Abstract
Ivabradine selectively reduces heart rate (HR) by inhibiting the cardiac pacemaker If current, thus prolonging the duration of spontaneous depolarization in the sinus node. The activity of ivabradine under conditions of enhanced sympathoadrenergic activity has been addressed by investigating the effects of repeated oral administration in mice with sympathoadrenergic activation due to either stress, cardiac-restricted overexpression of β2-adrenergic receptors (β2AR), or β-agonist administration. HR and left ventricular fractional shortening (FS) were determined by echocardiography.
Initial experiments showed that the conscious restrained state was associated with stress-mediated sympathetic activation, while sympathetic withdrawal occurred under anaesthetized conditions. In wild-type mice, ivabradine reduced HR under both conscious and anaesthetized states, with a similar degree in absolute reduction under both states. FS was unchanged by the treatment.
Ivabradine was similarly effective in reducing HR in the β2AR transgenic mice. Further, ivabradine at 10 mg kg−1 day−1 reduced the maximal HR increase in response to the β-agonist isoproterenol, without modifying the response of contractile parameters.
These data indicate that oral administration of ivabradine in mice reduces HR while ventricular performance is maintained. This specific HR-reducing action of ivabradine is well preserved under conditions that are associated with significant activation of the sympathoadrenergic system.
Keywords: Sinus node inhibitor, transgenic mice, sympathetic nervous system, echocardiography
Introduction
An increase in heart rate (HR) is a common occurrence in cardiac pathophysiology, particularly in heart failure, mediated by β-adrenergic receptors (βARs) following activation of the sympathetic nervous system (Cohn, 1990; Kaye et al., 1995; Borer et al., 2003). Although an elevated HR may initially compensate for insufficient cardiac output, sustained tachycardia usually leads to adverse haemodynamic consequences. The long-term use of β-blockers in heart failure is associated with improved contractile function, which is likely due to the prevention of altered gene expression and cardiomyocyte loss and to the partial reversal of downregulated βARs (Bristow, 2000). However, additional benefit from β-blockade arises from a reduction in HR with prolonged diastolic coronary perfusion period and reduction in energy expenditure. Thus, an elevated HR may impair ventricular diastolic filling, compromise coronary blood flow and increase myocardial oxygen demand. Indeed, there is evidence that the beneficial action of β-blockade in dogs or patients with heart failure is at least partly due to its HR-lowering effect (Packer et al., 1996; Nagatsu et al., 2000). Thus, HR reduction per se emerges as a potential therapeutic target for heart disease.
Ivabradine (S16257) is a novel pharmacological agent specifically inhibiting the hyperpolarization-activated pacemaker If current that underlies the rate of spontaneous diastolic depolarization in sinoatrial pacemaker cells (Thollon et al., 1994; Bucchi et al., 2002). Previous studies have established the selective HR-reducing activity of ivabradine devoid of negative inotropic effect in various species, including the rat, pig, dog and human, when given intravenously (Thollon et al., 1994; Simon et al., 1995; Monnet et al., 2001; Colin et al., 2002; Camm & Lau, 2003) or orally (Borer et al., 2003). Antianginal and anti-ischaemic effects of ivabradine have been demonstrated in patients with stable angina (Borer et al., 2003). Although ivabradine is able to reduce HR under conditions of exercise (Simon et al., 1995; Monnet et al., 2001; Colin et al., 2002; Borer et al., 2003), there has been limited information on the efficacy of ivabradine in HR reduction under other conditions of enhanced sympathoadrenergic activity, a situation commonly seen under diseased conditions. In this study, we have investigated the effects of oral ivabradine on HR in the mouse with enhanced sympathoadrenergic activity due to (1) stress-evoked sympathetic activation, (2) cardiac specific overexpression of β2AR and (3) β-agonist stimulation.
