Abstract
The effects of balloon injury on the reactivity of ipsilateral and contralateral carotid arteries were compared to those observed in arteries from intact animals (control arteries).
Carotid arteries were obtained from Wistar rats 2, 4, 7, 15, 30 or 45 days after injury and mounted in an isolated organ bath. Reactivity to angiotensin II (Ang II), phenylephrine (Phe) and bradykinin (BK) was studied. Curves were constructed in the absence or presence of endothelium or after incubation with 10 μM indomethacin, 500 μM valeryl salicylate or 0.1 μM celecoxib.
Phe, Ang II and BK maximum effects (Emax) were decreased in ipsilateral arteries when compared to control arteries. No differences were observed among pD2 or Hill coefficient.
Emax to Phe (4 and 7 days) and to Ang II (15 and 30 days) increased in the contralateral artery. In addition, Phe or Ang II reactivity was not significantly different in aorta rings from control or carotid-injured animals.
The increased responsiveness of contralateral artery was not due to changes in carotid blood flow or resting membrane potential. The endothelium-dependent inhibitory component is not present in the contraction of contralateral arteries and it is not related to superoxide anion production.
Indomethacin decreased contralateral artery responsiveness to Phe and Ang II. Valeryl salicylate reduced the Ang II response in contralateral and control arteries. Celecoxib decreased the Phe Emax of contralateral artery.
In conclusion, decreased endothelium-derived factors and increased prostanoids appear to be responsible for the increased reactivity of contralateral arteries after injury.
Keywords: Balloon catheter, contralateral artery, angiotensin II, phenylephrine, carotid, vascular reactivity
Introduction
Balloon catheter angioplasty is a widely accepted procedure for treatment of peripheral and coronary obstructive disease to restore blood flow. Despite its widespread application, there is still uncertainly regarding the effects of the procedure on smooth muscle cells and arterial responsiveness, as the technique may also cause extensive endothelial injury (Schwartz et al., 1995).
In vitro and in vivo studies have confirmed the involvement of the renin—angiotensin system in the growth of the neointimal layer after balloon injury. Ang II stimulates DNA synthesis in the media and neointima after angioplasty (Hutchinson et al., 1999). Powell et al. (1989) and others (Hanson et al., 1991; Farhy et al., 1992; Rakugi et al., 1994; Fernandez-Alfonso et al., 1997) reported that angiotensin-converting enzyme inhibitors or AT1 antagonist are able to prevent balloon-induced neointima formation in rats. The Ang II-induced DNA synthesis observed in the media and the neointima after angioplasty is partly mediated by α1-adrenoceptor activation (Bruijns et al. 1998).
Conversely, the renin–angiotensin system also influences the noradrenergic system. Ang II facilitates sympathetic transmission, inhibits norepinephrine reuptake (Starke, 1977) and releases catecholamine from the adrenal medulla (Feldberg & Lewis, 1964). Additionally, the renin–angiotensin system modulates the kallikrein–kinin system, a regulatory system involved in a multitude of physiologic and pathologic conditions. In addition, stimulation of Ang 1–7 or AT2 receptor has been shown to activate tissue kallikrein, increasing formation (Tschöpe et al., 2002).
There are reports that vascular injury can cause changes in tissues of normal appearance located at a distance from the injury site. Renal artery manipulation was reported to cause a centripetal spread of endothelial cell replication along the periureteric collateral artery (Hollenberg & Odori, 1987). Reidy (1990) observed that focal endothelial cell loss and formation of platelet thrombi in rat aorta caused a widespread increase in smooth muscle cell replication at distant aortic sites. In patients with acute myocardial infarction, the coronary vasodilator response is significantly reduced in areas of the myocardium that are not directly supplied by the impaired artery (Uren et al., 1994).
Immunohistochemical examination of the contralateral artery, 1 day after balloon catheter-induced endothelial injury, revealed a substantial increase in the density of protein gene product 9.5 (PGP) – substance P (SP) – and calcitonin-gene-related peptide (CGRP)-containing nerves innervating the carotid artery and vasa vasorum compared to arteries from intact animals, which was no longer observed twenty-eight days after surgery (Milner et al., 1997).
In the present study, we examined the effect of balloon catheter-induced injury on the responsiveness of ipsilateral artery and distant vessel (contralateral carotid artery) to Ang II, Phe and BK at days 2, 4, 7, 15, 30 and 45 after surgery and compared the responsiveness to those of carotid arteries from intact animals (control arteries).
Methods
Surgery
All experimental procedures conformed to International Guidelines and Ethical Animal Committee of the Ribeirão Preto Campus, University of São Paulo, Brazil.
Adult male Wistar rats (3.5–4 months) were divided into three groups: control, sham-operated and animals that underwent unilateral balloon catheter injury. Care was taken for all the animals to be of the same age on the day of the experiment.
Balloon angioplasty was carried out as previously described (Clowes et al., 1983). In brief, general anaesthesia was installed using the short-lasting anaesthetic tribromoethanol (2.5 mg kg−1, i.p.). The left common carotid artery (ipsilateral to injury) was exposed and the external carotid artery was ligated for access of the 2F Fogarty balloon catheter (Baxter, the Netherlands), which was passed three times along the common carotid artery and distended saline. The catheter was removed, the external carotid artery was ligated, and the wound was closed. Sham-operated rats underwent anaesthesia, the left common carotid artery was exposed and the external carotid artery was ligated without performing catheterisation.
Vascular studies
At various time points after injury (2, 4, 7, 15, 30 and 45 days), rats were anaesthetised with tribromoethanol i.p. (2.5 mg kg−1). and killed. The carotid or aorta arteries were removed and immediately placed in Krebs–Henseleit solution, which was composed of the following (mmol l−1): NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; KH2PO4,1.2; MgSO4·7H2O, 1.2; NaHCO3, 25; C6H12O6, 11.6; pH 7.4, at 37°C. Carotid artery rings (4 mm) or aorta rings (6 mm) were mounted in organ chambers bubbled with a carbogenic mixture (95% O2 and 5% CO2) and connected to force transducers (Letica, Spain).
Preliminary experiments showed that a 1 g resting tension was at the optimal plateau of the length–tension relationship for 90 mM KCl. Cumulative concentration–response curves were obtained using 0.1 nM–10 μM Phe or 0.01 nM–1 μM Ang II. Response to 90 mM KCl was also recorded. Concentration–relaxation curves to 0.1 nM–10 μM BK were performed in arteries previously contracted using 0.1 μM Phe. In some preparations, the endothelium was mechanically removed or the rings were incubated with 10 μM indomethacin for 45 min, 500 μM valeryl salicylate for 1 h (Bhattacharyya et al., 1995) or 0.1 μM celecoxib for 1 h (Penning et al., 1997).
Results were expressed as gram (g) of tension (Phe or Ang II curves) or % relaxation (BK curves) and were represented as mean±s.e.m. of 6–10 animals. Pharmacological parameters, that is, the agonist potency (pD2), maximum effect (Emax) and the Hill coefficient were derived from the concentration–effect curves (Kenakin, 1996).
