Abstract
To investigate whether S-nitrosothiols, in addition to NO, mediate bradykinin-induced vasorelaxation, porcine coronary microarteries (PCMAs) were mounted in myographs.
Following preconstriction, concentration–response curves (CRCs) were constructed to bradykinin, the NO donors S-nitroso-N-penicillamine (SNAP) and diethylamine NONOate (DEA-NONOate) and the S-nitrosothiols L-S-nitrosocysteine (L-SNC) and D-SNC. All agonists relaxed PCMAs. L-SNC was ≈5-fold more potent than D-SNC.
The guanylyl cyclase inhibitor ODQ and the NO scavenger hydroxocobalamin induced a larger shift of the bradykinin CRC than the NO synthase inhibitor L-NAME, although all three inhibitors equally suppressed bradykinin-induced cGMP responses.
Complete blockade of bradykinin-induced relaxation was obtained with L-NAME in the presence of the large- and intermediate-conductance Ca2+-activated K+-channel (BKCa, IKCa) blocker charybdotoxin and the small-conductance Ca2+-activated K+-channel (SKCa) channel blocker apamin, but not in the presence of L-NAME, apamin and the BKCa channel blocker iberiotoxin.
Inhibitors of cytochrome P450 epoxygenase, cyclooxygenase, voltage-dependent K+ channels and ATP-sensitive K+ channels did not affect bradykinin-induced relaxation.
SNAP-, DEA-NONOate- and D-SNC-induced relaxations were mediated entirely by the NO-guanylyl cyclase pathway. L-SNC-induced relaxations were partially blocked by charybdotoxin+apamin, but not by iberiotoxin+apamin, and this blockade was abolished following endothelium removal. ODQ, but not hydroxocobalamin, prevented L-SNC-induced increases in cGMP, and both drugs shifted the L-SNC CRC 5–10-fold to the right.
L-SNC hyperpolarized intact and endothelium-denuded coronary arteries.
Our results support the concept that bradykinin-induced relaxation is mediated via de novo synthesized NO and a non-NO, endothelium-derived hyperpolarizing factor (EDHF). S-nitrosothiols, via stereoselective activation of endothelial IKCa and SKCa channels, and through direct effects on smooth muscle cells, may function as an EDHF in porcine coronary microarteries.
Keywords: Bradykinin, coronary artery, endothelium-derived hyperpolarizing factor, nitric oxide, S-nitrosothiol
Introduction
Bradykinin relaxes coronary arteries in an endothelium-dependent manner. This effect is mediated via bradykinin type 2 (B2) receptors. B2 receptor activation results in NO synthesis by endothelial NOS, and NO relaxes vascular smooth muscle cells through guanylyl cyclase activation and subsequent cGMP generation (Danser et al., 2000). NOS inhibitors, however, do not completely block bradykinin-induced vasorelaxation, suggesting the existence of either NO-storage sites (Davisson et al., 1996a; Danser et al., 1998) or a non-NO ‘endothelium-derived hyperpolarizing factor' (EDHF) (McGuire et al., 2001; Busse et al., 2002).
EDHF-mediated responses in different arteries have been linked to the release of K+, the generation of cytochrome-P450 products from arachidonic acid (epoxyeicosatrienoic acids, EETs), and to the production of H2O2 (Campbell et al., 1996; Randall et al., 1996; Mombouli &Vanhoutte, 1997; Edwards et al., 1998; 2001; Fisslthaler et al., 1999; Matoba & Shimokawa, 2003). The identity of EDHF and its contribution to overall relaxation differs between species, between vascular beds and between vessels of different sizes (Hwa et al., 1994).
EDHF-mediated relaxation depends on the activation of intermediate- and small-conductance Ca2+-activated K+-channels (IKCa, SKCa) (Busse et al., 2002). These channels are located on endothelial cells (Bychkov et al., 2002; Sollini et al., 2002), and (as a consequence of endothelial hyperpolarization), may be responsible for the subsequent relaxation that is generally attributed to the release of an EDHF (Edwards et al., 1998; 2001). This EDHF induces smooth muscle hyperpolarization by activating inwardly rectifying K+ channel (KIR) channels, Na+-K+-ATPase and/or large-conductance Ca2+-activated K+ (BKCa) channels (Busse et al., 2002; Archer et al., 2003). With regard to the latter, it is important to note that NO itself is capable of inducing hyperpolarization via activation of Ca2+-activated K+ channels in vascular smooth muscle (Bolotina et al., 1994).
Bradykinin induces release of NO-containing factors (e.g., S-nitrosothiols) from cellular storage sites (Davisson et al., 1996a; Danser et al., 1998; Tom et al., 2001). Depletion of NO storage sites occurs only after repeated exposure to bradykinin or following prolonged inhibition of NOS (Davisson et al., 1996a; Danser et al., 1998). S-nitrosothiols induce relaxation through decomposition to NO (Rafikova et al., 2002), or by activating stereoselective recognition sites (Davisson et al., 1996b). These recognition sites could either be cysteine residues within Ca2+-activated K+ channels (Lang et al., 2003) or a novel class of receptors which specifically recognize L-S-nitrosocysteine (L-SNC) and structurally similar S-nitrosothiols such as L-S-nitroso-β,β-dimethylcysteine (Travis et al., 1997).
In the present study, we set out to investigate the possibility that S-nitrosothiols act as an EDHF in porcine coronary microarteries (PCMAs). PCMAs rather than large porcine coronary arteries were used, because the contribution of EDHF to vasorelaxation is larger in smaller vessels (Hwa et al., 1994; Danser et al., 2000). We compared the relaxant effects of L-SNC to those of bradykinin and the NO donors S-nitroso-N-acetylpenicillamine (N-acetyl-3-(nitrosothio)-D-valine or SNAP) and diethylamine NONOate (DEA-NONOate), both in the absence and presence of an inhibitor of NOS, an inhibitor of guanylyl cyclase, and inhibitors of a wide range of EDHF candidates. To rule out residual NO (i.e., non-EDHF)-mediated effects as much as possible we also made use of the NO scavenger hydroxocobalamin. Guanylyl cyclase activation by NO or NO-containing factors was quantified by measuring cGMP generation. To verify the stereoselectivity of L-SNC-induced effects, parallel experiments were performed with D-S-nitrosocysteine (D-SNC). Finally, electrophysiological measurements were performed in intact and endothelium-denuded porcine coronary arteries to verify direct hyperpolarization by bradykinin and L-SNC.
