Abstract
During mast cell degranulation, histamine is released in large quantities. Human eosinophils were found to express histamine H4 but not H3 receptors. The possible effects of histamine on eosinophils and the receptor mediating these effects were investigated in our studies.
Histamine (0.01–30 μM) induced a rapid and transient cell shape change in human eosinophils, but had no effects on neutrophils. The maximal shape change was at 0.3 μM histamine with EC50 at 19 nM. After 60 min incubation with 1 μM histamine, eosinophils were desensitized and were refractory to shape change response upon histamine restimulation. Histamine (0.01–1 μM) also enhanced the eosinophil shape change induced by other chemokines.
Histamine-induced eosinophil shape change was mediated by the H4 receptor. This effect was completely inhibited by H4 receptor-specific antagonist JNJ 7777120 (IC50 0.3 μM) and H3/H4 receptor antagonist thioperamide (IC50 1.4 μM), but not by selective H1, H2 or H3 receptor antagonists. H4 receptor agonists imetit (EC50 25 nM) and clobenpropit (EC50 72 nM) could mimic histamine effect in inducing eosinophil shape change.
Histamine (0.01–100 μM) induced upregulation of adhesion molecules CD11b/CD18 (Mac-1) and CD54 (ICAM-1) on eosinophils. This effect was mediated by the H4 receptor and could be blocked by H4 receptor antagonists JNJ 7777120 and thioperamide.
Histamine (0.01–10 μM) induced eosinophil chemotaxis with an EC50 of 83 nM. This effect was mediated by the H4 receptor and could be blocked by H4 receptor antagonists JNJ 7777120 (IC50 86 nM) and thioperamide (IC50 519 nM). Histamine (0.5 μM) also enhanced the eosinophil shape change induced by other chemokines.
In conclusion, we have demonstrated a new mechanism of eosinophil recruitment driven by mast cells via the release of histamine. Using specific histamine receptor ligands, we have provided a definitive proof that the H4 receptor mediates eosinophil chemotaxis, cell shape change and upregulation of adhesion molecules. The effect of H4 receptor antagonists in blocking eosinophil infiltration could be valuable for the treatment of allergic diseases. The histamine-induced shape change and upregulation of adhesion molecules on eosinophils can serve as biomarkers for clinical studies of H4 receptor antagonists.
Keywords: Histamine, histamine H4 receptor, eosinophils, cell shape change, chemotaxis, adhesion molecules
Introduction
Histamine is a biogenic amine playing an important role in the regulation of different physiological systems in the body. Histamine is synthesized from L-histidine by histidine decarboxylation in specific cell types, such as mast cells, basophils, enterochromaffin-like cells and neurons. The diverse biological effects of histamine are mediated through different histamine receptors, which are all G-protein-coupled receptors.
Four different histamine receptors, namely, the H1, H2, H3 and H4 receptors, have been identified (Hill et al., 1997 for a review). The H4 receptor is a new member of the histamine receptor family identified recently, and it has low homology with other histamine receptors. Its closest member in the histamine receptor family is the H3 receptor, which shares only a 35% amino-acid homology with the H4 receptor. Pharmacological properties of the H4 receptor have been studied using H4 receptor-transfected cells (Oda et al., 2000; Liu et al., 2001a, 2001b; Morse et al., 2001; Nguyen et al., 2001; Zhu et al., 2001). H1- and H2 receptor-specific ligands do not bind to the H4 receptor. In contrast, H3 receptor agonists and antagonists, such as clobenpropit, imetit, R-α-methylhistamine and thioperamide, show various degrees of crossreactivity with the H4 receptor. Recently, antagonists specific for the H3 or H4 receptor have been generated and they are valuable tools for dissecting the biological roles of H3 and H4 receptors (Shah et al., 2002; Jablonowski et al., 2003; 2004; Fung-Leung et al., 2004).
The four histamine receptors are distinct in their expression profiles and they mediate different biological effects. The H1 receptor mediates symptoms of allergic reactions, including smooth muscle contractions, vasodilation and sensory nerve activation, the H2 receptor enhances gastric acid secretion in the stomach, and the H3 receptor regulates the release of histamine and neurotransmitters by neurons (Hill et al., 1997). Expression of H4 receptors is restricted to cells of haematopoietic lineage, in particular, mast cells, basophils and eosinophils (Oda et al., 2000; Liu et al., 2001a; Morse et al., 2001; Zhu et al., 2001). However, the physiological role of H4 receptor is still unclear. We recently reported that the H4 receptor mediates mast cell chemotaxis (Hofstra et al., 2003). The chemotactic effects of histamine on eosinophils have also been suggested in early studies (Clark et al., 1975). Recently, eosinophil chemotaxis toward histamine was found to be blocked by thioperamide, and was therefore suggested to be mediated by the H4 receptor (O'Reilly et al., 2002). However, a direct proof of the H4 receptor involvement and a detailed analysis of the mechanisms of H4 receptor-induced cellular responses in eosinophils have not been pursued.
Eosinophils are bone marrow-derived granulocytic leukocytes, which normally reside in tissues, especially in the respiratory and intestinal systems and in the uterus. Eosinophil numbers in the blood stream are relatively low, and the control of eosinophil migration toward tissues has been attributed to adhesion molecules and chemokines (Lukacs, 2001; Tachimoto et al., 2002). Eosinophils are important effector cells in the late phase allergic response, and they have been implicated in the pathogenesis of allergic diseases (Bousquet et al., 1990). Activation of eosinophils results in the release of toxic granule proteins that are thought to cause airway epithelial damage and the development of bronchial hyper-reactivity in asthma.
In this report, we show that eosinophils express histamine H4, but not H3 receptors. Since mast cells are the major producers of histamine and both mast cells and eosinophils are known effector cells in allergic reactions, the possible role of mast cells in the recruitment of eosinophils via histamine was investigated. We show that eosinophils respond to histamine in changing cell shape, upregulation of adhesion molecules and chemotaxis. Using H4 receptor-specific antagonists, we show that all these responses are mediated by the H4 receptor expressed on eosinophils.
