Abstract
Experiments were performed to elucidate the mechanism by which alterations of extracellular pH (pHo) change membrane potential (EM) in rat mesenteric and pulmonary arteries.
Changing pHo from 7.4 to 6.4 or 8.4 produced a depolarisation or hyperpolarisation, respectively, in mesenteric and pulmonary arteries. Anandamide (10 μM) or bupivacaine (100 μM) reversed the hyperpolarisation associated with alkaline pHo, shifting the EM of both vessels to levels comparable to that at pH 6.4. In pulmonary arteries, clofilium (100 μM) caused a significant reversal of hyperpolarisation seen at pH 8.4 but was without effect at pH 7.4.
K+ channel blockade by 4-aminopyridine (4-AP) (5 mM), tetraethylammonium (TEA) (10 mM), Ba2+ (30 μM) and glibenclamide (10 μM) depolarised the pulmonary artery. However, shifts in EM with changes in pHo remained and were sensitive to anandamide (10 μM), bupivacaine (100 μM) or Zn2+ (200 μM).
Anandamide (0.3–60 μM) or bupivacaine (0.3–300 μM) caused a concentration-dependent increase in basal tone in pulmonary arteries.
RT–PCR demonstrated the expression of TASK-1, TASK-2, THIK-1, TRAAK, TREK-1, TWIK-1 and TWIK-2 in mesenteric arteries and TASK-1, TASK-2, THIK-1, TREK-2 and TWIK-2 in pulmonary arteries. TASK-1, TASK-2, TREK-1 and TWIK-2 protein was demonstrated in both arteries by immunostaining.
These experiments provide evidence for the presence of two-pore domain K+ channels in rat mesenteric and pulmonary arteries. Collectively, they strongly suggest that modulation of TASK-1 channels is most likely to have mediated the pH-induced changes in membrane potential observed in these vessels, and that blockade of these channels by anandamide or bupivacaine generates a small increase in pulmonary artery tone.
Keywords: Two-pore, potassium channels, mesenteric, pulmonary, pH, myocytes
Introduction
Potassium (K+) channel alpha subunits with two pore (2P) forming regions were first described in yeast by Ketchum et al. (1995) and a human homologue was subsequently discovered (Lesage et al., 1996). Two such alpha subunits are believed to form the K+ channel (Lopes et al., 2001) and at least 12 functional channels have so far been identified (Patel & Honoré, 2001). For descriptive purposes, 2P-domain K+ channels are divided into families largely according to their electrophysiological and/or pharmacological properties and designated by acronyms such as TWIK (Tandem of P domains in Weak Inward rectifier K+ channel) and TASK (TWIK-related Acid-Sensitive K+ channel) (Patel & Honoré, 2001). The TASK family includes TASK-1, 2, 3, 4 and 5, although TASK-5 does not seem to produce a functional channel when expressed in artificial systems and the TWIK family comprises two members, designated TWIK-1 and 2, respectively (Kim & Gnatenco, 2001).
During an investigation of membrane potential changes in small, isolated pulmonary arteries using sharp microelectrodes (Weston, unpublished), it was noted that modifying the pH of the superfusing Tyrodes solution generated reproducible changes in the basal membrane potential of the vessel myocytes. 2P-domain K+ channels are not only believed to be important determinants of the basal membrane potential of excitable cells but also some, such as the members of the TASK family, can be modulated by fluctuations in extracellular pH (Lesage & Lazdunski, 2000).
In this paper, the effects of changing extracellular pH on the basal membrane potential of small pulmonary and mesenteric arterioles are described, together with a study of the presence of 2P-domain K+ channels in these vessels. The results suggest that TASK channels play a physiological role in regulating basal membrane potential and tone in these vessels and suggest that these channels could represent a novel target for the treatment of cardiovascular disease. A preliminary account of some of the findings has been reported (Gardener et al., 2003; Johnson et al., 2003).
Methods
Second- and third-order mesenteric (100–200 μm internal diameter) and main and second-order pulmonary arteries (250–350 μm internal diameter) were dissected from male Sprague–Dawley rats (150–200 g) previously killed by stunning followed by cervical dislocation.
Electrophysiology
Intact, third-order mesenteric and second-order pulmonary arteries were pinned to the Sylgard base of a thermostatically controlled bath and superfused (10 ml min−1) at 37°C with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered Tyrode's solution (NaCl 140 mM, KCl 4.7 mM, MgCl2.6H2O 1.0 mM, CaCl2 1.3 mM, HEPES 10 mM, glucose 10 mM), adjusted to pH 7.4 with 3 M NaOH and gassed with 100% O2. Solutions were made more acidic (pH 6.4) or alkaline (pH 8.4) by the addition of 3 M HCl or 3 M NaOH, respectively. Vessels were impaled from the adventitial side using microelectrodes filled with 3 M KCl (resistance 40–80 MΩ).
Experiments were performed using a conventional high impedance amplifier (Intra 767; WPI Instruments, U.S.A.). Interference (50 Hz) at the amplifier output was selectively removed using an active processing circuit (Humbug; Digitimer, U.K.), after which the signals were digitised and analysed using a MacLab system (ADI Instruments). Since the membrane potential changes induced by modifying extracellular pH were relatively small, only data obtained from stable, continuous recordings are described in this paper. Thus, if the basal membrane potential at pH 7.4 did not lie in the region of −50 mV and if the electrode became dislodged during an experiment, the data were not used for analysis and a new impalement or vessel segment was used. Where appropriate, the endothelium was removed by passing deionized water down the vessel lumen, and the success of this procedure was demonstrated by a lack of acetylcholine-induced hyperpolarization (see Chen et al., 1988).
Myography
Small second-order pulmonary arteries up to 2 mm in length were dissected on ice and suspended horizontally between two 40 μm stainless steel wires in 5 ml baths of a multimyograph (Mulvaney–Halpern type model 610, JP Trading, Aarhus, Denmark). Vessels were maintained in Krebs physiological salt solution (PSS) at 37°C (NaCl 118 mM, KCl 3.4 mM, CaCl2 2.5 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25 mM, glucose 11.1 mM and was bubbled with 21% O2 : 5% CO2 : 74% N2, pH 7.5). Arteries were normalised to a transmural pressure equivalent to 30 mmHg, a procedure which has been shown to give optimal responses (Ozaki et al., 1998). Subsequent to normalisation and 30 min equilibration, vessels were stimulated for 2 min on three successive occasions (2 min between responses) with 75 mM K+ depolarising PSS (equimolar substitution of NaCl by KCl) and the largest response was used as the reference contraction. Drug effects on resting tension were examined by obtaining concentration–effect curves following cumulative application of anandamide and bupivacaine. Drug responses were calculated as a percentage of 75 mM K+-induced tension (%TK).
