Abstract
Tachykinin NK2 receptors are expressed in the gastrointestinal tract of both laboratory animals and humans. Experimental data indicate a role for these receptors in the regulation of intestinal motor functions (both excitatory and inhibitory), secretions, inflammation and visceral sensitivity. In particular, NK2 receptor stimulation inhibits intestinal motility by activating sympathetic extrinsic pathways or NANC intramural inhibitory components, whereas a modulatory effect on cholinergic nerves or a direct effect on smooth muscle account for the NK2 receptor-mediated increase in intestinal motility. Accordingly, selective NK2 receptor antagonists can reactivate inhibited motility or decrease inflammation- or stress-associated hypermotility. Intraluminal secretion of water is increased by NK2 receptor agonists via a direct effect on epithelial cells, and this mechanism is active in models of diarrhoea since selective antagonists reverse the increase in faecal water content in these models. Hyperalgesia in response to intraluminal volume signals is possibly mediated through the stimulation of NK2 receptors located on peripheral branches of primary afferent neurones. NK2 receptor antagonists reduce the hyper-responsiveness that occurs following intestinal inflammation or application of stressful stimuli to animals. Likewise, NK2 receptor antagonists reduce intestinal tissue damage induced by chemical irritation of the intestinal wall or lumen. In healthy volunteers, the selective NK2 antagonist nepadutant reduced the motility-stimulating effects and irritable bowel syndrome-like symptoms triggered by intravenous infusion of neurokinin A, and displayed other characteristics that could support its use in patients. It is concluded that blockade of peripheral tachykinin NK2 receptors should be considered as a viable mechanism for decreasing the painful symptoms and altered bowel habits of irritable bowel syndrome patients.
Keywords: Neurokinin A, substance P, nepadutant, saredutant, irritable bowel syndrome, intestinal motility, intestinal secretion, intestinal distension, visceral sensitivity, inflammation
Introduction
Mammalian tachykinins (TKs), which include substance P (SP), neurokinin A (NKA) and its elongated forms neuropeptide-gamma and neuropeptide-kappa, neurokinin B (NKB), and the recently discovered hemokinin-1 and its elongated forms endokinin A and B, are a family of peptides which act through the stimulation of tachykinin NK1, NK2 or NK3 receptors (Patacchini et al., 2004). Although SP, NKA, and NKB are the preferential agonists for NK1, NK2, and NK3 receptors, respectively, their selectivity is limited, and all TKs are capable of behaving as full agonists at all tachykinin receptor types (Maggi, 1995). Therefore, besides NKA, neuropeptide-gamma and neuropeptide-kappa, NKB and SP can also behave as potent agonists at NK2 receptors.
Irritable bowel syndrome (IBS) is a functional intestinal disorder characterised by changes in bowel habits, which range from constipation to diarrhoea, associated with abdominal discomfort or overt pain (Drossman et al., 2002; Talley & Spiller, 2002). These symptoms can be attributed to modifications of the sensory-motor function of the intestine, where the substrate(s) involved could reside within the intestinal wall, in extrinsic ganglia, or in the central nervous system (Camilleri, 2001). As has recently been pointed out (Drossman et al., 2003), psychological distress, affective disorders, and narcotic abuse have to be considered as important ethiopathogenetic factors for IBS; however, there is little doubt that such factors also trigger peripheral changes. In accordance with this concept, stress can reactivate a previous peripheral inflammation (Collins, 2001), thus establishing a link between psychological factors and post-infectious (or post-dysenteric) IBS (Spiller et al., 2000). Current pharmacological treatments are aimed at controlling specific symptoms (e.g., tricyclic antidepressants for pain, 5-HT3 antagonists or spasmolytics for diarrhoea, 5-HT4 agonists for constipation) and the improvement obtained is relatively modest (Mertz, 2003). Therefore, a single drug that could potentially affect all of these symptoms would represent an important therapeutic achievement (Kirkup et al., 2001).
TKs are considered as key mediators in the communication between neurones (sensory neurones, in particular) and effector cells (smooth muscle, glands, immune cells), and among TK receptors we have identified the NK2 type as the most promising for developing an antagonist for the treatment of IBS (Lecci et al., 2002a). This review will focus on evidence establishing a potential link between NK2 receptors and IBS.
Distribution of TKs and NK2 receptors in the gastrointestinal tract
Distribution of TKs
Three genes encoding for tachykinin have been discovered: (i) TAC1 encodes for both SP and NKA and it is responsible for the wide co-localisation of these peptides, TAC3 encodes for NKB, and TAC4 encodes for haemokinin and endokinin A and B (Patacchini et al., 2004). TAC1 can generate four different mRNAs (alpha-TAC1, beta-TAC1, gamma-TAC1, delta-TAC1), two of which (beta-TAC1, gamma-TAC1) produce both SP and NKA (or its elongated forms; see above), while the other two produce SP only. Since gamma-TAC1 and beta-TAC1 are the most abundant mRNAs expressed in the gastrointestinal tract, SP and NKA are often co-localised in and co-released from the same cells (Sternini et al., 1989).
The gastrointestinal tract contains the highest density of TKs among peripheral organs. The TKs SP and NKA, in particular, are expressed in extrinsic primary afferent neurones (such as capsaicin-sensitive neurones, that is, TRPV-1-expressing) and in several populations of intrinsic neurones such as myenteric cholinergic excitatory motor neurones of both circular and longitudinal muscle layers, myenteric ascending interneurones (cholinergic), myenteric intrinsic primary afferent neurones (cholinergic), and submucosal intrinsic primary afferent neurones (cholinergic) (Costa et al., 1996; Furness, 2000; Furness & Sanger, 2002). In addition, non-neuronal gastrointestinal expression of TKs can occur in enterochromaffin and immune cells (Holzer & Holzer-Petsche, 2001), and even in smooth muscle cells (Khan & Collins, 1994).
Distribution of TK NK2 receptors
All the three TK receptors are expressed in the gastrointestinal tract. Schematically, NK2 receptors have been thought to be mainly expressed on effector cells (mainly smooth muscle), NK3 receptors on neurones, and NK1 receptors on both effector cells (smooth muscle, interstitial cells of Cajal, enterocytes, and glands) and neurones. However, as will be discussed below for NK2 receptors, there are large species-, segment-, and disease-related variations in the expression of tachykinin receptors, so that the above scheme appears insufficient to explain the complex effects induced by TKs at the intestinal level.
The intestinal distribution of TK NK2 receptors has been studied using immunohistochemical methods involving NK2 receptor antibodies and visualising the distribution of NK2 receptor mRNA. In the guinea-pig intestine, TK NK2 receptors were found to be localised to both the longitudinal and circular smooth muscle layers, and the deep muscular plexus, where a dense network of intramuscular interstitial cells of Cajal are located (Portbury et al., 1996; Vannucchi et al., 2000). NK2 receptor expression has also been detected in nerve fibre varicosities within the myenteric ganglia (identified as belonging to descending interneurones expressing nitric oxide synthase and bombesin), in the submucous ganglia, in nerve fibres within the mucosa (caecum and proximal colon), on enterocytes and on mucosal glands (Portbury et al., 1996; Vannucchi et al., 2000). Conflicting results have been presented regarding the expression of NK2 receptors on nerve fibres (SP-containing excitatory motor neurones) within smooth muscle layers, which was detected in one study (Vannucchi et al., 2000) but not in another (Portbury et al., 1996). In rats, in addition to expression in both muscular layers and muscularis mucosa, NK2 receptors were found to be localised to nerve terminals within both myenteric and submucous plexuses (Grady et al., 1996), whereas mice did not express NK2 receptors in the submucous plexuslatter location (Vannucchi & Faussone-Pellegrini, 2000). In human specimens (ileum and colon), NK2 receptors have been identified on smooth muscle layers muscularis mucosa and lamina propria, in the latter of which NK2 receptor mRNA was detected within inflammatory cells (Renzi et al., 2000), but this study failed to show NK2 receptor expression on neurones, nerve fibres, and enterocytes (Renzi et al., 2000). However, it should be mentioned that the failure to detect NK2 receptors on human enterocytes contrasts with functional data showing that a selective NK2 receptor agonist, [betaAla8]NKA(4–10), increased the short-circuit current (Isc; indicative of increased chloride, and therefore water secretion) in isolated human colonic mucosa, and the effect was blocked by a selective NK2 receptor antagonist (Tough et al., 2003). It is also worth considering that extramurally located NK2 receptors can affect gastrointestinal functions. In particular, at the peripheral level, NK2 receptors located on primary afferent neurones exert a facilitatory modulation on the activity of both N- and L-type Ca2+ channels (Sculptoreanu & De Groat, 2003). Furthermore, the possibility of NK2 receptor-mediated modulation of sympathetic nerves cannot be excluded (Shinkai & Takayanagi, 1993). Finally, supraspinal NK2 receptors located in hippocampal and hypothalamic areas might modulate the gastrointestinal responses that follow emotional stimuli (Desvignes et al., 2003). In this context, however, it should be pointed out that most of the preclinical evidence for the possible involvement of NK2 receptors in IBS symptoms has been obtained through the use of nepadutant (MEN11420), a cyclic peptide-selective NK2 receptor antagonist that does not cross the blood–brain barrier to any pharmacologically significant extent (Catalioto et al., 1998). Likewise, experiments carried out with the non-peptide NK2 receptor antagonist saredutant (SR48968) have demonstrated gastrointestinal effects (e.g., Croci et al., 1997) when the doses administered were lower than those required to exert supraspinal effects (e.g., Desvignes et al., 2003). Therefore, this article will consider peripherally located NK2 receptors as a possible therapeutic target for IBS.
Role of NK2 receptors in the control of intestinal motility: inhibitory effects
Constipation and inhibited motility constitute the main bowel habit in one population of IBS patients (constipation-predominant IBS), or appear as a phase in the bowel habit in another patient population (diarrhoea–constipation alternating IBS). Constipated IBS patients benefit from prokinetic drugs such as 5HT4 receptor agonists (e.g., Mertz, 2003), and therefore drugs that reactivate an inhibited intestinal motility can be considered for the treatment of these populations of IBS patients.
Inhibitory motor effects mediated through the stimulation of NK2 receptors are not readily evident in most preparations, because concomitant excitatory effects overshadow them. Several investigations have revealed that NK2 receptor-mediated inhibitory motor effects are present in the intestines of various animal species, including humans. Despite this, no systematic studies have been performed to clarify the physiological conditions under which the stimulation of NK2 receptors leads to the inhibition of intestinal motility, and the exact mechanisms underlying these inhibitory effects.
Sympathetic mechanisms
In the rat duodenum, there is evidence that TKs induce, through the stimulation of NK2 receptors, smooth muscle relaxation involving the activation of sympathetic mechanisms (Giuliani et al., 1988). In fact, intravenous administration of TKs induced a composite duodenal motor response, consisting of a transient relaxation followed by a contraction made up of a phasic and a tonic component. The evidence for the involvement of NK2 receptors in duodenal relaxation was deduced from the order of potency of TKs (e.g., NKA (4–10)=NKA>SP>NKB), and from the inability of an NK1 receptor antagonist, [D-Pro9, D-Trp7,9]-SP, to antagonise the effect. NKA-induced relaxation was reduced by the co-administration of phentolamine and propranolol, pretreatment with guanethidine, or the surgical ablation of coeliac and superior mesenteric ganglia, but not by hexamethonium, indicating the involvement of postganglionic sympathetic prevertebral neurones (Giuliani et al., 1988). Although the vast majority of TK-binding sites in sympathetic ganglia correspond to NK1 receptors, NK2 receptors have also been detected at this level (Messenger & Gibbins, 1998). Interestingly, colonic distension induced SP release in the guinea-pig inferior mesenteric ganglia that was resistant to hexamethonium, indicating that ganglionic SP release was not due to a conventional reflex arc, but rather to an axon reflex (Parkman et al., 1993). The evidence that distension-induced SP release in the inferior mesenteric ganglion originated from mechanosensitive afferent fibres was obtained in capsaicin-pretreated animals in which SP release induced by colonic distension was abolished (Ma & Szurszewski, 1996).
