Abstract
The selective oestrogen (ER) receptor modulator, raloxifene, is widely used in the treatment of postmenopausal osteoporosis, but may also possess cardioprotective properties. We investigated whether it directly suppresses myocyte contractility through Ca2+ channel antagonism in a similar way to 17β-oestradiol.
Cell shortening and Ca2+ transients were measured in single guinea-pig ventricular myocytes field-stimulated (1 Hz, 37°C) in a superfusion chamber. Electrophysiological recordings were performed using single electrode voltage-clamp.
Raloxifene decreased cell shortening (EC50 2.4 μM) and the Ca2+ transient amplitude (EC50 6.4 μM) in a concentration-dependent manner. At a concentration of 1 μM, raloxifene produced a 33±2% (mean±s.e.m) and 24±2% reduction, respectively (P<0.001, n=14 for both parameters).
These inhibitory actions were not observed in myocytes that had been incubated with the specific antagonist, ICI 182,780 (10 μM) (n=11).
Raloxifene (1 μM) shortened action potential durations at 50 and 90% repolarisation (P<0.05 and <0.001, respectively; n=27) and decreased peak L-type Ca2+ current by 45%, from −5.1±0.5 pA/pF to −2.8±0.3 pA/pF (P<0.001, n=18).
Raloxifene did not significantly alter sarcoplasmic reticulum Ca2+ content, as assessed by integrating the Na+/Ca2+ exchanger currents following rapid caffeine application.
The present study provides evidence for direct inhibitory actions of raloxifene on ventricular myocyte contractility, mediated through Ca2+ channel antagonism.
Keywords: Raloxifene; selective oestrogen receptor modulator; SERM; Ca2+ channel; calcium; excitation contraction coupling; EC coupling; cardiac myocyte; sarcoplasmic reticulum; action potential; ICI 182,780
Introduction
Cardiovascular disease is a major cause of death among postmenopausal women in the Western world (Mosca et al., 1997). Although observational studies have suggested that hormone replacement therapy may be cardioprotective (Wenger et al., 1993; Hayward et al., 2000), recent large trials using oral conjugated oestrogens and medroxyprogesterone acetate have not confirmed this (Grady et al., 2002). Consequently, there has been increasing interest in the use of alternative postmenopausal agents, which possess the benefits of traditional hormone replacement therapy while also being cardioprotective. Raloxifene, widely used in the treatment and prevention of postmenopausal osteoporosis, may be such an agent (Riggs & Hartmann, 2003). Being a selective oestrogen receptor (ER) modulator, it can act as an agonist or antagonist at the ER, depending on the target tissue (Dardes & Jordan, 2000; Lonard & Smith, 2002; Riggs & Hartmann, 2003). It therefore has the potential to exert beneficial oestrogenic effects on the cardiovascular system and bone, without producing harmful effects on the uterus and mammary glands (Delmas et al., 1997).
The potential mechanisms through which raloxifene may afford cardioprotection are poorly understood and are likely to involve direct as well as indirect actions, such as favourable changes in the lipid profile (Walsh et al., 1998). Animal studies investigating the effects of raloxifene on atherosclerosis have produced contradictory results (Bjarnason et al., 1997; Clarkson et al., 1998), while studies investigating the direct vascular actions of raloxifene have demonstrated beneficial effects. For example, raloxifene has been shown to exert acute, nongenomic, ER-mediated actions on rabbit coronary arteries and human umbilical vein endothelial cells through rapid activation of nitric oxide synthase (Figtree et al., 1999; Simoncini et al., 2002). In addition, raloxifene improves endothelial dysfunction and reduces blood pressure in spontaneously hypertensive male rats (Wassmann et al., 2002). Few studies have addressed the issue as to whether raloxifene exerts any direct actions on the heart. The finding that raloxifene reduces myocardial ischaemia-reperfusion injury in the canine heart is similar to that observed with the mammalian oestrogen, 17β-oestradiol, and highlights the similarities in myocardial action between these two compounds (Jovanovic et al., 2000; Zhai et al., 2000; Ogita et al., 2002).
It has long been known that 17β-oestradiol can exert direct actions on the heart through inhibition of the L-type calcium current (ICa,L), although these effects have tended to occur at supra-physiological concentrations (Jiang et al., 1992; Meyer et al., 1998). In the present study, we tested the hypothesis that raloxifene acts as a Ca2+ channel antagonist on the heart and suppresses ventricular myocyte contractility in a similar way to 17β-oestradiol. We report on novel direct cardiac actions of raloxifene that appear to be mediated via the ER and which result in decreased cell shortening.
