Abstract
At the mouse neuromuscular junction, adenosine (AD) and the A1 agonist 2-chloro-N6-cyclopentyl-adenosine (CCPA) induce presynaptic inhibition of spontaneous acetylcholine (ACh) release by activation of A1 AD receptors through a mechanism that is still unknown. To evaluate whether the inhibition is mediated by modulation of the voltage-dependent calcium channels (VDCCs) associated with tonic secretion (L- and N-type VDCCs), we measured the miniature end-plate potential (mepp) frequency in mouse diaphragm muscles.
Blockade of VDCCs by Cd2+ prevented the effect of the CCPA. Nitrendipine (an L-type VDCC antagonist) but not ω-conotoxin GVIA (an N-type VDCC antagonist) blocked the action of CCPA, suggesting that the decrease in spontaneous mepp frequency by CCPA is associated with an action on L-type VDCCs only.
As A1 receptors are coupled to a Gi/o protein, we investigated whether the inhibition of PKA or the activation of PKC is involved in the presynaptic inhibition mechanism. Neither N-(2[p-bromocinnamylamino]-ethyl)-5-isoquinolinesulfonamide (H-89, a PKA inhibitor), nor 1-(5-isoquinolinesulfonyl)-2-methyl-piperazine (H-7, a PKC antagonist), nor phorbol 12-myristate 13-acetate (PHA, a PKC activator) modified CCPA-induced presynaptic inhibition, suggesting that these second messenger pathways are not involved.
The effect of CCPA was eliminated by the calmodulin antagonist N-(6-aminohexil)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) and by ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester ɛ6TΔ-BM, which suggests that the action of CCPA to modulate L-type VDCCs may involve Ca2+-calmodulin.
To investigate the action of CCPA on diverse degrees of nerve terminal depolarization, we studied its effect at different external K+ concentrations. The effect of CCPA on ACh secretion evoked by 10 mM K+ was prevented by the P/Q-type VDCC antagonist ω-agatoxin IVA.
CCPA failed to inhibit the increases in mepp frequency evoked by 15 and 20 mM K+. We demonstrated that, at high K+ concentrations, endogenous AD occupies A1 receptors, impairing the action of CCPA, since incubation with 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, an A1 receptor antagonist) and adenosine deaminase (ADA), which degrades AD into the inactive metabolite inosine, increased mepp frequency compared with that obtained in 15 and 20 mM K+ in the absence of the drugs. Moreover, CCPA was able to induce presynaptic inhibition in the presence of ADA. It is concluded that, at high K+ concentrations, the activation of A1 receptors by endogenous AD prevents excessive neurotransmitter release.
Keywords: Adenosine, A1 adenosine receptors, presynaptic inhibition, L-type VDCC, PKA, PKC, Ca2+-calmodulin, mammalian neuromuscular junction
Introduction
Synaptic transmission is subject to a variety of modulatory influences that can modify the probability of neurotransmitter secretion. The neuromodulator adenosine (AD) is known to decrease ACh release at the neuromuscular junction by activation of A1 AD receptors coupled to a pertussis toxin-sensitive G protein (Gi1–3, Go) (Ginsborg & Hirst, 1972; Gross et al., 1989; Hamilton & Smith, 1991; Zhu & Ikeda, 1993; Mynlieff & Beam, 1994). These G proteins can cause inhibition of the adenylate cyclase-cAMP signal-transduction pathway, or can activate phospholipase C, with subsequent activation of protein kinase C (PKC) (Linden, 1991; Hille, 1994; Zink et al., 1995; Marquardt, 1998; Nyce, 1999). On the other hand, G protein-coupled receptors can reduce neurotransmitter release by directly inhibiting calcium currents, rather than by acting through a downstream soluble intracellular messenger (Hille, 1992; Herlitze et al., 1996; Ikeda, 1996; Song et al., 2000).
AD is a physiological constituent of all body fluids, including the fluid filling the extracellular space. At the synaptic cleft, the AD concentration is closely matched to the intracellular concentration through AD transporters (see the review by Fredholm, 1995). In addition, AD can be formed extracellularly when ATP, which is released together with ACh, is hydrolyzed to AD via the ectonucleotidase cascade (Ribeiro & Sebastião, 1987; Meriney & Grinnell, 1991; Redman & Silinsky, 1994).
At the frog neuromuscular junction, it has been shown that the inhibitory effect of AD upon evoked and spontaneous neurotransmitter release is not related to a reduction in calcium influx through the N-type voltage-dependent calcium channels (VDCCs), suggesting that AD exerts its action at a site distal to the locus of Ca2+ entry (Silinsky, 1984; Silinsky & Solsona, 1992; Redman & Silinsky, 1994). On the other hand, Hamilton & Smith (1991) showed that, at rat neuromuscular synapses, AD inhibits the motor nerve terminal Ca2+ current in response to nerve stimulation, but the mechanism(s) by which AD induces presynaptic inhibition of spontaneous neurotransmitter release remains unknown. We have previously shown that, at the mammalian neuromuscular junction, L- and N-type VDCCs are involved in spontaneous ACh release (Losavio & Muchnik, 1997). At resting membrane potential, these channels open stochastically, which allows Ca2+ influx into the nerve terminal and fusion of single vesicles to the presynaptic membrane. One plausible mechanism is that AD reduces Ca2+ influx through the VDCCs associated with spontaneous secretion, as occurs in mammalian evoked release. An alternative mechanism is that AD might directly impair transmitter exocytosis. Most of the reports of the effect of presynaptic modulators upon spontaneous release show that they modulate the release machinery downstream of Ca2+ influx (Silinsky, 1984; Prince & Stevens, 1992; Scanziani et al., 1992; Scholz & Miller, 1992; Silinsky & Solsona, 1992; Capogna et al., 1996; Dittman & Regehr, 1996).
The aim of the present work was to investigate the mechanism responsible for the presynaptic depression of spontaneous quantal secretion at the mammalian synapse induced by AD. Unlike the situation at the frog neuromuscular synapse (Redman & Silinsky, 1994), we demonstrate that AD and the specific AD A1 receptor agonist CCPA exert their modulatory effects by exclusively decreasing the L-type calcium channel-sensitive component of mepp frequency. This effect seems to be independent of direct inhibition of protein kinase A (PKA) or activation of PKC, but does seem to involve an action on Ca2+-calmodulin. We also studied the effect of CCPA at different external K+ concentrations and found that, at high concentrations (15 and 20 mM K+), endogenous AD blocked the effect of CCPA by occupying the A1 receptors.
