Abstract
We have studied the effects of acute and chronic treatment with the anticonvulsant lamotrigine (LTG) on basal and stimulated extracellular 5-hydroxytryptamine (5-HT), dopamine (DA) and their metabolites in the hippocampus of freely moving rats using in vivo microdialysis.
Acute LTG (10 and 20 mg kg−1) decreased extracellular 5-HT, but had no effect on its metabolite 5-hydroxyindoleacetic acid (5-HIAA). Dialysate DA was also decreased by LTG as were its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). When transmitter release was stimulated by either 50 μM veratridine or 100 mM K+, marked increases in the release of both transmitters occurred, but LTG was entirely without effect on this.
In chronic experiments, rats were dialysed after 2, 4, 7, 14 and 21 days of LTG treatment (5 mg kg−1, twice daily). During this period a progressively different response to the drug was seen. After 2 days, basal extracellular 5-HT was significantly greater in treated rats than control rats. This effect persisted up to 14 days, but by 21 days 5-HT levels had returned to control values. 5-HIAA levels were unaltered and there was no effect of LTG on veratridine or K+ stimulated 5-HT release.
Similarly, DA concentrations significantly increased after 2–7 days of LTG treatment, but returned and remained at basal values thereafter. During the treatment period LTG had no effect on extracellular DOPAC, but HVA followed a similar pattern to its parent transmitter. As with 5-HT, at no time point did LTG have any effect on stimulated DA release.
These neurochemical findings observed in these experiments are considered in relation to the use of LTG in bipolar disorder.
Keywords: Lamotrigine, 5-hydroxytryptamine, dopamine, hippocampus, dialysis
Introduction
Lamotrigine (LTG) is a comparatively novel antiepileptic agent (Btaiche & Woster, 1995; Bazil, 2002) whose mechanism of action is considered to be a reduction in glutamate release resulting from an inhibitory effect on type IIA sodium channels (Xie et al., 2001; Remy et al., 2003) and consequent neurotransmitter exocytosis (Lees & Leach, 1993; Ahmad et al., 1995). Since its introduction as an antiepileptic, LTG has found considerable success in the treatment of some patients suffering from mania and manic-depression (Bowden et al., 2003; Goldsmith et al. 2003). It has been suggested that LTG may be particularly effective in the acute treatment of bipolar depression (Calabrese et al., 2002). Interestingly, both valproate and carbamazepine, both used in bipolar illness, have been shown to alter 5-hydroxytryptamine (5-HT) and dopamine (DA) release in several brain regions (Whitton & Fowler, 1991; Biggs et al., 1992; Okada et al., 1997; 1998; Ichikawa & Meltzer, 1999).
Although there is a fair literature on the clinical aspects of this use of LTG, there is comparatively little on the possible underlying neurochemical basis of this therapeutic effect and to our knowledge few, if any, in vivo studies have been carried out to study this. To this end, one logical approach would be to investigate the effects of LTG on serotonergic and dopaminergic neurotransmission in the CNS, given that these two transmitters are strongly implicated in mania and depression. Shiah et al. (1998) reported the effect of LTG on 5-HT1A receptors in healthy human males using body temperature and plasma cortisol as an index of 5-HT1A receptor function. LTG was found to have no apparent effect on 5-HT1A receptors on this basis, possibly indicating that LTG may have little, if any, effect on the serotonergic system. However, the authors themselves conceded that their study was limited by the use of a low LTG dose, small sample size and lack of a placebo control. In vitro, using human platelets and rat brain synaptosomes LTG was observed to inhibit the uptake of 5-HT in a manner that appeared to be independent of any effect on sodium channels (Southam et al., 1998). These authors concluded that LTG must be interacting with the 5-HT transporter and observed similar findings for DA and noradrenaline. In addition Sotham et al. (1998) found that LTG inhibited p-chloroamphetamine-induced 5-HT syndrome in vivo, which they concluded supports an inhibitory effect of LTG on 5-HT reuptake in the whole animal. Subsequently, it has been found that in LTG-treated children suffering from intractable epilepsy plasma 5-HT was significantly decreased, while urinary 5-HT and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA) were unaltered by the drug (Jovic et al., 1999). The relevance of these observations to 5-HT function in the CNS is unclear, however, since the correlation between plasma 5-HT and CNS serotonergic activity is essentially nonexistent. Vinod & Subhash (2002) observed that oral administration of LTG for 7 days led to a downregulation of CNS 5-HT1A receptors in the rat clearly indicating potential changes in serotonergic transmission as a result of LTG treatment.