Methods
Cell isolation and indo-1 loading
Experiments were performed on single left ventricular myocytes isolated from adult male guinea pigs (550–750 g) by enzymatic digestion as previously described (MacLeod & Harding, 1991). We chose to study male, rather than female, animals to avoid the potential problems associated with varying expression of the ER that occurs throughout the oestrus cycle. Myocytes were stored in Dulbecco's modified Eagle's medium solution at room temperature and loaded with 10 μM of the acetoxymethyl ester form of the Ca2+-sensitive fluorescent dye, indo-1 (Molecular Probes, Eugene, OR, U.S.A.) for 25 min. Once loaded, cells were not used for at least another 30 min to allow the intracellular indo-1-AM to be de-esterified. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Cell shortening and intracellular Ca2+ measurements
Cells were superfused with normal Tyrode, NT (containing in mM: NaCl 140, KCl 6, CaCl2 2, MgCl2 1, glucose 10, N-2-hydroxyethylpiperazine-N′′-2- ethanesulphonic acid (HEPES) 10, pH adjusted to 7.4. using NaOH) in a low volume chamber mounted on the stage of an inverted microscope. Two platinum electrodes, placed on either side of the chamber, were used to field stimulate the myocytes at a rate of 1 Hz. Myocytes were directly visualised using a × 40 oil immersion objective (Nikon) and a single rod shaped cell with clear striations, clean edges and good contractions when stimulated was chosen. Cell shortening was monitored at one end with a video edge-detector system and the indo-1 fluorescence emission ratio (i.e. ratio of light emitted at wavelengths 405 and 485 nm) was simultaneously recorded using a pair of photomultiplier tubes.
Once steady-state contractions were achieved, raloxifene (or 17β-oestradiol) was added to the superfusate and the new steady-state contractions and indo-1 ratio were recorded. Experiments were tightly paired so that changes in cell shortening and the indo-1 ratio, Δindo-1 ratio (i.e. peak minus resting indo-1 ratios), before and after the addition of raloxifene could be compared. This allowed the Δindo-1 ratio during field stimulation to be used as a qualitative measure of the effects of raloxifene, on the Ca2+ transient, even though the relationship between the indo-1 ratio and intracellular-free Ca2+ concentration is not linear. In addition, use of actual indo-1 ratios, rather than calculated Ca2+ concentrations, avoided the potential errors that can result from dye compartmentalisation during calibration experiments (Spurgeon et al., 1990).
All experiments were performed at 37°C and each bath of cells was changed following every exposure to raloxifene in order to avoid residual effects of incomplete washout of the drug.
Effects of ICI 182,780
The specific ER antagonist ICI 182,780 (Tocris, UK) was used to investigate whether the effects of raloxifene on cell shortening and the Ca2+ transient were mediated via the ER. Indo-1 loaded myocytes were incubated with 10 μM ICI 182,780 at room temperature for 1 h before being placed in the superfusion chamber. Cell shortening and the indo-1 ratio were measured in the continuing presence of 10 μM ICI 182,780 before and after the addition of 1 μM raloxifene. The concentration of ICI 182,780 chosen (10 μM) has previously been shown to be effective in inhibiting the acute relaxing effects of raloxifene on coronary arteries (Figtree et al., 1999).
Electrophysiology
Electrophysiological parameters were measured in control solution and then in the presence of 1 μM raloxifene using an Axoclamp-2B system and pCLAMP software (Axon Instruments, Foster City, CA, U.S.A.). Action potentials were elicited under current-clamp using 1 nA pulses (5 or 10 ms duration at 1 Hz) and action potential durations at 50 and 90% repolarisation (APD50 and APD90, respectively) were determined. ICa,L was recorded under voltage-clamp (discontinuous switch mode with the switching rate set between 4 and 6 kHz) using a holding potential of −40 mV. Care was taken to monitor the switching period to avoid false-clamp and optimise clamp speed. Test pulses of 200 ms duration, ranging from −45 to +50 mV, were imposed and ICa,L determined by subtracting the current obtained during cadmium application (100 μM) from the one elicited before cadmium application. High-resistance microelectrodes were used to limit dialysis of the cells. These were pulled from borosilicate glass (Clark Electromedical Instruments, Reading, U.K.) and had resistances between 20 and 30 MΩ when filled with solution containing: KCl 2 M; 1,2-di(2-aminoethoxy)ethane-NNN′N′-tetra-acetic acid (EGTA), 0.1 mM; HEPES, 5 mM, pH 7.2.
Steady-state activation parameters of ICa,L were obtained from the relationship between membrane conductance and the imposed potential. Steady-state inactivation parameters were analysed with double-pulse protocols. Conditioning pulses (200 ms duration) ranging from −55 to +50 mV were imposed from a holding potential of −50 mV. At 5 ms after the end of each conditioning pulse, a test pulse to +5 mV (200 ms duration) was applied to elicit the Ca2+ current.
Sarcoplasmic reticulum Ca2+ load was measured using rapid application of caffeine and integrating the resulting inward Na+/Ca2+ exchanger current (Varro et al., 1993). Myocytes were voltage-clamped at −80 mV and subjected to a train of 10 prepulses to +30 mV. In total 10 ms after the last pre-pulse, 10 mM caffeine was rapidly applied to the superfusing solution to produce a sudden release of Ca2+ from the sarcoplasmic reticulum and elicit the Na+/Ca2+ exchanger current. Caffeine application was maintained for 6 s to prevent sarcoplasmic reticular Ca2+ resequestration, and thereby ensure that the Na+/Ca2+ exchanger essentially removed all the Ca2+ released from the sarcoplasmic reticulum.
Drugs
Raloxifene hydrochloride (LY 139481) was a gift from Eli Lilly (Indianapolis, IN, U.S.A.). 17β-oestradiol was purchased from Sigma-Aldrich, U.K. All drugs were analytical grade and dissolved in dimethyl sulphoxide (DMSO) to make a 100 mM stock solution. The maximum final concentration of DMSO used (0.05%) had no significant effect on cell shortening or the Δindo-1 ratio (parameters in NT with 0.05% DMSO measured 103.4±4.1 and 96.4±2.9%, respectively, compared with equivalent parameter in NT alone; P=nonsignificant using paired t-test, n=7).