Methods
CF1 mouse diaphragm muscles were used. Mice (males, 30–40 g) were anesthetized with sodium thiopental (50 mg kg−1) intraperitoneally and the left hemidiaphragm was excised and transferred to a 5 ml chamber superfused (3 ml min−1) with Ringer Krebs solution (mM: NaCl 135, KCl 5, CaCl2 2, MgCl2 1, D-glucose 11, HEPES 5, pH 7.3–7.4, bubbled with O2), In some experiments, the KCl concentration of the Ringer Krebs was raised to 10, 15 or 20 mM, by adding KCl to control Krebs solution. All recordings were made at room temperature (20–23°C) using conventional intracellular recording techniques. Mepps were recorded at the end-plate region of the muscle fibers using borosilicate glass microelectrodes (WP Instruments) with a resistance of 5–10 MΩ, filled with 3 M KCl. Muscle fibers with a resting membrane potential less negative than −60 mV (except for preparations incubated in high K+) or mepps with a rise time greater than 1 ms were rejected. In each experimental group, the muscles were allowed to equilibrate in the respective solution for at least 20 min, after observing that the mepps represented a period of stable spontaneous neurotransmitter release. Mepp frequency in each solution was recorded for 100 s from at least 10 different neuromuscular junctions and the values were averaged.
In order to decrease [Ca2+]i, nerve terminals were loaded with EGTA-AM (Molecular Probes), a cell-permeant buffer, as follows. The preparation was immersed in a Ca2+-free Ringer solution containing EGTA-AM dissolved in dimethylsulfoxide (DMSO) (3 × 10−7 mol (30 mg of muscle)−1). Preparations were incubated under these conditions for 2 h at room temperature, and then rinsed for 40 min with Ca2+-free Ringer solution (Kijima & Tanabe, 1988). The final DMSO concentration was 0.08% (v v−1). In control experiments, this concentration of DMSO did not affect any of the parameters studied (data not shown).
The data given in Results represent mean values±s.e.m. and n represents the number of animals (only the left hemidiaphragm was used from each mouse for a given experiment). To overcome the problem of variability in the spontaneous release of different muscles, in each experiment, mepp frequency was expressed as a percentage of the frequency obtained in the same muscle in control Ringer solution. The frequencies were measured by direct observation from the oscilloscope screen or as mepp amplitudes, acquired through an A/D converter controlled by computer, and analyzed using WCP software (Dagan Corp.). The statistical significance of differences between means was evaluated by one-way analysis of variance (ANOVA) followed by Tukey's test. Differences were considered to be significant when P<0.05 (*).
The following compounds were used: the specific AD A1 receptor agonist CCPA (Sigma, St Louis, MO, U.S.A.; 500 nM); the selective AD A1 receptor antagonist DPCPX (Sigma; 0.1 μM); the enzyme that converts endogenous AD into inosine, ADA (Sigma; 0.5 U ml−1); nitrendipine (Sigma; 5 μM, used as an L-type VDCC blocker in a darkened room to prevent photo-oxidation of the compound); ω-CgTx (Alomone Laboratories; 5 μM, used as an N-type VDCC blocker); ω-Aga (Alomone Laboratories, Jerusalem, Israel; 100 nM, used as an P/Q-type VDCC blocker); H-89 (Sigma; 1 μM, an inhibitor of PKA); forskolin (Alomone Laboratories; 20 μM, an activator of PKA; H-7 (Sigma; 10 μM, an inhibitor of PKC); PMA (Sigma; 200 nM, an activator of PKC); W-7 (Sigma; 10 μM, an inhibitor of calmodulin).
Results
Effect of AD and CCPA on spontaneous acetylcholine release
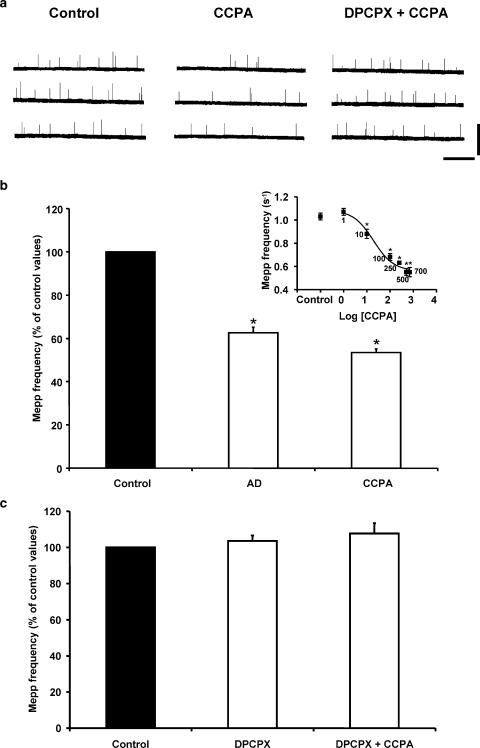
Figure 1a and b illustrates the presynaptic effects of AD (100 μM) and the specific AD A1 receptor agonist CCPA (500 nM) on spontaneous ACh release. These nucleosides decreased the mepp frequency to 62.6±2.6% (n=6) and 53.5±1.6% (n=8) of the control values, respectively, an effect that was totally reversible upon washout. The inset shows the effect of CCPA as a function of its concentration: maximal effect was observed at 500 nM. The presynaptic effect of CCPA did not occur when preparations were preincubated with the A1 selective AD receptor antagonist DPCPX (Figure 1a and c). DPCPX (0.1 μM) alone did not modify mepp frequency, suggesting that the basal efflux of AD is minimal and does not affect spontaneous neurotransmitter release.
Figure 1.
AD and CCPA inhibit spontaneous ACh release in mouse skeletal muscle. (a) Mepps recorded from diaphragm muscle fibers bathed with control Ringer solution (Vm: −74.4 mV), with 500 nM CCPA (Vm: −73.5 mV), and with 500 nM CCPA in the presence of the A1-selective AD receptor antagonist DPCPX (0.1 μM, Vm: −73.9 mV). Calibration: 3 mV, 2.5 s. Mepp amplitude was not modified by CCPA and/or DPCPX. (b) Summary graph bar of the presynaptic inhibition induced by AD and CCPA. The effect of CCPA on mepp frequency (s−1) as a function of concentration is shown in the inset. Control: 1.03±0.03 (n=8); CCPA: 1 nM: 1.07±0.03 (n=4); 10 nM: 0.88±0.04 (n=4), 100 nM: 0.68±0.03 (n=4), 250 nM: 0.63±0.01 (n=3), 500 nM: 0.55±0.03 (n=8), 700 nM: 0.55±0.04 (n=4). EC50: 19.4 nM. (c) DPCPX prevented the modulatory effect of CCPA. In (b, c), data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
Effect of AD on VDCCs
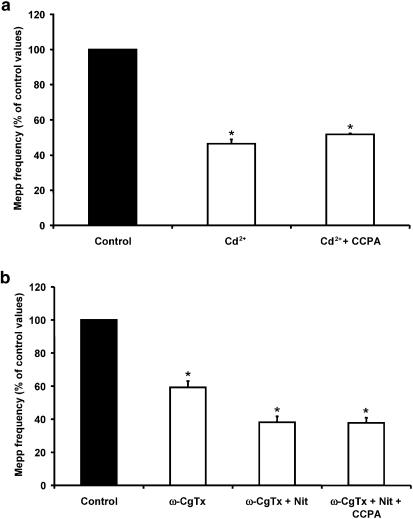
One of the possible mechanisms by which AD could induce presynaptic inhibition of spontaneous ACh release would be via a reduction in Ca2+ influx through the VDCCs that regulate this type of secretion. To verify this hypothesis, we first analyzed the effect of CCPA on nerve terminals previously incubated with a universal VDCC blocker such as Cd2+. In Figure 2a, it can be observed that 100 μM Cd2+ reduced the mepp frequency to 46.4±2.5% (n=5) of the control values and the addition of CCPA did not induce a significant reduction in spontaneous ACh secretion (51.7±0.6%). Since we have demonstrated that L- and N-type VDCCs are involved in tonic ACh secretion at the mammalian neuromuscular junction (Losavio & Muchnik, 1997), we thought it worthwhile to investigate the effect of CCPA in the presence of the specific channel blockers (Figure 2b). The application of 5 μM ω-CgTx, an N-type VDCC blocker, reduced mepp frequency to 59.2±3.8% (n=4) of the control values and the addition of 5 μM nitrendipine, an L-type VDCC blocker, induced a further reduction in spontaneous ACh secretion (to 38.2±3.5%). Introduction of 500 nM CCPA to the bathing solution did not significantly modify the values obtained with both blockers (37.8±3.1%), suggesting that VDCCs play a role in the mechanism of action of AD. This result prompted us to examine the effect of CCPA upon the individual VDCCs that are involved in the genesis of spontaneous secretion. We next investigated the effects of specific Ca2+-channel blockers and CCPA on mepp frequency.