Methods
Materials
Human cell lines HMC-1, HL60.15 and primary HUVEC cells were purchased from American Type Culture Collection (Rockville, MD, U.S.A.). RNeasy kit was from Qiagen (Valencia, CA, U.S.A.). RT reaction kits and ExpressHyb solution were from Invitrogen (Carlsbad, CA, U.S.A.). The H3 receptor-specific antagonist JNJ 6379490, 7-methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-imidazo[1,2-a]pyridine, as well as the H4 receptor-specific antagonist JNJ 7777120, 1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine, were in-house synthesized and their histamine receptor specificities were described previously (Shah et al., 2002; Jablonowski et al., 2003). All chemokines and cytokines were purchased from R&D systems (Minneapolis, MN, U.S.A.). All antibodies were purchased from BD PharMingen (San Diego, CA, U.S.A.). All other reagents were purchased from Sigma (St Louis, MO, U.S.A.).
Purification of different human hematopoietic cell types
Eosinophils and neutrophils were purified from blood samples collected from healthy volunteers. Briefly, platelet-rich plasma was removed by centrifugation of heparinized whole blood. Polymorphonuclear leukocytes (PMNL), which are enriched with neutrophils and eosinophils, were separated from PBMC by centrifugation at 2000 r.p.m. for 20 min over a discontinuous plasma-Percoll gradient (density 1.082 g ml−1). Red blood cells in PMNL were removed by hypotonic shock lysis. PMNL were stained with hematoxylin and eosin (H&E) and a differential cell count was performed. Eosinophil counts ranged from 2 to 10% of the total PMNL number. Human neutrophils were purified from PMNL by positive selection using anti-CD16-conjugated microbeads in a magnetic cell separation system (AutoMACS from Miltenyi Biotec, Auburn, CA, U.S.A.). PMNL were incubated with anti-CD16-conjugated microbeads in phosphate-buffered saline (PBS) containing 0.5% BSA and 2 mM EDTA, which selectively bind to neutrophils. Neutrophils were purified by passage of the cell suspensions through a magnetic field in the AutoMACS system. Eosinophils were purified from the PMNL by negative selection. PMNL were incubated with a cocktail of anti-CD16-, anti-CD3-, anti-CD19- and anti-CD14-conjugated microbeads in PBS containing 0.5% BSA and 2 mM EDTA, which selectively bind to neutrophils, T cells, B cells and monocytes, respectively, in the PMNL suspension. Eosinophils were purified by removing cells bound to microbeads in the AutoMACS system, resulting in eosinophil populations of >97.5% purity according to H&E stain. Purified eosinophils were washed once in buffer (PBS containing 10 mM Ca2+ and Mg2+, 10 mM HEPES, 10 mM glucose and 0.1% BSA, pH 7.2–7.4) and used immediately for experiments.
Human CD4+ T cells were purified from PBMC. Human PBMC were separated from PMNL over a discontinuous plasma-Percoll gradient (density 1.082 g ml−1). CD4+ T cells in PBMC were purified by positive selection using anti-CD4-conjugated microbeads in AutoMACS system. CD4+ T cells were stimulated for 7 days with immobilized anti-CD3 (culture plates were coated with 5 μg ml−1 of anti-CD3 in PBS overnight and then rinsed twice with PBS before use) and 2 μg ml−1 soluble anti-CD28 in the presence of cytokines or antibodies for T cell differentiation. For type I helper T-cell differentiation, 10 ng ml−1 human IL-12 and 10 μg ml−1 anti-IL-4 were added in culture medium. For type II helper T cell differentiation, 10 ng ml−1 human IL-4 and 10 μg ml−1 of anti-IL-12 and anti-IFN-γ were added in culture medium. Human IL-2 was added in cultures at 20 U ml−1 on day 4 of T cell stimulation. T cells on day 7 were collected for RNA preparation. Type I or type II helper T cells were characterized by their production of IFN-γ or IL-4, respectively. To confirm the effector cell types after the 7-day differentiation, aliquots of T cells were restimulated overnight with immobilizd anti-CD3 and culture supernatants were tested for IL-4 or IFN-γ by ELISA.
CD8+ T cells in PBMC were purified by positive selection using anti-CD8-conjugated microbeads in AutoMACS system. Purified CD8+ T cells were stimulated overnight with immobilized anti-CD3 and anti-CD28 in RPMI 1640 medium. Cells after overnight activation were harvested for RNA preparation.
Monocytes in PBMC were purified by positive selection using anti-CD14-conjugated microbeads in AutoMACS system. Dendritic cells were generated from blood monocytes by culturing purified monocytes for 10 days in the presence of 500 U ml−1 IL-4 and 800 U ml−1 GM-CSF to reach the immature dendritic cell phenotype. Immature dendritic cells were then treated with 100 U ml−1 TNF-α for 24 h to drive the cells to the mature dendritic cells phenotype. Mature dendritic cells were used for RNA preparation.
Differentiation of human eosinophilic cell line
Human HL60.15 cell line was cultured in RPMI 1640 medium containing 10% FCS and differentiated into eosinophils by treating cells with 0.5 μM butyric acid and 10 ng ml−1 IL-5 for 2 days.
Detection of H4 and H3 receptor RNA expression
Total RNA was extracted from purified human cells using the RNeasy kit (Qiagen) and reverse transcribed to cDNA using the RT reaction kit (Invitrogen). H4 receptor RNA was detected by RT–PCR using human H4 receptor-specific primers 5′-ATGCCAGATACTAATAGCACA and 5′-CAGTCGGTCAGTATCTTCT. The amplified PCR band for H4 receptor is 1170 bp. H3 receptor RNA was detected by using human H3 receptor-specific primers 5′-ATGGAGCGCGCGCCGCCCGACGGG and 5′-ATGAAGAAGAAAACATGTCTG. The amplified PCR band for H3 receptor is 1120 bp.