Statistical Analysis and curve fitting
Results are expressed as mean±s.e. m and analysed using the Student's t-test, repeated measures or one-way ANOVA with Bonferroni post-test as appropriate. A value of P<0.05 was considered significant.
The concentration – response relationships of raloxifene and the percentage inhibition of cell shortening and the Δindo-1 ratio were analysed by nonlinear regression (GraphPad Prism software, San Diego, CA, U.S.A.) using a modified Hill equation
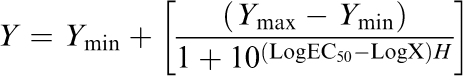 |
where Y is the response (percentage inhibition of cell shortening or the Δindo-1 ratio) to a particular concentration of raloxifene, X. The minimum inhibition (Ymin) is the percentage change with raloxifene at which there was no significant difference from baseline values in NT and the maximum inhibition (Ymax) is taken as 100%. This allowed the EC50 (raloxifene concentration producing 50% inhibition) and Hill coefficient (H) to be determined.
The relationships between membrane potentials and Ca2+ channel activation or inactivation were fitted with the Boltzmann function:
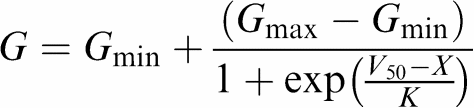 |
where G is the conductance at membrane potential X, Gmin and Gmax are the minimum and maximum conductances respectively and K is the slope factor. This allowed the membrane potential, V50, when half the channels are activated (d∞ variable) or inactivated (f∞ variable) to be determined in the presence and absence of raloxifene.
Results
Effects of raloxifene on cell shortening and the Ca2+ transient
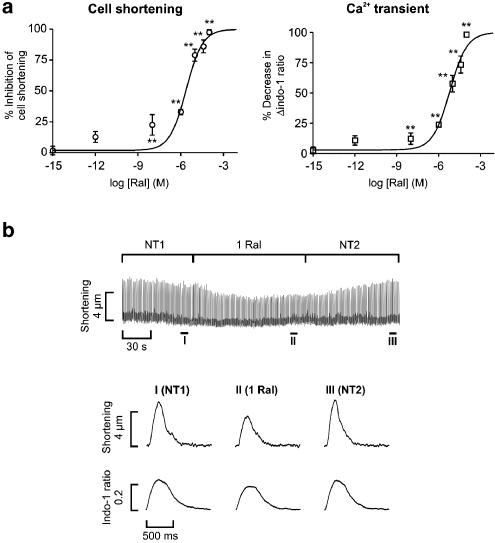
Raloxifene inhibited cell shortening and the Δindo-1 ratio during field stimulation in a concentration–dependent manner (Figure 1). The EC50 of raloxifene effect on cell shortening and the Δindo-1 ratio were 2.4 μM and 6.4 μM, respectively, with corresponding H values of 0.75 and 0.71. A concentration of 1 μM raloxifene (similar to the EC50 for cell shortening) was subsequently used for the majority of the following experiments. Representative recordings showing the acute actions of 1 μM raloxifene on cell shortening and the Δindo-1 ratio are shown in Figure 1.
Figure 1.
Acute effects of raloxifene on cell shortening and the Ca2+ transient. (a) Concentration–response curves: parameters are expressed as a percentage decrease (mean±s.e.m) relative to the initial value in control solution. Sigmoidal curves have been fitted using nonlinear regression, giving EC50 values of 2.4 μM for cell shortening and 6.4 μM for the Ca2+ transient (n=5–14 cells at each concentration, **P<0.01). (b) Continuous recording of cell shortening showing the effect of 1 μM raloxifene (1 Ral). NT1 and NT2 are the periods in control solution (normal Tyrode) before and immediately after addition of raloxifene. Magnified averaged traces of six consecutive contractions in parts I–III of the continuous trace plotted at a faster sweep speed showing twitch and Ca2+ transient morphologies.
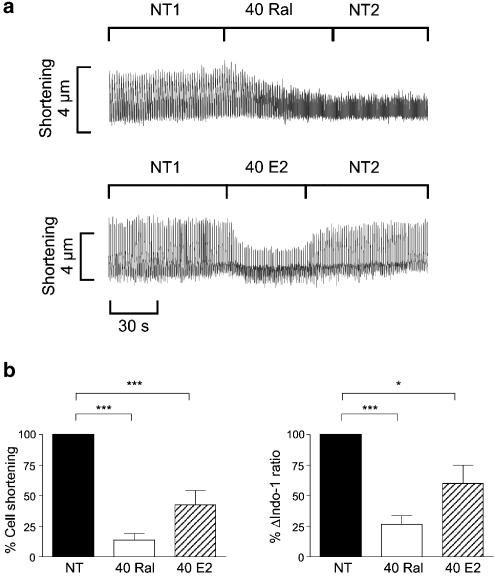
Similar experiments on field stimulated myocytes were performed with 17β-oestradiol in order to compare the acute actions of the two oestrogenic compounds. As the acute cardiac actions of 17β-oestradiol have previously been demonstrated to occur at supra-physiological concentrations (Jiang et al., 1992; Meyer et al., 1998), we chose to use a concentration of 40 μM in these experiments. Both raloxifene and 17β-oestradiol significantly decreased cell shortening and the Δindo-1 ratio (Figure 2). However, the speed of action of raloxifene appeared to be slower than that of 17β-oestradiol, as can be seen in the continuous traces. The effect of 40 μM raloxifene on cell shortening was irreversible, in contrast to that of 17β-oestradiol, which was quickly reversed upon washout with control solution.
