Abstract
We determined (1) the inhibitory potency of zoniporide against the native Na+/H+ exchanger isoform 1 (NHE1) that is expressed in adult rat ventricular myocytes and platelets, and (2) the cardioprotective efficacy of zoniporide in isolated, blood-perfused adult rat hearts subjected to cardioplegic arrest, hypothermic ischaemia (150 min at 25°C) and normothermic reperfusion (60 min at 37°C).
In isolated myocytes, in which NHE1 activity was determined directly by measurement of H+ efflux rate following intracellular acidification, zoniporide produced a dose-dependent inhibition of such activity (IC50 73 nM at 25°C). A comparable NHE1-inhibitory potency was retained at 37°C.
In platelets, in which the rate of cell swelling was used as a surrogate index of NHE1 activity, this was again inhibited by zoniporide (IC50 67 nM at 25°C).
In the isolated heart model, administration of zoniporide (loading bolus of 1 mg kg−1 i.v. plus continuous infusion at 1.98 mg kg−1 h−1 i.v.) to the support animal achieved a free plasma drug concentration of ⩾1 μM. At this dose, zoniporide afforded significant cardioprotective benefit relative to vehicle treatment, with improved preservation of left ventricular end-diastolic and developed pressures and coronary perfusion pressure during reperfusion. Myocardial myeloperoxidase activity was also attenuated by zoniporide treatment, indicating reduced neutrophil accumulation.
These data show that zoniporide (1) is a potent inhibitor of native NHE1 activity in ventricular myocytes and platelets, and (2) affords significant cardioprotective benefit during ischaemia and reperfusion in an experimental model that mimics several distinctive features of human cardioplegic arrest with cardiopulmonary bypass.
Keywords: Na+/H+ exchanger, zoniporide, ischaemia, cardioplegia, cardiopulmonary bypass
Introduction
There is substantial preclinical evidence that activity of the cardiac sarcolemmal Na+/H+ exchanger (NHE), a membrane glycoprotein that is encoded by the NHE1 gene (Fliegel & Dyck, 1995), contributes significantly to myocardial injury and dysfunction during ischaemia and reperfusion (Avkiran, 1999; Karmazyn et al., 1999). This evidence has led to the clinical development of highly selective pharmacological inhibitors of NHE1, as potential therapeutic agents for cardioprotection in ischaemic heart disease. The results from clinical trials to date have been largely disappointing, probably due, at least in part, to an inadequate reflection of the preclinical knowledge base in clinical trial design (Avkiran & Marber, 2002). Nevertheless, some encouraging findings have emerged from the Guard in Ischemia Against Necrosis (GUARDIAN) trial (Théroux et al., 2000). Retrospective analysis of data from this trial has revealed that, in a subgroup of high-risk patients who underwent coronary artery bypass graft (CABG) surgery, intravenous treatment with cariporide, a benzoylguanidine NHE1-selective inhibitor, significantly reduced the incidence of death and myocardial infarction (Boyce et al., 2003). Since no significant benefit of treatment was observed in the other patient cohorts included in the GUARDIAN study (Théroux et al., 2000), the reasons behind the apparently selective protection afforded by cariporide in the CABG surgery setting are unclear. Important contributory factors are likely to include the unique opportunity in CABG surgery to achieve preischaemic treatment with cariporide and to institute timely reperfusion (Avkiran & Marber, 2002), both of which have been demonstrated to be prerequisites to achieve maximum cardioprotective efficacy with NHE1 inhibitors in preclinical studies (Avkiran, 1999, 2001). Other distinctive characteristics of myocardial ischaemia in the surgical setting include the concomitant use of established cardioprotective interventions (such as hypothermia and cardioplegia) and the application of cardiopulmonary bypass, with the attendant inflammatory response that arises from blood contact with synthetic surfaces. Indeed, in the GUARDIAN study, the large majority of the patients who underwent CABG surgery received cardioplegia, mostly under hypothermic conditions, and these patients were maintained on cardiopulmonary bypass for an average duration of around 100 min (Klatte et al., 2001; Boyce et al., 2003).
Zoniporide is a newer pyrazolylguanidine NHE inhibitor; relative to cariporide, it possesses (1) greater potency in inhibiting human NHE1, when expressed exogenously in transfected hamster fibroblasts; and (2) greater selectivity for NHE1 over the NHE2 and NHE3 isoforms (Marala et al., 2002). However, while the potency of cariporide as an inhibitor of the native sarcolemmal NHE that is expressed in myocardial cells has been established under both normothermic and hypothermic conditions (Hoshino & Avkiran, 2001), comparable information is not available for zoniporide. Furthermore, although zoniporide has been shown to protect the myocardium during normothermic ischaemia and reperfusion in several experimental settings (Knight et al., 2001; Tracey et al., 2003), its efficacy in surgically relevant animal models has not been established.
On the basis that myocardial protection during cardiac surgery may provide a viable clinical application for NHE1 inhibitors, we performed the present study with the following principal objectives: (1) To determine the potency of zoniporide as an inhibitor of native sarcolemmal NHE activity in rat ventricular myocytes, under both hypothermic and normothermic conditions. (2) To examine the cardioprotective efficacy of zoniporide in isolated, blood-perfused rat hearts, an experimental model that incorporates several key characteristics of human cardiac surgery with cardiopulmonary bypass, such as extracorporeal circulation, cardioplegic arrest, hypothermic ischaemia, and timely and documented reperfusion.
Methods
This investigation was performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986, published by Her Majesty's Stationery Office, London, and conforms with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996). Zoniporide (CP-597,396; [1-(quinolin-5-yl)-5-cyclopropyl-1H-pyrazole-4-carbonyl] guanidine) was provided by Pfizer Inc. and was dissolved in Tyrode's solution (isolated myocyte studies) or saline (isolated heart studies) to obtain the desired concentrations.
Isolated myocyte studies
Sarcolemmal NHE activity and its inhibition by zoniporide were assessed in ventricular myocytes, essentially as we have described in previous studies (Yasutake et al., 1996; Yokoyama et al., 1998; Gunasegaram et al., 1999; Avkiran & Yokoyama, 2000; Hoshino & Avkiran, 2001; Haworth et al., 2003).
Isolation of ventricular myocytes
Adult male Wistar rats (Bantin & Kingman Universal Ltd, 200–250 g) were anaesthetised with sodium pentobarbitone (60 mg kg−1 i.p.) and injected with heparin (1000 IU kg−1 i.v.), and their hearts were excised for the isolation of ventricular myocytes by enzymatic digestion, as we have described in detail previously (Yasutake et al., 1996).
Determination of sarcolemmal NHE activity
Intracellular pH (pHi) was monitored in single myocytes loaded with the pH-sensitive fluoroprobe carboxy-seminaphthorhodafluor-1 (cSNARF-1), using an established microepifluorescence technique. The myocytes were superfused with bicarbonate-free Tyrode's solution (in mM: NaCl 137, KCl 5.4, CaCl2 1.8, MgCl2 0.5, N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10, and glucose 10; adjusted to pH 7.4 at 25 or 37°C) throughout each experiment, thus enabling the rate of acid efflux (JH), which was calculated from the equation JH=βi dpHi/dt (where dpHi/dt is the rate of recovery of pHi and βi is the intrinsic buffering power), to be used as the indicator of sarcolemmal NHE activity.