Methods
Animals
Transgenic (TG) mice with cardiac-restricted overexpression of β2AR by 200-fold and their nontransgenic (NTG) littermates of both genders were used (Milano et al., 1994). Animals of 3–4 months age had a genetic background of C57BLK/SJL crossing and were individually genotyped. The TG mice have a life-time tachycardia phenotype due to constitutively activated β2-adrenergic signalling, with a marked increase in receptor density (Bond et al., 1995; Du et al., 1996). The NTG and TG mice were housed one to three per cage, with a 12 h/12 h day/night cycle, and with free access to water and chow diet. Animals assigned to treated or untreated group had a similar male/female ratio and the group size was eight for all experiments. Experimental procedures used in this study were approved by a local animal ethics committee.
Ivabradine treatment and assay of plasma drug levels
Ivabradine hydrochloride (MW 505.1) (3-(3-{[((7S)-3,4-dimethoxybicyclo[4,2,0]octa-1,3,5-trien-7-yl) methyl]methylamino}propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride) was supplied by the Institut de Recherches Internationales Servier (France). The drug was added to drinking water and the amount of water consumed by animals was measured weekly to ensure the desired dose of drug was taken. Untreated animals were similarly monitored. Fresh drug solution was prepared weekly.
Blood was collected during treatment to assess the plasma levels of ivabradine in the NTG and TG mice using a validated method involving liquid chromatography with fluorimetric detection (Klippert et al., 1998). The detection limit of the assay was 2.50 ng ml−1 (equal to 0.005 μM l−1).
Echocardiography
A Hewlett-Packard Sonos 5500 ultrasound machine and a 15 MHz linear transducer were used for echocardiography, as described previously (Du et al., 2000; Gao et al., 2000). Short-axis 2-D image-guided M-mode traces of the left ventricle (LV) were acquired and HR was measured digitally from the duration of consecutive beats at a sweep speed of 100 mm s−1. Fractional shortening (FS) of the LV was calculated as [(LVDd−LVDs)/LVDd] × 100%, where LVDd and LVDs refer to LV diastolic and systolic diameters, respectively. Absolute thickening of the LV wall during contraction was used as an alternative estimation of contractile function (Du et al., 2000). Animals were trained on three separate sessions 3 days prior to echocardiographic examination to acclimatise them to experimental conditions of conscious echocardiography, as described by others (Yang et al., 1999).
Statistics
Results are means±s.e.m. Between-group differences were compared by analysis of variance (ANOVA) followed by unpaired t-test. A P-value <5% was considered statistically significant.
Results
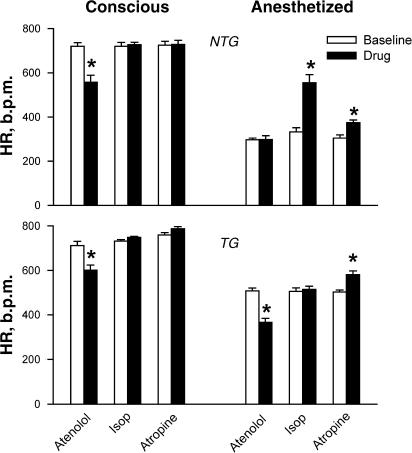
We initially evaluated sympathetic and parasympathetic activities in conscious NTG and TG mice restrained for echocardiography or anaesthetized with ketamine/xylazine (KX, 80/20 mg kg−1, respectively, i.p). Cardiac autonomic activities were determined from the effects of the β-agonist isoproterenol (Abbott Laboratory Ltd, 4 μg kg−1, i.p.), the β1-antagonist atenolol (Sigma Co, 5 mg kg−1, i.p.) and the muscarinic antagonist atropine (Pharmacia & Upjohn, 1.2 mg kg−1, i.p.). In restrained conscious mice of both genotypes, HR ranged between 710 and 760 b.p.m., a maximal level for this species (P=NS for the comparison between NTG and TG mice), and neither isoproterenol or atropine altered HR. However, the β-blocker atenolol significant reduced HR in both NTG and TG groups (Figure 1). NTG mice anaesthetized with KX had a markedly lower HR compared with the conscious state and responded to both isoproterenol and atropine with significant increase in HR, but did not respond to atenolol. KX-anaesthetized TG mice had significantly higher basal HR level than the NTG group (P<0.001). These mice did not respond to isoproterenol but had HR reduction in response to atenolol, indicating an intrinsic adrenergic activation that kept a higher HR under the conditions studied. Atropine under KX-anaesthetized conditions significantly increased HR-suggesting a parasympathetic activation. Thus, conscious mice subjected to echocardiographic examination had maximal sympathetic activation and parasympathetic withdrawal due to stress. Therefore, in all subsequent experiments under anaesthetized conditions, atropine was added to minimize the vagal effect.
