Abstract
In phenylephrine (1 μM)-precontracted rat superior mesenteric arteries (MA), hydrogen peroxide (H2O2, 0.3 and 1 mM) caused a biphasic response: a transient contraction followed by a relaxation. In the presence of thromboxane A2/prostaglandin H2 (TP) receptor antagonist (SQ 29548), the contractile component of the biphasic response was abolished. The relaxation response to H2O2 was smaller in spontaneously hypertensive rats (SHR) when compared with normotensive Wistar–Kyoto rats (WKY).
The mechanisms for the attenuated relaxation to H2O2 in the SHR were studied. KCl (40 mM) prevented the relaxation response. Calcium-dependent K+ channel (KCa) blockers (tetraethylammonium chloride, TEA; iberiotoxin, and charybdotoxin) showed a greater inhibition of H2O2 relaxation in SHR than in WKY, whereas voltage-dependent K+-channel (Kv) blocker 4-aminopyridine was more effective in inhibiting the relaxation in WKY than in SHR.
H2O2 (1 mM) greatly enhanced the frequency and intensity of the spontaneous transient outward K+ currents in SHR MA, and the effects of H2O2 were inhibited by iberiotoxin, while in WKY MA the K+ currents induced by H2O2 were mainly of the Kv type. The consequence of the activation of different types of K+ channel was that the net increase in mean outward K+ current density in response to H2O2 was smaller in SHR than in WKY, which may account for the attenuated relaxation response to H2O2 in the SHR.
The contractile responses of MA to TEA, iberiotoxin, and charybdotoxin were greater in SHR than in WKY.
In summary, an attenuated relaxation response to H2O2 was found in SHR MA when compared to WKY. In contrast to the activation of Kv channels in WKY, H2O2 markedly enhanced KCa activity in SHR, resulting in an attenuation of the increase in mean outward K+ current density in response to H2O2. These results suggest that alteration in K+ channel activation by reactive oxygen species may play a role in the development of hypertension in SHR.
Keywords: Hydrogen peroxide, mesenteric artery, potassium channels, reactive oxygen species, relaxation, spontaneously hypertensive rats
Introduction
The role of reactive oxygen species (ROS) in hypertension is gaining increasing attention. Patients with uncontrolled hypertension showed a higher level of ROS (superoxide and hydrogen peroxide, H2O2), and effective control of high blood pressure also lowered the high level of ROS to normal level (Prabha et al., 1990). Even among the normotensives, persons with a family history of hypertension exhibited a higher plasma H2O2 level than those without such a family history (Lacy et al., 1998). In the spontaneously hypertensive rats (SHR), a widely used animal model of human essential hypertension, a higher level of ROS and lipid peroxidation in mesenteric arterioles and myocardium was reported (Ito et al., 1992; Suzuki et al., 1995). Polymorphonuclear leukocytes of SHR produced more superoxide than that of WKY (Ohmori et al., 2000). In other hypertensive animal models, a higher production of ROS was found in deoxycorticosterone acetate-salt hypertensive rats, Dahl hypertensive rats, and in angiotensin II- or renovascular ligation-induced hypertensive rats (Rajagopalan et al., 1996; Swei et al., 1997; Harrison et al., 1999; Somers et al., 2000). These results suggest that ROS may participate in the development or maintenance of hypertension.
Functional alterations in the artery of hypertensive subjects are characterized by an enhanced contractile response, or a blunted relaxation response, or both, to certain vasoactive agents. A combination of an augmented contraction and a weakened relaxation may contribute to an elevation of vascular tone, which in turn can result in an increase in peripheral arterial resistance and therefore high blood pressure. It is well known that ROS affect vascular function. Studies on the involvement of ROS in hypertension have focused on the contractile reactivity to ROS. SHR aorta showed a higher contractile response to exogenously applied H2O2 or superoxide or lipid peroxides (Auch-Schwelk et al., 1989; Rodriguez-Martinez et al., 1998; Hibino et al., 1999; Garcia-Cohen et al., 2000). In smaller vessels, we have previously found that H2O2 induces a greater contraction in SHR mesenteric artery (MA) than WKY (Gao & Lee, 2001).