Experimental protocol
Myocytes (n=8–10 per group) were maintained at 25 or 37°C throughout and were subjected to intracellular acidosis by transient (6 min at 25°C and 3 min at 37°C) exposure to 20 mM NH4Cl and its subsequent washout for 8 min. The longer exposure to NH4Cl at 25°C was necessitated by the higher resting pHi and attenuated intracellular acidification that is observed at this temperature (Hoshino & Avkiran, 2001). JH values were determined during the first 120 s of recovery from intracellular acidosis. When used, zoniporide (0.001, 0.003, 0.01, 0.03, 0.1, 0.3, or 1.0 μM) was present throughout NH4Cl washout.
Isolated heart studies
The effects of hypothermic cardioplegic arrest and subsequent normothermic reperfusion on myocardial viability and function were assessed, in the absence or presence of zoniporide treatment, using an isolated blood-perfused heart preparation, which we have described in detail previously (Hearse et al., 1999; Sutherland & Hearse, 2000; Clements-Jewery et al., 2002).
Support rat preparation
Adult male Wistar rats (Bantin & Kingman Universal Ltd, 340–430 g) were anaesthetised with sodium pentobarbitone (60 mg kg−1 i.p.), and given heparin (1000 IU kg−1 i.v.). The left femoral artery and right femoral vein were exposed by blunt dissection and cannulated, to enable supply of arterial blood to the perfused donor heart and its return back into the support animal's venous circulation, respectively. An extracorporeal circuit was established, primed with 12 ml of the plasma substitute Gelofusine ® (B. Braun Medical Ltd), and maintained for 10 min prior to aortic cannulation of the donor heart. Blood from the arterial limb was passed through a peristaltic pump (Gilson Minipuls 3) and flow was increased gradually over 10 min to a value of 2.4 ml min−1. Blood was passed through a steel cannula (for connection of the donor heart) and returned, by gravity, via a reservoir and gauze filter to the venous inflow line of the support rat. The support animal was placed supine on a thermostatically controlled surface to maintain a body temperature of 36–37°C (monitored by a rectal thermometer), and was allowed to breathe a mixture of 95% O2/5% CO2 through a face-mask. Anaesthesia was maintained with sodium pentobarbitone (0.6–3 mg) administered every 15 min into the venous reservoir. Gelofusine® was added to the extracorporeal circuit to replace fluid loss, when required; this was necessary to maintain venous return and thus minimize changes to the cardiac output and blood pressure of the support animal. The support animal's blood pressure was monitored by means of a pressure transducer attached to the arterial line.
Isolated heart perfusion
A second male Wistar rat (237–310 g) was then anaesthetised with sodium pentobaribitone (60 mg kg−1 i.p.), given heparin (1000 IU kg−1 i.v.) and the heart excised for blood perfusion. The aorta was cannulated and perfused in the Langendorff mode with arterial blood at 37°C at a coronary flow rate of 2.4 ml min−1, which maintains stable electrical and mechanical function of the isolated heart (Hearse et al., 1999; Sutherland & Hearse, 2000; Clements-Jewery et al., 2002). After left atrial excision, a collapsed compliant balloon was inserted through the mitral valve into the left ventricle, for the measurement of intraventricular pressure. The balloon was inflated with water to achieve a left ventricular end diastolic pressure (LVEDP) of 4–8 mmHg at baseline, and this balloon volume was maintained thereafter. Hearts were paced via the right atrium at 360 beats min−1 and coronary perfusion pressure (CPP) was monitored via a pressure transducer connected to a side arm of the aortic cannula.
Experimental protocols
In preliminary dose-ranging experiments, support animals received zoniporide as a loading bolus of 1 mg kg−1 i.v. followed by continuous infusion at 1.98 mg kg−1 h−1 i.v. (n=3) or a loading bolus of 2 mg kg−1 i.v. followed by continuous infusion at 3.96 mg kg−1 h−1 i.v. (n=5). Administration of zoniporide was initiated 10 min after the start of blood circulation through the extracorporeal circuit and blood samples were obtained immediately before and 20 and 80 min after the start of drug administration (the latter two time-points correspond to the onset and 60 min after the onset of ischaemia, respectively, in subsequent experiments), for the following assays: (1) analysis of plasma concentrations of zoniporide and its active metabolite CP-703,160, for which blood was collected into lithium heparin-containing tubes (Becton-Dickinson), centrifuged at 10,000 g for 10 min, and the supernatant stored at −20°C until subsequent analysis by liquid chromatography and tandem mass spectrometry. (2) Determination of the NHE1-inhibitory efficacy of the relevant concentrations by application of the platelet swelling assay (see below), for which blood was collected into potassium ethylenediaminetetraacetic acid (EDTA)-containing tubes (Becton-Dickinson) and used within 4 h.
In subsequent ischaemia/reperfusion experiments, isolated hearts were perfused with arterial blood for an initial 10 min period, during which atrial pacing and the baseline LVEDP were established. Hearts were then randomized, in a blinded manner, to one of two groups (n=10 per group). In the drug treatment group, zoniporide was administered as a loading bolus of 1 mg kg−1 i.v. followed by continuous infusion at 1.98 mg kg−1 h−1 i.v. thereafter. In the control group, an equivalent volume of saline (0.9% NaCl w v−1) was given as a bolus followed by continuous infusion. In both groups, 20 min after the start of drug/vehicle administration, the arterial inflow was diverted away from the aortic cannula to bypass the isolated heart, which was then perfused with St Thomas' Hospital No. 2 cardioplegic solution (in mM: NaCl 110, KCl 16, MgCl2 16, CaCl2 1.2 and NaHCO3 10; pH 7.8 at 25°C) for 2 min, at a perfusion pressure of 45 mmHg. During cardioplegia infusion, the coronary effluent was collected and its volume was noted. The heart was then immersed in a chamber filled with the cardioplegic solution (25°C) and global ischaemia maintained for 150 min. At the end of the ischaemic period, coronary reperfusion with arterial blood at 37°C was initiated and maintained for a further 60 min. Left ventricular pressure (LVP) and CPP were monitored throughout the protocol, at the end of which the heart was snap-frozen in liquid N2 for subsequent measurement of tissue myeloperoxidase (MPO) activity.
In all experiments, blood samples were collected from the arterial limb of the extracorporeal circuit prior to connection of the donor heart to the circuit, at the onset of ischaemia, 60 min after the onset of ischaemia and at the end of reperfusion, for analysis of PO2, PCO2 pH, haematocrit and electrolyte levels, using a Stat Profile 9 Blood Gas Analyser (Nova Biomedical). Additionally, blood samples collected at the onset and 60 min after the onset of ischaemia were again used for determination of the plasma concentrations of zoniporide and its active metabolite CP-703,160.