Measurement of eosinophil shape change using flow cytometry
Human PMNL samples were used to study the eosinophil shape change response. PMNL were prepared as described above and cells were resuspended in assay buffer (PBS containing 10 mM Ca2+ and Mg2+, 10 mM HEPES, 10 mM glucose and 0.1% BSA, pH 7.2–7.4). Aliquots of cells (5 × 105 PMNL in 80 μl assay buffer) were pretreated with histamine receptor analogues (diphenhydramine, ranitidine, thioperamide, JNJ 7777120 or H3 receptor antagonist) for 10 min at room temperature before the addition of histamine or chemokines in 1.2-ml polypropylene cluster tubes (Costar, Cambridge, MA, U.S.A.) in a final volume of 100 μl. The tubes were placed in a 37°C water bath for 10 min (or as indicated), after which they were transferred to an ice-water bath, and 250 μl of ice-cold fixative (2% paraformaldehyde in PBS) was added to terminate the reaction and to maintain the cell shape change. The cell shape change was analyzed with the flow cytometer (Becton Dickinson, Mountain View, CA, U.S.A.). Eosinophils in PMNL were gated based on their high autoflourescence relative to that of neutrophils. Cell shape change was monitored in forward scatter signals. To identify eosinophils and neutrophils in PMNL, cells were stained on ice for 30 min with saturating concentrations of FITC-conjugated anti-CCR3 or anti-CD16 antibodies, which are specific for eosinophils or neutrophils, respectively. Samples after antibody staining were analyzed in flow cytometry.
Detection of cell surface expression of adhesion molecules
Purified eosinophils were used to study cell surface expression of adhesion molecules. Eosinophils were resuspended in PBS containing 10 mM Ca2+ and Mg2+, 10 mM HEPES, 10 mM glucose and 0.1% BSA, pH 7.2–7.4. Aliquots of cells (5 × 105 PMNL) were pretreated with histamine receptor analogues (as described above) for 10 min before the addition of histamine or chemokines in 1.2-ml polypropylene cluster tubes (Costar, Cambridge, MA, U.S.A.) in a final volume of 100 μl. The tubes were placed in a 37°C water bath for 10 min (or as indicated), after which they were transferred to an ice-water bath, and 250 μl of ice-cold fixative (0.5% paraformaldehyde in PBS) was added. Samples were then incubated on ice for 30 min with saturating concentration of either FITC- or phycoerytherine-conjugated anti-CD11a, anti-CD11b or anti-CD54 antibodies, washed and then analyzed by flow cytometry.
In vitro chemotaxis assay
Transwells (Costar, Cambridge, MA, U.S.A.) with 5 μm pore size were coated with 100 μl of 100 ng ml−1 human fibronectin (Sigma) for 2 h at room temperature. After removal of excess fibronectin, 600 μl of RPMI-1640 medium containing 0.5% BSA and different concentrations of histamine (0.01–100 μM) was added to the bottom chamber. Eosinophils (2 × 105 well−1) were added to the top chamber. Histamine receptor analogues (diphenhydramine, ranitidine, thioperamide, JNJ 7777120 or JNJ 6379490) were added to both the top and bottom chambers to a final concentration of 10 μM or at other concentration as stated in figure legends. The plates were incubated for 2 h at 37°C, and the number of cells migrated to the bottom chamber was counted for 1 min using flow cytometery.
Statistics
Experimental data are presented as mean±standard deviation (s.d.) from the number (n) of independent samples. The IC50 or EC50 values were calculated from the concentration–effect curves by nonlinear regression analysis using GraphPad Prism (GraphPad Software Inc., Philadelphia, U.S.A.). Statistical significance (P-value) was determined by the Student's t-test.
Results
Eosinophils express H4 receptors but not H3 receptors
Expression of H4 receptors in different purified human hematopoietic cell types and cell lines were studied. Significant levels of H4 receptor mRNA were detected in eosinophils and dendritic cells by RT–PCR (Figure 1a). In contrast to the H4 receptor expression in eosinophils, H3 receptors were not detected in these cells (Figure 1b). Minute expression of H4 receptor was found in CD4+ Th1 and Th2 effector cells, but was not detected in neutrophils, monocytes and activated CD8+ T cells (Figure 1a). H4 receptor expression was also detected in eosinophilic precursor cell line HL60.15, and its expression was significantly increased when cells were induced by IL-5 to differentiate into eosinophils. The human HMC-1 mast cell line expressed a detectable level of H4 receptors.
Figure 1.
H4 receptor expression is restricted to eosinophils and dendritic cells. (a) RT–PCR detection of H4 receptor mRNA in different purified cell types and cell lines. Total RNA from different cell types were reversed transcribed and used as templates for PCR. (b) Human eosinophils express H4 but not H3 receptors. H3 or H4 receptor mRNA in human eosinophils was detected by RT–PCR using specific primers. For both (a) and (b), 25 PCR cycles were performed for the amplification of the H4 receptor. Human SK-N-MC cells transfected with the H1, H2, H3 or H4 receptor were used as controls for specificity of histamine receptor detection. G3PDH mRNA in RNA samples was amplified with specific primers as controls in PCR reactions.
H4 receptors mediate eosinophil shape change
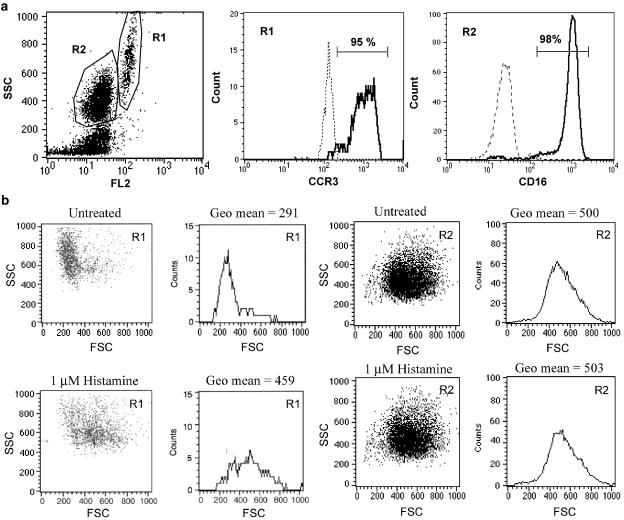
The analysis of eosinophil cell shape change by flow cytometry, known as gated autofluorescence forward scatter (GAFS) assay, allows a quantitative measurement of cell shape change induced by chemoattractants (Sabroe et al., 1999). The possible effects of histamine on the cell shape of human eosinophils were studied by flow cytometry. PMNL enriched with neutrophils and eosinophils were prepared from human blood samples, and the response of these cells to histamine was studied. Eosinophils had high levels of autoflourescence and could be distinguished from neutrophils by flow cytometry (Figure 2a). As shown in Figure 2a, the cell population in PMNL with high levels of autoflourescence was highly enriched with CCR3+ eosinophils, whereas the population with low autoflourescence was composed mainly of CD16+ neutrophils. Histamine at 1 μM induced a significant cell shape change on eosinophils, but had no effects on neutrophils (Figure 2b).