Vasomotor effects of ROS are not limited to vasoconstriction, because some ROS can also relax precontracted arteries. Activation of K+ channels has been suggested as one of the mechanisms for the relaxation response. For example, H2O2 was found to induce a relaxation response in porcine coronary artery by opening calcium-dependent K+ channels (KCa) (Barlow & White, 1998), and in feline cerebral artery by activating ATP-dependent K+ channels (KATP) (Wei et al., 1996). In the MA from normotensive rats, we have found that relaxation response to H2O2 was through the activation of voltage-dependent K+ channels (Kv) (Gao et al., 2003). The relaxation response to H2O2 in the arteries of hypertensives such as SHR remains unknown.
In hypertension, a decreased K+ channel activity may contribute to an increase in vessel contractility (Martens & Gelband, 1998). In SHR, an impaired Kv activity was found in the aorta (Cox, 1996) and an impaired KCa and KATP activity was found in the MA (Ohya et al., 1996; Borges et al., 1999). Since ROS have emerged as a significant determinant of K+ channel activity (Liu & Gutterman, 2002) and production of ROS is higher in hypertension, we hypothesized that the vascular relaxation response to H2O2 is altered in SHR MA, and that this alteration is related to changes in K+ channel activity. In this study, we have used a combined pharmacological and electrophysiological approach to examine the relaxation response to H2O2 in SHR MA and the underlying mechanisms related to this response.
Methods
Animals
Male SHR and Wistar–Kyoto rats (WKY) 6–8 months old were obtained from the rat colonies maintained at the McMaster University Central Animal Facilities. These colonies originated from the Charles River strains, and we have maintained these inbred colonies at our institute for more than 20 years. The care and the use of these animals were in accordance with the guidelines of the Canadian Council on Animal Care.
Reactivity experiments
The detailed procedure for preparing mesenteric arterial rings and the components of Krebs solution have been described in our previous reports (Gao & Lee, 2001; Gao et al., 2003). Briefly, the rats were anaesthetized with sodium pentobarbitol (50 mg kg−1, i.p.) and then exanguinated by bleeding from the abdominal aorta. Following the dissection of the MA, the endothelium was mechanically removed by rubbing the internal surface of the ring with a fine wooden stick, and successful removal of endothelium was verified by the absence of relaxation response to 1 μM carbamycholine chloride. After 1 h of equilibration (resting tension 1.5 g), the MA ring (approximately 4 mm long) was precontracted with phenylephrine (PHE, 1 μM, to 70–80% of the maximal contraction) in the presence or absence of thromboxane A2/prostaglandin H2 (TP) receptor antagonist (SQ 29548, 30 μM, 25 min). H2O2 was added when the precontraction to PHE had reached a plateau. In the experiments without TP receptor antagonist, each ring was exposed to one concentration of H2O2; in the presence of TP receptor antagonist, each ring was exposed to one cumulative series (0.1, 0.3, and 1 mM) of H2O2. Catalase, superoxide dismutase, or dimethylsulphoxide (DMSO) was introduced for 5–10 min, whereas K+ channel blockers were added 20–25 min before exposure to H2O2. Maximal contraction and/or relaxation response to H2O2 was expressed as a percentage of the precontraction value by PHE. The inhibitory effect of K+ channel blockers was expressed as a percentage of the control. The contractile response to K+ channel blockers was expressed as a percentage of KCl contraction (which is not different between SHR and WKY, data not shown).
Patch clamp experiments
Membrane K+ currents were recorded at room temperature using the perforated whole cell patch clamp with conventional configuration as previously described (Gao et al., 2003). Access resistance ranged from 11 to 44 MΩ (70% compensated), and whole cell capacitance ranged from 8 to 33 pF. The electrode solution contained (in mM) 140 KCl, 1 MgCl2, 0.4 CaCl2, 20 HEPES, 1 EGTA and 0.3 mg ml−1 nystatin (pH 7.2). Membrane currents were filtered at 1 kHz and sampled at 2 kHz. Drug was applied using a micropuffer (Picospritzer™ II, General Valve Corp, Fairfield, NJ, U.S.A.). Currents were standardized according to the membrane area (using cell capacitance). There was no significant difference in cell capacitance between SHR and WKY (data not shown). The mean outward K+ current density was calculated using Axopatch 200B and pCLAMP8 software (Axon Instrument, Foster City, CA, U.S.A.) by integrating the currents over a fixed time interval (170–340 ms following onset of voltage pulse) and dividing by cell capacitance.