In the case of DA, in animal studies, interest has in part been directed to a possible effect of LTG in models of Parkinson's disease (PD). LTG was not seen to alter rotations in rats treated with either reserpine or 6-hydroxydopamine following a challenge with a DA agonist, suggesting that LTG would have little value in PD patients or that it significantly alters dopaminergic transmission (Loschmann et al., 1995). Kaur & Starr (1996) observed that LTG decreased spontaneous motor activity in normal mice, which might indicate decreased dopaminergic transmission in the basal ganglia. LTG has been found to decrease striatal tissue content of DA and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and this was reflected by decreased activity of the DA synthetic enzyme tyrosine hydroxylase (Vriend & Alexiuk, 1997). These observations might also reflect a decrease in dopaminergic transmission.
Given the interest in the use of LTG in bipolar disorder and mania, we considered it to be of interest to look at the effects of LTG on two parameters that may be relevant to these conditions. In the present study, we have therefore investigated the effects of LTG on extracellular 5-HT, DA and their metabolites. In order to do this, we have given LTG both acutely and chronically under both basal and stimulated conditions using in vivo microdialysis in the hippocampus of the freely moving rat. This brain region was chosen in the light of its association with a number of affective psychiatric disorders, including bipolar disorder (Phillips et al., 2003).
Methods
Animals and microdialysis procedures
All procedures were performed in accordance with the Animals (Scientific Procedures) Act 1986. Male Wistar rats (270 – 330 g; Harlan Olac), six per group in all experiments, were anaesthetised with isoflourane and implanted with concentric dialysis probes of a construction previously described (Whitton et al., 1992). Probes were implanted into the ventral hippocampus (A – 5.0 mm, L – 5.0 mm from bregma and 7.5 mm below dura) using stereotaxic coordinates taken from the atlas of Paxinos & Watson (1982). The following day, hippocampi were perfused with artificial cerebrospinal fluid (aCSF) of a composition previously described (Whitton et al., 1992) but without the addition of the 5-HT reuptake inhibitor citalopram. Following a 1 h equilibration period, four 30 min samples were taken to establish basal transmitter release prior to systemic drug injection or infusion for various periods via the dialysis probe.
Acute study
In these investigations, LTG was given as a single injection acutely on the day of the experiment (LTG dose 10 – 20 mg kg−1). 5-HT and DA release were evoked by the presence of either 50 μM veratridine or 100 mM K+ in the aCSF. In the latter case, the osmolarity of the aCSF was maintained by the removal of an equivilent quantity of NaCl. In these experiments the veratridine or 100 mM K+ was infused twice: firstly after sample 4 (S1) and then after sample 10 (S2) with LTG being given after sample 7. Using peak release values, S2/S1 ratios were then taken, converted to percentages and used to determine the effects of LTG on evoked transmitter release.
Chronic studies
In these experiments, LTG was Given chronically for periods up to 21 days (LTG dose 2.5 –5 mg. kg−1 twice daily). During chronic experiments different groups of rats were used for each time point (2, 4, 7, 14 and 21 days of treatment), and the final dose of LTG was given on the day before rats were dialysed. Rats administered 2.5 mg kg−1 LTG (twice daily) were only dialysed after 21 days of treatment. After chronic LTG treatment, only one single pulse of either of the two stimulants was infused after collection of sample 4. The possibility of using the same rats during the time course of the chronic experiments, although attractive, was discarded. We have previously studied the effects of treatments over just 3 days on monoamine and amino-acid release in the hippocampus using the same probes. After day 3 recoveries of substances, especially amino acids, fell dramatically and gross histological examination revealed very extensive gliosis in the entire vicinity of the probe. The alternative to this would be to have a guide cannula in place, and insert the dialysis probe into the same animal repeatedly for each sampling time point. Although we have not attempted such a procedure before, it is our view that the repeated tissue trauma involved would outweigh more than the advantages. The doses of LTG were chosen on the basis of our previous experience with the drug (e.g. Ahmad et al., 1995), and values were shown to be effective in a number of animal models of neurological disorders following an extensive review of the literature. In general, the doses of LTG used in the present study are at the lower end of those generally employed. Furthermore, it is important to note that these doses give plasma concentrations that are within the upper therapeutic range for LTG used in human patients in the clinic (Ahmad et al., unpublished data; Bowden et al., 2003).
