofacial pain, the effectiveness of selective COX-2 inhibitors in this model calls for a more intense investigation of their effects in more chronic conditions that are considered to be particularly difficult to treat.
On the other hand, the lack of effect of the LT synthesis inhibitor MK 886 against both phases of formalin-induced orofacial nociception, at doses which reduce substantially articular incapacitation caused by LPS or Freund's complete adjuvant in rat knee joints (Tonussi & Ferreira, 1999), seems to rule out the possible contribution of those eicosanoids derived from the lipoxygenase pathway to this response. It has been shown that macrophages from chronically inflamed tissues enhance production of LTs after stimulation, at the expense of PG synthesis (Beusenberg et al., 1994). These findings suggest that LTs may play a significant role in chronic inflammatory reaction, whereas acute inflammatory hyperalgesia is generally unresponsive to the inhibition of LT synthesis (Higgs et al., 1988; Griswold et al., 1991; Muller-Peddinghaus et al., 1993; Amann et al., 1996).
The present study describes for the first time the participation of BK, TNF-α, IL-1β, IL-6, IL-8, PG and sympathetic amines in the orofacial nociception induced by formalin in the rat. Mechanical hyperalgesia induced by carrageenan and LPS in the rat hind-paw depends on nociceptor sensitisation via BK-triggered synthesis and release of TNF-α, which in turn activates two distinct signalling pathways: one requiring IL-1β and IL-6 expression and PG generation, and another which is PG independent and involves IL-8 expression and sympathetic activation (Cunha et al., 1992; Ferreira et al., 1993). It remains to be seen if formalin-induced orofacial nociception is based on this same mechanistic sequence of events, and/or if other mechanisms/mediators also contribute to this phenomenon.
In conclusion, although differences between craniofacial pain and other types of pain have been shown (Fried et al., 2001), the mediators involved in orofacial nociception elicited by formalin in rats did not differ greatly from those elicited by formalin in rodent paws. It is possible that more substantial differences could be found in more chronic models. However, it does appear that nociception in this model is more susceptible to inhibition by BK B2 receptor antagonist and selective COX-2 inhibitors, which could constitute more effective therapeutic tools in orofacial nociception, than by BK B1 receptor antagonist or nonselective COX inhibitors.
Acknowledgments
J.G.C. was the recipient of a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Brazil.
Abbreviations
- BK
bradykinin
- COX
cyclooxygenase
- CINC
cytokine-induced neutrophil chemoattractant
- DALBK
des-Arg9-Leu8-BK
- IL
interleukin
- LT
leukotriene
- 5-LOX
5-lipoxygenase
- PG
prostaglandins
- TNFα
tumor necrosis factor α
References
- AMANN R., SCHULIGOI R., LANZ I., PESKAR B.A. Effect of a 5-lipoxygenase inhibitor on nerve growth factor-induced thermal hyperalgesia in the rat. Eur. J. Pharmacol. 1996;306:89–91. doi: 10.1016/0014-2999(96)00255-5. [DOI] [PubMed] [Google Scholar]
- BEICHE F., SCHEUERER S., BRUNE K., GEISSLINGER G., GOPPELT-STRUEBE M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- BEUSENBERG F.D., HOOGSTENDEN H.C., BONTA I.L., VAN AMSTERDAN J.G. Cyclic AMP enhancing drugs modulate eicosanoid release from human alveolar macrophages. Life Sci. 1994;54:1269–1274. doi: 10.1016/0024-3205(94)00854-x. [DOI] [PubMed] [Google Scholar]
- BIANCHI M., PANERAI A.E. Effects of lornoxicam, piroxicam, and meloxicam in a model of thermal hindpaw hyperalgesia induced by formalin injection in rat tail. Pharmacol Res. 2002;45:101–105. doi: 10.1006/phrs.2001.0921. [DOI] [PubMed] [Google Scholar]