MPO Assay
MPO (MPO) activity in myocardial tissue samples was measured, as an index of neutrophil accumulation, using methodology that we have described in detail previously (Clements-Jewery et al., 2002). In brief, the tissue samples were homogenized in 0.5% hexadecyltrimethylammonium bromide (HETAB) in 50 mM potassium phosphate buffer (pH 6), in the ratio 1 ml HETAB/100 mg tissue. The homogenate was then centrifuged at 13,000 g for 20 min at 4°C and the protein concentration in the supernatant was determined using the bicinchoninic acid method. To measure supernatant MPO activity, 50 μl of each sample was added to 50 μl of o-dianisidine dihydrochloride (0.025% in phosphate buffer with 0.5% HETAB) in a 96-well plate. The reaction was started by addition of 50 μl of 0.01% hydrogen peroxide and the increase in optical density at 510 nM was measured over 3 min, using a microplate reader (Molecular Devices). MPO activity was quantified by reference to a standard curve, established using serial dilutions of a commercially available preparation of human neutrophil MPO (Sigma), and expressed as mU/mg protein.
Platelet swelling assay
We determined the effect of zoniporide (added in vitro or administered to the support animal, as described above) on platelet swelling, essentially as described previously (Knight et al., 2001). Whole blood in potassium EDTA-containing tubes was centrifuged at 150 g for 10 min at room temperature, and platelet-rich plasma (PRP) that comprised the upper two-thirds of the plasma layer was used for the assessment of platelet swelling. Propionate medium (in mM: sodium propionate 140, HEPES 20, glucose 10, KCl 5, MgCl2 1 and CaCl2 1; pH 6.7) was placed in the wells of a 96-well plate, to which samples of PRP were subsequently added. The reduction in optical density at 680 nM was measured over 5 min using a microplate reader (Molecular Devices) and the rate constant calculated from the initial slope was used as the surrogate index for platelet NHE1 activity. When added in vitro, zoniporide was present in the propionate medium at concentrations ranging from 0.001 to 1 μM.
Statistical analysis
Data are expressed as mean±s.e.m. Experiments in isolated myocytes were carried out in a randomized manner, such that a random selection of zoniporide concentrations were studied on any given day. Experiments in the blood-perfused heart model (including the determination of plasma drug concentrations) were carried out in a randomized and blinded manner, such that the operator was unaware of the nature of treatment (zoniporide or vehicle). For multigroup comparisons of data, analysis of variance followed by Dunnett's test (for comparisons of treatment groups with a control group) or the Bonferroni t-test (for comparisons of each group with every other) was used. For comparisons between a treatment group and a control group, the unpaired t-test was used. P<0.05 was considered significant. Dose–response curves and IC50 values were obtained by nonlinear regression analysis, using GraphPad Prism 4 software.
Results
NHE1 inhibitory potency of zoniporide
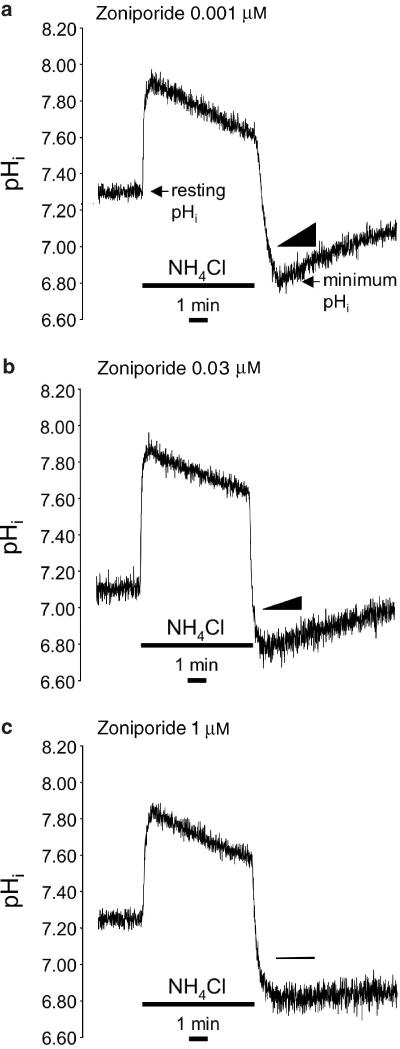
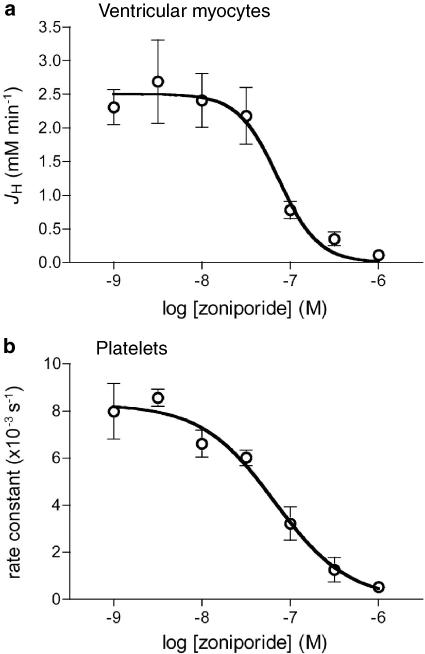
We determined the potency of zoniporide as an inhibitor of the native NHE1 expressed in two different adult rat cell types, namely ventricular myocytes (believed to be the main target of NHE1 inhibitors in producing their cardioprotective effects) and platelets. Figure 1 shows representative recordings of pHi in three individual ventricular myocytes maintained at 25°C and subjected to intracellular acidosis by transient exposure to NH4Cl, in the presence of different concentrations of zoniporide during NH4Cl washout. There was no significant difference between groups in resting pHi (7.27±0.06, 7.27±0.05, 7.28±0.05, 7.21±0.05, 7.27±0.05, 7.21±0.05 and 7.27±0.04, in the groups that received 0.001, 0.003, 0.01, 0.03, 0.1, 0.3 or 1.0 μM zoniporide, respectively) or the extent of intracellular acidosis (minimum pHi) achieved upon NH4Cl washout (6.85±0.04, 6.90±0.03, 6.84±0.04, 6.80±0.05, 6.77±0.04, 6.78±0.03 and 6.81±0.04, in the groups that received 0.001, 0.003, 0.01, 0.03, 0.1, 0.3 or 1.0 μM zoniporide, respectively). As is apparent in the recordings shown in Figure 1, zoniporide produced a dose-dependent reduction in the rate of pHi recovery from intracellular acidosis in ventricular myocytes. Figure 2a illustrates JH, a direct indicator of sarcolemmal NHE activity in the bicarbonate-free conditions employed in the present study (Yasutake et al., 1996), in seven groups of myocytes (n=8–10 per group) that were exposed to 0.001–1 μM zoniporide during recovery from intracellular acidosis. Zoniporide produced a dose-dependent inhibition of sarcolemmal NHE activity at 25°C, with an IC50 of 73 nM. To confirm that zoniporide inhibits sarcolemmal NHE activity with similar potency under normothermic conditions, in additional experiments we determined the effects of a limited range of concentrations (0.01, 0.1 and 1 μM) on JH in ventricular myocytes (n=10 per group) that were maintained at 37°C. As predicted from our previous work (Hoshino & Avkiran, 2001), JH values were greater at the normothermic temperature; once again, JH was not significantly inhibited by 0.01 μM zoniporide (4.73±0.85 mM min−1) but was reduced by around 57% (to 2.04±0.49 mM min−1) by 0.1 μM zoniporide and by around 82% (to 0.84±0.21 mM min−1) by 1 μM zoniporide. Figure 2b illustrates the effects of zoniporide on the rate constant of platelet swelling in propionate medium, an established surrogate index of NHE1 activity in this cell type (Rosskopf et al., 1991); in this assay also, zoniporide exerted a dose-dependent inhibitory effect, with an IC50 of 67 nM. These findings confirm that zoniporide is a potent inhibitor of native NHE1 activity in rat ventricular myocytes (at both hypothermic and normothermic temperatures) and platelets, and indicate that a free plasma concentration of ⩾1 μM should be targeted in subsequent experiments, in order to achieve effective inhibition of NHE1 activity in both the perfused myocardium and circulatory cells.