RT–PCR
Total RNA extraction was performed using the RNeasy mini kit (Qiagen) and eluted using RNase-free water. Total RNA was subjected to treatment with DNase 1 (Life Technologies, Paisley, U.K.) prior to reverse transcription using Superscript II RNase H− reverse transcriptase (Life Technologies). PCR reactions were performed using custom oligonucleotides (Genosys, Cambridge, U.K.) (see Table 1 ). PCR reactions were carried out using the following:- 0.3 μM primers, 0.25 mM dNTPs, 1 × Taq buffer (5 μl 10 × buffer in 50 μl final reaction volume), cDNA, 0.25 μl ExTaq (5 U μl−1) and nuclease-free water (Promega, Southampton, U.K.) to 50 μl. Reactions were subjected to a hot start for 5 min and cycled 35 times through 94°C for 30 s, 55°C (GAPDH), 58°C (TASK-1, TASK-2, TREK-1, TWIK-2 and THIK-1), 60°C (TRAAK and TREK-2) or 68°C (touchdown PCR, −1°C decrease per cycle for the first 15 cycles) (TWIK-1 and TASK-3) for 30 s and 1 min at 72°C, followed by an extension step of 72°C for 7 min. Products were electrophoresed at 10 V cm−1 on a 1% w v−1 agarose gel containing 0.5 μg ml−1 ethidium bromide bathed in 0.5 × TBE (44.5 mM Trizma base, 56 mM boric acid, 0.99 mM ethylenediaminetetra-acetic acid (EDTA) in milliQ purified water) containing 0.5 μg ml−1 ethidium bromide and visualised under UV light. Product identity was confirmed by sequencing using a TA cloning kit (Invitrogen) and a big dye terminated sequencing kit (v1.0) (Applied Biosciences).
Table 1.
Primer sequences used for PCR amplification of two-pore domain K+ channels
| Genbank no. | Primer: name | Sequence (5′ → 3′) |
|---|---|---|
| X02231 | rGAPDH 5′ | CTACATGGCCTCCAAGGAGTAAG |
| rGAPDH 3′ | GAGGGAAGAGAGCTTATGGTAG | |
| AF031384 | rTASK-1 5′ | GGTGCTCATCGGTTTCGTGTC |
| rTASK-1 3′ | CAGGCTACCGCTTAGGCAGCTC | |
| AF319542/AF084830 | rTASK-2 5′ | CTCATCAAACAGATTGGGAAGAAG |
| rTASK-2 3′ | GGAAGATGAGTGGGTGGTAGTC | |
| AF192366 | rTASK-3 5′ | CTGGAGCTGGTAATCCTGCAGTCTGAG |
| rTASK-3 3′ | AAGCTCCAATCTTCGCACTGGG | |
| AF287301 | rTHIK-1 5′ | GATGACGACCCCAGCCACAAC |
| rTHIK-1 3′ | GTCTGTTTGATCAGGATGGAGATGACG | |
| AF302842 | rTRAAK 5′ | CTGGAGCAGCCTCACGAGCAG |
| rTRAAK 3′ | ATGGCTTCTAACTTGCTCCAGCTCTCC | |
| AF325671 | rTREK-1 5′ | CCATAGGATTTGGAAACATCTCCCCAC |
| rTREK-1 3′ | CAATCATGCTCAGAACAGCTGCAAAG | |
| NM_023096 | rTREK-2 5′ | GCTGGCATCAATTACCGAGAATG |
| rTREK-2 3′ | GTTGTCCTCAGAAGCGCCCTG | |
| NM_021688 | rTWIK-1 5′ | CCATGCCGTTCTGCTGGGATTC |
| rTWIK-1 3′ | GGAAAAGGACAGTTGGTCATGCTCC | |
| NM_053806 | rTWIK-2 5′ | GTTTGGGCTTTCTGACTTTGTG |
| rTWIK-2 3′ | GCTAAGCTGCTTGTCTCATGC |
Western blotting
Whole rat brain was homogenised in extraction buffer (20 mM Trizma base, 2.5 mM sucrose, 5 mM EDTA, 1 mM ethylene-bis(oxyethylenenitrilo)tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT) in distilled water plus protease inhibitor cocktail P2714 (Sigma, Dorset, U.K.; one vial per 100 ml of extraction buffer) and 1 mM phenylmethylsulphonylfluoride (PMSF) added on the day of use. For TREK-1 blots, whole brain lysates were centrifuged at 100,000 × g for 30 min to isolate membrane and contractile proteins from the cytosolic fraction. Samples were quantified with a modified Bradford assay (Bradford, 1976) using a commercially available Coomassie Brilliant Blue G-250-containing protein estimation buffer (Biorad, Hertfordshire, U.K.) in conjunction with a UV spectrophotometer. A595 values were compared with those on a standard curve constructed with bovine serum albumin (BSA). Protein samples were mixed with a Laemmli sample buffer (Laemmli, 1970) and were loaded onto 12% v v−1 acrylamide gels and electrophoresed at 120 V, followed by electrophoretic transfer to polyvinylidene difluoride membranes at 80 V as previously described (Laemmli, 1970; Towbin et al., 1979). Membranes were subsequently blocked with 5% w v−1 dried nonfat milk solution in 1 × TwTBS (0.1% v v−1 Tween 20 in tris-buffered saline (TBS) (Trizma base 2 mM, NaCl 15 mM) pH 8.0) prior to addition of primary antibodies. Primary antibodies (anti-TASK-1, -TASK-2, -TREK-1 and -TWIK-2, Alomone) were applied overnight at 4°C at a concentration of 3 μg ml−1 for TASK-1, TASK-2 and TREK-1 and 1.5 μg ml−1 for TWIK-2 in 1 × TwTBS. Secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit, Jackson ImmunoResearch, Cambridge, U.K.) were used at a final concentration of 40 ng ml−1 in 1 × TwTBS. An ECL+ chemiluminescence detection system (Amersham) was used to visualise antibody labelling.