Carotid blood flow
Animals were anaesthetised with 7 mg kg−1 ketamine + 0.4 mg kg−1 xylazine, i.p. (suggested anaesthetic protocol for flow measurements – Guideline for use of anaesthetics from Transonic Systems Inc., U.S.A.) and the common carotid arteries were exposed. A noninvasive transit-time flow probe (model 1.5RB; Transonic Systems, Inc., Ithaca, NY, U.S.A.) was placed around the carotid arteries. The flow probe was connected to a flow meter (model T-206; Transonic system, U.S.A.) and carotid blood flow was recorded using a computerised acquisition system (Dataq, U.S.A.). The system provided actual volume flow measurements with a resolution of 0.05 ml.min−1. The carotid was minimally manipulated to avoid vasospasm.
Resting membrane potential (RMP)
Isolated carotids were placed in a 2 ml perfusion chamber with Krebs–Henseleit solution gassed with 95% CO2/5% O2 at a rate of 3 ml.min−1. Micropipettes from borosilicate glass capillaries were prepared using an horizontal puller (Narishige, Model PN3) filled with 2 M KCl (tip resistance 20–40 MΩ and tip potential <6 mV), (1B120F-6, World Precision Instruments, U.S.A.). The microelectrodes were mounted in Ag/AgCl half-cells on a Leitz micromanipulator and connected to an electrometer (Intra 767, World Precision Instruments, U.S.A.). Impalements were made in smooth muscle cells. Electrical signals were continuously monitored on an oscilloscope (54645-A, Hewlett Packard, U.S.A.) and recorded on a potentiometric chart recorder (2210, LKB, Sweden).
Successful implantation of the electrode was demonstrated by a sharp voltage drop upon entry into the cell, a stable 3 mV potential for at least 1 min after impalement, a sharp return to zero upon exiting the cell and a minimal change (<10%) in microelectrode resistance following impalement. Isolated arteries were initially equilibrated for 1 h.
Superoxide analyses
Production of superoxide anion was measured by the coelenterazine-enhanced chemiluminescence technique, as previously described by Laurindo et al. (2002), with a final coelenterazine concentration of 10 μM. Rats were anaesthetised, killed and the carotid arteries were removed and cut into 0.5 mm segments which were incubated in Krebs-HEPES bicarbonate buffer and bubbled with 95%O2/5% CO2 at 37°C for 30 min.
Artery rings were exposed to potassium phosphate buffer containing 0.1 mM N-methyl-L-arginine (L-NMMA) and 0.1 mM diethylenetriamine pentaacetic acid (DTPA) during the experiments. After 2 min equilibration, photon emission was continuously recorded for 4 min in a Biolumat LB9505 (Berthold, Germany). The specific chemiluminescence was expressed as c.p.m. minus background. mg−1 dry weight min−1.
Drugs
Phe (Sigma, U.S.A.), Ang II (Sigma, U.S.A.), BK (Sigma, U.S.A.), ketamine (União Química, Brazil), xylazine (Calier Laboratory, Brazil), coelenterazine (ProLume, U.S.A.), L-NMMA (Calbiochem, U.S.A.), DTPA (Sigma, U.S.A.), indomethacin (Calbiochem, U.S.A.) valeryl salicilate (Cayman Chemical, U.S.A.) and celecoxib (Pfizer, U.S.A.).
Statistical analyses
pD2 (−log EC50), Emax and Hill coefficient were determined from concentration–response curves using the Prism software (version 2.0,. GraphPad Software Inc., U.S.A.). Parameters were analysed using one-way ANOVA and Bonferroni's post-test or unpaired ‘t' test, P<0.05, using the Prism software.
Results
Initially, the arterial reactivity of the arteries from intact animals was compared to that of sham operated animals (2, 4, 7, 15, 30 and 45 days after sham -surgery). Phe, Ang II and BK evoked similar concentration-dependent responses in carotid arteries from intact or sham-operated rats. No differences were observed in the pD2 and Emax values for Phe, Ang II or BK between ipsilateral and contralateral arteries from sham animals or between those and the arteries from intact animals (data not shown). Thus, thereafter, arteries from intact rats were used as control arteries to avoid unnecessary animal suffering.
In arteries from injured rats, Phe and Ang II evoked concentration-dependent contractions in ipsilateral and contralateral arteries removed 2, 4, 7, 15, 30 and 45 days after balloon catheter surgery. The injury significantly reduced the maximum response to Phe and Ang II in ipsilateral arteries when compared to control arteries from intact animals (Table 1). The pD2 values for Phe and Ang II were not different for the control and ipsilateral (injured) arteries (Table 2).
Table 1.
Emax values for agonists in rat carotid artery after endothelial injury
| Days after injury | Phe (g) | Ang II (g) | BK (% relaxation) | |||
|---|---|---|---|---|---|---|
| Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | |
| Control | 0.28±0.02 | 0.28±0.01 | 52.12±6.34 | |||
| 2 | 0.22±0.03 | 0.17±0.02a | 0.24±0.02 | 0.24±0.03 | 49.16±4.31 | 19.19±3.25a |
| 4 | 0.37±0.03a | 0.16±0.04a | 0.26±0.03 | 0.16±0.01a | 57.74±6.86 | 21.79±3.48a |
| 7 | 0.35±0.03a | 0.14±0.02a | 0.24±0.02 | 0.13±0.02a | 52.50±5.96 | 27.31±8.99a |
| 15 | 0.32±0.02 | 0.20±0.02a | 0.35±0.04a | 0.16±0.01a | 50.95±6.89 | 44.27±6.10 |
| 30 | 0.27±0.03 | 0.17±0.03a | 0.36±0.03a | 0.12±0.02a | 53.88±4.69 | 55.43±0.70 |
| 45 | 0.26±0.03 | 0.16±0.03a | 0.29±0.04 | 0.10±0.03a | 55.13±6.03 | 50.40±9.51 |
Anova and Bonferroni's post test, P<0.05.
vs control group.
Table 2.
pD2 values (−log EC50) for agonists in rat carotid artery after endothelial injury
| Days after injury | Phe | Ang II | BK | |||
|---|---|---|---|---|---|---|
| Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | |
| Control | 8.64±0.20 | 9.12±0.13 | 6.61±0.50 | |||
| 2 | 8.72±0.30 | 8.13±0.19 | 8.76±0.16 | 9.08±0.18 | 6.45±0.14 | ND |
| 4 | 8.26±0.10 | 8.50±0.53 | 9.45±0.25 | 8.92±0.12 | 6.23±0.33 | 6.97±0.18 |
| 7 | 8.09±0.19 | 7.83±0.40 | 9.03±0.33 | 8.74±0.25 | 6.08±0.17 | 6.85±0.20 |
| 15 | 8.33±0.19 | 8.20±0.14 | 8.66±0.12 | 9.10±0.27 | 6.45±0.18 | 7.41±0.13 |
| 30 | 8.39±0.27 | 7.89±0.32 | 8.91±0.16 | 8.32±0.17 | 6.06±0.12 | 6.16±0.41 |
| 45 | 8.12±0.17 | 8.11±0.37 | 8.88±0.13 | 8.63±0.19 | 6.21±0.28 | 6.73±0.13 |
ANOVA: not significant. ND: not determined.