Chemicals
The following chemicals were used: H2O2 and DMSO (BDH Inc., Toronto, Canada); 4-AP, charybdotoxin, catalase, iberiotoxin, PHE, superoxide dismutase, tetraethylammonium chloride (TEA, Sigma, U.S.A.); SQ 29548 (RBI, Sigma, U.S.A.). SQ 29548 was dissolved in absolute ethanol and diluted in 50% ethanol; charybdotoxin and iberiotoxin were dissolved in oxygen-free water. All other agents were dissolved in deionized water and prepared freshly before use.
Statistical analysis
The results are expressed as mean±s.e.m., where n represents the number of rats. Statistical analysis was performed using one-way ANOVA and unpaired Student's t-test. The differences are considered significant when P<0.05.
Results
At 6–8 months of age, body weight of SHR (380±4 g n=28) was lighter than WKY (401±7 g, n=19; P<0.05). Systolic blood pressure measured with the tail-cuff compression method was significantly higher in the SHR (195±4 mmHg) than in WKY (130±3 mmHg; P<0.01).
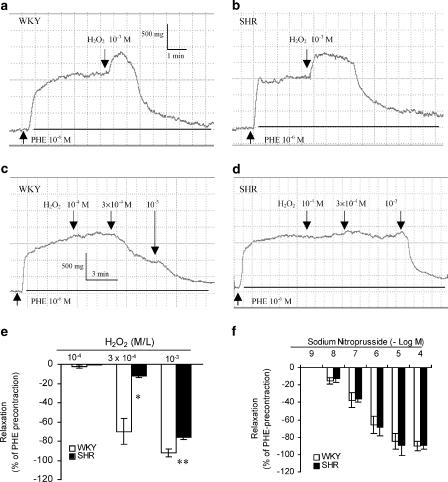
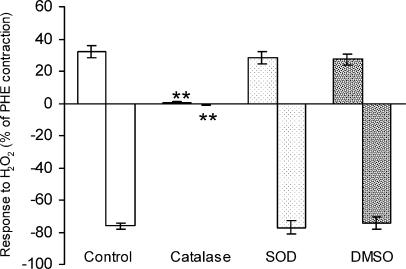
H2O2 (1 mM) induced a biphasic response in WKY and SHR MA: a transient contraction followed by a relaxation (which is endothelium independent) in PHE-precontracted tissues (Figure 1a,b). Removal of endothelium did not affect the contractile component to 1 mM H2O2 (e.g. maximal contractile response in SHR as % of PHE precontraction in the presence or absence of endothelium was 31.5±1.4 versus 32.1±3.8, n=6–7, P>0.05). In the presence of thromboxane A2/prostaglandin H2 (TP) receptor antagonist (SQ 29548, 30 μM), only the relaxation response was present because the contractile response was inhibited (Figure 1c,d). SQ 29548 did not affect PHE precontraction (data not shown) or the relaxation to H2O2 (maximal relaxation to 1 mM H2O2 in the absence or presence of SQ 29548 : 69.9±5.1% versus 76.1±1.9%, P>0.05). In endothelium-denuded MA of WKY and in the presence of SQ 29548, the relaxation response to H2O2 was concentration dependent, with a large relaxation response at 300 μM (Figure 1c). In SHR, 300 μM H2O2 only induced a small relaxation response and maximal relaxation to 1 mM H2O2 was also significantly smaller than that in WKY (Figure 1d,e). The relaxation response to sodium nitroprusside was similar between SHR and WKY (Figure 1f). We also examined the effects of free radical scavengers on H2O2-induced response in the SHR MA without TP receptor antagonist SQ 29548. Catalase (H2O2 scavenger, 1000 u ml−1) abolished the biphasic response to H2O2, while superoxide dismutase (superoxide scavenger, 150 u ml−1) and DMSO (hydroxyl radical scavenger, 5 mM) had no effects (Figure 2).
Figure 1.