Figure 2.
Histamine induces eosinophil shape change. (a) Eosinophils were distinguished from neutrophils in human PMNL by gating on cells with high levels of autoflourescence in flow cytometry analysis. The majority of the cell population with high autoflourescence, gated as R1 group, was CCR3+ eosinophils. The cell population with low autoflourescence, gated as R2 group, was CD16+ neutrophils. Histograms shown in solid lines are antibody-stained samples whereas those in broken lines are unstained controls. (b) Histamine induced cell shape change on eosinophils but not on neutrophils. Human PMNL were treated with 1 μM histamine for 10 min and the change in cell shape was monitored by flow cytometry. Human eosinophils or neutrophils were gated in flow cytometry analysis based on their difference in autoflourescence. The cell size in histamine-treated samples was compared to that of the untreated control samples. The means of cell size in forward scattered signal (FSC) are shown.
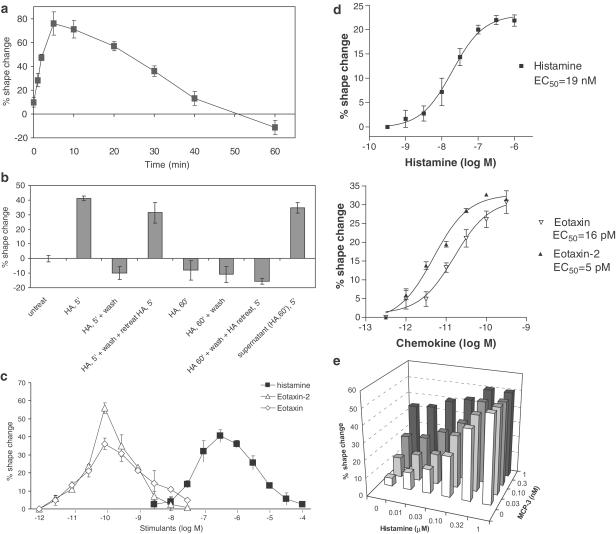
Histamine induced a rapid and transient cell shape change on eosinophils that could be detected by flow cytometry as early as 1 min after histamine treatment, with a maximal change at 5 min, and a gradual return to the original cell shape after 40 min (Figure 3a). The disappearance of histamine effects overtime was not due to the loss of histamine activity in the cell supernatants. These cell supernatants could still trigger a normal cell shape change on freshly prepared eosinophils (Figure 3b). The eosinophil shape change was not maintained when histamine was removed. As shown in Figure 3b, eosinophils treated with histamine for 5 min followed by washing did not retain any of the cell shape change. However, these cells were still fully capable of remounting shape change response when retreated with histamine (Figure 3b). In contrast, eosinophils after 60-min incubation with histamine not only returned to original cell shape but also lost their response to histamine restimulation. These cells did not respond to histamine anymore even when they were washed free of histamine before restimulation (Figure 3b).
Figure 3.
Kinetics and potency of histamine in triggering eosinophil shape change. (a) Kinetics of histamine induced eosinophil shape change. Human PMNL were treated with 1 μM histamine and the change in eosinophil cell shape at different time points was studied by flow cytometry. The percentage of cell shape change was calculated based on the increase in FSC from those of untreated samples. Data are mean±s.d., n=3. (b) The shape change response of human eosinophils to histamine under different conditions was studied. Data are mean±s.d., n=3. (c) Titration of histamine effects on human eosinophil shape change and its comparison with chemokines. Human PMNL were treated with different concentrations of histamine or chemokines (eotaxin-2 or eotaxin) for 10 min. Eosinophil shape change was monitored by flow cytometry. Data are mean±s.d., n=3. (d) Determination of EC50 values of histamine and chemokines on eosinophil shape change. Human PMNL were treated with different concentrations of histamine or chemokines (eotaxin and eotaxin-2). Eosinophil shape change was monitored by flow cytometry. Data shown are a representative of six repeated experiments and each data point is mean±s.d., n=3. EC50 values were calculated with the GraphPad Prism program. (e) Histamine enhances eosinophil shape change when combined with chemokines MCP-3. Human PMNL were treated with histamine in combination with chemokines MCP-3 for 10 min. Eosinophil shape change was monitored by flow cytometry. Data shown are a representative of four repeated experiments.
Eosinophil shape change was induced by histamine in a concentration-dependent manner (Figure 3c). The optimal concentration of histamine for maximal shape change on eosinophils was 0.3 μM. This shape change became less obvious when histamine concentration was higher than 0.3 μM, and no shape change was found at 100 μM histamine. The EC50 of histamine on eosinophil shape change was 19 nM, whereas the EC50 of chemokines eotaxin and eotaxin-2 were 16 and 5 pM, respectively (Figure 3d).
Possible synergistic effects between histamine and chemokines in mediating eosinophil shape change were studied. A titration of both histamine and chemokine MCP-3 and their combined effects on eosinophil shape change were monitored (Figure 3e). Partial additive effects were shown when either histamine or MCP-3 was at suboptimal concentration range. This effect was histamine or MCP-3 concentration dependent, and no further enhancement was found when they reached their maximal effective doses. Similar additive effects were also observed when histamine was combined with eotaxin-2 in eosinophil shape change studies (data not shown).
