Abstract
To determine biological functions of platelet-activating factor (PAF) in chronic inflammation, we have investigated the kinetics of angiogenesis, inflammatory cells recruitment and cytokine production in sponge-induced granuloma in wild type and PAF receptor-deficient mice (PAFR-KO).
Angiogenesis as determined by morphometric analysis and hemoglobin content was significantly higher in the implants of PAFR-KO mice at all time points. Treatment with PAF receptor antagonist UK74505 (30 mg kg−1) also increased angiogenesis in sponge implants.
Neutrophils and macrophages accumulation, as determined by myeloperoxidase and N-acetylglucosaminidase activities in the supernatant of implanted sponges were markedly decreased in PAFR-KO mice. Surprisingly, the levels of the proinflammatory chemokines, keratinocyte-derived chemokine and chemokine monocyte chemoattractant protein 1 were higher in the implants of the transgenic animals.
We have shown that angiogenesis was stimulated in PAFR-KO mice whereas inflammation was decreased, indicating that PAF is an endogenous regulator of new blood vessels formation in the inflammatory microenvironment induced by the sponge implant.
Keywords: Neovascularization, chemokines, growth factors, sponge implants
Introduction
There is compelling evidence that persistent unregulated angiogenesis is an important component in a range of pathophysiological processes, chronic inflammation, tumor growth, diabetic retinopathy, and atherosclerosis. (Folkman, 1995; Griffioen & Molema, 2000).
Many models both in vitro and in vivo have been used to characterize the mechanisms involved in the formation of new blood vessels and in the associated cell trafficking in order to develop drugs and strategies to control or prevent the pathological consequences. In one in vivo model, the subcutaneous implantation of a sponge induces a chronic granulomatous response including an intense angiogenesis and infiltration of inflammatory cells (Lage & Andrade, 2000). This sponge model has been used to study the protein (cytokines, growth factors, chemokines) and nonprotein mediators involved in the vascular and inflammatory aspects of granuloma formation (Ford et al., 1989; Andrade et al., 1992). There is good evidence that one group of nonprotein substances, the lipid mediators such as prostaglandins, leukotrienes and platelet-activating factor (PAF), is involved in angiogenesis and in the recruitment and activation of inflammatory cells (Ben Ezra, 1978; Kanayasu et al., 1989; Andrade et al., 1992; Tsopanoglou et al., 1994; Jackson et al., 1998).
Here, we chose to assess the contribution of one of the lipid mediators, PAF, to these effects, because although there is evidence for exogenous PAF as an effective inducer of angiogenesis in vivo (Andrade et al., 1992; Jackson et al., 1998) and as a growth inducer for tumors (Bussolino et al., 1995; Kume & Shimizu, 1997), evidence for endogenous PAF in these conditions is less convincing. We have, therefore, used animals with gene inactivation of the receptor for PAF – PAF receptor knock out (PAFR-KO) – to analyze the range of activities exhibited by endogenous PAF in the sponge model of angiogenesis. We measured neovascularization, accumulation of inflammatory cells and production of proinflammatory cytokines. We have also evaluated the effects of the PAF receptor antagonist UK74505 on the vascularization of the sponge implants. UK74505 is a long-acting selective PAF receptor antagonist in animals and human (Alabaster et al., 1991; Parry et al., 1994). We found that although signs of inflammation were reduced in the PAFR-KO mice, neovascularization was increased, relative to the results in the wild-type (WT) mice of the same strain.
Methods
Balb/c mice lacking the PAFR were generated as described previously by Ishii et al. (1998). These PAFR-KO mice were bred at least eight generations into Balb/c mice and were provided by Professor Takao Shimizu (Department of Biochemistry and Molecular Biology, University of Tokyo, Tokyo, Japan). Mice have been bred and maintained at the bioscience unit of Institute Gonçalo Muniz (Fundação Oswaldo Cruz, Salvador, Brazil).
Pharmacological reactivity of cutaneous vasculature to PAF in PAFR-KO and WT mice
This was carried out in the dorsal skin of nonimplant-bearing mice to establish the phenotype in response to the mediator in KO animals. The principle underlying the fluorimetric assay we used is that measurement of fluorochrome-generated emission in the bloodstream following the application of a fluorescent dye in a vascular compartment reflects the local blood flow. Thus, exogenous application of vasoactive substances would alter the fluorescence intensity in the systemic circulation (Andrade et al., 1997; Lage & Andrade, 2000; Machado et al., 2001). A sterile solution (10 μl) of sodium fluorescein (Sigma, U.S.A., 10%) was injected intradermally 2 min after local application of PAF (1 μg) or vehicle (10 μl). Blood samples (5 μl) were withdrawn from the tail vein at 1, 3, 5, 7, 10, 15, 20, 25 and 30 min after the dye injection. The blood samples were mixed in 1 ml of isotonic saline, centrifuged for 5 min and the supernatant was kept for fluorescence determination in a Jenway fluorimeter (model 6200) at an excitation/emission of 485/520. The results were expressed in terms of half-time (t1/2; time taken for the fluorescence to reach 50% of the peak in the systemic circulation). At least three different PAFR-KO and WT animals were used to establish the effect of the mediator.
