Abstract
Acetyl-KIFMK-amide (KIFMK) restores fast inactivation to mutant sodium channels having a defective inactivation gate. Its binding site with sodium channels could be considered to be the cytoplasmic linker (III–IV linker) connecting domains III and IV of the sodium channel α subunit. There is a close resemblance of the amino-acid sequences between the III–IV linker and the activation loop of the insulin receptor (IR). This resemblance of the amino-acid sequences suggests that KIFMK may also modulate insulin signalling. In order to test this assumption, we studied the effects of KIFMK and its related (KIYEK, KIQMK, and DIYET) and unrelated (LPFFD) peptides on tyrosine phosphorylation or dephosphorylation of IR in vitro.
Purified IR was phosphorylated in vitro with insulin in the presence of various synthetic peptides and lignocaine. The phosphorylation level of IR was then evaluated after SDS–PAGE separation, followed by Western blot analysis with antiphosphotyrosine antibody.
KIFMK and KIYEK inhibited insulin-stimulated autophosphorylation of IR. Lignocaine showed similar effects, but at a higher order of concentration. KIYEK and DIYET, but not KIFMK, dephosphorylated the phosphorylated tyrosine residues. The structurally unrelated peptide LPFFD had no effect either on phosphorylation or dephosphorylation of IR.
These results indicate that KIFMK, KIYEK, and lignocaine bind with the autophosphorylation sites of IR.
The present findings also suggest that KIFMK and lignocaine bind with the III–IV linker of sodium channel α subunit.
Keywords: Insulin receptor, KIFMK, lignocaine, lidocaine, phosphorylation, sodium channel, tyrosine kinase
Introduction
The local anaesthetic lignocaine blocks not only the voltage-dependent sodium channels (Strichartz & Ritchie, 1987), but also insulin-stimulated glucose transport or insulin-stimulated glycogenesis (Clausen et al., 1973; Nordenberg et al., 1981; Morgan et al., 1985; Kolaczynski et al., 1994; Karniel & Beitner, 2000). No residues in IR structure are elucidated as important determinants for binding lignocaine. In sodium channels, some residues of the segments 6 of domains IV (DIV-S6), III (DIII-S6), and I (DI-S6), but not of domain II (DII-S6), are considered to be forming local anaesthetic binding sites (Ragsdale et al., 1994; Nau et al., 1999; Wang et al., 2000; 2001; Yarov-Yarovoy et al., 2001).
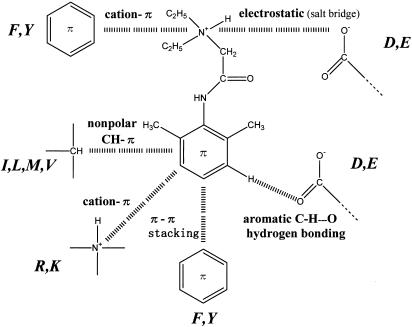
Kuroda et al. (1996; 2000) studied the interactions between local anaesthetics and sodium channel inactivation gate-related peptides (G1484–K1495;), which are dissected from the III–IV linker connecting DIII-S6 and DIV-S1 of rat brain type IIA sodium channel. Based on the amino-acid sequence between K1480 and M1501 of the III–IV linker (Figure 1), they showed that interactions between tertiary amine-type local anaesthetics and the III–IV linker can be stabilized greatly by a variety of combinations of noncovalent interactions. The noncovalent interactions expected are electrostatic (salt bridge), π–π stacking (Hunter & Sanders, 1990), cation–π (Dougherty, 1996), nonpolar C–H–π (Padmanabhan et al., 1998), and aromatic C–H…O hydrogen bonding (Olson et al., 2001) interactions (Figure 2).
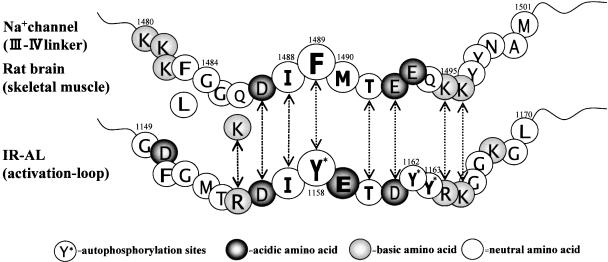
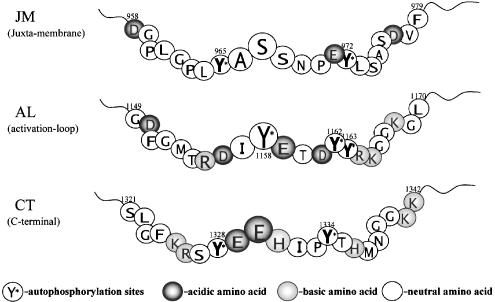
Figure 1.