Methods
Drugs
Bradykinin, SNAP, DEA-NONOate, L-cysteine, D-cysteine, NaNO2, 9,11-dideoxy-11α,9α-epoxy-methano-prostaglandin F2α (U46619), Nω-nitro-L-arginine methyl ester HCl (L-NAME), Nω-nitro-L-arginine (L-NA), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), hydroxocobalamin, indomethacin, diclofenac, glibenclamide, 4-aminopyridine, charybdotoxin, apamin, iberiotoxin, ouabain, BaCl2, sulfaphenazole, miconazole and 3-isobutyl-1-methyl-xanthine were from Sigma-Aldrich (Zwijndrecht, The Netherlands). D-Arg[Hyp3,Thi5,D-Tic7,Oic8]-bradykinin (Hoe140) was a kind gift of Dr W. Linz, Hoechst, Frankfurt, Germany. Indomethacin, glibenclamide, ouabain and ODQ were dissolved in dimethylsulfoxide. Sulfaphenazole and miconazole were dissolved in ethanol. Hydroxocobalamin was dissolved in methanol. All other chemicals were dissolved in water.
Tissue collection
Pig hearts (n=123) were collected at the local slaughterhouse. Epicardial arteries (diameter ≈1.5 mm) and tertiary branches of the left anterior descending coronary artery (PCMAs; diameter 337±8.4 μm) were removed and either used directly or stored overnight in cold, oxygenated Krebs bicarbonate solution of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 8.3; pH 7.4.
Organ bath studies
PCMAs were cut into segments of ≈2 mm length and mounted in microvascular myographs (J.P. Trading, Aarhus, Denmark) with separated 6-ml organ baths containing Krebs bicarbonate solution aerated with 95% O2/5% CO2 and maintained at 37°C. Changes in contractile force were recorded with a Harvard isometric transducer. Following a 30-min stabilization period, the internal diameter was set to a tension equivalent to 0.9 times the estimated diameter at 100 mmHg effective transmural pressure (Mulvany & Halpern, 1977).
The normalized vessel segments were exposed to 30 mM KCl twice. In some vessels, the endothelium was removed by gently rubbing a hair through the lumen of the mounted artery. Endothelial integrity or removal was verified by observing relaxation (or lack thereof) to 10 nM substance P after preconstriction with 10 nM of the thromboxane-A2 analogue U46619. The maximal contractile response to KCl was determined by exposing the tissue to 100 mM KCl. Thereafter, vessels were allowed to equilibrate in fresh organ bath fluid for 30 min in the absence or presence of one or more of the following inhibitors: the NOS inhibitor L-NAME (100 μM), the NO scavenger hydroxocobalamin (200 μM), the guanylyl cyclase inhibitor ODQ (10 μM), the COX inhibitor indomethacin (10 μM), the IKCa+BKCa channel inhibitor charybdotoxin (100 nM), the SKCa channel inhibitor apamin (100 nM), the BKCa channel inhibitor iberiotoxin (100 nM), the voltage-dependent K+ channel (Kv) inhibitor 4-aminopyridine (5 mM), the ATP-sensitive K+ channel (KATP) inhibitor glibenclamide (1 μM), the KIR inhibitor BaCl2 (30 μM), the Na+-K+-ATPase inhibitor ouabain (0.5 mM), the cytochrome P450 epoxygenase inhibitors sulfaphenazole or miconazole (10 μM) or the B2 receptor antagonist Hoe140 (1 μM). Vessels were then preconstricted with U46619, and concentration-response curves (CRCs) were constructed to bradykinin, SNAP, DEA-NONOate, L-SNC or D-SNC. L-SNC and D-SNC were prepared immediately prior to the experiment and stored in the dark below 0°C. In short, 50 μl of a 0.2 M solution of L- and D-cysteine was mixed with 50 μl 0.2 M NaNO2. The subsequent addition of 10 μl 1 M HCl resulted in a stable 0.1 M solution (pH≈5) of the respective SNC isomers (Davisson et al., 1996b). Preliminary studies with NaNO2, L- and D-cysteine (n=3 each) revealed that, separately, these drugs did not exert relaxant effects in preconstricted PCMAs (data not shown).
Cyclic GMP measurement
To study bradykinin- and L-SNC-induced cGMP production, vessel segments (5–10 mg) were exposed to bradykinin (1 μM) or L-SNC (10 or 100 μM) in 10 ml oxygenated Krebs bicarbonate solution for 1 min at 37°C in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methyl-xanthine (100 μM), following a 30-min preincubation in the absence (control) or presence of hydroxocobalamin, ODQ, Hoe140 and/or L-NAME at the above concentrations. Tissues were subsequently frozen in liquid nitrogen, and stored at −80°C. To determine cGMP, frozen tissues were homogenized in 0.5 ml 0.1 M HCl using a stainless-steel ultraturrax (polytron). Homogenates were centrifuged at 3300 g, and cGMP was measured in 300 μl supernatant by ELISA following acetylation (R&D Systems, Minneapolis, U.S.A.). Experiments were performed in quadruplicate, and results are expressed as pmol mg−1 protein. The lower limit of detection was 0.1 pmol mg−1 protein.
Electrophysiological measurements
Freshly isolated epicardial artery segments (≈40 mm length) were excised, slit and mounted in heated (37°C) chambers and maintained in modified Tyrode's solution (in mM: NaCl 132, KCl 4, CaCl2 1.6, MgCl2 1.2, NaH2PO4 0.36, NaHCO3 23.8, Ca2+-EDTA 0.05, glucose 10; gassed with 20% O2/5% CO2/75% N2, pH 7.4) containing the NOS inhibitor L-NA (300 μM), the COX inhibitor diclofenac (10 μM), and U46619 (1 μM) to mimic conditions in the organ chamber experiments as closely as possible. Both endothelium intact and endothelium-denuded segments were used. Smooth muscle membrane potential was recorded by impaling cells through the intima as described (Fisslthaler et al., 1999). Bradykinin (100 nM) and L-SNC (50 μM) were applied as bolus injections into the bath.
Data analysis
Data are given as mean±s.e.m. Contractile responses are expressed as a percentage of the contraction to U46619. CRCs were analyzed as described using the logistic function described by de Lean et al. (1978) to obtain pEC50 (−10log EC50) values (Table 1). L-NAME, ODQ, hydroxocobalamin and/or ouabain+BaCl2 increased basal tone by 10–40%, whereas 4-aminopyridine increased basal tone by 80%. In such cases the concentration of U46619 (range 10–30 nM) was adjusted to obtain a preconstriction corresponding to ≈95% of the maximal contractile response in all vessels. Statistical analysis was by paired t-test, once one-way ANOVA, followed by Dunnett's post hoc evaluation, had revealed that differences existed between groups. P<0.05 was considered significant.
