Abstract
The gastrointestinal (GI) tract is exposed to a large array of proteases, under both physiological and pathophysiological conditions. The discovery of G protein-coupled receptors activated by proteases, the protease-activated receptors (PARs), has highlighted new signaling functions for proteases in the GI tract, particularly in the domains of inflammation and pain mechanisms. Activation of PARs by selective peptidic agonists in the intestine or the pancreas leads to inflammatory events and changes in visceral nociception, suggesting that PARs could be involved in the modulation of visceral pain and inflammation. PARs are present in most of the cells that are potentially actors in the generation of irritable bowel syndrome (IBS) symptoms. Activation of PARs interferes with several pathophysiological factors that are involved in the generation of IBS symptoms, such as altered motility patterns, inflammatory mediator release, altered epithelial functions (immune, permeability and secretory) and altered visceral nociceptive functions. Although definitive studies using genetically modified animals, and, when available, pharmacological tools, in different IBS and inflammatory models have not yet confirmed a role for PARs in those pathologies, PARs appear as promising targets for therapeutic intervention in visceral pain and inflammation processes.
Keywords: Inflammation, pain, visceral hypersensitivity, irritable bowel syndrome, thrombin, trypsin, tryptase, proteases
Introduction
Proteases represent 2% of the human genome and are present at particularly high levels in the gastrointestinal (GI) tract (Caughey, 1995). Thus, it is not surprising if, in addition to their role in protein degradation and/or digestion, certain proteases exert also a role as signaling molecules, regulating cell functions by cleaving receptors activated upon proteolysis. Those receptors constitute a family of G protein-coupled receptors (four members have been cloned thus far), which are called protease-activated receptors (PARs). Activation of those receptors has been shown to interfere with inflammatory and nociceptive pathways in different tissues (Vergnolle et al., 2001b). Understanding the functional role of PARs in a system as exposed to proteases as the GI tract represents an important and exciting challenge that could lead to define new pharmacological targets for GI diseases in general, and in particular for pathologies associating inflammatory and visceral pain disorders.
PAR structure and activation
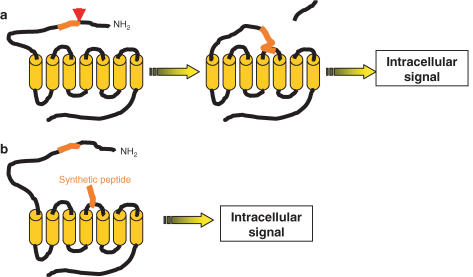
PARs are activated by a unique mechanism that first involves recognition of an extracellular domain of the receptor situated on the N-terminus, by a protease (Rasmussen et al., 1991; Vu et al., 1991; Ishihara et al., 1997; Xu et al., 1998; Coughlin, 1999). Some proteases such as thrombin, bind the receptors. Others, such as trypsin or tryptase, do not need to bind the receptor in order to cleave it. Then, cleavage by proteolysis of this recognized site occurs, which exposes a new N-terminus domain that acts as a tethered ligand domain interacting with domains situated in the second extracellular loop of the receptor, to induce an intracellular signal (see Figure 1a). PARs respond to a variety of proteases, although thrombin for PAR1, PAR3 and PAR4, and trypsin for PAR2 and PAR4 are usually regarded as the main activators of PARs. Proteases of the coagulation cascade such as factor Xa and VIIa can activate PAR2 and PAR4 (Dery et al., 1998; Coughlin, 1999; Hollenberg & Compton, 2002). However, co-factors such as tissue factor are needed to enhance the ability of factor VIIa to activate PAR2. Cathepsin G released from neutrophils triggers PAR4 activation and can activate PAR1. Tryptase released from mast cells can activate PAR2, while bacterial proteases such as gingipains can activate PAR1, PAR2 and PAR4 (Dery et al., 1998; Coughlin, 1999; Hollenberg & Compton, 2002). Proteases released by dust mites are also able to activate PAR2 (see Table 1) (Lourbakos et al., 2001; Asokananthan et al., 2002). Soluble forms of integral membrane proteases such as membrane-type serine protease 1 can also activate PARs in general, and PAR2 in particular. Since PAR2 and membrane-type serine protease 1 have similar tissue distribution, this further suggests a role for intra-membrane-type serine protease 1 as an endogenous activator of PAR2. Although some proteases are common to the activation of different PARs, a single protease agonist activates distinct receptors with different potencies. For instance, thrombin can activate PAR1, PAR3 and PAR4, with a highest potency for PAR1, then a lower potency for PAR3, and is finally weaker for the activation of PAR4 (for reviews, see Dery et al., 1998; Coughlin, 1999; Hollenberg & Compton, 2002). In vivo activation of PARs requires the release of active proteases in the vicinity of the receptors. Depending on the physiological or pathophysiological situation, different proteases might activate the same receptor. Determining which proteases are endogenous activators of PARs in selected pathologies would be of great importance for the potential use of protease inhibitors as therapeutic options.
Figure 1.
Mechanism of activation of PARs. (a) Proteases cleave the extracellular N-terminus domain to release a new N-terminus domain that acts as a tethered ligand binding and activating the receptor to induce an intracellular signal. (b) Synthetic peptides corresponding to the tethered ligand domain can mimic the effects of the proteolytically cleaved N-terminal domain to specifically activate the receptor.
Table 1.
Structure and activation of protease-activated receptors
| Number of amino acids | Tethered ligand sequence | Activating protease | Peptidic selective agonists | Antagonists | |
|---|---|---|---|---|---|
| PAR1 | 425 aa | Human: SFLLRMouse/rat: SFFLR | Thrombin, trypsin, cathepsin G, granzyme A, factor VIIa, factor Xa, gingipain, plasmin | TFLLR ApfFRChaCitY | RWJ-56110RWJ-58259SCH-79797SCH-73754FR-171113BMS-200261‘Merck isoxazole 1'Trans-cinnamoyl-(p-F-Phe)-(p-guanidino-Phe)-LRR |
| PAR2 | 397 aa | Human: SLIGKVMouse/rat: SLIGRL | Trypsin, tryptase, factor Xa, factor VIIa, tissue factor, acrosin, trypsin IV, membrane-type serine protease 1, dust mite proteases | SLIGRLTrans-cinnamoyl-LIGRLO 2-furoyl-LIGRLO | |
| PAR3 | 374 aa | Human: TFRGAPMouse: SFNGGP | Thrombin, trypsin | ||
| PAR4 | 385 aa | Human: GYPGQVMouse: GYPGKF | Thrombin, trypsin, cathepsin G, factor VIIa, factor Xa, gingipain, trypsin IV | GYPGKFGYPGFKGYPGQVAYPGKF | Trans-cinnamoyl-YPGKF Palmitoyl-SGRRYGHALR |
With the exception of PAR3, synthetic peptides corresponding to the tethered ligand domain released upon proteolytic activation of PAR1, PAR2 and PAR4 can directly activate the receptor (see Figure 1b) (Hollenberg & Compton, 2002). Those peptides constitute important pharmacological tools to understand the functions of PARs. Pharmacological studies have determined optimum peptidic sequences for selectivity and potency of PAR activation (see Table 1). For example, the tethered ligand peptide SFLLR, which corresponds to the human PAR1, has been shown to also activate PAR2, but a simple replacement of the serine residue by a threonine residue (TFLLR peptide) gives a more selective peptidic agonist for PAR1 activation. Synthetic peptide corresponding to the mouse PAR4 (GYPGKF) constitutes a selective agonist for mouse PAR4, but substitution of the G residue by an A residue (AYPGKF peptide) creates a selective PAR4 agonist, at least 10 times more potent than the tethered ligand peptide (Faruqi et al., 2000).
