Abstract
Depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) are two related forms of short-term synaptic plasticity of GABAergic and glutamatergic transmission, respectively. They are induced by calcium concentration increases in postsynaptic cells and are mediated by the release of a retrograde messenger, which reversibly inhibits afferent synapses via presynaptic mechanisms.
We review here:
The evidence accumulated during the 1990s that has led to the conclusion that DSI/DSE rely on retrograde signaling.
The more recent research that has led to the identification of endocannabinoids as the retrograde messengers responsible for DSI/DSE.
The possible mechanisms by which presynaptic type 1 cannabinoid receptors reduce synaptic efficacy during DSI/DSE.
The possible modes of induction of DSI/DSE by physiological activity patterns, and the partially conflicting evaluations of the calcium concentration increases required for cannabinoid synthesis.
Finally, the relation between DSI/DSE and other forms of long- and short-term synaptic inhibition, which were more recently associated with the production of endocannabinoids by postsynaptic cells.
Keywords: DSI, DSE, endocannabinoids, CB1 receptors, short-term synaptic plasticity, GABAergic transmission, glutamatergic transmission, retrograde messengers
The law of dynamic polarization: a rule with many exceptions
The classical concept of interneuronal communication holds that information flows from the axon terminals of the presynaptic neuron to the dendrites of its postsynaptic partner. This idea was stated by Santiago Ramon y Cayal under the name of ‘law of dynamic polarization'.
With hindsight, it is clear that the law of dynamic polarization cannot be considered as stringent as a physics law, and that it suffers many exceptions. Already, Cajal had recognized that, for example, many neurons throughout the animal kingdom lack axons; in such neurons, the law of dynamic polarization can clearly not hold. Nevertheless, this law has guided the thinking of neuroscientists up to the present time, so that exceptions from it have been slow to be recognized and accepted.
Certain sets of neurons, including interneurons, are now recognized as being connected via dendro-dendritic or axo-axonal electrical synapses (review: Galarreta & Hestrin, 2001). In addition, it is well established that neurons (e.g. in the olfactory bulb, Shepherd & Greer, 2001, or for the thalamic GABAergic F-terminals, Sherman & Guillery, 2001) are able to release neurotransmitters from structures closely resembling ‘classical' axonal synaptic terminals, but which are classified as dendritic according to present identification criteria.
Finally, many central neurons appear to be capable of releasing neurotransmitter-like substances from nonsynaptic areas of their somatodendritic compartment. These substances are able to modulate neurotransmitter release from afferent presynaptic terminals and include dopamine (Cheramy et al., 1981; Jaffe et al., 1998), dynorphin (Drake et al., 1994), glutamate and GABA (Zilberter et al., 1999; Zilberter, 2000), oxytocin and vasopressin (Kombian et al., 1997). Since they act in a direction which is opposite to that proposed by Cajal, they can be defined as ‘retrograde messengers' (for a thorough review of retrograde signaling in the nervous system, see, Alger, 2002).
Endocannabinoids are an especially important class of retrograde messengers. Unlike the above compounds, which act primarily as ordinary neurotransmitters or neurohormones, and incidentally as retrograde messengers, so far endocannabinoids have been found to act primarily or exclusively as retrograde messengers in the mammalian brain. This special adaptation could be due to the fact that, following synthesis from membranous, lipidic precursors (Di Marzo et al., 1998; Piomelli et al., 2000), they are not stored in vesicles like the above transmitters, but rather released presumably by diffusing across membranes. Moreover, they have recently been discovered to play a prominent role both in short-term and in long-term synaptic plasticity, as well as to directly control the rate of firing of presynaptic cells. In this review, we focus on the retrograde, short-term inhibitory actions of cannabinoids on afferent GABAergic and glutamatergic transmission, which are respectively known as depolarization-induced suppression of inhibition, or DSI, and depolarization-induced suppression of excitation, or DSE.
DSI and DSE
DSI/DSE are two closely related forms of short-term plasticity, which share the same modes of induction, the same type of retrograde messengers (i.e. endocannabinoids) and similar mechanisms of expression.
DSI was first reported more than a decade ago, at the GABAergic synapses onto cerebellar Purkinje cells and onto hippocampal CA1 pyramidal cells, and has been extensively described in the intervening period. Consequently, the main body of the literature in the field concerns the modulation of GABAergic transmission. By contrast, the first descriptions of DSE are very recent (Kreitzer & Regehr, 2001a; Ohno-Shosaku et al., 2002b) so that comparatively little is known on DSE. Due to the broad similarity between the two forms of synaptic plasticity, we will treat DSI and DSE together. However, a few discrepancies do exist, and they will be pointed out as they come.
Original reports of DSI concerned stellate/basket cell inputs onto cerebellar Purkinje cells (Llano et al., 1991; Vincent et al., 1992), as well as GABAergic inputs onto CA1 pyramidal cells (Pitler & Alger, 1992; Pitler & Alger, 1994; DSE was found at parallel and climbing fiber inputs of Purkinje cells (Kreitzer & Regehr, 2001a) and at glutamatergic inputs onto CA1 pyramidal cells (Ohno-Shosaku et al., 2002b). By definition, DSI (as well as DSE) is triggered by postsynaptic depolarization. Following several intervening steps, postsynaptic depolarization induces a transient inhibition of afferent synaptic currents, which is fully reversible in a range of a few tens of seconds at room temperature (Figure 1). It was quickly recognized that the calcium concentration rise that follows postsynaptic depolarization serves as a signal for DSI/DSE. Three lines of evidence indicate that this increase in intracellular calcium is both a necessary and a sufficient condition for induction: (1) blockers of calcium entry block DSI (Llano et al., 1991; Lenz et al., 1998); (2) postsynaptic application of calcium chelating agents like EGTA and/or BAPTA prevent DSI/DSE (Pitler & Alger, 1992; Glitsch et al., 2000; Kreitzer & Regehr, 2001a; Ohno-Shosaku et al., 2002a); (3) postsynaptic calcium uncaging is able to trigger synaptic depression (Wang & Zucker, 2000; Wilson & Nicoll, 2001).
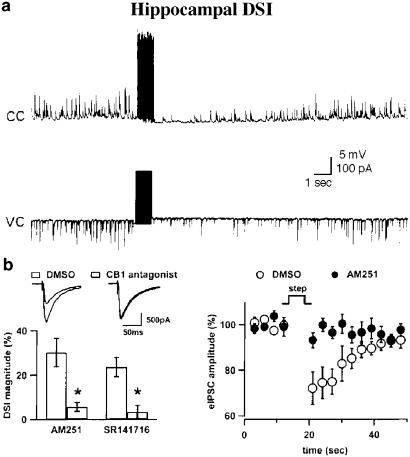
Figure 1.