The amino-acid sequences of the III–IV linker (K1480–M1501) of rat brain type IIA sodium channel and of the activation loop (G1149–L1170) of human IR. In human skeletal muscle (hSkM1) sodium channel, F1483 and Q1486 are replaced, respectively, by Leu and Lys (K1308 in hSkM1). The similarities of the amino acids between the III–IV linker and the activation loop of IR are indicated by arrows.
Figure 2.
Plausible intermolecular interactions between lignocaine and various kinds of amino acids.
The insulin receptor (IR) is an α2β2 heterotetrameric glycoprotein possessing intrinsic protein-tyrosine kinase activity. Binding of insulin to the α subunit activates a tyrosine-specific phosphotransferase activity. This leads to the autophosphorylation of the specific tyrosine residues in the cytoplasmic domain of the β subunit. The region of autophosphorylation, which is mainly responsible for activation of substrate phosphorylation, consists of three tyrosine residues at Y1158, Y1162, and Y1163 on the activation loop of the β subunit (Cherqui et al., 1990; Murakami & Rosen, 1991; Zhang et al., 1991). We compared the amino-acid sequence of this activation loop (Ebina et al., 1985) with that of the III–IV linker of rat brain type IIA sodium channels (Noda et al., 1986) in Figure 1. Surprisingly, the amino-acid sequence between D1487 and K1496 of the III–IV linker closely resembles that of the IR activation loop between the D1156 and K1165. In human skeletal muscle (hSkM1) sodium channels (George et al., 1992), F1483 and Q1486 are replaced, respectively, by Leu and Lys (K1308 in hSkM1). The Lys residue well matches R1155 of IR, because both Lys and Arg are basic amino acids. Thus, these findings suggest that if a local anaesthetic binding site for the sodium channel was the III–IV linker, local anaesthetics are also expected to bind to the activation loop of IR, and especially the autophosphorylation sites. In our previous investigation, we verified that this is indeed true, since clinically used doses of lignocaine inhibited insulin signalling in skeletal muscle in vivo and in vitro (Hirose et al., 2002). These results give not only firm evidence that the local anaesthetic binding site in IR is situated within the activation loop of the IR β subunit, but also that a local anaesthetic binding site for the sodium channel is located on the III–IV linker.
A synthetic peptide containing the IFM sequence KIFMK (acetyl-KIFMK-amide) is known to restore fast inactivation to mutant sodium channels having a defective inactivation gate (Eaholtz et al., 1994). Eaholtz et al. (1999) showed that both the N- and C-terminal Lys residues as well as the exact Ile–Phe–Met sequence are important determinants to restore fast inactivation. They also showed that the peptide competes with the intrinsic inactivation particle of the III–IV linker. Thus, KIFMK could be considered to bind to the III–IV linker, especially at its DIFMTEE moiety (Figure 1), and occludes the pore instead of the intrinsic IFM motif (Kuroda et al., 1999; Maeda et al., 2001). By considering the close resemblance of the amino-acid sequences between the III–IV linker and the activation loop (Figure 1), it might now be expected that KIFMK would also modulate insulin signalling.
Presently, to investigate the effect of KIFMK on insulin signalling, we studied tyrosine phosphorylation of IR β subunit in vitro. Some other related (KIYEK, KIQMK, DIYET) and unrelated (LPFFD) peptides and lignocaine were also studied for comparison purposes.
Methods
Materials
Antiphosphotyrosine antibody (4G10) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY, U.S.A.). IR from rat liver, which was purified by affinity chromatography on wheat germ agglutinin, and chemicals including lignocaine hydrochloride were from Sigma Chemical Co. (St Louise, MO, U.S.A.).
Synthesis and purification of peptides
Peptides were synthesized automatically by the solid-phase method using Fmoc chemistry on an Applied Biosystems 433A peptide synthesizer, their N-termini were acetylated (denoted by Ac-) and their C-termini amidated (denoted by –NH2). After cleavage with TFA, the peptides were purified on a reverse-phase C18 HPLC column using a gradient 90% A, 10% B–60% A, 40% B in 30 min, where A is 0.1% trifluoroacetic acid (TFA) in water and B is 0.1% TFA in acetonitrile. Peptides were characterized by ion-spray mass spectrometry on a Perkin-Elmer SCIEX API III mass spectrometer. KIFMK, Ac-KIFMK-NH2: m/z calculated 706.42 (monoisotope), 706.95 (av.), found 707.0 (MH+); KIYEK, Ac-KIYEK-NH2: m/z calculated 720.42 (monoisotope), 720.87 (av.), found 721.0 (MH+); KIQMK, Ac-KIQMK-NH2: m/z calculated 687.41 (monoisotope), 687.91 (av.), found 688.0 (MH+); DIYET, Ac-DIYET-NH2: m/z calculated 680.30 (monoisotope), 680.71 (av.), found 680.5 (MH+); LPFFD, Ac-LPFFD-NH2: m/z 678.34 (monoisotope), 678.79 (av.), found 680.0 (MH+).