Preparation of cannulated sponge discs and implantation
Polyether–polyurethane sponge discs, 5 mm thick × 8 mm diameter (Vitafoam Ltd, Manchester, U.K.) were used as the matrix for fibrovascular tissue growth. A 12 mm length polyvinyl tubing (PE 20, Biovida, Brazil) was secured with silk sutures (Ethicon Ltd, U.K.) to the center of each disc in such a way that the tube was perpendicular to the disc face. Its open end was sealed with removable plugs. The cannulated sponge discs were soaked overnight in 70% v v−1 ethanol and sterilized by boiling in distilled water for 15 min before the implantation surgery. For this, the animals were anesthetized with 2,2,2-tribromoethanol (1 mg kg−1; i.p. Aldrich/U.S.A.), the dorsal hair shaved and the skin wiped with 70% ethanol. The cannulated sponge discs were aseptically implanted into a subcutaneous pouch, which had been made with curved artery forceps through a 1 cm long dorsal mid-line incision. The cannula was exteriorized through a small incision in a subcutaneous neck pouch. The animals were housed individually and provided with chow pellets and water ad libitum. The light/dark cycle was 12 : 12 h, with lights on at 07 : 00 and lights off at 19 : 00. Efforts were made to avoid any unnecessary distress to the animals. Housing and anesthesia concurred with the guidelines established by our local Institutional Animal Welfare Committee.
The implant-bearing mice were killed by cervical dislocation at 7, 10 and 14 days postimplantation, and the sponge discs carefully removed, dissected free from adherent tissue, weighed and analyzed for the angiogenic and inflammatory processes and histological assessment.
Assessment of the effects of the PAF receptor antagonist UK74505 on the sponge-induced angiogenesis
The PAF receptor antagonist UK74505 (30 mg kg−1 body weight) was given orally for 6 days from day 1 postimplantation in wild-type Balb/c mice (n=5) to verify whether the effect of this antagonist would mimic the effects of the genetic deletion of PAF receptor on angiogenesis. A control group of mice (n=6) received vehicle (saline 50 μl) in the same protocol. The animals were killed on day 7, the implants removed and processed for hemoglobin (Hb) determination.
Quantification of angiogenesis by Hb measurement
The extent of the vascularization of the sponge implants was assessed by the amount of Hb detected in the tissue using the Drabkin method (Drabkin & Austin, 1932). This approach has been modified and used to determine angiogenesis in several models and tissues, including the sponge model (Passaniti et al., 1992; Hu et al., 1995; Lage & Andrade, 2000). Each implant was homogenized (Tekmar TR-10, OH, U.S.A.) in 2.0 ml of Drabkin reagent (Labtest, São Paulo, Brazil) and centrifuged at 10,000 × g for 15 min. The supernatants were filtered through a 0.22 μm filter (Millipore). Hb in the samples was determined spectrophotometrically by measuring absorbance at 540 nm using an ELISA plate reader and compared against a standard curve of Hb. The content of Hb in the granulation tissue was expressed as μg Hb per mg wet tissue.
Morphometric analysis and blood vessel quantitation of the sponge implants
To examine the degree of neovascularization in the implants of control (WT) and PAFR-KO mice a total of 18 sponge discs (three for each time point for each group) was harvested and stained with Giemsa. Microscopic images of cross-sections (5 μm) were obtained with a planapochromatic objective × 40 in light microscopy. The images were digitized through a microcamera JVC TK-1270/JGB and transferred to an image analyzer (Kontron Eletronics, Carl Zeiss – KS300 version 2). A countable microvessel was defined as a structure with a lumen that contained red blood cells or not.
To establish the minimal representative microscopic fields per sample, blood vessels were counted in 50 fields (× 400 magnification, that is, × 40 objective lens and × 10 ocular lens; 0.053 mm2 per field) from a randomly chosen slide in each group as described by Moro et al. (2003). Then, groups of 5, 10, 20, 30, 40 and 50 fields were formed taken randomly with reposition. Mean and standard deviation were calculated from each group. Standard deviations decreased as the sample size increased. The minimal representative number of 15 microscopic fields per treatment was obtained when the increment in the number of fields did not result in considerable reduction in respective standard deviation value. The results were expressed as mean±s.e.m. of the total number of vessels/field. The vascular area was calculated by measuring each vessel. The sum of these areas was then divided by the number of vessels in order to obtain the mean±s.e.m. of vascular area (μm2).
Tissue extraction and determination of myeloperoxidase (MPO) and N-acetyl-β-D-glucosaminidase (NAG) activities
The extent of neutrophil accumulation in the implants was measured by assaying MPO activity in whole tissue as described previously (Bailey, 1988; Souza et al., 2000). After processing the supernatant of the sponge implant for Hb determination (see above), a part of the corresponding pellet was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M Na3PO4, 0.015 M NaEDTA) and centrifuged at 12,000 × g for 20 min. The pellets were then resuspended in 0.05 M sodium phosphate buffer (pH 5.4) containing 0.5% hexa-1,6-bis-decyltrimethylammonium bromide (HTAB, Sigma). The suspensions were freeze–thawed three times using liquid nitrogen and finally centrifuged at 10,000 × g for 10 min. MPO activity in the resulting supernatant was assayed by mixing 25 μl of 3,3′-5,5′-tetramethylbenzidine (TMB, Sigma), prepared in dimethyl sulfoxide (DMSO, Merck) in a final concentration of 1.6 mM with 100 μl H2O2, dissolved in phosphate buffer (pH 5.4) containing 0.5% HTAB in a final concentration of 0.003% (v v−1) and 25 μl of the supernatant from tissue sample processing. The assay was carried out in a 96-well microplate and was started by incubating the supernatant sample and the TMB solution for 5 min at 37°C. Then, the H2O2 was added and incubation continued for another 5 min. The reaction was terminated by adding 100 μl 4 M H2SO4 and was quantified colorimetrically at 450 nm in a spectrophotometer (Emax – Molecular Devices). Results were expressed as change in OD per g of wet tissue (implant).