Table 1.
pEC50 values of bradykinin, SNAP, L-SNC, D-SNC or DEA-NONOate in the absence or presence of several inhibitors
| pEC50 | |||||
|---|---|---|---|---|---|
| Inhibitor | Bradykinin | SNAP | L-SNC | D-SNC | DEA-NONOate |
| None | 8.2±0.1 (45) | 7.1±0.1 (14) | 6.5±0.1 (22)# | 6.0±0.1 (18) | 6.6±0.1 (4) |
| Hoe140 | <6 (3)* | ||||
| Indomethacin | 8.0±0.1 (5) | ||||
| ODQ | 7.2±0.2 (15)* | 5.7±0.1 (11)* | 5.3±0.1 (9)* | 5.3±0.1 (13)* | 5.8±0.1 (4)† |
| Hydroxocobalamin | 6.8±0.2 (16)* | 6.5±0.2 (11)* | 5.4±0.2 (8)† | 5.5±0.1 (13)* | 5.9±0.1 (4)† |
| L-NAME | 7.7±0.1 (23)† | 7.5±0.3 (5) | |||
| Apamin | 8.5±0.2 (7) | 7.2±0.1 (4) | |||
| Charybdotoxin | 8.2±0.2 (8) | ||||
| Iberiotoxin | 8.4±0.2 (6) | 7.1±0.1 (4) | |||
| Charybdotoxin+apamin | 7.6±0.3 (7) | 7.2±0.1 (6) | 6.0±0.1 (10)* | 5.9±0.1 (13) | 6.5±0.1 (4) |
| Iberiotoxin+apamin | 8.8±0.2 (6) | 7.1±0.2 (5) | |||
| Ouabain+BaCl2 | 8.7±0.1 (6) | 6.7±0.1 (4)† | |||
| Glibenclamide | 7.2±0.1 (5) | ||||
| 4-Aminopyridine | 7.1±0.5 (4) | ||||
| No endothelium | 7.3±0.1 (9)† | ||||
| No endothelium+charybdotoxin+apamin | 7.0±0.1 (4) | ||||
| ODQ+hydroxocobalamin | <5 (5)*‡ | 4.6±0.3 (5)*§ | 4.3±0.2 (12)*‡ | ||
| ODQ+charybdotoxin+apamin | 5.6±0.2 (5)* | 5.3±0.1 (5)* | 5.2±0.1 (5)* | ||
| Hydroxocobalamin+charybdotoxin+apamin | 6.3±0.2 (6)† | 5.3±0.2 (5)† | 5.7±0.1 (5)† | ||
| ODQ+hydroxocobalamin+charybdotoxin+apamin | <5 (3)*‡ | 4.5±0.2 (5)*‡ | 4.3±0.2 (8)*‡ | ||
Data are mean±s.e.m. (n value);
P<0.01 vs none;
P<0.05 vs none;
P<0.01 vs hydroxocobalamin or ODQ;
P<0.05 vs hydroxocobalamin or ODQ;
P<0.05 vs D-SNC.
Results
Mechanism of bradykinin-induced relaxation
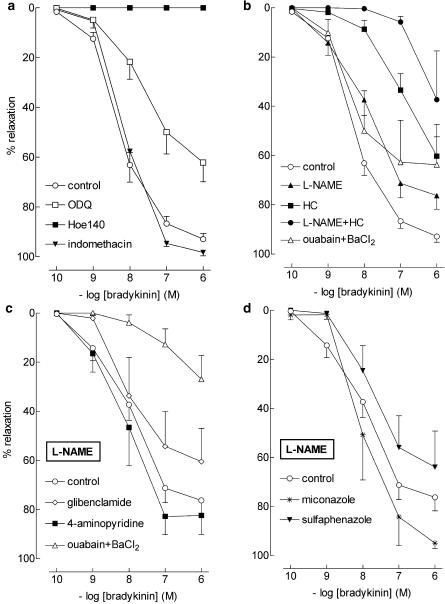
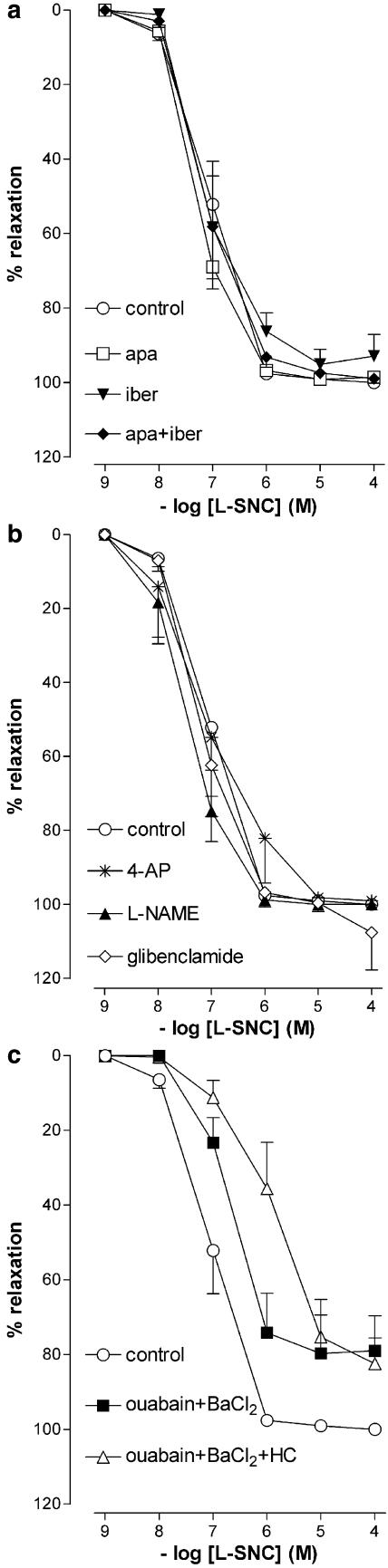
Bradykinin relaxed preconstricted vessel segments in a concentration-dependent manner (pEC50=8.2±0.1, n=45; Figure 1). Bradykinin-induced relaxations were unaffected by indomethacin and abrogated by Hoe140. L-NAME shifted the bradykinin CRC ≈5-fold to the right, whereas ODQ and hydroxocobalamin induced a ≈10-fold rightward shift (see Table 1 for pEC50 values). Apamin, iberiotoxin and charybdotoxin, separately or in combination, did not significantly affect the bradykinin CRC (Figure 2, Table 1), nor did ouabain+BaCl2 (Figure 1, Table 1).
Figure 1.
