Abstract
The possibility that thromboxane (TXA2) receptor stimulation causes differential block of the SKCa and IKCa channels which underlie EDHF-mediated vascular smooth muscle hyperpolarization and relaxation was investigated in the rat isolated mesenteric artery.
Acetylcholine (30 nM–3 μM ACh) or cyclopiazonic acid (10 μM CPA, SERCA inhibitor) were used to stimulate EDHF-evoked smooth muscle hyperpolarization. In each case, this led to maximal hyperpolarization of around 20 mV, which was sensitive to block with 50 nM apamin and abolished by repeated stimulation of mesenteric arteries with the thromboxane mimetic, U46619 (30 nM–0.1 μM), but not the α1-adrenoceptor agonist phenylephrine (PE).
The ability of U46619 to abolish EDHF-evoked smooth muscle hyperpolarization was prevented by prior exposure of mesenteric arteries to the TXA2 receptor antagonist 1 μM SQ29548.
Similar-sized smooth muscle hyperpolarization evoked with the SKCa activator 100 μM riluzole was also abolished by prior stimulation with U46619, while direct muscle hyperpolarization in response to either levcromakalim (1 μM, KATP activator) or NS1619 (40 μM, BKCa activator) was unaffected.
During smooth muscle contraction and depolarization to either PE or U46619, ACh evoked concentration-dependent hyperpolarization (to −67 mV) and complete relaxation. These responses were well maintained during repeated stimulation with PE, but with U46619 there was a progressive decline, so that during a third exposure to U46619 maximum hyperpolarization only reached –52 mV and relaxation was reduced by 20%. This relaxation could now be blocked with charybdotoxin alone. The latter responses could be mimicked with 300 μM 1-EBIO (IKCa activator), an action not modified by exposure to U46619.
An early consequence of TXA2 receptor stimulation is a reduction in the arterial hyperpolarization and relaxation attributed to EDHF. This effect appears to reflect a loss of SKCa activity.
Keywords: Acetylcholine, endothelium-derived hyperpolarizing factor, EDHF, endothelium, K-channels, mesenteric artery, U46619, phenylephrine, small conductance calcium-activated potassium channels
Introduction
Thromboxane A2 (TXA2) is a powerful vasoconstrictor and mitogenic agent, which has both physiological and pathophysiological roles within the cardiovascular system. The ability of TXA2 potently to stimulate sustained background contraction of vascular smooth muscle is also commonly employed in studies investigating endothelium-dependent dilator pathways (e.g. Fisslthaler et al., 1999; McNeish et al., 2001). However, stimulation of resistance arteries with high concentrations of the TXA2 mimetic U46619 is associated with a selective loss of the subsequent relaxation caused by endothelium-derived hyperpolarizing factor (EDHF; Plane & Garland, 1996).
EDHF causes vascular smooth muscle hyperpolarization leading to relaxation, which is independent of the action of both nitric oxide (NO) and prostacylin. The hyperpolarization reflects the action of a factor (EDHF) and/or the spread of current from the endothelium through heterocellular (myo-endothelial) gap junctions. Physiologically, EDHF appears to provide an important route to vasodilatation, which assumes increasing importance over NO with decreasing arterial size, predominating in resistance arteries and arterioles and operating in vivo (Yamamoto et al., 2001; Busse et al., 2002; Parkington et al., 2002). Therefore, the possibility that an autacoid like TXA2 could influence the ability of the endothelium locally to modulate the arterial diameter through EDHF is clearly of applied interest.
Whatever the precise factor or structures underlying the EDHF pathway in different arteries, a characteristic is a susceptibility to block with a combination of the potassium channel blockers apamin and charybdotoxin, but not with apamin and iberiotoxin (see Dora & Garland, 1998; Busse et al., 2002). This reflects a pivotal role for small (SKCa) and intermediate (IKCa) conductance, calcium-activated potassium channels, which are restricted to the endothelium (Edwards et al., 1998; Doughty et al., 1999; Walker et al., 2001). Activation of these K channels results in hyperpolarization of the endothelial cells, causing smooth muscle hyperpolarization and relaxation (Busse et al., 2002). We have recently reported that the contribution made to the EDHF response by SKCa and IKCa activation can vary depending on the extent of smooth muscle depolarization and contraction (Crane et al., 2003). While this may reflect a differential localization of these channels within the endothelial cells, it raises the possibility that their respective activities may be modulated independently. The block of EDHF-evoked smooth muscle hyperpolarization and relaxation previously reported with U46619 was both complete and selective, as it did not occur in arteries stimulated with the α1-adrenergic agonist phenylephrine (PE) (Plane & Garland, 1996). The fact that the smooth muscle cells were still able to contract and relax in this previous study suggested a primary action of U46619 against endothelial cell K channels.