In vitro phosphorylation of IR in the presence of peptides or lignocaine
Purified IR (1 μg of protein) was phosphorylated, with 100 nM of insulin for 10 min at 37°C in a 50 μl of incubation buffer (50 mM HEPES, pH 7.4, 125 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 5 mM MnCl2, 5 mM dithiothreitol, 1 mM phenylmethylsulphonyl fluoride, and 0.2 mM of ATP). Peptides (LPFFD, KIQMK, DIYET, KIFMK, KIYEK; 0.04, 0.4 or 4 mM) or lignocaine (0.4, 4 or 40 mM) were also added before incubation. The high concentration of lignocaine (40 mM) is almost equivalent to a clinical concentration (1% lignocaine) for local anaesthesia. After the incubation, the samples were prepared for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), by adding Laemmli sample buffer and boiling for 5 min, followed by Western blot analysis with antiphosphotyrosine antibody.
In vitro analysis of the effect of peptides or lignocaine on phosphorylated tyrosine residues of IR
Purified IRs (1 μg of protein) were incubated in the incubation buffer for 0, 10, 20, or 30 min at 37°C. Insulin (100 nM) was added at 0 min into all samples. The reactions were terminated by adding Laemmli sample buffer at 0 or 10 min after insulin stimulation in the samples that had been scheduled for 0 or 10 min incubation. In the samples that had been scheduled for 20 or 30 min incubation, either 40 mM of lignocaine or 4 mM of synthetic peptide (LPFFD, KIQMK, DIYET, KIFMK, KIYEK) was added at 11 min after insulin stimulation, and then Laemmli sample buffer was added at 20 or 30 min after insulin stimulation to terminate the reactions. All samples were boiled for 5 min, followed by Western blot analysis with antiphosphotyrosine antibody.
Statistical analysis
Data were analysed by one-way analysis of variance with Turkey post hoc analysis, using SPSS (SPSS Inc., Chicago, IL, U.S.A.). The statistical significance was established at the P<0.05 level. All values are reported as means±s.d.
Results
Inhibition of insulin-stimulated tyrosine phosphorylation of IR by peptides or lignocaine in vitro
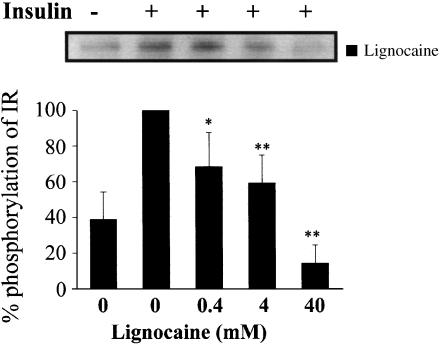
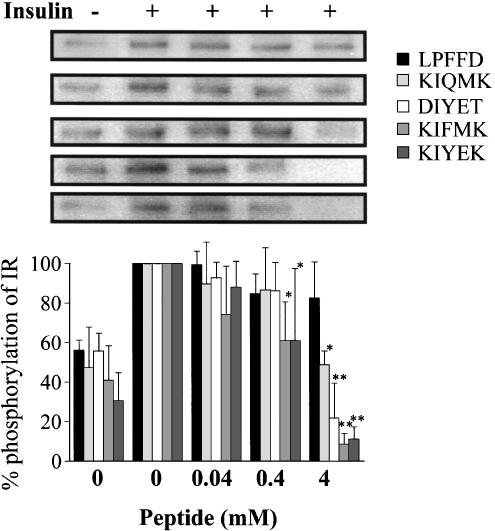
Previously, we showed that, during insulin stimulation, 4 and 40 mM of lignocaine suppressed phosphorylation of IR in vitro (Hirose et al., 2002). Presently, in order to investigate more precisely the dose-dependent modulation of phosphorylation, we tried the experiments further by employing 0.4 mM of lignocaine, as shown in Figure 3, where the insulin-stimulated responses of IR without lignocaine at 10 min was taken as the control and considered to be 100%. Although 0.4 and 4 mM of lignocaine could significantly suppress the insulin-stimulated autophosphorylation, almost complete suppression of the autophosphorylation was achieved at 40 mM of lignocaine. On the other hand, both KIFMK and KIYEK induced complete suppression of insulin-stimulated autophosphorylation of IR at 4 mM (Figure 4). Although 0.4 mM of KIFMK and KIYEK showed significant suppression of phosphorylation, the same concentration of neither KIQMK nor DIYET induced the suppression (Figure 4). However, 4 mM of KIQMK and DIYET showed significant suppression of phosphorylation, which is comparable to 0.4 and 4 mM of lignocaine, respectively. The control peptide LPFFD had no effect on insulin-stimulated autophosphorylation. These results showed that KIFMK and KIYEK suppressed the insulin-stimulated autophosphorylation of IR, and at a 10-fold lower concentration than did lignocaine, KIQMK, or DIYET.