Relaxations of PCMAs, preconstricted with U46619, to bradykinin in absence (control; a, b) or presence of 100 μM L-NAME (c, d) with one or more of the following inhibitors: 1 μM Hoe140, 10 μM ODQ, 10 μM indomethacin, 100 μM L-NAME, 200 μM hydroxocobalamin (HC), 0.5 mM ouabain, 30 μM BaCl2, 1 μM glibenclamide, 5 mM 4-aminopyridine, 10 μM miconazole or 10 μM sulfaphenazole. Data (mean±s.e.mean; n=5–45) are expressed as a percentage of the contraction induced by U46619.
Figure 2.
Relaxations of PCMAs, preconstricted with U46619, to bradykinin in absence (control; a, b) or presence of 100 μM L-NAME (c, d) with one or more of the following inhibitors: 100 nM charybdotoxin (char), 100 nM apamin (apa), or 100 nM iberiotoxin (iber). Data (mean±s.e.mean; n=5–45) are expressed as a percentage of the contraction induced by U46619.
In the presence of L-NAME, apamin and charybdotoxin, when given separately, did not affect the bradykinin CRC (pEC50's 7.8±0.3 and 8.2±0.2, respectively, n=6 for each; Figure 2), nor did glibenclamide, 4-aminopyridine, sulfaphenazole and miconazole (pEC50's 7.8±0.5, 8.0±0.3, 7.8±0.4 and 7.9±0.6, respectively, n=5 for each; Figure 1). In contrast, when given in addition to L-NAME, charybdotoxin+apamin fully blocked the bradykinin-induced responses (Figure 2, n=5), whereas hydroxocobalamin (n=5) and ouabain+BaCl2 (n=5) shifted the bradykinin CRC >100-fold (P<0.01; Figure 1) to the right. Iberiotoxin, without (n=6) or with (n=6) apamin, reduced the maximum effect of bradykinin in the presence of L-NAME (P<0.01; Figure 2), without altering its pEC50 (7.8±0.5 and 8.1±0.2, respectively).
Thus, NO and/or NO-containing factors as well as Ca2+-activated K+-channels (BKCa, IKCa and SKCa), KIR channels, and Na+-K+-ATPase are involved in the bradykinin-induced relaxation, and the NO-induced effects are mediated, at least in part, via activation of guanylyl cyclase. No evidence for a role of Kv channels, KATP channels, COX products, or cytochrome P450 epoxygenase products was obtained.
Mechanism of NO-induced relaxation
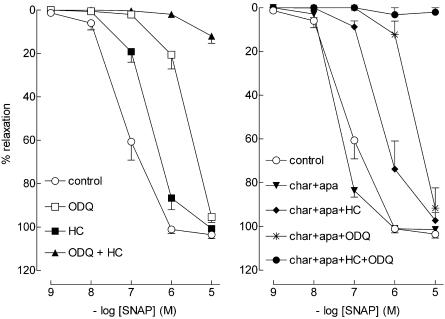
SNAP and DEA-NONOate relaxed preconstricted coronary microvessels in a concentration-dependent manner (Figure 3 and 4; Table 1). Both hydroxocobalamin and ODQ shifted the SNAP and DEA-NONOate-induced CRC to the right and, in combination, completely blocked SNAP-induced relaxation. Charybdotoxin+apamin, either as combination or together with hydroxocobalamin or ODQ, did not elicit a rightward shift in the SNAP or DEA-NONOate CRC.
Figure 3.
Relaxations of PCMAs, preconstricted with U46619, to SNAP in the absence (control) or presence of one or more of the following inhibitors: 10 μM ODQ, 200 μM hydroxocobalamin (HC), 100 nM charybdotoxin (char) or 100 nM apamin (apa). Data (mean±s.e.mean; n=5–14) are expressed as a percentage of the contraction induced by U46619.
Figure 4.
Relaxations of PCMAs, preconstricted with U46619, to DEA-NONOate in the absence (control) or presence of one or more of the following inhibitors: 10 μM ODQ, 200 μM hydroxocobalamin (HC), 100 nM charybdotoxin (char) or 100 nM apamin (apa). Data (mean±s.e.mean; n=4) are expressed as a percentage of the contraction induced by U46619.
Thus, the relaxation induced by exogenous NO depends entirely on activation of guanylyl cyclase, and does not involve Ca2+-activated K+-channels.
Mechanism of S-nitrosothiol-induced relaxation
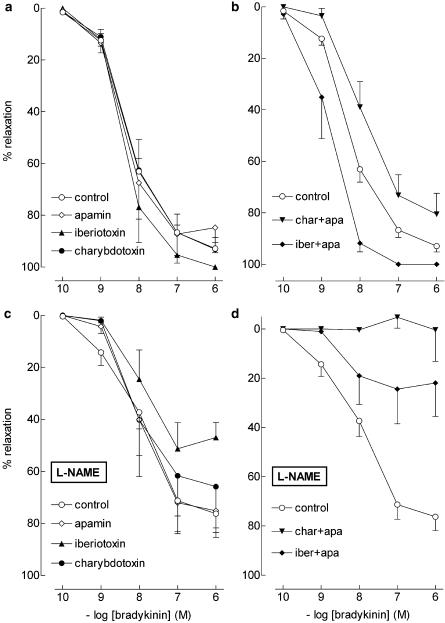
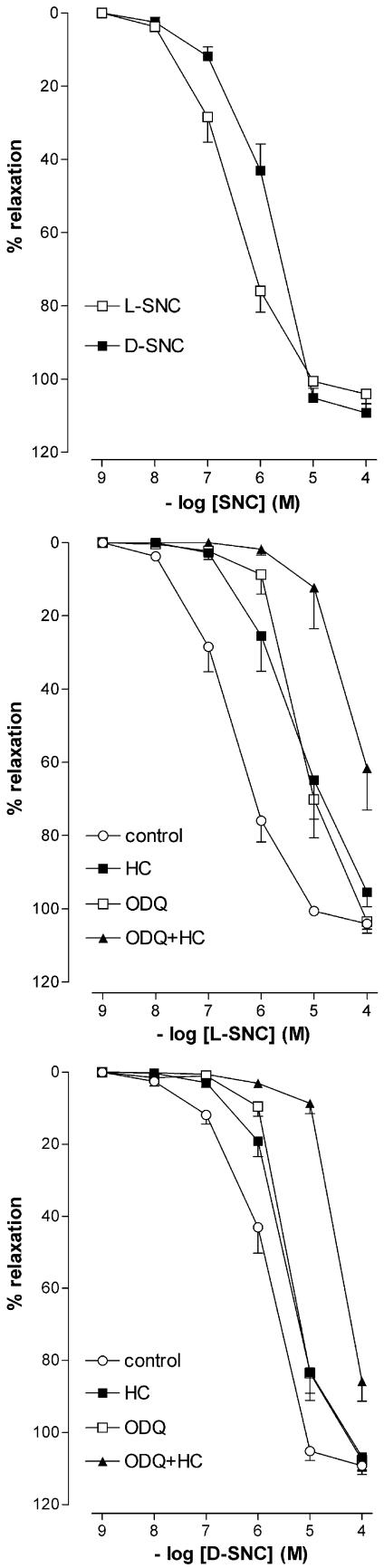
L-SNC and D-SNC relaxed preconstricted PCMAs in a concentration-dependent manner. L-SNC was 5 times more potent than D-SNC (P<0.05; Figure 5, Table 1). ODQ and hydroxocobalamin shifted the CRCs of both L-SNC and D-SNC 5–10-fold to the right (P=NS for the difference in rightward shift between L-SNC and D-SNC) and, when combined, caused a further rightward shift (Table 1).