- BODDEKE E.W.G.M. Involvement of chemokines in pain. Eur. J. Pharmacol. 2001;429:115–119. doi: 10.1016/s0014-2999(01)01311-5. [DOI] [PubMed] [Google Scholar]
- BONGENHIELM U., BOISSONADE F.M., WESTERMARK A., ROBINSON P.P., FRIED K. Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain. 1999;82:283–288. doi: 10.1016/S0304-3959(99)00064-0. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CARTMELL T., POOLE S., TURNBULL A.V., ROTHWELL N.J., LUHESHI G.N. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J. Physiol. 2000;526:653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG D.J., DESJARDINS P.J., CHEN E., POLIS A.B., MCAVOY M., MOCKOVIAK S.H., GEBA G.P. Comparison of the analgesic efficacy of rofecoxib and enteric-coated diclofenac sodium in the treatment of postoperative dental pain: a randomized, placebo-controlled clinical trial. Clin. Ther. 2002;24:490–503. doi: 10.1016/s0149-2918(02)85126-8. [DOI] [PubMed] [Google Scholar]
- CHAPMAN V., DICKENSON A.H. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur. J. Pharmacol. 1992;219:427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- CHAU T.T., LEWIN A.C., WALTER T.L., CARLSON R.P., WEICHMAN B.M. Evidence for a role of bradykinin in experimental pain models. Agents Actions. 1991;34:235–238. doi: 10.1007/BF01993290. [DOI] [PubMed] [Google Scholar]
- CHOI H.S., LEE H.J., JUNG C.Y., JU J.S., PARK J.S., AHN D.K. Central cyclooxygenase-2 participates in interleukin-1β-induced hyperalgesia in the orofacial formalin test of freely moving rats. Neurosci. Lett. 2003;352:187–190. doi: 10.1016/j.neulet.2003.08.065. [DOI] [PubMed] [Google Scholar]
- CLAVELOU P., PAJOT J., DALLEL R., RABOISSON P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci. Lett. 1989;103:349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., ABOTT F.V., MELZACK R. Effects of peripheral anti-sympathetic treatments in the tail-flick, formalin and autotomy tests. Pain. 1984;18:13–23. doi: 10.1016/0304-3959(84)90122-2. [DOI] [PubMed] [Google Scholar]
- CORREA C.R., CALIXTO J.B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br. J. Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTANI F., SEGUY F., DANTZER R. Behavioral effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- CUNHA F.Q., LORENZETTI B.B., POOLE S., FERREIRA S.H. Interleukin-8 as a mediator of sympathetic pain. Br. J. Pharmacol. 1991;104:765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., MAZZON E., SAUTEBIN L., DUGO L., SERRAINO I., DE SARRO A., CAPUTI A.P. Protective effects of Celecoxib on lung injury and red blood cell modification induced by carrageenan in the rat. Biochem. Pharmacol. 2002;63:785–795. doi: 10.1016/s0006-2952(01)00908-x. [DOI] [PubMed] [Google Scholar]
- DE LEO J.A., YEZIERSKI R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A., IKEJIMA T., WARNER S.J.C., ORENCOLE S.F., LONNEMANN G., CANNON J.G., LIBBY P. Interleukin-1 induces interleukin-1. Induction of circulating interleukin-1 in rabbits in vivo and in human mononuclear cells in vitro. J. Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- DIRIG D.M., KONIN G.P., ISAKSON P.C., YAKSH T.L. Effect of spinal cyclooxygenase inhibitors in rat using the formalin test and in vitro prostaglandin E2 release. Eur. J. Pharmacol. 1997;331:155–160. doi: 10.1016/s0014-2999(97)01053-4. [DOI] [PubMed] [Google Scholar]
- DUARTE I.D.G., NAKAMURA M., FERREIRA S.H. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res. 1988;21:341–343. [PubMed] [Google Scholar]