Figure 1.
Validation of experimental conditions for measurement of HR by echocardiography in NTG (upper panel) and TG (lower panel) mice under conscious and restrained conditions or anaesthetized with a mixture of ketamine and xylazine (KX, 80/20 mg kg−1, respectively, i.p.). Autonomic nervous control of HR under the experimental conditions was examined by the use of the β-blocker atenolol (5 mg kg−1, i.p.), the β-agonist isoproterenol (4 μg kg−1 i.p.) and the muscarinic angatonist atropine (1.2 mg kg−1 i.p.). N=8 for all groups. *P<0.01 vs baseline.
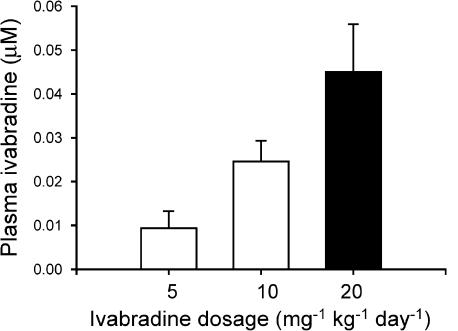
To evaluate the plasma level of ivabradine achieved by the oral dosing, plasma samples were prepared from three groups of NTG mice treated with ivabradine at 5, 10 and 20 mg kg−1, respectively, for 1 week. Ivabradine levels in plasma were detectable (>2.50 ng ml−1, 0.005 μM l−1) in most samples and in proportion to the oral dosages (Figure 2).
Figure 2.
Plasma levels of ivabradine in NTG mice receiving drug treatment at different doses for 1 week. Plasma concentration of ivabradine was dose-dependent. N=8 per group.
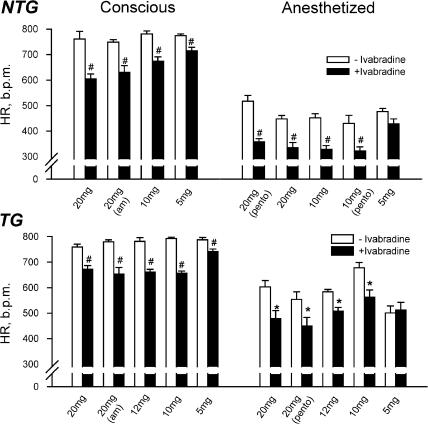
We then assessed the effect of ivabradine on HR in NTG and TG mice treated for 1 week with ivabradine at three doses of 5, 10 and 20 mg kg−1 day−1. Echocardiography was performed under both conscious and anaesthetized (ketamine/xylazine/atropine, KXA, at 80/20/0.6 mg kg−1, respectively, i.p.) conditions. Untreated NTG and TG animals served as controls. A significant HR-lowering effect of ivabradine was evident at oral doses ⩾5 mg kg−1 day−1 (Figure 3), leading to plasma ivabradine levels of approximately 0.01 μM or higher. Although the mice treated with ivabradine at 5 mg kg−1 day−1 showed the least reduction in HR in comparison with higher dosages, overall a dose-effect relation was not observed under our experimental conditions. The HR-reducing activity of ivabradine was similar in KXA or pentobarbitone anaesthetized states, or when animals were studied in the morning or afternoon (Figure 3), indicating an action independent of anaesthetics and a lack of circadian variation in efficacy. Ventricular contractile function, estimated by FS and LV wall thickening, was not altered in the NTG or TG mice treated with ivabradine at any dose compared with untreated counterparts (data not shown). Although HR reduction might be more pronounced at 20 mg kg−1 day−1 (drug concentration in drinking water was 200 mg l−1), this dose was associated with a palatability problem leading to a 40% reduction in water intake. Unchanged water consumption was observed in mice treated at 5 or 10 mg kg−1 day−1.