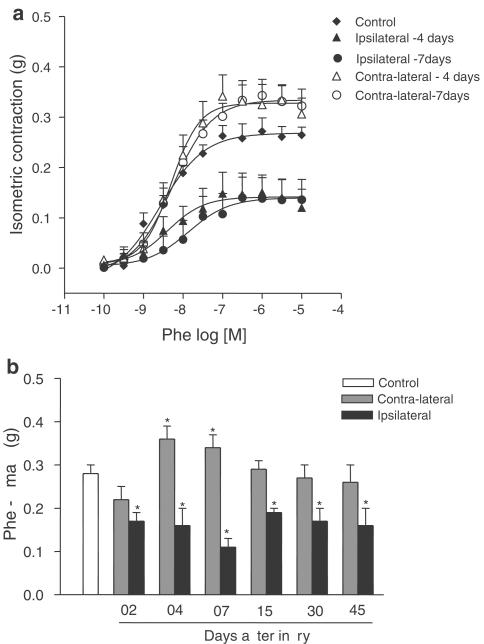
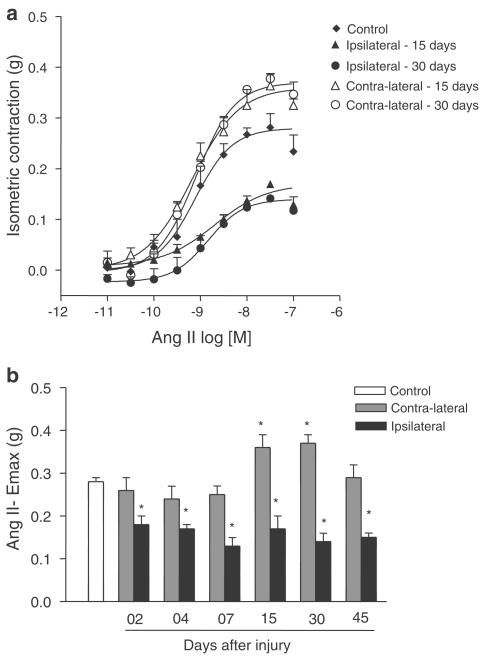
In contrast, an increase in the Emax to Ang II and Phe was observed in contralateral when compared to control arteries. The Emax to Phe was increased in contralateral arteries at days 4 and 7 after injury (Figure 1a and Table 1). The Emax to Ang II was increased in contra-lateral arteries at days 15 and 30 after injury (Figure 2a and Table 1). There were no differences in Emax to Phe and Ang II between contralateral arteries and control arteries on the other days studied (Figure 1b and 2b, respectively).
Figure 1.
(a) Phe concentration–effect curves from control carotid arteries and contralateral and ipsilateral arteries 4 and 7 days after the injury; (b) Emax values (g) for Phe obtained in control, contralateral and ipsilateral carotid arteries (2, 4, 7, 15, 30 and 45 days after the injury). One-way ANOVA followed by Bonferroni's post test, P<0.05, *significantly different from control group.
Figure 2.
(a) Ang II concentration–effect curves from control carotid arteries and contralateral and ipsilateral carotid arteries taken 15 and 30 days after the injury; (b) Ang II Emax values (g) from control, contralateral and ipsilateral arteries (2, 4, 7, 15, 30 and 45 days after the injury). One-way ANOVA followed by Bonferroni's post test, P<0.05, *significantly different from control group.
BK induced significant relaxation (52%) in control carotid arteries precontracted with Phe (EC50=0.1 μM). BK-induced relaxation was decreased in ipsilateral vessels on days 2, 4 and 7 after removal of the endothelium by balloon injury. Relaxation was re-established at days 15, 30 and 45 after injury (Table 1). The maximal relaxation induced by BK in contralateral vessel was similar to that observed in control arteries (Table 1).
There were no differences in the pD2 values for Ang II, Phe and BK among control, ipsilateral and contralateral arteries (Table 2). The analysis of the concentration–response curves showed that Hill coefficients were not significantly different from unity (data not shown).
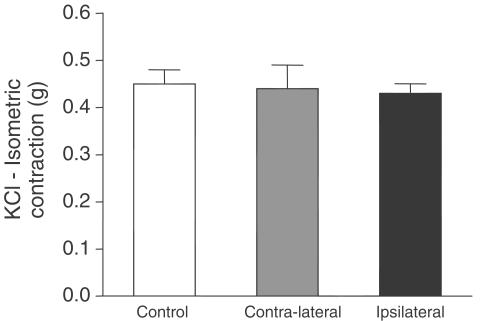
Vasoconstrictions of ipsilateral or contralateral arteries in response to a high potassium concentration (90 mM) were not different from those observed in control arteries (Figure 3).
Figure 3.
KCL-induced contraction (90 mM) in isolated carotid from control or injured animals killed 15 days after the injury. One-way ANOVA (not significant).
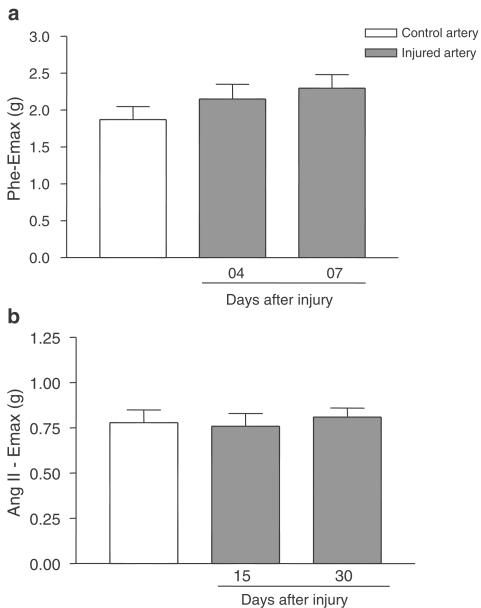
To investigate whether this increased contraction effect was selective for the contralateral carotid or if it also occurred in other vessels, we studied the contractions evoked by Phe or Ang II in aorta rings from carotid injured rats at the times when increased Emax was observed in the contralateral arteries.
The maximal responses to Phe in the aorta artery at 4 and 7 days after the lesion to the carotid artery (Figure 4a) were similar to that of the aorta artery of the control animal (2.15 ±0.20; 2.30 ±0.18 and 1.87±0.21 g, respectively, not significant). Similarly, the maximum force developed in response to Ang II in control aorta arteries (1.07±0.10 g) was not different from that of the aorta, artery of carotid-injured animals 15 days or 30 days after injury (1.18±0.13, 1.15±0.07 g, respectively) (Figure 4b). There were no significant differences in the pD2 values of Phe and Ang II in isolated aorta as shown by the curves from control or injured animals (data not shown).