Figure 1.
Representative recordings of intracellular pH (pHi) in single adult rat ventricular myocytes subjected to intracellular acidosis by transient (6 min) exposure to 20 mM NH4Cl at 25°C. The recordings are from myocytes that were exposed to zoniporide at (a) 0.001 μM, (b) 0.03 μM or (c) 1 μM, during NH4Cl washout. In (a), the points at which resting pHi and minimum pHi were measured are also indicated. The slopes of the triangles in each panel illustrate the initial rates of pHi recovery (dpHi/dt).
Figure 2.
Dose–response curves for the inhibition by zoniporide of native NHE1 activity in (a) adult rat ventricular myocytes (n=8–10 cells per concentration) and (b) adult rat platelets (n=3 separate assays). In myocytes, the rate of H+ efflux (JH) following intracellular acidification was used as a direct index of NHE1 activity. In platelets, the rate of cell swelling in propionate medium was used as a surrogate index of NHE1 activity. The IC50 values were 73 nM in (a) and 67 nM in (b).
Preliminary dose-ranging study in cardiopulmonary bypass model
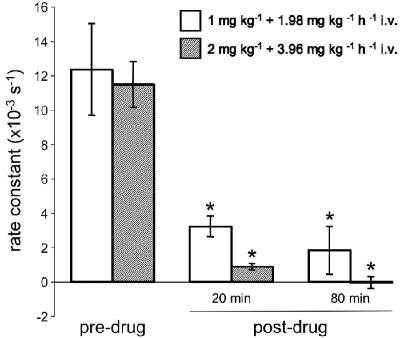
In order to determine the optimal dosing schedule to achieve an effective plasma drug concentration, in initial experiments we administered zoniporide as either a loading bolus of 1 mg kg−1 i.v. followed by continuous infusion at 1.98 mg kg−1 h−1 i.v. (n=3) or a loading bolus of 2 mg kg−1 i.v. followed by continuous infusion at 3.96 mg kg−1 h−1 i.v. (n=5). Table 1 shows the plasma concentrations of zoniporide and its active metabolite CP-703,160 at 20 and 80 min after the start of drug administration. With the lower dose, the total plasma concentration of active drug (i.e. zoniporide plus CP-703,160) was approximately 2.2 μM after 20 min and 2.6 μM after 80 min. Similarly, with the higher dose, the total plasma concentration of active drug was approximately 3.0 μM after 20 min and 4.0 μM after 80 min. Since plasma protein binding in the rat amounts to 54% (Tracey et al., 2003), with the lower dose, the above values translate into free active drug concentrations of approximately 1.0 and 1.2 μM, respectively, at 20 and 80 min after the start of administration of zoniporide. Such concentrations should be sufficient to afford effective inhibition of NHE1 activity, as indicated by our studies in rat ventricular myocytes and platelets with in vitro addition of zoniporide (see above). Indeed, the platelet swelling assay, carried out using blood obtained before and 20 and 80 min after the start of zoniporide administration, revealed a significant reduction in the rate constant (Figure 3), which is consistent with an effective NHE1-inhibitory concentration being achieved in the circulation with either dosing schedule. On the basis of these studies, we selected the dose of 1 mg kg−1 i.v. loading bolus followed by continuous infusion at 1.98 mg kg−1 h−1 i.v. for use in our efficacy study, which was designed to assess the cardioprotective efficacy of zoniporide.
Table 1.
Plasma concentrations of zoniporide and CP-703,160 in the dose-ranging study
| Dosing protocol | n | Zoniporide (μM) | CP-703,160(μM) | ||
|---|---|---|---|---|---|
| 20 min | 80 min | 20 min | 80 min | ||
| 1 mg kg−1+1.98 mg kg−1 h−1 i.v | 3 | 2.01±0.06 | 2.13±0.11 | 0.21±0.03 | 0.46±0.04 |
| 2 mg kg−1+3.96 mg kg−1 h−1 i.v | 5 | 2.63±0.30 | 3.20±0.16 | 0.37±0.04 | 0.72±0.08 |
Figure 3.
The rate of swelling in propionate medium, used as a surrogate index of NHE1 activity, in platelet samples obtained before (predrug) and 20 and 80 min after (postdrug) the start of zoniporide administration to the support animal, as a loading bolus of 1 mg kg−1 i.v. plus continuous infusion at 1.98 mg kg−1 h−1 i.v. (n=3) or as a loading bolus of 2 mg kg−1 i.v. plus continuous infusion at 3.96 mg kg−1 h−1 i.v. (n=5). *P<0.05 versus predrug.
Cardioprotective efficacy of zoniporide
Table 2 shows basal values for functional parameters in the isolated heart, haemodynamic parameters in the support rat and biochemical parameters in arterial blood. There was no significant difference between the two groups in any of these parameters. Indeed, the values for parameters related to support rat haemodynamics and blood biochemistry did not differ significantly between the groups throughout the experimental protocol.
Table 2.
Basal values for functional, haemodynamic and biochemical parameters in the isolated heart, support rat and arterial blood
| Parameter | Experimental group | |
|---|---|---|
| Control (n=10) | Zoniporide (n=10) | |
| Isolated heart | ||
| CPP (mmHg) | 89±4 | 95±6 |
| LVEDP (mmHg) | 5±0 | 5±0 |
| LVSP (mmHg) | 149±5 | 149±4 |
| LVDP (mmHg) | 144±4 | 145±4 |
| Support animal | ||
| Arterial systolic BP (mmHg) | 104±3 | 109±4 |
| Arterial diastolic BP (mmHg) | 84±3 | 86±4 |
| Heart rate (beats min−1) | 385±9 | 398±7 |
| Arterial blood | ||
| pH | 7.37±0.02 | 7.39±0.03 |
| PO2 (mmHg) | 204±15 | 204±11 |
| PCO2 (mmHg) | 48±3 | 47±2 |
| [K+] (mM) | 4.2±0.1 | 4.3±0.1 |
| [Na+] (mM) | 145±1 | 146±1 |
| Haematocrit (%) | 32±1 | 30±1 |
The values for basal parameters were noted either immediately before (isolated heart function; support rat haemodynamics) or immediately after (blood biochemistry) cardioplegic arrest of the isolated heart.