Figure 3.
In vitro phosphorylation of purified IR in the presence or absence of lignocaine. Purified IR was incubated in buffer with or without 100 nM insulin, and with or without lignocaine for 10 min at 37°C. The results displayed on the top panel represent typical immunoblots. *P<0.05, **P<0.01 vs insulin-stimulated tyrosine phosphorylation without lignocaine, n=4 for each lane.
Figure 4.
In vitro phosphorylation of purified IR in the presence or absence of peptide (LPFFD, KIQMK, DIYET, KIFMK, or KIYEK). Purified IR was incubated in buffer with or without 100 nM insulin, and with or without peptide for 10 min at 37°C. Four results of each peptide displayed on the top panel represent typical immunoblots. *P<0.05, **P<0.01 vs insulin-stimulated tyrosine phosphorylation without peptide, n=4 for each lane.
Dephosphorylation of tyrosine residues of IR by peptides or lignocaine in vitro
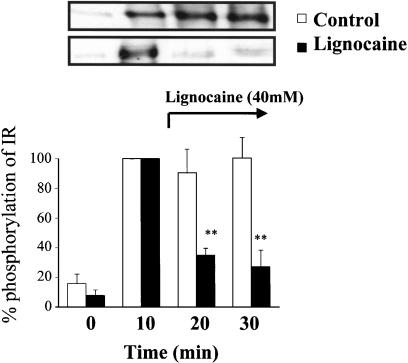
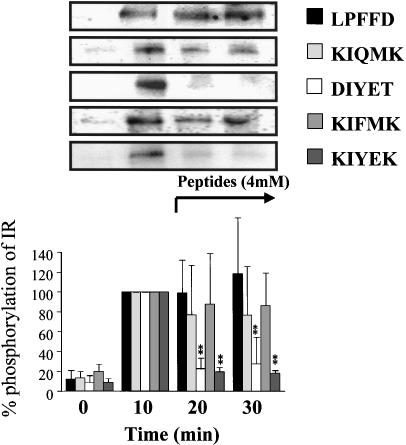
The control panel in Figure 5 shows that the maximum level of tyrosine phosphorylation of IR was reached at 10 min after insulin stimulation, and the level was kept for at least 30 min in the incubation buffer. Therefore, the insulin-stimulated response of IR at 10 min was considered to be 100%. Lignocaine (40 mM) lowered this phosphorylation level when it was added at 11 min after insulin stimulation (Figure 5). In a similar manner, when KIYEK (4 mM) or DIYET (4 mM) was added at 11 min after insulin stimulation, a maximum level of tyrosine phosphorylation was also lowered (Figure 6). In contrast, KIQMK and KIFMK were less efficient to lower the phosphorylation level of tyrosine. The two KIYEK and DIYET peptides and lignocaine almost completely reversed the already present increased phosphorylation of IR observed at 10 min. As expected, LPFFD had no effect on the insulin-stimulated autophosphorylation. These results show that KIYEK and DIYET, which contain the IYE sequence present on the activation loop of IR (Figure 1), have dephosphorylation effects on the autophosphorylated IR. In contrast, KIFMK, which contains the IFM sequence in the III–IV linker of the sodium channel (Figure 1), was effective to suppress autophosphorylation, but not effective to dephosphorylate the autophosphorylated IR. These differences in the dephosphorylation effect between KIYEK and KIFMK may be ascribed to the molecular mechanisms of dephosphorylation. The ‘IYE (Ile–Tyr–Glu)' sequence appears to be necessary for dephosphorylation, because DIYET could also dephosphorylate the phosphotyrosine residues.
Figure 5.