Figure 3.
Reduction in HR by oral ivabradine in the NTG (N=8 per group) and TG (N=8 per group) mice studied under conscious and anaesthetized conditions. All data obtained were collected in the afternoon unless indicated as a.m. (morning). Note that this effect of ivabradine is independent of the time (i.e. morning or afternoon) and anaesthetics tested. Pento=pentobarbitone. HR levels were significantly higher in TG than NTG mice under anesthetized conditions. *P<0.05, #P<0.01 vs untreated group.
To confirm the long-term effect of ivabradine at 10 mg kg−1 day−1, in one group of TG mice, we extended the treatment to 2 months and observed that the HR reduction by ivabradine was well maintained at the end of the 2-month period (conscious: 686±9 vs 761±11 b.p.m., P<0.001; anaesthetized with KXA: 508±29 vs 582±18 b.p.m., P<0.05). FS was similar between treated and untreated TG mice in both conscious (53±1 vs 52±1%) and anaesthetized states (43±2 vs 40±3%, both P=NS).
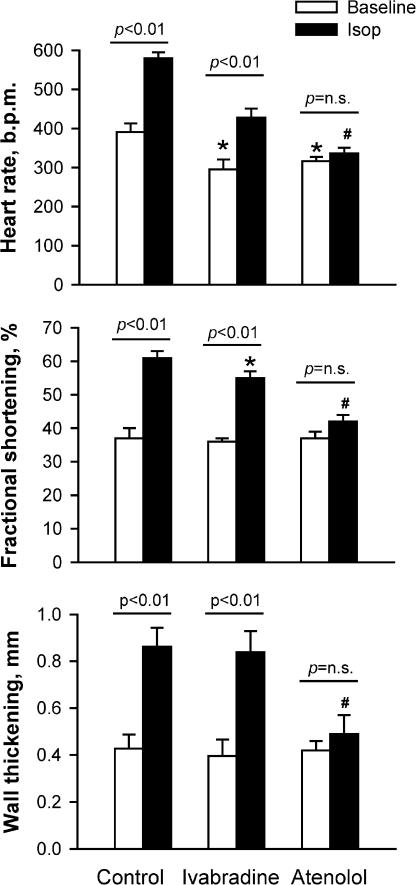
In a further experiment, we examined the effect of ivabradine on the HR response to isoproterenol (4 μg kg−1) in two groups of NTG mice: with one group treated with ivabradine for 1 week at 10 mg kg−1 day−1 and another group with atenolol for 1 week at 2 mg kg−1 day−1. Animals were anesthetized with KXA and echocardiography was performed at baseline and 3 min after injection with isoproterenol. Baseline HR was lower in ivabradine-treated than in untreated animals by approximately 20%, but FS and wall thickening were not different (Figure 4). In untreated animals, both HR and FS increased by about 50% and wall thickening doubled in response to isoproterenol (both P<0.001). Although the ivabradine-treated mice were able to respond to isoproterenol with a 45% increase in HR, the absolute increment and the maximum level of HR were significantly lower than in control mice (both P<0.01). In the presence of isoproterenol, FS level was only slightly, although significantly, lower and wall thickening was unchanged compared with the untreated mice. The responses of HR, FS and wall thickening to isoproterenol were abolished by treatment with atenolol. These data suggest that ivabradine selectively reduces HR without changes in ventricular performance under basal and β-adrenergic stimulated conditions, while the HR-reducing effect of atenolol is associated with a reduced LV contractility, consistent with its negative inotropic effects.
Figure 4.