Monoamine and amino-acid analysis
Dialysates were analysed for 5-HT, 5-HIAA, DA, DOPAC and HVA using high-performance liquid chromatography (HPLC) followed by electrochemical detection as previously described (Whitton & Fowler, 1991; Biggs et al., 1992). Basal hippocampal dialysate concentrations of 5-HT , 5-HIAA, DA, DOPAC and HVA were 96.3±6.4, 9830±103, 54.6±4.2, 801±68 and 1643±119 fmol 10 μl−1, respectively corrected for 18 – 20% in vitro recovery (mean±s.e.m. n=70). These values are close to those we have found in the hippocampus in previous studies (Whitton & Fowler, 1991; Biggs et al., 1992; Whitton et al., 1994b).
Statistics
Statistics were performed using one-way ANOVA with repeated measures followed by Dunnett's test or in some cases Student's t-test.
Drugs
LTG isethionate (Glaxo-Wellcome, Ware, U.K.) was dissolved in distilled water and administered in a volume of 1 ml kg−1. Control rats were given an equal volume of the vehicle. Veratridine (Sigma, Poole, U.K.) was dissolved in the aCSF and infused directly into the hippocampus via the dialysis probe.
Results
Acute study
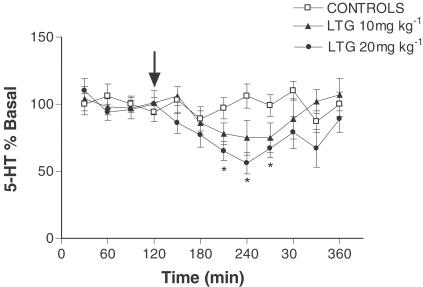
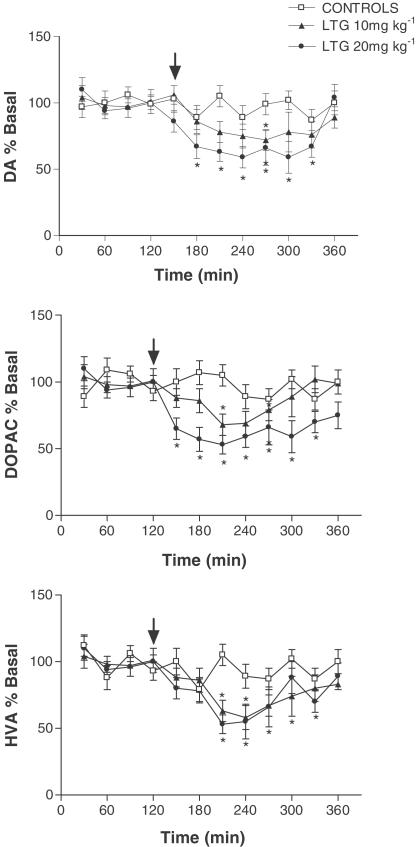
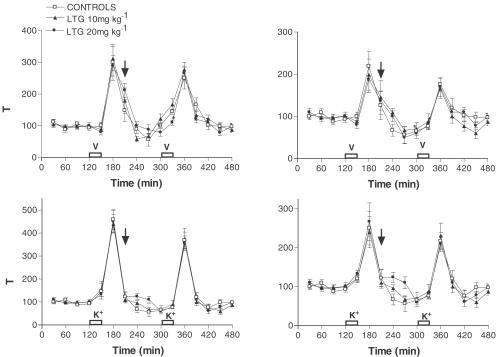
Acute LTG administration caused a dose-dependent decrease in hippocampal extracellular 5-HT leading to a maximal reduction of around 50% (F (2, 11)=8. 60, P<0.0017; Figure 1). Acute LTG had no effect on extracellular 5-HIAA suggesting no significant change in 5-HT metabolism (data not shown). In a manner similar to that seen with 5-HT, LTG dose-dependently decreased extracellular DA levels (F (2, 11)=9.02, P<0.0014; Figure 2). However, unlike 5-HT, where metabolism did not appear to be altered, LTG had a clear effect on hippocampal DA metabolism. Both extracellular DOPAC (F (2, 11)=10.68, P<0.0006) and HVA (F (2, 11)=7.09, P<0.0042) were dose-dependently decreased following LTG treatment, suggesting a reduction in DA metabolism (Figure 2). When either veratridine or high K+ were added to the aCSF rapid increases in 5-HT release were observed up to around 300 – 400% above basal, with levels returning to prestimulus values within 30–60 min, and this was associated with a concomitant decrease in 5-HIAA (Figure 3). However, unlike its effect on basal 5-HT release LTG had no effect on evoked 5-HT release (F (2, 15)=0.363, P<0.698 and F (2, 15)=0.282, P<0.756 in veratridine and K+-treated rats, respectively) or 5-HIAA levels (Figure 3). When veratridine or high K+ were infused to evoke DA release, levels rose by 200 – 250% with comparatively little effect on its metabolites. As with 5-HT, LTG had no effect on evoked release of DA (F (2, 15)=0.164, P<0.849 and F (2, 15)=1.312, P<0.284 in veratridine and K+-treated rats, respectively) or extracellular levels of DOPAC or HVA (Figure 3). Both veratridine and K+ infusion produced a period (15 – 30 min) of behavioural stimulation in the rats comprising increased locomotion, grooming, rearing and in some animals occasional ‘wet dog shakes'. No other changes in behaviour were observed during any of the experiments reported.