Figure 4.
(a) Phe Emax (g) in aorta rings from control or operated rats 4 and 7 days after the injury in carotid artery. One-way ANOVA: not significant. (b) Ang II Emax (g) in aorta rings from control or operated rats 15 and 30 days after the injury in carotid artery. One-way ANOVA: not significant.
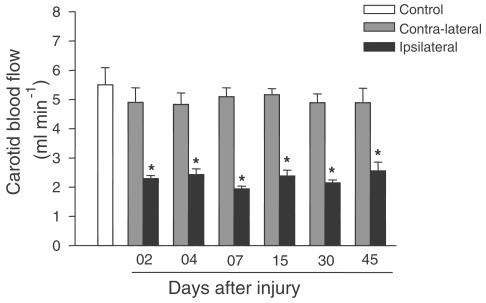
To establish the role of blood flow in the contractile effect, we measured the blood flow in carotid arteries. In control animals, carotid blood flow was 5.5±0.9 ml min−1.The blood flow values for contralateral arteries were similar to the values from control animals; however, blood flow was decreased in the ipsilateral arteries of injured rats (Figure 5).
Figure 5.
Carotid blood flow (ml min−1) in control, contralateral or ipsilateral arteries (2, 4, 7, 15, 30 and 45 days after the injury). One-way ANOVA followed by Bonferroni's post test, P<0.05, * significantly different from control group.
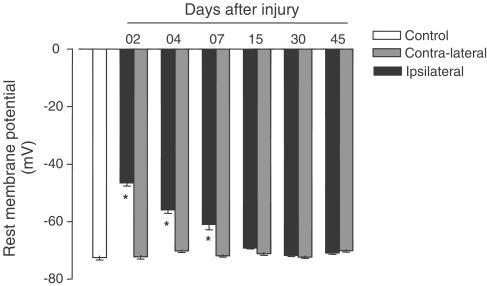
The electrophysiological study showed that the resting membrane potentials (RMP) of the contralateral artery and the control animal artery did not differ statistically from each other. In contrast, RMP were significantly lower in ipsilateral arteries 2, 4 and 7 days after surgery than in intact artery; furthermore, at 15 days after injury the resting potential membrane of the arteries returned to normal (Figure 6).
Figure 6.
Resting potential membrane (mV) of control, contralateral or ipsilateral arteries (2, 4, 7, 15, 30 and 45 days after the injury). One-way ANOVA followed by Bonferroni's post test, P<0.05, * significantly different from control group.
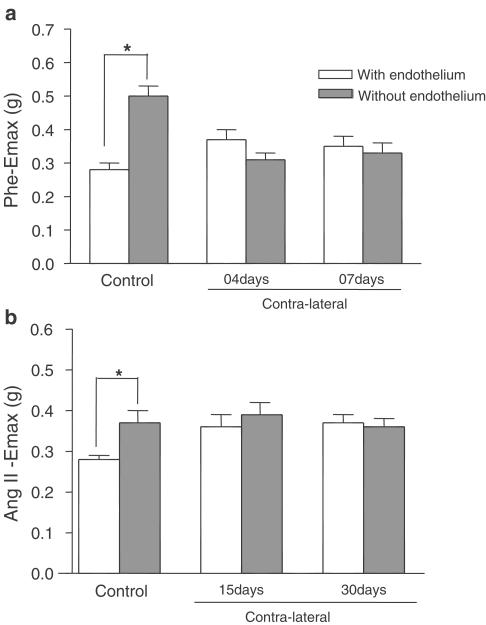
To evaluate the role of the endothelium in increased contraction in the contralateral artery, the arteries were denuded mechanically before the experiments. The absence of endothelium significantly increased the contractions caused by Phe or Ang II in vessels from the control group. However, in the contralateral arteries, the absence of endothelium did not affect the Phe or Ang II response (Figures 7a and 7b), suggesting that endothelium-derived factors are decreased in contralateral arteries 4, 7, 15 and 30 days after injury.
Figure 7.
(a) Phe Emax (g) of control or contralateral (4 and 7 days after the injury) arteries with or without endothelium; (b): Ang II Emax (g) of control or contralateral (15 and 30 days after the injury) arteries with or without endothelium. Unpaired ‘t' test, P<0.05, *significantly different from respective group with endothelium.
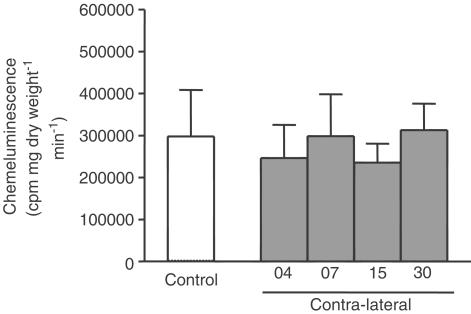
Superoxide production was measured to demonstrate the reduction of the endothelium-derived factors in the contralateral artery. Figure 8 demonstrates the chemiluminescence of coelenterazine in the control and contralateral carotid rings under basal conditions. The chemiluminescence activity of the control or contralateral arteries was not significantly different at 4, 7, 15 or 30 days after injury.
Figure 8.
Coelenterazine chemiluminescence's response (cpm mg.dry weight−1 min−1) in control or contralateral carotid arteries 4, 7, 15 or 30 days after the injury. One-way ANOVA: not significant.
To evaluate the possible role played by prostanoids in the Ang II or Phe effect in the contralateral artery indomethacin (a nonselective cyclooxygenase inhibitor), valeryl salicylate (selective cyclooxygenase 1 inhibitor) and celecoxib (selective cyclooxygenase 2 inhibitor) were employed. The baseline tone in isolated arteries was increased in the presence of indomethacin and valeryl salicylate.
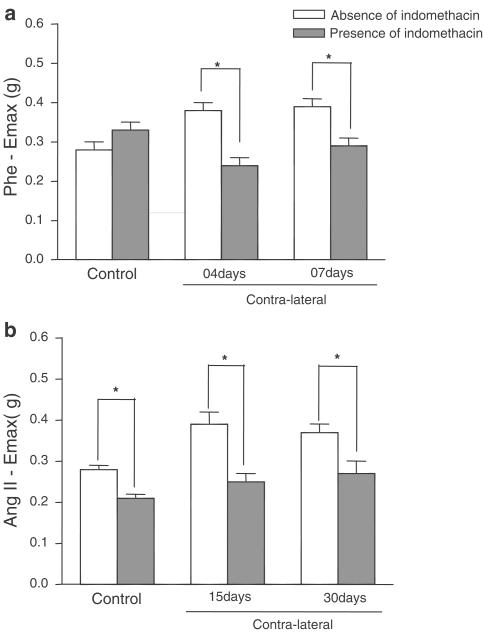
Indomethacin did not significantly alter the Phe responses in the control carotid artery. In contrast, it depressed maximal contraction to adrenergic agonist in contralateral arteries (4 and 7 days after injury) (Figure 9a) and it reduced the Ang II-induced contraction in control and contralateral carotid arteries (Figure 9b).