Effects of oral ivabradine (10 mg kg−1 day−1 for 1 week) and atenolol (2 mg kg−1 day−1 for 1 week) on HR, fractional shortening (FS) and wall thickening in anaesthetized NTG mice and the response to administration of the β-agonist isoprotorenol (Isop, 4 μg kg−1, i.p.). Animals were anaesthetized with a mixture of ketamine/xylazine/atropine. In ivabradine-treated mice, HR levels at baseline and during β-agonist stimulation were about 25% lower than control, whereas FS and wall thickening were largely unaffected. In the mice treated with atenolol, responses of HR, FS and wall thickening to isoproterenol were abolished. N=8 per group. *P<0.05 vs respective control values. #P<0.05 vs respective values of ivabradine-treated group.
Discussion
We aimed to demonstrate the HR-reducing effect of ivabradine given orally in the mouse under conditions that were associated with sympathoadrenergic activation, including restraint-mediated stress, cardiac-restricted overexpression of β2AR, and the use of the β-agonist isoproterenol. Ivabradine reduced HR under all these conditions. However, a correlation between plasma levels of ivabradine and HR-lowering potency was not evident. The efficacy observed was up to a 20% reduction in HR at doses of 10 and 20 mg kg−1 day−1, limited by the palatability of ivabradine being administered via drinking water, and not associated with changes in FS. This is consistent with the lack of negative inotropic effect of this drug reported in other animal species as well as in humans (Simon et al., 1995; Thollon et al., 1997; Monnet et al., 2001; Colin et al., 2002). Further, this study has provided evidence that ivabradine reduces HR well under conditions where HR is elevated by sympathoadrenergic activation.
Among the time-dependent ionic currents that determine the rate of diastolic depolarization, the hyperpolarization-activated inward rectifier current If is considered to be the most important (Catterall et al., 1991; DiFrancesco, 1991; 1995). It is interesting to observe that in the mice treated orally with ivabradine for 1 week, reduction in HR was detectable with ivabradine plasma level in the range of 0.01–0.04 μM. In vitro patch-clamp studies showed that the IC50 for the If blockade by ivabradine is 1.5–2.2 μM (Bois et al., 1996; Thollon et al., 1997; Bucchi et al., 2002).
HR as well as If current are directly modulated by sympathetic and parasympathetic activities (DiFrancesco et al., 1989; DiFrancesco & Tortora, 1991; Guth & Dietze, 1995). It has been shown that If current of sinoatrial node pacemaker cells is affected by adrenergic stimulation (DiFrancesco & Tortora, 1991) and that ivabradine is a use-dependent If current blocker (Bois et al., 1996; Bucchi et al., 2002). Therefore, it is expected that the efficacy of ivabradine on HR would be modulated by alterations in the sympathetic activity. In the present study, we provided evidence for a complete sympathetic withdrawal in anaesthetized NTG mice, and a full activation of sympathetic nervous activity in restrained conscious mice. Interestingly, ivabradine effectively reduced HR under both settings, with similar degree of absolute HR reduction. We further showed a HR-lowering action of ivabradine under conditions in which HR was driven by an enhanced β-adrenergic signalling pathway due either to transgenic overexpression of β2AR in the heart or administration of isoproterenol. Ivabradine differs from other bradycardiac agents, such as β-blockers, in that its HR-lowering activity is maintained under both basal and stressed conditions in the NTG mice as well as in the β2-AR TG mice. Thus, at least in the mouse, the selective HR-lowering activity of ivabradine appears independent of autonomic nervous activity that might alter HR levels and its sensitivity to drugs. This is in keeping with previous reports that ivabradine reduces HR in dogs and in patients with documented stable angina both at rest and during exercise (Monnet et al., 2001; Colin et al., 2002; Borer et al., 2003), unlike β-antagonists that lower HR largely depending on the level of sympathetic tone (Colin et al., 2002).
In the clinical setting, activation of the sympathetic nervous system is a hallmark of heart failure and the consequent HR elevation could lead to adverse consequences (Cohn, 1990). Persistent rapid pacing in larger laboratory species can cause cardiomyopathy and heart failure (Packer et al., 1986; Calderone et al., 1991). Although β-blockers yield a number of changes that may ultimately contribute to the overall efficacy in the setting of heart failure, reduction in HR by β-blockers can reduce myocardial oxygen consumption and prolong diastolic period with increased coronary blood flow. Packer et al. (1996) have shown, in heart failure patients, that the higher the pretreatment HR levels, the greater the efficacy of the β-blocker carvedilol. The adverse outcome in trials of β-antagonists with intrinsic sympathomimetic activity may be related to insufficient reduction in HR than that of drugs without such activity (Cleland et al., 1996). In an experimental heart failure model, preventing HR reduction by pacing largely abolished the beneficial effects of β-blockade (Nagatsu et al., 2000).
