Abstract
Cholecystokinin (CCK) is a brain-gut peptide; it functions both as a neuropeptide and as a gut hormone. Although the pancreas and the gallbladder were long thought to be the principal peripheral targets of CCK, CCK receptors are found throughout the gut. It is likely that CCK has a physiological role not only in the stimulation of pancreatic and biliary secretions but also in the regulation of gastrointestinal motility. The motor effects of CCK include postprandial inhibition of gastric emptying and inhibition of colonic transit. It is now evident that at least two different receptors, CCK1 and CCK2 (formerly CCK-A and CCK-B, respectively), mediate the actions of CCK. Both localization and functional studies suggest that the motor effects of CCK are mediated by CCK1 receptors in humans. Since CCK is involved in sensory and motor responses to distension in the intestinal tract, it may contribute to the symptoms of constipation, bloating and abdominal pain that are often characteristic of functional gastrointestinal disorders in general and irritable bowel syndrome (IBS), in particular. CCK1 receptor antagonists are therefore currently under development for the treatment of constipation-predominant IBS. Clinical studies suggest that CCK1 receptor antagonists are effective facilitators of gastric emptying and inhibitors of gallbladder contraction and can accelerate colonic transit time in healthy volunteers and patients with IBS. These drugs are therefore potentially of great value in the treatment of motility disorders such as constipation and constipation-predominant IBS.
Keywords: Cholecystokinin, CCK1 receptor antagonist, colon, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is the most commonly identified functional gastrointestinal disorder. It is characterized by recurrent and often disabling abdominal pain associated with altered defecation (Thompson et al., 1999). Cholecystokinin (CCK) is a peptide known to be a potent regulator of gastrointestinal motility. Its effects include stimulation of postprandial gallbladder contraction, inhibition of gastric emptying, and inhibition of colonic transit (Crawley & Corwin, 1994). Alterations in CCK release and in tissue responses to the peptide have been implicated in the pathogenesis of IBS (Kellow et al., 1988). These studies indicate that either exaggerated release or increased sensitivity to CCK could contribute to the symptoms (Simren et al., 2001). This paper reviews our present knowledge of CCK and CCK receptors and summarizes the available data on the involvement of CCK1 receptors in the regulation of colonic motility. We also report promising clinical investigations that indicate the beneficial effects of CCK1 receptor blockade in IBS patients.
CCK
The actions of CCK include stimulation of exocrine and endocrine secretion, motility and growth in the gastrointestinal tract, and regulation of satiety, anxiety, pain and behavior in the central and peripheral nervous systems. It is therefore a prototype of a class of agents known as brain-gut peptides, functioning both as a neuropeptide and as a gut hormone (Crawley & Corwin, 1994; Noble et al., 1999). Although the pancreas and gallbladder were long thought to be the principal targets of CCK in the gastrointestinal tract, CCK receptors are actually found throughout the gut (Crawley & Corwin, 1994; Noble et al., 1999). It is therefore likely that CCK also has a physiological role in the regulation of gastrointestinal motility.
There are two principal sources of CCK: endocrine I cells in the duodenal wall that are in contact with the lumen of the intestine, and peptidergic nerves both in the enteric nervous system (ENS) and in the central nervous system (CNS). In the periphery, CCK-containing neurons are found in the myenteric plexus, submucosal plexus and muscle layers of the small intestine and colon, and in the celiac plexus and the vagus nerve (Liddle, 1997).
CCK, initially characterized as a 33-amino-acid peptide, is present in a variety of biologically active molecular forms, all derived from a 115-amino-acid precursor prepro-CCK (Deschenes et al., 1984). They include CCK-58, CCK-39, CCK-33, CCK-22, sulfated CCK-8 and CCK-7, unsulfated CCK-8 and CCK-7, CCK-5, and CCK-4 (Rehfeld & Hansen, 1986). All of these, as well as the closely related peptide gastrin and the amphibian skin peptide caerulein, share a common amidated tetrapeptide Trp-Met-Asp-Phe-NH2 at the C-terminal. Within the CCK/gastrin family of peptides, the characteristic CCK-like activity depends on the sulfated Tyr residue at the seventh position. If the Tyr residue is not sulfated, or if another amino-acid residue is present at this location, the peptide behaves as a gastrin analogue and loses its CCK-like potency by a factor of about 1000 (Wank, 1998) (Figure 1).
Figure 1.
Structural similarities of members of the CCK/gastrin peptide family: CCK, gastrin and the amphibian skin peptide caerulein. All of these peptides share a common feature, the same amidated tetrapeptide Trp-Met-Asp-Phe-NH2 at the C-terminal. The characteristic CCK-like activity depends on the sulfated Tyr residue at the seventh position.
CCK receptors
The biological actions of CCK are mediated by two distinct receptors originally denoted CCK-A (where ‘A' indicated alimentary type) and CCK-B (‘B' for brain type), based on their anatomical location. They are now termed CCK1 and CCK2, respectively, because of evidence indicating overlapping areas of localization (Noble et al., 1999). The existence and anatomical distribution of these CCK receptors was subsequently confirmed by molecular cloning. It had long been known that the gastrin and CCK2 receptors were similar, so it was no surprise when molecular biological investigations showed that a single gene encodes both the brain and stomach CCK2/gastrin receptors (Lee et al., 1993).
The CCK1 receptor has an approximately 1000-fold greater affinity for CCK than for gastrin, while the CCK2 receptor has the same high affinity for both CCK and gastrin. In addition, while the CCK1 receptor responds to sulfated CCK with a 1000-fold greater potency than non-sulfated CCK, the CCK2 receptor does not discriminate between the two (Noble et al., 1999). The CCK1 receptor, like the CCK2 receptor, belongs to the class A, rhodopsin-like family of G-protein-coupled receptors (Archer et al., 2003) (Figure 2). The receptor activates a Gq/11-mediated pathway leading to activation of phospholipase C. High agonist concentrations can also activate adenylyl cyclase via a Gs-mediated pathway (Wu et al., 1997).
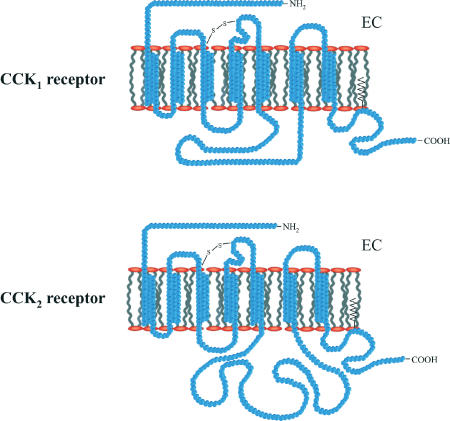
Figure 2.
Predicted membrane topology of CCK1 and CCK2 receptors. Both belong to the family of G-protein-coupled receptors with seven transmembrane domains. A large portion of the amino-acid sequence is conserved. EC=extracellular side of the membrane.