- DUBUISSON D., DENNIS S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- EUCHENHOFER C., MAIHOFNER C., BRUNE K., TEGEDER I., GEISSLINGER G. Differential effect of selective cyclooxygenase-2 inhibitor NS 398 and diclofenac on formalin-induced nociception in the rat. Neurosci. Lett. 1998;248:25–28. doi: 10.1016/s0304-3940(98)00325-5. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., POOLE S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLENFANT R.L., NAKAMURA-CRAIG M., HENDERSON B., HIGGS G.A. Inhibition by neuropeptides of interleukin-1β-induced prostaglandin-independent hyperalgesia. Br. J. Pharmacol. 1989;98:41–43. doi: 10.1111/j.1476-5381.1989.tb16860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN K.B., ABBOTT F.V. Techniques for assessing the effects of drugs on nociceptive responses. Neuromethods. 1989;13:145–148. [Google Scholar]
- FRIED K., BONGENHIELM U., BOISSONADE F.M., ROBINSON P.P. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–165. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- FUCHS P.N., RINGKAMP M., SHIOTANI M., RAJA S.N. Sympathectomy decreases formalin-induced nociceptive responses independent of changes in peripheral blood flow. Exp. Neurol. 1999;155:95–102. doi: 10.1006/exnr.1998.6967. [DOI] [PubMed] [Google Scholar]
- GRISWOLD D.E., MARSHALL P., MARTIN L., WEBB E.F., ZABKO-POTAPOVICH B. Analgesic activity of SK&F 105809, a dual inhibitor of arachidonic acid metabolism. Agents Actions. 1991;32:113–117. doi: 10.1007/978-3-0348-7405-2_15. [DOI] [PubMed] [Google Scholar]
- HAEUSLER G. Pharmacology of β-blockers: classical aspects and recent developments. J. Cardiovasc. Pharmacol. 1990;16 Suppl. 5:S1–S9. [PubMed] [Google Scholar]
- HIGGS G.A., FOLLENFANT R.L., GARLAND L.G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: effects on acute inflammatory responses. Br. J. Pharmacol. 1988;94:547–551. doi: 10.1111/j.1476-5381.1988.tb11559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IÑIGUEZ M.A., PUNZÓN C., FRESNO M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J. Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- KHAN A.A., BRAHIM J.S., ROWAN J.S., DIONNE R.A. In vivo selectivity of a selective cyclooxygenase 2 inhibitor in the oral surgery model. Clin. Pharmacol. Ther. 2002;72:44–49. doi: 10.1067/mcp.2002.125560. [DOI] [PubMed] [Google Scholar]
- LAZAROV N.E. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog. Neurobiol. 2001;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- LORENZETTI B.B., VEIGA F.H., CANETTI C.A., POOLE S., CUNHA F.Q., FERREIRA S.H. Cytokine-induced neutrophil chemoattractant-1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivity in rats. Eur. Cytokine Netw. 2002;13:456–461. [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH L. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J. Neurosci. 1995;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS B. Autonomic mechanisms in oral sensations. Proc. Finn. Dent. Soc. 1989;85:365–373. [PubMed] [Google Scholar]
- MORRISON B.W., CHRISTENSEN S., YUAN W., BROWN J., AMLANI S., SEIDENBERG B. Analgesic efficacy of the cyclooxygenase-2-specific inhibitor rofecoxib in post-dental surgery pain: a randomized, controlled trial. Clin. Ther. 1999;21:943–953. doi: 10.1016/S0149-2918(99)80016-2. [DOI] [PubMed] [Google Scholar]
- MULLER-PEDDINGHAUS R., KOHLSDORFER C., THEISEN-POPP P., FRUCHTMANN R., PERZBORN E., BECKERMANN B., BUHNER K., AHR H.J., MOHRS K.H. BAY X1005, a new inhibitor of leukotriene synthesis: in vivo inflammation pharmacology and pharmacokinetics. J. Pharmacol. Exp. Ther. 1993;267:51–57. [PubMed] [Google Scholar]