Infiltration of mononuclear cells was quantitated by measuring the levels of lysosomal enzyme N-acetylglucosaminidase present in high levels in activated macrophages (Bailey, 1988). Part of the pellet remaining after the Hb measurement was kept for this assay. These pellets were homogenized in NaCl solution (0.9% w v−1) containing 0.1% v v−1 Triton X-100 (Promega) and centrifuged (3000 × g; 10 min 4°C). Samples of the resulting supernatant (100 μl) were incubated for 10 min with 100 μl p-nitrophenyl-N-acetyl-β-D-glucosaminide (Sigma), prepared in citrate/phosphate buffer (pH 4.5) in a final concentration of 2.24 mM. The reaction was terminated by the addition of 100 μl 0.2 M glycine buffer pH 10.6. Hydrolysis of the substrate was determined by measuring the color absorption at 405 nm. NAG activity was expressed as change in OD per g wet tissue.
Measurement of VEGF, TNF-α, CXCL2(KC) and CCL2(MCP-1/JE) production in the sponge implants
The implants of both groups of animals (PAFR-KO and WT), removed at days 7, 10 and 14 postimplantation, were homogenized in 500 μl of phosphate-buffered-saline pH 7.4 containing 0.05% Tween-20 and centrifuged at 12,000 × g for 30 min. The amount of the cytokines, vascular endothelial growth factor (VEGF) and tumor necrosis factor-α (TNF-α), and the chemokines, keratinocyte-derived chemokine (CXCL2(KC)) and chemokine monocyte chemoattractant protein 1 (CCL2(MCP-1/JE)), in each sample was measured in 50 μl of the supernatant using Immunoassay Kits (R and D Systems, U.S.A.) and following the manufacturer's protocol. Briefly, dilutions of cell-free supernatants were added in duplicate to ELISA plates coated with a specific murine polyclonal antibody against the cyto/chemokine, followed by the addition of a second polyclonal antibody against the cyto/chemokine. After washing to remove any unbound antibody–enzyme reagent, a substrate solution (a 1 : 1 solution of hydrogen peroxide and tetramethylbenzidine) was added to the wells. The reaction was terminated with 50 μl well−1 of 1 M H2SO4. Plates were read at 492 nm in a spectrophotometer (Emax – Molecular Devices). Standards were 0.5-log10 dilutions of recombinant murine chemokines from 7.5 to 1000 pg ml−1 (100 μl). The results were expressed as pg per mg wet tissue.
Statistical analysis
Results are presented as mean±s.e.m. Comparisons between two groups were carried out using Student's t-test for unpaired data. Comparisons between three or more groups were carried out using one-way analysis of variance (ANOVA) and differences between groups assessed using Newman–Keuls post-test. A P-value less that 0.05 was considered significant.
Results
Response of skin blood vessels to PAF in PAFR-KO and WT mice
Vasomotor responses of cutaneous vasculature in PAFR-KO mice were assessed after acute administration of PAF (1 μg) and compared with the response of WT animals (Figure 1). The bolus dose of the mediator applied in the skin vascular bed of WT mice caused a significant (P< 0.05; n=5) increase in the t1/2 value of the fluorescence peak when compared with the mean value of vehicle injection (saline 10 μl). In contrast, administration of exogenous PAF in the skin of PAFR-KO mice produced no increase in t1/2 values. This lack of vasoconstrictor response to PAF confirmed the loss of the receptors in these animals.
Figure 1.
Lack of vasoconstrictor response to PAF in the skin of PAFR-KO mice. Topical application of PAF (1 μg) in the skin of WT mice produced increase in t1/2 values of the fluorescence peak. The mediator caused no changes in t1/2 values when applied in the skin of PAFR-KO mice. Results are expressed as mean±s.e.m. of three to four animals in each group. *P< 0.05.
Histological assessments
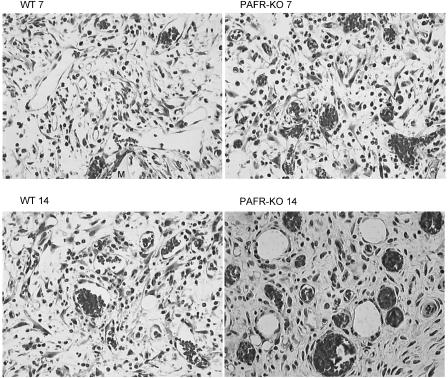
After Giemsa staining, sponge implants of both groups presented fibrovascular tissue growth at the three time points studied. The histologic changes during the development of the granulation tissue induced by sponge implants are illustrated in Figure 2 at day 7 (a) and day 14 (b). At day 7, the fibrovascular tissue of the implants of WT and PAFR-KO mice comprised of neutrophils, macrophages, fibroblasts and capillaries embedded in extracellular matrix. By day 14, the stroma consisting of more mature collagen was densely packed with fibroblasts, leukocytes and newly formed blood vessels containing red blood cells indicative of functional vessels. When histological sections from the implants of PAFR-KO mice were compared with sections from WT mice, the former showed much less cellular accumulation and a higher number of congested blood vessels at all time points (Figure 2a and b).