Immunostaining
After dissection, arteries were PLP-fixed (4% w v−1 paraformaldehyde in phosphate-buffered saline (PBS); McLean & Nakane, 1974) for 30 min and then placed overnight in cryoprotectant (30% w v−1 sucrose in PBS). Small artery segments were embedded in OCT® compound (R.A. Lamb Ltd, Eastbourne, U.K.), rapidly frozen and 4 μm cryostat sections were transferred to silanated slides.
Tissue sections were blocked with 1% w v−1 BSA in PBS (NaCl 145 mM, Na2HPO4 8.4 mM, NaH2PO4 2 mM) pH 7.4, containing 5% v v−1 normal goat serum (Jackson ImmunoResearch), incubated with primary antibodies overnight at 4°C (12 μg ml−1 for TASK-1, TASK-2 and TREK-1 and 6 μg ml−1 for TWIK-2) and washed in PBS. Secondary antibodies (goat anti-rabbit conjugated to Texas Red 13 μg ml−1 (Jackson ImmunoResearch)) were applied for 40 min at room temperature together with a blue nuclear label (4,6-diamidino-2-phenylindole (DAPI) 6 μg ml−1 final concentration). Slides were viewed using an epifluorescence microscope.
Reagents and drugs
Acrylamide/Bis solution (40% 37.5 : 1), BIORAD protein assay dye reagent concentrate (estimation buffer), Biorad, Hertfordshire U.K.; ECL+, Amersham Pharmacia Biotech, Buckinghamshire U.K.; PVDF (polyvinylidene difluoride) membrane ‘Immobilon-P', Millipore, Hertfordshire U.K.; ExTaq, 2.5 mM dNTPs and Taq buffer, TaKaRa Biomedicals, U.K.; all other compounds, Sigma, Dorset, U.K.
Statistics
Data are displayed as mean±s.e.m. Where appropriate, data were statistically analysed using a paired or unpaired t-test. Significance was determined at P<0.05.
Results
Effects of changing extracellular pH
The basal membrane potential of myocytes in intact third-order mesenteric and second-order pulmonary arteries at pH 7.4 was −52.4±0.8 and −51.5±0.5 mV, respectively (n=4). On superfusing with Tyrode's solution at pH 6.4, the basal membrane potential of myocytes in both vessels became significantly depolarised reaching a new steady-state level within 1–2 min (mesenteric, −48.4±0.4 mV, n=4; pulmonary, −43.8±0.6 mV; P<0.05, n=3, see Figure 1). Subsequent superfusion with Tyrode's at pH 8.4 resulted in a significant negative shift in membrane potential which again reached a steady state within 1–2 min (mesenteric, −56.0±1.4 mV, n=4; pulmonary, −61.4±0.9 mV, n=3; P<0.05, see Figure 1).
Figure 1.
Effects of changes in extracellular pH on membrane potential in mesenteric (a) and pulmonary arteries (b). Vessels were initially bathed in Tyrode's solution of pH 7.4. Reduction of extracellular pH (to pH 6.4) caused a marked depolarisation, while addition of alkaline Tyrode (pH 8.4) caused hyperpolarisation. Application of anandamide (10 μM) at either pH 8.4 or 7.4 returned EM to that seen with acidic pH0 (pH 6.4). (a1 and b1) Representative traces from mesentric (a1) and pulmonary (b1) arteries. (a2 and b2) Mean data ±s.e.m. derived from separate experiments (◊; n=3 or 4) on mesenteric and pulmonary arteries, respectively.
Effects of anandamide, bupivacaine and clofilium on pH-induced changes in membrane potential
The effects of 10 μM anandamide (a putative inhibitor of TASK-1 channels; Maingret et al., 2001) were examined at both pH 8.4 and 7.4 and the results of a typical experiment are summarised in Figure 1. On addition of anandamide to the superfusing Tyrode's solution at pH 8.4, the membrane potential of both mesenteric and pulmonary myocytes depolarised within 1–3 min to levels not significantly different from those in Tyrode's solution at pH 6.4 (pH 8.4 plus anandamide; mesenteric, −48.6±0.5 mV, n=4; pulmonary −45.5±0.3 mV, n=3). On removal of the anandamide at pH 8.4, the effects of this cannabinoid on membrane potential were reversed within 3–4 min. Anandamide (10 μM) also depolarised both mesenteric and pulmonary myocytes at pH 7.4 (to −47.4±0.6 mV, n=4 and −46.8±3.3 mV, n=3, respectively; see Figure 1) to values that were similar to those attained in Tyrode's solution alone at pH 6.4.
The effects of 100 μM bupivacaine (another putative inhibitor of TASK channels; Kindler et al., 1999) were examined using an experimental design identical to that employed in the anandamide experiments. The resting membrane potential (EM) at pH 7.4 of both third-order mesenteric and second-order pulmonary arteries was again significantly altered by changes in extracellular pH to both 6.4 and 8.4 (pH 7.4 EM −51.7±0.6 and −52.0±0.7 mV; pH 6.4 EM −47.5±1.4 and −43.2±0.5 mV; pH 8.4 EM −57.1±0.9 and −61.5±1.2 mV, respectively, P<0.05 n=4). Addition of 100 μM bupivacaine to the superfusing Tyrode's solution at pH 8.4 shifted the membrane potential to a level comparable to that seen with Tyrode's solution at pH 6.4 (pH 8.4 EM −57.1±0.9 and −61.5±1.2 mV; pH 8.4 plus bupivacaine: EM −42.7±0.8 and −47.1±1.7 mV, respectively, n=4). At pH 7.4, however, 100 μM bupivacaine produced a depolarisation that was less than that seen in Tyrode's solution alone at pH 6.4 (pH 7.4 plus bupivacaine: EM −49.3±0.8 and −48.1±0.6 mV, respectively, n=4). The results of a typical experiment are summarised in Figure 2.
Figure 2.