Typical tracings showing the response to H2O2 in PHE (10–6 M)-precontracted mesenteric arteries from WKY and SHR. In the absence of the TP receptor antagonist SQ 29548, H2O2 induced a biphasic response: a transient contraction followed by a relaxation in WKY (a) and SHR (b). In the presence of SQ 29548, only the relaxation response was present (c, d). Mean concentration–response relationship for H2O2 (in the presence of SQ 29548) and for nitroprusside are given in (e) and (f), respectively (n=4–6 rats). Arteries were denuded of endothelium. *P<0.05, **P<0.01 compared with respective WKY.
Figure 2.
Effects of catalase (1000 U ml−1), superoxide dismutase (SOD, 150 U ml−1), and dimethylsulphoxide (DMSO, 5 mM), on the biphasic response to H2O2 (10–3 M) in PHE-precontracted MA of SHR (without TP receptor antagonist). Endothelium was removed in all the arteries. Contraction (upward bars) and the following relaxation response (downward bars) were expressed as a percentage of PHE-precontraction. Results (mean±s.e.) were from four to six rats. Catalase almost eliminated the H2O2 response, while SOD or DMSO had no effects. **P<0.01 compared with control.
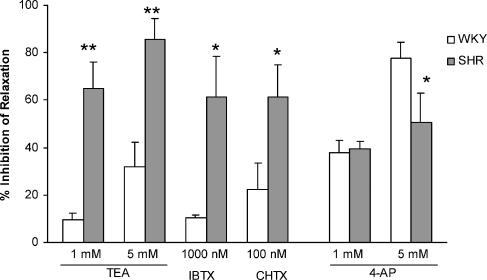
The mechanisms for the attenuated relaxation response to H2O2 were studied. The relaxation response to 0.3 mM H2O2 was abolished and that to 1 mM H2O2 was greatly inhibited (by 97.6±12%) in SHR by 40 mM KCl, indicating that activation of K+ channels may be responsible for the relaxation response. In the following experiments, we examined the inhibitory effects of K+ channel blockers on the relaxation response to 1 mM H2O2. KCa channel blockers (TEA, iberiotoxin, and charybdotoxin) showed a greater inhibition of the H2O2-induced relaxation in SHR than in WKY, while Kv channel blocker 4-AP exhibited a higher inhibition of relaxation in WKY than in SHR (Figure 3).
Figure 3.
Inhibitory effects of KCa blockers (tetraethylammonium chloride (TEA), iberiotoxin (IBTX), and charybdotoxin (CHTX)) and Kv blocker (4-AP) on H2O2-induced relaxation in the mesenteric arteries of SHR and WKY in the absence of SQ 29548. Results (mean±s.e.) are from five to seven rats. *P<0.05, **P<0.01 versus respective WKY.
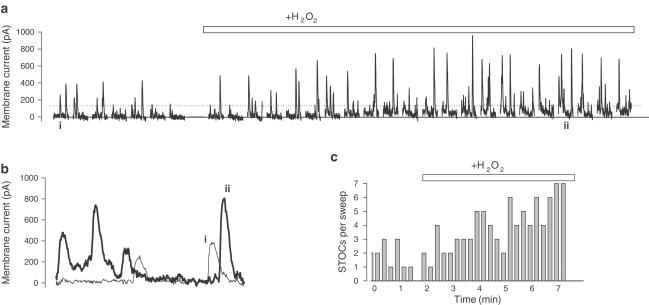
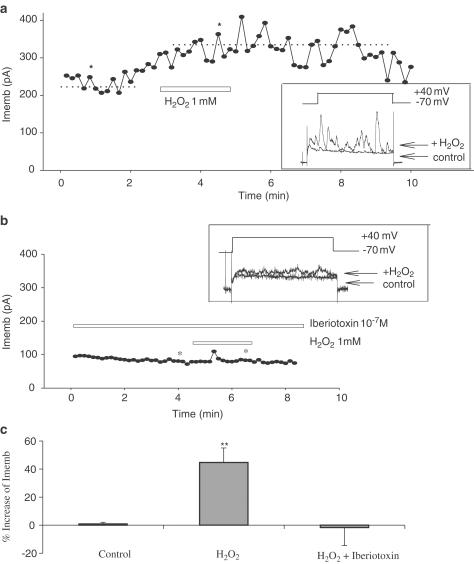
Whole cell patch clamp studies were carried out using single smooth muscle cells freshly isolated from the artery. In tissues from WKY, stepwise depolarizing pulses mainly induced Kv currents; H2O2 significantly enhanced these currents. In tissues from SHR, stepwise depolarization evoked multiple spontaneous transient outward K+ currents (STOCs) superimposed on the sustained Kv currents, and H2O2 markedly increased the frequency and intensity of the STOCs (Figure 4a–c). The consequence of the activation of different K+ channels by H2O2 was that the net increase in mean outward K+ current density was smaller in SHR than in WKY (in pA/pF; SHR: 11±3.5; WKY: 22.5±4.4; n=4 rats for each strain; P<0.05), which may account for the attenuated relaxation response to H2O2 in the SHR. The H2O2-induced increment of K+ currents in SHR was inhibited by the selective large conductance KCa channel blocker iberiotoxin (0.1 μM) (Figure 5a,b,c).