The understanding of the physiological and pathophysiological role of PARs has been hampered by difficult pharmacologic intervention to inhibit the activation of PARs. Different strategies and extensive chemical synthetic efforts have been employed to develop PAR antagonists (reviewed in Derian et al., 2003). Several PAR1 antagonists are now available (see Table 1), and their use has demonstrated the prime role for PAR1 in thrombotic and restenotic events. However, no studies have reported the use of PAR2 or PAR3 antagonists in bioassays or in vivo studies. Two peptidic PAR4 antagonists have been tested in platelet assays (see Table 1), but their pharmacological properties in other tissues and bioassays still have to be established. Gene-deletion approach with the use of PAR-deficient mice appears as an alternate reliable approach to understand the role of PARs.
Proteases and PARs in the GI tract
PARs have been detected in several cell types throughout the entire GI tract and pancreas (see Figure 3 and Table 2). PAR1, PAR2 and PAR4 have been detected in enterocytes (Kong et al., 1997; Buresi et al., 2001; Mule et al., 2004), where PAR1 and PAR2 have been shown to be functional both on the apical and baso-lateral membranes (Kong et al., 1997; Chin et al., 2003). PAR1 and PAR2 are also expressed in human colon cancer cell lines where their activation modulates proliferation and motility (Darmoul et al., 2001; 2003). PAR1 and PAR2 have been detected in enteric neurons (extrinsic and intrinsic submucosal and myenteric neurons), where they co-express with neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) (reviewed in Vergnolle et al., 2003c). Although its expression on enteric neurons has not been clearly established, functional PAR4 seems to be present on enteric neurons, which respond to PAR4 agonists by evoking a depolarizing response in the guinea-pig small intestine (Gao et al., 2002). PAR1 and PAR2 have also been shown to be expressed in intestinal myofibroblasts (Seymour et al., 2001; 2003) and in mast cells (D'Andrea et al., 2000). The fact that PAR1, PAR2 and PAR4 agonists cause contraction and/or relaxation in isolated GI segments suggest that those receptors are expressed in smooth muscle cells (Cocks et al., 1999; Kawabata et al., 2000a,2000b; Gao et al., 2002; Mule et al., 2002b; 2004; Zhao & Shea-Donohue, 2003). However, only the expression of PAR2 in intestinal myocytes and PAR1 in irradiated intestinal smooth muscle cells has been clearly established (Corvera et al., 1997; Wang et al., 2002). PAR1, PAR2 and PAR4 expression both on endothelial surfaces and leukocytes has also been demonstrated (Hou et al., 1998; Vergnolle et al., 2002). Messengers of RNA for PAR1 and PAR2 have been found in parotid, sublingual and submaxillary glands, and PAR2 protein has been detected in the pancreatic duct epithelium and pancreatic acinar cells (Nguyen et al., 1999; Kawabata et al., 2000c,2000d; 2002; Kawabata, 2003).
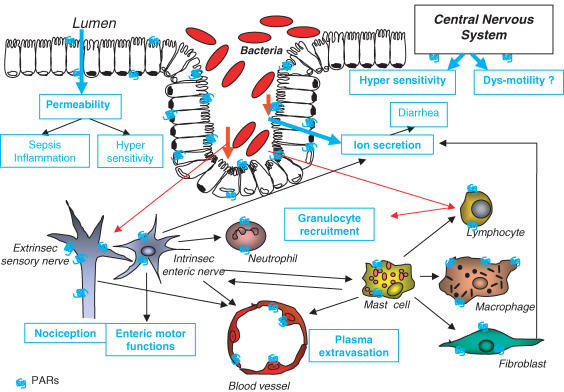
Figure 3.
Interactions of PARs with components of the pathogenesis of IBS. PAR1 and PAR2 are present on enterocytes, where their activation potentially induces an increase in permeability, which could lead to bacterial translocation, sepsis and inflammation, as well as hypersensitivity. Direct activation of PAR1 and PAR2 on enteric neurons interferes with nociceptive and motor functions. Activation of PARs on cells involved in inflammatory responses (mast cells, neutrophils, lymphocytes, macrophages, fibroblasts, endothelium or neuropeptides containing neurons) could lead to the generation of inflammatory mediators and provokes further signs of inflammation such as granulocyte recruitment and plasma extravasation. Ion secretion and potentially water flux disturbances (diarrhea) could be evoked by PAR activation on enterocytes, fibroblasts or enteric nerves. PAR activation in the central nervous system modulates nociceptive responses to peripheral stimulation, playing a potential role in hypersensitivity states and eventually (although never studied) in enteric motor dysfunctions. Activation of PARs on enterocytes, enteric neurons or lymphocytes by bacterial proteases from luminal or infiltrated pathogens could also participate in the generation of symptoms associated with IBS.
Table 2.