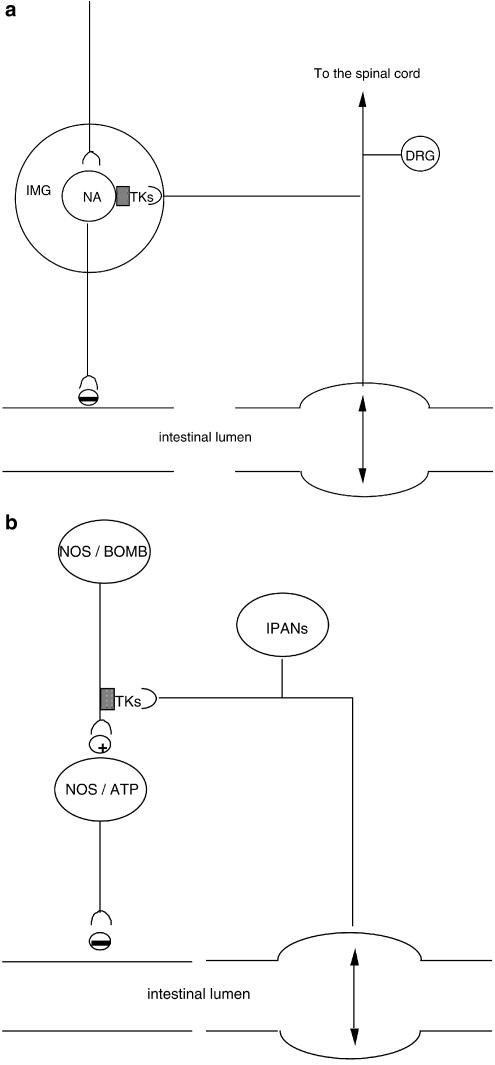
The activation of sympathetic mechanisms could account for the inhibitory effect induced by intraperitoneal administration of NKA or [Nle10]-NKA(4–10) on the upper intestine transit in rats (Holzer, 1985; Chang et al., 1999). Likewise, we speculate that NK2 receptor-activated sympathetic mechanisms may be involved in the inhibition of intestinal motility in pathophysiological conditions. In this context, it has recently been found that both yohimbine and nepadutant reduced the inhibition of rat small intestine motility induced by abdominal surgery (a model of surgical ileum) (Toulouse et al., 2001). Moreover, in dogs fed a standard meal, nepadutant prevented the delay in gastric emptying induced by noxious colorectal distension without affecting normal gastric emptying (Bueno and Fioramonti, unpublished results). Figure 1a provides a schematic drawing on the organisation of NK2 receptor-activated sympathetic mechanisms in extrinsic ganglia.
Figure 1.
Schematic drawings representing possible anatomical arrangements accounting for the inhibition of intestinal motility triggered by NK2 receptor stimulation. Grey squares represent TK NK2 receptors. (a) Sympathetic mechanism activated by NK2 receptor agonist and intestinal distension. Intestinal distension induces the release of TKs from collaterals of capsaicin-sensitive dorsal root ganglion (DRG) neurones innervating the inferior mesenteric ganglion (IMG). Stimulation of NK2 receptors activates postganglionic noradrenergic (NA) neurones, which inhibit intestinal motility. (b) NANC inhibitory mechanisms activated by NK2 receptor agonist and intestinal distension. TKs, possibly released from intramural primary afferent neurones (IPANs) following intestinal distension, act on NK2 receptors located on varicosities of nitric oxide (NO) synthase and/or bombesin-expressing descending interneurones, which in turn, activate inhibitory motor neurons releasing NO and ATP.
NANC mechanisms
Beyond the activation of extrinsic inhibitory mechanisms, NK2 receptor stimulation also triggers inhibitory electrical and motor responses through the activation of intramural NANC mechanisms (Figure 1b). Superfusion with NKA or [betaAla8]NKA(4–10) of the circular muscle of the guinea-pig colon pretreated with a NK3 receptor antagonist, atropine, guanethidine, indomethacin, and nifedipine produced a slow depolarisation with a series of inhibitory junction potentials superimposed (Zagorodnyuk & Maggi, 1995). Both excitatory and inhibitory responses were abolished by the selective NK2 receptor antagonist GR94800, whereas NK1 receptor antagonists were not effective. Tetrodotoxin abolished NKA-induced inhibitory junction potentials but not depolarisation, indicating that the excitatory response was due to a direct effect of the agonist on NK2 receptors located on smooth muscle, whereas the inhibitory response was indirectly mediated by the activation of NANC inhibitory mechanisms. In accordance with this hypothesis, the inhibitory response was reduced by either L-nitroarginine or apamin and abolished by co-administration of the drugs. The localisation of NK2 receptors on inhibitory descending myenteric interneurones expressing nitric oxide synthase (Portbury et al., 1996) offers an anatomical correlate to this functional response. Interestingly, the peristaltic activity of isolated rabbit distal colon was biphasically affected by NK2 receptor antagonists (SR48968, or MEN10627, a close analogue of nepadutant), since propulsion velocity was increased by low concentrations but decreased by high, but still pharmacologically specific, concentrations of these antagonists (Onori et al., 2000). The involvement of an NK2 receptor-triggered nitrergic mechanism is supported by the effect of L-nitroarginine, which slightly increased propulsion velocity on its own but abrogated the excitatory effect induced by NK2 receptor antagonists. This inhibitory pathway, activated by TKs, is conveyed through nicotinic synapses, since hexamethonium reduced the excitatory effect of NK2 antagonists, without altering basal propulsion velocity. This could be the main mechanism whereby the blockade of nitric oxide synthase decreases the threshold dose of NKA required to increase the myoelectrical activity of the small intestine in conscious rats (Schmidt et al., 2002) and augments the contractile effect of [betaAla8]NKA(4–10) in the colon of anaesthetised rats (Carini et al., 2001). Interestingly, the intravenous administration of a low dose of [betaAla8]NKA(4–10) during phase III of the migrating motor complex immediately abolished it in conscious dogs; however, since this effect persisted following pretreatment with SR48968, the involvement of NK2 receptors is doubtful (Basilisco & Phillips, 1994). In guanethidine-pretreated, anaesthetised guinea-pigs, the atropine-sensitive colonic propulsion induced by balloon distension was facilitated by nepadutant, MEN10627, and SR48968 (although the effect of the latter NK2 receptor antagonist was not statistically significant), and this facilitation was no longer evident in apamin-treated animals (Lecci et al., 1998). These results further indicate that the NK2 receptor-triggered NANC inhibitory mechanism can also be manifested in in vivo preparations.
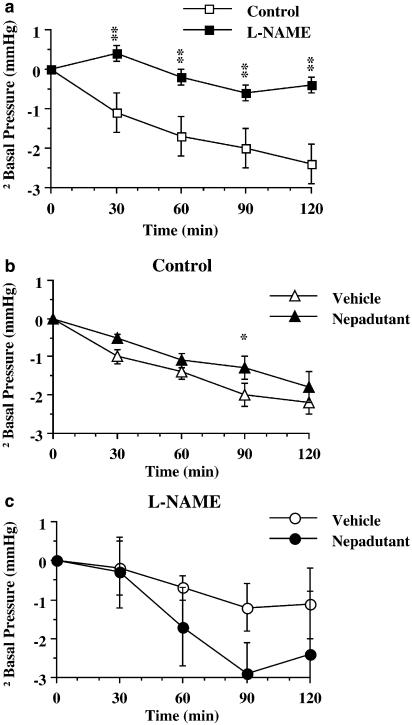
An additional excitatory action exerted by NK2 receptor antagonists on intestinal smooth muscle consists of a slight but reliable decrease in compliance. This effect, which was shared by atropine, has been detected in atropine-sensitive, isolated guinea-pig ileal peristalsis following incubation with SR48968, GR94800, or MEN10376 (Holzer & Maggi, 1994). The involvement of sympathetic mechanisms could be excluded because the effect of the NK2 receptor antagonists persisted when they were co-administered with isoproterenol. Since the decrease in muscle compliance produced by NK2 receptor antagonists was always associated with an increase in the pressure threshold for activation of peristaltic contractions, this effect has been considered as a kind of artefact due to a certain degree of impairment of peristalsis. However, in anaesthetised rats, during analysis of non-propulsive colonic motility with a balloon kept at a fixed distance from the anal sphincter, it was found that administration of nepadutant (or atropine) still increased the basal smooth muscle tone (Figure 2b), compared to vehicle-treated preparations (Carini et al., 2001). This effect occurred even in the absence of a reflex background motility (Carini et al., 2001), thus excluding any relationship with ascending excitatory or descending inhibitory reflexes. On the other hand, a neural pathway is likely to be involved since activation of muscular NK2 receptors did increase smooth muscle tone, as evidenced by the smooth muscle relaxation produced by NK2 receptor antagonists (MEN10627, GR94800, SR48968) in atropine- and guanethidine-pretreated isolated rat small intestine (Maggi & Giuliani, 1996), or by a decrease in spontaneous contractions in rat proximal colon (Mulè et al., 2000). The possibility that the decreased intestinal smooth muscle compliance observed following administration of NK2 receptor antagonists (in the absence of atropine) is attributable to the blockade of TK-mediated nitric oxide production is supported by the results obtained in preparations pretreated with nitric oxide synthase inhibitors (Figure 2). As previously described (Ciccocioppo et al., 1994) in L-nitroarginine methylester-pretreated rats, colonic muscle accommodation was dramatically decreased compared to controls (Figure 2a); in these preparations nepadutant did not decrease compliance, rather there was a trend towards facilitating accommodation (Figure 2c). The neuronal circuitries underlying the NK2 receptor-mediated regulation of smooth muscle tone are unknown, but the involvement of nitric oxide suggests an arrangement similar to that described in Figure 1b.
Figure 2.
(a) Effect of L-nitroargininemethylesther (L-NAME, 1.85 μmol kg−1, i.v.) on basal intraluminal colonic pressure in response to balloon distension (0.5 ml) in anaesthetised rats. (b) Effect of nepadutant (100 nmol kg−1, i.v.) in control preparations. (c) Effect of nepadutant in L-NAME-pretreated rats. Each point and bar represents the mean and s.e.m. of 12 experiments. Fisher LSD test: **P<0.01 vs Control; *P<0.05 vs Vehicle. Panel (b) has been taken from Carini et al. (2001).
Finally, NK2 receptor-mediated inhibitory mechanisms have also been demonstrated in human colon preparations, since the atropine-resistant component of the ‘off' contraction induced by electrical field stimulation was enhanced by prolonged exposure to high, desensitising concentrations of NKA but not SP (Kolbel et al., 1994). Although this result argues against an involvement of muscular NK2 receptors in the atropine-resistant ‘off' contraction, the possibility that muscular and neuronal NK2 receptors are subject to a different degree of desensitisation cannot be ruled out. Indeed, we have collected evidence in isolated intestinal preparations, indicating that desensitisation of the direct myotropic effect induced by NK2 receptor stimulation is minimal, if any.
Role of NK2 receptors in the control of intestinal motility: excitatory effects
Increased intestinal motility characterises diarrhoea-predominant IBS patients, who benefit from drugs that reduce intestinal motility such as 5HT3 receptor antagonists (Mertz, 2003); therefore drugs inhibiting motility can be considered for treating these patients.