Figure 4.
H2O2 (1 mM) augmented STOCs in freshly isolated smooth muscle cells from SHR MA. Depolarizing pulses (to +40 mV, 1 s duration, from a holding potential of −70 mV), delivered at 15-s intervals, evoked voltage-dependent K+ currents upon which STOCs were superimposed. An average representation of the voltage-dependent K+ currents was obtained and subtracted digitally off-line from all the traces, leaving primarily the STOCs shown in panel a. Application of H2O2 (1 mM) markedly increased the amplitude and the frequency of the STOCs. Panel b shows representative current sweeps obtained before and during application of H2O2 (indicated in panel a). Panel c indicates the number of STOCs obtained per sweep (as determined by crossing of the dotted line in panel a).
Figure 5.
Effects of KCa channel blocker iberiotoxin on H2O2-induced membrane K+ currents (Imemb) in freshly isolated MA smooth muscle cells of SHR. (a) H2O2 markedly augmented K+ currents in control cells (dotted lines show the average before and during application of H2O2); (b) incubation with iberiotoxin abolished the effects of H2O2. Inserts in (a) and (b) show representative current sweeps obtained before and during the application of H2O2 from the sweeps indicated by asterisks; (c) percent increase of Imemb by H2O2 and the inhibitory effects of iberiotoxin (n=3–5 rats). **P<0.01 versus control.
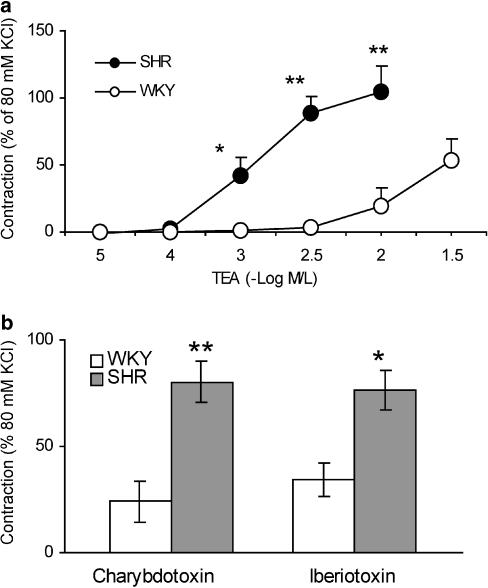
Concentration–contraction relation to TEA was constructed in arteries at resting tension. TEA (1 mM) caused a significant contraction in SHR but not in WKY arteries (Figure 6a). Although higher concentrations of TEA (⩾3 mM) contracted WKY MA, the amplitude of contraction was much lower than that of SHR. 4-AP induced a similar contraction in the two strains (data not shown). Contraction to a single concentration of iberiotoxin (10–6 M) and charybdotoxin (10–7 M) was also higher in SHR MA than in WKY (Figure 6b).
Figure 6.
Contractile responses evoked by KCa channel blockers in SHR MA at resting tension. (a) Concentration–contraction response curve for TEA; (b) contraction to iberiotoxin and to charybdotoxin in SHR and WKY mesenteric arteries. Results (mean±s.e.) were from four to 12 rats. *P<0.05, **P<0.01 compared with WKY.