Figure 2.
Comparison of raloxifene and 17β-oestradiol actions on cell shortening and the Ca2+ transient. (a) Representative continuous traces showing the decrease in cell shortening following the addition of 40 μM raloxifene (40 Ral) or 17β-oestradiol (40 E2) to the superfusing solution. NT1 and NT2 are the periods in control solution before and immediately after addition of the oestrogenic compounds, respectively. (b) Bar graphs showing cell shortening and Δindo-1 ratio in the presence of 40 μM raloxifene and 17β-oestradiol, expressed as a percentage of initial values. (n=5 in each group, *P<0.05, ***P<0.001)
Effects after myocyte incubation with ICI 182,780
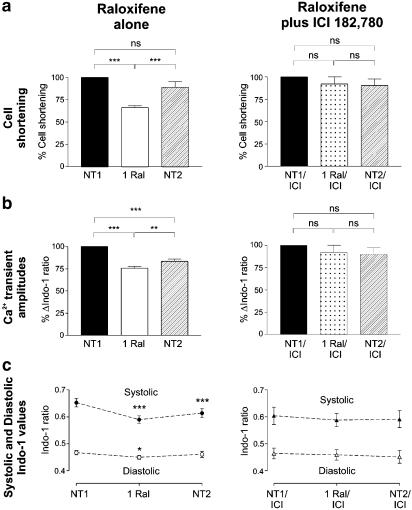
We next investigated whether the inhibitory actions of raloxifene on myocyte contractility were mediated through interactions with the ER using the specific ER antagonist, ICI 182,780. Control experiments were first performed to determine whether 10 μM ICI 182,780 had any direct actions of its own on cell contractility. We found that this concentration of the ER antagonist had no significant effects on cell shortening or Ca2+ transient parameters (amplitudes and kinetics) when acutely applied (comparing paired data before and after the addition of ICI 182,780, n=9) or after a 1 h incubation period (comparing unpaired data between cells incubated with and without ICI 182,780, n=12).
Raloxifene (1 μM) decreased cell shortening by 33±2% and the Ca2+ transient amplitude by 24±2% of control values (P<0.001, n=14 for both parameters, Figure 3). The suppression of cell shortening was reversible upon washout with NT, but the decrease in Ca2+ transient amplitude was only partially reversible despite an NT washout period in excess of 10 min. Raloxifene significantly decreased both diastolic and systolic indo-1 ratios compared with control values in NT. The decrease in diastolic indo-1 ratio was fully reversible upon washout with NT, whereas the change in systolic ratio was only partially reversible. Myocyte incubation with ICI 182,780 for 1 h completely abolished the inhibitory actions of raloxifene on cell shortening and the indo-1 ratios, suggesting that these effects were mediated through the ER.
Figure 3.
Effects of raloxifene on cell shortening and the indo-1 ratios before (left) and after (right) myocyte incubation with ICI 182,780. 1 μM raloxifene (1 Ral) decreased cell shortening (a), Ca2+ transient amplitudes (b) and systolic and diastolic indo-1 ratios (c) compared with initial values in control solution (NT1). These effects are no longer present in myocytes that have been preincubated 10 μM ICI 182,780 (ICI) for 1 h (n=11–14 in each group, NS=nonsignificant, *P<0.05, **P<0.01, ***P<0.001)
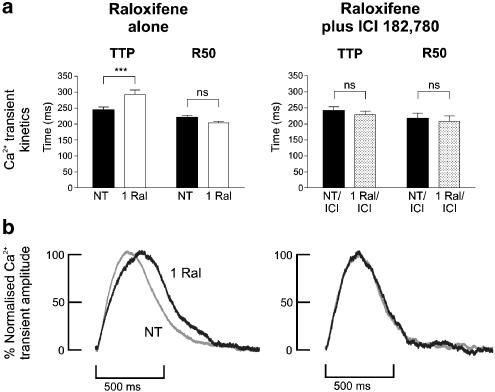
Raloxifene significantly increased the time taken to reach peak Ca2+ transient from 244±10 to 293±14 ms (P<0.001, n=14). However, this effect on Ca2+ transient kinetics was no longer observed after myocyte incubation with ICI 182,780 (Figure 4). Raloxifene did not significantly alter the time-to-50% decay of the Ca2+ transient (R50) in the absence or presence of ICI 182,780.
Figure 4.
Effects of 1 μM raloxifene on Ca2+ transient kinetics. (a) Bar graphs showing the effects of raloxifene (1 Ral) on the time-to-peak indo-1 ratio (TTP) and time-to-50% Ca2+ transient decay (R50) in freshly isolated myocytes (left) and in myocytes that have been preincubated with 10 μM ICI 182,780 for 1 h (right). (n=14, ***P<0.001). (b) Representative traces of Ca2+ transients in normal tyrode (NT, grey traces) and in the presence of raloxifene (black traces). Traces have been normalised to the same amplitude to facilitate comparison. Raloxifene can be seen to prolong TTP in the myocyte on the left, but not in the myocyte preincubated with ICI 182,780 on the right.