The physiological and pathophysiological significance of CCK receptors can be investigated in either CCK-deficient or CCK-receptor-deficient animal models. The drawback of such studies is the tendency for adaptation to obscure the changes in function. For example, in CCK-deficient mice, pancreatic function does not change greatly because the lack of CCK is compensated by other mechanisms (Lacourse et al., 1999). The formation of gallstones in CCK1-receptor knockout mice suggests, however, that gallbladder control is either monofactorial or that CCK1 receptors are involved in a single pathway controlling gallbladder motility (Sato et al., 2003). Limited amounts of data are also available in CCK2-receptor-deficient mice obtained through gene targeting (Nagata et al., 1996) and in Otsuka Long-Evans Fatty Tokushima (OLEFT) rats which have no functional CCK1 receptors (Kobayashi et al., 1996). However, no information is available to indicate whether gastrointestinal motility is affected in either CCK1- or CCK2-receptor null rats, mice or humans. On the other hand, a substantial modification in the central dopaminergic system has been reported in CCK1-receptor-deficient rats (Feifel et al., 2003). These data are extremely important since they reveal the interaction of CCK with other major transmitter systems that may also affect colonic motility and sensation.
Species variability in the localization of CCK1 and CCK2 receptors also needs special attention. For example, in rodents, pain perception in the spinal cord is primarily mediated by CCK2 receptors (Wiesenfeld-Hallin et al., 2002). In contrast, the majority of the receptors involved in this process in primates are CCK1 receptors (Ghilardi et al., 1992). Furthermore, a recent study suggests that CCK1 receptor blockade potentiates opiate analgesia (Simpson et al., 2002). These observations carry the message that data from animal studies can be extrapolated to humans only at a rather limited level. Nonetheless, the nociceptive, antianalgesic effects of CCK mediated by CCK1 receptors provide a further and potentially important pharmacological target for the development of drugs to treat gastrointestinal motor disorders related to pain sensation.
CCK1 receptor antagonists
A specific approach for evaluating the importance of CCK in the regulation of gastrointestinal function is to establish whether blockade of the CCK receptor lessens or abolishes the response to endogenous stimulants thought to act through CCK release. Immunoneutralization, the administration of specific, high-affinity, anti-CCK antibodies, is one approach to this (Reidelberger et al., 1994), but in most cases the use of a specific and competitive receptor blocker is certainly better. A clear definition of the role of CCK in the physiology of gastric motor activity was hampered for a long time by the lack of specific and potent nonpeptide antagonists of CCK receptors. The development of such compounds has stimulated a broad investigation into the physiological actions of CCK and its role in certain diseases (Scarpignato, 1992; D'Amato & Rovati, 1997). At least 10 classes of CCK receptor antagonists are now available (D'Amato & Rovati, 1997). In this review, we refer only to those for which advanced clinical data are available. (Figure 3).
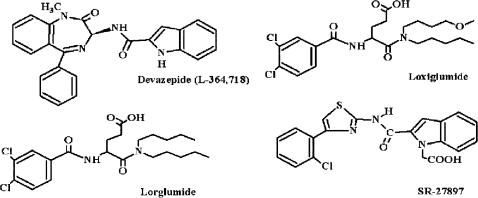
Figure 3.
Chemical structures of CCK1 receptor antagonists: devazepide, lorglumide, loxiglumide and lintitript.
Among the amino-acid derivatives, proglumide was the first to be discovered, more than 35 years ago in Rotta Research Laboratorium SpA. However, its low potency and specificity (it also effectively binds CCK2 receptors) stimulated the synthesis of glutaramic acid derivatives, the most promising of which were the compounds CR-1409 (Rotta Research Laboratorium SpA) and CR-1505 (Rotta Research Laboratorium SpA), lorglumide, and loxiglumide, respectively. These are potent, specific, and competitive antagonists of CCK1 receptors. They are active after oral administration and are able to antagonize the effects of both endogenous and exogenous CCK. Since loxiglumide is a racemic mixture, both isomeric forms could be obtained. The dextro isomer dexloxiglumide is about twice as potent as the parent compound because the anti-CCK activity is specific to the R form whereas the S form is almost ineffective (D'Amato & Rovati, 1997).
The first selective nonpeptide CCK1 receptor antagonist asperlicin was discovered during screening of microbial fermentation media in 1985 by scientists at Merck. Chemical modification of asperlicin, retaining its benzodiazepine skeleton, has led to the discovery of a line of potent and selective CCK1 receptor antagonists, devazepide (also known as L364,718 or MK-329) being the most potent and widely studied among these (Chang & Lotti, 1986). The CCK1 receptor antagonist activity of lintitript (also known as SR-27,897) was discovered through random screening of a large chemical library at Sanofi. The selectivity and potency of this compound for the CCK1 receptor has also been well characterized (Gully et al., 1993).
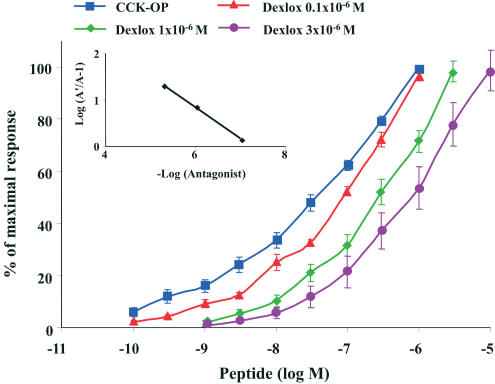
Among the CCK1 receptor antagonists, dexloxiglumide is of particular interest in the present context since this compound is under clinical development for IBS treatment. Functional studies, both in vitro and in vivo, confirm that dexloxiglumide is a highly potent CCK1 receptor antagonist. In rat pancreatic acinar cells, it displaced the concentration–response curve for CCK-8 to the right without affecting the maximum response, suggesting a competitive antagonism. Schild analysis gave a straight line with a slope (0.90±0.36) that was not significantly different from unity. The calculated pA2 for dexloxiglumide was 6.41±0.38 (Revel et al., 1999). On isolated human gallbladder, the compound had a similar affinity for CCK1 receptors when the effects of three CCK1 antagonists (dexloxiglumide, lorglumide, and amiglumide) were compared. All the three antagonists showed competitive inhibition of CCK-8-induced gallbladder contractions with pA2 values of 7.00, 6.95, and 6.71 for lorglumide, dexloxiglumide, and amiglumide, respectively (Maselli et al., 2001) (Figure 4). These results are in line with those obtained using other CCK1 receptor antagonists (Herranz, 2003).
Figure 4.
Inhibition of CCK-induced contractions of human gallbladder by CCK1 receptor blockade. Effects of dexloxiglumide at 0.1 μM (red), 1 μM (green), 3 μM (purple) on the contractile responses produced by CCK-OP (blue) in isolated human gallbladder. Each point represents the mean and standard error of the values obtained from 35 individual experiments. Adapted from Maselli et al. (2001).
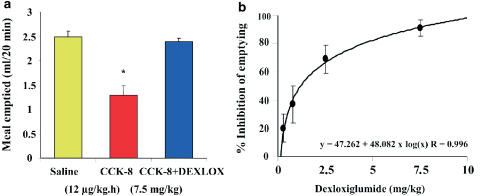
In vivo experiments have confirmed the results from the in vitro studies: intravenous dexloxiglumide, like other CCK1 receptor antagonists, reduced rat pancreatic exocrine secretion induced by submaximal CCK-8 stimulation (0.5 nmol kg−1 h−1) in a dose-dependent manner with an ID50 of 0.64 mg kg−1 (Revel et al., 1999). In chronic studies, both exogenous CCK and endogenous CCK, released by intraduodenal trypsin inhibitor camostate (Ono Pharmaceutical Co Ltd), increased the weight of the pancreas, the total pancreatic protein and DNA, and the trypsin and amylase content. Dexloxiglumide (25 mg kg−1 i.p.) administered together with the CCK agonist caerulein (1 μg kg−1) reduced the peptide-induced increase in pancreatic weight, protein, and enzyme content in rats. Similarly, when dexloxiglumide was given together with camostate (200 mg kg−1), all the observed changes were reduced by the antagonist (Varga et al., 1998). When CCK receptor subtype selectivity was tested in vivo in rats, dexloxiglumide, at doses sufficient to completely block CCK1 receptor-mediated inhibition of gastric emptying (ID50 1.14 mg kg−1), was ineffective against the pentagastrin-induced gastric acid secretion mediated by CCK2 receptors (Scarpignato et al., 1996) (Figure 5). A range of selective and potent CCK1 receptor antagonists similar to dexloxiglumide have now been characterized and are available for further studies (Herranz, 2003).