Specific histamine receptor ligands were used to determine the histamine receptor responsible for eosinophil shape change. The H4 receptor-specific antagonist JNJ 7777120 and the H3/H4 receptor antagonist thioperamide at 10 μM blocked the histamine-induced eosinophil shape change completely (Figure 4a). In contrast, the H3 receptor antagonist JNJ 6379490, the H1 receptor antagonist diphenhydramine and the H2 receptor antagonist ranitidine did not show any inhibitory effects (Figure 4a). The IC50 of JNJ 7777120 and thioperamide in blocking 1 μM histamine-induced eosinophil shape change was 0.3 μM and 1.4 μM, respectively, whereas the H3 receptor-specific antagonist was ineffective up to 30 μM (Figure 4b). The H3/H4 receptor agonist imetit and the H4 receptor-specific agonist clobenpropit could mimic histamine effect in triggering a shape change in eosinophils with EC50 of 25 and 72 nM, respectively (Figure 4c). Thus, histamine-induced eosinophil shape change appeared to be mediated by the H4 receptor.
Figure 4.
Histamine-induced eosinophil shape change is mediated by the H4 receptor. (a) Histamine induced eosinophil shape change was blocked by the H4 receptor antagonist JNJ 7777120, the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Human PMNL were pretreated with 10 μM of different histamine receptor antagonists, followed by 10-min treatment with 1 μM histamine. Eosinophil shape change was monitored by flow cytometry. The percentage of cell shape change was calculated based on the increase in FSC from those of untreated samples. Data shown are a representative of three repeated experiments. Data are mean±s.d. and n=3. Statistical significance (P-value) was determined by the Student's t-test. (b) Determination of IC50 values of JNJ 7777120, thioperamide and H3 receptor antagonist on histamine-induced eosinophil shape change. Human PMNL were pretreated with different antagonists for 10 min before inducing cell shape change with 1 μM histamine. Eosinophil shape change was monitored by flow cytometry. The percentage of inhibition was calculated based on the decrease in shape change compared to samples treated with1 μM histamine only. Data shown are a representative of four repeated experiments and each data point is a mean±s.d. and n=3. IC50 values were calculated with the GraphPad Prism program. (c) Concentration-dependent effects of histamine, H3/H4 receptor agonist imetit and H4 receptor agonist clobenpropit on human eosinophil shape change. Data are mean±s.d. and n=3. EC50 values were calculated with the GraphPad Prism program.
Histamine upregulates cell surface adhesion molecules through H4 receptors
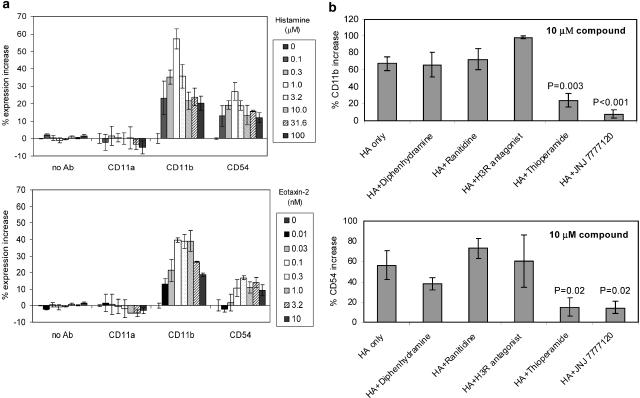
The effect of histamine on adhesion molecule expression on eosinophil cell surface was studied in flow cytometry using specific antibodies. Cell surface expression of CD11b/CD18 (Mac-1) and CD54 (ICAM-1) on eosinophils was induced by histamine in a concentration-dependent manner (Figure 5a). The increase in cell surface expression of adhesion molecules could be detected at 10 min of histamine treatment. The optimal histamine concentration for maximal upregulation of CD11b/CD18 and CD54 was 1 μM. Interestingly, the expression of CD11a/CD18 (LFA-1), another member of the β2-intergrin as CD11b/CD18, was not induced by histamine (Figure 5a).
Figure 5.
Histamine-induced adhesion molecule expression on eosinophils is mediated by the H4 receptor. (a) Cell surface expression of adhesion molecules CD11b/CD18 and CD54 on eosinophils was upregulated by histamine. Human PMNL were treated with different concentrations of histamine or chemokine eotaxin-2 for 10 min at 37°C. Cell samples were fixed with paraformaldehyde and stained with FITC-conjugated antibodies specific for CD11b, CD11a or CD54. Expression of adhesion molecules on eosinophils was monitored by flow cytometry. The percentage of upregulation was calculated based on the increase in expression levels from those of untreated samples. Data are mean±s.d. and n=3. (b) Histamine-induced adhesion molecule upregulation on eosinophils was blocked by the H4 receptor antagonist JNJ 7777120 and the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Human PMNL were pretreated with 10 μM of different histamine receptor antagonists for 10 min, followed by 10-min treatment with 1 μM histamine at 37°C. Cell samples were fixed with paraformaldehyde and stained with FITC-conjugated antibodies specific for CD11b or CD54. Data are mean±s.d. and n=3. Statistical significance (P-value) was determined by the Student's t-test.
The histamine receptor responsible for adhesion molecule upregulation on eosinophils was investigated using different specific histamine receptor antagonists. As shown in Figure 5b, 10 μM of either JNJ 7777120 or thioperamide abolished the upregulation of CD11b/CD18 and CD54 expression. In contrast, diphenhydramine, ranitidine and the H3 receptor antagonist JNJ 6379490 were all ineffective in blocking the upregulation of adhesion molecules on eosinophils.