Despite a marked HR-lowering activity of ivabradine, studies in other species have shown that this drug does not have any negative inotropic effect (Gardiner et al., 1995; Simon et al., 1995; Colin et al., 2002). In the present study, this was found to be the case in the mouse, as estimated from an unchanged FS and wall thickening under various conditions. Therefore, ivabradine is unique in that it selectively reduces HR with no negative inotropic action in vivo in mice under the conditions studied. While β-blockers are clearly beneficial in heart failure, the accompanying negative inotropic activity of β-blockade is a concern in patients with decompensated heart failure (Cleland et al., 1996; Bristow, 2000; Borer et al., 2003). Perhaps the more suitable situation that favours the use of HR-lowering agents would be cases with profound reduction in cardiac function and with marked sensitivity to β-blockade. On the other hand, patients with decompensated heart failure showed clear haemodynamic benefits from the acute use of β-agonists, but the tachycardia is an unwanted effect. As shown in Figure 4, combined use of ivabradine and isoproterenol achieved significant inotropic enhancement, but with the chronotropic action to isoproterenol diminished. Thus, this combination is likely to yield better haemodynamic benefit to heart failure patients.
From its known mechanism of action, ivabradine should have no significant effect on potential non-sinoatrial node pacemaker sites and therefore the theoretical basis for unmasked ectopic activity exists. Electrophysiological studies have shown that ivabradine lowers HR without causing changes in conductivity, refractoriness or repolarization duration of atria-ventricular node, and ventricular Purkinje system (Thollon et al., 1997; Camm & Lau, 2003). This specificity of ivabradine on sinoatrial node differs from β-blockers that suppress the automaticity of both sinoatrial node and the ectopic sites. While ivabradine has been safely used, both clinically and experimentally, under conditions of acute myocardial ischaemia (Monnet et al., 2001; Borer et al., 2003), a situation associated with increased risk of ectopic arrhythmias, arrhythmia was not the end point in these studies. As we are aware, there has been no study to test whether ivabradine affects ectopic automaticity. Further study is required to address this question especially in the setting of an augmented sympathoadrenergic activity.
With the generation of a large number of gene-targeted mouse strains, the mouse has become one of the most commonly used animal species for research on heart disease. Of interest are the reports of tachycardiac phenotypes being associated with cardiomyopathy and heart failure in several strains of mice (Emanueli et al., 1999; Engelhardt et al., 1999; Du et al., 2000; Liggett et al., 2000; Hardt et al., 2002). The demonstration of the HR-lowering action of oral ivabradine in the mouse indicates the possibility of studying the role of an increased HR in the development of cardiomyopathy phenotypes by administrating HR-reducing agents.
In summary, this study has shown that ivabradine, when given orally, reduces HR without influencing LV contractile function. This action of ivabradine in HR reduction is well preserved under conditions that are associated with significant activation of the sympathoadrenergic system.
Acknowledgments
This work was supported by Institut de Recherches Internationales Servier, France.