Atropine-sensitive motility
As previously mentioned, TKs are co-stored in excitatory motor neurones projecting to the circular and longitudinal muscle layers; given this arrangement, it could be expected that TKs play an important role in the ascending excitatory reflex along the whole gastrointestinal tract. Furthermore, the prevalence of muscular expression of NK2 over NK1 receptors (Southwell & Furness, 2001) predicts that NK2 receptor antagonists should have dramatic depressant effects on intestinal motility. However, in most intestinal segments, the contribution of TKs to intestinal propulsion is barely detectable if acetylcholine muscarinic receptors are viable. This could be for a variety of reasons. First, short pulses of electrical stimulation are sufficient to activate cholinergic transmission, whereas the TK contribution can only be measured following long train stimulation (Maggi et al., 1997). Second, muscarinic transmission is more efficient in terms of electromechanical coupling compared to that mediated by tachykinins (Maggi et al., 1997), and, comparing TK NK1 and NK2 receptors, the transmission triggered by the former is faster (Maggi et al., 1994). This probably occurs because both M3 and NK1 receptors are expressed on interstitial cells of Cajal, and these cells specialise in the transmission of impulses from motor neurones to smooth muscle cells (see Lecci et al., 2002b, for a review). Owing to the greater efficiency of cholinergic transmission, small- or large-intestine peristalsis is only mildly affected by NK2 receptor antagonists in the absence of atropine. In the guinea-pig small intestine, NK2 receptor antagonists only evoked an increase in the pressure threshold for activation of peristaltic contractions (Holzer & Maggi, 1994; Holzer et al., 1998), whereas in the guinea-pig colon these compounds also transiently decreased the propulsion velocity (Tonini et al., 2001). Likewise, in the rabbit colon, MEN10627 or SR48968 produced a small decrease (10–20%) in propulsion velocity at high concentrations only (Onori et al., 2000). Similarly, NK2 receptor antagonists had no effect on the amplitude of distension-induced atropine-sensitive duodenal or colonic contractions in anaesthetised (Giuliani et al., 1996; Carini et al., 2001) and conscious (Croci et al., 1997) rats, and guinea-pigs (Lecci et al., 1998). A small (40%) and transient (45 min) reduction of the amplitude of distension-induced colonic contractions by NK2 receptor antagonists was detected in naloxone-pretreated guinea-pigs (Santicioli et al., 1997). In agreement with studies on distension-induced intestinal contractions, NK2 receptor antagonists had no effect per se on rat intestinal transit or faecal excretion (Croci et al., 1994; 1997; Tramontana et al., 1994). On the other hand, an inhibitory effect of NK2 receptor antagonists can be detected on atropine-sensitive abnormal motility, even in the absence of inflammation or laxatives; this is the case in stress-induced intestinal motility. Indeed, MEN10627 reduced restraint stress-induced output of faecal pellets in rats (Evangelista, 2001). Finally, it should be noted that intestinal motor effects induced by the stimulation of other receptors (e.g., serotonin or protease-activated receptors PAR-2 and PAR-4) are mediated in part by TKs acting through NK2 receptors (De Ponti et al., 2001; Zhao & Shea-Donohue, 2003; Mulè et al., 2004).
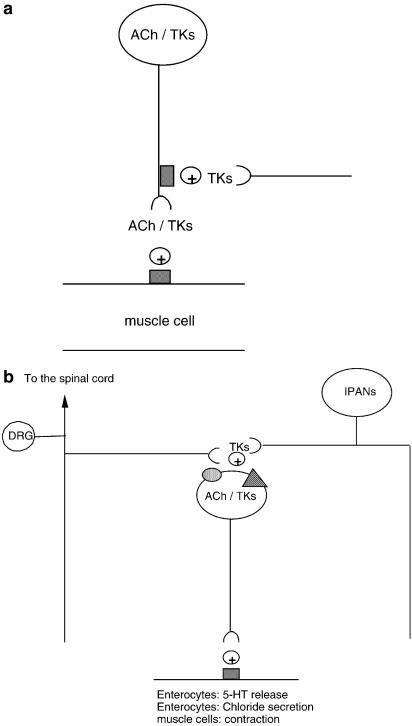
Even if NK2 receptor antagonists have only a small or no effect on normal intestine motility and propulsion, NK2 receptor agonists can enhance atropine-sensitive motility. This has been assessed through the increase in intestinal transit and faecal excretion induced by administration of [betaAla8]NKA(4–10) (Croci et al., 1994; Tramontana et al., 1994), or through the inhibitory effects exerted by NK2 receptor antagonists on the enhanced intestinal motility induced by a variety of stimuli causing the release of endogenous TKs. In this context, it has been shown that NK2 receptor antagonists reduce the faecal output and/or intestinal hypermotility induced by Salmonella enteritidis endotoxin (Croci et al., 1994), castor oil (Croci et al., 1997), acetic acid enema (Carini et al., 2001), Escherichia coli toxin STa, or Clostridium difficile toxin (Lecci et al., 2001). The NK2 receptor-mediated enhancement of motility could be attributed to the stimulation of muscular receptors, since a loss of jejunal circular muscle NK2 receptors (probably due to internalisation resulting from massive receptor stimulation) was observed to occur 14 days after Nippostrongylus brasiliensis infestation in rats (Faussone-Pellegrini et al., 2002). The ability of TKs to enhance atropine-sensitive small- or large-intestine motility can be explained by the stimulation of muscular or prejunctional receptors located on cholinergic excitatory motor neurones (Figure 3a). In fact, although there is little direct evidence showing that TKs acting through NK2 receptors enhance acetylcholine release during peristalsis, several results converge to indicate that some of the motor effects of NK2 receptor agonists are atropine-sensitive. One piece of direct evidence that TKs enhance acetylcholine release through the stimulation of NK2 receptors has been obtained in guinea-pig airways (D'Agostino et al., 2000). Low doses of NKA have also been shown to enhance rat small-intestine transit in an atropine-sensitive manner (Holzer, 1985), and part of the contractile effect induced by NKA in rat, cat, and human isolated colonic muscle is decreased by atropine (Chang et al., 1991; Hellstrom et al., 1991; Kolbel et al., 1994). Furthermore, TKs potentiate atropine-sensitive contraction induced by electrical field stimulation in the isolated rat rectum (Maggi et al., 1988), and the contractile effect induced by [betaAla8]NKA-(4–10)- is reduced by atropine in rat rectum in vivo (Carini et al., 2001). Figure 3a is a schematic drawing showing one possible anatomical arrangement of NK2 receptors that accounts for the enhancement of atropine-sensitive motility.
Figure 3.
Schematic drawings representing possible anatomical arrangements accounting for the excitation of intestinal motility or secretion triggered by NK2 receptor stimulation. Grey squares represent TK NK2 receptors, dotted ovals NK1 receptors, and dashed triangles NK3 receptors. (a) The presence of prejunctional receptors on excitatory motor neurones containing acetylcholine (ACh) and tachykinins (TKs) and postjunctional receptors on smooth muscle cells could account for both the enhancement of cholinergic transmission by NK2 receptor agonists and the inhibitory interaction occurring between muscarinic and NK2 receptor antagonists. (b) In some cases, the selective stimulation of NK1 or NK3 receptors evokes responses that are reduced by selective NK2 receptor antagonists. Therefore, tachykinins (TKs) released from extrinsic (DRG) or intramural (IPANs) primary afferent neurones stimulate NK1 or NK3 receptors located on the somata or dendrites of motor or secretomotor neurones expressing TKs. At the postjunctional level, TKs act through NK2 receptors to elicit the final response.
Atropine-resistant motility
The interaction occurring between contractile mechanisms activated by cholinergic muscarinic and TK NK2 receptors is particularly evident in integrated models of intestinal motility. In guinea-pig small or large intestine, as well as in rabbit colon in vitro, peristalsis was abolished by co-incubation with muscarinic and NK2 receptor antagonists at concentrations having little or no effect when these antagonists were given alone (Holzer & Maggi, 1994; Onori et al., 2000; Tonini et al., 2001). These results are consistent with a role for TKs in mediating atropine-resistant excitatory junction potentials and associated contractions in several intestinal segments of various animal species (Lecci et al., 2002b), including dogs (Tichenor et al., 2003), and as cholinergic cotransmitters of the migrating motor complex in the isolated murine colon (Brierley et al., 2001). Figure 3a is a schematic drawing showing one possible anatomical arrangement of NK2 receptors that accounts for the involvement in atropine-resistant motility.
In most of the cases, TK NK1 and NK2 receptors co-operate in producing excitatory neuro-muscular transmission (Maggi, 2000), and the co-operation between NK1 and NK2 receptors can occur within a single smooth muscle cell (Bayguinov et al., 2003). This co-operation is also evident in in vivo models, where the blockade of either NK1 or NK2 receptors produces an inhibitory effect on the amplitude of distension-induced atropine-resistant contractions, comparable to that produced by the co-administration of both NK1 and NK2 receptor antagonists (Giuliani et al., 1993; 1996; Lecci et al., 1998). However, even in intestinal segments where functional co-operation between NK1 and NK2 receptors has been described, intracellular mechanisms activated by NK1 or NK2 receptors show a clear specialisation. For instance, in the guinea-pig colon, both NK1 and NK2 receptor antagonists reduce atropine-resistant contractions in vivo (Giuliani et al., 1993), suggesting that the stimulation of both NK1 and NK2 receptors is necessary to maintain the NANC contractile function. Indeed, in isolated colonic strips, NK1-mediated depolarisation and contraction are nifedipine-sensitive, whereas nifedipine only abolished NK2 receptor-mediated action potentials, without affecting the amplitude of the associated depolarisation and contraction (Zagorodnyuk et al., 1994). This could be the reason why it is possible to encounter experimental conditions or intestinal segments in which the functional role of NK2 predominates over that of NK1 receptors. For instance, in the rat duodenum, contractions induced by selective NK1 receptor agonists are consistently reduced by selective NK2 receptor antagonists, whereas the opposite does not occur, thus suggesting that in this organ NK2 receptors are the end mechanism through which NK1 agonists produce contractions (Giuliani et al., 1996). A similar hierarchy in the functional importance of NK receptors, with NK2 predominating over NK1 or NK3 receptors, has been described in the stimulation of water secretion in the rat colon (Eutamene et al., 1995), and in eliciting serotonin release from guinea-pig colonic mucosa (Kojima et al., 2004), respectively. A schematic drawing showing a possible anatomical arrangement that accounts for the blockade by NK2 receptor antagonists of NK1 or NK3 receptor-mediated functions is represented in Figure 3b. In guinea-pig small intestine, hexamethonium-resistant peristalsis can be reactivated in the presence of naloxone: under these conditions, NK2 but not NK1 receptor antagonists have an inhibitory effect on the peristalsis, whereas either antagonist can abolish naloxone-triggered, atropine-resistant peristalsis (Holzer et al., 1998). Other examples where the functional importance of NK2 receptors predominates over that of NK1 receptors include both dog and human colon. These organs are endowed with both NK1 and NK2 receptors coupled to contractile mechanisms (Parlani et al., 1996; Cao et al., 2000). However, only NK2 and not NK1 receptor antagonists inhibit electrical field stimulation-induced contractions (Cao et al., 2000; Tichenor et al., 2003).
There are also cases, or particular experimental conditions, where the contribution of TK-mediated, NK2 receptor-induced neurotransmission to gastrointestinal smooth muscle can be seen when muscarinic receptors are still viable. For instance, in guanethidine-pretreated dog rectal preparations, the TK-mediated component of electrical field stimulation-induced contraction was evident, even without atropine pretreatment (Tichenor et al., 2003). Likewise, in the human colon pretreated with a nitric oxide synthase inhibitor, atropine had no appreciable effect on the amplitude of contraction induced by electrical field stimulation; this response was completely inhibited by NK2 receptor antagonists (Cao et al., 2000).
The physiological significance of atropine-resistant motility can be questioned; however, recent findings in scleroderma patients have shown the presence, and the antimuscarinic activity, of specific muscarinic M3 receptor autoantibodies. Incubation of mouse colonic strips with the serum from scleroderma patients inhibited carbachol-induced contraction. In contrast, tachykinergic transmission was unaffected, suggesting that a specific deficit of muscarinic transmission could be responsible for the symptoms and intestinal motility abnormalities observed in these patients (Goldblatt et al., 2002). It must be noted that these symptoms, some of which, including dysphagia, nausea, abdominal bloating, constipation, diarrhoea, and faecal incontinence, also characterise IBS, could be mediated by an enhancement in tachykininergic tone (see below), which replaced the deficit in muscarinic transmission.
Most recently, a further NK2 receptor-triggered effect that could increase both intestinal motility and visceral sensitivity has been described in the guinea-pig colon (Kojima et al., 2004). CGRP evoked an atropine-resistant but tetrodotoxin-sensitive 5-HT release from colonic mucosa by stimulating neurones located in the myenteric plexus. The release of 5-HT induced by CGRP was inhibited by NK2 or NK3 receptor antagonists. On the other hand, both [betaAla8]NKA-(4–10) and the selective NK3 receptor agonist senktide were capable of inducing 5-HT release, although the effect of the former agonist was tetrodotoxin-resistant, whereas the effect of the latter was sensitive to the toxin. Furthermore, the effect of senktide was inhibited by SR48968, thus suggesting that NK2 receptors were the common end mechanism through which CGRP and TKs acting through NK3 receptors evoke the release of 5-HT (Figure 3b). Given the importance of CGRP-expressing sensory neurones and 5-HT release in the triggering of the peristaltic reflex induced by mucosal stimulation (Grider et al., 1998), and the well-known sensitising effects of 5-HT on intestinal afferents, NK2 receptors could have a crucial role in the hypermotility and increased visceral sensitivity associated with noxious mucosal stimuli.