Figure 9.
Effect of indomethacin (10 μM) on Emax of Phe (a) or Ang II (b) in control or contralateral arteries. Unpaired ‘t' test, P<0.05, *significantly different from respective group in the absence of inhibitor.
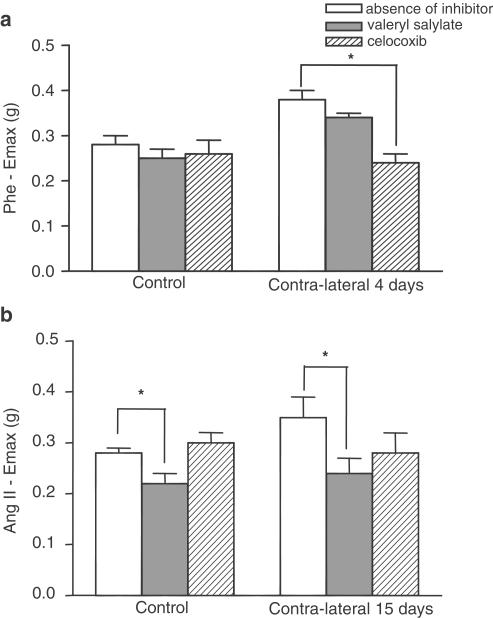
The selective cyclooxygenase 1 inhibitor, valeryl salicylate, did not affect the Phe Emax in control or contralateral artery 4 days after the injury, while celecoxib (a selective cyclooxygenase 2 inhibitor) reduced the adrenergic response in the contralateral artery without affecting the control artery response (Figure 10a).
Figure 10.
Effect of valeryl salicylate (500 μM) or celecoxib (0.1 μM) on Emax of Phe(a) or Ang II (b) in control or contralateral arteries. Unpaired ‘t' test, P<0.05, *significantly different from respective group in the absence of inhibitor.
Valeryl salicylate decreased the Ang II Emax in control or contralateral artery 15 days after the injury, but celecoxib did not alter the peptide maximum response in control or contralateral arteries (Figure 10b).
Discussion and conclusions
This study compared the effects of balloon catheter-induced injury on the vascular reactivity of the ipsilateral and contralateral carotid arteries with the vascular reactivity of control arteries from intact animals.
The vascular responses (Emax, pD2 and Hill coefficient values) to the three agonists used (Ang II, Phe and BK) in the ipsilateral and contralateral arteries of sham-operated rats were not significantly different from those of arteries from intact animals. These findings indicate that surgical procedures, such as the dissection of the vagus nerve and ligation of the external carotid artery, did not affect the vascular reactivity. Thus, vessels isolated from intact animals were used as controls to compare to injured animals.
The pD2 values for Ang II, Phe or BK were similar in control, ipsilateral and contralateral arteries, suggesting that balloon catheter surgery does not affect agonist potency, as previously described by Lemay et al. (2000). However, the endothelium injury-induced balloon caused an immediate increase in the endothelin-1 contractile potency and it could be related to an increase in affinity for the endothelin-1 receptor subtype mediating vasoconstriction, an increase in agonist bio-availability at this smooth muscle cell receptor or the loss of some type of physiological antagonism, an intrinsic mechanism which normally exists to selectively oppose the contractile actions of this peptide (Douglas et al., 1994). Hill coefficients from all curves were similar to unity, suggesting a homogeneous response and the positive cooperative phenomenon does not affect the response (Kenakin, 1996).
Data demonstrate that balloon catheter injury decreased the maximum contractions evoked by Ang II or Phe in the ipsilateral artery. These results are in agreement with those of Major et al. (1985), Joly et al. (1992) and Antonnacio et al. (1994), who all demonstrated a reduction in the Emax value for several agonists in injured arteries.
The decreased Ang II-induced contraction in the ipsilateral artery may be the consequence of changes in the activities of AT2 receptors. Activation of AT2 has been associated with vasodilator effects (Tschöpe et al., 2002) and AT2 receptors re-expresssion was reported to occur 48–72 h after injury (Hutchinson et al., 1999). This effect may be the consequence of an increased production of inducible nitric oxide in the ipsilateral vessel wall, as suggested by several studies (Major et al., 1985; Joly et al., 1992; Douglas et al., 1994; Hansson et al., 1994).
The decreased response to Phe could also be related to the desensitisation to exogenous ligands (Candipan et al., 1994), to a decrease in α1-adrenoceptors from day 3 up to 10 weeks after injury (Bruijns et al., 1998) or increased production of inducible nitric oxide in injured vessel (Major et al., 1985; Joly et al., 1992; Douglas et al., 1994; Hansson et al., 1994; Yan & Hansson, 1998).
The vascular hyporeactivity to Phe and Ang II described above was not selective for these agonists. The BK-induced relaxation was reduced during the early phases of injury (2, 4 and 7 days), possibly due to the absence of endothelium (Clowes et al., 1983). After 15 days, BK-induced relaxation returned to control levels. Similar results were reported for carbachol-induced relaxations in the rat carotid (Major et al., 1985) or for BK relaxation of the pig coronary, after angioplasty (Thollon et al., 2002). At 15 days after balloon catheterisation, the injured vessel develops a neointima layer, in which the smooth muscle cells are primarily of the synthetic rather than the contractile phenotype (Campbell et al., 1988) with a different shape and organisation of smooth muscle cells (Majesky et al., 1992). These cells could induce vasodilatation, explaining the recovery of relaxation obtained.
The balloon injury did not interfere with the intrinsic contractility of muscle cells since the maximal responses to a high potassium concentration were not influenced by balloon catheter, as previously described by Douglas et al. (1994) and Lemay et al. (2000).
We also demonstrate that balloon injury promoted an increase in the responsiveness of contralateral arteries, which was agonist-dependent and occurred at different postinjury times. These results could indicate the existence of a compensatory mechanism in the contralateral artery that was activated after the balloon catheter procedure. Several examples of vascular changes in normal-appearing tissues, which take place at a distance from the injury site, have been reported (Segal & Duling, 1986; Hollenberg & Odori, 1987; Reidy, 1990; Milner et al., 1997). Compensatory changes may also occur in the contralateral sympathetic neurons of the superior cervical ganglion and in their terminals in the pineal gland following unilateral ganglionectomy (Dornay et al., 1995).
Evidence exists to demonstrate that balloon catheter dilatation may induce transient changes in the innervations of the contralateral artery. At 1 day after the surgery, there was a substantial increase in the density of PGP, SP and CGRP-containing nerves innervating the vasa vasorum and vessel wall in the contralateral artery, which were no longer observed 28 days after surgery (Milner et al., 1997).