Figure 1.
Effect of acute LTG on extracellular 5-HT in the hippocampus. Data are the mean±s.e.m. of six animals in each group. Administration of LTG is indicated by the arrow. *Denotes significant difference from first four basal values.
Figure 2.
Effect of acute LTG on extracellular DA, DOPAC and HVA in the hippocampus. Data are the mean±s.e.m. of six animals in each group. Administration of LTG is indicated by the arrow. *Denotes significant difference from first four basal values.
Figure 3.
Effect of acute LTG on veratridine (a; upper panel) or 100 mm K+-evoked (b; lower panel) 5-HT and DA in the hippocampus. Data are the mean±s.e.m. of six animals in each group. Administration of LTG is indicated by the arrow.
Chronic study
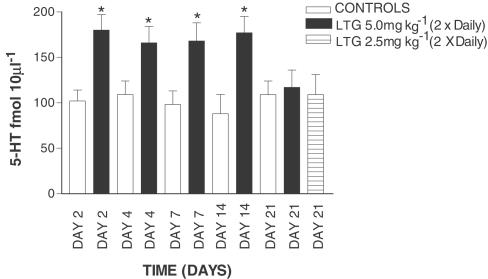
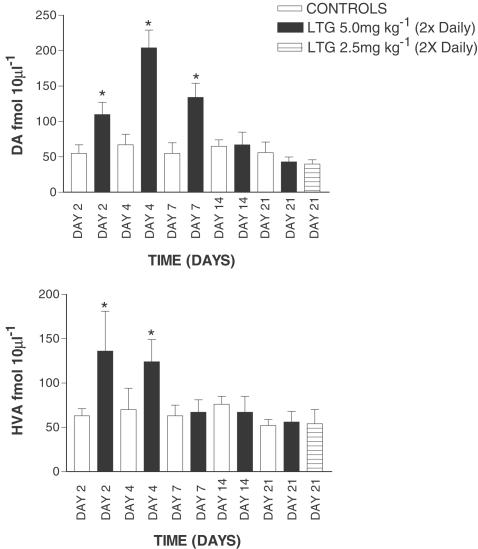
In contrast to the observations following acute LTG treatment, the effects of ‘chronic' treatment were very different, even after a short period of time. The data shown in all chronic and subchronic experiments represent the mean of the first four samples from each rat. After 2 days of LTG treatment (5 mg kg−1, twice daily) 5-HT levels were significantly higher in drug-treated controls compared with vehicle-treated controls (Figure 4). This pattern continued up to 14 days of LTG treatment but by 21 days basal dialysate 5-HT in drug-treated rats was not significantly different from control values (Figure 4). LTG given at a lower dose (2.5 mg kg−1) over 21 days produced a similar lack of effect on dialysate 5-HT at day 21 (Figure 4). A broadly similar picture to that seen with 5-HT was also found with hippocampal dialysate DA. As shown in Figure 5, after 2 days of LTG treatment DA levels were significantly higher than in control rats. This effect continued up to 7 days, after which DA levels returned to basal values (Figure 5). Evidence of some change in DA metabolism was also apparent as dialysate HVA largely parallelled the LTG-induced changes in the parent transmitter (Figure 5). The possibility that these changes in either 5-HT or DA levels during chronic LTG treatment are pharmacokinetic, perhaps the result of LTG reaching a steady state over the period of the experiment is highly unlikely. We have observed that during treatment with 5 mg kg−1 LTG, twice daily, plasma, whole brain and extracellular LTG concentrations are all maximal by day 4 of treatment, and show very little fluctuation there after (up to 21 days; Ahmed et al., unpublished data). At no point of time was LTG observed to have any significant effect on either 50 μM veratridine or 100 mMK+-evoked increases in either 5-HT or DA (data not shown). Thus, the effects of the drug were entirely restricted to basal release of the two transmitters studied.