Figure 5.
Relaxations of PCMAs, preconstricted with U46619, to L-SNC or D-SNC in the absence (control) or presence of 10 μM ODQ and/or 200 μM hydroxocobalamin (HC). Data (mean±s.e.mean; n=4–18) are expressed as a percentage of the contraction induced by U46619.
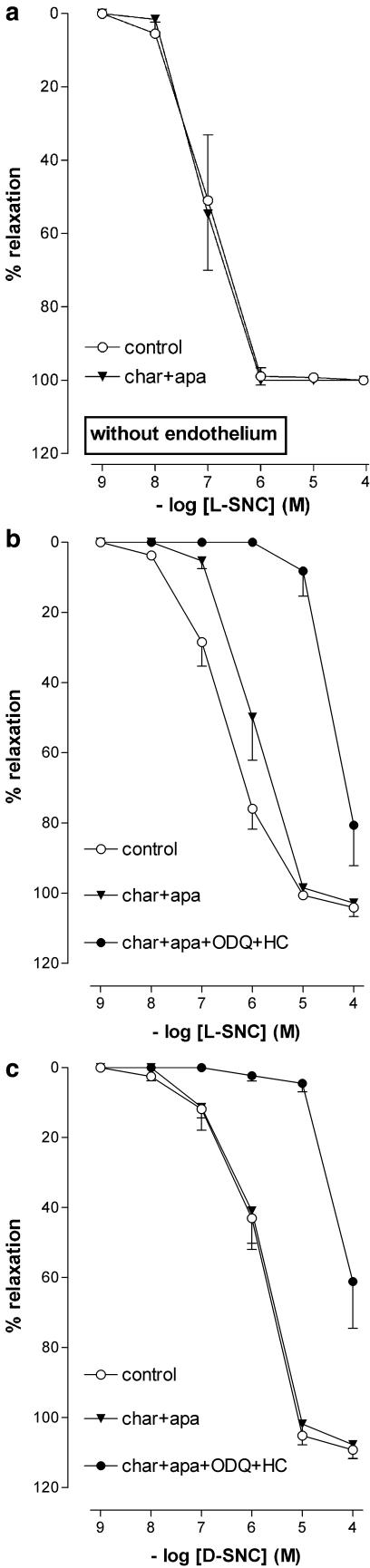
Charybdotoxin+apamin shifted the L-SNC CRC, but not the D-SNC CRC, 5–10 fold to the right (Figure 6, Table 1). Endothelium-denudation shifted the L-SNC CRC 5–10 fold to the left, and abolished the rightward shift induced by charybdotoxin+apamin (Figure 6, Table 1). Charybdotoxin+apamin did not have additional effects on top of ODQ, hydroxocobalamin (Table 1) or ODQ+hydroxocobalamin (Figures 5 and 6, Table 1) with either L-SNC or D-SNC.
Figure 6.
Relaxations of PCMAs without (a) or with (b, c) endothelium, preconstricted with U46619, to L-SNC or D-SNC in the absence (control) or presence of one or more of the following inhibitors: 10 μM ODQ, 200 μM hydroxocobalamin (HC), 100 nM charybdotoxin (char) or 100 nM apamin (apa). Data (mean± s.e.mean; n=4-18) are expressed as a percentage of the contraction induced by U46619.
Glibenclamide, 4-aminopyridine, L-NAME, apamin, iberiotoxin, and iberiotoxin+apamin did not affect the L-SNC CRC (Figure 7, Table 1).
Figure 7.
Relaxations of PCMAs, preconstricted with U46619, to L-SNC in the absence (control) or presence of one or more of the following inhibitors: 100 nM iberiotoxin (iber), 100 nM apamin (apa), 5 mM 4-aminopyridine (4-AP), 100 μM L-NAME, 1 μM glibenclamide, 0.5 mM ouabain or 30 μM BaCl2. Data (mean±s.e.mean; n=4–9) are expressed as a percentage of the contraction induced by U46619.
Ouabain+BaCl2 shifted the L-SNC CRC five-fold to the right (Figure 7, Table 1) but did not exert an additional effect on top of hydroxocobalamin (pEC50 5.9±0.2, n=4; Figures 5 and 7).
Thus, S-nitrosothiol-induced relaxation occurs in a stereoselective manner, and is mediated via activation of guanylyl cyclase, endothelial IKCa and SKCa channels, KIR channels and the Na+-K+-ATPase. Neither NOS, Kv channels, KATP channels, nor BKCa channels appear to mediate this response.
Cyclic GMP
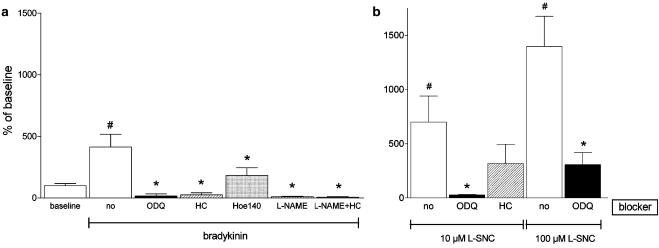
Baseline cGMP levels were 9.4±2.7 pmol mg−1 protein (n=16). Bradykinin increased the microvascular cGMP levels four- to 5- fold (Figure 8; P<0.05 vs control). Hoe140 largely prevented this increase, whereas L-NAME, hydroxocobalamin and ODQ reduced the microvascular cGMP content following bradykinin stimulation to levels below baseline. The combination of hydroxocobalamin and L-NAME did not further decrease cGMP levels. L-SNC increased cGMP >5-fold (P<0.05 vs control). ODQ fully prevented the increase induced by 10 μM L-SNC, and a similar tendency was observed for hydroxocobalamin (P=NS). ODQ did not fully prevent the increase induced by 100 μM L-SNC.
Figure 8.