Figure 5.
Effect of CCK1 receptor blockade on gastric emptying of liquids in rats. (a) Inhibitory action of 7.5 mg kg−1 dexloxiglumide, administered intravenously 15 min before the agonist, on the CCK-induced delay in gastric emptying. (b) Dose-dependent inhibition of the CCK-induced delay in gastric emptying by dexloxiglumide (DEXLOX). Each column or point represents the mean and standard error of the values obtained from 8 to 10 individual experiments (*P<0.05). The curve shows a computer-generated logarithmic plot of percentage inhibition as a function of the antagonist dose. Adapted from Scarpignato et al. (1996).
CCK1 receptor heterogeneity
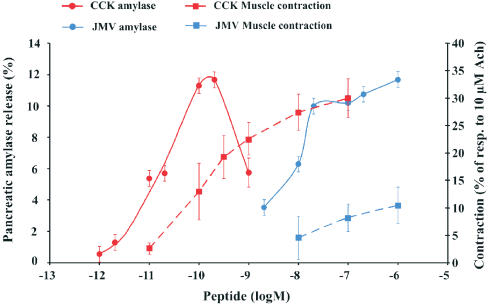
Although the nucleotide sequences of cloned cDNAs of CCK1 receptors from pancreas and from gastric and gallbladder smooth muscle are identical (De Weerth et al., 1993), their affinity states are different (Maubach et al., 1991; Moran et al., 1994; Taniguchi et al., 1995; Varga et al., 1988; 1998; Kisfalvi et al., 2001) (Figure 6). The available evidence suggests that the CCK1 receptor exists in both high- and low-affinity states (Bianchi et al., 1994; Rivard et al., 1994; Tsunoda & Owyang, 1995) and that CCK occupancy of these results in the initiation of different intracellular events and consequent biological responses. The CCK analogue JMV-180, which is an agonist at the high-affinity CCK1 receptors and an antagonist towards the low-affinity ones, is a useful tool for functionally distinguishing the two receptor states (Rivard et al., 1994; Tsunoda & Owyang, 1995; Tsunoda et al., 1996).
Figure 6.
Concentration-dependent effect of CCK-8 (red) and JMV-180 (blue) on amylase release from rat pancreatic acini (solid line, left axis) and on contractile activity of isolated rat pyloric rings (broken line, right axis). Amylase release is calculated as a percentage of the initial amylase content. Values for the contractile activity are expressed as a percentage of the contractile effect of 10−5 M acetylcholine on the same preparation. Each point represents the mean and standard error of the values obtained from at least five to six individual experiments. Adapted from Kisfalvi et al. (2001).
Binding of the peptide to high-affinity CCK1 receptors leads to synchronized activation of tyrosine kinase, phosphatidylinositol 3-kinase, and phospholipase D (Rivard et al., 1994; Tsunoda & Owyang, 1995), while occupancy of low-affinity receptors induces activation of phospholipase C and enhanced phosphoinositide breakdown (Bianchi et al., 1994; Tsunoda et al., 1996). Whereas CCK1 receptors in the pancreas are present in both high- and low-affinity states, those in gastrointestinal smooth muscle exist only in the low-affinity state (Maubach et al., 1991; Taniguchi et al., 1995; Varga et al., 1998; Kisfalvi et al., 2001) and mediate CCK-induced contraction (Figure 6). In line with these results, Moran et al. (1994) suggested that CCK-8 induces pyloric contraction and delays gastric emptying via interaction with receptors functionally similar to low-affinity pancreatic receptors. Furthermore, similar results have been obtained with gallbladder smooth muscle from guinea-pig and rabbit (Maubach et al., 1991; Taniguchi et al., 1995), suggesting that the absence of high-affinity CCK1 receptors on gastrointestinal smooth muscle cells might be a general phenomenon, at least in rodents.
Data regarding CCK1-receptor affinity states in nerves are controversial. It has been reported that activation of gastric mechanosensitive vagal afferent fibers (Schwartz et al., 1994) is mediated by low-affinity CCK1 receptors in rats. More recent studies, however, have confirmed the presence of both high- and low-affinity receptors on vagal nerves in the same species (Li et al., 1999; Lu & Owyang, 1999; Simasko et al., 2002).
We have to emphasize at this point that it is very difficult to predict ab initio the functional consequences of CCK1 receptor stimulation. In guinea-pig pancreas, both high- and low-affinity CCK1 receptors mediate the stimulation of bicarbonate and fluid secretion (Szalmay et al., 2001). In rat pancreas, high-affinity CCK1 receptors induce pancreatic growth while activation of low-affinity CCK receptors results in interstitial pancreatitis and cell destruction (Varga et al., 1988). In the rat acinar cell line AR4-2J, both high- and low affinity-receptors elicit growth-promoting effects (Hoshi & Logsdon, 1993). Finally, in human Panc-1 cells transfected with CCK1 receptors, activation of both high- and low-affinity CCK receptors leads to an arrest of cell proliferation (Detjen et al., 1997). In tissues where more than one cell type bears CCK1 receptors, the functional responses can be quite complex and may actually derive from a mixture of opposing effects.
CCK1 receptor polymorphism
Although CCK2 receptor polymorphism is well characterized (Kopin et al., 2000), CCK1 receptor polymorphism is relatively unexplored. The CCK1 receptor gene is mapped to chromosomal location 4p15.2-15.1 (Inoue et al., 1997) and polymorphisms have been detected both in the coding regions and in the promoter region. First, two missense variants were identified in the coding region of the gene: a G-to-C base mutation in exon 1, resulting in a glycine-to-arginine substitution in codon 21, and a G-to-A base mutation in exon 5 that introduced an isoleucine for valine in codon 365 (Inoue et al., 1997). Marchal-Victorion et al. (2002) characterized the isoleucine for valine mutation in codon 365 and demonstrated a decreased level of expression (26%) and reduced efficacy in generating inositol phosphates (25%). The authors suggested, therefore, that in humans bearing this or other mutations, decreases in CCK1 receptor expression and coupling efficiency may influence CCK-induced regulation of satiety, and might be involved in the development of type II diabetes mellitus and obesity (Marchal-Victorion et al., 2002). Novel polymorphisms (201A>G, 246G>A in the promoter region, 1260T>A, 1266T>C in intron 1 within the 3′ mRNA splice acceptor site consensus sequence, and Leu306Leu in exon 5) were found in addition to the variants (608G>A in intron 1, 3849C>T [Ile296Ile] in exon 5) reported previously (Tachikawa et al., 2001). The analysis suggested that the 201A allele frequency was higher in the schizophrenic group, especially in the paranoid type (Tachikawa et al., 2001). CCK1 receptor polymorphism has also been described in other studies: no correlation was found with panic disorder (Ise et al., 2003), but there were indications of an association with Parkinson's disease (Wang et al., 2003) and with chronic alcoholism (Wang et al., 2003).