In the present study, we simultaneously measured the changes in smooth muscle membrane potential and tension to investigate the possibility that lower concentrations of U46619 may act differentially against endothelial cell K channels and thus initiate a progressive block of the EDHF pathway. Our data are consistent with an initial and irreversible block by U46619 of SKCa channels in the endothelium. Some of these results have been presented in preliminary form to the Physiological Society (Crane & Garland, 2003).
Methods
Male Wistar rats (200–250 g) were killed by cervical dislocation and exsanguination (Animals Scientific Procedure Act 1986, U.K.). The mesentery was quickly removed and placed in Krebs buffer at room temperature. A segment (2 mm in length) of a third-order branch of the superior mesenteric artery was carefully cleared of adherent tissue and mounted in Krebs buffer at 37°C in a small vessel myograph (model 400A, Danish Myotechnology, Denmark), at a tension equivalent to that generated at 0.9 times the diameter of the vessel at 100 mmHg (Garland & McPherson, 1992). The functional viability of endothelial cells was then assessed by the ability of 1 μM acetylcholine (ACh) to induce over 95% relaxation following contraction of the arterial segments, with a submaximal concentration of PE (3 μM). The artery was superfused at 3–4 ml min−1 with oxygenated Krebs buffer at 37°C. Drugs were then applied under static conditions at 37°C and mixed by continuous gassing with 5% CO2 in 95% O2. All experiments were performed in the presence of the NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM). Individual smooth muscle cells were impaled with sharp glass electrodes (backfilled with 2 M KCl, tip resistances approximately 80 MΩ), to enable measurement of smooth muscle membrane potential and tension simultaneously (Garland & McPherson, 1992).
PE (0.1–1 μM) or U46619 (1 nM–0.1 μM) were applied cumulatively, to evoke smooth muscle depolarization and contraction. In each experiment, the concentration of agonist was titrated to match the extent of depolarization and contraction as closely as possible between each artery and each exposure to either PE and/or U46619 in that artery. This minimized any effect due to the extent of smooth muscle stimulation per se. ACh (10 nM–3 μM; cumulatively), or in some cases 300 μM 1-EBIO, was then applied. Following washout for 45–60 min, this cycle was repeated at least twice. In some experiments, a final stimulation with PE was used to assess any cross-depression of ACh responses due to prior exposure to U46619.
U46619 experiments involving KCa channel inhibitors and the thromboxane receptor antagonist, SQ29548
KCa channel inhibitors or the TXA2 antagonist SQ29548 (1 μM) were applied directly to a static bath at least 20 min before and throughout concentration–response determinations to U46619, PE and/or ACh.
Experiments involving cyclopiazonic acid (CPA) and K-channel activator induced smooth muscle hyperpolarization
Smooth muscle hyperpolarization was recorded to the cumulative application of 10 nM–3 μM ACh or single concentrations of CPA (10 μM), NS1619 (40 μM BKCa channel opener), levcromakalim (1 μM KATP channel opener), riluzole (100 μM SKCa channel opener) or 1-EBIO (300 μM IKCa channel opener). In some experiments, these agents were added after repeated exposure and washout with U46619. In experiments using 50 nM apamin or 100 nM charybdotoxin, toxins were added to the bath at least 20 min before membrane potential changes were recorded.
Solutions and drugs
In all experiments, arterial segments were maintained at 37°C in oxygenated Krebs buffer of the following composition (mM): NaCl 118.0, NaHCO3 25.0, KCl 3.6, MgSO4·7H2O 1.2, KH2PO4 1.2, glucose 11.0, CaCl2 2.5. The drugs used were all from Sigma except for charybdotoxin, apamin, iberiotoxin, from Latoxan, 1-ethyl-2-benzimidazolinone (1-EBIO) from Aldrich, and U46619 from Calbiochem. Charybdotoxin was dissolved in 0.9% saline. 1-EBIO (0.1 M), NS1619 (0.1 M), CPA (0.1 M), levcromakalim (0.01 M), and riluzole (0.01 M) were each dissolved in dimethylsulfoxide (DMSO) to make a stock solution for subsequent dilution. Control experiments indicated that DMSO had no direct action in the final concentrations applied. All other stock solutions were dissolved in distilled water.
Analysis of data
Data are given as mean±s.e.m. of n replicates, with n=1 for a separate artery, unless stated otherwise. Repeated-measures ANOVA tests are used in cases where protocols were repeated twice. Student's t-tests (paired and unpaired) and ANOVA with Bonferroni multiple comparisons were also used, as indicated. The significance level was P<0.05.
Results
Modulation of EDHF evoked hyperpolarization by U46619 in the mesenteric artery
The outside diameter of the mesenteric arteries ranged between 200 and 300 μm. As previously reported, ACh evoked reproducible, concentration- and endothelium-dependent smooth muscle hyperpolarization (McPherson & Angus, 1991; Garland & McPherson, 1992), increasing the resting potential from −53±1 to −75±2 mV (n=5). Over the 3–4 h of an experiment, the magnitude of hyperpolarization decreased slightly with repeated exposure of the tissue to ACh, so that by the third concentration–response determination the maximum increase obtained with 3 μM ACh was reduced by 6 mV, to −69±3 mV, n=5, (P<0.05).