Role of NK2 receptors in the control of intestinal secretions
Altered intestinal secretions could be responsible for symptoms such as diarrhoea and the passage of mucus in IBS patients (Talley & Spiller, 2002). As occurs with other TK-mediated effects, the role of TK receptors involved in pro-secretory effects is subject to species-related variations. Thus, NK1 and NK3 receptors mediated TK-induced secretions in guinea-pigs (Frieling et al., 1999), whereas NK1, NK2, and NK3 receptors could all trigger Isc secretory current in rat descending colon (Cox et al., 1993). NK2 receptor activation also induces bicarbonate secretion and increased mucosal permeability in the rat duodenum (Hallgren et al., 1997; 1998). Increased water secretion in the rat colon can be elicited through the selective stimulation of NK1 or NK2 receptors, although NK2 receptors seem to play a pivotal role in this response, since the selective NK2 receptor antagonist SR48968 inhibits intestinal water secretion induced by both NK1 and NK2 receptor agonists (Eutamene et al., 1995), thus suggesting that NK2 receptors are the final common pathway for increasing secretory responses by TKs (Figure 3b). However, this hypothesis contrasts with the finding showing that, in the rat colonic mucosa, the activation of Isc current by [betaAla8]NKA-(4–10) is largely reduced by indomethacin pretreatment, whereas the effect of SP is not (Patacchini et al., 2001). A further discrepancy concerns the effect of nerve blockade on NK2 receptor-mediated secretory responses, since in one study tetrodotoxin blocked the effect of a selective NK2 receptor agonist (Eutamene et al., 1995), whereas in another this treatment had no effect (Cox et al., 1993). In the rat small intestine, both indomethacin and increased intraluminal pressure enhanced the bicarbonate secretory response induced by NKA through the stimulation of NK2 receptors, whereas lidocaine had no effect (Hallgren et al., 1998). Colonic overdistension (2 ml, producing intraluminal pressure corresponding to 60–70 mmHg), or intraperitoneal administration of interleukin-1beta induced colonic water secretion that was antagonised by NK2 receptor antagonists (MEN10,627 and SR48968, respectively) (Eutamene et al., 1995; 1997). The secretory response induced by the cytokine was inhibited by L-nitroarginine, further implicating nitric oxide production in NK2 receptor-mediated responses (Eutamene et al., 1995), whereas overdistension-induced secretion was abolished in capsaicin-pretreated rats, indicating that the secretory effect was due to TKs released from primary afferent nerves (Eutamene et al., 1997). A NK2 receptor antagonist (GR83074) also reduced cholera toxin-induced chloride rat jejunal secretion; since the effect of this toxin was tetrodotoxin-sensitive but capsaicin-resistant (Turvill et al., 2000), this would imply that TKs can be released to induce intestinal secretion through several independent pathways. Reduced faecal water content following administration of NK2 receptor antagonists has been also detected in a number of diarrhoea rat models, including those induced by castor oil (Croci et al., 1997), Salmonella toxin (Croci et al., 1994), and Escherichia coli toxin STa, or Clostridium difficile toxin (Lecci et al., 2001). Importantly, NK2 receptor antagonists had no effect on basal water secretion, showing that these drugs are unlikely to induce constipative symptoms.
NK2 receptor stimulation also increased rat gastric mucous secretion, and GR83704 inhibited both NKA- and protease-activated receptor-2-induced effects; since the effect of this latter stimulus was prevented by pretreatment with capsaicin, NK2 receptor stimulation must have occurred following TK release from primary afferent neurones (Kawabata et al., 2001). The role of NK2 receptors on mucous secretion is not limited to the stomach, since colonic mucin release induced by partial restraint stress is reduced by MEN10627 pretreatment (Evangelista, 2001).
Altered ion fluxes through the intestinal mucosa are accompanied by permeability changes; thus, in the rat duodenum, NKA increased intestinal permeability (Lordal et al., 1996) through the stimulation of NK2 receptors, and this effect was reduced by concomitant infusion of vasoactive intestinal peptide, but enhanced by lidocaine, suggesting that neural mechanisms reduced the secretory effect of NKA through release of vasoactive intestinal peptide from secretomotor neurones (Hallgren et al., 1998).
Recently, it has been reported that increases in Isc current in human colonic mucosa can be induced by either selective NK1 or NK2 receptor activation; however, unlike in rats, the effect of each agonist is selectively reduced by the respective antagonist (Tough et al., 2003). As mentioned above, no expression of NK2 receptors was found in human intestinal mucosa, although immunoreactivity was localised to inflammatory cells located beneath enterocytes (Renzi et al., 2000); it is unknown, however, whether NK2 receptor-induced secretory effects are mediated through inflammatory cells or rather whether the expression of NK2 receptors on enterocytes has remained undetected.
There are several studies associating the intestinal levels of TKs with diarrhoea symptoms in humans. A recent trial investigated the correlation between symptoms of diarrhoea and jejunal levels of SP or its mRNA in asymptomatic or symptomatic healthy volunteers challenged with Cryptosporidium parvum, and acquired immunodeficiency syndrome patients with naturally occurring cryptosporidiosis (Robinson et al., 2003). Both the frequency and the intensity of SP mRNA expression were positively correlated with symptoms of diarrhoea, since low epithelial and lamina propria SP mRNA levels were detected in about half of the jejunal biopsies from volunteers developing mild gastrointestinal symptoms, and high SP mRNA levels were present in all patients with severe symptoms, whereas asymptomatic volunteers and prechallenge biopsies showed no SP mRNA. Likewise, it has previously been shown (Makridis et al., 1999) that intraluminal jejunal TK content (NKA, NKB, and neuropeptide-kappa, excluding SP) was higher in patients with malignant midgut carcinoma characterised by diarrhoea symptoms than in healthy subjects. By comparison, SP levels were also increased in carcinoid patients, but in this case the difference compared with controls was not significant.
Role of NK2 receptors in the control of intestinal inflammation
By definition, no overt inflammation accompanies IBS; however, microscopic signs of inflammatory and immune activation, including increased mucosal permeability (Spiller et al., 2000) and expression of interleukin-1beta (Gwee et al., 2003), have recently been detected in post-infectious IBS (Barbara et al., 2002). Although TK-mediated pro-inflammatory effects are typically associated with the stimulation of NK1 receptors (Evangelista et al., 2003), there is evidence that NK2 receptors also participate in this response. The protective effects of NK2 receptor antagonists (SR48968) on trinitrobenzensulphonic acid-induced ileitis in guinea-pigs and colitis in rats (Mazelin et al., 1998), and acetic acid-induced rectocolitis in guinea-pigs (nepadutant) have been detected (Cutrufo et al., 2000), but it is unknown whether these effects are attributable to a reduction in the irritant-induced increase in intestinal permeability (Hallgren et al., 1998) or to a specific effect on the activity of inflammatory cells. The observations that levels of NK2 receptor mRNA are increased in lamina propria inflammatory cells located in intestinal segments of patients with inflammatory bowel disease (Renzi et al., 2000), and that nepadutant reduces myeloperoxidase activity associated with guinea-pig colitis (Cutrufo et al., 2000) would support a specific effect on intestinal inflammatory cells.
Role of NK2 receptors in the control of visceral hypersensitivity
An increase in visceral sensitivity to rectosigmoidal balloon distension (Verne et al., 2001) and a decrease in the associated pain threshold is commonly found in patients with IBS (Mertz, 2003).
There are several studies documenting the role of TK NK2 receptors in the modulation of intestinal hypersensitivity and hyperalgesia; most of these studies have used colorectal balloon distension as a model for inducing pseudoaffective reflexes and assessing visceral sensitivity (Ness & Gebhart, 1988). Noxious colorectal distension induces contractions of abdominal muscles (referred to as abdominal cramps) and a reflex inhibition of proximal colon motility. Using this model, it was established that the selective NK2 (but not NK1) receptor agonist GR64349 decreased the volume threshold for inducing abdominal cramps. Accordingly, intracerebroventricular or intraperitoneal administration of high doses of SR48968 reduced the number of abdominal cramps induced by colorectal distension (Julia et al., 1994). Likewise, intravenous administration of SR48968 reduced the number of abdominal cramps induced by intraperitoneal acetic acid or intravenous administration of CGRP, whereas GR64349 mimicked the effect of acetic acid or CGRP (Julia & Bueno, 1997). These results suggested that NK2 receptor antagonists reduced intestinal nociception by acting at a central nervous system site. However, two considerations cast doubt on this interpretation: first, the selective NK2 receptor agonist GR64349 is a peptide and is unlikely to penetrate the blood–brain barrier; second, SR48968 possesses some affinity for opioid receptors (Martin et al., 1993), which could explain the analgesic effects observed following high doses of the compound. Indeed, it has recently been found that intrathecal administration of SR48968, upto doses causing locomotor impairment, did not reverse colonic hyperalgesia in response to mechanical stimuli induced by intracolonic zymosan or repetitive colorectal distension (Kamp et al., 2001; Gaudreau & Plourde, 2003). These results excluded a contribution of spinal NK2 receptors to the modulation of colonic hyperalgesia, but they did not rule out a possible role of supraspinal NK2 receptors. Other studies have provided further evidence for the antinociceptive effect exerted by SR48968 at the visceral level: this NK2 receptor antagonist attenuated, in a dose-dependent manner, the fall in blood pressure induced by noxious jejunal distension in anaesthetised rats, without altering the basal blood pressure or jejunal compliance (McLean et al., 1998). The doses at which SR48968 was active (ED50=0.7 mg kg−1) were lower than those previously found to reduce abdominal cramps elicited by colonic distension (Julia et al., 1994), but a supraspinal site of action cannot be excluded completely. Interestingly, when jejunal distension was applied in Nyppostrongilus brasiliensis-infested rats, the threshold pressure for eliciting the depressor cardiovascular reflex decreased and the magnitude of this response increased; in these animals, the effective doses of SR48968 (ED50=0.1 mg kg−1) were significantly lower than in control rats (McLean et al., 1997), supporting the possibility of a peripheral site of action. Further evidence of the involvement of peripheral TK NK2 receptors in visceral hyperalgesia was obtained from investigations of the effect of the peptide NK2 receptor antagonist nepadutant (Figure 4). In control rats, this antagonist decreased the number of abdominal cramps produced by noxious colorectal distension. When the procedure was repeated in rats with colonic inflammation (trinitrobenzene sulfonic acid-induced colitis), the volume threshold for inducing a nociceptive response decreased, and nepadutant reversed this visceral hyperalgesia (Figure 4a) (Toulouse et al., 2000). This effect was associated with a reduction in inflammation-induced c-fos and c-jun proto-oncogene expression (a cytochemical marker of nociception) in the spinal cord and in specific dorsal root ganglion neurones which project to the colon, respectively (Birder et al., 2003). Likewise, following acetic acid-induced colitis, nepadutant reversed the increased discharge induced by colonic distension in dorsal horn neurones receiving inputs from the colon (lamina X) (Laird et al., 2001). Interestingly, the effects of nepadutant were not restricted to models of inflammatory visceral hyperalgesia; at similar doses (100 μg kg−1, i.v.), nepadutant also reduced the restraint stress-induced decrease in the volume threshold for eliciting abdominal cramps following colorectal distension in rats (Figure 4b) (Toulouse et al., 2000). Furthermore, nepadutant (100 μg kg−1, i.v.) reversed the sensitisation of the gastric distension-induced hypotensive cardiovascular reflex that follows chronic oral administration of the herbicide diquat, without affecting the cardiovascular reflex induced by somatic pinching (Anton et al., 2001). In contrast to controls, gastric distension induced degranulation of mucosal mast cells and a parallel increase in blood histamine levels in diquat-treated rats and these effects too were prevented by nepadutant. On the other hand, nepadutant alone did not affect hypermastocytosis.