Although evidence for the possible significance of CCK1 receptor polymorphism is growing, no published data are available on the correlation between CCK receptor polymorphism and gastrointestinal motility. No other pharmacogenetic or pharmacogenomic studies have investigated the potential role of polymorphisms in genes encoding other regulatory systems, which might interact with CCK1 receptors in the control of gastrointestinal motility under normal conditions or in functional disorders. Such studies may, however, provide new opportunities for predicting how patients will respond to particular treatments according to their genetic make-up.
CCK in colon motility
CCK has been known to affect human colonic motility for more than three decades (Harvey & Read, 1972). In spite of the large number of functional investigations since then, only one study (Rettenbacher & Reubi, 2001) has provided data regarding the distribution of CCK1 receptors in human colon. This study, using receptor autoradiography, showed that the main target of CCK is the myenteric plexus, which has predominantly CCK1 receptors. In addition, CCK1 receptors were present at moderate-to-low density in the longitudinal muscle, while the circular muscle was negative for both CCK1 and CCK2 receptors. Neither CCK1 nor CCK2 receptors were expressed in the blood vessels, lymphoid tissue, mucosa, and muscularis mucosa. CCK receptors on nerve cells of the myenteric plexus had a high affinity for CCK over gastrin, which is characteristic of CCK1 receptors (Rettenbacher & Reubi, 2001). These data suggest that CCK affects colonic motility by two fundamentally different pathways: acting on neurons in the myenteric plexus and directly on the smooth muscle cells.
Direct contractile effects of CCK on longitudinal and circular colonic muscle have been demonstrated in a number of studies. Experiments on human colon specimens revealed a substantial variation in agonist-stimulated contractions in terms of the number of preparations that responded to CCK and also in the maximal responses obtained (D'Amato et al., 1990; 1991; Morton et al., 2002b). This may have been due to genetic factors, the extent of tissue damage, the ages of the patients, and the medication that they had received.
Using selective CCK1 receptor antagonists, it was also revealed that the contractile effects of CCK on human gallbladder and colon are solely mediated by CCK1 receptors (D'Amato et al., 1991; Morton et al., 2002b). A recent study, however, has indicated some heterogeneity of the CCK1 receptors in the colon in contrast to the homogeneity of those in the gallbladder (Morton et al., 2002a). A two-site analysis of the colon data revealed that one of the two sites is indistinguishable from that characterized in the gallbladder (Morton et al., 2002a). The molecular basis of the apparent receptor heterogeneity in the colon remains to be established, but it calls our attention to the fact that the actions of CCK on colonic smooth muscle are more complex than previously thought.
As observed in vitro, CCK also affects human colonic motility in vivo. It has long been suggested that endogenous CCK release increases colonic transit time (D'Amato & Rovati, 1997; Scarpignato & Pelosini, 1999). In the colon, the peptide stimulates electrical spike activity associated with segmenting contractions (Renny et al., 1983). In accordance with this observation, more recent data suggest that endogenous CCK exerts its inhibitory effect on propulsive motility in the ascending colon (Fosatti-Marchal et al., 1994). However, physiological concentrations of either endogenous or exogenous CCK in the circulation have been found not to affect phasic contractility, tone or transit in healthy subjects (Niederau et al., 1992; O'BRIEN et al., 1997), suggesting that CCK does not play a major physiological role in the control of interdigestive and postprandial human colonic motility. However, experiments with the CCK1 receptor antagonist loxiglumide indicate that this compound can accelerate colonic transit in normal volunteers (Meyer et al., 1989). This raises the possibility that a CCK antagonist can act as pro-kinetic compound and may therefore be useful in treating constipation. Indeed, it was found that loxiglumide was able to significantly improve chronic constipation in geriatric patients (Meier et al., 1993).
Furthermore, it has been shown that CCK infusion can provoke abnormal reactions of gallbladder motility in IBS patients (Kellow et al., 1987), higher pain scores in patients with functional abdominal pain (Roberts-Thomson et al., 1992), and it can also unmask dysmotility (Kellow et al., 1988). An amplified release of CCK in IBS patients has also been shown in one study (Sjolund et al., 1996), although it was not confirmed by another, more recent one (Simren et al., 2001). In addition, loxiglumide has been shown to interfere with the gastro-colonic reflex and ileal motility and is able to selectively slow colonic transit time in patients suffering from IBS (Barrow et al., 1994). In a recent study, motility patterns were compared between healthy volunteers and IBS patients with abdominal pain and frequent defecation or diarrhea (Chey et al., 2001). The motility index, the frequency of high-amplitude propagating complexes, and also the responses to CCK were significantly greater in this subset of IBS patients. The high-amplitude propagating complexes coincided with the appearance of pain in the vast majority of observations. The effects of CCK were profoundly inhibited by both CCK1 receptor blockade with loxiglumide and by muscarinic receptor blockade with atropine. These data indicate that the action of CCK on the colon in IBS is mediated at least in part via the enteric nervous system (Chey et al., 2001).
In summary, CCK1 receptors are present in the human colon both on the smooth muscle cells and also on neurons. CCK is effective at both sites and the CCK1 receptors are involved both in pain perception and in the regulation of motility offering multiple targets for potential beneficial effects. They are therefore important effectors in the control of colon function both in health and disease.
Clinical development of CCK1 receptor antagonists as a potential treatment for IBS
Since CCK is involved in sensory and motor responses to distention in the intestinal tract, it is conceivable that CCK may contribute to symptoms like constipation, bloating, and abdominal pain that are often characteristic of IBS. It is therefore, not surprising that CCK receptor antagonists are being developed for the treatment of different functional gastrointestinal disorders, including IBS (Scarpignato et al., 1993; D'Amato & Rovati, 1997; Varga, 2002).
So far, six CCK1 receptor antagonists have been tested in humans. Among these, to the best of our knowledge, only two are still under development for potential clinical applications. They are the two proglumide derivatives, loxiglumide and its active enantiomer dexloxiglumide (presently in phase III). No updated information is available for the indolyl derivative lintitript (Sanofi Synthelabo and reported to be in phase II). The substituted benzodiazepine derivatives devazepide (Merck & Co Inc) and FK-480 (Fujisawa Pharmaceutical Co Ltd), and the aspartic acid derivative 2-NAP (James Black Foundation, U.K.), have been discontinued because of gallstone formation and acute renal failure, respectively (D'Amato & Rovati, 1997). As we are concerned here with a potential clinical application, we will focus mainly on the effects of the two compounds still undergoing clinical development. It is hoped that these will provide a template for future therapeutic candidates and that they will help in defining the mechanistic role of CCK and its antagonists in this therapeutic area.
IBS is associated with increased sensitivity to gut distension, resulting in alterations of intestino-intestinal reflexes and pain perception. In a recent animal study, the blockade of CCK1 receptors by the CCK1 antagonist dexloxiglumide (5 and 20 mg kg−1) was investigated in colonic motor alterations (colonic spike bursts) and abdominal pain (abdominal contractions) induced by rectal distension in conscious rats under normal conditions and following intracolonic trinitrobenzene sulfonic acid-induced inflammation (Bonnafous et al., 2002). In control conditions, rectal distension progressively inhibited the occurrence of colonic spike bursts and increased the frequency of abdominal contractions. In both control and inflamed conditions, dexloxiglumide increased the threshold of the recto-colonic inhibitory reflex, and reduced hyperalgesia and the threshold of pain (Bonnafous et al., 2002). These data indicate that CCK1 receptor blockade can modulate rectal-distension associated viscero-motor and pain responses. In another experimental model in dogs, blockade of CCK1 receptors accelerated gastric emptying of a standard meal and reduced the inhibition of emptying rate induced by distension of the proximal colon (Fioramonti et al., 1996), indicating the potential therapeutic usefulness of CCK1 receptor antagonists in delayed gastric emptying and in IBS.