Discussion
In this study, we found that SHR MA showed an enhanced contractile response and an attenuated relaxation response to H2O2 as compared with WKY. Since we have already reported the possible mechanism underlying the enhanced contractile response of SHR MA to H2O2 (Gao & Lee, 2001), we have concentrated on the study of the relaxation component of SHR MA response to H2O2 in this study. Thus, we found that K+ channel activation was responsible for the relaxation to H2O2 in both SHR and WKY, but the main subtype of K+ channels activated by H2O2 was KCa in SHR versus Kv in WKY. Furthermore, the overall increase of mean current density by H2O2 was significantly smaller in SHR tissues compared to WKY (making SHR tissues more excitable), which might account for the attenuated relaxation to H2O2 in SHR MA.
We have also established that the attenuated relaxation response to H2O2 in SHR MA was not due to the enhanced contractile response that preceded the relaxation response, because the relaxation response was still smaller when the contractile component was inhibited with TP receptor antagonist. The attenuated relaxation to H2O2 was also not due to an altered myogenic property of SHR MA, because sodium nitroprusside produced a similar relaxation response in MA from both SHR and WKY. Therefore, the attenuated relaxation response to H2O2 was specific to SHR MA. Furthermore, the response to H2O2 was induced by H2O2 itself because catalase abolished this response, while superoxide dismutase and DMSO did not.
The finding that KCl prevented H2O2-induced relaxation in SHR MA suggests that the K+ channels are involved. Our patch clamp study results clearly showed that the main type of K+ channels activated by H2O2 was different between SHR and WKY: KCa predominated in SHR in contrast to Kv in WKY. The consequence of the activation of different K+ channels between SHR and WKY was a significantly smaller increment in mean outward K+ current density in SHR than in WKY, which may account for the attenuated relaxation in SHR MA to H2O2. To our knowledge, this is the first report to show that H2O2-induced relaxation was attenuated in SHR MA when compared with WKY.
The contribution of KCa channels in SHR MA was also assessed pharmacologically using muscle organ baths. The contractions evoked by TEA, iberiotoxin, or charybdotoxin were significantly higher in SHR MA than in WKY, suggesting an increased functional activity of KCa channels in the SHR vessels. This is consistent with the report by Rusch et al. (1992) in SHR aorta and that by Asano and Nomura (2002) in SHR femoral, mesenteric, and carotid artery, and in SHR aortic smooth muscle cells (England et al., 1993). In a separate study, we have performed a Western blot analysis of the large conductance KCa channel protein in the MA from SHR and WKY, and found that KCa was increased in SHR (unpublished data), similar to a previous report using SHR aorta (Liu et al., 1997). Therefore, the increased KCa current may be a result of both an increased number and an enhanced activity of KCa channels in SHR MA.
In recent years, ROS have been recognized as a significant determinant of K+ channel function (Liu & Gutterman, 2002). H2O2 certainly activated different types of K+ channel in SHR and WKY, based on the results from this study. The mechanisms underlying this difference may involve increased number and activity of KCa channels, altered Ca2+ handling, and/or a high cytosolic Ca2+ concentration (Nomura et al., 1997; Asano & Nomura, 2002). ROS may also increase the sensitivity of endoplasmic reticulum Ca2+ stores to some signaling molecules such as inositol 1,4,5-triphosphate, which generates Ca2+ oscillations (Hu et al., 2002), and may thus affect KCa channel function.
Although plasma level of H2O2 is usually at the nanomolar level (Lacy et al., 1998), local vascular concentration of H2O2 may be much higher. According to Schraufstatter & Cochrane (1997), local concentration of superoxide (which can be rapidly converted to H2O2 by superoxide dismutase) can reach 5–10 mM when polymorphonuclear leukocytes are stimulated. Vascular tissues are also a rich source of ROS including H2O2. In SHR, polymorphonuclear leukocytes are more capable of producing superoxide than those from WKY rats (Ohmori et al., 2000), and vascular tissues including mesenteric arterioles have a higher level of ROS (Suzuki et al., 1995; Wu et al., 2002). Therefore, local H2O2 might reach a level that can initiate K+ channel activation in the vasculature.