During perfusion with hypothermic (25°C) cardioplegic solution (2 min at a perfusion pressure of 45 mmHg), an identical volume (14±1 ml) was delivered to hearts in the control group and those in the zoniporide group. Cardioplegia produced a rapid arrest of mechanical activity in both groups. During the subsequent 150 min period of hypothermic (25°C) zero-flow global ischaemia, both groups of hearts developed ischaemic contracture. There was no significant difference between the groups in the time-to-peak contracture (114±8 min in the control group; 128±8 min in the zoniporide group) or the magnitude of the peak contracture (55±2 mmHg in both groups). LV pressure remained comparable in the two groups at the end of the ischaemic period (0 min time-point in Figure 4).
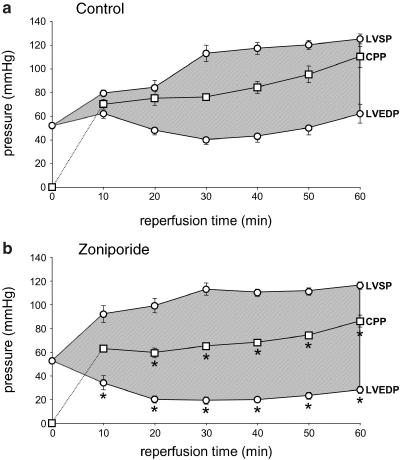
Figure 4.
The temporal profiles of LVEDP, LVSP and CPP, during 60 min of reperfusion of isolated rat hearts subjected to cardioplegic arrest and hypothermic ischaemia (150 min at 25°C). The shaded area indicates left ventricular developed pressure. The data are from (a) the control group (n=10 hearts), in which the support animals received vehicle (saline), and (b) the zoniporide group (n=10 hearts), in which the support animals received zoniporide as a loading bolus of 1 mg kg−1 i.v. plus continuous infusion at 1.98 mg kg−1 h−1 i.v. *P<0.05 versus control at corresponding time-point.
Figure 4 also profiles the changes in LV pressure (LVEDP and LVSP) and CPP that occurred during normothermic reperfusion, in the control group (Figure 4a) and the zoniporide group (Figure 4b). The most striking difference between the groups was in LVEDP, which was significantly lower in the zoniporide group throughout reperfusion. Interestingly, LVEDP tended to increase during the latter part (40–60 min) of the reperfusion period in both groups, albeit to a markedly smaller extent in the zoniporide group. There was no significant difference between the groups in LVSP during reperfusion and, after the first 20 min of reperfusion, LVSP was maintained at a relatively stable and comparable level in both groups. CPP was maintained at a significantly lower level throughout most of the reperfusion period in the zoniporide group, but tended to increase in both groups during the latter part (40–60 min) of reperfusion. This increase was smaller in the zoniporide group, such that, between 10 and 60 min of reperfusion, CPP increased by 23±4 mmHg in this group but by 40±6 mmHg in the control group (P<0.05).
The shaded area in Figure 4 illustrates left ventricular developed pressure (LVDP) (i.e. the difference between LVSP and LVEDP); this recovered faster in the zoniporide group, such that at 10 min of reperfusion the recovery of LVDP (relative to its preischaemic value) measured 40±9% in the zoniporide group but only 12±3% in the control group (P<0.05). The extent of the recovery of LVDP was also enhanced in the zoniporide group, such that at the end of the reperfusion period this measured 61±3% in the zoniporide group but only 43±5% in the control group (P<0.05).
Consistent with the functional benefits observed with zoniporide treatment, analysis of plasma samples obtained 20 and 80 min after the start of treatment (i.e. at the onset and 60 min after the onset of ischaemia) confirmed that the targeted free drug concentrations had indeed been achieved in the circulation (Table 3 ). As expected, neither zoniporide nor CP-703,160 could be detected in the plasma samples obtained from the control group at identical time-points (Table 3).
Table 3.
Plasma concentrations of zoniporide and CP-703,160 in the efficacy study
| Group | n | Zoniporide (μM) | CP-703,160 (μM) | ||
|---|---|---|---|---|---|
| 20 min | 80 min | 20 min | 80 min | ||
| Control | 10 | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| Zoniporide | 10 | 1.67±0.17 | 2.06±0.14 | 0.29±0.04 | 0.66±0.08 |
In the zoniporide group, the drug was administered as a loading bolus of 1 mg kg−1 i.v., followed by continuous infusion at 1.98 mg kg−1 h−1 i.v.; in the control group, an equivalent volume of vehicle (saline) was administered. <LLOQ: less than the lower limit of quantitation (0.03 μM).
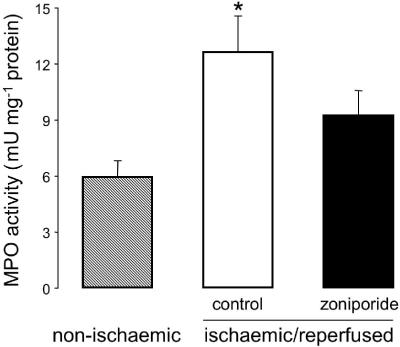
Figure 5 illustrates MPO activity in myocardial samples obtained at the end of the reperfusion period from the control and zoniporide groups; for comparison, MPO activity in nonischaemic myocardial samples (which were obtained from additional rats) is also shown. Relative to the nonischaemic samples, MPO activity was elevated in both groups of hearts subjected to in vitro blood perfusion and the ischaemia/reperfusion protocol, and there was no significant difference in MPO activity between these two groups. However, the difference from the non-ischaemic group was statistically significant only for the control group, indicating that treatment with zoniporide partially attenuated the increase in myocardial MPO activity that occurred during the experimental protocol.
Figure 5.
Myocardial MPO activity in nonischaemic hearts and in hearts subjected to cardioplegic arrest, hypothermic ischaemia (150 min at 25°C) and normothermic reperfusion (60 min at 37°C), with the administration of vehicle (control) or zoniporide (zoniporide) to the support animal. When used, zoniporide was administered as a loading bolus of 1 mg kg−1 i.v., followed by continuous infusion at 1.98 mg kg−1 h−1 i.v. *P<0.05 versus nonischaemic (n=6–10 hearts per group).
Discussion
The present study has shown, for the first time, that zoniporide is a potent inhibitor of sarcolemmal NHE activity in isolated rat ventricular myocytes, under both hypothermic and normothermic conditions. These findings are consistent with our previous data with cariporide, which was found to inhibit the sarcolemmal NHE with comparable potency at both 25 and 37°C (Hoshino & Avkiran, 2001). Comparison of the IC50 values obtained in the present study and in our previous work with cariporide (Hoshino & Avkiran, 2001) under similar assay conditions reveals that zoniporide (IC50 73 nM at 25°C) is more potent than cariporide (IC50 130 nM at 25°C) as an inhibitor of native sarcolemmal NHE activity in rat ventricular myocytes. Notably, the IC50 value for zoniporide that we have obtained in the present study is markedly greater than the IC50 value of 14 nM previously reported for the inhibition of human NHE1, expressed exogenously in transfected hamster fibroblasts (Marala et al., 2002). This difference is likely to have arisen from differences in assay conditions, in particular the extracellular Na+ concentration, rather than a species difference in the affinity of zoniporide for NHE1. In this context, it is worth noting that (1) the rate of 22Na+ uptake, the index of NHE activity in the fibroblast assay, is commonly measured in the presence of a trace amount of extracellular Na+ (Marala et al., 2002), and (2) extracellular Na+ is known to antagonize competitively the binding of benzoylguanidine inhibitors, such as HOE-694, cariporide and eniporide, to NHE1 (Baumgarth et al., 1998). Indeed, in guinea pig ventricular myocytes, the IC50 for HOE-694 has been estimated to be approximately 16-fold greater in the presence of an extracellular Na+ concentration of 150 mM, relative to the value obtained in the virtual absence of extracellular Na+ (Loh et al., 1996).