In humans, ingestion of fatty acid reduced the tolerance of intragastric liquid load by delaying gastric emptying, and this action could be effectively antagonized by CCK1 receptor blockade (Lal et al., 2004). In another study, duodenal lipid caused a dose-related appearance of nausea and other dyspeptic symptoms during gastric distention and release of CCK in healthy subjects and of patients with functional dyspepsia (Fried & Feinle, 2002). CCK1 receptor blockade abolished the increase in intragastric volume induced by duodenal lipid infusion, significantly increased the highest tolerable intragastric pressure and significantly attenuated symptoms severity, suggesting that CCK1 receptor blockade is able to modulate visceral hypersensitivity and to decrease dyspeptic symptoms (Feinle et al., 1999).
As described in detail above, it has long been known that endogenous CCK release increases colonic transit time (CTT) and that CCK1 receptor antagonists are able to shorten CCT (Meyer et al., 1989). However, by virtue of their activity as selective CCK1 receptor antagonists, these compounds also affect the function of the gallbladder. Owing to the potential contribution of bile stasis to the formation of gallstones, the inhibitory effect of dexloxiglumide on gallbladder emptying has been carefully evaluated in healthy volunteers with the aim of selecting doses of the antagonist that would provide maximal therapeutic effects on intestinal motility while minimizing the negative effects on gallbladder emptying.
In eight male volunteers the effect of dexloxiglumide (200 mg bid) on a liquid diet supplemented with soluble fiber induced changes in CTT (Meier et al., 1994). Ingestion of the liquid diet significantly increased mean CTT above normal values. Dexloxiglumide administration partly reversed this effect, thus confirming the involvement of CCK1 receptors. Its effects (200 mg kg−1 bid or tid) on gallbladder emptying were investigated in a subsequent study in which neither antagonist dose regimen was found to significantly impair postprandial gallbladder kinetics (Meier et al., 1997a, 1997b). These results suggest that the CCK1 antagonist dexloxiglumide, at putative therapeutic dose regimens that accelerate CTT, does not interfere with gallbladder contractions.
The therapeutic potential of CCK1 receptor antagonists as a treatment of functional gastrointestinal disorders has recently been explored in a proof-of-concept trial in patients suffering from IBS (D'Amato et al., 1999a, 1999b; 2001). This multi-centre, randomized, placebo-controlled, double-blind trial involved 405 IBS patients (328 females and 77 males) of all subtypes of altered bowel habit characteristics. Patients were prospectively stratified to investigate the safety and efficacy of the CCK1 receptor antagonist dexloxiglumide (200 mg tid). Dexloxiglumide treatment was well tolerated in all subtypes of patients. The proportion of responders after 12 weeks of treatment was statistically significantly higher to the CCK1 receptor antagonist than to placebo in the female constipation-predominant IBS (C-IBS) subgroup for whom the drug tended to normalize bowel function. In addition to proving the clinical efficacy of a CCK1 antagonist in IBS, this study also provided clinical evidence that this can be achieved at doses that do not promote gallstone formation.
Despite extensive research, the etiology and pathogenesis of gallstones remains uncertain and it is currently thought to involve an interplay among several processes, each with many endogenous and exogenous modifiers (Dowling, 2000). The present theory considers several genetic and environmental factors, which include, among others, the physicochemical properties of the bile, the motility of the biliary tree and gallbladder, and the enterohepatic circulation of bile salts and several possible dietary variables as being important in the genesis of gallstones (Meier et al., 1997b). On one hand, CCK1 receptor antagonist activity of dexloxiglumide on gallbladder might lead to ‘stasis' of bile, thereby possibly promoting gallstones formation. On the other hand, its ability to accelerate colonic transit would be expected to reduce the enterohepatic recirculation of deoxycholic acid and, as a consequence, lower its concentration in the bile salt pool and its detrimental effect on the solubility of cholesterol (Thomas et al., 2000). In addition, dexloxiglumide is also able to increase the biliary flow (Watanabe & Otsuki, 1994), which would lead, by a dilution effect on the bile, to an increase of the solubility of bile salts and cholesterol. It is therefore possible that its action to antagonize gallbladder contraction and its effects on intestinal motility (shortening transit time) and physicochemical properties of the bile might cancel each other out.
In conclusion, the discovery and development of CCK1 receptor antagonists has allowed a more precise definition of the role(s) of CCK among the major determinants of gastrointestinal function. The results obtained in clinical studies examining motility and symptoms are also promising. In these studies, CCK1 receptor antagonists are effective facilitators of gastric emptying and inhibitors of gallbladder contraction and have also been shown to accelerate colonic transit time in healthy volunteers and improve symptoms in patients with IBS. A very important clinical aspect of the potential clinical application of CCK1 antagonists is that a dose can be identified that accelerates colonic transit time without significantly inhibiting gallbladder emptying. These encouraging findings suggest that CCK1 receptor antagonists may offer an effective treatment for IBS and other disorders of colonic motility.
Acknowledgments
This work was supported in part by Hungarian Scientific Research Fund grants T034241 and T038244. We thank Dr. Martin Steward for his helpful suggestions during preparation of the manuscript.