Raloxifene effects on the action potential profile
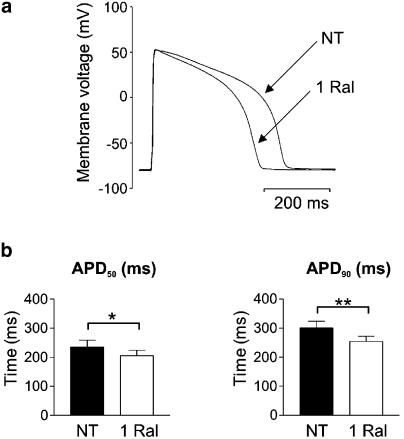
Raloxifene (1 μM) did not significantly alter the resting membrane potential, which was −75±1 mV (n=27) in both NT and in the presence of raloxifene. Figure 5 illustrates the effects of raloxifene on action potential waveform. Raloxifene decreased APD50 from 235±23 to 205±19 ms (P<0.05, n=27) and APD90 from 300±23 to 254±18 ms (P<0.01, Figure 5). These changes were fully reversible upon washout in NT (P=NS using paired t test between in NT before and after raloxifene addition; n=9).
Figure 5.
Effects of 1 μM raloxifene on action potential duration. (a) Sample recording of the membrane potential during steady–state stimulation at 1 Hz in normal Tyrode (NT) and in the presence of 1 μM Raloxifene (1 Ral). (b) Bar graphs showing significantly shortened times-to-50% and 90% repolarisation of the membrane (APD50 and APD90, respectively) in the presence of raloxifene (n=27, *P<0.05, **P<0.01).
Effects of raloxifene on the Ca2+ current
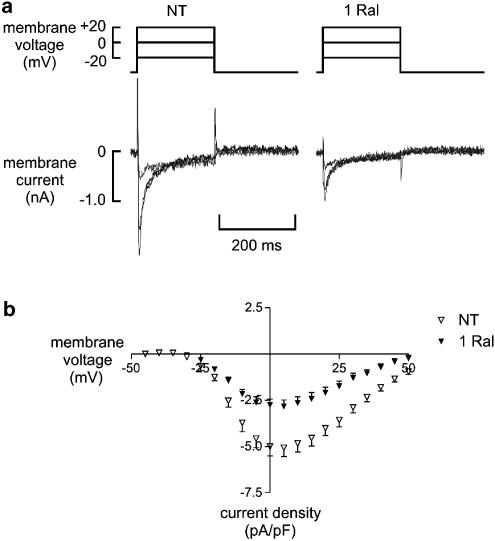
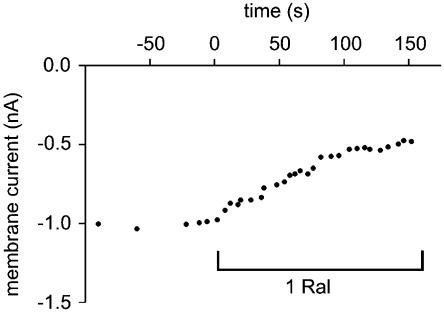
Raloxifene (1 μM) significantly decreased the peak ICa,L from −5.1±0. 5 to −2.8±0.3 pA/pF (P<0.001, n=18), that is, raloxifene inhibited peak ICa,L by 45% (Figure 6). This inhibition occurred over the range of voltages tested and was only partially reversible upon washout with NT. The raloxifene-induced inhibition of ICa,L took effect within 1 min (Figure 7). This could not be attributed to current rundown, which was found to be negligible over this period (0.0002±0.0001 pA/pF/min, n=7).
Figure 6.
Effects of 1 μM raloxifene on the L-type Ca2+ current (ICa,L). (a) Sample recordings showing ICa,L elicited at three depolarising potentials in normal Tyrode (NT) and in the presence of 1 μM raloxifene (1 Ral). (b) Current – voltage plot showing the mean peak ICa,L in NT (open triangle) and Ral (closed triangle) over the whole range of potentials tested (n=18).
Figure 7.
Time course of Ca2+ current inhibition by 1 μM raloxifene. Peak L-type Ca2+ current before and after the addition of 1 μM raloxifene to the superfusing solution.
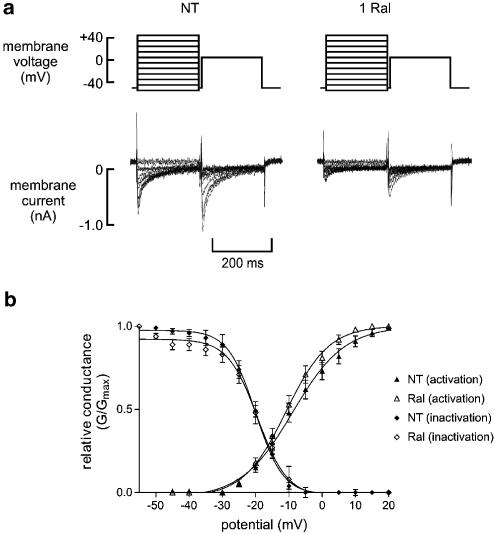
Raloxifene did not significantly shift the Ca2+ channel activation or inactivation curves (Figure 8). V50 for activation was −8.7±1.5 mV in NT and −10.4±1.7 mV after application of 1 μM raloxifene (P=NS, n=10), while V50 for inactivation was −20.9±1.5 and −19.2±1.7 mV respectively (P=NS).