Figure 4.
Effect of nepadutant (5–200 μg kg−1, i.v.) on the abdominal response to non-noxious rectal distension (0.4 ml) in trinitrobenzene sulphonic acid (TNBS)-treated rats (left panel) and in rats submitted to partial restraint stress (right panel). *P<0.01 vs Control or sham stress; +P<0.05, and ++P<0.01 vs NaCl (from Toulouse et al., 2000).
All these effects on visceral hypersensitivity imply a direct or indirect effect of NK2 receptor antagonists on the peripheral excitability of primary afferent fibres. We have proposed three different mechanisms whereby the stimulation of NK2 receptors could sensitise primary afferent fibres (Lecci et al., 2000). One possibility involves the expression of NK2 receptors on peripheral afferent fibres: this has been postulated to occur in rat urinary bladder on the basis of electrophysiological evidence (Morrison, 1999). This possibility has recently been supported by the finding that [betaAla8]neurokinin A-(4–10) enhanced the activity of L- and N-type calcium channels in freshly dissociated rat dorsal root ganglion neurones, an effect which was blocked by the selective NK2 receptor antagonist MEN10376 (Sculptoreanu & De Groat, 2003).
Another possible explanation for the inhibitory effect of NK2 receptor antagonists on visceral hypersensitivity involves the direct expression of these receptors on smooth muscle and/or epithelial cells. In fact, a direct coupling of these cells with primary afferent fibres through gap junctions and the ability of intracellular calcium signals generated by muscle stretch to invade afferent nerve terminals have been demonstrated (Ennes et al., 1999; Raybould et al., 1999). Although smooth muscle stretch and TK receptor stimulation activate a different repertoire of intracellular signals, both stimuli generate intracellular calcium waves in colonic myocytes (Young et al., 1999). Therefore, the possibility that the stimulation of smooth muscle NK2 receptors leads to activation of intracellular signals propagating from colonic muscle to sensory neurones cannot be excluded.
A third possibility for increasing the excitability of sensory nerves by NK2 receptor stimulation involves the release of mediators known to affect the excitability of neurones from other cells. As previously mentioned, NK2 receptor stimulation is capable of inducing histamine release from mucosal mast cells (Anton et al., 2001). There is also evidence that MEN10207 (100 nM), a first-generation NK2 receptor antagonist, decreases NKA-induced 6-keto-prostaglandin 2-alpha in canine ileum; however, the selective agonist [Nle10]NKA-(4–10) was not active in this respect, raising doubts about the antagonist selectivity in this preparation (Parrish et al., 1994). As mentioned above, recent results indicate that NK2 receptor agonists play an important role in the release of 5-HT from colonic enterocytes (Kojima et al., 2004). Given the established modulatory effect of 5-HT on intestinal afferent neurones (Tack & Sarnelli, 2002), the participation of 5-HT in the NK2 receptor-mediated increase in visceral sensitivity cannot be excluded.
Human in vitro studies
Functional changes in NK2 receptor-mediated colonic circular muscle contractions have been described in various intestinal diseases. In particular, the potency of NK2 receptor agonists and/or the maximal effect they produced was lower in specimens taken from patients with inflammatory bowel diseases, or diverticular disease compared to control preparations (Al-Saffar & Hellstrom, 2001; Menzies et al., 2001; Liu et al., 2002; Vrees et al., 2002). In sharp contrast, specimens from patients with idiopathic chronic constipation displayed supersensitivity to the contractile effects induced by selective NK2 receptor agonists (Menzies et al., 2001). Opposite results were reported in another study, where the potency of the contractile effect induced by the NK2 receptor agonist [Ala5, betaAla8]neurokinin A-(4–10) was markedly lower in colonic idiopathic chronic constipation specimens than in controls (Mitolo-Chieppa et al., 2001). In this study, the ‘off' contraction induced by electrical stimulation of colonic circular muscle strips from idiopathic chronic constipation patients was lower than in control strips; interestingly, incubation with a selective NK2 receptor agonist increased the amplitude of the ‘off' contraction in diseased but not in control strips, and this enhancement was antagonised by MEN10627 (Mitolo-Chieppa et al., 2001). On the other hand, the fact that MEN10627 did not significantly affect the ‘off' contraction in control preparations supports the previous results (see above), indicating that desensitising concentrations of NKA did not reduce the ‘off' contraction in the human colon in similar experimental conditions (Kolbel et al., 1994). Most recently, a selective impairment of tachykininergic NK2-mediated neuromuscular transmission has been detected in colonic circular muscle specimens taken from children affected by slow transit constipation (Stanton et al., 2003). These preparations displayed a normal contractile effect following carbachol or NKA challenge, thus indicating that the function of both muscarinic and TK receptors is not altered. However, in contrast to its effects in control preparations, SR48968 did not reduce electrical field stimulation-induced contraction in the circular muscle from diseased children, whereas the inhibitory effect induced by hyosciamine on this response was similar in control and diseased preparations (Stanton et al., 2003). These results imply that the deficit in NK2 receptor-mediated tachykininergic neuromuscular transmission observed in patients with constipation occurs mainly at the prejunctional level.
Overall, these results suggest that, in intestinal diseases characterised by inflammation and/or increased motility, an enhanced release of TKs downregulates NK2 receptor-mediated contractile mechanisms. On the other hand, findings from constipated patients are controversial since both post-junctional supersensitivity and desensitisation to NK2-mediated contractile mechanisms have been reported. Likewise, contrasting results regarding prejunctional mechanisms have been described: the NK2 receptor-mediated component of the contraction evoked by electrical field stimulation has been shown to be increased in one study (Mitolo-Chieppa et al., 2001) and reduced in another (Stanton et al., 2003).
Clinical studies
Currently, no selective NK2 receptor antagonists have been approved for the treatment of human diseases. Otilonium bromide, a spasmolytic drug particularly effective in reducing abdominal pain in IBS patients (Battaglia et al., 1998), possesses a moderate affinity (Ki about 7 μM) for human NK2 receptors, as evaluated by the displacement of labelled NKA from NK2 receptors expressed on CHO cells (Santicioli et al., 1999). However, since at similar concentrations otilonium also displays antimuscarinic and calcium channel blocker properties (Santicioli et al., 1999), the contribution of NK2 receptor antagonism to its therapeutic effect is unclear. Nepadutant (MEN11420) and saredutant (SR48968), two selective NK2 receptor antagonists, are being clinically developed for IBS treatment. In the isolated human colon circular muscle, nepadutant is a potent, selective, competitive, and reversible antagonist of NK2 receptor-mediated contractile and electrical events, whereas the behaviour of saredutant is pseudo-irreversible and non-competitive (Santicioli et al., 1997; Croci et al., 1998; Patacchini et al., 2000).
In the initial clinical study, intravenous administration of single ascending doses of nepadutant (0.1–32 mg) to healthy volunteers was safe and well-tolerated, and therefore a further phase I trial was scheduled in order to determine the pharmacodynamic effects of nepadutant on NKA-induced motor effects in the small intestine (Lordal et al., 2001). After having baseline motility recorded, volunteers received saline or nepadutant (8 mg i.v.), and then saline or NKA (25 pmol kg min−1) was intravenously infused for 4 h. NKA increased the fraction of time occupied by phase II-type intestinal motility, while phase I motility was decreased and phase III motility remained unchanged, that is, NKA shifted the motility pattern from a fasted to a fed state. Moreover, the frequency and amplitude of contractions, and the motility index of the phase II motility pattern, increased during NKA infusion. In the group receiving nepadutant, the motility changes induced by NKA were significantly inhibited, but normal motility was not affected. Several adverse events were recorded in the group receiving placebo and NKA; these included: flush, of either the face or whole body, borborhygmi, abdominal pain, headache, nausea, and vomiting. In contrast, NKA only induced flushes in nepadutant-treated patients, suggesting that intestinal, IBS-like adverse events are related to NK2 receptor stimulation (Lordal et al., 2001), whereas flushes are probably mediated by NK1 receptors (Newby et al., 1999). A further pharmacodynamic phase I study was carried out in a model mimicking the rectal hypersensitivity that occurs in IBS patients. It has recently been reported that intraluminal injection of glycerol induces a mild inflammation of the mucosa and affects perception during colorectal balloon distension (Bouin et al., 2001). Therefore, the effect of nepadutant (8 mg i.v.) was assessed in glycerol-treated volunteers. Nepadutant significantly increased compliance in glycerol-treated subjects and tended to reduce the sensitisation to the defecation stimulus produced by glycerol (M. Delavaux, unpublished results).
Overall, these results indicate that nepadutant antagonises intestinal motor effects and IBS-like symptoms induced by NKA without affecting basal motility. The dose of nepadutant that counteracts the effects of NKA also increases colorectal compliance following intrarectal glycerol, supporting preclinical findings indicating that endogenous TKs acting at NK2 receptors contribute to maintaining intestinal tone following inflammation.
Conclusions
Extensive preclinical studies support the concept that NK2 receptor antagonists can attenuate increased intestinal motility, secretion, and visceral sensitivity, by acting at the peripheral level. We speculate that the main mechanism whereby these antagonists reduce intestinal hypermotility involves the prejunctional modulation of the activity of cholinergic motoneurones, since, following inflammation, atropine abolished the enhancement of contractile activity induced by electrical field stimulation in vitro (Natale et al., 2003), or by colonic distension in vivo (Carini et al., 2001). On the other hand, NK2 receptor antagonists can potentially reactivate inhibited motility by modulating sympathetic and NANC inhibitory pathways. In vitro, low concentrations of NK2 receptor antagonists increase colonic propulsion velocity by reducing the activity of NANC inhibitory pathways (Onori et al., 2000). This result leads us to speculate that IBS phases characterised by constipation could be treated with low doses of antagonists, whereas diarrhoea-prevalent IBS patients might benefit from higher doses of NK2 receptor antagonists. TK NK2 receptor antagonists also reduce luminal water and mucin secretions associated with stress or inflammation; experimental studies suggest that these antisecretory effects could be due to the blockade of NK2 receptors expressed on enterocytes. Finally, NK2 receptor antagonists modulate the firing of pelvic afferents induced by mechanical stimulation and reduce the transmission of noxious stimuli to the spinal cord following inflammation or stress. Two hypotheses can be put forward to explain these effects: (i) endogenous TKs acting through NK2 receptors exert a direct modulatory effect on primary afferent neurones; (ii) endogenous TKs acting through NK2 receptors promote the release of other mediators (e.g., 5-HT) which in turn sensitise primary afferent neurones. As far as the first possibility is concerned, there is evidence for the presence of NK2 receptors on cultured rat sensory neurones (Brechenmacher et al., 1998). Furthermore, following antigen exposure, NK2 receptor agonists are capable of depolarising and switching on the firing of neurones from freshly isolated nodose ganglion of sensitised guinea-pigs (Moore et al., 2000). On the other hand, 5-HT3 receptors play a permissive role in the above-mentioned response (Moore et al., 2002), thus indicating that the two mechanisms proposed are not mutually exclusive. As mentioned before, NK2 receptor-triggered nerve sensitisation could also theoretically be mediated by the stimulation of muscle receptors where intracellular signals directly propagate to the neighbouring nerve terminal. This possibility is suggested when the dynamics of NK2 receptor-mediated contractile function on jejunal smooth muscle cells and the visceral hypersensitivity to jejunal distension that develops following parasitic infestation are considered (Faussone-Pellegrini et al., 2002).