Abbreviations
- CCK
cholecystokinin
- C-IBS
constipation-predominant IBS
- CTT
colonic transit time
- IBS
irritable bowel syndrome
References
- ARCHER E., MAIGRET B., ESCRIEUT C., PRADAYROL L., FOURMY D. Rhodopsin crystal: new template yielding realistic models of G-protein-coupled receptors. Trends Pharmacol. Sci. 2003;24:36–40. doi: 10.1016/s0165-6147(02)00009-3. [DOI] [PubMed] [Google Scholar]
- BARROW L., BLACKSHAW P.E., WILSON C.G., ROVATI L., ARNOLD R. Selective slowing of proximal colon transit in irritable bowel syndrome by cholecystokinin-receptor antagonist, loxiglumide. Eur. J. Gastroenterol. Hepatol. 1994;6:381–387. [Google Scholar]
- BIANCHI B.R., MILLER T.R., WITTE D.G., LIN C.W. Novel CCK analogues and bombesin: a detailed analysis between phosphoinositide breakdown and high-dose inhibition of pancreatic enzyme secretion in three rodent species. J. Pharmacol. Exp. Ther. 1994;268:996–1002. [PubMed] [Google Scholar]
- BONNAFOUS C., BUENO L., GRIFFIN P.H., SCHNEIER H., ROVATI L.C., D'Amato M. Influence of dexloxiglumide on visceromotor and pain response induced by rectal distension in rats. Gastroenterology. 2002;122 no. 4, Suppl. 1:A527. [Google Scholar]
- CHANG R.S., LOTTI V.J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEY W.Y., JIN H.O., LEE M.H., SUN S.W., LEE K.Y. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., CORWIN R.L. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- D'Amato M., ROVATI L.C. Cholecystokinin-A receptor antagonists: therapies for gastrointestinal disorders. Exp. Opin. Invest. Drugs. 1997;6:819–836. doi: 10.1517/13543784.6.7.819. [DOI] [PubMed] [Google Scholar]
- D'Amato M., STAMFORD I.F., BENNETT A. The effects of cholecystokinin octapeptide on human isolated alimentary muscle. Br. J. Pharmacol. 1990;100:126–130. doi: 10.1111/j.1476-5381.1990.tb12063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato M., STAMFORD I.F., BENNETT A. Studies of three non-peptide cholecystokinin antagonists (devazepide, lorglumide and loxiglumide) in human isolated alimentary muscle and guinea-pig ileum. Br. J. Pharmacol. 1991;102:391–395. doi: 10.1111/j.1476-5381.1991.tb12184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato M., WHORWELL P.J., THOMPSON D.G., SPILLER R.C., GIACOVELLI G., GRIFFIN P.H. The CCK-1 receptor antagonist dexloxiglumide is effective and safe in female patients with constipation predominant irritable bowel syndrome. Am. J. Gastroenterol. 2001;96:S31. [Google Scholar]
- D'Amato M., WHORWELL P.J., THOMPSON D.G., SPILLER R.C., GIACOVELLI G., ROVATI L.C. The efficacy and safety of the CCK-A receptor-antagonist dexloxiglumide in the treatment of IBS. Gut. 1999a;45 Suppl 5:A258. [Google Scholar]
- D'Amato M., WHORWELL P.J., THOMPSON D.G., SPILLER R.C., GIACOVELLI G., ROVATI L.C. The CCKA receptor-antagonist dexloxiglumide in the treatment of IBS. Gastroenterology. 1999b;116:A981. [Google Scholar]
- DE WEERTH A., PISEGNA J.R., WANK S.A. Guinea pig gallbladder and pancreas possess identical CCK-A receptor subtypes: receptor cloning and expression. Am. J. Physiol. 1993;265:G1116–G1121. doi: 10.1152/ajpgi.1993.265.6.G1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESCHENES R.J., LORENZ L.J., HAUN R.S., ROOS B.A., COLLIER K.J., DIXON J.E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc. Natl. Acad. Sci. U.S.A. 1984;81:726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DETJEN K., FENRICH M.C., LOGSDON C.D. Transfected cholecystokinin receptors mediate growth inhibitory effects on human pancreatic cancer cell lines. Gastroenterology. 1997;112:952–959. doi: 10.1053/gast.1997.v112.pm9041258. [DOI] [PubMed] [Google Scholar]
- DOWLING R.H. Review: pathogenesis of gallstones. Aliment Pharmacol. Ther. 2000;14 Suppl 2:39–47. doi: 10.1046/j.1365-2036.2000.014s2039.x. [DOI] [PubMed] [Google Scholar]
- FEIFEL D., SHILLING P.D., KUCZENSKI R., SEGAL D.S. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci. Lett. 2003;348:147–150. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- FEINLE C., MEIER O., D'Amato M., FRIED M. CCK-A receptor blockade improves dyspeptic symptoms due to duodenal lipid and gastric distension in functional dyspepsia. Gastroenterology. 1999;116:A992. [Google Scholar]
- FIORAMONTI J., D'Amato M., ROVATI L.C., BUENO L. Effects of dexloxiglumide on gastric emptying delayed by colonic distension in dogs. Neurogastroenterol. Motil. 1996;8:172. [Google Scholar]
- FOSATTI-MARCHAL S., COFFIN B., FLOURIE B., LEMANN M.C.F., JIAN R., RAMBAUD J.C. Effects of cholecystokinin octapeptide (CCK-OP) on the tonic and phasic motor activity of the human colon. Gastroenterology. 1994;106:A499. [Google Scholar]
- FRIED M., FEINLE C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51 Suppl I:i54–i57. doi: 10.1136/gut.51.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHILARDI J.R., ALLEN C.J., VIGNA S.R., MCVEY D.C., MANTYH P.W. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J. Neurosci. 1992;12:4854–4866. doi: 10.1523/JNEUROSCI.12-12-04854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLY D., FREHEL D., MARCY C., SPINAZZE A., LESPY L., NELIAT G., MAFFRAND J.P., LE FUR G. Peripheral biological activity of SR 27897: a new potent non-peptide antagonist of CCKA receptors. Eur. J. Pharmacol. 1993;232:13–19. doi: 10.1016/0014-2999(93)90722-t. [DOI] [PubMed] [Google Scholar]
- HARVEY R.F., READ A.E. Effects of cholecystokinin on colonic motility and symptoms in patients with the irritable bowel syndrome. Gut. 1972;13:837–838. [PubMed] [Google Scholar]
- HERRANZ R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med. Res. Rev. 2003;23:559–605. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- HOSHI H., LOGSDON C.D. Both low- and high-affinity CCK receptor states mediate trophic effects on rat pancreatic acinar cells. Am. J. Physiol. 1993;265:G1177–G1181. doi: 10.1152/ajpgi.1993.265.6.G1177. [DOI] [PubMed] [Google Scholar]
- INOUE H., IANNOTTI C.A., WELLING C.M., VEILE R., DONIS-KELLER H., PERMUTT M.A. Human cholecystokinin type A receptor gene: cytogenetic localization, physical mapping, and identification of two missense variants in patients with obesity and non-insulin-dependent diabetes mellitus (NIDDM) Genomics. 1997;42:331–335. doi: 10.1006/geno.1997.4749. [DOI] [PubMed] [Google Scholar]
- ISE K., AKIYOSHI J., HORINOUCHI Y., TSUTSUMI T., ISOGAWA K., NAGAYAMA H. Association between the CCK-A receptor gene and panic disorder. Am. J. Med. Genet. 2003;118B:29–31. doi: 10.1002/ajmg.b.10020. [DOI] [PubMed] [Google Scholar]
- KELLOW J.E., MILLER L.J., PHILLIPS S.F., ZINSMEISTER A.R., CHARBONEAU J.W. Altered sensitivity of the gallbladder to cholecystokinin octapeptide in irritable bowel syndrome. Am J Physiol. 1987;253:G650–G655. doi: 10.1152/ajpgi.1987.253.5.G650. [DOI] [PubMed] [Google Scholar]