Figure 2.
CCPA failed to induce presynaptic inhibition in the presence of Cd2+ and ω-CgTx+nitrendipine. (a) The universal VDCC blocker Cd2+ (100 μM) decreased mepp frequency and blocked the effect of AD. (b) The combined application of 5 μM ω-CgTx (an N-type VDCC antagonist) and 5 μM nitrendipine (Nit; an L-type VDCC antagonist) also diminished mepp frequency and prevented the inhibition induced by CCPA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
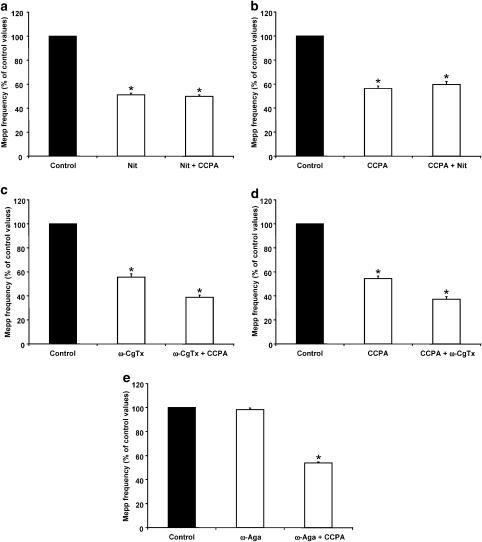
In Figure 3a, the effect of CCPA on the nitrendipine-sensitive component of mepp frequency can be observed: 5 μM nitrendipine reduced spontaneous secretion to 51.2±1.3% (n=5) of the control values. This effect of nitrendipine is greater in the absence than in the presence of ω-CgTx (see Figure 2 and Discussion, Losavio & Muchnik, 1998). Application of CCPA to preparations preincubated with nitrendipine did not show any further reduction in mepp frequency (49.8±1.3%). On reversing the order of administration, CCPA induced a presynaptic inhibition to 56.4±2.1% (n=5) of control values, and the addition of the L-type VDCC blocker did not have any further effect (59.6±2.6%; see Figure 3b). We obtained similar results when experiments were performed using 5 μM nifedipine as the L-type VDCC blocker (data not shown). These results clearly demonstrate that the specific activation of the A1 receptor reduces spontaneous ACh secretion that is associated with Ca2+ influx through L-type VDCCs.
Figure 3.
CCPA reduced the nitrendipine (Nit)- but not the ω-CgTx-sensitive component of mepp frequency. (a) Lack of effect of CCPA on spontaneous ACh secretion in the presence of 5 μM Nit. Mepp frequency recorded in CCPA and Nit was not significantly different from that obtained in Nit alone. (b) Lack of effect of Nit on spontaneous ACh secretion in the presence of CCPA. The effect of Nit was not observed when preparations were preincubated with CCPA. (c) Effect of CCPA on spontaneous ACh secretion in the presence of 5 μM ω-CgTx. CCPA induced a further reduction in mepp frequency when applied after ω-CgTx. (d) Effect of ω-CgTx on spontaneous ACh secretion in the presence of CCPA. ω-CgTx further reduced mepp frequency induced by CCPA. (e) Effect of CCPA on spontaneous ACh secretion in the presence of 100 nM ω-Aga. The P/Q-type VDCC blocker did not exert any change in spontaneous secretion and did not block the action of CCPA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
The effect of CCPA on N-type VDCCs is depicted in Figure 3c and d. When mepp frequency was recorded in the presence of 5 μM ω-CgTx, its value decreased to 55.6±2.8% (n=4) of control values, and the addition of CCPA induced a further reduction, which was significantly different from that obtained in ω-CgTx alone (38.8±1.9%, P<0.05). When the sequence of drug application was reversed, CCPA reduced mepp frequency to 54.5±2.1% (n=4) of control values, and subsequent application of ω-CgTx produced a further decrease to 37.2±2.2% (P<0.05 when compared to CCPA alone). The mepp frequency observed when preparations were exposed to the combined application of ω-CgTx and CCPA was very similar to that obtained when nitrendipine was applied after ω-CgTx, suggesting that the reduction of spontaneous release induced by CCPA was due to its effect on L-type VDCCs.
Spontaneous secretion is not related to Ca2+ influx through P/Q-type VDCCs (Losavio & Muchnik, 1997). As expected, 100 nM ω-Aga, a specific antagonist of P/Q-type VDCCs, did not modify mepp frequency (98.3±1.4% of control values, n=4) and did not alter the effect of CCPA (53.9±0.8% of control values, Figure 3e).
Mechanism of action of CCPA on L-type VDCCs
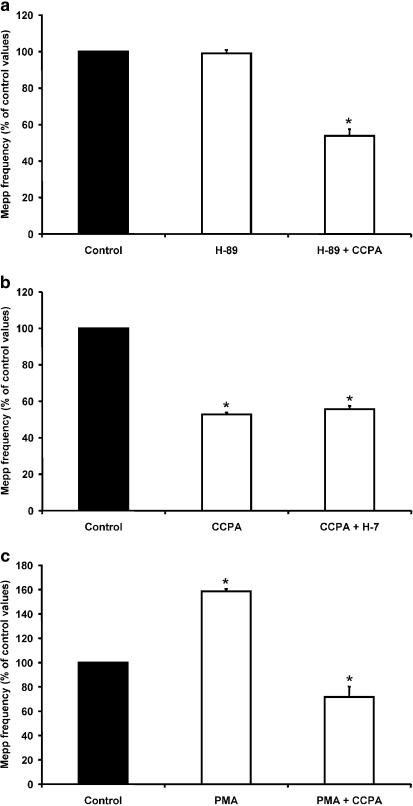
It has been reported that AD A1 receptors are negatively coupled to the adenylate cyclase-cAMP signal-transduction pathway (G protein Gi1–3), and that they also signal via phospholipase C (G protein Go) (Fredholm, 1995; Nyce, 1999). Thus, in order to test the hypothesis that AD decreases basal L-type VDCC activity by inhibiting the PKA pathway, we investigated whether a specific PKA blocker such as H-89 (Dezaki et al., 1996) altered (i.e., mimicked or blocked) the effect of CCPA on mepp frequency. In the absence of PKA inhibitors, the PKA activator forskolin (20 μM) increased spontaneous ACh release to ∼202% of control values. The addition of H-89 (1 μM) to the solution significantly reduced the stimulatory effect of forskolin (P<0.05), suggesting that H-89 inhibits PKA in our system (Table 1 ). We then studied the effect of CCPA in the presence of H-89, and found that CCPA still induced presynaptic inhibition to a degree similar to that induced in control solution (53.8±3.7% of control values, n=4, Figure 4a). These results suggest that CCPA does not modulate L-type VDCCs by decreasing the basal activity of PKA.