Abbreviations
- β2AR
β2-adrenergic receptor
- FS
fractional shortening
- HR
heart rate
- NTG
nontransgenic
- TG
transgenic
References
- BOIS P., BESCOND J., RENAUDON B., LENFANT J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br. J. Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOND R.A., LEFF P., JOHNSON T.D., MILANO C.A., ROCKMAN H.A., MCMINN T.R., APPARSUNDARAM S., HYEK M.F., KENAKIN T.P., ALLEN L.F., LEFKOWITZ R.J. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- BORER J.S., FOX K., JAILLON P., LEREBOURS G. Antianginal and antiischemic effects of ivabradine, an If inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- BRISTOW M. Mechanistic and clinical rationales for using β-blockers in heart failure. J. Card Fail. 2000;6 Suppl 1:8–14. [PubMed] [Google Scholar]
- BUCCHI A., BARUSCOTTI M., DIFRANCESCO D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J. Gen. Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDERONE A., BOUVIER M., LI K., JUNEAU C., DE CHAMPLAIN J., ROULEAU J.L. Dysfunction of the β- and α-adrenergic systems in a model of congestive heart failure. The pacing-overdrive dog. Circ. Res. 1991;69:332–343. doi: 10.1161/01.res.69.2.332. [DOI] [PubMed] [Google Scholar]
- CAMM A., LAU C. Electrophysiological effects of a single intravenous administration of ivabradine (s 16257) in adult patients with normal baseline electrophysiology. Drugs Res. Dev. 2003;4:83–89. doi: 10.2165/00126839-200304020-00001. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A., SCHEUER T., THOMSEN W., ROSSIE S. Structure and modulation of voltage-gated ion channels. Ann. N.Y. Acad. Sci. 1991;625:174–180. doi: 10.1111/j.1749-6632.1991.tb33840.x. [DOI] [PubMed] [Google Scholar]
- CLELAND J.G., BRISTOW M.R., ERDMANN E., REMME W.J., SWEDBERG K., WAAGSTEIN F. β Blocking agents in heart failure. Should they be used and how. Eur. Heart J. 1996;17:1629–1639. doi: 10.1093/oxfordjournals.eurheartj.a014745. [DOI] [PubMed] [Google Scholar]
- COHN J.N. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation. 1990;82:I59–I67. [PubMed] [Google Scholar]
- COLIN P., GHALEH B., HITTINGER L., MONNET X., SLAMA M., GIUDICELLI J.F., BERDEAUX A. Differential effects of heart rate reduction and β-blockade on left ventricular relaxation during exercise. Am. J. Physiol. 2002;282:H672–H679. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- DIFRANCESCO D. The contribution of the ‘pacemaker' current (If) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J. Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFRANCESCO D. The pacemaker current (If) plays an important role in regulating SA node pacemaker activity. Cardiovasc. Res. 1995;30:307–308. [PubMed] [Google Scholar]
- DIFRANCESCO D., DUCOURET P., ROBINSON R.B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989;243:669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DIFRANCESCO D., TORTORA P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DU X.J., GAO X.M., WANG B., JENNINGS G.L., WOODCOCK E.A., DART A.M. Age-dependent cardiomyopathy and heart failure phenotype in mice overexpressing β2-adrenergic receptors in the heart. Cardiovasc. Res. 2000;48:448–454. doi: 10.1016/s0008-6363(00)00187-5. [DOI] [PubMed] [Google Scholar]
- DU X.J., VINCAN E., WOODCOCK D.M., MILANO C.A., DART A.M., WOODCOCK E.A. Response to cardiac sympathetic activation in transgenic mice overexpressing β2-adrenergic receptor. Am. J. Physiol. 1996;271:H630–H636. doi: 10.1152/ajpheart.1996.271.2.H630. [DOI] [PubMed] [Google Scholar]
- EMANUELI C., MAESTRI R., CORRADI D., MARCHIONE R., MINASI A., TOZZI M.G., SALIS M.B., STRAINO S., CAPOGROSSI M.C., OLIVETTI G., MADEDDU P. Dilated and failing cardiomyopathy in bradykinin B2 receptor knockout mice. Circulation. 1999;100:2359–2365. doi: 10.1161/01.cir.100.23.2359. [DOI] [PubMed] [Google Scholar]