- KELLOW J.E., PHILLIPS S.F., MILLER L.J., ZINSMEISTER A.R. Dysmotility of the small intestine in irritable bowel syndrome. Gut. 1988;29:1236–1243. doi: 10.1136/gut.29.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISFALVI K., RACZ G., ZSIRKA-KLEIN A., PELOSINI I., SCARPIGNATO C., VARGA G. Different affinity states of CCK(1) receptors on pancreatic acini and gastric smooth muscle in the rat. J. Physiol. Paris. 2001;95:391–398. doi: 10.1016/s0928-4257(01)00053-5. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., OHTA M., MIYASAKA K., FUNAKOSHI A. Decrease in exploratory behavior in naturally occurring cholecystokinin (CCK)-A receptor gene knockout rats. Neurosci. Lett. 1996;214:61–64. doi: 10.1016/0304-3940(96)12881-0. [DOI] [PubMed] [Google Scholar]
- KOPIN A.S., MCBRIDE E.W., SCHAFFER K., BEINBORN M. CCK receptor polymorphisms: an illustration of emerging themes in pharmacogenomics. Trends Pharmacol. Sci. 2000;21:346–353. doi: 10.1016/s0165-6147(00)01526-1. [DOI] [PubMed] [Google Scholar]
- LACOURSE K.A., SWANBERG L.J., GILLESPIE P.J., REHFELD J.F., SAUNDERS T.L., SAMUELSON L.C. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am. J. Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- LAL S., MCLAUGHLIN L., BARLOW J., D'Amato M., GIACOVELLI G., VARRO A., DOCKRAY G.J., THOMPSON D.G.Cholecystokinin pathways modulate sensations induced by gastric distension in man Am. J. Physiol. 2004(in press) [DOI] [PubMed]
- LEE Y.M., BEINBORN M., MCBRIDE E.W., LU M., KOLAKOWSKI L.F., JR., KOPIN A.S. The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J. Biol. Chem. 1993;268:8164–8169. [PubMed] [Google Scholar]
- LI Y., ZHU J., OWYANG C. Electrical physiological evidence for high and low-affinity vagal CCK-A receptors. Am. J. Physiol. 1999;277:G469–G477. doi: 10.1152/ajpgi.1999.277.2.G469. [DOI] [PubMed] [Google Scholar]
- LIDDLE R.A. Cholecystokinin cells. Annu. Rev. Physiol. 1997;59:221–242. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- LU Y.X., OWYANG C. Duodenal acid-induced gastric relaxation is mediated by multiple pathways. Am. J. Physiol. 1999;276:G1501–G1506. doi: 10.1152/ajpgi.1999.276.6.G1501. [DOI] [PubMed] [Google Scholar]
- MARCHAL-VICTORION S., VIONNET N., ESCRIEUT C., DEMATOS F., DINA C., DUFRESNE M., VAYSSE N., PRADAYROL L., FROGUEL P., FOURMY D. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics. 2002;12:23–30. doi: 10.1097/00008571-200201000-00004. [DOI] [PubMed] [Google Scholar]
- MASELLI M.A., PIEPOLI A.L., PEZZOLLA F., GUERRA V., CARUSO M.L., MENNUNI L., LORUSSO D., MAKOVEC F. Effect of three nonpeptide cholecystokinin antagonists on human isolated gallbladder. Dig. Dis. Sci. 2001;46:2773–2778. doi: 10.1023/a:1012748017709. [DOI] [PubMed] [Google Scholar]
- MAUBACH K., PATEL M., SPRAGGS C.F. Interaction of gastrin/cholecystokinin agonists and antagonists on guinea pig gall-bladder. Br. J. Pharmacol. 1991;104 Suppl.:142. [Google Scholar]
- MEIER R., BEGLINGER C., GIACOVELLI G., D'Amato M. Effect of the CCK-A receptor antagonist dexloxiglumide on post-prandial gallbladder emptying and colonic transit time in healthy volunteers. Gastroenterology. 1997a;112:A788. [Google Scholar]
- MEIER R., BEGLINGER C., GIACOVELLI G., D'Amato M. Effect of the CCK-A receptor antagonist dexloxiglumide on post-prandial gallbladder emptying and colonic transit time in healthy volunteers. Gut. 1997b;41 Suppl 3:A193. [Google Scholar]
- MEIER R., BEGLINGER C., THUMSHIRN M., MEYER L., ROVATI L.C., GIACOVELLI G., D'Amato M., GYR K. Therapeutic effects of loxiglumide, a cholecystokinin antagonist, on chronic constipation in elderly patients: a prospective, randomized, double-blind, controlled trial. J. Gastrointest. Motil. 1993;5:129–135. [Google Scholar]
- MEIER R., D'Amato M., PULLWITT A., SCHNEIDER H., ROVATI L.C., BEGLINGER C. Effect of a CCK-A receptor antagonist in an experimental model of delayed colonic transit time in man. Gastroenterology. 1994;106:A538. [Google Scholar]
- MEYER B.M., WERTH B.A., BEGLINGER C., HILDEBRAND P., JANSEN J.B., ZACH D., ROVATI L.C., STALDER G.A. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet. 1989;2:12–15. doi: 10.1016/s0140-6736(89)90255-9. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., KORNBLUH R., MOORE K., SCHWARTZ G.J. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul. Pept. 1994;52:165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- MORTON M.F., HARPER E.A., TAVARES I.A., SHANKLEY N.P. Pharmacological evidence for putative CCK(1) receptor heterogeneity in human colon smooth muscle. Br. J. Pharmacol. 2002a;136:873–882. doi: 10.1038/sj.bjp.0704794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON M.F., WELSH N.J., TAVARES I.A., SHANKLEY N.P. Pharmacological characterization of cholecystokinin receptors mediating contraction of human gallbladder and ascending colon. Regul. Pept. 2002b;105:59–64. doi: 10.1016/s0167-0115(01)00383-4. [DOI] [PubMed] [Google Scholar]
- NAGATA A., ITO M., IWATA N., KUNO J., TAKANO H., MINOWA O., CHIHARA K., MATSUI T., NODA T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERAU C., FABER S., KARAUS M. Cholecystokinin's role in regulation of colonic motility in health and in irritable bowel syndrome. Gastroenterology. 1992;102:1889–1898. doi: 10.1016/0016-5085(92)90310-u. [DOI] [PubMed] [Google Scholar]
- NOBLE F., WANK S.A., CRAWLEY J.N., BRADWEJN J., SEROOGY K.B., HAMON M., ROQUES B.P. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- O'BRIEN M.D., CAMILLERI M., THOMFORDE G.M., WISTE J.A., HANSON R.B., ZINSMEISTER A.R. Effect of cholecystokinin octapeptide and atropine on human colonic motility, tone, and transit. Dig. Dis. Sci. 1997;42:26–33. doi: 10.1023/a:1018868601475. [DOI] [PubMed] [Google Scholar]
- REHFELD J.F., HANSEN H.F. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J. Biol. Chem. 1986;261:5832–5840. [PubMed] [Google Scholar]
- REIDELBERGER R.D., VARGA G., LIEHR R.M., CASTELLANOS D.A., ROSENQUIST G.L., WONG H.C., WALSH J.H. Cholecystokinin suppresses food intake by a nonendocrine mechanism in rats. Am. J. Physiol. 1994;267:R901–R908. doi: 10.1152/ajpregu.1994.267.4.R901. [DOI] [PubMed] [Google Scholar]
- RENNY A., SNAPE W.J., JR., SUN E.A., LONDON R., COHEN S. Role of cholecystokinin in the gastrocolonic response to a fat meal. Gastroenterology. 1983;85:17–21. [PubMed] [Google Scholar]
- RETTENBACHER M., REUBI J.C. Localization and characterization of neuropeptide receptors in human colon. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:291–304. doi: 10.1007/s002100100454. [DOI] [PubMed] [Google Scholar]