Effect of bupivacaine on changes in membrane potential with extracellular pH in mesenteric (a) and pulmonary arteries (b). Levcromakalim (LEV) was used as a positive control. Vessels were initially bathed in Tyrode's solution of pH 7.4. Reduction of extracellular pH (to pH 6.4) caused a marked depolarisation, while addition of alkaline Tyrode (pH 8.4) caused hyperpolarisation. Application of bupivacaine (100 μM) at pH 8.4 returned membrane potential to that seen in the presence of acidic Tyrode (pH 6.4), but was less effective at pH 7.4. (a1 and b1) Representative traces from mesenteric (a1) and pulmonary (b1) arteries are shown. (a2 and b2) Mean data±s.e.m. derived from separate experiments (◊; n=4) in mesenteric and pulmonary arteries are shown.
In a separate series of experiments the effects of 100 μM clofilium (a putative inhibitor of TASK-2 channels; Niemeyer et al., 2001) on membrane potential in de-endothelialised (confirmed by application of 10 μM ACh) pulmonary arteries were examined using the experimental design that was employed in the anandamide and bupivacaine experiments. As before, basal membrane potential at pH 7.4 was significantly altered by changes in extracellular pH to both 6.4 and 8.4 (pH 7.4 EM −50.4±0.5 mV; pH 6.4 EM −44.0±0.3 mV; pH 8.4 EM −58.1±1.2 mV, P<0.05). At pH 8.4, addition of 100 μM clofilium repolarised the vessels to a lesser extent than either anandamide or bupivacaine (pH 8.4 EM −58.1±1.2 mV; pH 8.4 plus clofilium: EM −54.6±1.0 mV, P<0.05 n=4) and was without effect on membrane potential at pH 7.4 (Figure 3).
Figure 3.
(a) Representative trace showing the effects of changes in extracellular pH on membrane potential in de-endothelialised pulmonary arteries. Vessels were initially bathed in Tyrode's solution of pH 7.4. Successful endothelial removal was assessed by application of 10 μM ACh. Reducing extracellular pH (to pH 6.4) caused marked depolarisation, while addition of alkaline Tyrode (pH 8.4) caused hyperpolarisation. This hyperpolarisation could be partially and reversibly inhibited by application of clofilium (100 μM). No effect could be seen upon application of clofilium (100 μM) when arteries where exposed to a Tyrode's solution of pH 7.4. (b) Mean±s.e.m. changes in membrane potential induced by alterations in pH0 and clofilium (100 μM) in four separate (◊) de-endothelialised pulmonary arteries.
Effects of anandamide, bupivacaine and zinc on pH-induced changes in pulmonary artery membrane potential in the presence of K+ channel inhibitors
In the absence of an endothelial cell layer, the possibility that other K+ channels located on the smooth muscle might contribute to the observed pH-dependent changes in membrane potential was examined with the use of an inhibitory cocktail comprising 10 μM glibenclamide, 30 μM Ba2+, 10 mM tetraethylammonium (TEA) and 5 mM 4-aminopyridine (4-AP). Resting membrane potential in the presence of Tyrode's solution at pH 7.4 was significantly depolarised by switching to Tyrode's at pH 7.4 containing the inhibitory cocktail (pH 7.4, −52.0±0.3 mV, n=4; pH 7.4 plus cocktail, −35.4±1.3 mV, P<0.05, n=4,) (Figure 4). Reduction of extracellular pH to 6.4 in the continued presence of drug cocktail further depolarised the vessel while switching to extracellular pH to 8.4 caused a marked hyperpolarisation (pH 6.4 plus cocktail, −30.8±0.5 mV, n=4; pH 8.4 plus cocktail, −58.9±0.6 mV, n=4). Addition of 10 μM anandamide or 200 μM Zn2+ (a known blocker of TASK-1 channels: Leonoudakis et al., 1998) reversed the hyperpolarisation caused by alkaline extracellular pH and returned membrane potential to a level comparable to that seen at pH 6.4 (pH 8.4 plus cocktail, −58.9±0.6 mV, n=4; pH 8.4 plus cocktail and anandamide, −35.7±1.5 mV, n=4; pH 8.4 plus cocktail, anandamide washout, −60.5±2.0 mV; pH 8.4 plus cocktail and Zn2+, −35.7±1.7 mV, n=4) (Figure 4).
Figure 4.
Effects of changes in extracellular pH on membrane potential in pulmonary arteries. Vessels were initially bathed in Tyrode's solution of pH 7.4 prior to the addition of K+ channel inhibitor cocktail (CT) comprising 10 mM TEA, 10 μM glibenclamide, 5 mM 4-AP and 30 μM Ba2+. Cocktail application caused marked depolarisation. Reduction of pH (to pH 6.4) in the presence of cocktail produced a further depolarisation, while addition of alkaline Tyrode (pH 8.4) caused hyperpolarisation. While maintaining vessels in alkaline Tyrode in the presence of the cocktail, application of anandamide (10 μM) or Zn2+ returned membrane potential to values seen with acidic pH0 (pH 6.4). Representative traces from pulmonary arteries (a). Mean data±s.e.m. derived from four separate experiments (◊) on pulmonary arteries (b).
In a separate series of experiments, the possible additive effects of the combined presence of anandamide and bupivacaine were studied on membrane hyperpolarisation seen with alkaline extracellular pH. In the continued presence of the inhibitory cocktail (10 μM glibenclamide plus 30 μM Ba2+ plus 10 mM TEA plus 5 mM 4-AP), acidic extracellular pH caused a depolarisation while alkaline extracellular pH caused a hyperpolarisation (pH 7.4 plus cocktail, −41.8±1.6 mV, n=4; pH 6.4 plus cocktail, −33.8±2.3 mV, n=4; pH 8.4 plus cocktail, −61.5±0.7 mV n=4). Addition of 10 μM anandamide returned the membrane potential to levels comparable to that seen at pH 6.4, while subsequent addition of bupivacaine had little further effect (pH 8.4 plus cocktail and anandamide, −32.8±2.4 mV, n=2; pH 8.4 plus cocktail, anandamide and bupivacaine –29.5.5±1.1 mV, n=2). Initial exposure to bupivacaine followed by anandamide produced similar results (pH 8.4 plus cocktail and bupivacaine, −42.5±1.4 mV, n=2; pH 8.4 plus cocktail, bupivacaine and anandamide, −37.6±1.5 mV, n=2) (data not shown).