Localization and function of protease-activated receptors in the GI tract
| Localization in the GI tract | Known functions | |
|---|---|---|
| PAR1 | Enterocytes | Increase permeability, apoptosis, chloride secretion, prostaglandin release |
| Human colon cancer epithelium | Proliferation and motility | |
| Myenteric neurons | Suppression of fast excitatory postsynaptic potential | |
| Submucosal neurons | Inhibition of chloride secretion, | |
| Fibroblasts | Prostaglandin release | |
| Mast cells | ||
| Smooth muscle | Relaxation/contraction | |
| Endothelium | Gap formation | |
| PAR2 | Enterocytes | Chloride secretion, prostaglandin production, eicosanoid production |
| Human colon cancer epithelium | Proliferation | |
| Myenteric neurons | Neuropeptide release, increased excitability, suppression of fast excitatory postsynaptic potential | |
| Submucosal neurons | Neuropeptide release, stimulate epithelial ion secretion, hyperexcitability | |
| Fibroblasts | Prostaglandin release, proliferation | |
| Mast cells | ||
| Smooth muscle | Relaxation/contraction | |
| Pancreatic duct epithelium | Ion channel activation | |
| Pancreatic acinar cells | Amylase secretion | |
| Endothelium/leukocyte interface | Rolling, adhesion, transmigration, gap formation | |
| PAR3 | Detected by RT–PCR in whole GI tissues (stomach and small intestine), but unidentified cell type | |
| PAR4 | Enterocytes | |
| Submucosa | Contraction of longitudinal muscle | |
| Enteric neurons | Depolarization | |
| Endothelium/leukocyte interface | Rolling, adhesion, transmigration |
The GI tract and pancreas are particularly exposed to a large array of proteases (see Figure 2). Trypsin is released in the upper GI tract lumen, and in pancreatic duct under its inactive (proactive) form trypsinogen, for physiological digestive purposes. On mucosal surfaces, a balance between proteolytic activity and the presence of protease inhibitors such as pancreatic secretory trypsin inhibitor (PSTI) is constantly present. PSTI, which is released by mucus-secreting cells throughout the GI tract, prevents premature activation of pancreatic proteases, and protects mucosal surfaces from exposure to active proteolytic enzymes (Marchbank et al., 1996; 1998). Thus, the balance between proteolytic activity in the center of the lumen and the presence of protease inhibitors at mucosal surfaces warrants in the upper intestine efficient digestive processes and mucosal protection. In the lower GI tract, trypsin is not released into the lumen for digestive purposes. However, inflammatory bowel disease (IBD) patients showed an increased trypsin activity in their colonic luminal content, suggesting that the balance between tryptic proteolytic activity and protease inhibitors is broken in this particular pathophysiological situation, and that trypsin is present in the lumen of the lower GI tract, associated with inflammatory conditions. Trypsinogen can also be synthesized by several different extrapancreatic cell types including endothelium (Koshikawa et al., 1997; 1998). Trypsinogen IV and trypsin IV, which have been shown to activate PAR2 and PAR4 (see Table 1), have been found in epithelial cell lines from prostate, colon and airways (Cottrell et al., 2003; 2004). Thus, it is conceivable that endothelium- or epithelium-derived trypsin can also be present on the basolateral side of the intestinal barrier. Tryptase, which is expressed by almost all subsets of human mast cells (Caughey, 1995), is released upon mast cell degranulation. Although tryptase is considered poorly diffusible, in pathologies such as intestinal inflammation, allergy, or even stress, large amounts of tryptase have been found in the vasculature and the gut lumen of patients or animals (Plebani et al., 1992; Miller, 1996; Santos et al., 1998; 1999; Gelbmann et al., 1999). Proteases of the coagulation cascade such as thrombin, factor VIIa and Xa are also potentially present in the GI tract during inflammation or tissue trauma. These events have been shown to lead to the presence of pro-thrombin and active thrombin into the intestinal lumen and deeper within the intestinal tissues (McHugh et al., 1996), where it could activate PARs. Cathepsin G, which is released upon neutrophil activation, can also be massively present in GI tissues associated with inflammatory conditions. The transepithelial migration of neutrophils towards mucosal surfaces upon inflammation (Edens et al., 2002) also renders plausible the presence of cathepsin G into the gut lumen. Finally, mucosal surfaces are constantly exposed to bacterial products, and particularly to bacterial proteases. Although no studies have reported the effects of intestinal bacteria (pathogens or nonpathogens) on PAR activation, studies have reported the possible activation of PAR1, PAR2 and PAR4 by proteases from pathogens such as Porphyromonas gingivalis (Lourbakos et al., 2001) or dust mites (Sun et al., 2001; Asokananthan et al., 2002). Thus, a variety of proteases may act on PARs and influence GI functions at several levels depending on the PAR-expressing target cells. Whether PARs are activated in physiological or pathophysiological settings might depend on the proximity of the receptors to digestive enzymes, inflammation-associated protease or proteases released by pathogen or nonpathogen organisms. However, several studies using different PAR agonists and antagonists point to a role for PARs in inflammatory and nociceptive mechanisms of the GI tract.
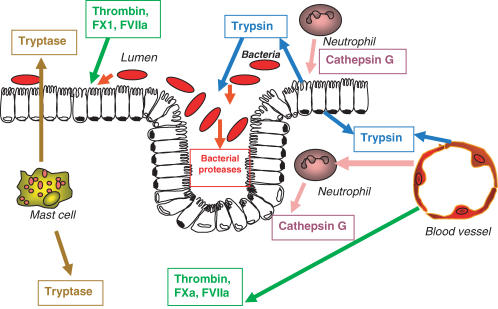
Figure 2.
Presence of proteases in the GI tract. Trypsin is released in the lumen of the GI tract for digestive purposes, but can also be present deeper into the tissues released from endothelial or epithelial cells. Proteases of the coagulation cascade such as thrombin, factor Xa and VIIa can be released into the lumen or the GI tissues upon tissue damage. When they degranulate, mast cells release massive amounts of tryptase both in the gut lumen and vasculature. Neutrophils present either in the tissues or translocated in the lumen release cathepsin G. Epithelial cells are exposed to bacterial proteases from the luminal side.
Proteases and PARs: role in inflammation of the GI tract
Direct injection of thrombin, trypsin, tryptase or selective agonists for PAR1 and PAR2 into the paw of rodents produces edema and granulocyte infiltration, two of the main features of inflammation (Cirino et al., 1996; Vergnolle et al., 1999a,1999b). Since those receptors and proteases are also highly present in the GI tract, they could be involved in GI inflammatory processes.