DSI and endocannabinoids in the hippocampus. (a) Whole-cell recordings from a single hippocampal CA1 cell. sIPSPs (upper trace) in current clamp and sIPSCs (lower trace) in voltage clamp are inhibited by a brief train of action potentials. Notice the fast reversibility of the inhibition (from Pitler & Alger, 1992). (b) Endocannabinoids mediate the DSI of extracellularly eIPSCs in the hippocampus. On the right, the time course of DSI in CA1 pyramidal cells is shown for the control DMSO vehicle (open circles) and after applying the CB1R antagonist AM251 (dots). On the left, bars show the amount of DSI inhibition by two distinct CB1R antagonists, AM251 and SR141716, with sample traces for control DSI and DSI in antagonist depicted above (from Wilson & Nicoll, 2001).
It is important to note here that, instead of applying postsynaptic depolarizations, it is possible to induce a postsynaptic production of endocannabinoids and to inhibit afferent synaptic transmission by activating postsynaptic receptors linked to G proteins. Strictly speaking, these forms of retrograde inhibition, which will be further described later, should not be called DSI/DSE, since they are not triggered by depolarization and since, in contrast to DSI/DSE, they do not seem to depend on postsynaptic calcium elevation (Maejima et al., 2001; Kim et al., 2002). They will therefore be considered separate from DSI/DSE throughout this review.
The presynaptic nature of the inhibitory effect on the afferent synaptic transmission, which defines DSI/DSE, was rapidly established, on the basis of the following observations: (1) The postsynaptic sensitivity to GABA, as measured either by the amplitude of miniature synaptic currents, or by that of responses to applications of exogenous neurotransmitter, is not modified during DSI (Llano et al., 1991; Pitler & Alger, 1992). (2) The frequency, but not the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) decreases during cerebellar DSI (Llano et al., 1991; Glitsch et al., 1996; (3) During DSI, the percentage of synaptic failures increases (Vincent et al., 1992; Alger et al., 1996; Diana & Marty, 2003), and so does the paired pulse ratio during DSI and DSE (Ohno-Shosaku et al., 1998; Wilson & Nicoll, 2001; Ohno-Shosaku et al., 2002b; Yoshida et al., 2002; Diana & Marty, 2003; Trettel & Levine, 2003; but see Alger et al., 1996; Varma et al., 2002)
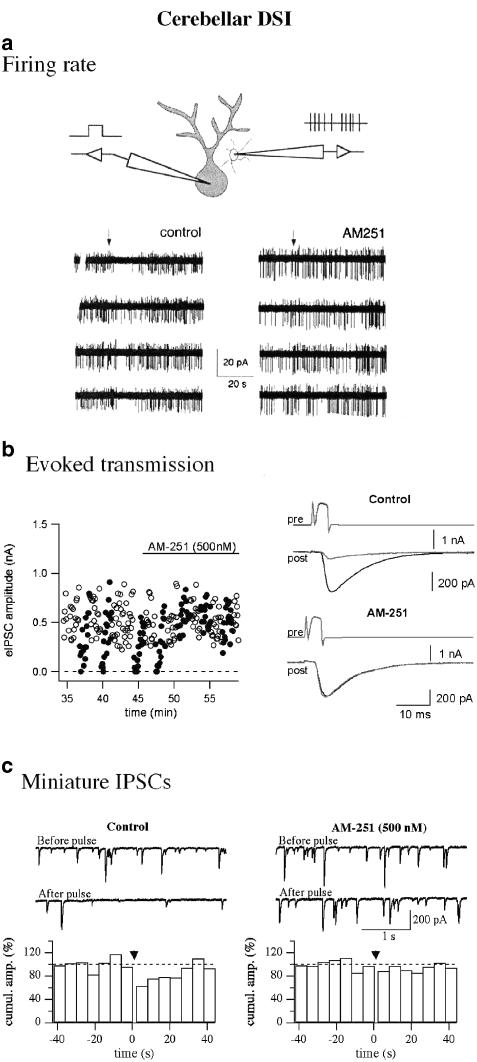
Figure 2.
Endocannabinoids mediate DSI in the cerebellum. The firing rate of presynaptic interneurons (a), the amplitude of eIPSCs (b), and the frequency of mIPSCs (c) are all reduced during DSI. The data in (a) and (b) were recorded in paired recordings from molecular layer presynaptic interneurons and postsynaptic Purkinje cells, as depicted in the scheme in (a). (a) In presynaptic cell attached recordings, the spontaneous firing rate of interneurons decreases after the depolarization of a simultaneously recorded Purkinje cell (left traces; depolarization time given at the arrow); this inhibition is blocked by the CB1R antagonist AM251 (right traces; from Kreitzer et al., 2002). (b) Presynaptic cells were here recorded with the perforated patch configuration and shortly depolarized to elicit eIPSCs in synaptically connected Purkinje cells. In the plot on the left, each open circle gives the amplitude of a single control IPSC, whereas filled circles show the IPSCs in the 90 s following a DSI induction protocol. In control, every depolarization reliably induces DSI, whereas after AM251 application the effect of depolarizations is completely prevented. Sample traces are shown on the right (from Diana et al., 2002). (c) The frequency of mIPSCs recorded from Purkinje cells is inhibited in control uring DSI, as shown by the sample traces and by the time plot on the left side of the figure. On the right, the effect of blocking the CB1R is shown: mIPSC frequency is unchanged after Purkinje cell depolarization (given at t=0 in both time plots; from Diana et al., 2002).
In view of the findings that a postsynaptic signal, a calcium concentration rise, was the trigger of DSI, and that a presynaptic modification, a reduction of transmitter release, was the end effect, the existence of a retrograde messenger was soon proposed (Llano et al., 1991). Early studies proposed that glutamate was released postsynaptically, and that it activated presynaptic metabotropic glutamate receptors (mGluRs) of group II (in the cerebellum: Glitsch et al., 1996) or of group I (in the hippocampus: Morishita et al., 1998). However, the glutamate hypothesis remained tentative because inhibition through mGluR antagonists was only partial. In retrospect, it is possible to explain a large part of the glutamate results on the basis of a modulatory role of postsynaptic mGluR receptors deriving from the convergence of mGluR and CB1R pathways on presynaptic terminals (see later).
The pace of research in the DSI field abruptly changed in the year 2001. Wilson and Nicoll (2001) proposed that during hippocampal DSI, the postsynaptic calcium rise led to the release of endocannabinoids and a consequent activation of presynaptic type 1 cannabinoid receptors (CB1Rs). Wilson and Nicoll could demonstrate a complete block of DSI with low doses of a specific antagonist of CB1Rs (Figure 1b), which contrasted with the weak and poorly specific block previously obtained with mGluR antagonists. In addition hippocampal DSI was soon shown to be absent in CB1R-deficient mice (Wilson et al., 2001). In a short period of time, the endocannabinoid- and CB1R-related nature of DSI has been confirmed in the hippocampus and extended to several systems: for GABAergic synapses onto cerebellar Purkinje cells (Kreitzer & Regehr, 2001b; Diana et al., 2002; Yoshida et al., 2002; Figure 2a), cultured rat hippocampal neurons (Ohno-Shosaku et al., 2001), hippocampal dentate granule cells in particular pathological conditions (Chen et al., 2003), cortical pyramidal cells (Trettel & Levine, 2003) and neurons of the substantia nigra (Yanovsky et al., 2003).