Effects of anandamide, bupivacaine and clofilium on vessel tone
In small pulmonary arteries anandamide (n=6) and bupivacaine (n=5) each significantly increased baseline tone in a gradual, concentration-dependent manner with the maximum effect being reached in 2–3 min (Figure 5). At the highest concentration employed, anandamide 60 μM increased tension to 6.1±2.7%TK, while bupivacaine 300 μM increased tension to 5.6±1.5%TK. Anandamide vehicle dimethyl sulphoxide, (DMSO) produced no change in baseline tension (data not shown). Clofilium (0.3–100 μM) was without effect on resting tension (data not shown).
Figure 5.
Effect of cumulative drug addition on resting tension elicited by anandamide and bupivacaine in small pulmonary arteries. Tension is presented as a mean percentage of that induced by 75 mM KCI PSS±s.e.m. (n=5–6).
2P-domain K+ channel mRNA expression in rat mesenteric and pulmonary arteries
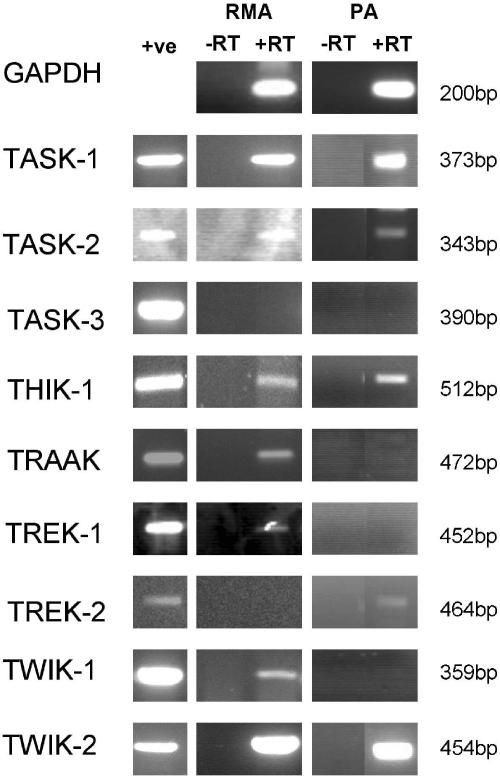
RT–PCR results demonstrated the presence of mRNA encoding TASK-1, TASK-2, THIK-1, TRAAK, TREK-1, TWIK-1 and TWIK-2 in mesenteric arteries (Figure 6), but TASK-3 and TREK-2 were not detected. Pulmonary arteries expressed mRNA for TASK-1, TASK-2, THIK-1, TREK-2 and TWIK-2 (Figure 6), but not TASK-3, TRAAK, TREK-1 and TWIK-1. Control reactions performed with DNase-treated but not reverse-transcribed total RNA and in the absence of a cDNA template were all negative.
Figure 6.
Agarose gel electrophoresis of RT–PCR amplified with primers specific for GAPDH, TASK-1, TASK-2, TASK-3, THIK-1, TRAAK, TREK-1, TREK-2, TWIK-1 and TWIK-2. cDNA derived from rat brain or kidney was used as a positive control (+ve). Rat mesenteric and pulmonary artery mRNA was DNase treated and reverse transcribed. −RT controls were performed on DNase-treated mRNA that was not reverse transcribed. Products were sequenced to confirm identity.
Expression of 2P-domain K+ channel protein and antibody specificity
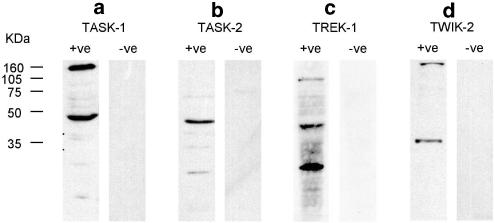
Western blots using anti-TASK-1, TASK-2, TREK-1 and TWIK-2 were performed on protein samples derived from rat brain (Figure 7). TASK-1 bound to a protein of about 47 kDa in rat brain samples, corresponding to its calculated molecular weight of 45 kDa, while binding was ablated by preincubation with the control peptide. In rat brain protein samples, TASK-2 antibodies labelled a band of approximately 45 kDa that was completely ablated by preincubation with the control peptide. Anti-TREK-1 antibodies labelled a band of approximately 45 kDa as well as a band at about 25 kDa. TWIK-2 antibodies labelled bands of 35 kDa in rat brain samples (theoretical weight of TWIK-2 is 35 kDa).
Figure 7.
Western blots of rat brain protein samples probed with anti -TASK-1, -TASK-2, -TREK-1 and -TWIK-2. Blots were performed with the primary antibody (+ve) or primary antibody that had been incubated with the antigenic peptide (−ve) prior to use. Theoretical weights of TASK-1, TASK-2, TREK-1 and TWIK-2 are 45, 55, 45 and 35 kDa, respectively.
Location of 2P-domain channel proteins in mesenteric and pulmonary arteries
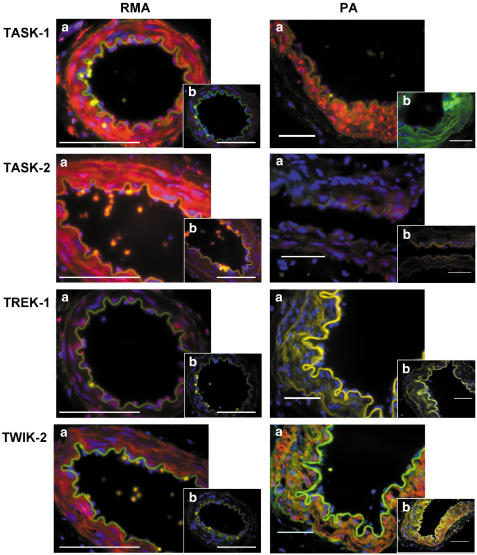
The intravascular distribution of TASK-1, TASK-2, TREK-1 and TWIK-2 protein was demonstrated by immunostaining sections of mesenteric and pulmonary arteries (Figure 8). TASK-1 and TASK-2 labelling was widespread throughout the vessels in both the myocyte and the endothelial cell layers. TWIK-2 staining was also evident throughout the myocytes but the endothelial cell layer was less well stained. TREK-1 appeared to be located intracellularly. Labelling of all these proteins was ablated by preincubation of the antibody with the corresponding antigenic peptide. Immunostaining in rat pulmonary arteries demonstrated the presence of TASK-1, TASK-2 and TWIK-2, whereas TREK-1 was absent. Staining for TASK-1 and TASK-2 was prominent in the myocytes but to a lesser extent in the endothelium. TWIK-2 was also present in the smooth muscle but was essentially absent from the endothelium.