Figure 2.
Representative histological sections of sponge implants stained with Giemsa (7 and 14 days) from WT and PAFR-KO mice. The fibrovascular stroma at day 7 in both implants sections is composed of blood vessels, macrophages, neutrophils, lymphocytes, fibroblasts within a loose fibrotic extracellular matrix. The number of vessels is increased in PAFR-KO. By day 14, the implants of both groups consisting of more mature collagen were densely packed with fibroblasts, leukocytes and newly formed blood vessels containing red blood cells indicative of functional vessels. The section of PAFR-KO implant exhibits a higher number of congested blood vessels as compared with the section of WT implant. Original magnification × 66; M=sponge matrix.
Kinetics of implant-induced angiogenesis in WT and PAFR-KO mice
The sponge matrix was well tolerated by all animals. No sign of infection or rejection was observed in the implant compartment during the 14-day period of the experiment.
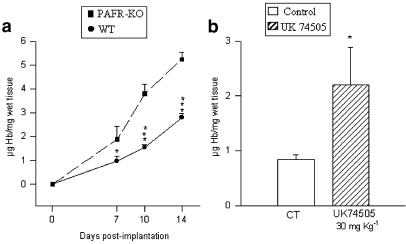
Sponge Hb content, an index of neovascularization, increased steadily in both groups but was significantly higher in PAFR-KO mice at all time points (Figure 3a). In WT implants, the Hb values (μg mg−1 wet tissue) increased from 0.98±0.19 (n=6) at day 7 to 2.78±0.19 (n=7) by day 14 postimplantation, almost three-fold during the period. Over the same time intervals, the Hb levels in the PAFR-KO group started from a higher level at day 7 (1.88±0.53; n=5) and increased by almost the same proportion (three-fold) by day 14 (5.23±0.31; n=5). Morphometric analysis of the vascularity of the implants showed that in WT mice, at any time point, the number of vessels was lower than in implants of PAFR-KO mice. In both groups the number of vessels increased rapidly (day 7), but remained stable after day 10 (Table 1).
Figure 3.
Time course of angiogenesis in the sponge implant of PAFR-KO and WT mice and the effect of the PAF receptor antagonist UK74505 on angiogenesis of 7-day-old sponge implants (b). Angiogenesis as assessed by the Hb content in the implants was markedly increased in PAFR-KO mice (a) and in implants of the animals treated with the PAF receptor antagonist. Results are expressed as mean±s.e.m. of four to eight animals in each group. *P< 0.05; ***P<0.001.
Table 1.
Quantification of angiogenesis by morphometric analysis
| Number of vessels/field | Vascular area(μm2) | |||
|---|---|---|---|---|
| Days postimplantation | WT | PAFR-KO | WT | PAFR-KO |
| 7 | 17±2 | 25±2* | 330±19 | 400±23* |
| 10 | 17±1 | 22±2* | 453±28 | 567±34* |
| 14 | 16±1 | 21±1* | 458±33 | 609±63* |
Data represent mean±s.e.m. The number of vessels was obtained by counting 45 fields (three repetitions of 15 fields) of sponge implants of WT and PAFR KO mice. The vascular area was the sum of lumen area divided by the number of vessels.
P<0.05, significant difference between the values of PAFR-KO and WT mice.
Assessment of the effects of PAF receptor antagonist UK74505 on implant-induced angiogenesis
Similar to the findings in implants of PAFR-KO mice, angiogenesis was increased by treatment with the PAF receptor antagonist. The Hb content in 7-day-old implants from the treated group was 0.84±0.08; n=7 vs 2.2±0.7; n=5 in sponge implants of the vehicle-treated group (Figure 3c).
Kinetics of implant-induced inflammation and proangiogenic molecules in WT and PAFR-KO mice
The accumulation of neutrophils and macrophages were assessed by measuring MPO activity (a surrogate marker for neutrophils) and NAG activity (a surrogate marker for macrophages). As shown in Figure 4a and b, the levels of these activities were markedly lower in the implants of PAFR-KO mice as compared with those of WT animals with a more prominent effect on neutrophils. To determine the influence of PAF on the production of the chemokines CXCL2(KC) and CCL2(MCP-1/JE), mediators of inflammatory cells recruitment, the levels of these molecules were measured in the implant supernatant (Figure 4c and d). The levels of CXCL2(KC) reached a maximum on day 7 and fell progressively in the implants of both groups. Interestingly, the amount of this chemokine was significantly higher in the implants of PAFR-KO mice as compared with the WT group. The levels of CCL2(MCP-1/JE) were also higher in the implants of PAFR-KO mice, but the peak was attained on day 7 for the WT group and on day 10 for the PAFR-KO.
Figure 4.
Time course of inflammatory cell influx and chemokine production in sponge implant in PAFR-KO and WT mice. Neutrophils (a) and macrophages (b) accumulation in the implants were measured as MPO and NAG activities, respectively. In the implants of PAFR-KO mice, the levels of these activities were markedly lower as compared with those of WT mice. Conversely, the levels of the chemokines, CXCL2(KC) and CCL2(MCP1/JE) were higher in the implants of PAFR-KO mice (c and d). Results are expressed as mean±s.e.m. of four to eight animals in each group. *P< 0.05; **P<0.01; ***P<0.001.