Table 1.
Effects of activators and inhibitors of second-messenger pathways on mepp frequency
| Protein kinase A | Protein kinase C | ||
|---|---|---|---|
| Solution | Mepp frequency (% of control values) | Solution | Mepp frequency (% of control values) |
| Forskolin | 201.6±15.2 (4) | PMA | 158.5±2.3 (4) |
| Forskolin+H-89 | 122.4±5.2 (4) | PMA+H-7 | 96.8±1.7 (4) |
| H-89 | 99.0±1.9 (4) | H-7 | 95.6±2.8 (4) |
Figure 4.
cAMP-PKA and phospholipase C-PKC pathways are not involved directly in CCPA modulation. (a) The specific PKA inhibitor H-89 (1 μM) neither mimicked nor prevented the effects of CCPA. CCPA inhibited spontaneous ACh release in the presence of H-89. (b) PKC blocker H-7 (10 μM) did not reverse the presynaptic inhibition induced by CCPA. H-7 added to control saline did not alter mepp frequency (see Table 1). (c) The PKC activator PMA (200 nM) did not block the action of CCPA. CCPA reduced mepp frequency when applied after incubation with PMA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
We also investigated whether activation of A1 receptors, via G0 protein, could trigger the formation of inositol trisphosphate and diacylglycerol, which are known to activate PKC, leading to a decrease in the activation of L-type VDCCs. If this were the case, application of 10 μM H-7, a PKC blocker at that concentration (Fu et al., 1993; Plomp & Molenaar, 1996), should reverse the presynaptic inhibition induced by CCPA. Addition of H-7 to the solution containing CCPA did not modify the effect of the A1 analog on mepp frequency (55.6±1.9% of control values, n=4, Figure 4b). On the other hand, if stimulation of PKC is the reason for inactivation of L-type VDCCs, 200 nM PMA, a phorbol ester known to activate PKC at neuromuscular junctions (Shapira et al., 1987; Redman et al., 1997; Angleson & Betz, 2001), would occlude CCPA action. We found that application of PMA increased the mepp frequency to 158.5±2.3% of control values (n=4), and further application of CCPA induced a reduction of spontaneous release to 71.8±8.5% of control (Figure 4c). To evaluate the effect of H-7 in our system, we analyzed whether H-7 could attenuate the stimulatory effect of PMA. H-7 reduced the increase in mepp frequency induced by PMA (see Table 1). In combination, these results suggest that neither the cAMP-PKA pathway nor the phospholipase C-PKC pathway are involved directly in the modulation of spontaneous secretion by AD.
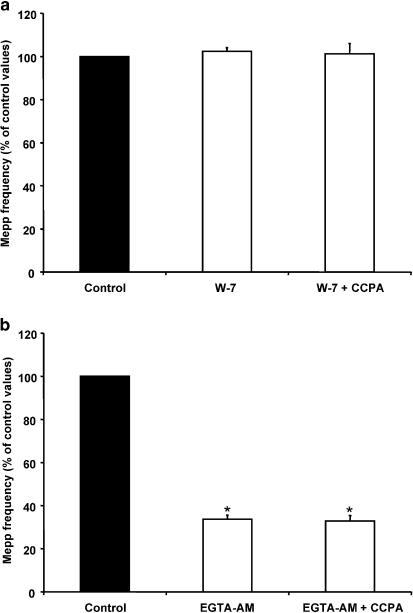
Recently, Ivanina et al. (2000) have demonstrated that both the Gβγ subunit of G protein and calmodulin directly bind to the cytosolic N terminus and the C terminus of the α1C subunit of L-type VDCCs, reducing the conductance of the channel. The inhibition occurs at basal cellular levels of Ca2+, and is eliminated by EGTA. Thus, in order to investigate whether activation of A1 receptors modulates L-type VDCCs in a calmodulin-dependent manner, we incubated the preparations with 50 μM W-7, an antagonist of calmodulin (Prior & Singh, 2000). W-7 itself did not modify the mepp frequency (102.4±1.7%, n=6), but prevented the expected decrease in mepp frequency with CCPA (101.3±4.8%, Figure 5a). We also performed experiments with the cell-permeant high-affinity Ca2+ chelator EGTA-AM. Muscles pretreated with EGTA-AM also blocked the inhibition induced by CCPA, suggesting that the modulation may be Ca2+-dependent (EGTA-AM: 33.7±1.7% of control values, n=4, EGTA-AM+CCPA: 32.9±2.5%, Figure 5b).
Figure 5.
Ca2+-calmodulin is involved in the presynaptic inhibition induced by CCPA. (a) W-7 (50 μM), an antagonist of calmodulin, did not alter mepp frequency, and prevented the effect of CCPA on spontaneous ACh release. (b) EGTA-AM decreased mepp frequency and blocked the action of CCPA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
Effect of CCPA at different external K+ concentrations
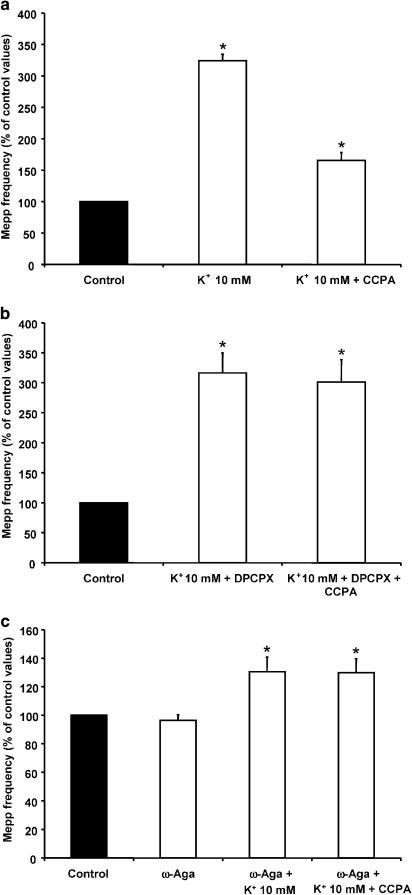
To investigate the effect of CCPA on diverse degrees of nerve terminal depolarization, we studied its effect at different external K+ concentrations (10, 15 and 20 mM). Figure 6a shows the increase in asynchronous ACh release when muscle fibres are bathed in 10 mM K+ (323.9±10.6% of control values, n=4). The addition of 500 nM CCPA reduced the mepp frequency to 165.6±12.4% of control. When preparations were pretreated with the specific A1 receptor blocker DPCPX (0.1 μM), the presynaptic modulation induced by CCPA was abolished (301.0±37.5% of control, n=4, Figure 6b). The increase in asynchronous ACh secretion induced by high K+ concentrations is due to Ca2+ influx through P/Q-type VDCCs (Losavio & Muchnik, 1997). Therefore, we investigated the effect of CCPA in the presence of 100 nM ω-Aga. Incubation with the P/Q-type antagonist reduced the effect of 10 mM K+ on mepp frequency (130.5±10.2%, n=4) and prevented the inhibitory effect of CCPA (129.8±9.9%), suggesting that, at high K+ concentrations, CCPA reduces asynchronous ACh secretion by modulating the P/Q-type VDCCs (Figure 6c).