Histamine mediates eosinophil chemotaxis through H4 receptors
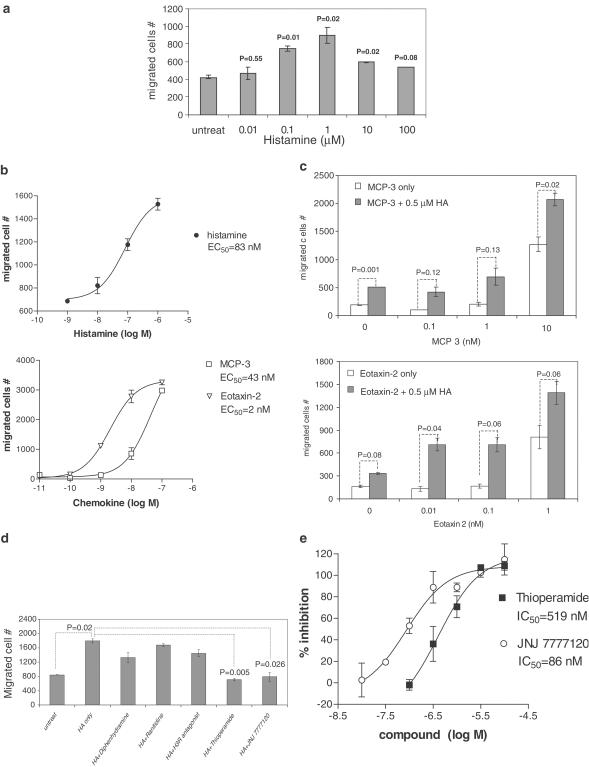
The chemotactic effects of histamine on eosinophils were investigated using purified human blood eosinophils. In vitro chemotaxis studies were performed in a Transwell system. Histamine induced eosinophil migration in a concentration-dependent manner with an EC50 of 83 nM (Figure 6a and b). A maximal chemotatic effect on eosinophils was achieved at 1 μM histamine (Figure 6a). Chemokines eotaxin-2 and MCP-3 were used in same assays for comparison. The EC50 of chemokines eotaxin-2 and MCP-3 on eosinophil chemotaxis was 2 and 43 nM, respectively (Figure 6b). The histamine effect on eosinophils was chemotactic, not chemokinetic, since disruption of the histamine concentration gradient abolished eosinophil migration completely (data not shown).
Figure 6.
Histamine-induced human eosinophil chemotaxis is mediated by the H4 receptor. (a) Titration of histamine effects on human eosinophil chemotaxis. Chemotaxis of purified human eosinophils toward different concentration of histamine was studied in a Transwell system. Human eosinophils were placed in the transwell and histamine was added in the lower chamber. Eosinophils migrated into the lower chambers after 2 h incubation were counted for 1 min by flow cytometry. Data shown are a representative of three repeated experiments. Data are mean±s.d. and n=3. Statistical significance (P-value) was determined by the Student's t-test. (b) Determination of EC50 values of histamine and chemokines on eosinophil chemotaxis. Human eosinophil chemotaxis was studied with a titration of histamine or chemokines eotaxin-2 or MCP-3. Data shown are a representative of two repeated experiments. Data are mean±s.d. and n=3. EC50 values were calculated with the GraphPad Prizm program. (c) Histamine enhanced chemokine-induced eosinophil chemotaxis. The effects of histamine (0.5 μM) on eosinophil chemotaxis induced by different concentrations of chemokine eotaxin-2 or MCP-3 were studied. Data shown are a representative from three repeated experiments. Data are mean±s.d. and n=3. Statistical significance (P-value) was determined by the Student's t-test. (d) Histamine-induced eosinophil chemotaxis was blocked by the H4 receptor antagonist JNJ 7777120 and the H3/H4 receptor antagonist thioperamide, but not by H1, H2 or H3 receptor antagonists. The H1, H2 and H3 receptor antagonists used in studies were diphenhydramine, ranitidine and JNJ 6379490, respectively. Histamine (10 μM) was added in the lower chamber, while 10 μM of different histamine receptor antagonists was added in both chambers. Data shown are a representative from four repeated experiments. Data are mean±s.d. and n=3. Statistical significance (P-value) was determined by the Student's t-test. (e) Determination of IC50 values of H4 receptor antagonists JNJ 7777120 and thioperamide in eosinophil chemotaxis assays. Histamine (1 μM) was added in the lower chamber, while different concentrations of JNJ 7777120 or thioperamide were added in both chambers. The percentage of inhibition was calculated based on the decrease in migrated cell numbers compared to samples treated with 1 μM of histamine only. Data shown are a representative from four repeated experiments. Data are mean±s.d. and n=3.
Possible synergistic effects between histamine and chemokines on eosinophil chemotaxis were studied. A titration of chemokines eotaxin-2 and MCP-3 was performed in the presence or absence of a suboptimal concentration of histamine, and the effect on eosinophil chemotaxis was studied. As shown in Figure 6c, addition of 0.5 μM histamine enhanced the chemotaxis of eosinophils induced by eotaxin-2 and MCP-3.
Histamine receptor antagonists were used to determine the histamine receptor responsible for eosinophil chemotaxis. Thioperamide and JNJ 7777120 at 10 μM inhibited completely the histamine-induced eosinophil chemotaxis, whereas the inhibitory effect of diphenhydramine, ranitidine or H3 receptor antagonist was minimal (Figure 6d). Both JNJ 7777120 and thioperamide showed a concentration-dependent effect in blocking 1 μM histamine-induced eosinophil chemotaxis with an IC50 of 86 and 519 nM, respectively (Figure 6e). The results suggest that the histamine-induced eosinophil chemotaxis is mediated by the H4 receptor.
Discussion
Since the discovery of the new histamine H4 receptor, accumulated information in the literature suggests that H4 receptor expression is restricted to cells of the hematopoietic lineage (Oda et al., 2000; Liu et al., 2001a; Morse et al., 2001; Zhu et al., 2001; Hofstra et al., 2003). However, the leukocyte cell types expressing H4 receptor are still controversial. We and other groups have previously shown that the H4 receptor is expressed in mast cells, basophils and eosinophils, and this cell type specificity is conserved in humans and mice (Oda et al., 2000; Liu et al., 2001a; Morse et al., 2001; Hofstra et al., 2003). In this report, we show that eosinophils expressed the H4 but not the H3 receptor. H4 receptors were also expressed at significant levels in dendritic cells and at low levels in CD4+ effector T cells. In contrast to other reports, we were unable to detect H4 receptor RNA message in neutrophils and monocytes (Oda et al., 2000; Morse et al., 2001; Zhu et al., 2001).