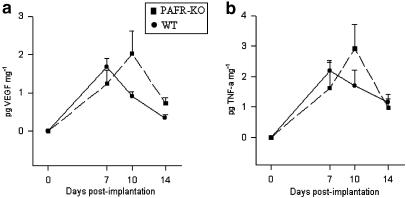
The levels of the proangiogenic cytokines, VEGF and TNF-α (Figure 5a and b) were measured in the implants of both groups of mice. These did not differ between the groups and exhibited similar biphasic profiles; that is, there was an initial increase by day 7 followed by a marked decrease by day 14.
Figure 5.
Time course of proangiogenic cytokines VEGF and production in sponge implant in PAFR-KO and WT mice. The levels of VEGF (a) and TNF-α (b) were similar in both groups. Results are expressed as mean±s.e.m. of four to eight animals in each group.
Discussion
We have studied the sequential development of angiogenesis, inflammation and cytokine production in the fibrovascular tissue induced by sponge implantation in mice lacking the PAF receptor (PAFR-KO). The absence of the PAF receptor gene in this strain was confirmed by the lack of vasoconstrictor response to exogenous application of PAF to the cutaneous vascular bed using a fluorescein diffusion method. This method has been shown to detect pharmacological responses of mature and newly formed vasculatures to local application of several vasomediators (Andrade et al., 1997; Lage & Andrade, 2000; Machado et al., 2002). The PAFR-KO mice also exhibited increased angiogenesis assessed by the Hb content (vascular index) of the sponges and by morphometric analysis. Both parameters have been extensively employed to study neovascularization in a variety of animal models, including the sponge implants (Passaniti et al., 1992; Hu et al., 1995). The apparent discrepancy between stable number of vessels and progressive increase in the Hb content may be explained by maturation and functional state of the blood vessels at later stages (days 10 and 14). Interestingly, increased angiogenesis was observed in implants of WT mice treated systemically with the PAF receptor antagonist, UK74505. This result rules out the possibility of developmental consequences of PAF receptor deficiency. However, this finding is in disagreement with the study by Jackson et al. (1998), which showed the inhibition of angiogenesis in the murine air pouch granuloma by Ro 24-4736, another PAF receptor antagonist. Possible explanations for this discrepancy may lie in the differences in the animal model, compound and dosing schedule. It is particularly relevant to note that the inflammatory stimulus (croton oil and Freunds adjuvant) used by Jackson et al. (1998) was more intense than that provided in our experiments (simple presence of sponge) and that they reported an increase in granuloma size, whereas in our experiments, no modification of granuloma size was apparent, after PAF antagonism (data not shown). It is possible that the effect of PAF as a pro- or antiangiogenic mediator could vary with the inflammatory environment in which the angiogenesis occurs. The vascular changes observed in the knockout mice were accompanied by decreased signs of inflammation. It is worth noting that angiogenesis, usually considered to be a direct consequence of inflammatory events such as cell infiltration and activation (Jackson et al., 1997), was in fact increased above normal in our PAFR-KO mice where leukocyte accumulation was decreased below normal. This finding is in marked contrast to the stimulation of angiogenesis induced by PAF in vitro and in vivo (Andrade et al., 1992; Bussolino et al., 1995; Camussi et al., 1995; Montrucchio et al., 2000a, 2000b). Another discrepant outcome of our results was that although there was increased angiogenesis in the implants of PAFR-KO mice, the levels of neither of the proangiogenic cytokines, VEGF and TNF-α, was changed in this strain. Both VEGF and TNF-α play important roles in angiogenesis (Frater-Schröder et al., 1987; Brown et al., 1997) and PAF stimulates the expression of growth factors and chemokines in a variety of tissues (Bussolino et al., 1995; Zhixing et al., 1995). It is clear that, in the PAFR-KO mice, the levels of VEGF or of TNF-α normally induced, that is, the same levels as in WT mice, were not rate limiting for angiogenesis and further that PAF was not a crucial stimulus for these cytokines to be produced. Of course there are other endogenous stimuli for the production of these cytokines. For instance, VEGF is abundantly produced by hypoxic tumor cells and activated endothelial cells, macrophages and other cells of the immune system (Brown et al., 1997) and VEGF output from mouse fibroblasts in culture was controlled by the products of cyclo-oxygenase-2(COX-2) (Williams et al., 2000). The delayed peak of the cytokines production in PAFR-KO mice is more apparent than real because VEGF or TNF-α did not differ from days 7 to 10.
In animals lacking PAF receptors throughout development and growth, compensatory pathways for the absence of its actions are likely to emerge. However, our results do suggest that changes in cytokine or chemokine generation and signaling and in PAF's effects are selectively influenced in the PAFR-KO strain.
PAF is an endogenous phospholipid capable of mediating many different biological events. Most of these events are concerned with inflammation, but PAF also has pathophysiological effects in the reproductive, nervous and cardiovascular systems (Montrucchio et al., 2000a, 2000b; Ishii & Shimizu, 2000). In addition, PAF plays important roles in cellular growth and transformation (Bennett et al., 1993; Roth et al., 1996; Kume & Shimizu, 1997). Our results clearly showed that the absence of the PAF receptor and therefore of the effects of its activating ligand, led to a decrease in the accumulation of leukocytes (neutrophils and macrophages) in the sponge implant.