Figure 4.
Effect of chronic LTG on extracellular 5-HT in the hippocampus. Data are the mean±s.e.m. of six animals in each group. Administration of LTG is indicated by the arrow. *Denotes significant difference from respective vehicle-treated rats using unpaired Student's t-test.
Figure 5.
Effect of chronic LTG on extracellular DA and HVA in the hippocampus. Data are the mean±s.e.m. of six animals in each group. Administration of LTG is indicated by the arrow. *Denotes significant difference from respective vehicle-treated rats using unpaired Student's t-test.
Discussion
The present findings show similar effects of LTG on both extracellular hippocampal 5-HT and DA. Acutely, LTG causes a dose-related decrease in both of these transmitters (Figures 1, 2). Chronic treatment with the drug reveals a somewhat more complex picture with both transmitters being elevated during the early stages of treatment, but in each case returning to basal levels within 21 days (5-HT; Figure 4) or less (DA; Figure 5). The acute effects of LTG in decreasing extracellular 5-HT, and particularly DA may, on the face of it at least, appear to be in line with what might be predicted for LTG as an anti-manic agent. LTG appears to act therapeutically, in epilepsy at least, as a result of inhibiting the release of neurotransmitters, in particular glutamate (Lees & Leach, 1993; Ahmad et al., 1998), very probably as a result of an inhibitory effect on type IIA sodium channels (Xie et al., 2001). Given that both hippocampal 5-HT and DA release are largely abolished by tetrodotoxin (e.g. Whitton et al., 1994a, b), indicating the well-established sodium channel involvement in their release process, this would seem a logical target for LTG in decreasing their release. The failure of LTG to alter evoked, particularly veratridine-evoked, 5-HT and DA release is therefore somewhat surprising. High K+-evoked transmitter ‘release' is almost certainly not restricted to a neuronal origin but also involves contributions from glial sources as well, which may not be sodium-dependent. Veratridine, however, acts on sodium channels and its effects should therefore be a prime target for LTG. Given that the effects of LTG have been ascribed to a particular effect on type IIA sodium channels (Xie et al., 2001), it may be the lack of selectivity of veratridine for specific sodium channel types that accounts for our present observations.
We are not clear as to why more prolonged treatment with LTG should, for the first few days, have such a different effect to acute treatment on basal extracellular 5-HT and DA, which declines thereafter. It could be that this initial effect constitutes a pharmacokinetic phenomenon since LTG concentrations in plasma, brain and extracellular fluid continue to rise in the first 4 days of chronic treatment, but are stable thereafter up to 21 days. However, we have also found that a single 10 mg kg−1 dose of LTG gives a lower peak plasma and brain LTG concentration than seen after 2 days of 5 mg kg−1 LTG administered twice daily (Ahmad et al., unpublished data). It would therefore seem that it is the sustained presence of LTG in the CNS during chronic treatment, even for a relatively short period of time, rather than the peak concentration of the drug, that determines the different effects observed between the acute and chronic dosing regimens. It therefore seems unlikely that different receptors mediate the responses to LTG seen in the acute and chronic studies. Since we have observed here progressive changes in basal levels of these transmitters up to day 21 of LTG treatment, a pharmacokinetic explanation does not, therefore, seem adequate. If this is the case, another possibility could be a progressive effect of LTG on the homoeostatic mechanisms that regulate the release of 5-HT and DA in the CNS.