Effect of lignocaine on in vitro insulin-stimulated tyrosine phosphorylation of IR at different time points. Purified IRs were incubated in buffer containing 100 nM insulin for 0, 10, 20, or 30 min at 37°C, respectively. One point was made for each time, resulting in different incubations with or without lignocaine. Lignocaine was added at 11 min after insulin stimulation in the samples of 20 or 30 min incubation. Results displayed on the top panel represent typical immunoblots. **P<0.01 vs insulin-stimulated tyrosine phosphorylation at 10 min incubation, n=4 for each lane.
Figure 6.
Effect of synthetic peptides on in vitro insulin-stimulated tyrosine phosphorylation of IR at different time points. Purified IRs were incubated in the buffer containing 100 nM insulin for 0, 10, 20, or 30 min at 37°C, respectively. One point was made for each time, resulting in different incubations with or without peptide. Each peptide was added at 11 min after insulin stimulation in the samples of 20 or 30 min incubation. Results displayed on the top panel represent typical immunoblots. **P<0.01 vs insulin-stimulated tyrosine phosphorylation at 10 min incubation, n=4 for each lane.
Discussion
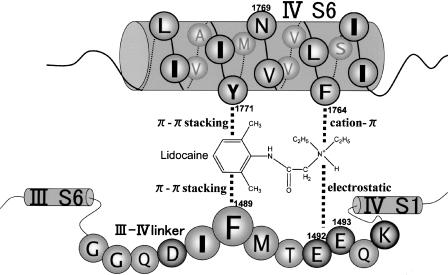
Taken together, the present and our previous works (Hirose et al., 2002) showed that lignocaine and the synthetic peptides KIFMK and KIYEK inhibited insulin-stimulated tyrosine phosphorylation. Lignocaine and KIYEK, but not KIFMK, also dephosphorylated phosphotyrosine residues. Thus, lignocaine and the peptides can be considered to occupy the site at which autophosphorylation occurs in IR. There are three clusters of autophosphorylation sites in IR (Figure 7): the juxtamembrane (Y965, Y972), activation loop (Y1158, Y1162, Y1163), and carboxy-terminal (Y1328, Y1334) subdomains (Cann & Kohanski, 1997). Among them, insulin-stimulated autophosphorylation of Y1158, Y1162, and Y1163 in the activation loop is critical for stimulation of kinase activity, and results in a dramatic change in the conformation of the activation loop. The conformation of the trisphosphorylated activation loop permits unrestricted access to the binding sites for ATP and protein substrates (Hubbard, 1997). Inspection of the amino-acid sequences of the three autophosphorylation sites shows that the activation loop is the most plausible binding site for the local anaesthetic and the peptides, because in addition to the aromatic side chains of Y1158, Y1162, and Y1163, these residues are surrounded by many basic (R1155, R1164, and K1165) and acidic (D1156, E1159, and D1161) amino acids which may be able to interact with lignocaine and the peptides, KIFMK or KIYEK, by various kinds of intermolecular forces (Figure 2). There is close resemblance between the amino-acid sequence of the activation loop of IR and that of the III–IV linker of the sodium channel (Figure 1). Thus, the present results mean that lignocaine and KIFMK can also bind stably with the III–IV linker of sodium channels, as argued by Kuroda et al (1996; 2000). However, electrophysiological studies clearly revealed that such local anaesthetics as etidocaine (Ragsdale et al., 1994) and cocaine (Wright et al., 1998) are binding with F1764 and Y1771 of DIV-S6 of the rat brain type IIA sodium channel. In addition, more recently, some residues arising from DIII-S6 and DI-S6 have also been shown to be the local anaesthetic binding sites. Yarov-Yarovoy et al. (2001) showed that mutations L1465A, N1466A, and I1469A in the segment DIII-S6 of the rat brain type IIA sodium channel decreased the affinity of the inactivated sodium channels for etidocaine. Lysine mutations of rat skeletal muscle μ1 sodium channel S1276 and L1280 (homologous to L1465 in the rat brain type IIA channel) in the segment DIII-S6 reduced the inactivated state affinity for bupivacaine (Wang et al., 2000). Moreover, lysine mutations of the same sodium channel at N434 in DI-S6 reduced the potency of bupivacaine (Nau et al., 1999). These observations show that a local anaesthetic binding site is composed of the amino-acid residues arising from the S6 segments of domains IV, III, and I. Accordingly, we suggest that the local anaesthetic interacts not only with the S6 segments but also with the III–IV linker, and we proposed a new model of interactions. Figure 8 schematically represents these interactions between lignocaine and the sodium channel, focused only on the amino-acid residues of DIV-S6 and the III–IV linker. In this model, the tertiary amine of lignocaine is interacting with the side chains of both F1764 and E1492 (Kuroda et al., 2000), respectively, by cation-π and electrostatic (salt bridge) interactions, while the aromatic ring is interacting with both the aromatic rings of Y1771 and F1489 by π–π stacking interactions. The space between DIV-S6 and the III–IV linker is considered to be forming a pore that allows Na+ ions to pass through from the extracellular side (right) to the cytoplasmic side (left).