Figure 8.
Effects of raloxifene on voltage-dependent activation and inactivation of the L-type Ca2+ current. (a), Sample traces showing currents elicited for calculation of inactivation parameters in normal Tyrode (NT) and after the application of 1 μM Raloxifene (1 Ral). Protocol plots are shown in the upper panel. (b) Boltzmann plots of steady-state activation and inactivation parameters.
Effects of raloxifene on sarcoplasmic reticulum Ca2+ content
We next investigated whether raloxifene exerts any additional effects on the sarcoplasmic reticulum Ca2+ load that may contribute to the decrease in cell shortening and Ca2+ transient amplitude. We found that there was no significant difference in the integrals of the Na+/Ca2+ exchanger currents obtained before compared with those obtained after the addition of 1 μM raloxifene (0.26±0.02 pC/pF compared with 0.24±0.03 pC/pF, respectively, n=13). Control experiments using two consecutive caffeine releases in NT (rather than a second release in the presence of raloxifene) revealed no significant change in SR Ca2+ load between the caffeine releases (results not shown).
Discussion
We have demonstrated for the first time that the widely used selective ER modulator, raloxifene, acutely suppresses ventricular myocyte contractility through Ca2+ channel antagonism. This appears to be similar to the known acute actions of the mammalian oestrogen, 17β-oestradiol, although importantly, the effects of raloxifene described here are observed at much lower concentrations.
The suppression of cell shortening and the Ca2+ transient produced by raloxifene appears to be mediated via the ER as incubation with the specific ER antagonist, ICI 182,780, completely abolished the inhibitory actions. Similarly, raloxifene also appears to reduce diastolic Ca2+ levels through an ER-dependent mechanism. Interactions of selective ER modulators with the ER are extremely complex and involve conformational changes in the ER-ligand structure that are distinct from those produced by 17β-oestradiol (Brzozowski et al., 1997; Pike et al., 1999; Lonard & Smith, 2002). It is therefore possible that, although both raloxifene and 17β-oestradiol both decrease ICa,L and reduce cardiac contractility, their mechanisms of action differ. The precise pathways linking ER activation with L-type Ca2+ channel activity are currently unknown and warrant further investigation. Although it is also unclear at present whether the acute Ca2+ channel antagonistic actions of 17β-oestradiol are mediated via the ER, the protective actions of this oestrogen in female ventricular myocytes on hypoxia-reoxygenation–induced Ca2+ loading does appear to be ER-mediated (Jovanovic et al., 2000). ICI 182,780 binds to the classical ER (ERα) with a similar binding affinity to raloxifene (Wijayaratne et al., 1999) and was used in our study as a competitive inhibitor of any acute ER-mediated actions of raloxifene. Our control experiments with ICI 182,780 alone showed that this compound does not significantly affect myocyte contractility acutely or after a 1 h incubation period. Although ICI 182,780 has been demonstrated to decrease levels of ERα expression when incubated with cells for 48 h (Wijayaratne et al., 1999), it is unlikely that this occurred to any significant degree in our experiments with an incubation period of 1 h.
During the mammalian cardiac action potential Ca2+ enters the cell predominantly through L-type Ca2+ channels and triggers further Ca2+ release from Ca2+ stores within the sarcoplasmic reticulum (Bers & Perez-Reyes, 1999). This process of Ca2+-induced Ca2+-release results in a rapid rise in intracellular free Ca2+ (the Ca2+ transient), culminating in myofilament shortening and cell contraction. A diminished Ca2+ transient amplitude may therefore result from a lower ICa,L or decreased sarcoplasmic reticulum Ca2+ content or both. The finding that sarcoplasmic reticulum Ca2+ content was unaltered in the presence of raloxifene suggests that inhibition of ICa,L is the main mechanism by which this compound decreases the Ca2+ transient amplitude and consequently cell shortening. Furthermore, a constant sarcoplasmic reticulum Ca2+ load coupled with reduced Ca2+ influx implies that Ca2+ efflux would also need to decrease in order to maintain steady-state Ca2+ balance in the presence of raloxifene (Eisner et al., 2000).
The profound inhibition of ICa,L by raloxifene over the range of potentials tested is similar to previously documented direct actions of 17β-oestradiol on cardiac myocytes (Jiang et al., 1992; Grohe et al., 1996; Nakajima et al., 1999a). However, raloxifene did not significantly shift the steady-state activation or inactivation curves. This contrasts with the known effects of 17β-oestradiol and dihydropyridine Ca2+ channel antagonists, which produce a leftward shift of the steady-state inactivation curves (Nakajima et al., 1999a,1999b; Porzig, 1990). Raloxifene therefore does not appear to preferentially exert inhibitory actions on activated or inactivated states of the L-type Ca2+ channel and consequently its mechanism of Ca2+ channel antagonism may be different to that of 17β-oestradiol.