Intestine
Recently, we have shown that luminal administration of selective peptidic agonists for PAR1 (TFFLR), PAR2 (SLIGRL) and PAR4 (AYPGKF) provoked a colonic inflammation within a few hours (from 4 to 24 h). This inflammation was characterized by an increased wall thickness, the presence of erythema and significant infiltration of granulocytes (Cenac et al., 2002; Ferazzini et al., 2004; Vergnolle et al., 2004). PAR2-induced colitis was dependent on sensory neuron activation, substance P and CGRP release (Cenac et al., 2003; Nguyen et al., 2003). Intracolonic administration of PAR2 also resulted in increased paracellular permeability, as observed by the passage of 51Cromium-EDTA from the lumen to the blood and by the presence of translocated bacteria in different intraperitoneal organs (Cenac et al., 2002). Tight junction blockers and inhibitors of myosin-light-chain kinase blocked this increased permeability without affecting the level of granulocyte recruitment (Cenac et al., 2003). This suggests that although increased permeability and granulocyte infiltration might both participate in the generation of the inflammatory response, they constitute separated events that can occur independently. Activation of PAR1 also caused increased intestinal permeability both in vivo (intracolonic administration of TFLLR) and in vitro (on enterocytes monolayers), by a mechanism involving the induction of apoptosis of the intestinal epithelium, and the activation of tyrosine and myosin-light-chain kinases (Chin et al., 2003). The fact that PAR1 activation can compromise the epithelial barrier function suggests that PAR1 could be implicated in the pathogenesis of a number of disorders affecting mucosal surfaces, including IBD and infectious diseases. Using PAR1-deficient mice, we have been able to show that PAR1 activation is implicated in the pathogenesis of trinitrobenzene sulfonic acid (TNBS)-induced colitis. PAR1−/− mice showed significantly less inflammatory damage than wild-type controls after the induction of TNBS colitis (Vergnolle et al., 2003a). Another recent study showed that PAR1 agonist-induced epithelial cell ion transport was altered after nematode infection, also suggesting a role for PAR1 in infectious intestinal diseases (Fernandez et al., 2003). The fact that PAR2-induced colonic inflammation is regulated by a neurogenic mechanism is in favor of a role for PAR2 in infectious intestinal diseases, if we consider the fact that enteric infections are largely mediated by neurogenic mechanisms (Spiller, 2002; Vergnolle et al., 2003c) and the fact that PAR2 can be activated by bacterial proteases (Lourbakos et al., 2001; Sun et al., 2001; Asokananthan et al., 2002). However, no study has reported yet such role for PAR2. In IBD models, however, activation of the enteric nervous system (ENS) and further release of neuropeptides protects against inflammatory damage (Collins, 2000). Thus, in the setting of chronic inflammation, PAR2-induced ENS activation might exert protective effects rather than pro-inflammatory effects. This hypothesis is further supported by the findings of Fiorucci et al. (2001), who have observed that daily systemic treatments with PAR2 agonists diminished inflammatory damage caused by the intracolonic injection of TNBS. These results suggest that PAR2 agonists might be beneficial in the setting of chronic inflammation such as IBD, where they might exert protective effects though ENS activation. However, the use of such agonists might also enhance visceral hypersensitivity (see next chapter), which then would be detrimental, potentially causing more pain to patients. The use of PAR2-deficient mice in different models of infectious colitis or IBD should help, in the very near future, to clarify the role of PAR2 in those pathologies. However, the overexpression of PAR2 observed in biopsies from ulcerative colitis patients strongly suggests a role for PAR2 in IBD (Kim et al., 2003). We have reported that treatment of mice with the PAR4 antagonist palmitoyl-SGRRYGHALR reduced inflammation induced by dextran sodium sulfate (Ferazzini et al., 2003). However, the specificity of such antagonist could still be questioned and only experiments performed with PAR4-deficient mice would completely clarify the role of PAR4 in IBD models.
Stomach
PAR2 can potentially modulate a variety of gastric functions, through its ability to induce the secretion of mucus and pepsinogen, to suppress acid output, to increase mucosal blood flow and to induce gastric strip contraction/relaxation (reviewed in Kawabata, 2003; Nishikawa & Kawabata, 2003). Except for pepsinogen secretion, all these effects of PAR2 in the stomach favor a protective role for PAR2 in gastric mucosa. As a matter of fact, treatment of rats with the selective PAR2 agonist SLIGRL has been shown to be protective in models of gastritis induced by indomethacin or ethanol and HCl (Kawabata et al., 2001a). Here again, this protective effect of PAR2 agonist has been shown to be mediated, at least in part, by the activation of sensory neurons (Kawabata et al., 2001a). The selective agonist for PAR1 also reveals protective effects for gastric mucosa, inhibiting acid secretion and increasing gastric mucosal blood flow (Kawabata, 2003). However, this protective effect of PAR1 activation does not seem to be mediated by a neurogenic mechanism, but rather by an enhancement of endogenous protective prostaglandin production (Kawabata, 2003).
Pancreas
Inflammation of the pancreas leads to the premature activation of trypsin, which can then signal to pancreatic acini and duct cells through the activation of PAR2 (Cottrell et al., 2003). Trypsin stimulates fluid and electrolyte secretion in the pancreatic ducts through a mechanism involving the activation of PAR2 (Nguyen et al., 1999). PAR2 selective agonist causes a prompt increase, followed by transient decrease of pancreatic juice secretion, and also facilitates amylase secretion (Kawabata et al., 2000d; 2002; Kawabata, 2003). These PAR2-induced effects in the pancreas may also be protective, although in vivo studies using PAR2-deficient mice and/or PAR2 antagonists still have to be performed to demonstrate such hypothesis.
Proteases and PARs: mediators of visceral perception
Afferent sensory fibers of the ENS convey sensory data to the central nervous system, and the presence of PAR1 and PAR2 on those fibers has suggested a role for those receptors in visceral nociception mechanisms.
PAR2
It has been shown that peripheral (intraplantar) administration of sub-inflammatory doses of the PAR2 agonist SLIGRL, and also trypsin and tryptase, provoked nociceptor activation at a spinal level, together with a severe and prolonged (>24 h) thermal and mechanical hyperalgesia (Vergnolle et al., 2001a). Transient receptor potential vanilloid-like 1 (TRPV1) mediated PAR2-induced thermal, but not mechanical hyperalgesia (N. Vergnolle, unpublished work). When injected into the colon lumen, sub-inflammatory doses of PAR2-activating peptide and trypsin caused visceral hyperalgesia, as observed by an increased number of abdominal contractions in response to colorectal distension (Coelho et al., 2002). The increased Fos expression observed in the superficial laminae of the dorsal horn in response to intracolonic administration of PAR2 agonist also suggests that visceral activation of PAR2 induces the activation of second-order neurons at a spinal level (Coelho et al., 2002). This was confirmed by a similar study, which showed that pancreatic activation of PAR2 also provoked an increased Fos expression in the superficial laminae of the dorsal horn (Hoogerwerf et al., 2001). Since in both cases (colonic lumen or pancreatic duct exposure to PAR2 agonists) increased Fos expression was observed primarily in laminae I and II of the dorsal horn, which contain nociceptive nerve terminals, this suggests that a central nociceptive signal is triggered by visceral activation of PAR2. Although these experiments showed that peripheral activation of PAR2 caused nociceptor activation, they did not unequivocally show that this effect was due to a direct activation of PAR2 on sensory neurons. The presence of functional PAR2 on dorsal root ganglia neurons (Steinhoff et al., 2000) and the fact that, in those cells, PAR2 agonists enhanced KCl- and capsaicin (TRPV1 agonist)-evoked release of CGRP (a spinal mediator of nociception) (Hoogerwerf et al., 2001) strongly suggest that PAR2 agonists directly activate primary afferents to induce a nociceptive signal. Other evidence suggesting that PAR2 agonists signal directly to neurons to induce hyperalgesia comes from a study by Reed et al. (2003), which showed that trypsin, tryptase and PAR2-activating peptide induced prolonged hyperexcitability of submucosal neurons isolated from the guinea-pig ileum. In a recent study, we have shown that not only peripheral but also spinal activation of PAR2 can participate in inflammatory visceral hyperalgesia (Vergnolle et al., 2003b). Intrathecal injection of the selective PAR2-activating peptide SLIGRL, but not the control peptide or their vehicle, increased (in a dose-dependent manner) the number of writhing behaviors in response to intraperitoneal injection of acetic acid. Intrathecal injections of tachykinin-1 receptor and CGRP receptor antagonists were able to block this PAR2-induced enhancement of visceral hyperalgesia, suggesting that spinal release of substance P and CGRP are involved in PAR2-induced spinal effect (Vergnolle et al., 2003b). These results suggest that PAR2 activation from the periphery, but also at a spinal level, might play an important role in states of hypersensitivity.