The expression of H4 receptor in eosinophils, mast cells, basophils and dendritic cells suggests a possible involvement of histamine and the H4 receptor in allergic responses. The establishment of a typical allergic response involves two different stages: the allergen sensitization stage and the allergic reaction stage. During the allergen sensitization stage, dendritic cells acquire antigens and migrate to draining lymph nodes for T cell activation. Histamine released from mast cells may affect dendritic cell function via the H4 receptor and influence T cell activation. At the stage of allergen challenge, exposure of mast cells to allergens leads to mast cell degranulation and the release of histamine. Histamine may enhance the accumulation of mast cells at sites of allergic reaction and recruit eosinophils as a late phase response. It has been shown previously that histamine H1 and H2 receptors are expressed differentially on type I and type II helper T cells, and they play a role in the modulation of T cell effector functions (Jutel et al., 2001). It is possible that the H4 receptor is another histamine receptor involved in the complicated process of allergic responses.
In this report, we show that histamine is a chemoattractant for eosinophils. Eosinophils respond to histamine with cell shape change, upregulation of adhesion molecules on the cell surface as well as chemotaxis. Using an H4 receptor-specific antagonist, we provided a definitive proof that all of these histamine effects on eosinophils were mediated by the H4 receptor. Chemotaxis is a directional cell movement up a chemoattractant gradient and requires an establishment of cell polarity, and thus a cell shape change toward a directional signal. Using the GAFS assay to measure eosinophil shape change, we demonstrated that histamine induced a rapid shape change in eosinophils in a concentration-dependent manner. Imetit is known to be an agonist for both H3 and H4 receptors, whereas clobenpropit behaves as an agonist for the H4 receptor, but as an antagonist for the H3 receptor (Oda et al., 2000; Liu et al., 2001a; Morse et al., 2001; Zhu et al., 2001). In our studies, both imetit and clobenpropit worked as agonists and mimicked histamine effects in inducing eosinophil shape change. The result further confirms that the eosinophil shape change induced by histamine is mediated by the H4 receptor.
The eosinophil shape change response to histamine was rapid but transient. A shape change was observed after 1-min incubation with histamine and a maximal response was reached at 5 min. Removal of histamine abolished the eosinophil shape change immediately. After short exposure to histamine, eosinophils were still fully capable of remounting a shape change response upon histamine restimulation. However, this eosinophil shape change response disappeared after incubation with histamine at high concentrations (>100 μM) or over a long period of time (>40 min). These eosinophils appeared to be desensitized and were no longer responsive to histamine upon restimulation. Receptor internalization has been reported to account for the desensitization of eosinophil response to eotaxin (Zimmermann & Rothenberg, 2003). Histamine has also been shown to induce internalization of H4 receptors (Nguyen et al., 2001). It is possible that the desensitization of the eosinophil response to histamine that we observed here is also the result of H4 receptor internalization.
Leukocyte chemoattractants are known to initiate a coordinated sequence of adhesive interactions between cells in circulating blood and in the microvascular endothelium. The phases of leukocyte migration are comprised of adhesion, spreading, diapedesis of the vessel endothelial cells and infiltration into tissues (Springer, 1994). Upregulation of adhesion molecules on the cell surface is essential for cell spreading and diapedesis in the process of cell migration. We show that cell surface expression of CD11b/CD18 (Mac-1) and CD54 (ICAM-1) was upregulated by histamine via the H4 receptor. This upregulation occurred within 10 min and is probably independent of protein synthesis. Although CD11a/CD18 (LFA-1) expression level was not induced by histamine, change in the avidity toward its ligands such as ICAM-1, ICAM-2 and ICAM-3 cannot be excluded.
The effect of H4 receptors in mediating eosinophil chemotaxis toward histamine was demonstrated in a Transwell in vitro system. Comparing to other typical eosinophil chemokines, histamine is a relatively weak chemotactic factor. The concentrations of histamine needed to trigger the eosinophil shape change and chemotatic response are higher than those needed for chemokines such as eotaxin-2 and MCP-3. In addition, the histamine half-life in serum is very short (around 1 min) and its serum concentration is in the range of 1 nM in normal conditions and may only reach 10 nM in a systemic allergic response (Church & Caulfield, 1993). Considering that the EC50 of histamine for eosinophil chemotaxis as measured in our studies is 83 nM, it is unlikely that histamine in the blood stream is able to trigger eosinophil migration. However, the histamine concentration in tissues may reach a much higher level, in particular, in areas where mast cell degranulation has occurred. Histamine released in tissues may form complexes with heparin sulfate to prolong its half-life and to interact with extracellular matrix for the establishment of a histamine concentration gradient. It is possible that eosinophils rely on different chemotactic factors in their path of migration through different compartments of the in vivo system. Histamine may exert its direct chemotactic effect in tissues to recruit eosinophils after their exit from blood circulation. We also explored the possible cooperative effect of histamine with other chemokines in mediating eosinophil chemotactic response. While additive effects were observed, we could not observe any synergistic effects between histamine and chemokines.
In an allergic reaction, large amounts of histamine are released from mast cells locally at sites of allergen exposure. Eosinophil infiltration follows as a late phase response, and these cells play a major role in the pathogenesis of allergy. The accumulated numbers of mast cells, basophils and eosinophils at sites of allergic reactions often correlate with disease severity (Bousquet et al., 1990; Macfarlane et al., 2000). Here, we demonstrated a new role of histamine in recruiting specific inflammatory cell types into sites of allergic response. Eosinophils are one of the key participants in chronic allergic diseases and therefore a better understanding of eosinophil recruitment may yield novel therapeutics for these disorders. Development of small molecule antagonists for H4 receptor might be valuable for the treatment of allergic inflammation. The H4 receptor-mediated eosinophil responses, such as cell shape change and upregulation of adhesion molecules, can serve as biomarkers in clinical studies of H4 receptor antagonists.