This decrease in proinflammatory cell infiltration in the PAFR-KO mice is fully compatible with the proinflammatory actions attributed to PAF in normal WT mice (Venable et al., 1993; Ford et al., 1989; Jackson et al., 1998). The decreased leukocyte accumulation was most clearly marked in the neutrophil component that was well below the corresponding levels in the WT mice by 7 days postimplantation and continued at a low level throughout the experimental period (14 days). Macrophage levels were decreased only towards the end of this period, between 10 and 14 days. Furthermore, the magnitude of the decrease in macrophage activity was much smaller than that in neutrophils, suggesting a degree of selectivity in the loss of underlying inflammatory signals.
It was therefore unexpected to find that in the PAF-KO mice, the levels of the relevant chemoattractant cytokines, the chemokines CXCL2(KC) and CCL2(MCP-1/JE), were higher than in the WT mice. These chemokines, in WT mice, serve as chemotatic agents for neutrophils and macrophages, respectively, and increased levels of chemokines are associated with increased accumulation of the relevant leukocyte, in vitro or in vivo (Rollins, 1997). Thus, in our PAFR-KO mice, the coincidence of decreased leukocyte accumulation and increased chemokine levels suggests that the leukocytes in the PAFR-KO mice are, in some way, defective in their response to the chemoattractant stimuli. Two possible explanations for such a defect are a fault in the chemokine receptors or a fault in the intracellular signaling pathways leading from the activated receptors to the eventual cellular kinesis. The fault in the receptor itself could be expressed as a reduced number of normal receptors or as abnormal receptors with reduced affinity for the ligands. Perhaps the more likely alternative, defective intracellular signaling, would be compatible with the intracellular actions that PAF is known to exhibit in cells from normal WT animals. If PAF were to act at the same receptor intracellularly as it activates in cell membrane, then the PAFR-KO mice could indeed be unable to transduce fully the activation of the chemokine receptors into cellular chemokinesis. A recent work (Marrache et al., 2002) provides evidence for a nuclear membrane receptor for PAF closely related to the cell membrane receptor in so far it was recognized by the same antibodies and the same antagonists. The activation of this internal PAF receptor caused the activation of the (extracellular signal-regulated kinases) extracellular-regulated kinase, nuclear factor-κB, and of inducible nitric oxide synthase and COX-2 pathways (Marrache et al., 2002). Decreased stimulation of such kinase cascades, consequent on the loss of internal PAF receptor in the PAFR-KO mice, could reasonably lead to the loss of coupling between chemokine receptors and cellular kinesis.
Apart from possible mechanisms of the defective response, the discrepancy between chemokine level and cell accumulation raises another interesting question – is the increased production of chemokines indicative of a loss of a negative feedback system in PAFR-KO mice? From existing experimental results, a normal concentration–response relation between chemokine concentration and cell accumulation has been assumed and generally observed. However, in our PAFR-KO mice, the production of the chemokines was higher than in WT mice even though one proinflammatory mediator, PAF, had been made ineffectual. A negative feedback signal emanating from the attracted leukocytes would appear to be a logical form of control of chemoattractant activity but there has been no model, so far, in which such a control has been implied or expressed. Nevertheless, in our model of angiogenesis, the implantation of the sponge and the loss of PAF signalling through the PAF receptor stimulated rather than inhibited the processes of neovascularization.
Our results may suggest that the accumulation of leukocytes may be a source of antiangiogenic signals under normal conditions, that is, in WT animals. Thus, when leukocytes do not accumulate in an angiogenic/inflammatory site, the process of angiogenesis proceeds unchecked, to a greater extent than in normal animals. Some support for this ‘leukocyte-dependent' inhibition of angiogenesis is provided by the finding of antiangiogenic angiostatin kringle 1–3 in leukocyte cultures (Scapini et al., 2002).
We undertook these experiments in PAFR-KO mice expecting to confirm the positive role of PAF in inflammation and angiogenesis. Our results have confirmed the proinflammatory effects of endogenous PAF but also have unexpectedly shown that endogenous PAF functions as an inhibitor of the process of angiogenesis in our sponge model. Moreover, the inflammatory cells accumulating at the site of neovascularization may serve as source of endogenous inhibitors of angiogenesis in addition to being sources of stimulators of this process. These unexpected roles of PAF and of the infiltrating inflammatory cells will need to be confirmed in other models using other proinflammatory stimuli before their overall relevance to the pathophysiology of angiogenesis can be fully assessed.
Acknowledgments
This work was supported by CNPq, FAPEMIG, PRONEX (Brazil).