Both 5-HT and DA release are controlled by multiple systems involving, particularly in the case of 5-HT, a number of autoreceptors as well as the effect of reuptake carriers and also regulation by other transmitters such as glutamate. Hippocampal 5-HT and DA release and metabolism are both partly under the control of glutamatergic NMDA receptors, stimulation of which leads to a decrease in extracellular levels of both transmitters (Bequet et al., 1990; Losher et al., 1991; Whitton et al., 1994a, b). Given that LTG decreases extracellular glutamate (Ahmad et al., 1995), this could lead to a secondary effect on the release of 5-HT and DA, but this might be expected to produce the opposite effect to the acute actions of LTG observed here. However, given the variety of ionotropic glutamatergic receptors, which can exert opposite effects on hippocampal 5-HT and DA release (Whitton et al., 1994a, b; Maione et al., 1997), it is difficult to predict the outcome of a net drop in extracellular glutamate on the release of 5-HT or DA. Although on the basis of the current data it is speculative, a possible explanation for the effects of chronic LTG on 5-HT and DA release, we believe, may involve up- or downregulation of receptors and/or uptake carriers, which regulate their extracellular levels. To establish this possibility it would have been necessary to challenge LTG-treated animals with a high dose of the drug during and after chronic treatment. However, the observations by Vinod & Subhash (2002) that 7-day treatment with LTG downregulates cortical, but not hippocampal, 5-HT1A receptors illustrate that such changes do occur. An alteration of 5-HT1A receptor density by LTG at the level of the raphe nuclei would have a profound effect on serotonergic transmission, as appears to be the case for at least some antidepressant and mood stabilising drugs such as lithium (Haddjeri et al., 2000). Similar arguments may also apply to the effects of LTG on extracellular DA also but a resolution to these speculations is beyond the scope of the present study.
As far as the therapeutic effects of LTG are concerned, the significance of the present data is necessarily speculative. With regard to its antiepileptic efficacy the most significant action of LTG is very probably its effect on glutamate release. However, both 5-HT and DA appear to play a role in the aetiology of some types of epilepsy (e.g. Deransart & Depaulis, 2002; Toczek et al., 2003), and the effects of LTG observed here could therefore have some significance in its antiepileptic profile. With regard to the role of LTG in bipolar disorder, any effect of the drug on either 5-HT or DA release could be of potential therapeutic significance, although it is the chronic effects that are likely to be the most relevant. The acute decrease in extracellular DA and its metabolites by LTG is in accordance with the observations by Vriend & Alexiuk (1997) that the drug reduces striatal content of DA, DOPAC and HVA, as well as the finding that, in mice, LTG decreases locomotor activity (Kaur & Starr, 1996). However, since there are, to our knowledge, no other studies on the chronic effects of LTG on extracellular DA and its metabolites, there is no way to know at present whether the observations cited above would have been sustained beyond the acute phase. With regard to bipolar illness an overall decrease in DA release or transmission would be considered a desirable outcome. Our chronic findings do not support a decrease in extracellular DA, at least in the hippocampus, but the presence of increased synaptic quantities of DA, as observed here, could lead to a downregulation in postsynaptic DA receptor density, and thereby a decrease in dopaminergic transmission and could account for the efficacy of LTG in this disorder (Dursun & Deakin, 2001; Bowden et al., 2003; Mahli et al., 2003).
Interestingly, two other drugs used in bipolar disorder, valproate and carbamazepine have both been shown to alter 5-HT and DA release in several brain regions (Whitton & Fowler, 1991; Biggs et al., 1992; Okada et al., 1997; 1998; Ichikawa & Meltzer, 1999).While the role of DA in this illness is more equivocal this is not so for 5-HT, which is far more firmly established. There is, overall, a general consensus that antidepressant effects of drugs mediated by 5-HT are dependent on long-term adaptive changes in the serotonergic transmission. While a number of widely used antidepressants are effective in the management of bipolar depression, their use carries some risk of antidepressant-induced mood instability (Mahli et al., 2003). Evidence suggests that LTG may be of particular use in the acute and prophylactic management of this disorder (Mahli et al., 2003). It may be that the long-term changes in extracellular 5-HT observed after LTG in the present study could have a contributory role in the therapeutic efficacy of the drug if such changes occur in humans (Lopez-Ibor, 1992).