Studies performed in healthy volunteers support the concept that NK2 receptors could be responsible for the IBS-like symptoms induced by NKA. Preliminary results also suggest a role for NK2 receptors in the regulation of rectal compliance following mucosal inflammation. Overall, these results encourage further assessment of the efficacy of this class of drugs in IBS patients.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CHO
Chinese ovary hamster
- 5-HT
5-hydroxytryptamine or serotonin
- IBS
irritable bowel syndrome
- NANC
non-adrenergic non-cholinergic
- NKA
neurokinin A
- NKB
neurokinin B
- SP
substance P
- TK
tachykinin
- TRPV-1
transient receptor potential vanilloid-1
References
- AL-SAFFAR A., HELLSTROM P.M. Contractile responses to natural tachykinins and selective tachykinin analogs in normal and inflamed ileal and colonic muscle. Scand. J. Gastroenterol. 2001;36:485–493. [PubMed] [Google Scholar]
- ANTON P.M., THEODOROU V., FIORAMONTI J., BUENO L. Chronic low-level administration of diquat increases the nociceptive response to gastric distension in rats: role of mast cells and tachykinin receptor activation. Pain. 2001;92:219–227. doi: 10.1016/s0304-3959(01)00257-3. [DOI] [PubMed] [Google Scholar]
- BARBARA G., DE GIORGIO R., STANGHELLINI V., CREMON C., CORINALDESI R. A role for inflammation in irritable bowel syndrome. Gut. 2002;51 Suppl I:i41–i44. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASILISCO G., PHILLIPS S.F. A selective NK2 antagonist blocks the increase of canine colonic tone and contractions induced by the NK2 selective agonist [betaAla8]neurokinin A-(4–10) Aliment. Pharmacol. Ther. 1994;8:527–533. doi: 10.1111/j.1365-2036.1994.tb00326.x. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA G., MORSELLI-LABATE A.M., CAMARRI E., FRANCAVILLA A., DE MARCO F., MASTROPAOLO G., NACCARATO R. Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment. Pharmacol. Ther. 1998;12:1003–1010. doi: 10.1046/j.1365-2036.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- BAYGUINOV O., HAGEN B., SANDERS K. Substance P modulates localized calcium transients and memmbrane current responses in murine colonic myocytes. Br. J. Pharmacol. 2003;138:1233–1243. doi: 10.1038/sj.bjp.0705139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRDER L.A., KISS S., DE GROAT W.C., LECCI A., MAGGI C.A. Effect of nepadutant, a neurokinin 2 tachykinin receptor antagonist, on immediate-early gene expression after trinitrobenzenesulfonic acid-induced colitis in the rat. J. Pharmacol. Exp. Ther. 2003;304:272–276. doi: 10.1124/jpet.102.042077. [DOI] [PubMed] [Google Scholar]
- BOUIN M., DELVAUX M., BLANC C., LAGIER E., DELISLE M.-B., FIORAMONTI J., BUENO L., FREXINOS J. Intrarectal injection of glycerol induces hypersensitivity to rectal distension in healthy subjects without modifying rectal compliance. Eur. J. Gastroenterol. Hepatol. 2001;13:573–580. doi: 10.1097/00042737-200105000-00018. [DOI] [PubMed] [Google Scholar]
- BRECHENMACHER C., LARMET Y., FELTZ P., RODEAU J.L. Cultured rat sensory neurones express functional tachykinin receptor subtypes 1, 2 and 3. Neurosci. Lett. 1998;241:159–162. doi: 10.1016/s0304-3940(98)00045-7. [DOI] [PubMed] [Google Scholar]
- BRIERLEY S.M., NICHOLS K., GRASBY D.J., WATERMAN S.A. Neural mechanisms underlying motor complex formation in mouse isolated colon. Br. J. Pharmacol. 2001;132:507–517. doi: 10.1038/sj.bjp.0703814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMILLERI M. Pathophysiology in irritable bowel syndrome. Drug News Perspect. 2001;14:268–278. doi: 10.1358/dnp.2001.14.5.704648. [DOI] [PubMed] [Google Scholar]
- CAO W., PRICOLO V.E., ZHANG L., BEHAR J., BIANCANI P., KIRBER M.T. Gq-linked NK2 receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology. 2000;119:51–61. doi: 10.1053/gast.2000.8552. [DOI] [PubMed] [Google Scholar]
- CARINI F., LECCI A., TRAMONTANA M., GIULIANI S., MAGGI C.A. Tachykinin NK2 receptors and enhancement of cholinergic transmission in the inflamed rat colon: an in vivo motility study. Br. J. Pharmacol. 2001;133:1107–1113. doi: 10.1038/sj.bjp.0704164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATALIOTO R.-M., CRISCUOLI M., CUCCHI P., GIACHETTI A., GIANNOTTI D., GIULIANI S., LECCI A., LIPPI A., PATACCHINI R., QUARTARA L., RENZETTI A.R., TRAMONTANA M., ARCAMONE F., MAGGI C.A. MEN 11420 (Nepadutant), a novel glycosylated bicyclic peptide tachykinin NK2 receptor antagonist. Br. J. Pharmacol. 1998;123:81–91. doi: 10.1038/sj.bjp.0701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG F.Y., LEE S.D., YEH G.H., WANG P.S. Rat gastrointestinal motor responses mediated by activation of neurokinin receptors. J. Gastroenterol. Hepatol. 1999;14:39–45. doi: 10.1046/j.1440-1746.1999.01808.x. [DOI] [PubMed] [Google Scholar]
- CHANG F.Y., SHARP D., OUYANG A. Multiple neurokinin receptor subtypes are present in the colon of cat and rat. Ann. N.Y. Acad. Sci. 1991;632:374–376. doi: 10.1111/j.1749-6632.1991.tb33128.x. [DOI] [PubMed] [Google Scholar]
- CICCOCIOPPO R., ONORI L., MESSORI E., CANDURA S.M., COCCINI T., TONINI M. Role of nitric oxide-dependent and -independent mechanisms in peristalsis and accommodation in the rabbit distal colon. J. Pharmacol. Exp. Ther. 1994;270:929–937. [PubMed] [Google Scholar]
- COLLINS S.M. Stress and the gastrointestinal tract. IV. Modulation of intestinal inflammation by stress: basic mechanism and clinical relevance. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- COSTA M., BROOKES S.J., STEELE P.A., GIBBINS I., BURCHER E., KANDIAH C.J. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- COX H.M., TOUGH I.R., GRAYSON K., YARROW S. Pharmacological characterization of neurokinin receptors mediating anion secretion in rat descending colon mucosa. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:172–177. doi: 10.1007/BF00164795. [DOI] [PubMed] [Google Scholar]
- CROCI T., AUREGGI G., MANARA L., EMONDS-ALT X., LE FUR G., MAFFRAND J.-P., MUKENGE S., FERLA G. In vitro characterisation of tachykinin NK2-receptors modulating motor responses of human colonic muscle strips. Br. J. Pharmacol. 1998;124:1321–1327. doi: 10.1038/sj.bjp.0701960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., EMONDS-ALT X., MANARA L. SR48968 selectively prevents faecal excretion following activation of tachykinin NK2 receptors in rats. J. Pharm. Pharmacol. 1994;46:383–385. doi: 10.1111/j.2042-7158.1994.tb03819.x. [DOI] [PubMed] [Google Scholar]
- CROCI T., LANDI M., EMONDS-ALT X., LE FUR G., MAFFRAND J.-P., MANARA L. Role of tachykinins in castor oil diarrhoea in rats. Br. J. Pharmacol. 1997;121:375–380. doi: 10.1038/sj.bjp.0701130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTRUFO C., EVANGELISTA S., CIRILLO R., CIUCCI A., LOPEZ G., MANZINI S., MAGGI C.A. Protective effect of the tachykinin NK2 receptor antagonist nepadutant in acute rectocolitis induced by diluted acetic acid in guinea-pigs. Neuropeptides. 2000;34:355–359. doi: 10.1054/npep.2000.0819. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO G., ERBELDING D., KILBINGER H. Tachykinin NK2 receptors facilitate acetylcholine release from guinea-pig isolated trachea. Eur. J. Pharmacol. 2000;396:29–32. doi: 10.1016/s0014-2999(00)00199-0. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., CREMA F., MORO E., NARDELLI G., CROCI T., FRIGO G.M. Intestinal motor stimulation by the 5-HT4 receptor agonist ML10302: differential involvement of tachykininergic pathways in the canine small bowel nd colon. Neurogastroenterol. Motil. 2001;13:543–553. doi: 10.1046/j.1365-2982.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- DESVIGNES C., ROUQUIER L., SOUILHAC J., MONS G., RODIER D., SOUBRIÉ P., STEINBERG R. Control by tachykinin NK2 receptors of CRF1 receptor-mediated activation of hippocampal acetylcholine release in the rat and guinea-pig. Neuropeptides. 2003;37:89–97. doi: 10.1016/s0143-4179(03)00019-2. [DOI] [PubMed] [Google Scholar]
- DROSSMAN D.A., CAMILLERI M., MAYER E., WHITEHEAD W.E. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- DROSSMAN D.A., RINGEL Y., VOGT B.A., LESERMAN J., LIN W., SMITH J.K., WHITEHEAD W. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology. 2003;124:754–761. doi: 10.1053/gast.2003.50103. [DOI] [PubMed] [Google Scholar]
- ENNES H.S., YOUNG S.H., GOLIGER J.A., MAYER E.A. Chemical signaling from colonic smooth muscle cells to DRG neurons in culture. Am. J. Physiol. 1999;276:C602–C610. doi: 10.1152/ajpcell.1999.276.3.C602. [DOI] [PubMed] [Google Scholar]
- EUTAMENE H., THEODOROU V., FIORAMONTI J., BUENO L. Implication of NK1 and NK2 receptors in rat colonic hypersecretion induced by interleukin 1beta: role of nitric oxide. Gastroenterology. 1995;109:483–489. doi: 10.1016/0016-5085(95)90336-4. [DOI] [PubMed] [Google Scholar]
- EUTAMENE H., THEODOROU V., FIORAMONTI J., BUENO L. Rectal distension-induced colonic net water secretion in rats involves tachykinins, capsaicin sensory, and vagus nerves. Gastroenterology. 1997;112:1595–1602. doi: 10.1016/s0016-5085(97)70041-6. [DOI] [PubMed] [Google Scholar]
- EVANGELISTA S. Involvement of tachykinins in intestinal inflammation. Curr. Pharm. Des. 2001;7:19–30. doi: 10.2174/1381612013398446. [DOI] [PubMed] [Google Scholar]
- EVANGELISTA S., PATACCHINI R., MAGGI C.A. Role of peripheral tachykinin receptors in neurogenic inflammation of the respiratory, genitourinary and gastrointestinal systems. Curr. Med. Chem. 2003;2:157–174. [Google Scholar]
- FAUSSONE-PELLEGRINI M.-S., GAY J., VANNUCCHI M.-G., CORSANI L., FIORAMONTI J. Alterations of neurokinin receptors and interstitial cells of Cajal during and after jejunal inflammation induced by Nippostrongylus brasiliensis in the rat. Neurogastroenterol. Motil. 2002;14:83–95. doi: 10.1046/j.1365-2982.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- FRIELING T., DOBREVA G., WEBER E., BECKER K., RUPPRECHT C., NEUNLIST M., SCHEMANN M. Different tachykinin receptors mediate chloride secretion in the distal colon through activation of submucosal neurones. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:71–79. doi: 10.1007/pl00005327. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., SANGER G.J. Intrinsic nerve circuits of the gastrointestinal tract: identification of drug targets. Curr. Opin. Pharmacol. 2002;2:616–622. doi: 10.1016/s1471-4892(02)00219-9. [DOI] [PubMed] [Google Scholar]
- GAUDREAU G.A., PLOURDE V. Role of tachykinin NK1, NK2 and NK3 receptors in the modulation of visceral hyperalgesia. Neurosci. Lett. 2003;351:59–62. doi: 10.1016/s0304-3940(03)00414-2. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., LECCI A., GIACHETTI A., MAGGI C.A. Tachykinins and reflexly evoked atropine-resistant motility in the guinea-pig colon in vivo. J. Pharmacol. Exp. Ther. 1993;265:1224–1231. [PubMed] [Google Scholar]