The increased response of the contralateral artery to Ang II and Phe may be related to an increase in receptor density after surgery. However, balloon injury did not alter the α1-adrenoceptor binding in contralateral arteries (Bruijns et al., 1998). There are no data concerning the density of Ang II receptors in contralateral arteries after balloon injury and this possibility cannot be excluded.
We hypothesised that if the increased maximal effect observed in the contralateral artery was related to a humoral phenomenon other vessels should be similarly affected. Phe and Ang II Emax values in aorta rings from rats with injured carotid arteries were not different from those in rings from control intact arteries, suggesting that humoral factors are not responsible for the increased reactivity of the contralateral artery and that this effect is vascular-bed dependent.
Hemodynamic stimuli such as altered flow or circumferential stress can induce arterial remodelling and changes in the characteristics of vessels (Reidy, 1990; Pasterkamp et al., 2000). The results of the current study suggest that the increased response of the contralateral artery is not related to blood flow. The decreased blood flow in the ipsilateral artery of the injured animals may be due to the ligation of the external carotid during the surgery and did not change the vessel response, since the ipsilateral arteries from sham animals demonstrated the same reduction (data nor shown).
The RMP may influence vascular reactivity. Accorsi-Mendonça et al. (2002) showed a correlation between Ang II potency and RMP in nonvascular muscle cells. Furthermore, endothelial hyperpolarisation increases the electrochemical driving force for Ca2+ influx into the endothelium and, thus, indirectly augments Ca2+-dependent formation of vasodilating factors (Luckhoff & Busse, 1990). In addition, the neointimal proliferation may be caused by the depolarised state of the smooth muscle cells (Dalle-Lucca et al., 2000).
Our data showed that injured carotids were significantly more depolarised than control muscle cells until 7 days after lesion; after 15 days, the RMP from vascular muscle cells are similar to control values, although Köhler et al. (2001) have suggested that the neointima cells have a reduced capacity to hyperpolarise and dilate because of the decreased expression of KCa channels. Our findings also demonstrate that there is no difference in RMP between control and contralateral carotids, suggesting that the Phe and Ang II increased contraction in the contralateral artery after injury is not caused by alterations in membrane potential.
To further understand the role of the endothelium in the increased contraction of the contralateral vessels, we examined the response to Phe and Ang II in arteries without intact endothelium. After ex vivo endothelium denudation, the effect of both agonists in control arteries is significantly increased. However, the endothelium-dependent inhibitory component of the response to Phe or Ang II is not present in the contraction of contralateral arteries, suggesting that endothelium-derived factors were decreased in contralateral arteries after the injury.
Endothelium-derived factors may be decreased due to the existence of strong oxidants such as the superoxide radical, which may change reactivity following its reaction with NO, thus reducing the bio-availability of NO (Hanafy et al., 2001; Lum & Roebuck, 2001). Vascular production of reactive oxygen species is reported to be increased 2 min after balloon injury (Azevedo et al., 2000). In smooth muscle cells, both superoxide and peroxide disrupt the sarcoplasmic reticulum Ca2+-ATPase and inactivate plasma membrane Ca2+ pumps leading to both short- and long-term effects on smooth muscle Ca2+ handling (Wolin et al., 2002). Thus, we evaluated superoxide production to analyse the participation of oxidative stress in contralateral artery reactivity. Our results showed that there was no difference in superoxide production between control or contralateral arteries, despite the difference in the arteries 7 and 30 days after balloon placement. These data suggest that superoxide does not participate in the increased reactivity observed in the contralateral artery.
The vascular responsiveness can be affected by prostaglandins that are molecules that can affect several physiological and pathological functions. The prostaglandin generation is enhanced in patients with atherosclerosis and after balloon angioplasty (Braden et al., 1991; Belton et al., 2000).
Prostaglandins are synthesized from arachidonic acid by the cyclooxygenase (COX) enzyme, of which there are two isoforms, COX1 and COX2 (Hla & Neilson, 1992). COX1 is constitutively expressed in most tissues and is largely responsible for TXA2 formation. COX2 is normally undetectable or is expressed in low amounts (Masferrer et al., 1994) and it is induced in the vascular smooth muscle and inflammatory cells of human atherosclerotic plaque (Schonbeck et al., 1999; Belton et al., 2000). Prostanoids are also related to the adrenergic response in cultured vascular rabbit smooth muscle cells (Nebigil & Malik, 1992), adrenergic-induced constriction of endothelium-denuded rat abdominal aorta (Asbún-Bojalil et al., 2002) and carotid artery (Furuhashi et al., 2000).
Normal, uninjured vessels showed COX1, but no COX2 expression. At 14 days after balloon injury, both COX1 and COX2 were expressed in the neointima. Balloon angioplasty resulted in a marked increase in prostaglandin excretion (Pritchard et al., 1994; Connolly et al., 2002).
Then, we analysed the prostaglandin role in increase responsiveness of contralateral artery. Indomethacin, a nonselective inhibitor, induced a decrease in Phe effect of contralateral artery without affect the response of control artery, suggesting that prostanoids are involved in the increased effect to Phe observed in the contralateral artery. Our data demonstrated that the COX2 isoform is involved in the prostaglandin production during adrenergic stimulation in contralateral artery 4 days after injury. The balloon catheter is an inflammatory response (Ross, 1999) and it is probable that the COX2 isoform is present in injured tissue.
The COX1-dedrived prostanoids are involved in Ang II effect in control artery since indomethacin and valeryl salicylate reduced the peptide Emax. The endothelium injury did not modify the involvement of prostanoids in the Ang II contraction since the response to the peptide in contralateral arteries was also decreased by indomethacin and valeryl salicylate.
These data suggest that some form of cellular communication generated by the focal mechanical injury might be responsible for vascular remodelling in other uninjured vessels. Vascular compensation and remodelling are poorly understood complex processes. To date, no other report exists that explains the altered reactivity seen in the contralateral artery, and therefore further studies are required to examine which of the above mechanisms is responsible for this phenomenon. Our results indicate that important information concerning possible compensatory mechanisms may be derived from the study of effects of balloon injury on the contralateral vessels. Although the basic molecular mechanisms involved in the response remain to be fully elucidated, these responses provide potential targets through which it may be possible to influence remodelling after angioplasty.
In conclusion, we report that after unilateral balloon catheter dilatation of the carotid artery there is an increase in Phe and Ang II, but not BK reactivity, in the contralateral artery when compared to the effect on control arteries. This phenomenon was not observed in aorta rings after the angioplasty in the carotid artery, suggesting that it is a vascular bed-specific effect, which may involve increased prostanoids or decreased endothelium-derived factors in this mediation.
Acknowledgments
We thank Míriam Cristina Contin de Melo and Mayara Santos Gomes for their technical assistance. This research was supported by FAPESP (99/05434-0).