Abbreviations
- DOPAC
dihydroxyphenylacetic acid
- DA
dopamine
- HVA
homovanillic acid
- HPLC
high-performance liquid chromatography
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine
- LTG
lamotrigine
References
- AHMAD S., FOWLER L.J., LEACH M.J., WHITTON P.S. Lamotrigine alters veratridine but not high K+ evoked amino acid release in the ventral hippocampus of the rat in vivo. Br. Pharmacol. Soc. 1995;115:116P. [Google Scholar]
- BAZIL C.W. New antiepileptic drugs. Neurology. 2002;8:71–81. doi: 10.1097/00127893-200203000-00002. [DOI] [PubMed] [Google Scholar]
- BEQUET D., FOUDON M., HERY F. In vivo evidence for an inhibitory glutamatergic control of serotonin release in the cat caudate nucleus: involvement of GABA neurons. Brain Res. 1990;519:82–88. doi: 10.1016/0006-8993(90)90063-h. [DOI] [PubMed] [Google Scholar]
- BIGGS C.S., PEARCE BR FOWLER L.J., WHITTON P.S. Regional effects of sodium valproate on extracellular concentrations of 5-hydroxytryptamine, dopamine and their metabolites in rat brain: an in vivo microdialysis study. J. Neurochem. 1992;59:1702–1708. doi: 10.1111/j.1471-4159.1992.tb11001.x. [DOI] [PubMed] [Google Scholar]
- BOWDEN C.L., CALABRESE J.R., SACHS G., YATHAM L.N., ASGHAR S.A., HOMPLAND M., MONTGOMERY P., EARL N., SMOOT T.M., DEVEUGH-GRIESS P. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch. Gen. Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- BTAICHE I.F., WOSTER P.S. Gabapentine and lamotrigine: novel antiepileptic drugs. Am. J. Health Syst. Pharm. 1995;52:61–69. doi: 10.1093/ajhp/52.1.61. [DOI] [PubMed] [Google Scholar]
- CALABRESE J.R., SHELTON M.D., RAPPORT D.J., KIMMEL S.E., ELHAJ O. Long-term treatment of bipolar disorder with lamotrigine. J. Clin. Psychiatry. 2002;10 Suppl:18–22. [PubMed] [Google Scholar]
- DERANSART C., DEPAULIS A. The control of seizures by the basal ganglia — a review of the experimental data. Epileptic Disord. 2002;4 Suppl 3:S61–S72. [PubMed] [Google Scholar]
- DURSUN S.M., DEAKIN J.F. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J. Psychopharmacol. 2001;15:297–301. doi: 10.1177/026988110101500409. [DOI] [PubMed] [Google Scholar]
- GOLDSMITH D., WAGSTAFF A., IBBOTSON T., PERRY C. Lamotrigine: a review of its use in bipolar disorder. Drugs. 2003;63:2029–2050. doi: 10.2165/00003495-200363190-00009. [DOI] [PubMed] [Google Scholar]
- HADDJERI N., SZABO S.T., DE MONTIGNY C., BLIER P. Increased tonic activation of rat forebrain 5-HT(1A) receptors by lithium addition to antidepressant treatments. Neuropsychopharmacolology. 2000;22:346–356. doi: 10.1016/S0893-133X(99)00138-4. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA J., MELTZER H.Y. Valproate and carbamazepine increase prefrontal dopamine release by 5-HT1A receptor activation. Eur. J. Pharmacol. 1999;380:R1–R3. doi: 10.1016/s0014-2999(99)00517-8. [DOI] [PubMed] [Google Scholar]
- JOVIC N.J., MIRKOVIC D., MAJKIC-SINGH N., MILOVANOVIC D.D. Plasma and urinary serotonin and 5-HIAA in children treated with lamotrigine for intractable epilepsy. Adv. Exp. Med. Biol. 1999;467:297–302. doi: 10.1007/978-1-4615-4709-9_38. [DOI] [PubMed] [Google Scholar]
- KAUR S., STARR M.S. Motor effects of lamotrigine in naive and dopamine-depleted mice. Eur. J. Pharmacol. 1996;304:1–6. doi: 10.1016/0014-2999(96)00134-3. [DOI] [PubMed] [Google Scholar]
- LEES G., LEACH M.J. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamactil) using primary neurological cultures from rat cortex. Brain Res. 1993;612:190–199. doi: 10.1016/0006-8993(93)91660-k. [DOI] [PubMed] [Google Scholar]
- LOPEZ-IBOR J.J. Serotonin and psychiatric disorders. Int. Clin. Psychopharm. 1992;7 Suppl:5–11. doi: 10.1097/00004850-199210002-00003. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., ANNIES R., HONACK D. The N-methyl-D-aspartate receptor antagonist MK-801 induces increases in serotonin and dopamine metabolism in several brain regions of rats. Neurosci. Lett. 1991;128:191–194. doi: 10.1016/0304-3940(91)90258-u. [DOI] [PubMed] [Google Scholar]