Cyclic GMP levels (expressed as % of baseline) in PCMAs after 1 min exposure to (a) bradykinin (1 μM) or (b) L-SNC (10 or 100 μM) under control conditions (no blocker) and in the presence of 10 μM ODQ, 200 μM hydroxocobalamin (HC), 1 μM Hoe140 and/or 100 μM L-NAME. Data are mean±s.e.mean (n=3–10). ♯P<0.05 vs control, *P<0.05 vs no blocker.
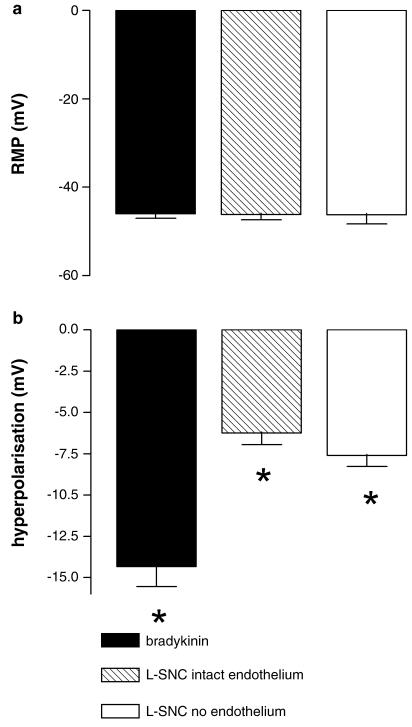
Electrophysiological measurements
Both bradykinin (n=6) and L-SNC (n=6) hyperpolarized vascular smooth muscle cells (P<0.01) in porcine coronary arteries (Figure 9). The effect of L-SNC was not affected by the removal of the endothelium (n=5).
Figure 9.
Hyperpolarization of smooth muscle cells by 100 nM bradykinin and 50 μM L-SNC in porcine coronary arteries with or without endothelium. (a) resting membrane potential (RMP). (b) change in membrane potential. Experiments were performed in the presence of 300 μM L-NA, 10 μM diclofenac and 1 μM U46619. Data are mean±s.e.mean of five to six separate experiments; *P<0.01 vs control. U46619 did not significantly affect RMP (–44.5±1.2 mV vs –42.7±1.7 mV, n=6), and in parallel experiments, using arterial rings from the same pig and following preconstriction with the same U46619 concentration (1 μM), 100 nM bradykinin relaxed the arteries by 89±9% (n=6).
Discussion
The present study shows that B2 receptor-mediated vasodilation in porcine coronary microarteries involves the NOS/NO/guanylyl cyclase/cGMP pathway and Ca2+-activated K+-channels, but not COX products or KATP channels. This is in full agreement with the concept that both NO and an EDHF that is not de novo synthesized NO determine bradykinin-induced relaxation. The two pathways appear to be interchangeable, since blocking each pathway separately (with L-NAME and charybdotoxin+apamin, respectively) only marginally affected bradykinin-mediated relaxation, whereas blocking both pathways together abrogated the effects of bradykinin. The modest effect of blocking NOS in the present study opposes earlier data in large porcine coronary arteries, where L-NAME alone induced a ≈10-fold rightward shift of the bradykinin CRC (Danser et al., 2000). Apparently, as has been suggested before, de novo synthesized NO is of greater importance in large arteries, and the contribution of EDHF is larger in microarteries (Hwa et al., 1994).
The BKCa channel inhibitor iberiotoxin, with or without the SKCa channel inhibitor apamin, reduced the maximum effect but not the potency of bradykinin in the presence of L-NAME. This finding, combined with the lack of effect of the Kv channel inhibitor 4-aminopyridine, suggests that the complete inhibition of bradykinin-induced relaxation obtained with charybdotoxin (a nonselective inhibitor of BKCa, IKCa and Kv channels) in the presence of apamin and L-NAME can be attributed to the blockade of all three types of KCa channels.
BKCa channels are located on vascular smooth muscle cells (Archer et al., 2003). Although endothelial EETs are believed to activate these channels (Edwards et al., 1998; Miura & Gutterman, 1998; Fisslthaler et al., 1999; Imig et al., 2001; Busse et al., 2002; Gauthier et al., 2002; Archer et al., 2003), the lack of effect of the cytochrome P450 epoxygenase inhibitors miconazole and sulfaphenazole excludes this possibility in our experimental setup.
IKCa and SKCa channels are expressed in endothelial cells (Bychkov et al., 2002; Sollini et al., 2002), and their activation results in endothelial hyperpolarization and the accumulation of K+ in the myo-endothelial space. This K+ is believed to subsequently hyperpolarize vascular smooth muscle cells by activating KIR channels and/or the Na+-K+-ATPase (Edwards et al., 1998; Edwards et al., 2001; Busse et al., 2002). The inhibitory effect of BaCl2 and ouabain towards bradykinin in the presence of L-NAME confirms this concept in PCMAs.
S-nitrosothiols as EDHF?
We propose that NO-containing/releasing factors, S-nitrosothiols in particular, act as an EDHF in PCMAs. The contribution of such factors is supported by our observations that the guanylyl cyclase inhibitor ODQ and the NO scavenger hydroxocobalamin inhibited the bradykinin-induced effects to a much greater degree than L-NAME, and that, in combination, L-NAME+hydroxocobalamin almost completely prevented bradykinin-induced relaxations. Since S-nitrosothiol-induced relaxations occur through activation of stereoselective recognition sites and/or via their decomposition to NO (Davisson et al., 1996b, 1997), we used both L-SNC and D-SNC to verify this proposal.
L-SNC was ≈5 times more potent than D-SNC. This difference disappeared in the presence of charybdotoxin+apamin (but not in the presence of iberiotoxin with or without apamin), suggesting that L-SNC, but not D-SNC, hyperpolarizes endothelial cells via IKCa and SKCa channel activation. The comparable rightward shift of the L-SNC CRC in the presence of ouabain+BaCl2 and charybdotoxin+apamin is in agreement with the concept that such hyperpolarization results in endothelial K+ release and subsequent smooth muscle cell hyperpolarization. In further support of this hypothesis, endothelium denudation abolished the effect of charybdotoxin+apamin towards L-SNC, and L-SNC reduced the membrane potential of smooth muscle cells in intact porcine coronary arteries.
Unexpectedly, however, the removal of the endothelium potentiated L-SNC five to 10- fold. This suggests that L-SNC, like other endothelium-dependent vasodilators, not only hyperpolarizes endothelial cells, but also induces the release of an endothelium-derived contractile factor (Sunano et al., 2001). Alternatively, endothelial denudation might uncover direct L-SNC-induced effects on smooth muscle cells, as evidenced by the fact that L-SNC also hyperpolarized endothelium-denuded coronary arteries. One such direct effect is BKCa channel activation via S-nitrosylation of cysteine residues (Lang et al., 2003). However, the lack of effect of charybdotoxin+apamin towards L-SNC in endothelium-denuded vessels does not support this concept in PCMAs.