Abbreviations
- Ang II
angiotensin II
- BK
bradykinin
- CGRP
calcitonin-gene-related peptide
- COX
cyclooxygenase enzyme
- Emax
maximum effect
- PGP
protein gene product 9.5
- Phe
phenylephrine
- RMP
resting membrane potential
- SP
substance P
References
- ACCORSI-MENDONÇA D., CORRÊA F.M.A., ANSELMO-FRANCI J.A., PAIVA T.B., de OLIVEIRA A.M. Angiotensin actions on the isolated rat uterus during the estrous cycle: influence of resting membrane potential and uterine morphology. Pharmacology. 2002;65:162–1689. doi: 10.1159/000058043. [DOI] [PubMed] [Google Scholar]
- ANTONNACIO M.J., NORMANDIN D., FERRE P. Reduced contractile function after balloon denudation of rat carotid arteries. Eur. J. Pharmacol. 1994;256:17–21. doi: 10.1016/0014-2999(94)90610-6. [DOI] [PubMed] [Google Scholar]
- ASBÚN-BOJALIL J., CASTILLO EF, ESCALANTE B.A., CASTILLO C. Does segmental difference in α1-adrenoceptor subtype explain contractile difference in rat abdominal and thoracic aorta. Vasc. Pharmacol. 2002;38:169–175. doi: 10.1016/s1537-1891(02)00164-7. [DOI] [PubMed] [Google Scholar]
- AZEVEDO L.C.P., PEDRO M.A., SOUZA L.C., SOUZA H.P., JANISZEWSKI M., LUZ P.L., LAURINDO F.R. Oxidative stress as a signaling mechanism of the vascular response to injury: the redox hypothesis of restenosis. Cardiovasc. Res. 2000;47:436–445. doi: 10.1016/s0008-6363(00)00091-2. [DOI] [PubMed] [Google Scholar]
- BELTON O., BYRNE D., KEARNEY D., LEAHY A., FITZGERALD D.J. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000;102:840–845. doi: 10.1161/01.cir.102.8.840. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYYA D.K., LECOMTE M., DUNN A.J., MORGANS D.J., SMITH W.L. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Arch. Biochem. Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- BRADEN G.A., KNAPP H.R., FITZGERALD G.A. Suppression of eicosanoid biosynthesis during coronary angioplasty by fish oil and aspirin. Circulation. 1991;84:679–685. doi: 10.1161/01.cir.84.2.679. [DOI] [PubMed] [Google Scholar]
- BRUIJNS R.H.J., VAN KLEEF E.M., SMITS J.F.M., DEMEY J.G.R., DAEMEN M.A.P. Effects of chemical sympathectomy on angiotensin II-induced neotintimal growth in the balloon-injured rat carotid artery. J. Vasc. Res. 1998;35:124–133. doi: 10.1159/000025574. [DOI] [PubMed] [Google Scholar]
- CAMPBELL G.R., CAMPBELL J.H., MANDERSON J.A., HORRIGAN S., RENNICK R.E. Arterial smooth muscle-a multifunctional mesenchymal cell. Arch. Pathol. Lab. Med. 1988;112:977–986. [PubMed] [Google Scholar]
- CANDIPAN R.C., HSIUN P.T., PRATT R., COOKE J.P. Vascular injury augments adrenergic neurotransmission. Circulation. 1994;89:777–784. doi: 10.1161/01.cir.89.2.777. [DOI] [PubMed] [Google Scholar]
- CONNOLLY E., BOUCHIER-HAYES D.J., KAYE E., LEAHY A., FITZGERALD D., BELTON O. Cyclooxygenase isozyme expression and intimal hyperplasia in a rat Model of balloon angioplasty. J. Pharmacol. Exp. Ther. 2002;300:393–398. doi: 10.1124/jpet.300.2.393. [DOI] [PubMed] [Google Scholar]
- CLOWES A.W., REIDY M.A., CLOWES M.M. Kinetics of cellular proliferation after arterial injury, I: smooth muscle growth in the absence of endothelium. Lab. Investig. 1983;49:327–333. [PubMed] [Google Scholar]
- DALLE-LUCCA S.L., DALLE-LUCCA J.J., BORGES A.C., IHARA S.S., PAIVA T.B. Abnormal proliferative response of the carotid artery of spontaneously hypertensive rats after angioplasty may be related to the despolarized state of its smooth muscle cells. Braz. J. Med. Biol. Res. 2000;33:919–927. doi: 10.1590/s0100-879x2000000800008. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., VICKERY-CALRK L.M., OHLSTEIN E.H. Functional evidence that balloon angioplasty results in transient nitric oxide synthase induction. Eur. J. Pharmacol. 1994;255:81–89. doi: 10.1016/0014-2999(94)90085-x. [DOI] [PubMed] [Google Scholar]
- DORNAY M., GILAD V.H., GILAD G.M. Compensatory changes in contralateral sympathetic neurons of the superior cervical ganglion and in their terminals in the pineal gland following unilateral ganglionectomy. J. Neurosci. 1985;5:1522–1526. doi: 10.1523/JNEUROSCI.05-06-01522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARHY R.D., HO K.L., CARRETERO O.A., SCICLI A.G. Kinins mediate the antiproliferative effect of ramipril in rat carotid artery. Biochem. Biophys. Res. Commun. 1992;182:283–288. doi: 10.1016/s0006-291x(05)80142-1. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G.P. The action of peptides on the adrenal medulla. Release of adrenaline by bradykinin and angiotensin. J. Physiol. Lond. 1964;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-ALFONSO M.S., MARTORANA P.A., LICKA I., VAN EVEN P., TROBISCH D., SCHOLKENS B.A., PAUL M. Early induction of angiotensin I-converting enzyme in rat carotid artery after balloon injury. Hypertension. 1997;30 Part 1:272–277. doi: 10.1161/01.hyp.30.2.272. [DOI] [PubMed] [Google Scholar]
- FURUHASHI N., MIYAZAKI S., KAMURA M., EMURA S., YASUDA K. Role of endothelium and vasoconstrictor prostanoids in norepinephrine-induced vasoconstriction in isolated rat common carotid arteries. Clin. Exp. Hypertens. 2000;22:543–554. doi: 10.1081/ceh-100100090. [DOI] [PubMed] [Google Scholar]
- HANAFY K.A., KRUMENACKER J.S., MURAD F. NO, nitrotyrosine, and GMP in signal transduction. Med. Sci. Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- HANSSON S.R., POWELL J.S., DODSON T., LUMSDEN A., KELLY A.B., ANDERSON J.S., CLOWES A.W., HARKER L. Effects of angiotensin converting enzyme inhibition with cilazapril on intimal hyperplasia uninjured arteries and vascular grafts in the baboon. Hypertension. 1991;18:II70–II76. doi: 10.1161/01.hyp.18.4_suppl.ii70. [DOI] [PubMed] [Google Scholar]
- HANSSON G.R., GENG Y.J., HOLM J., HARDHAMMAR P., WENNMALM A., JANNISCHE E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J. Exp. Med. 1994;180:733–738. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HLA T., NEILSON K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENBERG N., ODORI T. Centripetal spread of arterial collateral endothelial hyperplasia after artery stenosis in the rat. Circ. Res. 1987;60:398–401. doi: 10.1161/01.res.60.3.398. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON H.G., HEIN L., FIJINAGA M., PRATT R.E. Modulation of vascular development and injury by angiotensin II. Cardiovasc. Res. 1999;41:689–700. doi: 10.1016/s0008-6363(98)00267-3. [DOI] [PubMed] [Google Scholar]