Figure 6.
CCPA reduced the 10 mM K+-evoked increase in mepp frequency, acting on P/Q-type VDCCs. (a) CCPA reduced the asynchronous ACh release induced by 10 mM K+. (b) DPCPX prevented the modulatory effect of CCPA at 10 mM K+. (c) ω-Aga (100 nM) reduced the mepp frequency evoked by 10 mM K+-and prevented the presynaptic inhibition induced by CCPA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
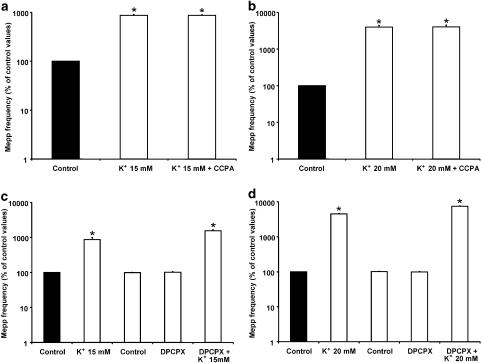
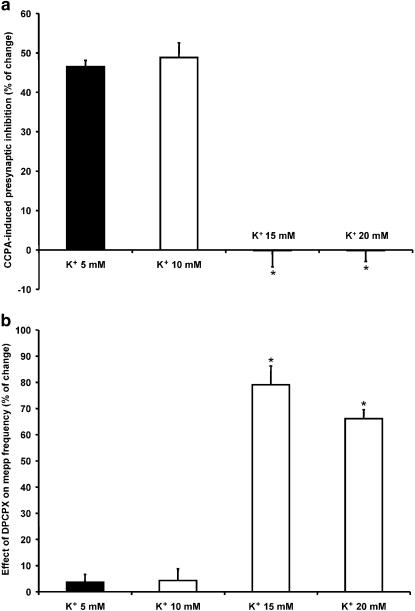
In 15 mM K+ (Figure 7a), asynchronous secretion increased to 870.7±37.7% of control values (n=5), but, interestingly, the addition of CCPA did not exert any modulatory effect (868.2±28.0% of control values). Similar results were obtained when the effect of CCPA on the mepp frequency induced by 20 mM K+ was investigated (20 mM K+: 3983.9±462.9% of control values; 20 mM K++CCPA: 4005.5±513.8%; n=4, Figure 7b). To evaluate whether the absence of an effect of CCPA upon tonic ACh secretion in 15 and 20 mM K+ was due to the fact that presynaptic A1 receptors had already been occupied by endogenous AD, 0.1 μM DPCPX was added to the solution before incubation in high K+ solutions. When mepp frequency was recorded in 15 and 20 mM K+, an increase to 1542.8±107.1% (n=5) and 7404.3±205.9% (n=5) of control values, respectively, was observed (Figure 7c and d). These values were significantly higher than those obtained in 15 mM and 20 mM K+ in the absence of the A1 AD receptor antagonist (P<0.05). Figure 8a and b summarizes the effect of CCPA and its antagonist DPCPX at different external K+ concentrations. The effect is expressed as the percentage change in mepp frequency compared to that in control solutions. At 5 and 10 mM K+, CCPA inhibited transmitter release by 46.5 and 48.8%, respectively, whereas, at 15 and 20 mM K+, CCPA failed to exert any inhibitory effect. DPCPX did not modify neurosecretion when it was added to 5 and 10 mM K+ solutions, but increased asynchronous ACh release by 79 and 66% in 15 and 20 mM K+.
Figure 7.
CCPA failed to reduce asynchronous ACh release induced by 15 and 20 mM K+. (a, b) Lack of effect of CCPA on the 15 and 20 mM K+-evoked increase in mepp frequency. CCPA did not affect mepp frequency when applied after 15 and 20 mM K+. (c, d) Effect of DPCPX on the mepp frequency induced by 15 and 20 mM K+. The blockade of A1 AD receptors by DPCPX caused a greater increase in mepp frequency compared to that obtained before the application of DPCPX in the same muscles. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
Figure 8.
Effect of exogenous and endogenous AD at different external K+ concentrations. (a) Effect of CCPA on mepp frequency at 5, 10, 15 and 20 mM K+, expressed as percentage change compared to control solutions. (b) Effect of DPCPX on mepp frequency at 5, 10, 15 and 20 mM K+, expressed as percentage change with respect to mepp frequency obtained in 5, 10, 15 and 20 mM K+ without DPCPX. *P<0.05.
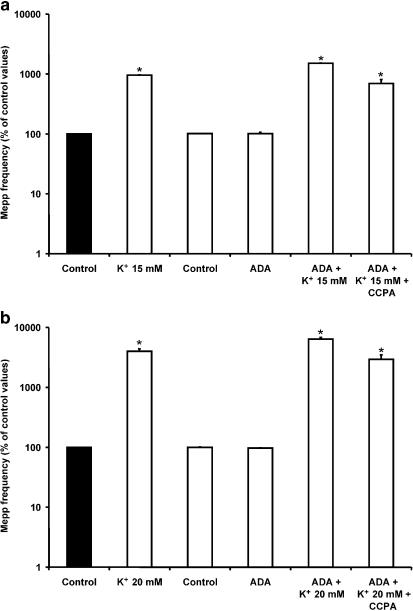
Consistent with these results, as shown in Figure 9a and b, ADA (0.5 U ml−1), the enzyme that inactivates endogenous AD by converting it into inosine (Redman & Silinsky, 1994), also increased the ACh secretion evoked by 15 and 20 mM K+ (15 mM K++ADA: 1507.5±12.3% of control values, n=4; 20 mM K++ADA: 6389.6±410.3%, n=4). Interestingly, in these conditions, CCPA was able to modulate spontaneous secretion (15 mM K++ADA+CCPA: 692.3±119.1% of control values; 20 mM K++ADA+CCPA: 2934.5±548.1%). All these results suggest that, at high K+ concentrations, endogenous AD induces presynaptic inhibition of asynchronous ACh release by occupying A1 receptors.
Figure 9.