Although a clear role for PAR2 in inflammation-induced somatic hyperalgesia and particularly in mast cell degranulation-induced hyperalgesia has been demonstrated using PAR2-deficient mice (Steinhoff et al., 2000), such role in visceral hyperalgesia has yet to be demonstrated.
PAR1
In contrast to what has been shown for PAR2, sub-inflammatory doses of PAR1 agonist did not provoke hyperalgesia when injected into the rat paw, but increased nociceptive threshold and significantly inhibited inflammatory hyperalgesia induced by intraplantar injection of carrageenan (Asfaha et al., 2002). Intraperitoneal injection of the selective PAR1 agonist TFLLR also inhibited visceral pain behaviors induced by the intraperitoneal injection of acetic acid, by a mechanism involving the activation of opioid receptors (Vergnolle et al., 2003a). Intracolonic administration of thrombin and TFLLR has also been shown to produce visceral analgesia, reducing the number of abdominal contractions in response to colorectal distension (Coelho & Bunnett, 2003). Here again, there are no evidences that such analgesic effect of PAR1 activation is due to a direct activation on primary afferents. Further in vitro studies would be necessary to determine whether or not PAR1 agonists could cause hyperpolarization of the sensory neuron membrane.
Proteases and PARs in the pathogenesis of irritable bowel syndrome (IBS)
The IBS is characterized by abdominal pain or discomfort associated with disturbed defecation and often bloating. Several pathophysiological factors are involved in the generation of symptoms of IBS: psychological factors, altered motility patterns, inflammatory mediator release, altered epithelial functions (permeability, ion exchanges and immune) and altered visceral nociceptive functions (hypersensitivity) (for a review, see Mayer & Collins, 2002). PARs are present in most of the cells that are potentially actors in the generation of IBS symptoms, and PAR activation interferes with several components of the pathogenesis of IBS (see Figure 3).
First, as discussed in the precedent paragraph, PARs are present on sensory neurons, where they can potentially interfere in a direct manner with the transmission of nociceptive signal. Activation of PARs on enteric neurons (PAR1 and PAR2) can also provoke the release of neuropeptides such as substance P and CGRP (Steinhoff et al., 2000; de Garavilla et al., 2001), which in turn activate their receptors present on endothelium to induce plasma extravasation. Such PAR-induced micro-inflammation might participate in the generation of IBS symptoms, as low levels of inflammation have been proposed to be involved in the pathogenesis of hypersensitivity (Collins, 2001; Collins et al., 2001; Bueno & Fioramonti, 2002). Activation of PARs on cells that are involved in inflammatory or immune responses, such as neutrophils, mast cells, lymphocytes, macrophages, or fibroblasts, might also be involved in causing hypersensitivity, by indirect stimulation of primary afferents (through the release of prostaglandins, neuropeptides or cytokines). Such indirect pro-algesic effect is in accordance with the hyperalgesia and nociceptor activation that have been demonstrated in response to visceral exposure to PAR2 agonists (Hoogerwerf et al., 2001; Coelho et al., 2002). However, PAR1 agonists did not cause hyperalgesia, but decreased nociceptive threshold and inhibited painful behaviors (Asfaha et al., 2002; Coelho & Bunnett, 2003; Vergnolle et al., 2003a). A possible explanation is that sub-inflammatory doses were used for PAR1 agonists to cause analgesia, while larger doses of TFLLR were necessary to cause inflammation.
Compromised intestinal barrier function has been associated with IBS in child and adult patients (Barau & Dupont, 1990; Spiller et al., 2000), suggesting that increased intestinal permeability plays a role in the generation of the symptoms associated with IBS. Since both PAR1 and PAR2 activation disrupted the integrity of the intestinal barrier, it can be hypothesized that PAR-induced increased permeability plays a role in visceral hypersensitivity states. As a matter of fact, a recent study has shown that a tight junction blocker (2,4,6 triaminopyrimidine – TAP) was able to inhibit PAR2-induced permeability, but also at the same time inhibited PAR2-induced rectal hypersensitivity (Moriez et al., 2003). The fact that PAR1 agonists induce increased permeability, and also analgesia, might also depend on the doses of PAR1 agonists used. In order to cause bacterial translocation and significant increase of 51CrEDTA passage from the lumen to the blood, doses of 200 μg per mouse were used (Chin et al., 2003), while doses of only 1–100 μg per animal were used in rats to observe analgesia (Coelho & Bunnett, 2003; Vergnolle et al., 2003a). This suggests that at low doses PAR1 activation induces analgesia independently of permeability dysfunctions, while high doses of PAR1 agonists might cause hyperalgesia through a mechanism dependent on the integrity of intestinal barrier.
Enterocytes that secrete electrolytes, such as chloride, promote the movement of water in the intestinal lumen, and can thereby regulate IBS symptoms such as diarrhea. Recent studies have demonstrated that PAR1 activation in human non-transformed intestinal epithelia cell line (SCBN) results in calcium-dependent chloride secretion through a pathway that involves MAP-kinase and cyclooxygenase (Buresi et al., 2001). In contrast, activation of PAR1 in isolated segments of mouse colon resulted in a decrease of neurally evoked ion secretion (Buresi et al., 2003). Thus, depending on the target cells where PAR1 is activated, this activation can lead to different diarrheal or anti-diarrheal symptoms. PAR2 activation in isolated intestinal segments also provoked chloride secretion (Vergnolle et al., 1998). Several cells could constitute the primary target for PAR2-induced chloride secretion: enterocytes which express functional PAR2 (Kong et al., 1997), fibroblasts which stimulate PGE2 release in response to PAR2 agonists (Seymour et al., 2003), or enteric neurons which have been shown to be involved at least in part in PAR2-induced ion transport in porcine ileum (Green et al., 2000). It is not yet known whether or not PAR1 and PAR2 activation participate in the generation of diarrhea symptoms associated with IBS, but considering the effects of PAR agonists on epithelial secretory functions PARs appear as potential therapeutic targets in the treatment of secretory dysfunctions.
Thrombin, trypsin and mast cell tryptase, as well as selective agonists for PAR1, PAR2 and PAR4, enhanced the excitability and firing of enteric neurons (Gao et al., 2002). In that study, the authors suggest that these PARs can be activated on inhibitory motor enteric neurons, which would lead to inhibited contractile and motor activity. Several studies have reported the effects of PAR agonists on motor functions of the GI tract (Kawabata et al., 2001b; Mule et al., 2002a,2002b; 2004; Zhao & Shea-Donohue, 2003), suggesting that PAR activation could participate in motor dysfunctions associated with IBS. Whether direct activation of PARs on smooth muscle cells or on enteric neurons is involved in PAR-induced GI motor changes still has to be determined.