- GIULIANI S., MAGGI C.A., ROVERO P., MELI A. Neurokinins induce a relaxation of rat duodenum in vivo by activating postganglionic sympathetic elements in prevertebral ganglia: involvement of an NK2 type of neurokinin receptors. J. Pharmacol. Exp. Ther. 1988;246:322–327. [PubMed] [Google Scholar]
- GIULIANI S., TRAMONTANA M., LECCI A., MAGGI C.A. Tachykinin receptors mediate atropine-resistant rat duodenal contractions in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:327–335. doi: 10.1007/BF00171064. [DOI] [PubMed] [Google Scholar]
- GOLDBLATT F., GORDON T.P., WATERMAN S.A. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology. 2002;123:1144–1150. doi: 10.1053/gast.2002.36057. [DOI] [PubMed] [Google Scholar]
- GRADY E.F., BALUK P., BOHM S., GAMP P.D., WONG H., PAYAN D.G., ANSEL J., PORTBURY A., FURNESS J.B., MCDONALD D.M., BUNNETT N.W. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIDER J.R., FOXX-ORENSTEIN A.E., JIN J.-G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea-pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- GWEE K.A., COLLINS S.M., READ N.W., RAJNAKOVA A., DENG Y., GRAHAM J.C., MCKENDRICK M.W., MOOCHHALA S.M. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLGREN A., FLEMSTROM G., HELLSTROM P.M., LORDAL M., HELLGREN S., NYLANDER O. Neurokinin A increases duodenal mucosal permeability, bicarbonate secretion and fluid output in the rat. Am. J. Physiol. 1997;273:G1077–G1086. doi: 10.1152/ajpgi.1997.273.5.G1077. [DOI] [PubMed] [Google Scholar]
- HALLGREN A., FLEMSTROM G., NYLANDER O. Interaction between neurokinin A, VIP, prostanoids in regulation of duodenal function. Am. J. Physiol. 1998;275:G95–G103. doi: 10.1152/ajpgi.1998.275.1.G95. [DOI] [PubMed] [Google Scholar]
- HELLSTROM P.M., SODER O., THEODORSSON E. Occurrence, release, and effects of multiple tachykinins in cat colonic tissue and nerves. Gastroenterology. 1991;100:431–440. doi: 10.1016/0016-5085(91)90213-5. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Stimulation and inhibition of gastrointestinal propulsion induced by substance P and substance K in the rat. Br. J. Pharmacol. 1985;86:305–312. doi: 10.1111/j.1476-5381.1985.tb09462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., HOLZER-PETSCHE U. Tachykinin receptors in the gut: physiological and pathological implications. Curr. Opin. Pharmacol. 2001;1:583–590. doi: 10.1016/s1471-4892(01)00100-x. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T.H., HEINEMANN A., BARTHO L. Tachykinin NK1 and NK2 receptor-mediated control of peristaltic propulsion in the guinea-pig small intestine in vitro. Neuropharmacology. 1998;37:131–138. doi: 10.1016/s0028-3908(97)00195-0. [DOI] [PubMed] [Google Scholar]
- HOLZER P., MAGGI C.A. Synergistic role of muscarinic acetylcholine and tachykinin NK2 receptors in intestinal peristalsis. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;349:194–201. doi: 10.1007/BF00169837. [DOI] [PubMed] [Google Scholar]
- JULIA V., BUENO L. Tachykininergic mediation of viscerosensitive responses to acute inflammation in rats: role of CGRP. Am. J. Physiol. 1997;272:G141–G146. doi: 10.1152/ajpgi.1997.272.1.G141. [DOI] [PubMed] [Google Scholar]
- JULIA V., MORTEAU O., BUENO L. Involvement of neurokinin 1 and 2 receptors in viscerosensitive response to rectal distension in rats. Gastroenterology. 1994;107:94–102. doi: 10.1016/0016-5085(94)90065-5. [DOI] [PubMed] [Google Scholar]
- KAMP E.H., BECK D.R., GEBHART G.F. Combinations of neurokinin receptor antagonists reduce visceral hyperalgesia. J. Pharmacol. Exp. Ther. 2001;299:105–113. [PubMed] [Google Scholar]
- KAWABATA A., KINOSHITA M., NISHIKAWA H., KURODA R., NISHIDA M., ARAKI H., ARIZONO N., ODA Y., KAKEHI K. The protease-activated receptor-2 agonist induces gastric mucous secretion and mucosal cytoprotection. J. Clin. Invest. 2001;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN I., COLLINS S.M. Fourth isoform of preprotachykinin messenger RNA encoding for substance P in the rat intestine. Biochem. Biophys. Res. Commun. 1994;114:533–540. doi: 10.1006/bbrc.1994.2000. [DOI] [PubMed] [Google Scholar]
- KIRKUP A.J., BRUNSDEN A.M., GRUNDY D. Receptors and transmission in the brain–gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- KOJIMA S., UEDA S., IKEDA M., KAMIKAWA Y. Calcitonin gene-related peptide facilitates serotonin release from guinea-pig colonic mucosa via myenteric neurons and tachykinin NK2/NK3 receptors. Br. J. Pharmacol. 2004;141:385–390. doi: 10.1038/sj.bjp.0705624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLBEL C.B., MAYER E.A., HOLTMANN G., LAYER P., PETSCHULAT W., ERHARD J. Effects of neurokinins on human colonic motility. Neurogastroenterol. Motil. 1994;6:119–127. [Google Scholar]
- LAIRD J.M.A., OLIVAR T., LOPEZ-GARCIA J.A., MAGGI C.A., CERVERO F. Response of rat spinal neurons to distension of inflamed colon: role of tachykinin NK2 receptors. Neuropharmacology. 2001;40:696–701. doi: 10.1016/s0028-3908(00)00205-7. [DOI] [PubMed] [Google Scholar]
- LECCI A., CARINI F., TRAMONTANA M., D'ARANNO V., MARINONI E., CREA A., BUENO L., FIORAMONTI J., CRISCUOLI M., GIULIANI S., MAGGI C.A. Nepadutant pharmacokinetics and dose–effect relationships as tachykinin NK2 receptor antagonist are altered by intestinal inflammation in rodent models. J. Pharmacol. Exp. Ther. 2001;299:247–254. [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., CARINI F., MAGGI C.A. Peripheral actions of tachykinins. Neuropeptides. 2000;34:303–313. doi: 10.1054/npep.2000.0825. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., DE GIORGIO R., MAGGI C.A. The role of tachykinin NK1 and NK2 receptors in atropine-resistant colonic propulsion in anaesthetized guinea-pigs. Br. J. Pharmacol. 1998;124:27–34. doi: 10.1038/sj.bjp.0701789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECCI A., SANTICIOLI P., MAGGI C.A. Pharmacology of transmission to gastrointestinal muscle. Curr. Opin. Pharmacol. 2002b;2:630–641. doi: 10.1016/s1471-4892(02)00225-4. [DOI] [PubMed] [Google Scholar]
- LECCI A., VALENTI C., MAGGI C.A. Tachykinin receptor antagonists in irritable bowel syndrome. Curr. Opin. Ivestig. Drugs. 2002a;3:589–601. [PubMed] [Google Scholar]
- LIU L., SHANG F., MARKUS I., BURCHER E. Roles of substance P receptors in human colon circular muscle: alterations in diverticular disease. J. Pharmacol. Exp. Ther. 2002;302:627–635. doi: 10.1124/jpet.102.034702. [DOI] [PubMed] [Google Scholar]
- LORDAL M., HALLGREN A., NYLANDER O., HELLSTROM P.M. Tachykinins increase vascular permeability in the gastrointestinal tract of the rat. Acta Physiol. Scand. 1996;156:489–494. doi: 10.1046/j.1365-201X.1996.457174000.x. [DOI] [PubMed] [Google Scholar]
- LORDAL M., NAVALESI G., THEODORSSON E., MAGGI C.A., HELLSTROM P.M. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin A in small intestine. Br. J. Pharmacol. 2001;134:215–223. doi: 10.1038/sj.bjp.0704217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA R.C., SZURSZEWSKI J.H. Modulation by opioid peptides of mechanosensory pathways supplying the guinea-pig inferior mesenteric ganglion. J. Physiol. 1996;491:435–445. doi: 10.1113/jphysiol.1996.sp021227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Principles of tachykininergic co-transmission in the peripheral and enteric nervous system. Regul. Pept. 2000;93:53–64. doi: 10.1016/s0167-0115(00)00177-4. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S. Evidence that tachykinin NK2 receptors modulate resting tone in rat isolated small intestine. Br. J. Pharmacol. 1996;118:1262–1268. doi: 10.1111/j.1476-5381.1996.tb15532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., SANTICIOLI P., PATACCHINI R., MELI A. Neural pathways and pharmacological modulation of defecation reflex. Gen. Pharmacol. 1988;19:517–523. doi: 10.1016/0306-3623(88)90157-7. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., ZAGORODNYUK V. Sequential activation of the triple excitatory transmission to the circular muscle of guinea-pig colon. Neuroscience. 1997;79:263–274. doi: 10.1016/s0306-4522(96)00659-8. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., ZAGORODNYUK V., GIULIANI S. Specialization of tachykinin NK1 and NK2 receptors in producing fast and slow atropine-resistant neurotransmission to the circular muscle of the guinea-pig colon. Neuroscience. 1994;63:1137–1152. doi: 10.1016/0306-4522(94)90579-7. [DOI] [PubMed] [Google Scholar]
- MAKRIDIS C., THEODORSSON E., AKERSTROM G., OBERG K., KNUTSON L. Increased intestinal non-substance P tachykinin concentrations in malignant midgut carcinoid disease. J. Gastroenterol. Hepatol. 1999;14:500–507. doi: 10.1046/j.1440-1746.1999.01888.x. [DOI] [PubMed] [Google Scholar]
- MARTIN C.A.E., EMONDS-ALT X., ADVENIER C. Inhibition of cholinergic neurotransmission in isolated guinea-pig bronchi by SR48968. Eur. J. Pharmacol. 1993;243:309–312. doi: 10.1016/0014-2999(93)90192-k. [DOI] [PubMed] [Google Scholar]
- MAZELIN L., THEDOROU V., MORE J., EMONDS-ALT X., FIORAMONTI J., BUENO L. Comparative effects of non peptide tachykinin receptor antagonists on experimental gut inflammation in rats and guinea-pigs. Life Sci. 1998;63:293–304. doi: 10.1016/s0024-3205(98)00271-9. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., GARCIA-VILLAR R., FIORAMONTI J., BUENO L. Effects of tachykinin receptor antagonists on rat jejunal distension pain response. Eur. J. Pharmacol. 1998;345:247–252. doi: 10.1016/s0014-2999(98)00040-5. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., PICARD C., GARCIA-VILLAR R., MORÉ J., FIORAMONTI J., BUENO L. Effects of nematode infection on sensitivity to intestinal distension: role of tachykinin NK2 receptors. Eur. J. Pharmacol. 1997;337:279–282. doi: 10.1016/s0014-2999(97)01275-2. [DOI] [PubMed] [Google Scholar]
- MENZIES J.R.W., MCKEE R., CORBETT A.D. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul. Pept. 2001;99:151–156. doi: 10.1016/s0167-0115(01)00244-0. [DOI] [PubMed] [Google Scholar]
- MERTZ H.R. Irritable bowel syndrome. New Engl. J. Med. 2003;349:2136–2146. doi: 10.1056/NEJMra035579. [DOI] [PubMed] [Google Scholar]
- MESSENGER J.P., GIBBINS I.L. Differential distribution of substance P binding sites in guinea-pig sympathetic ganglia. J. Auton. Nerv. Syst. 1998;69:103–114. doi: 10.1016/s0165-1838(98)00007-1. [DOI] [PubMed] [Google Scholar]
- MITOLO-CHIEPPA D., MANSI G., NACCI C., DE SALVIA M.A., MONTAGNANI M., POTENZA M.A., RINALDI R., LERRO G., SIRO-BRIGIANI G., MITOLO C.I., RINALDI M., ALTOMARE D.F., MEMEO V. Idiopathic chronic constipation: tachykinins as cotransmitters in colonic contraction. Eur. J. Clin. Invest. 2001;31:349–355. doi: 10.1046/j.1365-2362.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- MOORE K.A., OH E.J., WEINREICH D. 5-HT3 receptors mediate inflammation-induced unmasking of functional tachykinin responses in vitro. J. Appl. Physiol. 2002;92:2529–2534. doi: 10.1152/japplphysiol.00974.2001. [DOI] [PubMed] [Google Scholar]