We have additionally determined the potency of zoniporide in rat platelets, by determining their rate of swelling in propionate medium, which is an established surrogate index of NHE1 activity in this cell type (Rosskopf et al., 1991). The IC50 value that we obtained (67 nM) is very similar to the IC50 value reported previously in human platelets (59 nM), using an identical assay (Marala et al., 2002). This observation suggests that zoniporide does indeed inhibit NHE1 activity with similar potency in rats and humans.
Having established the inhibitory potency of zoniporide against the native NHE1 that is expressed in rat ventricular myocytes and platelets, we next determined the cardioprotective efficacy of this agent in isolated, blood-perfused rat hearts, using a dose that was selected rationally, on the basis of preliminary dose-ranging experiments with analysis of plasma drug concentrations. In this work, zoniporide was found to afford significant cardioprotective benefit, with improved preservation of diastolic and systolic left ventricular function, as reflected by the recoveries of LVEDP and LVDP, respectively, during reperfusion. Zoniporide treatment also improved the preservation of vascular function, with a significantly lower CPP during most of the reperfusion period. Although our findings clearly establish the cardioprotective efficacy of zoniporide treatment in this model of cardiopulmonary bypass, they do not allow delineation of the relative contributions of reduced stunning versus reduced infarction to the observed functional benefit. In this context, previous work in other models of ischaemia and reperfusion has shown that zoniporide treatment can attenuate myocardial stunning as well as limiting infarct size (Tracey et al., 2003).
In the vast majority of previous preclinical studies that have investigated the cardioprotective efficacy of NHE1 inhibitors, hearts have been subjected to ischaemia and reperfusion under normothermic conditions, to mimic the situation during acute coronary occlusion and nonsurgical revascularization in patients with coronary artery disease. In a recent study, however, Muraki et al. (2003) have investigated the effects of cariporide, used as a cardioplegia supplement, in a model of surgical reperfusion after acute coronary occlusion, in which global hypothermic ischaemia was superimposed on antecedent regional normothermic ischaemia. Additionally, a limited number of studies (Myers & Karmazyn, 1996; Shipolini et al., 1997; Yamauchi et al., 1997; Cox et al., 2002) have employed global hypothermic ischaemia alone, as encountered more commonly during nonemergency cardiac surgery, and have used NHE1 inhibitors as an adjunct or additive to established surgical cardioprotection techniques such as hyperkalaemic hypothermic cardioplegia. For example, our laboratory was the first to show that, in isolated rat hearts perfused with crystalloid solution, cariporide (used as an additive or adjunct to cardioplegia) provided additional cardioprotective benefit under the conditions of moderate hypothermia that are encountered during routine cardiac surgery (Shipolini et al., 1997). The present study is the first examination of the cardioprotective efficacy of zoniporide in a surgically relevant model and extends the findings of our previous work with cariporide, by showing that zoniporide provides significant cardioprotective benefit in blood-perfused hearts that are subjected to cardioplegic arrest and hypothermic ischaemia. Notably, in the present study, zoniporide was administered intravenously to the support animal, which mimics the mode of administration that has been used in the clinical examination of the cardioprotective efficacy of cariporide in patients undergoing CABG surgery (Boyce et al., 2003). The significant protection afforded by zoniporide in our model suggests that it may be possible to test the cardioprotective efficacy of intravenous administration of zoniporide in patients undergoing cardiac surgery, without any formulation concerns that may arise if this agent were to be used as an additive to the myriad of cardioplegic solutions that are currently in surgical use.
In relation to the potential application of NHE1 inhibitors for surgical myocardial protection, the maintained cardioprotective efficacy of zoniporide and cariporide under hypothermic conditions distinguishes these agents from other ion transport inhibitors, such as L-type calcium channel blockers. In this context, unlike zoniporide (present study) and cariporide (Shipolini et al., 1997), verapamil (Hearse et al., 1984) and nifedipine (Fukunami & Hearse, 1985) have been shown to provide no significant cardioprotective benefit over and above that afforded by hyperkalaemic cardioplegia in isolated rat hearts under conditions of moderate hypothermia, although both calcium channel blockers were effective at temperatures >30°C.
The principal mechanism by which NHE1 inhibitors are thought to afford protection during myocardial ischaemia and reperfusion is through the attenuation of intracellular Na+ and Ca2+ overload in cardiac myocytes (Avkiran, 2001). Nevertheless, the potential contribution of additional mechanisms, such as an altered neutrophil–endothelium interaction, cannot be precluded. Indeed, cariporide has been shown to inhibit the endothelial expression of intracellular adhesion molecule-1 during hypoxia and reoxygenation in vitro (Hattori et al., 2001) and to attenuate the myocardial accumulation of neutrophils during ischaemia and reperfusion in vivo (Gumina et al., 2000; Castella et al., 2002; Muraki et al., 2003). By design, the experimental model that we have used in the present study mimics the inflammatory response to extracorporeal blood circulation that is characteristic of cardiopulmonary bypass (Asimakopoulos & Taylor, 1998) and is associated with marked myocardial neutrophil accumulation (Clements-Jewery et al., 2002). We have found that the increase in myocardial MPO activity, measured at the end of the reperfusion period, was partially attenuated in the zoniporide group, indicating reduced myocardial neutrophil accumulation during the experimental protocol. Myocardial neutrophil accumulation has previously been associated with marked increases in coronary vascular resistance and LVEDP during reperfusion of isolated rat hearts subjected to normothermic ischaemia, with a marked functional benefit afforded by a monoclonal antibody against the neutrophil adhesion molecule CD-18 (Lefer et al., 1993). It is possible therefore that, in the present study, myocardial neutrophil accumulation contributed to vascular and contractile dysfunction (in particular, the increases in CPP and LVEDP during reperfusion) and that the attenuation of such accumulation was mechanistically involved in the cardioprotective benefit observed with zoniporide treatment. The concordant effects of zoniporide (present study) and cariporide (Gumina et al., 2000; Castella et al., 2002; Muraki et al., 2003) on myocardial neutrophil accumulation following ischaemia and reperfusion suggest that these effects arose from NHE1 inhibition, rather than from a non-specific effect of either drug. It is not clear, however, whether the zoniporide-induced reduction in myocardial neutrophil accumulation arose from the inhibition of NHE1 activity in cellular components within the ischaemic/reperfused myocardium (e.g. myocytes, endothelial cells) or those in the circulation (e.g. neutrophils). Furthermore, the possibility that reduced neutrophil accumulation occurred as a consequence of improved myocardial preservation (Baxter, 2002) cannot be excluded.