Figure 8.
Sections of rat mesenteric (RMA) and pulmonary artery (PA) stained with anti-TASK-1, -TASK-2, -TREK-1 and -TWIK-2. (a) Sections were stained with primary antibody and subsequently visualised using a Texas Red–conjugated secondary antibody. (b) Sections were labelled using the primary antibody that had been preincubated with the antigenic peptide. Nuclei were labelled blue with DAPI. Scale bars represent 100 μm.
Discussion
Existence of 2P-domain potassium channels in the vasculature
2P-domain potassium channels are present in a wide variety of tissues but only a few reports have described them in blood vessels (TASK-1 in pulmonary arteries, Gurney et al. (2002; 2003); TWIK-1 in mouse aorta, Arrighi et al. (1998) and TASK-4 in human aorta; Decher et al., 2001) with no reports of their presence in resistance arteries. In the present study, mRNAs encoding several of these channels were detected in both rat mesenteric and pulmonary arteries and their cellular distribution was shown using immunohistochemical techniques. Although there were some differences, the channels were present in both the myocytes and in the endothelial cells. Commercially available antibodies were employed in this aspect of the study and their use is supported by previous investigations using both anti-TASK-1 and anti-TASK-2.
Anti-TASK-1 antibodies are known to label a band of approximately 45 kDa and another band of about 150 kDa as stated by both the Alomone product literature (using rat brain membrane fractions) and Jones et al. (2002) (using protein samples derived from rat ventricle and atria). Our results are compatible with these data. In addition, studies using CHO cells transfected with mouse TASK-1 have shown that the anti-TASK-1 used in the present experiments binds specifically to the protein of interest with an absence of staining in nontransfected cells and isolated cardiac myocytes (Jones et al., 2002). Furthermore, Kindler et al. (2000) showed that the same antibody was specific for TASK-1 when used to probe sections taken from various regions of the rat central nervous system. Recently, this antibody was also used to probe rabbit pulmonary artery myocytes (Gurney et al., 2003). The anti-TASK-2 antibody used also binds specifically to TASK-2 in rat tissue (Gabriel et al., 2002) as well as in cultured Ehrlich cells (Niemeyer et al., 2001). Thus, the anti-TASK-1 and TASK-2 antibodies used in the present investigation are reliable tools with which to determine the distribution of these channels.
In the present study, anti-TWIK-2 labelled bands of the correct size in brain protein samples and these were ablated by the control peptide providing evidence for the specificity of this antibody. The anti-TREK-1 employed labelled bands of 45 kDa and approximately 20 kDa in Western blots performed on rat brain samples. The theoretical weight of TREK-1 is 45 kDa but the smaller detected band of approximately 20 kDa cannot be readily explained. However, a BLAST search using the 18 amino-acid sequence from human TREK-1 employed to generate the antibody shows the existence of a so-far unidentified 18 kDa protein that incorporates this sequence. The presence of such a protein in rat homogenates could possibly explain the smaller band detected in the present experiments. Although staining for this protein was absent from pulmonary arteries, there was some evidence of intracellular labelling in mesenteric artery sections and the significance of this is the subject of further study.
pH-induced changes of membrane potential: a role for the 2P-domain K+ channels
The membrane potential recordings from both rat mesenteric and pulmonary artery myocytes showed that altering extracellular pH triggered significant and reversible changes in membrane potential. Of the 2P-domain K+ channels present in the rat mesenteric and pulmonary arteries, members of the TASK family are known to be modulated by changes in extracellular pH. Predicted H+ dose–response curves indicate TASK-1, TASK-2 and TASK-3 channels are modulated by changes in pH between 8.4 and 6.4. Thus, TASK-1 is thought to be closed at pH 6.4, 50% open at pH 7.4 and 90% open at pH 8.4, while TASK-2 is thought to be closed at pH 6.4, 15% open at pH 7.4 and 50% open at pH 8.4 (O'Connell et al., 2002).
In the present study, mRNA for TASK-3 was not detected by RT–PCR in either pulmonary or mesenteric vessels. Based on the observed membrane potential changes, we adopted the hypothesis that they were mediated by either TASK-1 or TASK-2 channels or a combination of these.
pH-induced changes of membrane potential: possible involvement of TASK-1 channels
TASK-1 channels are selectively blocked by low concentrations of anandamide, while TASK-2 channels are essentially unaffected (Maingret et al., 2001). In both pulmonary and mesenteric vessel segments, 10 μM anandamide reversed the effects of increasing extracellular pH to 8.4 and returned the membrane potential to levels close to those observed at pH 6.4. At pH 7.4, anandamide also depolarised the myocytes to a membrane potential similar to that seen with extracellular pH at 6.4.
The local anaesthetic bupivacaine also inhibits TASK-1 channels (Kindler et al., 1999). In the present study, bupivacaine, like anandamide, inhibited hyperpolarisations caused by increasing extracellular pH to 8.4, while it had no effect at pH 6.4. However, in contrast to anandamide, addition of bupivacaine at pH 7.4 did not shift the membrane potential to a level comparable to that seen at pH 6.4. This pH dependency in the action of bupivacaine has also been observed on TASK-1 channels in a heterologous expression system in which bupivacaine produced 28% inhibition of TASK-1 currents at pH 8.4, 23% at pH 7.6 and 10% at pH 7.0 (Kindler et al., 1999). If it is assumed that bupivacaine (pKa 8.1) must be in its unionised form to block TASK-1 (see Kindler et al., 1999), then the results of the present study can also be explained in the same way. Thus, using the Henderson–Hasselbach equation (pKa=pH+log [ionised]/[non-ionised], 67% of 100 μM bupivacaine would be in its unionised form at pH 8.4, while at pH 7.4 the value would be only 17%. This would result in the failure of this agent to depolarise the membrane potential to the same extent as seen at pH 8.4.
pH-induced changes of membrane potential: possible involvement of the vascular endothelium, other K+ channels and TASK-2 channels
All the experiments so far discussed were carried out using endothelium-intact vessels. To test whether the endothelium played a role in the observed membrane potential changes in the vascular myocytes, a series of experiments was conducted using endothelium-denuded pulmonary arteries. Effective endothelium removal was tested using an acetylcholine challenge (Chen et al., 1988) and only vessels that did not respond to 10 μM acetylcholine were employed. There was no significant difference between the pH-induced changes in membrane potential in intact and denuded tissues. Thus, although 2P-domain channels were detected in the endothelial layer of both vessel types, their presence did not affect the magnitude of the observed myocyte membrane potential changes following fluctuations in extracellular pH.