- NAKAMURA M., FERREIRA S.H. A peripheral sympathetic component in inflammatory hyperalgesia. Eur. J. Pharmacol. 1987;135:145–153. doi: 10.1016/0014-2999(87)90606-6. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D. Interleukin-1β induced-desArg9 BK-mediated thermal hyperalgesia in the rat. Neuropharmacol. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- RIENDEAU D., PERCIVAL M.D., BRIDEAU C., CHARLESON S., DUBÉ D., ETHIER D., FALGUEYRET J.-P., FIRESEN R.W., GORDON R., GREIG G., GUAY J., MANCINI J., OUELLET M., WONG E., XU L., BOYCE S., VISCO D., GIRARD Y., PRASIT P., ZAMBONI R., RODGER I.W., GRESSER M., FORD-HUTCHINSON A.W., YOUNG R.N., CHAN C.-C. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J. Pharmacol. Exp. Ther. 2001;296:558–566. [PubMed] [Google Scholar]
- RUPNIAK N.M., BOYCE S., WEBB J.K., WILLIAMS A.R., CARLSON E.J., HILL R.G., BORKOWSKI J.A., HESS J.F. Effects of the bradykinin B1 receptor antagonist des-Arg9[Leu8]-bradykinin and genetic disruption of the B2 receptor on nociception in rats and mice. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., POOLE S., HADDAD J.J., MASSAAD C.A., JABBUR S.J., SAADÉ N.E.The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation Neuropharmacol. 200242864–872.(Issue 6) [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., CASTAÑO M.E.M., BARASCUB Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTIER F., GIROLAMI J.-P., BASCANDS J.-L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-κB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIBERT K., ZHA NG Y., LEAHY K., HAUSER S., MASFERRER J., PERKINS W., LEE L., ISAKSON P. Pharmacological and biochemical demonstration of the role of cyclooxygenase in inflammation and pain. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA F., KONISHI K., NAKAGAWA H. Identification of a common receptor for three types of rat cytokine-induced neutrophil chemoattractants (CINCs) Cytokine. 2000;12:1368–1373. doi: 10.1006/cyto.2000.0739. [DOI] [PubMed] [Google Scholar]
- SUFKA K.J., ROACH J.T. Stimulus properties and antinociceptive effects of selective bradykinin B1 and B2 receptor antagonists in rats. Pain. 1996;66:99–103. doi: 10.1016/0304-3959(96)02990-9. [DOI] [PubMed] [Google Scholar]
- TAL M., DEVOR M. Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res. 1992;579:148–151. doi: 10.1016/0006-8993(92)90753-v. [DOI] [PubMed] [Google Scholar]
- TJOLSEN A., BERGE O.G., HUNSKAAR S., ROSLAND J.H., HOLE K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- TONUSSI C.R., FERREIRA S.H. Tumor necrosis factor-α mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82:81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- TORRES-LÓPEZ J.E., ORTIZ M.I., CASTANEDA-HERNANDEZ G., ALONSO-LOPEZ R., ASOMOZA-ESPINOSA R., GRANADOS-SOTO V. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 2002;20:1669–1676. doi: 10.1016/s0024-3205(02)01491-1. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., MARTIN D., ULRICH P., TRACEY K.J., MAIER S.F. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., WIERTELAK E.P., OOEHLER L.E., SMITH K.P., MARTIN D., MAIER S.F. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J., ALLCHORNE A., SAFIEH-GARABEDIAN B., POOLE S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumor necrosis factor-α. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF C.J., SALTER M.W. Neuronal plasticity: increasing the gain in pain. Neuroscience. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- WORDLICZEK J., SZCZEPANIK A.M., BANACH M., TURCHAN J., ZEMBALA M., SIEDLAR M., PRZEWLOCKI W., PRZEWLOCKA B. The effect of pentoxifiline on post-injury hyperalgesia in rats and postoperative pain in patients. Life Sci. 2000;66:1155–1164. doi: 10.1016/s0024-3205(00)00419-7. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N. Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res. 1996;739:104–110. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N. The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth. Analg. 2002;94:962–967. doi: 10.1097/00000539-200204000-00035. [DOI] [PubMed] [Google Scholar]