Abbreviations
- CCL2(MCP-1/JE)
chemokine monocyte chemoattractant protein 1
- COX-2
cyclo-oxygenase-2
- CXCL2(KC)
keratinocyte-derived chemokine
- ERK
extracellular-regulated kinase
- Hb
hemoglobin
- MPO
myeloperoxidase
- NAG
N-acetyl-β-D-glucosaminidase
- NF-κB
nuclear factor-κB
- NOS
nitric oxide synthase
- TNF-α
tumor necrosis factor-α
- PAF
platelet-activating factor
- PAFR-KO
PAF receptor-deficient mice
- VEGF
vascular endothelial growth factor
- WT
wild type
References
- ALABASTER V.A., KEIR R.F., PARRY M.J., DE SOUZA R.N. UK74505, a novel and selective PAF antagonist, exhibits potent and long lasting activity in vivo. Agents Actions (Suppl.) 1991;34:221–227. [PubMed] [Google Scholar]
- ANDRADE S.P., MACHADO R.D.P., TEIXEIRA A.S., BELO A.V., TARSO A.M., BERALDO W.T. Sponge-induced angiogenesis in mice and the pharmacological reactivity of the neovasculature quantitated by a fluorimetric method. Microvasc. Res. 1997;54:253–261. doi: 10.1006/mvre.1997.2047. [DOI] [PubMed] [Google Scholar]
- ANDRADE S.P., VIEIRA L.B.G.B., BAKHLE Y.S., PIPER P.J. Effects of platelet activating factor (PAF) and other vasoconstrictors on a model of angiogenesis in the mouse. Int. J. Exp. Pathol. 1992;73:503–513. [PMC free article] [PubMed] [Google Scholar]
- BAILEY P.J. Sponge implants as models. Method Immunol. 1988;162:327–334. doi: 10.1016/0076-6879(88)62087-8. [DOI] [PubMed] [Google Scholar]
- BEN EZRA D. Neovasculogenic ability of prostaglandins, growth factors and synthetic chemoattractants. Am. J. Ophthalmol. 1978;86:455–461. doi: 10.1016/0002-9394(78)90289-1. [DOI] [PubMed] [Google Scholar]
- BENNETT S.A., LEITE L.C., BIRNBOIM H.C. Platelet activating factor, an endogenous mediator of inflammation, induces phenotypic transformation of rat embryo cells. Carcinogenesis. 1993;14:1289–1296. doi: 10.1093/carcin/14.7.1289. [DOI] [PubMed] [Google Scholar]
- BROWN L.F., DETMAR M., CLAFFEY K., NAGY J.A., FENG D., DVORAK A.M., DVORAK H.F. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Experientia Supplementum (Basel) 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- BUSSOLINO F., ARESE M., MONTRUCCHIO G., BARRA L., PRIMO L., BENELLI R., SANAVIO F., AGLIETTA M., GHIGO D., ROLA-PLESZZCZYNSKI M., BOSIA A., ALBINI A., CAMUSSI G. Platelet activating factor produced in vitro by Kaposi's sarcoma cells induces and sustains in vivo angiogenesis. J. Clin. Invest. 1995;96:940–952. doi: 10.1172/JCI118142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMUSSI G., MONTRUCCHIO G., LUPIA E., DE MARTINO A., PERONA L., ARESE M., VERCELLONE A., TONIOLO A., BUSSOLINO F. Platelet activating factor directly stimulates in vitro migration of endothelial cells and promotes in vivo angiogenesis by a heparin-dependent mechanism. J. Immunol. 1995;154:6492–6501. [PubMed] [Google Scholar]
- DRABKIN D.L., AUSTIN J.H. Spectrophotometric constants common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. 1932;98:719–733. [Google Scholar]
- FRATER-SCHRÖDER M., RISAU W., HALLMANN R., GAUTSCHI P., BÖHLEN P. Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKMAN J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- FORD H.R., HOFFMAN R.A., WING E.J., MAGEE M., MCINTYRE L., SIMMONS R. Characterization of wound cytokines in the sponge matrix model. Arch. Surg. 1989;124:1422–1428. doi: 10.1001/archsurg.1989.01410120068014. [DOI] [PubMed] [Google Scholar]
- GRIFFIOEN A.W., MOLEMA G. Angiogenesis: potentials for pharmacological intervention in the treatment of cancer, cardiovascular diseases and chronic inflammation. Pharmacol. Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- HU D.E., HILEY C.R., SMITHER R.L., GRESHAM G.A., FAN T.P. Correlation of 133Xe clearance, blood flow and histology in the rat sponge model for angiogenesis. Lab. Invest. 1995;72:601–610. [PubMed] [Google Scholar]
- ISHII S., KUWAKI T., NAGASE T., MAKI K., TASHIRO F., SUNAGA S., CAO W.H., KUME K., FUKUCHI Y., MIYAZAKI J., KUMADA M., SHIMIZU T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII S., SHIMIZU T. Platelet-activating factor (PAF) and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- JACKSON J.R., BOLOGNESE B., MANGAR C.A., HUBBARD W.C., MARSHALL L.A., WINKLER J.D. The role of platelet activating factor and other lipid mediators in inflammatory angiogenesis. Biochem. Biophy. Acta. 1998;1392:145–152. doi: 10.1016/s0005-2760(98)00012-5. [DOI] [PubMed] [Google Scholar]