- ENGELHARDT S., HEIN L., WIESMANN F., LOHSE M.J. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO X.M., DART A.M., DEWAR E., JENNINGS G., DU X.J. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc. Res. 2000;45:330–338. doi: 10.1016/s0008-6363(99)00274-6. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Acute and chronic cardiac and regional haemodynamic effects of the novel bradycardic agent, S16257, in conscious rats. Br. J. Pharmacol. 1995;115:579–586. doi: 10.1111/j.1476-5381.1995.tb14971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH B.D., DIETZE T. If current mediates β-adrenergic enhancement of heart rate but not contractility in vivo. Basic Res. Cardiol. 1995;90:192–202. doi: 10.1007/BF00805662. [DOI] [PubMed] [Google Scholar]
- HARDT S.E., GENG Y.J., MONTAGNE O., ASAI K., HONG C., YANG G.P., BISHOP S.P., KIM S.J., VATNER D.E., SEIDMAN C.E., SEIDMAN J.G., HOMCY C.J., VATNER S.F. Accelerated cardiomyopathy in mice with overexpression of cardiac Gsα and a missense mutation in the α-myosin heavy chain. Circulation. 2002;105:614–620. doi: 10.1161/hc0502.103012. [DOI] [PubMed] [Google Scholar]
- KAYE D.M., LEFKOVITS J., JENNINGS G.L., BERGIN P., BROUGHTON A., ESLER M.D. Adverse consequences of high sympathetic nervous activity in the failing human heart. J. Am. Coll. Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- KLIPPERT P., JEANNIOT J.P., POLVE S., LEFEVRE C., MERDJAN H. Determination of ivabradine and its N-demethylated metabolite in human plasma and urine, and in rat and dog plasma by a validated high-performance liquid chromatographic method with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1998;719:125–133. doi: 10.1016/s0378-4347(98)00406-x. [DOI] [PubMed] [Google Scholar]
- LIGGETT S.B., TEPE N.M., LORENZ J.N., CANNING A.M., JANTZ T.D., MITARAI S., YATANI A., DORN II G.W. Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- MILANO C.A., ALLEN L.F., ROCKMAN H.A., DOLBER P.C., MCMINN T.R., CHIEN K.R., JOHNSON T.D., BOND R.A., LEFKOWITZ R.J. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- MONNET X., GHALEH B., COLIN P., DE CURZON O.P., GIUDICELLI J.F., BERDEAUX A. Effects of heart rate reduction with ivabradine on exercise-induced myocardial ischemia and stunning. J. Pharmacol. Exp. Ther. 2001;299:1133–1139. [PubMed] [Google Scholar]
- NAGATSU M., SPINALE F.G., KOIDE M., TAGAWA H., DEFREITAS G., COOPER G.T., CARABELLO B.A. Bradycardia and the role of β-blockade in the amelioration of left ventricular dysfunction. Circulation. 2000;101:653–659. doi: 10.1161/01.cir.101.6.653. [DOI] [PubMed] [Google Scholar]
- PACKER D.L., BARDY G.H., WORLEY S.J., SMITH M.S., COBB F.R., COLEMAN R.E., GALLAGHER J.J., GERMAN L.D. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am. J. Cardiol. 1986;57:563–570. doi: 10.1016/0002-9149(86)90836-2. [DOI] [PubMed] [Google Scholar]
- PACKER M., BRISTOW M.R., COHN J.N., COLUCCI W.S., FOWLER M.B., GILBERT E.M., SHUSTERMAN N.H. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- SIMON L., GHALEH B., PUYBASSET L., GIUDICELLI J.F., BERDEAUX A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J. Pharmacol. Exp. Ther. 1995;275:659–666. [PubMed] [Google Scholar]
- THOLLON C., BIDOUARD J.P., CAMBARRAT C., LESAGE L., REURE H., DELESCLUSE I., VIAN J., PEGLION J.L., VILAINE J.P. Stereospecific in vitro and in vivo effects of the new sinus node inhibitor (+)-S 16257. Eur. J. Pharmacol. 1997;339:43–51. doi: 10.1016/s0014-2999(97)01364-2. [DOI] [PubMed] [Google Scholar]
- THOLLON C., CAMBARRAT C., VIAN J., PROST J.F., PEGLION J.L., VILAINE J.P. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br. J. Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X.P., LIU Y.H., RHALEB N.E., KURIHARA N., KIM H.E., CARRETERO O.A. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am. J. Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]