It is becoming increasingly apparent that myocardial injury arising from ischaemia and reperfusion during CABG surgery remains an important contributor to morbidity and mortality, and that therapies targeted at the attenuation of such injury are likely to improve clinical outcome (Klatte et al., 2001; Gavard et al., 2003; Mentzer, 2003). The recent Exchange Inhibition to Prevent Coronary Events in Acute Cardiac Conditions (EXPEDITION) trial with cariporide, whose results were presented at the American Heart Association Scientific Sessions in November 2003, has reconfirmed the indication from the GUARDIAN trial that NHE1 activity contributes significantly to myocardial injury during CABG surgery. However, serious adverse effects (increased incidence of cerebrovascular events and mortality) preclude the clinical application of the treatment modality used in the EXPEDITION trial. Our data indicate that zoniporide, a potent and selective inhibitor of NHE1 activity whose chemical structure is distinct from cariporide, affords significant cardioprotective benefit in an experimental model that reproduces many of the characteristics of myocardial ischaemia and reperfusion during CABG surgery with cardiopulmonary bypass. The safety and cardioprotective efficacy of zoniporide in CABG surgery await confirmation by further investigation.
Acknowledgments
This study was funded by a research grant from Pfizer Inc. We thank Phuong T. Huynh (Pfizer Global Research and Development) for assistance in the determination of zoniporide and CP-703,160 concentrations in plasma samples.
Abbreviations
- CABG
coronary artery bypass graft
- CPP
coronary perfusion pressure
- cSNARF-1
carboxy-seminaphthorhodafluor-1
- EDTA
ethylenediaminetetraacetic acid
- GUARDIAN
Guard in Ischemia Against Necrosis
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- HETAB
hexadecyltrimethylammonium bromide
- LV
left ventricular
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end-diastolic pressure
- LVP
left ventricular pressure
- LVSP
left ventricular systolic pressure
- MPO
myeloperoxidase
- NHE
Na+/H+ exchanger
- PRP
platelet-rich plasma
References
- ASIMAKOPOULOS G., TAYLOR K.M. Effects of cardiopulmonary bypass on leukocyte and endothelial adhesion molecules. Ann. Thorac. Surg. 1998;66:2135–2144. doi: 10.1016/s0003-4975(98)00727-9. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M. Protection of the ischaemic myocardium by Na+/H+ exchange inhibitors: potential mechanisms of action. Basic Res. Cardiol. 2001;96:306–311. doi: 10.1007/s003950170037. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M. Rational basis for use of sodium–hydrogen exchange inhibitors in myocardial ischemia. Am. J. Cardiol. 1999;83:10G–18G. doi: 10.1016/s0002-9149(99)00215-5. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M., MARBER M.S. Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J. Am. Coll. Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M., YOKOYAMA H. Adenosine A1 receptor stimulation inhibits α1-adrenergic activation of the cardiac sarcolemmal Na+/H+ exchanger. Br. J. Pharmacol. 2000;131:659–662. doi: 10.1038/sj.bjp.0703647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARTH M., BEIER N., GERICKE R. Bicyclic acylguanidine Na+/H+ antiporter inhibitors. J. Med. Chem. 1998;41:3736–3747. doi: 10.1021/jm981031w. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F. The neutrophil as a mediator of myocardial ischemia–reperfusion injury: time to move on. Basic Res. Cardiol. 2002;97:268–275. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- BOYCE S.W., BARTELS C., BOLLI R., CHAITMAN B., CHEN J.C., CHI E., JESSEL A., KEREIAKES D., KNIGHT J., THULIN L., THÉROUX P. Impact of sodium–hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J. Thorac. Cardiovasc. Surg. 2003;126:420–427. doi: 10.1016/s0022-5223(03)00209-5. [DOI] [PubMed] [Google Scholar]
- CASTELLA M., BUCKBERG G.D., TAN Z., IGNARRO L.J. Myocyte and endothelial effects of preconditioning the jeopardized heart by inhibiting Na/H exchange. J. Thorac. Cardiovasc. Surg. 2002;124:1113–1121. doi: 10.1067/mtc.2002.125485. [DOI] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. The isolated blood-perfused rat heart: an inappropriate model for the study of ischaemia- and infarction-related ventricular fibrillation. Brit. J. Pharmacol. 2002;137:1089–1099. doi: 10.1038/sj.bjp.0704977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX C.S., SAUER H., ALLEN S.J., BUJA L.M., LAINE G.A. Sodium/hydrogen-exchanger inhibition during cardioplegic arrest and cardiopulmonary bypass: an experimental study. J. Thorac. Cardiovasc. Surg. 2002;123:959–966. doi: 10.1067/mtc.2002.120715. [DOI] [PubMed] [Google Scholar]
- FLIEGEL L., DYCK J.R.B. Molecular biology of the cardiac sodium/hydrogen exchanger. Cardiovasc. Res. 1995;29:155–159. [PubMed] [Google Scholar]
- FUKUNAMI M., HEARSE D.J. Temperature-dependency of nifedipine as a protective agent during cardioplegia in the rat. Cardiovasc. Res. 1985;19:95–103. doi: 10.1093/cvr/19.2.95. [DOI] [PubMed] [Google Scholar]
- GAVARD J.A., CHAITMAN B.R., SAKAI S., STOCKE K., DANCHIN N., ERHARDT L., GALLO R., CHI E., JESSEL A., THÉROUX P. Prognostic significance of elevated creatine kinase MB after coronary bypass surgery and after an acute coronary syndrome: results from the GUARDIAN trial. J. Thorac. Cardiovasc. Surg. 2003;126:807–813. doi: 10.1016/s0022-5223(03)00735-9. [DOI] [PubMed] [Google Scholar]
- GUMINA R.J., AUCHAMPACH J., WANG R., BUERGER E., EICKMEIER C., MOORE J., DAEMMGEN J., GROSS G.J. Na+/H+ exchange inhibition-induced cardioprotection in dogs: effects on neutrophils versus cardiomyocytes. Am. J. Physiol. Heart. Circ. Physiol. 2000;279:H1563–H1570. doi: 10.1152/ajpheart.2000.279.4.H1563. [DOI] [PubMed] [Google Scholar]
- GUNASEGARAM S., HAWORTH R.S., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT1 versus AT2 receptors. Circ. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- HATTORI R., OTANI H., MORIGUCHI Y., MATSUBARA H., YAMAMURA T., NAKAO Y., OMIYA H., OSAKO M., IMAMURA H. NHE and ICAM-1 expression in hypoxic/reoxygenated coronary vascular endothelial cells. Am. J. Physiol. Heart. Circ. Physiol. 2001;280:H2796–H2803. doi: 10.1152/ajpheart.2001.280.6.H2796. [DOI] [PubMed] [Google Scholar]