Figure 7.
The amino-acid sequences for the three clusters (JM, AL, and CT) of autophosphorylation sites in IR: JM, juxtamembrane; AL, activation loop; CT, carboxy-terminal subdomains. Autophosphorylation sites are indicated by an asterisk.
Figure 8.
Schematic representation of the interactions of lignocaine with the III–IV linker and DIV-S6 of rat brain type IIA sodium channel.
The synthetic peptide KIFMK restores fast inactivation to mutant sodium channels having a defective inactivation gate by, for example, F1489Q mutation (Eaholtz et al., 1994). This observation can be explained in a similar manner as in lignocaine. In KIFMK, the two Lys residues can be considered to bind, respectively, with F1764 and Y1771 of DIV-S6 by cation–π interactions, while the same Lys residues are also binding, respectively, with F1489 and E1492 or E1493 of the III–IV linker by cation–π and electrostatic (salt bridge) interactions. McPhee et al. (1995) have mentioned that F1764A/V1774A mutations almost completely abolished fast inactivation of the rat brain type IIA sodium channel, remaining large sustained Na+ currents. For this mutant, KIFMK completely blocked the Na+ currents, but KIQMK was not competent to block these currents. Their results suggest that a bulky phenyl ring is necessary in the oligopeptide to block the sustained Na+ currents.
The importance of the phenyl ring in oligopeptides can also be deduced from the phosphorylation experiments of IR (Figure 4), because KIQMK showed the least potency to suppress phosphorylation of IR as compared with KIFMK, KIYEK, or DIYET. The bulky phenyl rings of Phe and Tyr in the oligopeptides or also of lignocaine appear to have served as a steric inhibitor of ATP action against tyrosine residues to become phosphorylated.
Many receptor kinases have highly conserved amino-acid residues within the catalytic domains, participating in ATP binding and phosphotransfer, and possess an activation loop containing three tyrosine autophosphorylation sites similar to the IR kinase (Hanks et al., 1988). The activation loop of insulin-like growth factor receptor (IGFR) has exactly the same amino-acid sequence as that of IR shown in Figure 1. A nerve growth factor receptor Trk has three tyrosine autophosphorylation sites (Y670, Y674, and Y675) in its amino-acid sequence of the activation loop, that is, RDIYSTDYYRV667-677 (Cunningham et al., 1997). This amino-acid sequence closely resembles that of the corresponding region of IR, that is, RDIYETDYYRK1155-1165. Therefore, we speculate that lignocaine and the small peptides employed presently or modified peptides from them could also inhibit the activity of these receptor tyrosine kinases through the interactions with the amino acids on the activation loop. We are currently studying the effects of lignocaine and small peptides on tyrosine phosphorylation/dephosphorylation of IGFR and Trk. A series of the synthetic protein tyrosine kinase inhibitors known as tyrphostins selectively inhibit receptor autophosphorylation such as insulin-like growth factor-1 receptor (IGF-1R) and IR (Levitzki & Gazit, 1995; Párrizas et al., 1997). Their binding sites with the receptors are considered to be the active centre of the receptors (Posner et al., 1994), but appear to be different from those of lignocaine and the oligopeptides studied presently. Thus, the present studies reported here and our current investigations for IGFR and Trk will facilitate the design of new protein tyrosine kinase inhibitors to diseases such as cancer, atherosclerosis, and psoriasis (Levitzki & Gazit, 1995), based on the molecular structures of local anaesthetics or of small synthetic peptides.
Acknowledgments
This study was supported in part by Grants-in-Aids for Scientific Research from the Ministry of Education, Science and Culture of Japan to M. Hirose and to Y. Kuroda.