Binding of endogenous AD to A1 AD receptors prevented the effect of CCPA at 15 and 20 mM K+. (a, b) Incubation of preparations with ADA (0.5 U ml−1), which degraded AD into the inactive metabolite inosine, increased mepp frequency compared to that obtained in 15 and 20 mM K+ before the application of ADA in the same muscles, and allowed the action of CCPA. Data are expressed as percentages of control values (black bars). Error bars indicate s.e.m. *P<0.05.
Discussion
We have shown that, at the mouse neuromuscular junction, the modulator AD and the specific AD A1 receptor agonist CCPA decreased spontaneous acetylcholine release by 37 and 43.4%, respectively. Among the mechanisms that could contribute to this modulation are the reduced entry of calcium through the channels associated with tonic secretion (L- and N-type VDCCs, Losavio & Muchnik, 1997) and direct impairment of neurotransmitter exocytosis. Our results provide evidence that presynaptic VDCCs play the main role in presynaptic inhibition of spontaneous transmitter release. In the light of this, it can be predicted that, at presynaptic receptors, AD should have the same effect as universal Ca2+ blockers such as the divalent cation Cd2+. We found that incubation of preparations with Cd2+ decreased mepp frequency and blocked the inhibitory effect of CCPA. The same result was observed when the effect of CCPA was studied in the presence of the specific N- and L-type VDCC antagonists ω-CgTx and nitrendipine (see Figure 2). A neuromodulatory action of CCPA via a decrease in Ca2+ influx was also observed at the mammalian neuromuscular junction when nerve terminals were stimulated electrically (Hamilton & Smith, 1991). In contrast, at frog neuromuscular junctions, this mechanism was not found to be associated with the inhibitory action of AD (Silinsky & Solsona, 1992; Redman & Silinsky, 1994; Robitaille et al., 1999), suggesting that, in this species, as in other tissues, the nucleoside inhibits tonic secretion by modulating the release machinery downstream of Ca2+ influx (Prince & Stevens, 1992; Scanziani et al., 1992; Scholz & Miller, 1992; Capogna et al., 1996; Dittman & Regehr, 1996; for a review see Wu & Saggau, 1997).
In order to investigate which of the VDCCs was involved in the presynaptic effect of CCPA, we studied the different blocking effects of specific VDCC blockers and CCPA on mepp frequency. Our experiments demonstrated that CCPA reduced spontaneous ACh release by exclusively modulating L-type VDCCs, since incubation with nitrendipine reduced spontaneous secretion and prevented CCPA-mediated inhibition. On reversing the order of administration of the drugs, CCPA prevented the effect of nitrendipine. AD has also been observed to modulate the basal L-type Ca2+ current in ferret isolated right ventricular myocytes (Qu et al., 1993), frog pituitary melanotrophs (Mei et al., 1996) and rabbit carotid body chemoreceptor cells (Rocher et al., 1999). On the other hand, the ω-CgTx-sensitive component of spontaneous secretion was not affected by CCPA, since when CCPA was added to a solution containing the toxin it induced a further reduction of the secretion. This value was similar to that obtained when preparations were exposed to both blockers. When the effect of ω-CgTx was evaluated after exposure to CCPA, it also produced a further decrease in spontaneous secretion, although this effect was smaller than that obtained when ω-CgTx was applied alone. If we assume that CCPA reduced the nitrendipine-sensitive component of spontaneous ACh secretion, these results are consistent with those reported in a previous paper when mepp frequency was studied in the presence of nifedipine and ω-CgTx in rat muscles. We found that simultaneous application of both antagonists reduced the mepp frequency to less than the arithmetic sum of the individual effects of nifedipine and ω-CgTx, suggesting that L- and N-type VDCCs act synergistically to control spontaneous secretion at individual release sites, with probable overlapping of their domains (Losavio & Muchnik, 1998).
Previous studies of neuromuscular junctions have shown that activation of A1 receptors is coupled to pertussis toxin (PTX)-sensitive G proteins (Gi/o class) (Hamilton & Smith, 1991; Mynlieff & Beam, 1994). Activation of Gi proteins by AD might cause the inhibition of the adenylate cyclase-cAMP signal-transduction pathway, leading to inactivation of L-type VDCCs, as has been shown in other cells (Ebersolt et al., 1983; Linden, 1991; Zink et al., 1995). However, in our experiments, this mechanism does not seem to be involved, since H-89, a specific PKA inhibitor, neither mimicked nor blocked the effect of CCPA. On the other hand, stimulation of Go proteins by AD might activate the phospholipase C–PKC pathway, leading in this case to a decrease in Ca2+ current, as was observed in isolated hippocampal neurons and dorsal root ganglion neurons (Doerner et al., 1988; Rane et al., 1989). Application of H7, a PKC blocker, did not reverse the presynaptic inhibition induced by CCPA, and did not by itself modify basal mepp frequency. Furthermore, PMA, an activator of PKC, known to induce an increase in spontaneous neurotransmitter release at the mouse neuromuscular junction via a mechanism independent of Ca2+ influx at the nerve terminal (Scornik et al., 1990), did not prevent the effect of CCPA, suggesting that activation of PKC did not have any effect on CCPA-induced depression of spontaneous release. All these results are in agreement with those reported by Hamilton & Smith (1991). These authors demonstrated that the observed reduction in the prejunctional Ca2+ current induced by AD could not be reversed by either cAMP analogues or the adenylate cyclase activator forskolin, and neither did PMA or a diacylglycerol analog have any effect. Recently, Hirsh & Silinsky (2002) showed that the inhibitory effect of 2-chloroadenosine on mepp frequency was independent of PKA inhibition.
Another alternative is that CCPA could inactivate L-type VDCCs by acting through the G protein βγ subunit via a membrane-delimited pathway, independently of soluble intracellular messengers (Hille, 1994; Herlitze et al., 1996; Ikeda, 1996; Dolphin, 1998). The activation of A1 AD receptors in cholinergic interneurons has been shown to reduce N-type Ca2+ currents via a membrane-delimited, Gi/o class G-protein pathway (Song et al., 2000). Although the inhibitory action of Gβγ protein seems to be selective for some types of Ca2+ channels, N- and P/Q-type VDCCs being the principal targets (Snutch & Reiner, 1992; Zhang et al., 1996; Jarvis & Zamponi, 2001), Ivanina et al. (2000) have demonstrated that, at basal cellular levels of Ca2+, G protein βγ subunits have an inhibitory effect on L-type VDCCs in a voltage-independent, but calmodulin-dependent manner. Here we propose that CCPA inhibits spontaneous ACh secretion by a mechanism that involves Ca2+-calmodulin, since treatment of preparations with the calmodulin antagonist W-7 and the intracellular Ca2+ chelator EGTA-AM eliminates the modulatory effect of CCPA.