The length and shape of the cardiac action potential are dependent upon concerted changes in depolarising (primarily Na+ and Ca2+) and repolarising (mainly K+) currents. In addition, APD is intricately related to sarcoplasmic reticulum Ca2+ content and Ca2+ transient amplitude (Terracciano et al., 1997; Han et al., 2002). Therefore, the shortened APD observed in the presence of raloxifene may solely be a function of ICa,L inhibition and a decreased Ca2+ transient amplitude. However, effects on repolarising K+ currents are also likely, particularly in view of the fact that shortening of the action potential was fully reversible upon washout of raloxifene, whereas inhibition of ICa,L was only partially reversible. Whether raloxifene directly modulates K+ currents is not known, although it does appear to reduce myocardial infarct size and the incidence of ventricular fibrillation during reperfusion partly via the opening of Ca2+-activated K+ channels (Ogita et al., 2002).
The observation that raloxifene no longer increased time to peak Ca2+ transient in myocytes that had been incubated with ICI 182,780 suggests that this effect is ER-dependent. Kinetics of the Ca2+ transient during field stimulation are determined by a variety of factors, including changes in ionic fluxes/membrane potential (both of which are affected by APD) and sarcoplasmic reticular handling of intracellular Ca2+. Consequently, the raloxifene-induced changes in time to peak Ca2+ transient may reflect effects of the compound on membrane Ca2+ and/or K+ currents. It is also possible that raloxifene exerts direct actions on sarcoplasmic reticular Ca2+/K+ fluxes, although our experiments were not specifically designed to test this. In support of the latter action, however, is the finding that the closely-related compound tamoxifen inhibits movement of Ca2+ out of the sarcoplasmic reticulum in isolated vesicles obtained from rat cardiac myocytes (Kargacin et al., 2000).
In conclusion, the present study provides evidence, at the level of single cardiac myocytes, that raloxifene directly affects cardiac function through Ca2+ channel antagonism. The clinical relevance of this effect remains to be determined.
Acknowledgments
We thank the British Heart Foundation for financial support.
Abbreviations
- APD
action potential duration
- DMSO
dimethyl sulphoxide
- EGTA, 1
2-di(2-aminoethoxy)ethane-NNN′N′-tetra-acetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2- ethanesulphonic acid
- ICa,L
L-type calcium channel
- ER
oestrogen receptor
References
- BERS D.M., PEREZ-REYES E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc.Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- BJARNASON N.H., HAARBO J., BYRJALSEN I., KAUFFMAN R.F., CHRISTIANSEN C. Raloxifene inhibits aortic accumulation of cholesterol in ovariectomized, cholesterol-fed rabbits. Circulation. 1997;96:1964. doi: 10.1161/01.cir.96.6.1964. [DOI] [PubMed] [Google Scholar]
- BRZOZOWSKI A.M., PIKE A.C., DAUTER Z., HUBBARD R.E., BONN T., ENGSTROM O., OHMAN L., GREENE G.L., GUSTAFSSON J.A., CARLQUIST M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- CLARKSON T.B., ANTHONY M.S., JEROME C.P. Lack of effect of raloxifene on coronary artery atherosclerosis of postmenopausal monkeys. J. Clin. Endocrinol. Metab. 1998;83:721. doi: 10.1210/jcem.83.3.4617. [DOI] [PubMed] [Google Scholar]
- DARDES R.C., JORDAN V.C. Novel agents to modulate oestrogen action. Br.Med.Bull. 2000;56:773–786. doi: 10.1258/0007142001903355. [DOI] [PubMed] [Google Scholar]
- DELMAS P.D., BJARNASON N.H., MITLAK B.H., RAVOUX A.C., SHAH A.S., HUSTER W.J., DRAPER M., CHRISTIANSEN C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N. Engl. J. Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- EISNER D.A., CHOI H.S., DIAZ M.E., O'NEILL S.C., TRAFFORD A.W. Integrative analysis of calcium cycling in cardiac muscle. Circ.Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- FIGTREE G.A., LU Y., WEBB C.M., COLLINS P. Raloxifene acutely relaxes rabbit coronary arteries in vitro by an estrogen receptor-dependent and nitric oxide-dependent mechanism. Circulation. 1999;100:1095–1101. doi: 10.1161/01.cir.100.10.1095. [DOI] [PubMed] [Google Scholar]
- GRADY D., HERRINGTON D., BITTNER V., BLUMENTHAL R., DAVIDSON M., HLATKY M., HSIA J., HULLEY S., HERD A., KHAN S., NEWBY L.K., WATERS D., VITTINGHOFF E., WENGER N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- GROHE C., KAHLERT S., LOBBERT K., MEYER R., LINZ K.W., KARAS R.H., VETTER H. Modulation of hypertensive heart disease by estrogen. Steroids. 1996;61:201–204. doi: 10.1016/0039-128x(96)00014-1. [DOI] [PubMed] [Google Scholar]
- HAN C., TAVI P., WECKSTROM M. Modulation of action potential by [Ca2+]i in modeled rat atrial and guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1047. doi: 10.1152/ajpheart.00573.2001. [DOI] [PubMed] [Google Scholar]