Taken together, the relaxant effects of L-SNC, like those of bradykinin, involve KCa channels, and they occur, at least in part, in a stereoselective manner.
The greater potency of L-SNC vs D-SNC is in agreement with previous in vivo studies (Davisson et al., 1996b, 1997), and may indicate the existence of binding sites that specifically recognize L-SNC and structurally related S-nitrosothiols. These binding sites may either be novel receptors or ‘nitrosation motifs' in functional proteins such as receptors and ion channels (Stamler et al., 1992b; Travis et al., 1997; Lang et al., 2000; 2003).
NO release from S-nitrosothiols?
The effects of L-SNC, at the concentrations used in the present study, are unlikely to be entirely due to its decomposition to NO, nor do they involve de novo NO generation by NOS. First, NO did not activate Ca2+-activated K+ channels in PCMAs, because the dilatory effects of the NO donors SNAP and DEA-NONOate were unaffected by charybdotoxin and apamin. Second, L-NAME did not affect L-SNC-mediated responses. Third, detectable NO production has been reported to occur at S-nitrosothiol concentrations above 100 μM only (Ceron et al., 2001), that is, at concentrations that are >100 times above the EC50 value of L-SNC in the present study. Fourth, ODQ, but not hydroxocobalamin, fully prevented the L-SNC-induced increases in cGMP. This suggests direct, NO-independent, activation of guanylyl cyclase by L-SNC, in agreement with a previous study in cultured vascular smooth muscle cells (Travis et al., 1996). Alternatively, the concentration of hydroxocobalamin used in the present study may have been too low to scavenge all NO generated following L-SNC application (Li & Rand, 1999).
Taken together, the following mechanisms may underlie L-SNC-induced vasorelaxation: direct activation of endothelial IKCa and SKCa channels, direct activation of guanylyl cyclase in smooth muscle cells, and decomposition to NO. Simultaneous inhibition of all mechanisms (with charybdotoxin+apamin, ODQ and hydroxocobalamin, respectively) did not fully prevent the relaxations induced by the highest concentration of L-SNC (100 μM). This could relate to the inability of hydroxocobalamin to scavenge all NO (Li & Rand, 1999) and/or the competitive inhibition of guanylyl cyclase by ODQ (Garthwaite et al., 1995), allowing full blockade of the cGMP increases and relaxations induced by 10 μM L-SNC, but not of those induced by a 10-fold higher L-SNC concentration (Figures 6 and 8).
Release of S-nitrosothiols?
Finally, despite the fact that L-SNC is capable of exerting EDHF-like effects, direct evidence demonstrating that L-SNC (or a related compound) mediates bradykinin-induced, EDHF-dependent relaxation is currently lacking. Previous studies support the existence of preformed pools of NO-containing factors (such as S-nitrosothiols) in endothelial and vascular smooth muscle cells (Rubanyi et al., 1991; Davisson et al., 1996a; Danser et al., 2000; Andrews et al., 2003). These pools become depleted after repeated exposure to endothelium-dependent agonists such as acetylcholine and bradykinin, following prolonged NOS inhibition, or after exposure to UV light (Davisson et al., 1996a; Danser et al., 2000; Andrews et al., 2003).
We did not measure S-nitrosothiol release following bradykinin stimulation in the present study. Such release may occur in a specific compartment (e.g., the myo-endothelial space, gap junctions, intraendothelial) that does not allow easy detection. Moreover, since it depends on preformed pools, it cannot be monitored by measuring the vascular S-nitrosothiol content following bradykinin stimulation. Similar difficulties exist with regard to EETs (Imig et al., 2001; Archer et al., 2003), and it has therefore been proposed that these cytochrome-P450 products contribute to the activation of endothelial K+ channels as second messengers (Busse et al., 2002), rather than being released from endothelial cells in large amounts.
Clinical perspective
S-nitrosylated proteins, the most abundant of which is albumin, are present in micromolar concentrations in normal subjects (Stamler et al., 1992a). They are thought to serve both as a source and a sink of NO, thereby buffering the concentration of free NO. A recent in vivo study showed that S-nitrosothiols induce dilator responses in human conduit and resistance arteries that are comparable with those of bradykinin and acetylcholine (Rassaf et al., 2002), and it has therefore been suggested (Schechter et al., 2002) that S-nitrosothiols provide a new pharmacological route for delivering NO regionally. Our data extend these findings, by implying not only that S-nitrosothiols may act by inducing hyperpolarization in microarteries (i.e., exert NO-independent effects), but also by showing that their effects occur in a stereoselective manner.
Acknowledgments
Research described in this article was partially supported by Philip Morris Inc.