In order to eliminate the possibility that the modulation of extracellular pH was affecting other K+ channels, experiments were performed (on vessels without a functional endothelium) in the presence of 4-AP to block delayed rectifier K+ channels, TEA to block Ca2+-sensitive K+ channels, glibenclamide to block KATP and Ba2+ to block KIR. Although the inhibitory cocktail generated an initial burst of spike-like potentials followed by a sustained depolarisation at pH 7.4, switching to extracellular pH of 6.4 and 8.4 still generated significant depolarisations and hyperpolarisations, respectively. At an extracellular pH of 8.4 in the continued presence of the K+ channel-blocking cocktail, anandamide, bupivacaine or Zn2+ all returned the membrane potential to levels similar to those seen at pH 6.4. The effects of addition of anandamide and bupivacaine together were also studied. The reversal of the hyperpolarisation associated with alkaline extracellular pH upon addition of either anandamide or bupivacaine alone was slightly enhanced with subsequent addition of bupivacaine or anandamide, respectively. As the concentrations of either drug used may not produce maximal channel block, this result is entirely consistent with an action of both compounds on the same ion channel.
The results of these experiments exclude the possibility that classical voltage-sensitive, Ca2+-sensitive, ATP-sensitive and inwardly-rectifying K+ channels play a significant role mediating the observed pH-induced changes in membrane potential. Instead, it seems likely that the observed electrical changes are mediated by other K+ channels of which members of the TASK family are the most likely candidates.
Clofilium, a putative, selective blocker of TASK-2 (Niemeyer et al., 2001) caused a small repolarisation of pulmonary arteries at pH 8.4, but had no effect on the membrane potential at pH 7.4. This is consistent with a TASK-2-mediated contribution to the observed pH-induced changes in membrane potential at pH 8.4. However, the pH dependency of these channels (Reyes et al., 1998) suggests that they would be unable to make a significant contribution to the electrical changes under more acid conditions.
Blood vessel tone: involvement of TASK-1 and TASK-2 channels
The changes in membrane potential putatively attributed to 2P-domain K+ channels were greatest in pulmonary arteries and it was decided to study the possible role of these channels on resting tone in these vessels. Both anandamide and bupivacaine each caused a concentration-dependent increase in basal tone, whereas clofilium was without effect, thus implicating TASK-1 but not TASK-2 channels in the regulation of pulmonary vascular tone. These findings are therefore consistent with the presence and functional significance of this channel in pulmonary artery at least.
Based on the effects of pH on membrane potential, it might be expected that a decrease in extracellular pH from 7.4 to 6.4 would also cause a contraction of the vessel, while increasing extracellular pH to 8.4 would cause a relaxation. However, we were unable to detect any effect of either acidic (pH 6.4) or alkaline (pH 8.4) conditions on basal pulmonary artery tone (data not shown).
Previous studies of normocapnic acidosis on intact lung or on pulmonary resistance in vivo have produced conflicting results (either a reduction, an increase or no effect on pulmonary vascular resistance – see Sweeney et al., 1999), while systemic vessels typically relax to normocapnic acidosis (rat cerebral artery – Peng et al. (1998); rat carotid artery – Sweeney et al., 1999). The membrane potential (in the region of −40 mV in pulmonary arteries) observed in the presence of an acidic extracellular pH was close to the activation threshold of voltage-gated Ca2+ channels in arterial myocytes (Clapp & Gurney, 1991). However, since acidic conditions reduce [Ca2+]i (despite the observed depolarisation) and decrease smooth muscle contractile protein responsiveness to [Ca2+]i (Peng et al., 1998), little or no change in tone would be anticipated on simply shifting extracellular pH from 7.4 to 6.4 in spite of the ensuing membrane depolarisation.
2P-domain K+ channels: functional significance
The present study has shown that several 2P-domain K+ channels are present in both the pulmonary and mesenteric vasculature of the rat. RT–PCR was performed on total RNA derived from whole arteries and does not provide data regarding the cellular distribution of mRNA encoding 2P-domain K+ channels. However, the RT–PCR results combined with (i) immunohistochemical staining techniques, (ii) the consistent electrophysiological changes and (iii) the pharmacology of the electrical events in intact and de-endothelialised vessels provides compelling evidence for the functional expression of these channels on myocytes from these vessels. The role of these channels located on the endothelium is a matter of further study, as is the possibility that these channels might be expressed on cells located in the adventitial layer although they seem unlikely to contribute to the results described above.
Although none of the channel modulators used should be regarded as selective, the results collectively suggest that TASK-1 channels in particular are of functional significance. Of note is the evidence that the potassium current designated IKN (Gurney et al., 2002) is carried by TASK-1 in pulmonary myocytes derived from rabbit pulmonary arteries (Gurney et al., 2003). This current, which is reduced in chronic pulmonary hypoxia (Osipenko et al., 1998) and is important in setting resting membrane potential in pulmonary vessels (Evans et al., 1998), implicates TASK-1 in hypoxic disease states.
Some evidence for the functional importance of TASK-2 was also obtained. In the present study, changing the extracellular pH was found to be an easy method of generating the observed changes and there was no qualitative difference between the responses of either pulmonary or mesenteric vessels. Whether TASK-1 and TASK-2 channels function as sensors of extracellular pH in vivo is a matter for conjecture. What is clear is that the opening and closing of the channel putatively designated as TASK-1 is capable of shifting the membrane potential by approximately 10 mV in a hyperpolarising or depolarising direction, respectively. Such shifts in membrane potential could markedly affect the general excitability of the vascular myocyte and further experiments are in progress to study the functional importance not only of TASK channels but also of the other 2P-domain K+ channels detected in the present study.