- MOORE K.A., UNDEM B.J., WEINREICH D. Antigen inhalation unmasks NK2 tachykinin receptor-mediated responses in vagal afferents. Am. J. Respir. Crit. Care Med. 2000;161:232–236. doi: 10.1164/ajrccm.161.1.9903091. [DOI] [PubMed] [Google Scholar]
- MORRISON J.F.B. The activation of bladder wall afferent nerves. Exp. Physiol. 1999;84:131–136. doi: 10.1111/j.1469-445x.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- MULÈ F., D'ANGELO S., TABACCHI G., SERIO R. Involvement of tachykinin NK2 receptors in the modulation of spontaneous motility in rat proximal colon. Neurogastroenterol. Motil. 2000;12:459–466. doi: 10.1046/j.1365-2982.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- MULÈ F., PIZZUTI R., CAPPARELLI A., VERGNOLLE N. Evidence for the presence of functional protease activated receptor 4 (PAR4) in the rat colon. Gut. 2004;53:229–234. doi: 10.1136/gut.2003.021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATALE L., PIEPOLI A.L., DE SALVIA M.A., DE SALVATORE G., MITOLO C.I., MARZULLO A., PORTINCASA P., MOSCHETTA A., PALASCIANO G., MITOLO-CHIEPPA D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur. J. Clin. Invest. 2003;33:704–712. doi: 10.1046/j.1365-2362.2003.01200.x. [DOI] [PubMed] [Google Scholar]
- NESS T.J., GEBHART G.F. Colorectal distension as a noxious visceral stimulus: physiologic characterisation of pseudoaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- NEWBY D.E., SCIBERRAS D.G., FERRO C.J., GERTZ B.I., SOMMERVILLE D., MAJUMDAR A., LOWRY R.C., WEBB D.J. Substance P-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. Br. J. Clin. Pharmacol. 1999;48:336–344. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONORI L., AGGIO A., TADDEI G., TONINI M. Contribution of NK2 tachykinin receptors to propulsion in the rabbit distal colon. Am. J. Physiol. 2000;278:G137–G147. doi: 10.1152/ajpgi.2000.278.1.G137. [DOI] [PubMed] [Google Scholar]
- PARKMAN H.P., MA R.C., STAPELFELDT W.H., SZURSZEWSKI J.H. Direct and indirect mechanosensory pathways from the colon to the inferior mesenteric ganglion. Am. J. Physiol. 1993;265:G499–G505. [Google Scholar]
- PARLANI M., CONTE B., CIRILLO R., MANZINI S. Characterization of tachykinin NK2 receptor on dog proximal colon. Antagonism by MEN10627 and SR48968. Eur. J. Pharmacol. 1996;318:419–424. doi: 10.1016/s0014-2999(96)00799-6. [DOI] [PubMed] [Google Scholar]
- PARRISH M.B., RAINSFORD K.D., JOHNSON D.M., DANIEL E.E. NK1 receptors mediate relaese of 6-keto-PGF1 alpha from ex vivo perfused canine ileum. J. Pharmacol. Exp. Ther. 1994;271:39–47. [PubMed] [Google Scholar]
- PATACCHINI R., COX H.M., STAHL S., TOUGH I.R., MAGGI C.A. Tachykinin NK2 receptor mediates contraction and ion transport in rat colon by different mechanisms. Eur. J. Pharmacol. 2001;415:277–283. doi: 10.1016/s0014-2999(01)00836-6. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., GIULIANI S., TURINI A., NAVARRA G., MAGGI C.A. Effect of nepadutant at tachykinin NK2 receptors in human intestine and urinary bladder. Eur. J. Pharmacol. 2000;398:389–397. doi: 10.1016/s0014-2999(00)00346-0. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., LECCI A., HOLZER P., MAGGI C.A. Newly discovered tachykinins raise new questions about their peripheral roles and the tachykinin nomenclature. Trends Pharmacol. Sci. 2004;25:1–3. doi: 10.1016/j.tips.2003.11.005. [DOI] [PubMed] [Google Scholar]
- PORTBURY A.L., FURNESS J.B., SOUTHWELL B.R., WONG H., WALSH J.H., BUNNETT N. Distribution of neurokinin-2 receptors in the guinea-pig intestinal tract. Cell Tissue Res. 1996;286:281–292. doi: 10.1007/s004410050698. [DOI] [PubMed] [Google Scholar]
- RAYBOULD H.E., GSCHOSSMAN J.M., ENNES H., LEMBO T., MAYER E.A. Involvement of stretch-sensitive calcium flux in mechanical transduction in visceral afferents. J. Auton. Nerv. Syst. 1999;75:1–6. doi: 10.1016/s0165-1838(98)00146-5. [DOI] [PubMed] [Google Scholar]
- RENZI D., PELLEGRINI B., TONELLI F., SURRENTI C., CALABRO A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am. J. Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON P., OKHUYSEN P.C., CHAPPELL C.L., WEINSTOCK J.V., LEWIS D.E., ACTOR J.K., WHITE C., JR Substance P expression correlates with severity of diarrhoea in Cryptosporidiosis. J. Infect. Dis. 2003;188:290–296. doi: 10.1086/376836. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., GIULIANI S., PATACCHINI R., TRAMONTANA M., CRISCUOLI M., MAGGI C.A. MEN11420, a potent and selective tachykinin NK2 receptor antagonist in the guinea-pig and human colon. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:678–688. doi: 10.1007/pl00005105. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., ZAGORODNYUK V., RENZETTI A.R., MAGGI C.A. Antimuscarinic, calcium channel blocker, and tachykinin NK2 receptor antagonist actions of otilonium bromide in the circular muscle of guinea-pig colon. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:420–427. doi: 10.1007/pl00005370. [DOI] [PubMed] [Google Scholar]
- SCHMIDT P.T., BOZKURT A., HELLSTROM P.M. Tachykinin-stimulated small bowel myoelectric pattern: sensitisation by NO inhibition, reversal by neurokinin receptor blockade. Regul. Pept. 2002;105:15–21. doi: 10.1016/s0167-0115(01)00369-x. [DOI] [PubMed] [Google Scholar]
- SCULPTOREANU A., DE GROAT W.C. Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in dorsal root ganglia neurons of the adult rat. J. Neurophysiol. 2003;90:21–31. doi: 10.1152/jn.00108.2003. [DOI] [PubMed] [Google Scholar]
- SHINKAI M., TAKAYANAGI I. Effect of tachykinins on the acetylcholine output stimulated by nicotine from guinea-pig bladder. Regul. Pept. 1993;46:399–401. doi: 10.1016/0167-0115(93)90099-t. [DOI] [PubMed] [Google Scholar]
- SOUTHWELL B.R., FURNESS J.B. Immunohistochemical demonstration of NK1 tachykinin receptor on muscle and epithelia in guinea-pig intestine. Gastroenterology. 2001;120:1140–1151. doi: 10.1053/gast.2001.23251. [DOI] [PubMed] [Google Scholar]
- SPILLER R.C., JENKINS D., HEBDEN J.M., WRIGHT T., SKINNER M., NEAL K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANTON M.P., HENGEL P.T., SOUTHWELL B.R., CHOW C.W., KECK J., HUTSON J.M., BORNSTEIN J.C. Cholinergic transmission to the circular muscle of children with slow transit constipation is unimpaired, but transmission via NK2 receptors is lacking. Neurogastroenterol. Motil. 2003;15:669–678. doi: 10.1046/j.1350-1925.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- STERNINI C., ANDERSON K., FRANTZ G., KRAUSE J.E., BRECHA N. Expression of substance P/neurokinin A-encoding preprotachykinin messenger ribonucleic acids in the rat enteric nervous system. Gastroenterology. 1989;97:348–356. doi: 10.1016/0016-5085(89)90070-x. [DOI] [PubMed] [Google Scholar]
- TACK J., SARNELLI G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut. 2002;51 Suppl I:i77–i80. doi: 10.1136/gut.51.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLEY N.J., SPILLER R. Irritable bowel syndrome: a little understood organic bowel disease. Lancet. 2002;360:555–564. doi: 10.1016/S0140-6736(02)09712-X. [DOI] [PubMed] [Google Scholar]
- TICHENOR S.D., BUXTON I.L.O., JOHNSON P., O'DRISCOLL K., KEEF K.D. Excitatory motor innervation in the canine rectoanal region: role of changing receptor populations. Br. J. Pharmacol. 2003;137:1321–1329. doi: 10.1038/sj.bjp.0704987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINI M., SPELTA V., DE PONTI F., DE GIORGIO R., D'AGOSTINO G., STANGHELLINI V., CORINALDESI R., STERNINI C., CREMA F. Tachykinin-dependent and independent components of peristalsis in the guinea pig isolated distal colon. Gastroenterology. 2001;120:938–945. doi: 10.1053/gast.2001.22526. [DOI] [PubMed] [Google Scholar]
- TOUGH I.R., LEWIS C.A., FOZARD J., COX H.M. Dual and selective antagonism of neurokinin NK1 and NK2 receptor-mediated responses in human colon mucosa. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:104–108. doi: 10.1007/s00210-002-0671-6. [DOI] [PubMed] [Google Scholar]
- TOULOUSE M., COELHO A.M., FIORAMONTI J., LECCI A., MAGGI C.A., BUENO L. Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br. J. Pharmacol. 2000;129:193–199. doi: 10.1038/sj.bjp.0703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOULOUSE M., FIORAMONTI J., MAGGI C.A., BUENO L. Role of NK2 receptors in gastric barosensitivity and in experimental ileus in rats. Neurogastroenterol. Motil. 2001;13:45–53. doi: 10.1046/j.1365-2982.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- TRAMONTANA M., MAGGI C.A., EVANGELISTA S. Spasmolytic effect of the NK2 receptor selective antagonist MEN10627 in rat small intestine. Jpn. J. Pharmacol. 1994;65:281–283. doi: 10.1254/jjp.65.281. [DOI] [PubMed] [Google Scholar]
- TURVILL J.L., CONNOR P., FARTHING J.G. Neurokinin 1 and 2 receptors mediate cholera toxin secretion in rat jejunum. Gastroenterology. 2000;119:1037–1044. doi: 10.1053/gast.2000.18147. [DOI] [PubMed] [Google Scholar]
- VANNUCCHI M.-G., CORSANI L., FAUSSONE-PELLEGRINI M.-S. Co-distribution of NK2 tachykinin receptors and substance P in nerve endings of guinea-pig ileum. Neurosci. Lett. 2000;287:71–75. doi: 10.1016/s0304-3940(00)01108-3. [DOI] [PubMed] [Google Scholar]
- VANNUCCHI M.-G., FAUSSONE-PELLEGRINI M.-S. NK1, NK2, and NK3 tachykinin receptor localisation and tachykinin distribution in the ileum of the mouse. Anat. Embryol. 2000;202:247–255. doi: 10.1007/s004290000106. [DOI] [PubMed] [Google Scholar]
- VERNE G.N., ROBINSON M.E., PRICE D.D. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- VREES M.D., PRICOLO V.E., POTENTI FM., CAO W. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Arch. Surg. 2002;137:439–445. doi: 10.1001/archsurg.137.4.439. [DOI] [PubMed] [Google Scholar]
- YOUNG S.H., ENNES H.S., MCROBERTS J.A., CHABAN V.V., DEA S.K., MAYER E.A. Calcium waves in colonic myocytes produced by mechanical and receptor-mediated stimulation. Am. J. Physiol. 1999;276:G1204–G1212. doi: 10.1152/ajpgi.1999.276.5.G1204. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Neuronal tachykinin receptors mediate release of non-adrenergic non-cholinergic inhibitory transmitters in the circular muscle of the guinea-pig colon. Neuroscience. 1995;69:643–650. doi: 10.1016/0306-4522(95)00271-j. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V., SANTICIOLI P., MAGGI C.A. Different Ca2+ influx pathways mediate tachykinin receptor-induced contraction in circular muscle of guinea-pig colon. Eur. J. Pharmacol. 1994;255:9–15. doi: 10.1016/0014-2999(94)90076-0. [DOI] [PubMed] [Google Scholar]
- ZHAO A., SHEA-DONOHUE T. PAR-2 agonists induce contraction of murine small intestine through neurokinin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G703–G969. doi: 10.1152/ajpgi.00064.2003. [DOI] [PubMed] [Google Scholar]