Abbreviations
- B2 receptor
bradykinin type 2 receptor
- BKCa
large conductance Ca2+-activated K+-channel
- CRC
concentration–response curve
- DEA-NONOate
diethylamine NONOate
- EDHF
endothelium-derived hyperpolarizing factor
- Hoe140
D-Arg[Hyp3,Thi5,D-Tic7,Oic8]-bradykinin
- IKCa
intermediate conductance Ca2+-activated K+-channel
- KIR
inwardly rectifying K+ channel
- L-NAME
Nω-nitro-L-arginine methyl ester HCl
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PCMAs
porcine coronary microarteries
- SKCa
small conductance Ca2+-activated K+-channel
- SNAP
S-nitroso-N-penicillamine
- D-SNC
D-S-nitrosocysteine
- L-SNC
L-S-nitrosocysteine
- U46619
9,11-dideoxy-11α,9α-epoxy-methano-prostaglandin F2α
References
- ANDREWS K.L., MCGUIRE J.J., TRIGGLE C.R. A photosensitive vascular smooth muscle store of nitric oxide in mouse aorta: no dependence on expression of endothelial nitric oxide synthase. Br. J. Pharmacol. 2003;138:932–940. doi: 10.1038/sj.bjp.0705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHER S.L., GRAGASIN F.S., WU X., WANG S., MCMURTRY S., KIM D.H., PLATONOV M., KOSHAL A., HASHIMOTO K., CAMPBELL W.B., FALCK J.R., MICHELAKIS E.D. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- BYCHKOV R., BURNHAM M.P., RICHARDS G.R., EDWARDS G., WESTON A.H., FELETOU M., VANHOUTTE P.M. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CERON P.I., CREMONEZ D.C., BENDHACK L.M., TEDESCO A.C. The relaxation induced by S-nitroso-glutathione and S-nitroso-N-acetylcysteine in rat aorta is not related to nitric oxide production. J. Pharmacol. Exp. Ther. 2001;298:686–694. [PubMed] [Google Scholar]
- DANSER A.H.J., DE VRIES R., SCHOEMAKER R.G., SAXENA P.R. Bradykinin-induced release of nitric oxide by the isolated perfused rat heart: importance of preformed pools of nitric oxide-containing factors. J. Hypertens. 1998;16:239–244. doi: 10.1097/00004872-199816020-00015. [DOI] [PubMed] [Google Scholar]
- DANSER A.H.J., TOM B., DE VRIES R., SAXENA P.R. L-NAME resistant bradykinin-induced relaxation in porcine coronary arteries is NO-dependent: effect of ACE inhibition. Br. J. Pharmacol. 2000;131:195–202. doi: 10.1038/sj.bjp.0703555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISSON R.L., BATES J.N., JOHNSON A.K., LEWIS S.J. Use-dependent loss of acetylcholine- and bradykinin-mediated vasodilation after nitric oxide synthase inhibition. Evidence for preformed stores of nitric oxide-containing factors in vascular endothelial cells. Hypertension. 1996a;28:354–360. doi: 10.1161/01.hyp.28.3.354. [DOI] [PubMed] [Google Scholar]
- DAVISSON R.L., TRAVIS M.D., BATES J.N., LEWIS S.J. Hemodynamic effects of L- and D-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ. Res. 1996b;79:256–262. doi: 10.1161/01.res.79.2.256. [DOI] [PubMed] [Google Scholar]
- DAVISSON R.L., TRAVIS M.D., BATES J.N., JOHNSON A.K., LEWIS S.J. Stereoselective actions of S-nitrosocysteine in central nervous system of conscious rats. Am. J. Physiol. 1997;272:H2361–H2368. doi: 10.1152/ajpheart.1997.272.5.H2361. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FELETOU M., GARDENER M.J., GLEN C.D., RICHARDS G.R., VANHOUTTE P.M., WESTON A.H. Further investigations into the endothelium-dependent hyperpolarizing effects of bradykinin and substance P in porcine coronary artery. Br. J. Pharmacol. 2001;133:1145–1153. doi: 10.1038/sj.bjp.0704157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GAUTHIER K.M., DEETER C., KRISHNA U.M., REDDY Y.K., BONDLELA M., FALCK J.R., CAMPBELL W.B. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- IMIG J.D., FALCK J.R., WEI S., CAPDEVILA J.H. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J. Vasc. Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- LANG D., MOSFER S.I., SHAKESBY A., DONALDSON F., LEWIS M.J. Coronary microvascular endothelial cell redox state in left ventricular hypertrophy: the role of angiotensin II. Circ. Res. 2000;86:463–469. doi: 10.1161/01.res.86.4.463. [DOI] [PubMed] [Google Scholar]
- LANG R.J., HARVEY J.R., MULHOLLAND E.L. Sodium (2-sulfonatoethyl) methanethiosulfonate prevents S-nitroso-L-cysteine activation of Ca2+-activated K+ (BK(Ca)) channels in myocytes of the guinea-pig taenia caeca. Br. J. Pharmacol. 2003;139:1153–1163. doi: 10.1038/sj.bjp.0705349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Effects of hydroxocobalamin and carboxy-PTIO on nitrergic transmission in porcine anococcygeus and retractor penis muscles. Br. J. Pharmacol. 1999;127:172–176. doi: 10.1038/sj.bjp.0702496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Pharmacol. Sci. 2003;92:1–6. doi: 10.1254/jphs.92.1. [DOI] [PubMed] [Google Scholar]
- MCGUIRE J.J., DING H., TRIGGLE C.R. Endothelium-derived relaxing factors: a focus on endothelium-derived hyper polarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- MIURA H., GUTTERMAN D.D. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ. Res. 1998;83:501–507. doi: 10.1161/01.res.83.5.501. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends Pharmacol. Sci. 1997;18:252–256. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- RAFIKOVA O., RAFIKOV R., NUDLER E. Catalysis of S-nitrosothiols formation by serum albumin: the mechanism and implication in vascular control. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5913–5918. doi: 10.1073/pnas.092048999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RASSAF T., PREIK M., KLEINBONGARD P., LAUER T., HEISS C., STRAUER B.E., FEELISCH M., KELM M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J. Clin. Invest. 2002;109:1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., JOHNS A., WILCOX D., BATES F.N., HARRISON D. Evidence that a S-nitrosothiol, but not nitric oxide, may be identical with endothelium-derived relaxing factor. J. Cardiovasc. Pharmacol. 1991;17 Suppl 3:S41–S45. [Google Scholar]
- SCHECHTER A.N., GLADWIN M.T., CANNON R.O. NO solutions. J. Clin. Invest. 2002;109:1149–1151. doi: 10.1172/JCI15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLLINI M., FRIEDEN M., BENY J.L. Charybdotoxin-sensitive small conductance K(Ca) channel activated by bradykinin and substance P in endothelial cells. Br. J. Pharmacol. 2002;136:1201–1209. doi: 10.1038/sj.bjp.0704819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S., JARAKI O., OSBORNE J., SIMON D.I., KEANEY J., VITA J., SINGEL D., VALERI C.R., LOSCALZO J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. U.S.A. 1992a;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992b;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- SUNANO S., NAKAHIRA T., KAWATA K., SEKIGUCHI F. Factors involved in the time course of response to acetylcholine in mesenteric arteries from spontaneously hypertensive rats. Eur. J. Pharmacol. 2001;423:47–55. doi: 10.1016/s0014-2999(01)01081-0. [DOI] [PubMed] [Google Scholar]
- TOM B., DE VRIES R., SAXENA P.R., DANSER A.H.J. Negative inotropic effect of bradykinin in porcine isolated atrial trabeculae: role of nitric oxide. J. Hypertens. 2001;19:1289–1293. doi: 10.1097/00004872-200107000-00014. [DOI] [PubMed] [Google Scholar]
- TRAVIS M.D., DAVISSON R.L., BATES J.N., LEWIS S.J. Hemodynamic effects of L- and D-S-nitroso-beta,beta-dimethylcysteine in rats. Am. J. Physiol. 1997;273:H1493–H1501. doi: 10.1152/ajpheart.1997.273.3.H1493. [DOI] [PubMed] [Google Scholar]
- TRAVIS M.D., STOLL L.L., BATES J.N., LEWIS S.J. L- and D-S-nitroso-beta,beta-dimethylcysteine differentially increase cGMP in cultured vascular smooth muscle cells. Eur. J. Pharmacol. 1996;318:47–53. doi: 10.1016/s0014-2999(96)00719-4. [DOI] [PubMed] [Google Scholar]