At low concentration (10−6–10−4 M), H2O2 only contracts vessels. This contraction was higher in SHR than in WKY at this range. We hypothesized that this may be one of the contributing mechanisms for ROS in SHR hypertension. At higher concentrations of H2O2 (0.3–1 mM), a transient contraction followed by a prolonged relaxation response was seen in precontracted vessels. The relaxation to higher concentrations of H2O2 is probably a compensatory means to prevent the vessel from overcontraction by H2O2 when a large amount of H2O2 is generated locally. This vasodilation may also help to flush out local accumulation of H2O2 by increasing blood flow. However, the dilation response to H2O2 is less in SHR than in normotensive rat WKY. We therefore speculated that the attenuated relaxation response to high concentration of H2O2 represents an impaired compensatory defense mechanism against oxidative stress in SHR.
In summary, we have found that a blunted relaxation response to H2O2 mediated through KCa activation was present in SHR MA. This blunted relaxation response to H2O2 may represent another defect in the handling of oxidative stress in SHR vessels. An understanding of the mechanisms of the attenuated relaxation to ROS in SHR may be helpful in the development of new therapeutic initiatives, such as antioxidants or specific channel blockers, in the management of hypertension.
Acknowledgments
We thank Drs. C.Y. Kwan and A.K. Grover for their discussion; Ms. Nada Albatish and Angela Demeter and Mr. Wen-bo Zhang for their technical assistance. Dr. Yu-Jing Gao was a Research Fellow supported by the Joint Canadian Hypertension Society/Medical Research Council of Canada Special Initiative Program. This study was supported by the Heart and Stroke Foundation of Ontario, Canada.
Abbreviations
- 4-AP
4-aminopyridine
- DMSO
dimethyl sulphoxide
- H2O2
hydrogen peroxide
- KATP
ATP-dependent potassium channels
- KCa
calcium-dependent potassium channels
- Kv
voltage-dependent potassium channels
- MA
mesenteric artery
- PHE
phenylephrine
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- STOC
spontaneous transient outward currents
- TEA
tetraethylammonium chloride
- WKY
Wistar–Kyoto rats
References
- ASANO M., NOMURA Y. Contribution of sarcoplasmic reticulum Ca2+ to the activation of Ca2+-activated K+ channels in the resting state of arteries from spontaneously hypertensive rats. J. Hypertens. 2002;20:447–454. doi: 10.1097/00004872-200203000-00020. [DOI] [PubMed] [Google Scholar]
- AUCH-SCHWELK W., KATUSIC Z.S., VANHOUTTE P.M. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- BARLOW R.S., WHITE R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- BORGES A.C.R., FERES T., VIANNA L.M., PAIVA T.B. Recovery of impaired K+ channels in mesenteric arteries from spontaneously hypertensive rats by prolonged treatment with cholecalciferol. Br. J. Pharmacol. 1999;127:772–778. doi: 10.1038/sj.bjp.0702581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX R.H. Comparison of K+ channel properties in freshly isolated myocytes from thoracic aorta of WKY and SHR. Am. J. Hypertens. 1996;9:884–894. doi: 10.1016/s0895-7061(96)00179-3. [DOI] [PubMed] [Google Scholar]
- ENGLAND S.K., WOOLDRIDGE T.A., STEKIEL W.J., RUSCH N.J. Enhanced single-channel K+ current in arterial membrane from genetically hypertensive rats. Am. J. Physiol. 1993;264:H1337–H1345. doi: 10.1152/ajpheart.1993.264.5.H1337. [DOI] [PubMed] [Google Scholar]
- GAO Y.J., HIROTA S., ZHANG D., JANSSEN L.J., LEE R.M.K.W. Mechanisms of hydrogen peroxide-induced biphasic response in rat mesenteric artery. Br. J. Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO Y.J., LEE R.M.K.W. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br. J. Pharmacol. 2001;134:1639–1646. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-COHEN E.C., MARIN J., DIEZ-PICAZO L.D., BAENA A.B., SALAICES M., RODRIGUEZ-MARTINEZ M.A. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J. Pharmacol. Exp. Ther. 2000;293:75–81. [PubMed] [Google Scholar]