- JACKSON J.R., SEED M.P., KIRCHER C.H., WILLOUGHBY D.A., WINKLER J.D. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- KANAYASU T., NAKAO-HAYASHI J., ASUWA N., MORITA I., ISHII T., ITO H., MUROTA S. Leukotriene C4 stimulates angiogenesis in bovine carotid artery endothelial cells in vitro. Biochem. Biophys. Res. Commun. 1989;159:572–578. doi: 10.1016/0006-291x(89)90032-6. [DOI] [PubMed] [Google Scholar]
- KUME K., SHIMIZU T. Platelet activating factor (PAF) induces growth stimulation, inhibition, and suppression of oncogenic transformation in NRK cells overexpressing the PAF receptor. J. Biol. Chem. 1997;272:22898–22904. doi: 10.1074/jbc.272.36.22898. [DOI] [PubMed] [Google Scholar]
- LAGE A.P., ANDRADE S.P. Assessment of angiogenesis and tumor growth in conscious mice by a fluorimetric method. Microvasc. Res. 2000;59:278–285. doi: 10.1006/mvre.1999.2217. [DOI] [PubMed] [Google Scholar]
- MACHADO R.D.P, FERREIRA M.A.N.D., BELO A.V., SANTOS R.A.S., ANDRADE S.P. Vasodilator effect of angiotensin-(1-7) in mature and sponge-induced neovasculature. Regul Peptides. 2002;107:105–113. doi: 10.1016/s0167-0115(02)00070-8. [DOI] [PubMed] [Google Scholar]
- MACHADO R.D.P, SANTOS R.A.S., ANDRADE S.P. Mechanisms of angiotensin-(1-7)-induced inhibition of angiogenesis. Am. J. Physiol. Regul. Integ. Comp. Physiol. 2001;280:R994–R1000. doi: 10.1152/ajpregu.2001.280.4.R994. [DOI] [PubMed] [Google Scholar]
- MARRACHE A.M., GOBEIL F., Jr, BERNIER S.G., STANKOVA J., ROLA-PLESZCZYNSKI M., CHOUFANI S., BKAILY G., BOURDEAU A., SIROIS Mg., VASQUEZ-TELLO A., FAN L., JOYAL J.S., FILEP J.G., VARMA D.R., RIBEIRO-DA-SILVA A., CHEMTOB S. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J. Immunol. 2002;169:6474–6481. doi: 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- MONTRUCCHIO G., ALLOATTI G., CAMUSSI G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 2000a;80:1669–1699. doi: 10.1152/physrev.2000.80.4.1669. [DOI] [PubMed] [Google Scholar]
- MONTRUCCHIO G., LUPIA E., BATTAGLIA E., DEL SORBO L., BOCCELLINO M., BIACONE L., EMANUELLI G., CAMUSSI G. Platelet-activating factor enhances VEGF-induced endothelial cell motility and neo-angiogenesis in a murine matrigel model. Arterioscler. Thromb. Vasc. Biol. 2000b;20:80–88. doi: 10.1161/01.atv.20.1.80. [DOI] [PubMed] [Google Scholar]
- MORO L., MARTINS A.S., ALVES C.M., SANTOS F.G.A., NUNES J.E.S., CARNEIRO R.A., CARVALHO R., VASCONCELOS A.C. Apoptosis in canine distemper. Arch. Virol. 2003;148:153–164. doi: 10.1007/s00705-002-0903-6. [DOI] [PubMed] [Google Scholar]
- PARRY M.J., ALABASTER V.A., CHEESEMAN H.E., COOPER K., DE SOUZA R.N., KEIR R.F. Pharmacological profile of UK-74,505, a novel and selective PAF antagonist with potent and prolonged oral activity. J. Lipid Mediat. Cell Signal. 1994;10:251–268. [PubMed] [Google Scholar]
- PASSANITI A., TAYLOR R.M., PILI R., GUO Y., LONG P.V., HANEY J.A., PAULY R.R., GRANT D.S., MARTIN G.R. A simple, quantitative method for assessing angiogenesis and antiangiogenesis agents using reconstituted basement membrane, heparin and fibroblast growth factor. Lab. Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- ROLLINS B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- ROTH M., NAUCK M., YOUSEFI S., TAMM M., BLASER K., PERRUCHOUD A.P., SIMON H.U. Platelet-activating factor exerts mitogenic activity and stimulates expression of interleukin 6 and interleukin 8 in human lung fibroblasts via binding to its functional receptor. J. Exp. Med. 1996;184:191–201. doi: 10.1084/jem.184.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAPINI P., NESI L., MORINI M., TANGHETTI E., BELLERI M., NOONAN D., PRESTA M., ALBINI A., CASSATELLA M.A. Generation of biologically active angiostatin kringle 1-3 by activated human neutrophils. J. Immunol. 2002;168 Suppl. 11:5798–5804. doi: 10.4049/jimmunol.168.11.5798. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOPANOGLOU N.E., PIPILI-SYNETOS E., MARAGOUDAKIS M.E. Leukotrienes C4 and D4 promote angiogenesis via a receptor-mediated interaction. Eur. J. Pharmacol. 1994;258:151–154. doi: 10.1016/0014-2999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- VENABLE M.E., ZIMMERMAN G.A., McINTYRE T.M., PRESCOTT S.M. Platelet-activating factor: a phospholipid autacoid with diverse actions. J. Lipid Res. 1993;34:691–702. [PubMed] [Google Scholar]
- WILLIAMS C.S., TSUJII M., REESE J., DEY S.K., DUBOIS R.N. Host cyclooxygenase-2 modulates carcinoma growth. J. Clin. Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHIXING P.K., KRAVCHENKO V.V., YE R.D. Platelet activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes. J. Biol. Chem. 1995;270:7787–7790. doi: 10.1074/jbc.270.14.7787. [DOI] [PubMed] [Google Scholar]