Nearly simultaneously with the Wilson and Nicoll paper, Kreitzer and Regehr (2001a) described DSE for the first time in Purkinje cells, and established that DSE is mediated by the release of endocannabinoids and the consequent activation of presynaptic CB1Rs. Later work extended these conclusions for glutamatergic transmission onto CA1 pyramidal cells (Ohno-Shosaku et al., 2002b).
Expression mechanisms of DSI/DSE
Maximum inhibition reached during DSI/DSE
DSI/DSE produce a powerful depression of synaptic transmission. The evaluation of maximum inhibition is best achieved in paired recordings because, in some preparations, only a part of the afferent synaptic input is sensitive to DSI/DSE and this fact limits the amount of total inhibition when the entire synapse population is tested for DSI. When restricting their measurements to endocannabinoid-sensitive synaptic connections, Wilson et al. (2001) found a maximum inhibition during hippocampal DSI close to 90%. A similar value was obtained from paired recordings in the cerebellum (Diana et al., 2002; Figure 2b), whereas in hippocampal cultured cells DSI reaches a somewhat smaller value (about 75% in DSI sensitive pairs: Ohno-Shosaku et al., 2001). In slices, these values correspond to the inhibition obtained with saturating concentration of a cannabinoid exogenous agonist, showing that standard DSI induction protocols are able to saturate presynaptic CBIRs. The maximal inhibition for DSE is slightly smaller than DSI for the synapses between cerebellar parallel fibers and Purkinje cells (Brenowitz & Regehr, 2003), whereas it is markedly smaller for the DSE of climbing fibers to Purkinje cell synapses (Kreitzer & Regehr, 2001a; Brenowitz & Regehr, 2003) and for hippocampal DSE (Ohno-Shosaku et al., 2002b; Chen et al., 2003). These differences could arise from a reduced number of functional CB1Rs, from a distinct location of CB1Rs on presynaptic glutamatergic terminals with respect to the postsynaptic endcannabinoid release machinery, or from a different sensitivity (possibly associated to a different isoform, see later) of endocannabinoid receptors to endogenous agonists (Ohno-Shosaku et al., 2002b).
Potential sites of action of endocannabinoids
What could be the presynaptic targets downstream of the CB1Rs? The existing literature on presynaptic effects of cannabinoids indicates that virtually every step in the process leading to vesicle release is a potential site of action for cannabinoids. These mechanisms have been extensively described in recent reviews (Ameri, 1999; Schlicker & Kathmann, 2001; Howlett et al., 2002) and are only briefly recalled hereafter. Each of these modes of operation has already been suggested for brain areas where CB1Rs are functional, although with distinct specificities from region to region:
N- and P/Q-type voltage-dependence conductances can be inhibited. In presynaptic terminals, such effects would lead to a reduced calcium entry per action potential, and hence to reduced transmitter release.
Potassium currents can be upregulated. This can lead to a shortening of the action potential duration (with a consequent reduction in the amount of calcium influx per spike), as well as to an increased threshold for action potential generation.
Finally, in many brain areas, the frequency of miniature currents decreases upon CB1R activation. This suggests an action on basic release pathways unrelated to calcium entry, given that the frequency of miniature synaptic currents is usually considered independent from calcium entry.
Multiple mechanisms contribute to cerebellar DSI
In view of the multiplicity of potential CB1R effects, it has to be expected that several processes contribute to reducing synaptic transmission during DSI/DSE. Cerebellar DSI is an example in point. It involves an inhibition of the presynaptic firing rate (Figure 2a; Kreitzer et al., 2002, and Diana & Marty, unpublished data), of evoked inhibitory postsynaptic currents (IPSCs) as measured in paired recordings (Figure 2b; Diana et al., 2002), and of mIPSCs (Figure 2c; Llano et al., 1991). We recently provided a quantitative evaluation of these three components: they account for 13.4, 23.2 and 63.4%, respectively, of the reduction of the overall afferent GABAergic transmission during DSI (Diana & Marty, 2003). Although the exact underlying mechanisms for each effect remain to be determined, it seems likely that they will turn out to be separate. All the three aforementioned mechanisms of cannabinoid action may be involved: an inhibition in P-type calcium channels, which are primarily responsible for presynaptic calcium entry in this system (Forti et al., 2000), could contribute to reducing action potential-driven calcium increases (Diana et al., 2002), and hence eIPSCs. An increase in potassium conductance appears to mediate the reduction in firing rate (Kreitzer et al., 2002), and could also have a role in the reduction of eIPSCs (Diana & Marty, 2003). Finally, mIPSC frequency is reduced by mechanisms that are presumably independent of conductance changes.
Inhibition of presynaptic firing expands the scope of DSI
An early report (Vincent & Marty, 1993), using paired recordings of neighboring Purkinje cells, showed that depolarization of one Purkinje cell not only inhibits its afferent IPSCs but also those of other Purkinje cells in as much as they share a common interneuron input. Thus, DSI can spread along the Purkinje cell layer from stimulated to unstimulated cells up to an intercellular distance of around 100 μm. These findings were interpreted as indicating that DSI was inhibiting the excitability of presynaptic interneurons.
The recent finding that presynaptic firing is inhibited during DSI (Kreitzer et al., 2002; Figure 2a), likely through an upregulation of inwardly rectifying GIRK potassium channels, confirms this interpretation and gives a firm mechanistic explanation for the spread of DSI. In addition, it expands the scope of DSI, which was hitherto envisaged as a mere modulation of synaptic strength, and which now appears as a direct way to modulate neuronal firing in the postsynaptic → presynaptic direction.
Hippocampal DSI involves the regulation of N-type calcium channels
The pattern for the DSI in the hippocampus appears, at first sight, simpler. CB1R activation does not seem to affect mIPSCs (Hoffman & Lupica, 2000; Chen et al., 2003, but see later) and, likewise, hippocampal DSI was reported to affect mIPSCs frequency only minimally (Pitler & Alger, 1994). Moreover, DSI spread between pyramidal cells takes place only at short distances (up to 20 μm), indicating that only passive diffusion of cannabinoids, but not a modulation of presynaptic firing, is involved (Wilson & Nicoll, 2001).
Thus, CB1R action on GABAergic interneurons could simply be explained by an inhibition of presynaptic calcium conductances (Hoffman & Lupica, 2000).