Acknowledgments
This study was supported by the British Heart Foundation.
Abbreviations
- 2P
two-pore
- 4-AP
4-aminopyridine
- EM
membrane potential
- EDTA
ethylenediaminetetra-acetic acid
- EGTA
ethylene-bis(oxyethylenenitrilo)tetraacetic acid
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
- DMSO
dimethyl sulphoxide
- DTT
dithiothreitol
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- pHo
extracellular pH
- PSS
physiological salt solution
- TBS
Tris-buffered saline
- TEA
tetraethylammonium
References
- ARRIGHI I., LESAGE F., SCIMECA J.C., CARLE G.F., BARHANIN J. Structure, chromosome localization, and tissue distribution of the mouse twik K+ channel gene. FEBS Lett. 1998;425:310–316. doi: 10.1016/s0014-5793(98)00260-9. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H., WESTON A.H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPP L.H., GURNEY A.M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991;418:462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- DECHER N., MAIER M., DITTRICH W., GASSENHUBER J., BRUGGEMANN A., BUSCH A.E., STEINMEYER K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett. 2001;492:84–89. doi: 10.1016/s0014-5793(01)02222-0. [DOI] [PubMed] [Google Scholar]
- EVANS A.M., OSIPENKO O.N., HAWORTH S.G., GURNEY A.M. Resting potentials and potassium currents during development of pulmonary artery smooth muscle cells. Am. J. Physiol. 1998;275:H887–H899. doi: 10.1152/ajpheart.1998.275.3.H887. [DOI] [PubMed] [Google Scholar]
- GABRIEL A., ABDALLAH M., YOST C.S., WINEGAR B.D., KINDLER C.H. Localization of the tandem pore domain K(+) channel KCNK5 (TASK-2) in the rat central nervous system. Mol. Brain Res. 2002;98:153–163. doi: 10.1016/s0169-328x(01)00330-8. [DOI] [PubMed] [Google Scholar]
- GARDENER M.J., JOHNSON I.T., EDWARDS G., WESTON A.H. Functional evidence for the presence of TASK-1 in the rat mesenteric artery. Br. J. Pharmacol. Proc. Supp. 2003.
- GURNEY A.M., OSIPENKO O.N., MACMILLAN D., KEMPSILL E.J. Potassium channels underlying the resting potential of pulmonary artery smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 2002;29:330–333. doi: 10.1046/j.1440-1681.2002.03653.x. [DOI] [PubMed] [Google Scholar]
- GURNEY A.M., OSIPENKO O.N., MACMILLAN D., MCFARLANE K.M., TATE R.J., KEMPSILL F.E.J. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ. Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- JOHNSON I.T., GARDENER M.J., EDWARDS G., WESTON A.H. Evidence for the involvement of TASK-1 in setting resting membrane potential and resting tension in the rat small pulmonary artery. Br. J. Pharmacol. Proc. Supp. 2003.
- JONES S.A., MORTON M.J., HUNTER M., BOYETT M.R. Expression of TASK-1, a pH-sensitive twin-pore domain K(+) channel, in rat myocytes. Am J. Physiol. Heart. Circ. Physiol. 2002;283:H181–H185. doi: 10.1152/ajpheart.00963.2001. [DOI] [PubMed] [Google Scholar]
- KETCHUM K.A., JOINER W.J., SELLERS A.J., KACZMAREK L.K., GOLDSTEIN S.A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- KIM D., GNATENCO C. TASK-5, a new member of the tandem-pore K+ channel family. Biochem. Biophys. Res. Commun. 2001;284:923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- KINDLER C.H., PIETRUCK C., YOST C.S., SAMPSON E.R., GRAY A.T. Localization of the tandem pore domain K+ channel TASK-1 in the rat central nervous system. Mol. Brain. Res. 2000;80:99–108. doi: 10.1016/s0169-328x(00)00136-4. [DOI] [PubMed] [Google Scholar]
- KINDLER C.H., YOST C.S., GRAY A.T. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LEONOUDAKIS D., GRAY A.T., WINEGAR B.D., KINDLER C.H., HARADA M., TAYLOR D.M., CHAVEZ R.A., FORSAYETH J.R., YOST C.S. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE F., GUILLEMARE E., FINK M., DUPRAT F., LAZDUNSKI M., ROMEY G., BARHANIN J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- LESAGE F., LAZDUNSKI M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- LOPES C.M.B., ZILBERBERG N., GOLDSTEIN S.A.N. Block of KCNK3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J. Biol. Chem. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- MAINGRET F., PATEL A.J., LAZDUNSKI M., HONORÉ E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN I.W., NAKANE P.K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- NIEMEYER M.I., CID L.P., BARROS L.F., SEPÚLVEDA F.V. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J. Biol. Chem. 2001;276:43166–43174. doi: 10.1074/jbc.M107192200. [DOI] [PubMed] [Google Scholar]
- O'CONNELL A.D., MORTON M.J., HUNTER M. Two-pore domain K+ channels – molecular sensors. Biochim. Biophys. Acta. 2002;1566:152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- OSIPENKO O.N., ALEXANDER D., MACLEAN M.R., GURNEY A.M. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br. J. Pharmacol. 1998;124:1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI M., MARSHALL C., AMAKI Y., MARSHALL B.E. Role of wall tension in hypoxic responses of isolated rat pulmonary arteries. Am. J. Physiol. 1998;275:L1069–1077. doi: 10.1152/ajplung.1998.275.6.L1069. [DOI] [PubMed] [Google Scholar]
- PATEL A.J., HONORÉ E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- PENG H.L., JENSEN P.E., NILSSON H., AALKJAER C. Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: is there a role for membrane potential. Am. J. Physiol. 1998;274:H655–H662. doi: 10.1152/ajpheart.1998.274.2.H655. [DOI] [PubMed] [Google Scholar]
- REYES R., DUPRAT F., LESAGE F., FINK M., SALINAS M., FARMAN N., LAZDUNSKI M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- SWEENEY M., O'REGAN R.G., MCLOUGHLIN P. Effects of changes in pH and pCO2 on wall tension in isolated rat intrapulmonary arteries. Exp. Physiol. 1999;84:529–539. [PubMed] [Google Scholar]
- TOWBIN H., STAEHELIN T., GORDON J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]