In order to study the effect of CCPA on different degrees of depolarization of nerve terminals, we investigated its action at different external K+ concentrations. We found that, at moderate levels of K+ (up to 10 mM K+), CCPA (exogenous AD) induced presynaptic inhibition of spontaneous ACh release. AD coming from the hydrolysis of released ATP through the ectonucleotidase pathway, in parallel with that released from both nerve and muscle cells (Cunha & Sebastião, 1991; 1993; Smith, 1991; Lloyd et al., 1993) (endogenous AD), does not appear to occupy most of the A1 receptors. In contrast, at high levels of K+ (15 and 20 mM), CCPA failed to exert its inhibitory effect, suggesting that under these conditions presynaptic A1 receptors were occupied by endogenous AD. The assumption that at high levels of neurotransmitter release endogenous AD exerts an inhibitory effect was confirmed by the fact that DPCPX, applied to the solutions containing high K+, induced an increase in mepp frequency when compared to values obtained in 15 and 20 mM K+ in the absence of the antagonist. Consistent with this notion, we found that ADA, which completely inactivates extracellular AD, mimicked the facilitatory effect of DPCPX and allowed the inhibitory action of CCPA (which is not metabolized by ADA).
It has previously been shown that ω-Aga (a P/Q-type VDCC blocker) inhibits the presynaptic Ca2+ currents and ACh release induced by either electrical or K+ depolarization at the mammalian neuromuscular junction (Protti & Uchitel, 1993; Bowersox et al., 1995; Losavio & Muchnik, 1997). We demonstrated that, at 10 mM K+, ω-Aga prevented the action of CCPA, suggesting that, in these conditions, AD inhibits mepp frequency by reducing Ca2+ influx through P/Q-type VDCCs. Taking into account the observation that AD decreases the presynaptic Ca2+ current in the rat when the nerve is electrically stimulated (Hamilton & Smith, 1991), we speculate that AD modulates P/Q-type VDCCs when ACh release is evoked. However, further investigations are needed to clarify whether AD induces presynaptic inhibition of P/Q-type VDCCs by a mechanism that involves calmodulin. It has been found that Ca2+-calmodulin may exert two opposing effects on P/Q-type VDCCs, initially facilitating and then inactivating channel opening (Lee et al., 2000; DeMaria et al., 2001).
In conclusion, our results are consistent with the hypothesis that, at mammalian neuromuscular junctions, activation of A1 receptors induces presynaptic modulation of spontaneous ACh release due to the modulation of VDCCs rather than inhibition of the release machinery downstream of Ca2+ influx. We found a differential inhibition of the calcium channels associated with tonic secretion: CCPA inhibits the nitrendipine-sensitive component of secretion, while the ω-CgTx-sensitive component is not affected. The inhibitory effect of CCPA is independent of PKA inhibition and PKC activation, but seems to be related to Ca2+-calmodulin. On the other hand, we demonstrated that, when spontaneous secretion was increased by exposing nerve terminals to high K+ concentrations, endogenous AD induced presynaptic inhibition as a means of preventing excessive neurotransmitter secretion by occupying the A1 AD receptors.
Acknowledgments
We thank Mrs Fernanda Rodriguez and Mrs Raquel Almirón for technical assistance, and Mr Agustín García Sanz for his invaluable contribution. This work was supported by grants from the Ministerio de Salud Pública and the Consejo de Investigaciones Científicas y Técnicas.
Abbreviations
- AD
adenosine
- ADA
adenosine deaminase
- CCPA
2-chloro-N6-cyclopentyl-adenosine
- DMSO
dimethylsulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- EGTA-AM
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
- H-7
1-(5-isoquinolinesulfonyl)-2-methyl-piperazine
- H-89
N-(2[p-bromocinnamylamino]-ethyl)-5-isoquinolinesulfonamide
- mepp
miniature end-plate potential
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- VDCC
voltage-dependent calcium channel
- W-7
N-(6-aminohexil)-5-chloro-1-naphthalenesulfonamide hydrochloride
- ω-Aga
ω-agatoxin IVA
- ω-CgTx
ω-conotoxin GVIA
References
- ANGLESON J.K., BETZ W.J. Intraterminal Ca2+ and spontaneous transmitter release at the frog neuromuscular junction. J. Neurophysiol. 2001;85:287–294. doi: 10.1152/jn.2001.85.1.287. [DOI] [PubMed] [Google Scholar]
- BOWERSOX S.S., MILJANICH G.P., SUGIURA Y., LI C., NADASDI L., HOFFMAN B.B., RAMACHANDRAN J., KO C.P. Differential blockade of voltage-sensitive calcium channels at the mouse neuromuscular junction by novel omega-conopeptides and omega-agatoxin-IVA. J. Pharmacol. Exp. Ther. 1995;273:248–256. [PubMed] [Google Scholar]
- CAPOGNA M., GÄHWILER B.H., THOMPSON S.M. Presynaptic inhibition of calcium-dependent and -independent release elicited with ionomycin, gadolinium, and α-latrotoxin in the hippocampus. J. Neurophysiol. 1996;75:2017–2028. doi: 10.1152/jn.1996.75.5.2017. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., SEBASTIÃO A.M. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur. J. Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., SEBASTIÃO A.M. Adenosine and adenine nucleotides are independently released from both the nerve terminals and the muscle fibres upon electrical stimulation of the innervated skeletal muscle of the frog. Pflügers Arch. 1993;424:503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- DEMARIA C.D., SOONG T.W., ALSEIKHAN B.A., ALVANIA R.S., YUE D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- DEZAKI K., KIMURA I., TSUNEKI H., KIMURA M. Enhancement by calcitonin gene-related peptide of non-contractile Ca2+-induced nicotinic receptor desensitization at the mouse neuromuscular junction. Br. J. Pharmacol. 1996;118:1971–1976. doi: 10.1111/j.1476-5381.1996.tb15632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMAN J., REGEHR W. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J. Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERNER D., PITLER T.A., ALGER B.E. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J. Neurosci. 1988;8:4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLPHIN A. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J. Physiol. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERSOLT C., PREMONT J., PROCHIANTZ A., PEREZ M., BOCKAERT J. Inhibition of brain adenylate cyclase by A1 adenosine receptors. Pharmacological characteristics and locations. Brain Res. 1983;267:123–129. doi: 10.1016/0006-8993(83)91045-4. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B. Adenosine receptors in the central nervous system. NIPS. 1995;10:122–128. [Google Scholar]