- HARRISON D., SCHIAVI P., FALGUI B. Renovascular hypertension, aortic superoxide production: effect of perindopril and indapamide. Am. J. Hypertens. 1999;12:50A. [Google Scholar]
- HIBINO M., OKUMURA K., IWAMA Y., MOKUNO S., OSANAI H., MATSUI H., TOKI Y., ITO T. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. J. Cardiovasc. Pharmacol. 1999;33:605–610. doi: 10.1097/00005344-199904000-00013. [DOI] [PubMed] [Google Scholar]
- HU Q., YU Z.X., FERRANS V.J., TAKEDA K., IRANI K., ZIEGELSTEIN R.C. Critical role of NADPH oxidase-derived reactive oxygen species in generation Ca2+ oscillation in human aortic endothelial cells stimulated by histamine. J. Biol. Chem. 2002;277:32546–32551. doi: 10.1074/jbc.M201550200. [DOI] [PubMed] [Google Scholar]
- ITO H., TORII M., SUZUKI T. A comparative study on defense systems for lipid peroxidation by free radicals in spontaneously hypertensive and normotensive rat myocardium. Comp. Biochem. Physiol. B. 1992;103:37–40. doi: 10.1016/0305-0491(92)90410-s. [DOI] [PubMed] [Google Scholar]
- LACY F., O'CONNOR D.T., SCHMID-SCHONBEIN G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- LIU Y., GUTTERMAN D.D. Oxidative stress and potassium function. Clin. Exp. Pharmacol. Physiol. 2002;29:305–311. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- LIU Y., PLEYTE K., KNAUS H.G., RUSCH N.J. Increased expression of Ca2+-sensitive channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- MARTENS J.R., GELBAND C.H. Ion channels in vascular smooth muscle: alterations in essential hypertension. Proc. Soc. Exp. Biol. Med. 1998;218:192–203. doi: 10.3181/00379727-218-44286. [DOI] [PubMed] [Google Scholar]
- NOMURA Y., ASANO M., ITO K., UYAMA Y., IMAIZUMI Y., WATANABE M. Potent vasoconstrictor action of cyclopiazonic acid and thapsigargin on femoral arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 1997;120:65–73. doi: 10.1038/sj.bjp.0700857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMORI M., KITOH Y., HARADA K., SUGIMOTO K., FUJIMURA A. Polymorphonuclear leukocytes (PMNs) functions in SHR, L-NAME- and DOCA/salt-induced hypertensive rats. J. Hypertens. 2000;18:703–707. doi: 10.1097/00004872-200018060-00007. [DOI] [PubMed] [Google Scholar]
- OHYA Y., SETOGUCHI M., FUJII K., NAGAO T., ABE I., FUJISHIMA M. Impaired action of levcromakalim on ATP-sensitive K+ channels in mesenteric artery cells from spontaneously hypertensive rats. Hypertension. 1996;27:1234–1239. doi: 10.1161/01.hyp.27.6.1234. [DOI] [PubMed] [Google Scholar]
- PRABHA P.S., DAS U.N., KORATKAR R., SAGAR P.S., RAMESH G. Free radical generation, lipid peroxidation and essential fatty acids in uncontrolled essential hypertension. Prostaglandins Leukot. Essent. Fatty Acids. 1990;41:27–33. doi: 10.1016/0952-3278(90)90127-7. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN S., KURZ S., MUNZEL T., TARPEY M., FREEMAN B.A., GRIENDLING K.K., HARRISON D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., GARCIA-COHEN E.C., BAENA A.B., GONZALEZ R., SALAICES M., MARIN J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br. J. Pharmacol. 1998;125:1329–1335. doi: 10.1038/sj.bjp.0702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSCH N.J., LUCENA R.G., WOOLDRIDGE T.A., ENGLAND S.K., COWLEY A.W., JR A Ca2+-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992;19:301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., COCHRANE C.G.Oxidants: types, sources, and mechanisms of injury The Lung Scientific Foundation 1997New York: Raven; 2251–2258.2nd edn. ed. Crystal R.G., West J.B., Weibel, E.R. & Barnes P.J [Google Scholar]
- SOMERS M.J., MAVROMATIS K., GALIS Z.S., HARRISON D.G. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., SWEI A., ZWEIFACH B.W., SCHMID-SCHONBEIN G.W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- SWEI A., LACY F., DELANO F.A., SCHMID-SCHONBEIN G.W. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- WU R., LAMONTAGNE D., DE CHAMPLAIN J. Antioxidative properties of acetylsalicylic acid on vascular tissues from normotensive and spontaneously hypertensive rats. Circulation. 2002;105:387–392. doi: 10.1161/hc0302.102609. [DOI] [PubMed] [Google Scholar]