Abbreviations
- ATP
adenosine triphosphate
- IR
insulin receptor
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulphate
References
- CANN A.D., KOHANSKI R.A. Cis-autophosphorylation of juxtamembrane tyrosines in the insulin receptor kinase domain. Biochemistry. 1997;36:7681–7689. doi: 10.1021/bi970170x. [DOI] [PubMed] [Google Scholar]
- CHERQUI G., REYNET C., CARON M., MELIN B., WICEK D., CLAUSER E., CAPEAU J., PICARD J. Insulin receptor tyrosine residues 1162 and 1163 control insulin stimulation of myristoyl-diacylglycerol generation and subsequent activation of glucose transport. J. Biol. Chem. 1990;265:21254–21261. [PubMed] [Google Scholar]
- CLAUSEN T., HARVING H., DAHL-HANSEN A.B. The relationship between the transport of glucose and cations across cell membranes in isolated tissues–VIII. The effect of membrane stabilizers on the transport of K+, Na+, and glucose in muscle, adipocytes and erythrocytes. Biochim. Biophys. Acta. 1973;298:393–411. doi: 10.1016/0005-2736(73)90367-2. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM M.E., STEPHENS R.M., KAPLAN D.R., GREENE L.A. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J. Biol. Chem. 1997;272:10957–10967. doi: 10.1074/jbc.272.16.10957. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY D.A. Cation–π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- EAHOLTZ G., COLVIN A., LEONARD D., TAYLOR C., CATTERALL W.A. Block of brain sodium channels by peptide mimetics of the isoleucine, phenylalanine, and methionine (IFM) motif from the inactivation gate. J. Gen. Physiol. 1999;113:279–293. doi: 10.1085/jgp.113.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAHOLTZ G., SCHEUER T., CATTERALL W.A. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron. 1994;12:1041–1048. doi: 10.1016/0896-6273(94)90312-3. [DOI] [PubMed] [Google Scholar]
- EBINA Y., ELLIS L., JARNAGIN K., EDERY M., GRAF L., CLAUSER E., OU J.-H., MASIARZ F., KAN Y.W., GOLDFINE I.D., ROTH R.A., RUTTER W.J. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985;40:747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- GEORGE A.L., KOMISAROF J., KALLEN R.G., BARCHI R.L. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann. Neurol. 1992;31:131–137. doi: 10.1002/ana.410310203. [DOI] [PubMed] [Google Scholar]
- HANKS S.K., QUINN A.M., HUNTER T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- HIROSE M., MARTYN J.A.J., KURODA Y., MARUNAKA Y., TANAKA Y. Mechanism of suppression of insulin signalling with lignocaine. Br. J. Pharmacol. 2002;136:76–80. doi: 10.1038/sj.bjp.0704691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD S.R. Crystal structures of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5573–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER C.A., SANDERS J.K.M. The nature of π–π interactions. J. Am. Chem. Soc. 1990;112:5525–5534. [Google Scholar]
- KARNIEL M., BEITNER R. Local anesthetics induce a decrease in the levels of glucose 1,6-bisphosphate, fructose 1,6-bisphosphate, and ATP, and in the viability of melanoma cells. Mol. Genet. Metab. 2000;69:40–45. doi: 10.1006/mgme.1999.2954. [DOI] [PubMed] [Google Scholar]
- KOLACZYNSKI J.W., MORALES L.M., MOORE J.R.J.H., CONSIDINE R.V., PIETRZKOWSKI Z., NOTO P.F., COLBERG J., CARO J.F. A new technique for biopsy of human abdominal fat under local anesthesia with lidocaine. Int. J. Obesity. 1994;18:161–166. [PubMed] [Google Scholar]
- KURODA Y., MAEDA Y., MIYAMOTO K., TANAKA K., KANAORI K., OTAKA A., FUJII N., NAKAGAWA T. 1H-NMR and circular dichroism spectroscopic studies on changes in secondary structures of the sodium channel inactivation gate peptides as caused by the pentapeptide KIFMK. Biophys. J. 1999;77:1363–1373. doi: 10.1016/S0006-3495(99)76985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURODA Y., MIYAMOTO K., TANAKA K., MAEDA Y., ISHIKAWA J., HINATA R., OTAKA A., FUJII N., NAKAGAWA T. Interactions between local anesthetics and Na+ channel inactivation gate peptides in phosphatidylserine suspensions as studied by 1H-NMR spectroscopy. Chem. Pharm. Bull. 2000;48:1293–1298. doi: 10.1248/cpb.48.1293. [DOI] [PubMed] [Google Scholar]