- FU W.M., YANG S.H., LIN-SHIAU S.Y. Potentiation of miniature endplate potential frequency by ATP in Xenopus tadpoles. Br. J. Pharmacol. 1993;108:236–241. doi: 10.1111/j.1476-5381.1993.tb13468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B.L., HIRST G.D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J. Physiol. 1972;224:629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS R.A., MACDONALD R.L., JASTROW T.R. 2-Chloroadenosine reduced the N calcium currents of cultured mouse sensory neurons in a pertussis toxin-sensitive manner. J. Physiol. 1989;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON B.R., SMITH D.O. Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J. Physiol. 1991;432:327–341. doi: 10.1113/jphysiol.1991.sp018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERLITZE S., GARCÍA D.E., MACKIE K., HILLE B., SCHEUER T., CATTERAL W.A. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- HILLE B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- HILLE B. Modulation of ion-channel function by G-protein-coupled receptors. TINS. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- HIRSH J.K., SILINSKY E.M. Inhibition of spontaneous acetylcholine secretion by 2-chloroadenosine as revealed by a protein kinase inhibitor at the mouse neuromuscular junction. Br. J. Pharmacol. 2002;135:1897–1902. doi: 10.1038/sj.bjp.0704653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKEDA S. Voltage-dependent modulation of N- type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–262. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- IVANINA T., BLUMENSTEIN Y., SHISTIK E., BARZILAI R., DASCAL N. Modulation of L-type Ca2+ channels By Gβγ and calmodulin via interactions with N and C termini of α1c. J. Biol. Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- JARVIS S.E., ZAMPONI G.W. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. TIPS. 2001;22:519–525. doi: 10.1016/s0165-6147(00)01800-9. [DOI] [PubMed] [Google Scholar]
- KIJIMA H., TANABE N. Calcium-independent increase of transmitter release at frog end-plate by trinitrobenzene sulphonic acid. J. Physiol. 1988;403:135–149. doi: 10.1113/jphysiol.1988.sp017243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE A., SCHEUER T., CATTERALL W.A. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca+ channels. J. Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEN J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- LLOYD H.G., LINDSTROM K., FREDHOLM B.B. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem. Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- LOSAVIO A., MUCHNIK S. Spontaneous acetylcholine release in mammalian neuromuscular junctions. Am. J. Physiol. 1997;273:C1835–C1841. doi: 10.1152/ajpcell.1997.273.6.C1835. [DOI] [PubMed] [Google Scholar]
- LOSAVIO A., MUCHNIK S. Role of L-type and N-type voltage-dependent calcium channels (VDCCs) on spontaneous acetylcholine release at the mammalian neuromuscular junction. Ann. N.Y. Acad. Sci. 1998;841:636–645. doi: 10.1111/j.1749-6632.1998.tb10995.x. [DOI] [PubMed] [Google Scholar]
- MARQUARDT D.L. Mast cell adenosine receptor characteristics and signaling. Adv. Exp. Med. Biol. 1998;431:79–82. doi: 10.1007/978-1-4615-5381-6_15. [DOI] [PubMed] [Google Scholar]
- MEI Y.A., LE FOLL F., VAUDRY H., CAZIN L. Adenosine inhibits L- and N- type calcium channels in pituitary melanotrophs. Evidence for the involvement of a G protein in calcium channel gating. J. Neuroendocrinol. 1996;8:85–91. doi: 10.1111/j.1365-2826.1996.tb00827.x. [DOI] [PubMed] [Google Scholar]
- MERINEY S.D., GRINNEL A.D. Endogenous adenosine modulates stimulation-induced depression at the frog neuromuscular junction. J. Physiol. 1991;443:441–455. doi: 10.1113/jphysiol.1991.sp018843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYNLIEFF M., BEAM K.G. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J. Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYCE J. Insight into adenosine receptor function using antisense and gene-knockout approaches. TIPS. 1999;20:79–83. doi: 10.1016/s0165-6147(99)01305-x. [DOI] [PubMed] [Google Scholar]
- PLOMP J.J., MOLENAAR P.C. Involvement of protein kinases in the upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats. J. Physiol. 1996;493:175–186. doi: 10.1113/jphysiol.1996.sp021373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINCE D.A., STEVENS C.F. Adenosine decreases neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR C., SINGH S. Factors influencing the low-frequency associated nicotinic ACh autoreceptor-mediated depression of ACh release from rat motor nerve terminals. Br. J. Pharmacol. 2000;129:1067–1074. doi: 10.1038/sj.bjp.0703161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROTTI D.A., UCHITEL O.D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993;5:333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- QU Y., CAMPBELL D.L., WHORTON A.R., STRAUSS H.C. Modulation of basal L-type Ca2+ current by adenosine in ferret isolated right ventricular myocytes. J. Physiol. 1993;471:269–293. doi: 10.1113/jphysiol.1993.sp019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANE S.G., WALSH M.P., MC DONALD J.R., DUNLAP K. Specifics inhibitors of protein kinase C block transmitter-induced modulation of sensory neuron calcium current. Neuron. 1989;3:239–245. doi: 10.1016/0896-6273(89)90037-8. [DOI] [PubMed] [Google Scholar]
- REDMAN R.S., SEARL T.J., HIRSH J.K., SILINSKY E.M. Opposing effects of phorbol esters on transmitter release and calcium currents at frog motor nerve endings. J. Physiol. 1997;501:41–48. doi: 10.1111/j.1469-7793.1997.041bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDMAN R.S., SILINSKY E.M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J. Physiol. 1994;477:127–177. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBEIRO J.A., SEBASTIÃO A.M. On the role, inactivation, and origin of endogenous adenosine at the frog neuromuscular junction. J. Physiol. 1987;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBITAILLE R., THOMAS S., CHARLTON M.P. Effects of adenosine on Ca2+ entry in the nerve terminal of the frog neuromuscular junction. Can. J. Physiol. Pharmacol. 1999;77:707–714. [PubMed] [Google Scholar]
- ROCHER A., GONZALEZ C., ALMARAZ L. Adenosine inhibits L-type Ca2+ current and catecholamine release in the rabbit carotid body chemoreceptor cells. Eur. J. Neurosci. 1999;11:673–681. doi: 10.1046/j.1460-9568.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- SCANZIANI M., CAPOGNA M., GÄHWILER B., THOMPSON S. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- SCHOLZ K., MILLER R. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- SCORNIK F., SZCZUPAK L., NICOLA SIRI L., UCHITEL O.D. Ca2+ role on the effect of phorbol esters on the spontaneous quantal release of neurotransmitter at the mouse neuromuscular junction. Brain Res. 1990;525:280–284. doi: 10.1016/0006-8993(90)90875-c. [DOI] [PubMed] [Google Scholar]
- SHAPIRA R., SILBERBERG S.D., GINSBURG S., RAHAMIMMOFF R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987;325:58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]
- SILINSKY E.M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J. Physiol. 1984;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M., SOLSONA C.S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J. Physiol. 1992;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D.O. Sources of adenosine released during neuromuscular transmission in the rat. J. Physiol. 1991;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNUTCH T.P., REINER P.B. Ca2+ channels: diversity of form and function. Curr. Opin. Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- SONG W.J., TKATCH T., SURMEIER D. Adenosine receptor expression and modulation of Ca2+ channels in rat striatal cholinergic interneurons. J. Neurophysiol. 2000;83:322–332. doi: 10.1152/jn.2000.83.1.322. [DOI] [PubMed] [Google Scholar]
- WU L.-G., SAGGAU P. Presynaptic inhibition of elicited neurotransmitter release. TINS. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- ZHANG J., ELLINOR P.T., ALDRICH R.W., TSIEN R.W. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- ZHU Y., IKEDA S.R. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J. Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- ZINK A., SCHERUBL H., HOFLICH M., HESCHELER J., RAUE F. Adenosine A1 receptors inhibit cAMP and Ca2+ mediated calcitonin secretion in C-cells. Horm. Metab. Res. 1995;27:408–414. doi: 10.1055/s-2007-979989. [DOI] [PubMed] [Google Scholar]