Finally, bi-directional interactions between the central nervous system and the gut-directed pathogenetic mechanisms are playing a major role in the development of IBS symptoms. Whether or not proteases and PARs are implicated in such interactions still has to be investigated. However, a recent study reports that central activation of PAR1 by thrombin or selective PAR1 agonist inhibits NMDA-mediated nociception (both somatic and visceral), by a pathway involving endothelin type A receptors (Fang et al., 2003). We also reported that spinal activation of PAR2 exacerbated visceral pain behaviors (Vergnolle et al., 2003b). These results suggest an important role for central activation of PARs in states of hypersensitivity, in accordance with a pro-algesic role for PAR2 activation and an analgesic role for PAR1 activation.
Conclusions
The development of drugs for IBS remains a therapeutic challenge. Scientists are constantly in search of potential new therapeutic targets for the treatment of IBS, and PARs appear as interesting receptors that clearly interfere with visceral nociceptive pathways. PARs are expressed in diverse cell types of the GI tract and their activation is associated with several pathophysiological factors that are involved in the generation of IBS symptoms, such as altered motility patterns, inflammatory mediator release, altered epithelial functions and altered visceral nociceptive functions. Further studies using genetically modified animals, and, when available, pharmacological tools, in different IBS and IBD models would definitively contribute to our understanding of the role of these receptors and their activating proteases in visceral pain and inflammation processes.
Acknowledgments
Dr Vergnolle is an Alberta Heritage Foundation for Medical Research Scholar. Her work is supported by the Canadian Association of Gastroenterology, the Crohn's and Colitis Foundation of Canada, and the Canadian Institute for Health Research.
Abbreviations
- CGRP
calcitonin gene-related peptide
- ENS
enteric nervous system
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- NK-1 R
tachykinin-1 receptor
- PARs
protease-activated receptors
- PAR1
protease-activated receptor-1
- PAR2
protease-activated receptor-2
- PAR3
protease-activated receptor-3
- PAR4
protease-activated receptor-4
- PSTI
pancreatic secretory trypsin inhibitor
References
- ASFAHA S., BRUSSEE V., CHAPMAN K., ZOCHODNE D.W., VERGNOLLE N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br. J. Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASOKANANTHAN N., GRAHAM P.T., STEWART D.J., BAKKER A.J., EIDNE K.A., THOMPSON P.J., STEWART G.A. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J. Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- BARAU E., DUPONT C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1990;11:72–77. doi: 10.1097/00005176-199007000-00015. [DOI] [PubMed] [Google Scholar]
- BUENO L., FIORAMONTI J. Visceral perception: inflammatory and non-inflammatory mediators. Gut. 2002;51 Suppl 1:i19–i23. doi: 10.1136/gut.51.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURESI M.C., HOLLENBERG M.D., MACNAUGHTON W.K. Activation of protease-activated receptor-1 (PAR-1) inhibits neurally evoked chloride secretion in the mouse colon. Can. J. Gastroenterol. 2003;17:61A. doi: 10.1152/ajpgi.00112.2004. [DOI] [PubMed] [Google Scholar]
- BURESI M.C., SCHLEIHAUF E., VERGNOLLE N., BURET A., WALLACE J.L., HOLLENBERG M.D., MACNAUGHTON W.K. Protease-activated receptor-1 stimulates Ca(2+)-dependent Cl(−) secretion in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G323–G332. doi: 10.1152/ajpgi.2001.281.2.G323. [DOI] [PubMed] [Google Scholar]
- CAUGHEY G.H. Mast Cell Proteases in Immunology and Biology 1995New York: Marcel-Decker; 305–329.ed. Caughey, G.H. pp [Google Scholar]
- CENAC N., COELHO A., NGUYEN C., COMPTON S., ANDRADE-GORDON P., MACNAUGHTON W.K., WALLACE J.L., HOLLENBERG M.D., BUNNETT N.W., GARCIA-VILLAR R., BUENO L., VERGNOLLE N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENAC N., GARCIA-VILLAR R., FERRIER L., LARAUCHE M., VERGNOLLE N., BUNNETT N.W., COELHO A.M., FIORAMONTI J., BUENO L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- CHIN A.C., VERGNOLLE N., MACNAUGHTON W.K., WALLACE J.L., HOLLENBERG M.D., BURET A.G. Proteinase-activated receptor-1 induces apoptosis and increases intestinal permeability. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11104–11109. doi: 10.1073/pnas.1831452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M., SORRENTINO L., MARAGANORE J., STONE S. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- COELHO A., BUNNETT N.W. Intestinal activation of proteinase-activated receptor-1 (PAR1) reduces visceral nociception associated to rectal distension (RD) in rats. Gastroenterology. 2003;124:A-1. [Google Scholar]
- COELHO A.M., VERGNOLLE N., GUIARD B., FIORAMONTI J., BUENO L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- COLLINS S.M.Altered neuromuscular function in the inflamed bowel Inflammatory Bowel Disease 2000Philadelphia: Saunders WB; 5th edn. ed. Kirsner, J.B [Google Scholar]
- COLLINS S.M. Peripheral mechanisms of symptom generation in irritable bowel syndrome. Can. J. Gastroenterol. 2001;15 Suppl B:14B–16B. doi: 10.1155/2001/690794. [DOI] [PubMed] [Google Scholar]
- COLLINS S.M., PICHE T., RAMPAL P. The putative role of inflammation in the irritable bowel syndrome. Gut. 2001;49:743–745. doi: 10.1136/gut.49.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGUE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTRELL G.S., AMADESI S., GRADY E.F., BUNNETT N.W.Trypsin IV: a novel agonist of protease-activated receptors 2 and 4 J. Biol. Chem. 2004(in press) [DOI] [PubMed]
- COTTRELL G.S., AMADESI S., SCHMIDLIN F., BUNNETT N. Protease-activated receptor 2: activation, signalling and function. Biochem. Soc. Trans. 2003;31:1191–1197. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA M.R., ROGAHN C.J., ANDRADE-GORDON P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech. Histochem. 2000;75:85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- DARMOUL D., GRATIO V., DEVAUD H., LEHY T., LABURTHE M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am. J. Pathol. 2003;162:1503–1513. doi: 10.1016/S0002-9440(10)64283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARMOUL D., MARIE J.C., DEVAUD H., GRATIO V., LABURTHE M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br. J. Cancer. 2001;85:772–779. doi: 10.1054/bjoc.2001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GARAVILLA L., VERGNOLLE N., YOUNG S.H., ENNES H., STEINHOFF M., OSSOVSKAYA V.S., D'ANDREA M.R., MAYER E.A., WALLACE J.L., HOLLENBERG M.D., ANDRADE-GORDON P., BUNNETT N.W. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br. J. Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERIAN C.K., MARYANOFF B.E., ANDRADE-GORDON P., ZHANG H.C. Design and evaluation of potent peptidic-mimetic PAR1 antagonist. Drug Dev. Res. 2003;59:355–366. [Google Scholar]