- KURODA Y., OGAWA M., NASU H., TERASHIMA M., KASAHARA M., KIYAMA Y., WAKITA M., FUJIWARA Y., FUJII N., NAKAGAWA T. Locations of local anesthetic dibucaine in model membranes and the interaction between dibucaine and a Na+ channel inactivation gate peptide as studied by 2H- and 1H-NMR spectroscopies. Biophys. J. 1996;71:1191–1207. doi: 10.1016/S0006-3495(96)79327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITZKI A., GAZIT A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- MAEDA Y., NAKAGAWA T., KURODA Y. Helix-stabilizing effects of the pentapeptide KIFMK and its related peptides on the sodium channel inactivation gate peptides. J. Peptide Res. 2001;58:413–423. doi: 10.1034/j.1399-3011.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- MCPHEE J.C., RAGSDALE D.S., SCHEUER T., CATTERALL W.A. A critical role for transmembrane segment IVS6 of the sodium channel α subunit in fast inactivation. J. Biol. Chem. 1995;270:12025–12034. doi: 10.1074/jbc.270.20.12025. [DOI] [PubMed] [Google Scholar]
- MORGAN M.S., DARROW R.M., NAFZ M.A., VARANDANI P.T. Role of membranes and energy-producing reactions in cellular processing of insulin in primary cultures of rat hepatocytes. Biochem. Biophys. Res. Commun. 1985;132:749–756. doi: 10.1016/0006-291x(85)91196-9. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M.S., ROSEN O.M. The role of insulin receptor autophosphorylation in signal transduction. J. Biol. Chem. 1991;266:22653–22660. [PubMed] [Google Scholar]
- NAU C., WANG S.-Y., STRICHARTZ G.R., WANG G.K. Point mutations at N434 in D1-S6 of μ1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol. Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- NODA M., IKEDA T., KAYANO T., SUZUKI H., TAKESHIMA H., KURASAKI M., TAKAHASHI H., NUMA S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- NORDENBERG J., KLEIN S., BEERY E., KAPLANSKY M., BEITNER R. Changes in the levels of glucose 1,6-diphosphate and ATP and in the activities of phosphofructokinase and phosphoglucomutase induced by local anesthetics in the isolated rat diaphragm muscle. Int. J. Biochem. 1981;13:1005–1009. doi: 10.1016/0020-711x(81)90006-9. [DOI] [PubMed] [Google Scholar]
- OLSON C.A., SHI Z., KALLENBACH N.R. Polar interactions with aromatic side chains in α-helical peptides: CH···O H-bonding and cation–π interactions. J. Am. Chem. Soc. 2001;123:6451–6452. doi: 10.1021/ja015590v. [DOI] [PubMed] [Google Scholar]
- PADMANABHAN S., JIMENEZ M.A., LAURENTS D.V., RICO M. Helix-stabilizing nonpolar interactions between tyrosine and leucine in aqueous and TFE solutions: 2D-1H NMR and CD studies in alanine–lysine peptides. Biochemistry. 1998;37:17318–17330. doi: 10.1021/bi9813678. [DOI] [PubMed] [Google Scholar]
- PÁRRIZAS M., GAZIT A., LEVITZKI A., WERTHEIMER E., LEROITH D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- POSNER I., ENGEL M., GAZIT A., LEVITZKI A. Kinetics of inhibition by tyrphostins of the tyrosine kinase activity of the epidermal growth factor receptor and analysis by a new computer program. Mol. Pharmacol. 1994;45:673–683. [PubMed] [Google Scholar]
- RAGSDALE D.S., MCPHEE J.C., SCHEUER T., CATTERALL W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- STRICHARTZ G.R, RITCHIE J.M.The action of local anesthetics on ion channels of excitable tissues Local Anesthetics 1987New York: Springer-Verlag; 21–52.ed. Strichartz, G.R. pp [Google Scholar]
- WANG S.-Y., BARILE M., WANG G.K. Disparate role of Na+ channel D2-S6 residues in batrachotoxin and local anesthetic action. Mol. Pharmacol. 2001;59:1100–1107. doi: 10.1124/mol.59.5.1100. [DOI] [PubMed] [Google Scholar]
- WANG S.-Y., NAU C., WANG G.K. Residues in Na+ channel D3-S6 segment modulate both batrachotoxin and local anesthetic affinities. Biophys. J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT S.N., WANG S.-Y., WANG G.K. Lysine point mutations in Na+ channel D4-S6 reduce inactivated channel block by local anesthetics. Mol. Pharmacol. 1998;54:733–739. [PubMed] [Google Scholar]
- YAROV-YAROVOY V., BROWN J., SHARP E.M., CLARE J.J., SCHEUER T., CATTERALL W.A. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J. Biol. Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- ZHANG B., TAVARE J.M., ELLIS L., ROTH R.A. The regulatory role of known tyrosine autophosphorylation sites of the insulin receptor kinase domain. J. Biol. Chem. 1991;266:990–996. [PubMed] [Google Scholar]