Acknowledgments
We thank Dr Mani Neelakandha and Dr Cheryl Grice for supplies of the H4 receptor antagonist JNJ 7777120; Dr Tim Lovenberg for providing us with the different histamine receptor constructs as well as the human SK-N-MC cells transfected with different histamine receptors; Dr Nicholas Carruthers for giving us the H3 receptor antagonistic compound JNJ 6379490; and Dr Didier Leturcq and Ms Juli DeGraw for sharing with us the RNA samples from purified human monocytes, dendritic cells and activated CD8+ T cells.
Abbreviations
- PMNL
polymorphonuclear leukocytes
- PBMC
peripheral blood mononuclear cells
References
- BOUSQUET J., CHANEZ P., LACOSTE J.Y., BARNEON G., GHAVANIAN N., ENANDER I., VENGE P., AHLSTEDT S., SIMONY-LAFONTAINE J., GODARD P., MICHEL F.-B. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- CHURCH M.K., CAULFIELD J.P.Mast cell and basophil function Allergy 1993New York: Raven Press Ltd; ed. Holgate, S.T. & Church, M.K. Chapter 5.6 [Google Scholar]
- CLARK R.A., GALLIN J.I., KAPLAN A.P. The selective eosinophil chemotactic activity of histamine. J. Exp. Med. 1975;142:1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNG-LEUG W.-P., THURMOND R.L., LING P., KARLSSON L.H4 receptor antagonists – the new antihistamines Curr Opin Investing Drugs 2004. in press [PubMed]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International union of pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- HOFSTRA C.L., DESAI P.J., THURMOND R.L., FUNG-LEUNG W.-P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- JABLONOWSKI J.A., GRICE C.A., CHAI W., DVORAK C.A., VENABLE J.D., KWOK A.K., LY K.S., WEI J., BAKER S.M., DESAI P.J., JIANG W., WILSON S.J., THURMOND R.L., KARLSSON L., EDWARDS J.P., LOVENBERG T.W., CARRUTHERS N.I. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J. Med. Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- JABLONOWSKI J.A., CARRUTHERES N.I., THURMOND R.L.Histamine H4 receptor ligands and their potential therapeutic uses Mini Rev Med Chem. 2004. in press [DOI] [PubMed]
- JUTEL M., WATANABE T., KLUNKER S., AKDIS M., THOMET O.A., MALOLEPSZY J., ZAK-NEJMARK T., KOGA R., KOBAYASHI T., BLASER K., AKDIS C.A. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- LIU C., MA X., JIANG X., WILSON S.J., HOFSTRA C.L., BLEVITT J., PYATI J., LI X., CHAI W., CARRUTHERS N., LOVENBERG T.W. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol. Pharmacol. 2001a;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- LIU C., WILSON S.J., KUEI C., LOVENBERG T.W. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J. Pharmacol. Exp. Ther. 2001b;299:121–130. [PubMed] [Google Scholar]
- LUKACS N.W. Role of chemokines in the pathogenesis of asthma. Nat. Rev. Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- MACFARLANE A.J., KON O.M., SMITH S.J., ZEIBECOGLOU K., KHAN L.N., BARATA L.T., MCEUEN A.R., BUCKLEY M.G., WALLS A.F., MENG Q., HUMBERT M., BARNES N.C., ROBINSON D.S., YING S., KAY A.B. Basophils, eosinophils and mast cells in atopic and nonatopic ashtma and in late-phase allergic reactions in the lung and skin. J. Allergy Clin. Immunol. 2000;105:99–107. doi: 10.1016/s0091-6749(00)90184-2. [DOI] [PubMed] [Google Scholar]
- MORSE K.L., BEHAN J., LAZ T.M., WEST R.E., JR., GREENFEDER S.A., ANTHES J.C., UMLAND S., WAN Y., HIPKIN R.W., GONSIOREK W., SHIN N., GUSTAFSON E.L., QIAO X., WANG S., HEDRICK J.A., GREENE J., BAYNE M., MONSMA F.J., JR Cloning and characterization of a novel human histamine receptor. J. Pharmacol. Exp. Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- NGUYEN T., SHAPIRO D.A., GEORGE S.R., SETOLA V., LEE D.K., CHENG R., RAUSER L., LEE S.P., LYNCH K.R., ROTH B.L., O'DOWD B.F. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- ODA T., MORIKA W.A.N., SAITO Y., MASUHO Y., MATSUMOTO S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- O'REILLY M., ALPERT R., JENKINSON S., GLADUE R.P., FOO S., TRIM S., PETER B., TREVETHICK M., FIDOCK M. Identification of a histamine H4 receptor on human eosinophils – role in eosinophil chemotaxis. J. Recept. Signal Transduct. Res. 2002;22:431–448. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- SABROE I., HARTNELL A., JOPLING L.A., BEL S., PONATH P.D., PEASE J.E., COLLINS P.D., WILLIAMS T.J. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 1999;162:2946–2955. [PubMed] [Google Scholar]
- SHAH C., MCATEE L., BREITENBUCHER J.G., RUDOLPH D., LI X., LOVENBERG T.W., MAZUR C., WILSON S.J., CARRUTHERS N.I. Novel human histamine H3 receptor antagonists. Bioorg. Med. Chem. 2002;12:3309–3312. doi: 10.1016/s0960-894x(02)00738-2. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- TACHIMOTO H., EBISAWA M., BOCHNER B.S. Cross-talk between integrins and chemokines that influences eosinophil adhesion and migration. Int. Arch. Allergy Immunol. 2002;128:18–20. doi: 10.1159/000059414. [DOI] [PubMed] [Google Scholar]
- ZHU Y., MICHALOVICH D., WU H., TAN K.B., DYTKO G.M., MANNAN I.J., BOYCE R., ALSTON J., TIERNEY L.A., LI X., HERRITY N.C., VAWTER L., SARAU H.M., AMES R.S., DAVENPORT C.M., HIEBLE J.P., WILSON S., BERGSMA D.J., FITZGERALD L.R. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol. Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN N., ROTHENBERG M.E. Receptor internalization is required for eotaxin-induced responses in human eosinophils. J. Allergy Clin. Immunol. 2003;111:97–105. doi: 10.1067/mai.2003.3. [DOI] [PubMed] [Google Scholar]