- DERY O., CORVERA C., STEINHOFF M., BUNNETT N. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- EDENS H.A., LEVI B.P., JAYE D.L., WALSH S., REAVES T.A., TURNER J.R., NUSRAT A., PARKOS C.A. Neutrophil transepithelial migration: evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J. Immunol. 2002;169:476–486. doi: 10.4049/jimmunol.169.1.476. [DOI] [PubMed] [Google Scholar]
- FANG M., KOVACS K.J., FISHER L.L., LARSON A.A. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J. Physiol. 2003;549:903–917. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARUQI T.R., WEISS E.J., SHAPIRO M.J., HUANG W., COUGHLIN S.R. Structure function analysis of protease activated receptor 4 tethered ligand peptides: determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- FERAZZINI M., SANTI S., MACNAUGHTON W.K., HOLLENBERG M.D., WALLACE J.L., VERGNOLLE N. Proteinase-activated receptor-4 (PAR4) is implicated in the pathogenesis of dextran sodium sulfate colitis. Gastroenterology. 2003;124:A-487. [Google Scholar]
- FERAZZINI M., SANTI S., MACNAUGHTON W.K., HOLLENBERG M.D., WALLACE J.L., VERGNOLLE N. Proteinase-activated receptor-4 (PAR4) is implicated in the pathogenesis of dextran sodium sulfate colitis. Gastroenterology. 2004;124:A-487. [Google Scholar]
- FERNANDEZ M., AUYEUNG K.J., MADDEN K., ZHAO A., SULLIVAN C.A., URBAN J.J., KINKELMAN F.D., SHEA-DONOHUE T. Nematode infection alters protease-activated receptor (PAR)-induced epithelial cell responses in murine small intestine. Gastroenterology. 2003;124:A-83. [Google Scholar]
- FIORUCCI S., MENCARELLI A., PALAZZETTI B., DISTRUTTI E., VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L., MORELLI A., CIRINO G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO C.Y., LIU S.M., HU H.Z., GAO N.A., KIM G.Y., XIA Y., WOOD J.D. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- GELBMANN C.M., MESTERMANN S., GROSS V., KOLLINGER M., SCHOLMERICH J., FALK W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45:210–217. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN B.T., BUNNETT N.W., KULKARNI-NARLA A., STEINHOFF M., BROWN D.R. Intestinal type-2 proteinase-activated receptors: expression in opioid-sensitive secretomotor neural circuits that mediate epithelial transport. J. Pharm. Exp. Ther. 2000;295:410–416. [PubMed] [Google Scholar]
- HOLLENBERG M.D., COMPTON S.J. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- HOOGERWERF W.A., ZOU L., SHENOY M., SUN D., MICCI M.A., LEE-HELLMICH H., XIAO S.Y., WINSTON J.H., PASRICHA P.J. The proteinase-activated receptor 2 is involved in nociception. J. Neurosci. 2001;21:9036–9042. doi: 10.1523/JNEUROSCI.21-22-09036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOU L., HOWELLS G.L., KAPAS S., MACEY M.G. The protease-activated receptors and their cellular expression and function in blood-related cells. Br. J. Haematol. 1998;101:1–9. doi: 10.1046/j.1365-2141.1998.00696.x. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A., ZENG D., KAHN M., ZHENG Y., TIMMONS C., TRAM T., COUGHLIN S. Protease-activated receptor-3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAWABATA A. Gastrointestinal functions of proteinase-activated receptors. Life Sci. 2003;74:247–254. doi: 10.1016/j.lfs.2003.09.011. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KINOSHITA M., NISHIKAWA H., KURODA R., NISHIDA M., ARAKI H., ARIZONO N., ODA Y., KAKEHI K. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J. Clin. Invest. 2001a;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K. Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br. J. Pharmacol. 2000a;131:578–584. doi: 10.1038/sj.bjp.0703590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K., ARAKI H. Characterization of the protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle. Life Sci. 2000b;67:2521–2530. doi: 10.1016/s0024-3205(00)00835-3. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAGATA N., KAWAO N., MASUKO T., NISHIKAWA H., KAWAI K. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 2001b;133:1213–1218. doi: 10.1038/sj.bjp.0704211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIDA M., NAGATA N., SAKAGUCHI Y., KAWAO N., NISHIKAWA H., ARIZONO N., KAWAI K. Protease-activated receptor-2 (PAR-2) in the pancreas and parotid gland: immunolocalization and involvement of nitric oxide in the evoked amylase secretion. Life Sci. 2002;71:2435–2446. doi: 10.1016/s0024-3205(02)02044-1. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., MORIMOTO N., NISHIKAWA H., KURODA R., ODA Y., KAKEHI K. Activation of protease-activated receptor-2 (PAR-2) triggers mucin secretion in the rat sublingual gland. Biochem. Biophys. Res. Commun. 2000c;270:298–302. doi: 10.1006/bbrc.2000.2404. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., NISHIKAWA H., KURODA R., KAWAI K., HOLLENBERG M.D. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br. J. Pharmacol. 2000d;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM J.A., CHOI S.C., YUN K.J., KIM D.K., HAN M.K., SEO G.S., YEOM J.J., KIM T.H., NAH Y.H., LEE Y.M. Expression of protease-activated receptor 2 in ulcerative colitis. Inflamm. Bowel Dis. 2003;9:224–229. doi: 10.1097/00054725-200307000-00002. [DOI] [PubMed] [Google Scholar]
- KONG W., MCCONALOGUE K., KHITIN L., HOLLENBERG M.D., PAYAN D., BOHM S., BUNNETT N. Luminal trypsin may regulate enterocytes through proteinase-activated receptor-2. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHIKAWA N., HASEGAWA S., NAGASHIMA Y., MITSUHASHI K., TSUBOTA Y., MIYATA S., MIYAGI Y., YASUMITSU H., MIYAZAKI K. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am. J. Pathol. 1998;153:937–944. doi: 10.1016/S0002-9440(10)65635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHIKAWA N., NAGASHIMA Y., MIYAGI Y., MIZUSHIMA H., YANOMA S., YASUMITSU H., MIYAZAKI K. Expression of trypsin in vascular endothelial cells. FEBS Lett. 1997;409:442–448. doi: 10.1016/s0014-5793(97)00565-6. [DOI] [PubMed] [Google Scholar]
- LOURBAKOS A., POTEMPA J., TRAVIS J., D'ANDREA M.R., ANDRADE-GORDON P., SANTULLI R., MACKIE E.J., PIKE R.N. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect. Immun. 2001;69:5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHBANK T., CHINERY R., HANDY A., POULSON R., ELIA G., PLAYFORD R. Distribution and expression of pancreatic secretory trypsin inhibitor and its possible role in epithelial restitution. Am. J. Pathol. 1996;148:715–722. [PMC free article] [PubMed] [Google Scholar]
- MARCHBANK T., FREEMAN T.C., PLAYFORD R.J. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59:167–174. doi: 10.1159/000007485. [DOI] [PubMed] [Google Scholar]