- JOLY G.A., SCHINI V.B., VANHOUTTE P.M. Balloon injury and interleukin-1 β induce nitric oxide synthase activity in rat carotid arteries. Circ. Res. 1992;71:331–338. doi: 10.1161/01.res.71.2.331. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Dose–response curves Molecular Pharmacology: a short course 1996Cambridge: Blackwell Science Inc; 22–42.ed. Kenakin, T. [Google Scholar]
- KÖHLER R., BRAKEMEIER S., KUHN M., BEHRENS C., REAL R., DEGENHARDT C., ORZECHOWSKI H.D., PRIES A.R., PAUL M., HOYER J. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ. Res. 2001;89:174–179. doi: 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- LAURINDO F.R., DE SOUZA H.P., PEDRO M., DE A., JANISZEWSKI M. Redox aspects to vascular response to injury. Methods Enzymol. 2002;352:432–454. doi: 10.1016/s0076-6879(02)52039-5. [DOI] [PubMed] [Google Scholar]
- LEMAY J., HOU Y., TREMBALY J., DE BLOIS D. Angiotensin I-converting enzyme activity and vascular sensitivity to angiotensin I in rat injured carotid artery. Eur. J. Pharmacol. 2000;394:301–309. doi: 10.1016/s0014-2999(00)00071-6. [DOI] [PubMed] [Google Scholar]
- LUCKHOFF A., BUSSE R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- LUM H., ROEBUCK K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- MAJESKY M.W., GIACHELLII C.M., REIDY M.A., SCHWARTZ S.M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair if arterial injury. Circ. Res. 1992;71:759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- MAJOR T.C., OVERHISER R.W., PANEK R.L. Evidence for NO involvement in regulating vascular reactivity in balloon-injured rat carotid artery. Am. J. Physiol. 1985;269:H966–H988. doi: 10.1152/ajpheart.1995.269.3.H988. [DOI] [PubMed] [Google Scholar]
- MASFERRER J.L., ZWEIFEL B.S., MANNING P.T., HAUSER S.D., LEAHY K.M., SMITH W.G., ISAKSON P.C., SEIBERT K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER P., CROWE R., LOESCH A., ANGLIN S., BURNSTOCK G., MCEWAN J.R. Neurocompensatory responses to balloon-catheter-induced injury of the carotid artery. J. Vasc. Res. 1997;34:31–34. doi: 10.1159/000159199. [DOI] [PubMed] [Google Scholar]
- NEBIGIL C., MALIK K.U. Prostaglandin synthesis elicited by adrenergic stimuli is mediated via alpha-2C and alpha-1A adrenergic receptors in cultured smooth muscle cells of rabbit aorta. J. Pharmacol. Exp. Ther. 1992;260:849–858. [PubMed] [Google Scholar]
- PASTERKAMP G., DE KLEIJN D.P.V., BORST C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc. Res. 2000;45:843–852. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- PENNING T.D., TALLEY J.J., BERTENSHAW S.R., CARTER J.S., COLLINS P.W., DOCTER S., GRANETO M.J., LEE L.F., MALECHA J.W., MIYASHIRO J.M., ROGERS R.S., ROGIER D.J., YU S.S., ANDERSON G.D., BURTON E.G., COGBURN J.N., GREGORY S.A., KOBOLDT C.M., PERKINS W.E., SEIBERT K., VEENHUIZEN A.W., ZHANG Y.Y., ISAKSON P.C. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3- (trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib) Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- POWELL J.S., CLOZEL J.P., MÜLLER R.K.M., KUHN H., HEFTI F., HOSANG M., BAUMGARTNER H.R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989;245:186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- PRITCHARD K.A., JR, ÓBANION M.K., MIANO J.M., VLASSIC N., BHATIA U.G., YOUNG D.A., STEMERMAN M.B. Induction of cyclooxygenase-2 in rat vascular smooth muscle cells in vitro and in vivo. J. Biol. Chem. 1994;289:8504–8509. [PubMed] [Google Scholar]
- RAKUGI H., WANG D.S., DZAU V.J., PRATT R.E. Potential importance of tissue angiotensin-converting enzyme inhibition in preventing neointima formation. Circulation. 1994;90:449–455. doi: 10.1161/01.cir.90.1.449. [DOI] [PubMed] [Google Scholar]
- REIDY M.A. Proliferation of smooth muscle cells at sites distant from vascular injury. Arteriosclerosis. 1990;10:298–305. doi: 10.1161/01.atv.10.2.298. [DOI] [PubMed] [Google Scholar]
- ROSS R. Atheroesclerosis–an inflammatory disease. New. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ R.S., DE BLOIS D., O'BRIEN E.R.M. The intima. Soil for atherosclerosis and restenosis. Circ. Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- SCHONBECK U., SUKHOVA G.K., GRABER P., COULTERM S., LIBBY P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am. J. Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL S.S., DULING B.R. Communication between feed arteries and micro vessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ. Res. 1986;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- STARKE K. Regulation of noradrenaline release by presynaptic receptor systems. Rev. Physiol. Biochem. Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- THOLLON C., FOUNERT-BOURGUINON M.P., SABOUREAU D., LESAGE L., REURE H., VANHOUTTE P.M., VILAINE J.P. Consequences of reduced production of NO on vascular reactivity of porcine coronary arteries after angioplasty: importance of EDHF. Br. J. Pharmacol. 2002;136:1153–1161. doi: 10.1038/sj.bjp.0704828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSCHOPE C., SCHULTHEISS H.P., WALTHER T. Multiple interactions between the rennin–angiotensin and the kallikrein–kinin systems: role of ACE inhibition and AT1 receptor blockade. J. Cardiovasc. Pharmacol. 2002;39:478–487. doi: 10.1097/00005344-200204000-00003. [DOI] [PubMed] [Google Scholar]
- UREN N.G., CRAKE T., LEFROY D.C., DE SILVA R., DAVIES G.J., MASERI A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardium infarction. N. Engl. J. Med. 1994;331:222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- WOLIN M.S., GUPTE S.A., OECKLER R.A. Superoxide in the vascular system. J. Vasc. Res. 2002;39:191–207. doi: 10.1159/000063685. [DOI] [PubMed] [Google Scholar]
- YAN Z., HANSSON G.K. Overexpression of inducible nitric oxide synthase by neointimal smooth muscle cells. Circ. Res. 1998;82:21–29. doi: 10.1161/01.res.82.1.21. [DOI] [PubMed] [Google Scholar]