Specifically, a reduction of N-type channels was proposed, because these channels mediate all the afferent IPSCs onto CA1 cells originating from the subfamily of hippocampal interneurons that are cannabinoid-sensitive (Wilson et al., 2001). These interneurons, which most likely correspond to the previously identified family of CB1R- and cholecystokinin (CCK)-expressing cells (Katona et al. 1999; Tsuo et al., 1999), are thus the selective targets of hippocampal DSI (Wilson et al., 2001). The high degree of selectivity of CB1R expression in the hippocampus could also explain the apparent absence of effects of cannabinoids on hippocampal GABAergic mIPSCs, as follows. mIPSCs arising from CB1R-sensitive synapses could be a small percentage of the overall population recorded from CA1 cells, so that the effect of exogenous and endogenous cannabinoid agonists could turn out to be statistically insignificant.
By contrast, only a negligible percentage of cerebellar molecular layer interneurons are insensitive either to CB1R agonists or to DSI (Diana et al., 2002; Diana & Marty, 2003), attesting a more uniform distribution of CB1Rs in the presynaptic GABAergic cells of this structure.
Even though inhibition of N-type calcium channels is likely to play a major role in hippocampal DSI, the possibility remains that potassium conductances may also be involved, similarly to what we reported in the cerebellum (Diana & Marty, 2003). In particular, evidence was obtained indicating the participation of potassium conductance upstream of presynaptic calcium entry, at some still unidentified step (Varma et al., 2002).
Expression mechanisms of DSE
Compared to DSI, little is known on the presynaptic mechanisms underlying DSE. The available information comes essentially from the cerebellum.
In Purkinje cells, miniature EPSCs were reported to be decreased by CB1R agonists in one study (Levenes et al., 1998), but not in a subsequent publication (Takahashi & Linden, 2000), whereas evoked glutamatergic transmission is inhibited both at parallel and at climbing fibers (Levenes et al., 1998; Takahashi & Linden, 2000). CB1R agonists inhibit action potential-mediated calcium transients at parallel fiber synaptic terminals by modulating potassium conductances (Daniel & Crepel, 2001). Likewise, calcium transients are inhibited at climbing fiber terminals during DSE (Kreitzer & Regehr, 2001a).
From these data, it can be concluded that DSE is mediated at least in part by a reduction in presynaptic calcium signal for each incoming action potential.
Calcium dependence of DSI/DSE
Calcium dependence of DSI
Standard protocols used to induce DSI involve rather long-lasting voltage steps to the postsynaptic cell, which may lead to abnormally high intracellular calcium rises. It is therefore important to evaluate quantitatively the dose–response curve of retrograde inhibition as a function of intracellular calcium in order to assess possible physiological roles for DSI.
Three different groups have approached this question with diverging results. In Purkinje cells, two papers provide strikingly different estimates for the calcium concentration required to obtain half-maximum DSI. Glitsch et al. (2000) found 40 and 200 nM for somatic and dendritic DSI, respectively, whereas Brenowitz & Regehr (2003) found 15 μM for dendritic DSI. This 100-fold difference has, of course, nontrivial consequences regarding the physiological relevance of DSI. The lower calcium range, if correct, would imply that endocannabinoid production could occur tonically and that it could be finely modulated by slight changes in afferent synaptic transmission; conversely, the higher estimate would suggest that the endocannabinoid machinery only responds to high or very high levels of activity.
The discrepancy could arise from one or several of the following factors. First, it appears that a given voltage step was more efficient in eliciting DSI in one than in the other study. Thus, 200 ms long pulses nearly saturated DSI in the Glitsch et al. study but only elicited a modest inhibition in the Brenowitz & Regehr report. This suggests that differences in experimental protocols might have made endocannabinoid production more efficient in one case than in the other. Recent results indicate that differences in intracellular solutions may lead to differences in DSI (compare Diana et al., 2002 with Diana & Marty, 2003). Moreover, two different aspects of DSI were evaluated in the two papers: spontaneous inhibitory postsynaptic currents (sIPSCs) in Glitsch et al. (2000), and extracellularly evoked IPSCs in Brenowitz & Regehr (2003). Secondly, discrepancies may have arisen from the difficulty of obtaining precise calibration curves for the calcium-measuring fluorescent dyes in Purkinje cells, due to the very large endogenous buffering capacity of these cells (Fierro & Llano, 1996). Another factor that can account for part of the discrepancy is the fact that the intracellular calcium concentration is not homogeneous during DSI, and the two groups may not have necessarily taken their measurements from equivalent cell regions.
Indirect support in favour of the hypothesis of a high sensitivity of DSI to calcium is given by the fact that cerebellar is blocked only by extremely high intracellular concentrations of BAPTA (40 mM: Glitsch et al., 2000), whereas, by comparison, full block of hippocampal DSI is achieved with 10 mM BAPTA (Pitler & Alger, 1992). However, it is clear that further investigations will be required in order to determine the true calcium sensitivity of cerebellar DSI.
A third report, which was performed in hippocampal pyramidal cells, gave an intermediate value of 4 μM for half saturation (Wang & Zucker, 2000). In this paper, voltage steps were not applied, and calcium elevations were instead achieved by uncaging. This technique allows uniform concentration increases in the recorded cell. It therefore avoids possible confusions resulting from the large calcium concentration gradients, which are elicited by voltage steps in highly complex neurons such as pyramidal or Purkinje cells.
Calcium dependence of DSE
A first study (Kreitzer & Regehr, 2001a) reported a lower sensitivity to postsynaptic depolarizations of climbing fiber than of parallel fiber terminals onto Purkinje cells. However, a more recent work (Brenowitz & Regehr, 2003) showed that the two excitatory synapses share the same half-saturation value; it furthermore reported that this was the same as that obtained for GABAergic synapses in the same paper: 15 μM.
On the contrary, hippocampal DSE is more difficult to induce than DSI. Very long depolarizations are needed to obtain a weak depression of glutamatergic transmission (Ohno-Shosaku et al., 2002b). This has been explained on the basis of a smaller sensitivity of presynaptic CB1Rs to cannabinoid agonists in glutamatergic terminals compared to GABAergic synapses and/or of a lower expression of these receptors on the former terminals (Ohno-Shosaku et al., 2002b). The pattern for hippocampal DSE is further complicated by the uncertainties on the CB receptor isoform mediating the cannabinoid response. In fact, although this is still a disputed issue (Marsicano & Lutz, 1999: Marsicano et al., 2003), some of the presently available evidence suggests that CB1Rs are not present on glutamatergic terminals onto CA1 pyramidal cell (Katona et al., 1999; Tsuo et al., 1999; Hajos et al., 2000), and that a so far uncloned CB receptor might provide the sensitivity to cannabinoids (Hajos et al., 2001; Breivogel et al., 2001). Independently of this issue, the take home message remains that, in the hippocampus, cannabinoid-mediated retrograde inhibition appears to pertain mainly to GABAergic synapses (Wagner & Alger, 1996). This is further illustrated by a recent report, showing that in an animal model of febrile seizures, DSI but not DSE is selectively enhanced through an increase in the expression of presynaptic CB1Rs on hippocampal, CCK-positive interneurons (Chen et al., 2003).