- REVEL L., MAKOVEC F., CASTANER J. Dexloxiglumide. CCK1 (CCK(A)) receptor antagonist, treatment of irritable bowel syndrome. Drugs Future. 1999;24:725–728. [Google Scholar]
- RIVARD N., RYDZEWSKA G., LODS J.S., MARTINEZ J., MORISSET J. Pancreas growth, tyrosine kinase, PtdIns 3-kinase, and PLD involve high-affinity CCK-receptor occupation. Am. J. Physiol. 1994;266:G62–G70. doi: 10.1152/ajpgi.1994.266.1.G62. [DOI] [PubMed] [Google Scholar]
- ROBERTS-THOMSON I.C., FETTMAN M.J., JONSSON J.R., FREWIN D.B. Responses to cholecystokinin octapeptide in patients with functional abdominal pain syndromes. J. Gastroenterol. Hepatol. 1992;7:293–297. doi: 10.1111/j.1440-1746.1992.tb00983.x. [DOI] [PubMed] [Google Scholar]
- SATO N., MIYASAKA K., SUZUKI S., KANAI S., OHTA M., KAWANAMI T., YOSHIDA Y., TAKIGUCHI S., NODA T., TAKATA Y., FUNAKOSHI A. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig. Dis. Sci. 2003;48:1944–1947. doi: 10.1023/a:1026110002713. [DOI] [PubMed] [Google Scholar]
- SCARPIGNATO C. Cholecystokinin antagonists and motilides: pharmacology and potential in the treatment of gastroesophageal reflux disease and other digestive motor disorders. Front. Gastrointest. Res. 1992;20:90–128. [Google Scholar]
- SCARPIGNATO C., KISFALVI I., D'Amato M., VARGA G. Effect of dexloxiglumide and spiroglumide, two new CCK-receptor antagonists, on gastric emptying and secretion in the rat: evaluation of their receptor selectivity in vivo. Aliment Pharmacol. Ther. 1996;10:411–419. doi: 10.1111/j.0953-0673.1996.00411.x. [DOI] [PubMed] [Google Scholar]
- SCARPIGNATO C., PELOSINI I. Management of irritable bowel syndrome: novel approaches to the pharmacology of gut motility. Can. J. Gastroenterol. 1999;13:50A–65A. doi: 10.1155/1999/183697. [DOI] [PubMed] [Google Scholar]
- SCARPIGNATO C., VARGA G., CORRADI C. Effect of CCK and its antagonists on gastric emptying. J. Physiol. Paris. 1993;87:291–300. doi: 10.1016/0928-4257(93)90035-r. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ G.J., MCHUGH P.R., MORAN T.H. Pharmacological dissociation of responses to CCK and gastric loads in rat mechanosensitive vagal afferents. Am. J. Physiol. 1994;267:R303–R308. doi: 10.1152/ajpregu.1994.267.1.R303. [DOI] [PubMed] [Google Scholar]
- SIMASKO S.M., WIENS J., KARPIEL A., COVASA M., RITTER R.C. Cholecystokinin increases cytosolic calcium in a subpopulation of cultured vagal afferent neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1303–R1313. doi: 10.1152/ajpregu.00050.2002. [DOI] [PubMed] [Google Scholar]
- SIMPSON K.H., SERPELL M., MCCUBBINS T.D., PADFIELD N.L., EDWARDS N., MARKHAM K., EASTWOOD D., BLOCK R., ROWBOTHAM D.J., IVERSEN L., GIBSON K.A multi-dose study: management of neuropathic pain in patients using CCK antagonist devazepide (DEVACADE) as an adjunct to strong opioids In World Congress of 10th IASP 2002San Diego, CA, U.S.A; (abstract) [Google Scholar]
- SIMREN M., ABRAHAMSSON H., BJORNSSON E.S. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOLUND K., EKMAN R., LINDGREN S., REHFELD J.F. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand. J. Gastroenterol. 1996;31:1110–1114. doi: 10.3109/00365529609036895. [DOI] [PubMed] [Google Scholar]
- SZALMAY G., VARGA G., KAJIYAMA F., YANG X.S., LANG T.F., CASE R.M., STEWARD M.C. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J. Physiol. 2001;535:795–807. doi: 10.1111/j.1469-7793.2001.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHIKAWA H., HARADA S., KAWANISHI Y., OKUBO T., SUZUKI T. Linked polymorphisms (−333G>T and −286A>G) in the promoter region of the CCK-A receptor gene may be associated with schizophrenia. Psychiatry Res. 2001;103:147–155. doi: 10.1016/s0165-1781(01)00276-1. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI H., NAGASAKI M., TAMAKI H. Effects of cholecystokinin (CCK)-JMV-180 on the CCK receptors of rabbit pancreatic acini and gallbladder smooth muscle. Jpn. J. Pharmacol. 1995;67:219–224. doi: 10.1254/jjp.67.219. [DOI] [PubMed] [Google Scholar]
- THOMAS L.A., VEYSEY M.J., BATHGATE T., KING A., FRENCH G., SMEETON N.C., MURPHY G.M., DOWLING R.H. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806–815. doi: 10.1053/gast.2000.16495. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.G., LONGSTRETH G.F., DROSSMAN D.A., HEATON K.W., IRVINE E.J., MULLER-LISSNER S.A. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUNODA Y., OWYANG C. High-affinity CCK receptors are coupled to phospholipase A2 pathways to mediate pancreatic amylase secretion. Am. J. Physiol. 1995;269:G435–G444. doi: 10.1152/ajpgi.1995.269.3.G435. [DOI] [PubMed] [Google Scholar]
- TSUNODA Y., YOSHIDA H., OWYANG C. Structural requirements of CCK analogues to differentiate second messengers and pancreatic secretion. Am. J. Physiol. 1996;271:G8–19. doi: 10.1152/ajpgi.1996.271.1.G8. [DOI] [PubMed] [Google Scholar]
- VARGA G. Dexloxiglumide Rotta Research Lab. Curr. Opin. Investig. Drugs. 2002;3:621–626. [PubMed] [Google Scholar]
- VARGA G., KISFALVI K., PELOSINI I., D'Amato M., SCARPIGNATO C. Different actions of CCK on pancreatic and gastric growth in the rat: effect of CCK(A) receptor blockade. Br. J. Pharmacol. 1998;124:435–440. doi: 10.1038/sj.bjp.0701811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA G., PAPP M., SOLOMON T.E., SCARPIGNATO C., TOTH I.E., SZABO D. Caerulein in supramaximal doses fails to stimulate pancreatic growth, but it forces secretory granulopoiesis. Acta. Physiol. Hung. 1988;71:99–106. [PubMed] [Google Scholar]
- WANG J., SI Y.M., LIU Z.L., YU L. Cholecystokinin, cholecystokinin-A receptor and cholecystokinin-B receptor gene polymorphisms in Parkinson's disease. Pharmacogenetics. 2003;13:365–369. doi: 10.1097/00008571-200306000-00008. [DOI] [PubMed] [Google Scholar]
- WATANABE N., OTSUKI M. A cholecystokinin receptor antagonist, loxiglumide, stimulates biliary secretion in conscious rats. Eur. J. Pharmacol. 1994;264:331–336. doi: 10.1016/0014-2999(94)90670-x. [DOI] [PubMed] [Google Scholar]
- WANK S.A. G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am. J. Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- WIESENFELD-HALLIN Z., XU X.J., HOKFELT T. The role of spinal cholecystokinin in chronic pain states. Pharmacol. Toxicol. 2002;91:398–403. doi: 10.1034/j.1600-0773.2002.910619.x. [DOI] [PubMed] [Google Scholar]
- WU V., YANG M., MCROBERTS J.A., REN J., SEENSALU R., ZENG N., DAGRAG M., BIRNBAUMER M., WALSH J.H. First intracellular loop of the human cholecystokinin-A receptor is essential for cyclic AMP signaling in transfected HEK-293 cells. J. Biol. Chem. 1997;272:9037–9042. doi: 10.1074/jbc.272.14.9037. [DOI] [PubMed] [Google Scholar]