- LOSCHMANN P.A., EBLEN F., WULLNER U., WACHTEL H., KOCKGETHER T. Lamotrigine has no antiparkinsonian activity in rat models of Parkinson's disease. Eur. J. Pharmacol. 1995;284:129–134. doi: 10.1016/0014-2999(95)00380-4. [DOI] [PubMed] [Google Scholar]
- MAHLI G.S., MITCHELL P.B., SALIM S. Bipolar depression: management options. CNS Drugs. 2003;17:9–25. doi: 10.2165/00023210-200317010-00002. [DOI] [PubMed] [Google Scholar]
- MAIONE S., ROSSI F., BIGGS C.S., FOWLER L.J., WHITTON P.S. AMPA receptors modulate extracellular 5-hydroxytryptamine concentration and metabolism in rat striatum in vivo. Neurochem. Int. 1997;30:299–304. doi: 10.1016/s0197-0186(96)00101-5. [DOI] [PubMed] [Google Scholar]
- OKADA M., HIRANO T., MIZUNO K., CHIBA T., KAWATA Y., KIRYU K., WADA K., TASAKI H., KANEKO S. Biphasic effects of carbamazepine on the dopaminergic system in rat striatum and hippocampus. Epilepsy Res. 1997;28:143–153. doi: 10.1016/s0920-1211(97)00042-9. [DOI] [PubMed] [Google Scholar]
- OKADA M., HIRANO T., MIZUNO K., KAWATA T., WADA K., MURAKAMI T., TASAKI H., KANEKO S. Effects of carbamazepine on hippocampal serotonergic system. Epilepsy Res. 1998;31:187–198. doi: 10.1016/s0920-1211(98)00025-4. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain In Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- PHILLIPS M.L., DREVETS W.C., RAUCH S.L., LANE R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- REMY S., URBAN B.W., ELGER C.E., BECK H. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur. J. Neurosci. 2003;17:2648–2658. doi: 10.1046/j.1460-9568.2003.02710.x. [DOI] [PubMed] [Google Scholar]
- SHIAH I.S., YATHAM L.N., LAM R.W., ZIS A.P. Effects of lamotrigine on the 5-HT1A receptor function in healthy human males. J. Affect. Disord. 1998;49:157–162. doi: 10.1016/s0165-0327(98)00008-1. [DOI] [PubMed] [Google Scholar]
- SOUTHAM E., KIRBY D., HIGGINS G.A., HAGAN R.M. Lamotrigine inhibits monoamine uptake in vitro and modulates 5-hydroxytryptamine uptake in rats. Eur. J. Pharmacol. 1998;358:19–24. doi: 10.1016/s0014-2999(98)00580-9. [DOI] [PubMed] [Google Scholar]
- TOCZEK M.T., CARSON R.E., LANG L., MA Y., SPANAKI M.V., DER M.G., FAZILAT S., KOPYLEV L., HERSCOVITCH P., ECKLEMAN W.C., THEODORE W.H. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- VINOD K.Y., SUBHASH M.N. Lamotrigine induced selective changes in 5-HT(1A) receptor mediated response in rat brain. Neurochem. Int. 2002;40:315–319. doi: 10.1016/s0197-0186(01)00088-2. [DOI] [PubMed] [Google Scholar]
- VRIEND J., ALEXIUK N.A. Lamotrigine inhibits the in situ activity of tyrosine hydroxylase in striatum of audiogenic seizure prone and audiogenic seizure resistant Balb/c mice. Life Sci. 1997;61:2467–2474. doi: 10.1016/s0024-3205(97)00981-8. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., BIGGS C.S., PEARCE B.R., FOWLER L.J. MK-801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J. Neurochem. 1992;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., FOWLER L.J. The effect of valproic acid on 5-hydroxytryptamine and 5-hydroxyindoleacetic acid concentration in hippocampal dialysates in vivo. Eur. J. Pharmacol. 1991;200:167–169. doi: 10.1016/0014-2999(91)90681-f. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., RICHARDS D.A., BIGGS C.S., FOWLER, L.J. N-methyl-D-aspartate receptors modulate extracellular 5-hydroxytryptamine concentration in rat hippocampus and striatum in vivo. Neurosci. Lett. 1994a;169:215–218. doi: 10.1016/0304-3940(94)90395-6. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., MAIONE S., BIGGS C.S., FOWLER L.J. N-methyl-D-aspartate receptors modulate extracellular dopamine concentration and metabolism in rat hippocampus and striatum. Brain Res. 1994b;635:312–316. doi: 10.1016/0006-8993(94)91453-2. [DOI] [PubMed] [Google Scholar]
- XIE X., DALE T.J., JOHN V.H., CATER H.L., PEAKMAN T.C., CLARE J.J. Electrophysiological and pharmacological properties of the human brain type IIA Na+ channel expressed in a stable mammalian cell line. Pflugers Arch. 2001;441:425–433. doi: 10.1007/s004240000448. [DOI] [PubMed] [Google Scholar]