Physiological implications
Until now most DSI/DSE studies have focused on studying the basic mechanisms underlying these phenomena; this has implied, in most cases, the use of massive stimulation protocols, which cannot be regarded as physiological. Accordingly, studies concerning the synaptic induction of DSI/DSE are just starting.
Physiological induction protocols of hippocampal DSI
Early work showed that high-frequency trains of action potentials in hippocampal CA1 pyramidal cells, induced either with somatic depolarizations (Pitler & Alger, 1992, Figure 1a) or in conditions that elicit epileptiform burst discharges (Mg2+-free saline: Beau & Alger, 1998), could induce a significant degree of DSI. Back-propagating dendritic action potentials are likely to play an important role in triggering the increases in intracellular calcium necessary for DSI in these conditions (Morishita & Alger, 2001). Combining back-propagating action potentials with synaptic activation of mGluRs (Nakamura et al., 1999) was shown to induce increases in intracellular calcium in the micromolar range, thus well in the order of magnitude shown by Wang & Zucker (2000) to be required for DSI induction.
These data indicate that, in the hippocampus, calcium increases sufficient for obtaining DSI could be obtained in physiological conditions (for a more extensive discussion of this issue, see, Freund et al., 2003).
It must be mentioned, nonetheless, that a more recent paper, in which experiments very similar to those of Pitler & Alger, 1992 were performed, challenges this view (Hampson et al., 2003). We have noticed two main differences between these reports, although we cannot say if they can explain these contradictory results: the age of the animals used (1-week-old rats in Hampson et al., 2003 and adult rats in Pitler & Alger, 1992) and the main anion in the postsynaptic solution used (CH3SO3− in Hampson et al., Cl− in Pitler & Alger).
Functional consequences of the specific localization of CB1Rs in the hippocampus
It was stressed earlier that CB1Rs in hippocampal GABAergic cells are expressed only in one kind of interneurons, which are identified also by their positivity to CCK. These CCK-expressing interneurons have been proposed to participate in the transmission to the hippocampal region of serotonergically- and cholinergically controlled emotional and motivational physiological states (Freund, 2003). Importantly in this context, DSI is greatly enhanced by the activation of muscarinic receptors (Martin & Alger, 1999; Kim et al., 2002; Ohno-Shosaku et al., 2003). We invite interested readers to look at the extensive review by Freund et al. (2003), for a discussion of the possible importance of DSI in relation to this fact and to the network rhythms of the hippocampal formation.
The potential relevance for cognitive processes of the predominance of DSI over DSE in the hippocampus is illustrated by the facilitation of LTP induction in CA1 pyramidal cells during DSI (Carlson et al., 2002).
Finally, we already mentioned that in a novel animal model of human fever-induced seizures, CB1R expression is upregulated on CCK-positive GABAergic terminals (Chen et al., 2003); this induces a specific increase of DSI with respect to control animals, whereas DSE is not modified. This study opens the way for a role of DSI in pathological states.
Functional implications of DSI in the cerebellum
In the cerebellum, separate or combined synaptic activation of climbing and parallel fibers leads to local or widespread dendritic calcium increases in Purkinje cells via voltage-dependent calcium channels and intracellular calcium stores (Tank et al., 1988; Finch & Augustine, 1998; Takechi et al., 1998; Wang et al., 2000). However, it is unclear whether these signals are sufficiently large to produce endocannabinoids.
Therefore, at the moment little is known on the relation between synaptically induced calcium transients and DSI/DSE in Purkinje cells. Paradoxically, the only report concerning the effects of high-frequency, glutamatergic fiber stimulation on GABAergic transmission has shown an inhibition after climbing fiber activation which is not dependent on CB1Rs, but rather on presynaptic ionotropic glutamate receptors (Satake et al., 2000).
Thus, we still have some way to go before being able to assign a clear physiological function to cerebellar DSI. Nevertheless, on a purely speculative ground, we will propose two possible roles for cerebellar DSI, one at the level of control of single cell activity and another on a larger, network scale.
CB1Rs are very strongly expressed in Purkinje cell pinceaux (Tsuo et al., 1998). This suggests that the possibility by CB1Rs of sensing Purkinje cell firing at this strategic location could influence the inhibitory input from presynaptic basket cells, thus controlling the output of the cerebellar cortex. Such a role would require a high sensitivity of cerebellar DSI to calcium, as indicated by some estimates (see above), so that modest changes in intracellular calcium levels associated with the level of Purkinje cell activity would lead to a finely tuned release of endocannabinoids.
On a larger scale, GABAergic interneurons can dictate the synchronized activity of large groups of neurons in cortical areas, thus generating specific network rhythms thought to be important in cognitive processes (Traub et al., 1999). This general pattern may apply to the synchronized activity of Purkinje cells (Isope et al., 2002). The inhibition of presynaptic firing occurring during DSI tends to simultaneously remove the GABAergic synaptic potentials from groups of neighboring Purkinje cells. This could transiently dissociate the time of firing of these Purkinje cells from the coordinated rythms of larger areas of the cerebellar cortex, with likely important consequences on the cerebellar output.
A neuroprotective role for DSE?
Exogeneous cannabinoids are known to have a neuroprotective role; a recent study has shown that mice in which CB1Rs had been selectively eliminated from glutamatergic principal cells were much more subject than control mice to neurotoxic events induced by kainate (Marsicano et al., 2003; for a review Piomelli et al., 2000).
We suggest that this neuroprotective role could come from DSE by the following mechanism: intracellular calcium increases in response to excitatory ionotropic receptor activation can trigger excitotoxicity and neuronal death following, for example, ischemic brain injury; the accumulation of intracellular calcium during such noxious events could lead to cannabinoid production, to CB1R activation and, finally, to a DSE-like presynaptic inhibition of glutamatergic transmission with neuroprotective effects.
On the control of DSI/DSE by neuromodulators and the identity of the retrograde molecule
So far, we have only discussed the direct pathway that starts with postsynaptic depolarization and leads to DSI/DSE via calcium-dependent production of endocannabinoids. In this section we will discuss a parallel pathway leading from activation of G-protein-coupled receptors to inhibition of afferent synaptic potentials, and the links between this pathway and DSI/DSE.