- MAYER E.A., COLLINS S.M. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- MCHUGH K.J., SVENSJO E., PERSSON C.G. Exudative and absorptive permeability in different phases of an experimental colitis condition. Scand. J. Gastroenterol. 1996;31:900–905. doi: 10.3109/00365529609051999. [DOI] [PubMed] [Google Scholar]
- MILLER H.R. Mucosal mast cells and the allergic response against nematode parasites. Vet. Immunol. Immunopathol. 1996;54:331–336. doi: 10.1016/s0165-2427(96)05696-6. [DOI] [PubMed] [Google Scholar]
- MORIEZ R., CENAC N., BUENO L. Delayed rectal hypersensitivity to intracolonic PAR-2-activating peptide and taurocholate is linked to increased epithelial paracellular permeability in rats. Gastroenterology. 2003;124:A-250. [Google Scholar]
- MULE F., BAFFI M.C., CERRA M.C. Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon. Br. J. Pharmacol. 2002a;136:367–374. doi: 10.1038/sj.bjp.0704746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULE F., BAFFI M.C., FALZONE M., CERRA M.C. Signal transduction pathways involved in the mechanical responses to protease-activated receptors in rat colon. J. Pharmacol. Exp. Ther. 2002b;303:1265–1272. doi: 10.1124/jpet.102.041301. [DOI] [PubMed] [Google Scholar]
- MULE F., PIZZUTI R., CAPPARELLI A., VERGNOLLE N. Evidence for the presence of functional protease activated receptor 4 (PAR(4)) in the rat colon. Gut. 2004;53:229–234. doi: 10.1136/gut.2003.021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN C., COELHO A., GRADY E.F., WALLACE J.L., HOLLENBERG M.D., CENAC N., GARCIA-VILLAR R., BUENO L., STEINHOFF M., BUNNETT N.W., VERGNOLLE N. Proteinase-activated receptor-2-induced colitis is mediated by a neurogenic mechanism. Can. J. Physiol. Pharmacol. 2003;81:920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., MOODY M.W., STEINHOFF M., OKOLO C., KOH D.S., BUNNETT N.W. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J. Clin. Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWA H., KAWABATA A. Modulation of gastric function by proteinase-activated receptors. Drug Dev. Res. 2003;60:9–13. [Google Scholar]
- PLEBANI M., DI MARIO F., BATTISTEL M., BASSO D., MANTOVANI G., GIACOMINI A., BURLINA A. Measurement of tryptase in endoscopic gastroduodenal biopsies: distribution and relationship with ulcer disease. Clin. Chim. Acta. 1992;206:107–114. doi: 10.1016/0009-8981(92)90011-e. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN U., VOURET-CRAVIARI V., JALLAT S., SCHLESINGER Y., PAGES G., PAVIRANI A., LECOCQ J., POUYSSEGUR J., VAN OBBERGHEN-SCHILLING E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- REED D.E., BARAJAS-LOPEZ C., COTTRELL G., VELAZQUEZ-ROCHA S., DERY O., GRADY E.F., BUNNETT N.W., VANNER S. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea pig submucosal neurons. J. Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS J., BAYARRI C., SAPERAS E., NOGUEIRAS C., ANTOLIN M., MOURELLE M., CADAHIA A., MALAGELADA J.R. Characterisation of immune mediator release during the immediate response to segmental mucosal challenge in the jejunum of patients with food allergy. Gut. 1999;45:553–558. doi: 10.1136/gut.45.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS J., SAPERAS E., NOGUEIRAS C., MOURELLE M., ANTOLIN M., CADAHIA A., MALAGELADA J.R. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- SEYMOUR M.L., BINION D.G., HOLLENBERG M.D., MACNAUGHTON W.K. Expression of proteinase-activated receptor-2 in primary human myofibroblasts and stimulation of prostaglandin E2 synthesis. Inflamm. Res. 2001;50:S171. doi: 10.1139/y05-046. [DOI] [PubMed] [Google Scholar]
- SEYMOUR M.L., ZAIDI N.F., HOLLENBERG M.D., MACNAUGHTON W.K. PAR1-dependent and independent increases in COX-2 and PGE2 in human colonic myofibroblasts stimulated by thrombin. Am. J. Physiol. Cell Physiol. 2003;284:C1185–C1192. doi: 10.1152/ajpcell.00126.2002. [DOI] [PubMed] [Google Scholar]
- SPILLER R.C. Role of nerves in enteric infection. Gut. 2002;51:759–762. doi: 10.1136/gut.51.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPILLER R.C., JENKINS D., THORNLEY J.P., HEBDEN J.M., WRIGHT T., SKINNER M., NEAL K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S., TOGNETTO M., AMADESI S., ENNES H., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G., MITCHELL S., WILLIAMS L., GEPPETTI P., MAYER E., BUNNETT N. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- SUN G., STACEY M.A., SCHMIDT M., MORI L., MATTOLI S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J. Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., BUNNETT N.W., SHARKEY K.A., BRUSSEE V., COMPTON S., GRADY E.F., CIRINO G., GERARD N., BASBAUM A., ANDRADE-GORDON P., HOLLENBERG M.D., WALLACE J.L. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat. Med. 2001a;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., CELLARS L., CHAPMAN K. Proteinase-activated receptor-1 agonists attenuate visceral pain. Gastroenterology. 2003a;124:A-252. [Google Scholar]
- VERGNOLLE N., CELLARS L., CHAPMAN K. Spinal activation of proteinase-activated receptor-2 exacerbates peripheral hyperalgesia. Neurogastoenterol. Motil. 2003b;15:571–590. [Google Scholar]
- VERGNOLLE N., CELLARS L., HOLLENBERG M.D., WALLACE J.L., ANDRADE-GORDON P. Proteinase-activated receptor-1 (PAR1) is implicated in the pathogenesis of TNBS colitis. Gastroenterology. 2004;124:A-83. [Google Scholar]
- VERGNOLLE N., DERIAN C.K., D'ANDREA M.R., STEINHOFF M., ANDRADE-GORDON P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential pro-inflammatory role for proteinase-activated receptor-4 (PAR-4) J. Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., FERAZZINI M., D'ANDREA M.R., BUDDENKOTTE J., STEINHOFF M. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 2003c;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., SHARKEY K., WALLACE J.L. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR-2)-activating peptides in the rat paw. Br. J. Pharmacol. 1999a;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br. J. Pharmacol. 1999b;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766s–7771s. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001b;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- VU T., HUNG D., WHEATON V., COUGHLIN S. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WANG J., ZHENG H., OU X., FINK L.M., HAUER-JENSEN M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am. J. Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU W., ANDERSEN H., WHITMORE T., PRESNELL S., YEE D., CHING A., GILBERT T., DAVIE E., FOSTER D. Cloning and characterization of human protease-activated receptor-4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO A., SHEA-DONOHUE T. PAR-2 agonists induce contraction of murine small intestine through neurokinin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G696–G703. doi: 10.1152/ajpgi.00064.2003. [DOI] [PubMed] [Google Scholar]