- HAWORTH R.S., MCCANN C., SNABAITIS A.K., ROBERTS N.A., AVKIRAN M. Stimulation of the plasma membrane Na+/H+ exchanger NHE1 by sustained intracellular acidosis: evidence for a novel mechanism mediated by the ERK pathway. J. Biol. Chem. 2003;278:31676–31684. doi: 10.1074/jbc.M304400200. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., FERRARI R., SUTHERLAND F.J. Cardioprotection: intermittent ventricular fibrillation and rapid pacing can induce preconditioning in the blood-perfused rat heart. J. Mol. Cell Cardiol. 1999;31:1961–1973. doi: 10.1006/jmcc.1999.1027. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., YAMAMOTO F., SHATTOCK M.J. Calcium antagonists and hypothermia: the temperature dependency of the negative inotropic and anti-ischemic properties of verapamil in the isolated rat heart. Circulation. 1984;70:I54–I64. [PubMed] [Google Scholar]
- HOSHINO K., AVKIRAN M. Effects of moderate hypothermia on sarcolemmal Na+/H+ exchanger activity and its inhibition by cariporide in cardiac ventricular myocytes. Brit. J. Pharmacol. 2001;134:1587–1595. doi: 10.1038/sj.bjp.0704405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARMAZYN M., GAN X.T., HUMPHREYS R.A., YOSHIDA H., KUSUMOTO K. The myocardial Na+–H+ exchange: structure, regulation, and its role in heart disease. Circ. Res. 1999;85:777–786. doi: 10.1161/01.res.85.9.777. [DOI] [PubMed] [Google Scholar]
- KLATTE K., CHAITMAN B.R., THÉROUX P., GAVARD J.A., STOCKE K., BOYCE S., BARTELS C., KELLER B., JESSEL A. Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J. Am. Coll. Cardiol. 2001;38:1070–1077. doi: 10.1016/s0735-1097(01)01481-4. [DOI] [PubMed] [Google Scholar]
- KNIGHT D.R., SMITH A.H., FLYNN D.M., MACANDREW J.T., ELLERY S.S., KONG J.X., MARALA R.B., WESTER R.T., GUZMAN-PEREZ A., HILL R.J., MAGEE W.P., TRACEY W.R. A novel sodium–hydrogen exchanger isoform-1 inhibitor, zoniporide, reduces ischemic myocardial injury in vitro and in vivo. J. Pharmacol. Exp. Ther. 2001;297:254–259. [PubMed] [Google Scholar]
- LEFER D.J., SHANDELYA S.M., SERRANO C.V., BECKER L.C., KUPPUSAMY P., ZWEIER J.L. Cardioprotective actions of a monoclonal antibody against CD-18 in myocardial ischemia–reperfusion injury. Circulation. 1993;88:1779–1787. doi: 10.1161/01.cir.88.4.1779. [DOI] [PubMed] [Google Scholar]
- LOH S.-H., SUN B., VAUGHAN-JONES R.D. Effect of Hoe 694, a novel Na+–H+ exchange inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte. Br. J. Pharmacol. 1996;118:1905–1912. doi: 10.1111/j.1476-5381.1996.tb15623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARALA R.B., BROWN J.A., KONG J.X., TRACEY W.R., KNIGHT D.R., WESTER R.T., SUN D., KENNEDY S.P., HAMANAKA E.S., RUGGERI R.B., HILL R.J. Zoniporide: a potent and highly selective inhibitor of human Na+/H+ exchanger-1. Eur. J. Pharmacol. 2002;451:37–41. doi: 10.1016/s0014-2999(02)02193-3. [DOI] [PubMed] [Google Scholar]
- MENTZER R.M. Does size matter? What is your infarct rate after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2003;126:326–328. doi: 10.1016/s0022-5223(03)00753-0. [DOI] [PubMed] [Google Scholar]
- MURAKI S., MORRIS C.D., BUDDE J.M., ZHAO Z.Q., GUYTON R.A., VINTEN-JOHANSEN J. Blood cardioplegia supplementation with the sodium–hydrogen ion exchange inhibitor cariporide to attenuate infarct size and coronary artery endothelial dysfunction after severe regional ischemia in a canine model. J. Thorac. Cardiovasc. Surg. 2003;125:155–164. doi: 10.1067/mtc.2003.65. [DOI] [PubMed] [Google Scholar]
- MYERS M.L., KARMAZYN M. Improved cardiac function after prolonged hypothermic ischemia with the Na+/H+ exchange inhibitor HOE 694. Ann. Thorac. Surg. 1996;61:1400–1406. doi: 10.1016/0003-4975(96)00088-4. [DOI] [PubMed] [Google Scholar]
- ROSSKOPF D., MORGENSTERN E., SCHOLZ W., OSSWALD U., SIFFERT W. Rapid determination of the elevated Na+–H+ exchange in platelets of patients with essential hypertension using an optical swelling assay. J. Hypertens. 1991;9:231–238. [PubMed] [Google Scholar]
- SHIPOLINI A.R., GALIÑANES M., EDMONDSON S.J., HEARSE D.J., AVKIRAN M. Na+/H+ exchanger inhibitor HOE-642 improves cardioplegic myocardial preservation under both normothermic and hypothermic conditions. Circulation. 1997;96:II266–II273. [PubMed] [Google Scholar]
- SUTHERLAND F.J., HEARSE D.J. The isolated blood and perfusion fluid perfused heart. Pharmacol. Res. 2000;41:613–627. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- THÉROUX P., CHAITMAN B.R., DANCHIN N., ERHARDT L.R.W., MEINERTZ T., SCHROEDER J.S., TOGNONI G., WHITE H.D., WILLERSON J.T., JESSEL A. Inhibition of the sodium–hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations: main results of the GUARDIAN trial. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- TRACEY W.R., ALLEN M.C., FRAZIER D.E., FOSSA A.A., JOHNSON C.G., MARALA R.B., KNIGHT D.R., GUZMAN-PEREZ A. Zoniporide: a potent and selective inhibitor of the human sodium–hydrogen exchanger isoform 1 (NHE-1) Cardiovasc. Drug Rev. 2003;21:17–32. doi: 10.1111/j.1527-3466.2003.tb00103.x. [DOI] [PubMed] [Google Scholar]
- YAMAUCHI T., ICHIKAWA H., SAWA Y., FUKUSHIMA N., KAGISAKI K., MAEDA K., MATSUDA H., SHIRAKURA R. The contribution of Na+/H+ exchange to ischemia–reperfusion injury after hypothermic cardioplegic arrest. Ann. Thorac. Surg. 1997;63:1107–1112. doi: 10.1016/s0003-4975(96)01390-2. [DOI] [PubMed] [Google Scholar]
- YASUTAKE M., HAWORTH R.S., KING A., AVKIRAN M. Thrombin activates the sarcolemmal Na+/H+ exchanger: evidence for a receptor-mediated mechanism involving protein kinase C. Circ. Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA H., YASUTAKE M., AVKIRAN M. 1-Adrenergic stimulation of sarcolemmal Na+/H+ exchanger activity in rat ventricular myocytes: evidence for selective mediation by the α1A-adrenoceptor subtype. Circ. Res. 1998;82:1078–1085. doi: 10.1161/01.res.82.10.1078. [DOI] [PubMed] [Google Scholar]