The G-protein coupled receptor pathway of retrograde inhibition
Activation of group I mGluRs in Purkinje cells leads to a depression of parallel fiber EPSPs (Levenes et al., 2001). This effect was shown to be mediated by the calcium-dependent release of a retrograde messenger. Similar to DSI, the messenger was initially proposed to be glutamate, but more recent evidence suggests endocannabinoids. A following study showed that activation of group I mGluR receptors, either by application of an exogenous agonist or by repetitive stimulation of parallel fibers, led to a retrograde inhibition of climbing fiber EPSPs (Maejima et al., 2001), and that these effects were blocked by CB1R antagonists; hence these effects were proposed to be mediated by endocannabinoids. Likewise, brief repetitive stimulation of parallel fibres at high frequency leads to a transient self-inhibition: this was shown to depend on activation of mGluRs (Neale et al., 2001) and, following the development of endocannabinoid research, can now be ascribed to the synthesis and the action of endocannabinoids (Brown et al., 2003).
The newly found connection between group I mGluR receptors and the cannabinoid system in the cerebellum offered possible explanations for the apparent interactions between mGluR activation and DSI. Indeed, Varma et al. (2001) (and later Ohno-Shosaku et al., 2002a) confirmed that group I mGluR activation also led to the production of endocannabinoids in hippocampal CA1 cells, and showed that this pathway, although not mediating DSI, had a powerful modulatory effect on the phenomenon. Further work extended these findings by showing that the activation of muscarinic receptors of the m1 and m3 subtypes had effects similar to those of group I mGluR activation (Martin & Alger, 1999; Kim et al., 2002; Ohno-Shosaku et al., 2003).
In summary, these studies show that the activation of group I mGlu or of muscarinic receptors can lead both to an enhancement of DSI when using low agonist concentrations, which by themselves do not alter synaptic efficacy, but also to a direct production of endocannabinoids at higher agonist concentrations, which can directly activate presynaptic CB1 receptors and inhibit transmission. In the former case, DSI was suggested to be facilitated through an increased production of endocannabinoids rather than via changes of basal calcium levels and/or changes in the maximal levels of calcium reached during depolarizations.
Mechanisms linking activation of group 1 mGluR and m1/m3 receptors to endocannabinoid synthesis
Group 1 mGluRs and m1/m3 muscarinic receptors are positively coupled to phospholipase C (PLC): PLC increases the intracellular levels of IP3 and, importantly, of diacylglycerol (DAG), which is itself a direct precursor of 2-arachidonylglycerol (2-AG) through a specific lipase (see Di Marzo et al., 1998; Piomelli et al., 2000). 2-AG and anandamide are two of the putative endocannabinoids presumed to mediate the effects of CB1Rs in the CNS (Devane et al., 1992; Mechoulam et al., 1995; Sugiura et al., 1995; for a review Di Marzo et al., 1998; Piomelli et al., 2000).
Thus, it appears possible that muscarinic and group 1 mGluR receptor activation preferentially leads to 2-AG production, and that this compound, rather than anandamide, could be the active endocannabinoid involved in the retrograde effects exerted by activation of these receptors. Indeed, a recent study has shown that the heterosynaptic inhibition of GABAergic transmission by glutamatergic inputs onto CA1 cells is blocked by inhibitors of PLC and of DAG lipase, and furthermore, that DSI is not affected by the same inhibitors (Chevaleyre & Castillo, 2003). These results indicate that the endocannabinoid synthesis pathways are likely different for DSI and for group 1 mGluR-induced retrograde signaling. In agreement with this idea, DSI is strictly dependent on intracellular calcium increase, while the production of endocannabinoids via muscarinic and group 1 mGluR receptors is not (Maejima et al., 2001; Kim et al., 2002; Chevaleyre & Castillo, 2003). Two possibilities can be proposed at this stage. One is that there are two or more alternative metabolic pathways leading to 2-AG (as suggested in the review by Freund et al., 2003). The first pathway would take a route involving PLC and DAG lipase, and would be responsible for at least part of the group 1 mGluR-and m1/m3-activated retrograde signaling; then, a distinct pathway would still lead to 2-AG synthesis and would be responsible also for DSI/DSE. A second possibility is that 2-AG is involved in the group 1 mGluR- and m1/m3-activated retrograde signaling, but that another endocannabinoid is responsible for DSI/DSE (anandamide or, else, other newly found molecules: Freund et al., 2003). In both schemes, a group 1 mGluR/muscarinic-triggered cascade should be able to exert a modulatory role on the production of the endocannabinoid mediating DSI, given the aforementioned facilitatory effect of these receptors on the phenomenon.
Interestingly, 2-AG and anandamide can be produced by the same system (cultured cells: Stella & Piomelli, 2001) in response to different sets of activated receptors. Moreover, in in vivo experiments in the striatum, dopamine D2 receptor stimulation leads to production of anandamide (but not of 2-AG; Giuffrida et al., 1999), whereas Schaeffer collateral stimulation produces 2-AG (but not anandamide) in hippocampal slices (Stella et al., 1997). So, the nature of the synthesized endocannabinoid is likely to depend on the stimulation pattern, on the specific receptor which is activated, and on the specific brain region considered.
Endocannabinoids and long-term depression (LTD)
It was recently discovered that endocannabinoids are responsible for the induction of long-term synaptic depression (LTD) of GABAergic (in the amygdala: Marsicano et al., 2002, and in the hippocampus: Chevaleyre & Castillo, 2003) and glutamatergic (in the striatum: Gerdeman et al., 2002, in the nucleus accumbens: Robbe et al., 2002 and in the cortex: Sjöström et al., 2003) transmission.
In two cases (Robbe et al., 2002; Chevaleyre & Castillo, 2003), the production of cannabinoids was clearly shown to follow the activation of postsynaptic group I mGlu receptors; in these areas it is tempting to speculate that 2-AG may be the endocannabinoid mediating this process.
Another situation applies in the striatum. Here, the requirement for group 1 mGluR activation during LTD induction has been known for some time (Calabresi et al., 1992; Sung et al., 2001), but the exogenous activation of group 1 mGluR receptors does not inhibit the incoming glutamatergic transmission (Sung et al., 2001), implying that the endocannabinoids required for LTD cannot be produced in this case by mere activation of group 1 mGluRs. Thus, a cannabinoid other than 2-AG may mediate LTD, and in particular anandamide could be a plausible candidate. This is suggested by the fact that, as already discussed, anandamide is selectively produced following dopaminergic D2 receptor activation in the striatum (Giuffrida et al., 1999) and that D2 receptors are indeed necessary for LTD induction (Calabresi et al., 1997).
An interesting difference between LTD phenomena and DSI/DSE is that the former forms of plasticity require either long (several minutes in the nucleus accumbens) or repetitive (hippocampal, striatal and amygdalar LTD) induction protocols. An activation of CBIRs in the order of minutes seems to be required for LTD induction: in fact, in the hippocampus the CB1R antagonist AM251 reduces LTD by half also if applied 5 min after the induction protocol (Chevaleyre & Castillo, 2003). This contrasts with the short single stimuli leading to DSI/DSE, where the cannabinoid signaling is presumably functional for only a few seconds, and where the final result is a fast-recovering modulation of the synaptic input.