- HAYWARD C.S., KELLY R.P., COLLINS P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc.Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- JIANG C., POOLE-WILSON P.A., SARREL P.M., MOCHIZUKI S., COLLINS P., MACLEOD K.T. Effect of 17 beta-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br.J.Pharmacol. 1992;106:739–745. doi: 10.1111/j.1476-5381.1992.tb14403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVANOVIC S., JOVANOVIC A., SHEN W.K., TERZIC A. Low concentrations of 17beta-estradiol protect single cardiac cells against metabolic stress-induced Ca2+ loading. J. Am. Coll. Cardiol. 2000;36:948–952. doi: 10.1016/s0735-1097(00)00798-1. [DOI] [PubMed] [Google Scholar]
- KARGACIN M.E., ALI Z., WARD C.A., POLLOCK N.S., KARGACIN G.J. Tamoxifen inhibits Ca uptake by the cardiac sarcoplasmic reticulum. Pflugers Arch. Eur. J. Physiol. 2000;440:573–579. doi: 10.1007/s004240000318. [DOI] [PubMed] [Google Scholar]
- LONARD D.M., SMITH C.L. Molecular perspectives on selective estrogen receptor modulators (SERMs): progress in understanding their tissue-specific agonist and antagonist actions. Steroids. 2002;67:15–24. doi: 10.1016/s0039-128x(01)00133-7. [DOI] [PubMed] [Google Scholar]
- MACLEOD K.T., HARDING S.E. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J. Physiol. 1991;444:481–498. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER R., LINZ K.W., SURGES R., MEINARDUS S., VEES J., HOFFMANN A., WINDHOLZ O., GROHE C. Rapid modulation of L-type calcium current by acutely applied oestrogens in isolated cardiac myocytes from human, guinea-pig and rat. Exp. Physiol. 1998;83:305–321. doi: 10.1113/expphysiol.1998.sp004115. [DOI] [PubMed] [Google Scholar]
- MOSCA L., MANSON J.E., SUTHERLAND S.E., LANGER R.D., MANOLIO T., BARRETT-CONNOR E. Cardiovascular disease in women : a statement for healthcare professionals from the american heart association. Circulation. 1997;96:2468–2482. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., IWASAWA K., OONUMA H., MORITA T., GOTO A., WANG Y., HAZAMA H. Antiarrhythmic effect and its underlying ionic mechanism of 17beta-estradiol in cardiac myocytes. Br. J. Pharmacol. 1999a;127:429–440. doi: 10.1038/sj.bjp.0702576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA T., IWASAWA K., OONUMA H., IMUTA H., HAZAMA H., ASANO M., MORITA T., NAKAMURA F., SUZUKI Ji., SUZUKI S., KAWAKAMI Y., OMATA M., OKUDA Y. Troglitazone Inhibits voltage-dependent calcium currents in guinea pig cardiac myocytes. Circulation. 1999b;99:2942–2950. doi: 10.1161/01.cir.99.22.2942. [DOI] [PubMed] [Google Scholar]
- OGITA H., NODE K., ASANUMA H., SANADA S., LIAO Y., TAKASHIMA S., ASAKURA M., MORI H., SHINOZAKI Y., HORI M., KITAKAZE M. Amelioration of ischemia- and reperfusion-induced myocardial injury by the selective estrogen receptor modulator, raloxifene, in the canine heart. J. Am. Coll. Cardiol. 2002;40:998–1005. doi: 10.1016/s0735-1097(02)02056-9. [DOI] [PubMed] [Google Scholar]
- PIKE A.C., BRZOZOWSKI A.M., HUBBARD R.E., BONN T., THORSELL A.G., ENGSTROM O., LJUNGGREN J., GUSTAFSSON J.A., CARLQUIST M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORZIG H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev. Physiol. Biochem. Pharmacol. 1990;114:209–262. doi: 10.1007/BFb0031020. [DOI] [PubMed] [Google Scholar]
- RIGGS B.L., HARTMANN L.C. Selective estrogen-receptor modulators - mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- SIMONCINI T., GENAZZANI A.R., LIAO J.K. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105:1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- SPURGEON H.A., STERN M.D., BAARTZ G., RAFFAELI S., HANSFORD R.G., TALO A., LAKATTA E.G., CAPOGROSSI M.C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am.J.Physiol. 1990;258:H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- TERRACCIANO C.M., TWEEDIE D., MACLEOD K.T. The effects of changes to action potential duration on the calcium content of the sarcoplasmic reticulum in isolated guinea-pig ventricular myocytes. Pflugers Arch. 1997;433:542–544. doi: 10.1007/s004240050312. [DOI] [PubMed] [Google Scholar]
- VARRO A., NEGRETTI N., HESTER S.B., EISNER D.A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993;423:158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- WALSH B.W., KULLER L.H., WILD R.A., PAUL S., FARMER M., LAWRENCE J.B., SHAH A.S., ANDERSON P.W. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- WASSMANN S., LAUFS U., STAMENKOVIC D., LINZ W., STASCH J.P., AHLBORY K., ROSEN R., BOHM M., NICKENIG G. Raloxifene improves endothelial dysfunction in hypertension by reduced oxidative stress and enhanced nitric oxide production. Circulation. 2002;105:2083–2091. doi: 10.1161/01.cir.0000014618.91633.67. [DOI] [PubMed] [Google Scholar]
- WENGER N.K., SPEROFF L., PACKARD B. Cardiovascular health and disease in women. N. Engl. J. Med. 1993;329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- WIJAYARATNE A.L., NAGEL S.C., PAIGE L.A., CHRISTENSEN D.J., NORRIS J.D., FOWLKES D.M., MCDONNELL D.P. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- ZHAI P., EURELL T.E., COTTHAUS R., JEFFERY E.H., BAHR J.M., GROSS D.R. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2766–H2775. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]