Whether distinct endocannabinoids and/or different temporal patterns of CB1Rs activation also convey specific informations to the presynaptic terminals is still a matter for speculation. In addition, the exact nature of the endocannabinoids involved in synaptic plasticity is still unknown. Such identification will only be possible when the endogenous metabolic pathways of synthesis will be identified in situ and when specific blockers will be developed.
Conclusion
It is clear that research on endocannabinoids has a bright future waiting ahead. We are still at a very early stage, but there is every reason to believe that endocannabinoids play a fundamental role in the functioning of the mammalian brain. DSI/DSE is presumably only a small component of it. This component is nevertheless of primary importance, because it has suddenly put retrograde signaling in the limelight, and it has revealed a kind of neuronal signaling that is fundamentally different from that exerted by classical neurotransmitters. One of the greatest challenges at this stage is to Figure 1a establish the physiological role of DSI/DSE, but it can be hoped that, with research now starting to concentrate on this issue, a clear answer will be available soon.
Abbreviations
- 2-AG
2-Arachidonyl glycerol
- CB1Rs
type 1 cannabinoid receptors
- CCK
cholecystokinin
- DAG
diacylglycerol
- DSE
depolarization-induced suppression of excitation
- DSI
depolarization-induced suppression of inhibition
- LTD
long-term depression
- mGluR
metabotropic glutamate receptors
- PLC
phospholipase C
- eIPSCs
evoked inhibitory postsynaptic currents
- sIPSCs
spontaneous inhibitory postsynaptic currents
- mIPSCs
miniature inhibitory postsynaptic currents
References
- ALGER B.E. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- ALGER B.E., PITLER T.A., WAGNER J.J., MARTIN L.A., MORISHITA W., KIROV S.A., LENZ R.A. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J. Physiol. 1996;496:197–209. doi: 10.1113/jphysiol.1996.sp021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMERI A. The effects of cannabinoids on the brain. Prog. Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- BEAU F.E., ALGER B.E. Transient suppression of GABAa-receptor-mediated IPSPs after epileptiform burst discharges in CA1 pyramidal cells. J. Neurophysiol. 1998;79:659–669. doi: 10.1152/jn.1998.79.2.659. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., Di MARZO V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- BRENOWITZ S.D., REGEHR W.G. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J. Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN S.P., BRENOWITZ S.D., REGEHR W.G. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat. Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- CALABRESI P., MAJ R., PISANI A., MERCURI N.B., BERNARDI G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALABRESI P., SAIARDI A., PISANI A., BAIK J.H., CENTONZE D., MERCURI N.B., BERNARDI G., BORRELLI E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J. Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON G., WANG Y., ALGER B.E. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- CHEN K., RATZLIFF A., HILGENBERG L., GULYAS A., FREUND T.F., SMITH M., DINH T.P., PIOMELLI D., MACKIE K., SOLTESZ I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- CHERAMY A., LEVIEL V., GLOWINSKI J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- CHEVALEYRE V., CASTILLO P.E. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- DANIEL H., CREPEL F. Control of Ca(2+) influx by cannabinoid and metabotropic glutamate receptors in rat cerebellar cortex requires K(+) channels. J. Physiol. 2001;537:793–800. doi: 10.1111/j.1469-7793.2001.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., BISOGNO T., DE PETROCELLIS L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- DIANA M.A., MARTY A. Characterization of depolarization-induced suppression of inhibition using paired interneuron-Purkinje cell recordings. J. Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIANA M.A., LEVENES C., MACKIE K., MARTY A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J. Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE C.T., TERMAN G.W., SIMMONS M.L., MILNER T.A., KUNKEL D.D., SCHWARTZKROIN P.A., CHAVKIN C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIERRO L., LLANO I. High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J. Physiol. 1996;496:617–625. doi: 10.1113/jphysiol.1996.sp021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCH A.E., AUGUSTINE G. Local calcium signalling by inositol-1,4,5-triphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- FORTI L., POUZAT C., LLANO I. Action potential-evoked Ca2+ signals and calcium channels in axons of developing rat cerebellar interneurons. J. Physiol. 2000;527:33–48. doi: 10.1111/j.1469-7793.2000.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND T.F. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- FREUND T.F., KATONA I., PIOMELLI D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- GALARRETA M., HESTRIN S. Electrical synapses between GABA-releasing interneurons. Nat. Rev. Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- GERDEMAN G.L., RONESI J., LOVINGER D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- GIUFFRIDA A., PARSONS L.H., KERR T.M., RODRIGUEZ D.E., FONSECA F., NAVARRO M., PIOMELLI D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- GLITSCH M., LLANO I., MARTY A. Glutamate as a candidate retrograde messenger at interneurone-Purkinje cell synapses of rat cerebellum. J. Physiol. 1996;497:531–537. doi: 10.1113/jphysiol.1996.sp021786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLITSCH M., PARRA P., LLANO I. The retrograde inhibition of IPSCs in cerebellar Purkinje cells is highly sensitive to intracellular [Ca2+] Eur. J. Neurosci. 2000;12:987–993. doi: 10.1046/j.1460-9568.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- HAJOS N., KATONA I., NAIEM S.S., MACKIE K., LEDENT C., MODY I., FREUND T.F. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- HAJOS N., LEDENT C., FREUD T.F. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- HAMPSON R.E., ZHUANG S., WEINER J.L., DEADWYLER S.A. Functional significance of cannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the hippocampus. J. Neurophysiol. 2003;90:55–64. doi: 10.1152/jn.01161.2002. [DOI] [PubMed] [Google Scholar]
- HOFFMAN A.F., LUPICA C.R. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- ISOPE P., DIEUDONNE S., BARBOUR B. Temporal organization of activity in the cerebellar cortex: a manifesto for synchrony. Ann. NY Acad. Sci. 2002;978:164–174. doi: 10.1111/j.1749-6632.2002.tb07564.x. [DOI] [PubMed] [Google Scholar]
- JAFFE E.H., MARTY A., SCHULTE A., CHOW R.H. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J. Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATONA I., SPERLAGH B., SIK A., KAFALVI A., VIZI E.S., MACKIE K., FREUND T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM J., ISOKAWA M., LEDENT C., ALGER B.E. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J. Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMBIAN S.B., MOUGINOT D., PITTMANN Q.J. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- KREITZER A.C., REGEHR W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001a;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- KREITZER A.C., REGEHR W.G. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J. Neurosci. 2001b;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREITZER A.C., CARTER A.G., REGEHR W.G. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- LENZ R.A., WAGNER J.J., ALGER B.E. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CAI cells. J. Physiol. 1998;512:61–73. doi: 10.1111/j.1469-7793.1998.061bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENES C., DANIEL H., CREPEL F. Retrograde modulation of transmitter release by postsynaptic subtype 1 metabotropic glutamate receptors in the rat cerebellum. J. Physiol. 2001;537:125–140. doi: 10.1111/j.1469-7793.2001.0125k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENES C., DANIEL H., SOUBRIE P., CREPEL F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J. Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLANO I., LERESCHE N., MARTY A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- MAEJIMA T., HASHIMOTO K., YOSHIDA T., AIBA A., KANO M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- MARSICANO G., LUTZ B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- MARSICANO G., GOODENOUGH S., MONORY K., HERMANN H., EDER M., CANNICH A., AZAD S.C., CASCIO M.G., GUTIERREZ S.O., VAN DER STELT M., LOPEZ-RODRIGUEZ M.L., CASANOVA E., SCHUTZ G., ZIEGLGANSBERGER W., DI MARZO V, BEHL C., LUTZ B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- MARSICANO G., WOTJAK C.T., AZAD S.C., BISOGNO T., RAMMES G., CASCIO M.G., HERMANN H., TANG J., HOFMANN C., ZIEGLGANSBERGER W., DI MARZO V., LUTZ B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- MARTIN L.A., ALGER B.E. Muscarinic facilitation of the occurrence of depolarization-induced suppression of inhibition in rat hippocampus. Neurosciencce. 1999;92:61–71. doi: 10.1016/s0306-4522(98)00745-3. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHAATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWEE R.G., GRIFFIN G., BAYEWITCH M., BARG J., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MORISHITA W., ALGER B.E. Direct depolarization and antidromic action potentials transiently suppress dendritic IPSPs in hippocampal CA1 pyramidal cells. J. Neurophysiol. 2001;85:480–484. doi: 10.1152/jn.2001.85.1.480. [DOI] [PubMed] [Google Scholar]
- MORISHITA W., KIROV S.A., ALGER B.E. Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1. J. Neurosci. 1998;18:4870–4882. doi: 10.1523/JNEUROSCI.18-13-04870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA T., BARBARA J.-G., NAKAMURA K., ROSS W.N. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- NEALE S.A., GARTHWAITE J., BATCHELOR A.M. mGlu1 receptors mediate a post-tetanic depression at parallel fibre-Purkinje cell synapses in rat cerebellum. Eur. J. Neurosci. 2001;14:1313–1319. doi: 10.1046/j.0953-816x.2001.01769.x. [DOI] [PubMed] [Google Scholar]
- OHNO-SHOSAKU T., MAEJIMA T., KANO M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- OHNO-SHOSAKU T., MATSUI M., FUKUDOME Y., SHOSAKU J., TSUBOKAWA H., TAKETO M.M., MANABE T., KANO M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in hte hippocampus. Eur. J. Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- OHNO-SHOSAKU T., SAWADA S., YAMAMOTO C. Properties of depolarization-induced suppression of inhibitory transmission in cultured rat hippocampal neurons. Pflügers Arch. 1998;435:273–279. doi: 10.1007/s004240050512. [DOI] [PubMed] [Google Scholar]
- OHNO-SHOSAKU T., SHOSAKU J., TSUBOKAWA H., KANO M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur. J. Neurosci. 2002a;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- OHNO-SHOSAKU T., TSUBOKAWA H., MIZUSHIMA I., YONEDA N., ZIMMER A., KANO M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 2002b;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIOMELLI D., GIUFFRIDA A., CALIGNANO A., RODRIGUEZ DE FONSECA F. The endocannabinoid system as a target for therapeutic drugs. TiPS. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- PITLER T.A., ALGER B.E. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J. Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITLER T.A., ALGER B.E. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- ROBBE D., KOPF M., REMAURY A., BOCKAERT J., MANZONI O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATAKE S., SAITOW F., YAMADA J., KONISHI S. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat. Neurosci. 2000;3:551–558. doi: 10.1038/75718. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMANN M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- SHEPHERD G.M., GREER C.A.Olfactory bulb The Synaptic Organization of the Brain 2001NewYork: Oxford University Press; 159–203.ed. Shepherd, G.M. pp [Google Scholar]
- SHERMAN S.M., GUILLERY R.W. Exploring the Thalamus. San Diego: Academic Press; 2001. [Google Scholar]
- SJÖSTRÖM P.J., TURRIGIANO G.G., NELSON S.B. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- STELLA N., PIOMELLI D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur. J. Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- STELLA N., SCHWEITZER P., PIOMELLI D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUKAGAWA A., NAKANE S., SHINODA A., ITOH K., YAMASHITA A., WAKU K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- SUNG K.-W., CHOOI S., LOVINGER D.M. Activation of group 1 mGluRs is necessary for induction of long-term depression at striatal synapses. J. Neurophysiol. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K.A., LINDEN D.J. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J. Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- TAKECHI H., EILERS J., KONNERTH A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- TANK D.W., SUGIMORI M., CONNOR J.A., LLINAS R.R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242:773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- TRAUB R.D., JEFFERYS J.G.R., WHITTINGTON M.A. Fast Oscillations in Cortical Circuits. Boston: MIT Press; 1999. [Google Scholar]
- TRETTEL J., LEVINE E.S. Endocannabinoids mediate rapid retrograde signaling at interneuron → pyramidal neuron synapses of the neocortex. J. Neurophysiol. 2003;89:2334–2338. doi: 10.1152/jn.01037.2002. [DOI] [PubMed] [Google Scholar]
- TSUO K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- TSUO K., MACKIE K., SANUDO-PENA M.C., WALKER J.M. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- VARMA N., BRAGER D.H., MORISHITA W., LENZ R.A., LONDON B., ALGER B.E. Presynaptic factors in the regulation of DSI expression in hippocampus. Neuropharmacology. 2002;43:550–562. doi: 10.1016/s0028-3908(02)00168-5. [DOI] [PubMed] [Google Scholar]
- VARMA N., CARLSON G.C., LEDENT C., ALGER B.E. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J. Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT P., MARTY A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993;11:885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- VINCENT P., ARMSTRONG C.M., MARTY A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J. Physiol. 1992;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER J.J., ALGER B.E. Increased neuronal excitability during depolarization-induced suppression of inhibition in rat hippocampus. J. Physiol. 1996;495:107–112. doi: 10.1113/jphysiol.1996.sp021577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J., ZUCKER R.S. Photolysis-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J. Physiol. 2000;533:757–763. doi: 10.1111/j.1469-7793.2001.t01-1-00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S.-H., DENK W., HÄUSSER M. Coincidence detection in single dendritic spines mediated by calcium release. Nat. Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., KUNOS G., NICOLL R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- YANOVSKY Y., MADES S., MISGELD U. Retrograde signaling changes the venue of postsynaptic inhibition in rat substantia nigra. Neuroscience. 2003;122:317–328. doi: 10.1016/s0306-4522(03)00607-9. [DOI] [PubMed] [Google Scholar]
- YOSHIDA T., HASHIMOTO K., ZIMMER A., MAEJIMA T., ARAISHI K., KANO M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J. Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILBERTER Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J. Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILBERTER Y., KAISER K.M., SAKMANN B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]