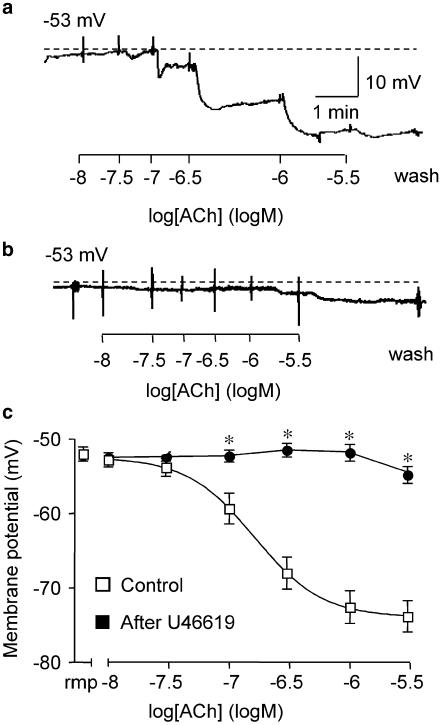
In marked contrast, if mesenteric arteries were repeatedly stimulated with U46619, followed by washout, and then stimulated with ACh, the normal, robust hyperpolarization was progressively blocked. After three to four separate exposures to U46619 (up to 0.1 μM in each case), the maximum membrane potential attained in the presence of 3 μM ACh was only −55±1 mV. This was not markedly different from the resting potential of −52±1 mV in this series, n=8, and represented a marked reduction in hyperpolarization of around 22 mV (from −74±2 mV, n=13, Figure 1).
Figure 1.
Effect of repeated exposure to U46619 on ACh-evoked hyperpolarization. Representative, original traces show: (a) a typical ACh-evoked smooth muscle hyperpolarization in the mesenteric artery; (b) the depressed response after three repeated exposures and washout of U46619 (30 nM–0.1 μM); (c) ACh cumulative concentration–response curves for smooth muscle hyperpolarization under control conditions, n=13, and after 3–4 repeated exposures and washout of U46619, n=8, are shown in the right segment (*P<0.05, Student's t -test). Resting membrane potential (rmp) for each group is shown in the left segment of the graph.
The ability of U46619 to block subsequent endothelium-dependent hyperpolarization to ACh appeared to be receptor mediated, as the smooth muscle hyperpolarization to ACh was not depressed if the TXA2 receptor antagonist SQ29548 (1 μM) was also present during repeated stimulation with 0.1 μM U46619. The concentration of antagonist was sufficient to block completely the contraction to U46619 in these arteries. With 1 μM SQ29548 present, the first exposure to ACh increased the membrane potential to −80±1 mV, n=6, while by the third exposure to ACh, the maximum potential attained was −70±4 mV. This potential was not different from time-matched controls which had not been exposed to SQ29548, −69±3 mV.
Influence of U46619 on smooth muscle hyperpolarization evoked with CPA and various K-channel activators
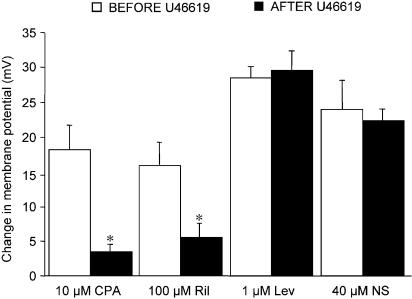
Either CPA, which blocks SERCA, or the putative SKCa channel activator riluzole, each evoked smooth muscle hyperpolarization with a similar maximum to that in response to ACh. CPA (10 μM) increased the membrane potential from a resting value of −54±2 to −72±3 mV (n=4) and 100 μM riluzole from −55±2 mV to −71±3 mV (n=5). As with ACh, the extent of hyperpolarization to each agent was reduced markedly after repeated exposure, followed by washout, to U46619, to −56±1 (n=6) and −58±2 mV (n=5), respectively. In contrast, hyperpolarization either to the KATP activator, 1 μM levcromakalim (n=4) or the BKCa channel activator 40 μM NS1619 (n=5), was unaffected by similar, repeated exposures to U46619. These data are summarized in Figure 2. Consistent with our recent data (Crane et al., 2003), very little smooth muscle hyperpolarization was recorded in control experiments in response to the IKCa channel activator 300 μM 1-EBIO (5±2 increase to 56±2 mV, n=6).
Figure 2.
Influence of U46619 exposure on smooth muscle hyperpolarization evoked by CPA and K-channel activators in mesenteric arteries. Smooth muscle hyperpolarization to 10 μM CPA and 100 μM Riluzole (Ril) was significantly reduced after repeated prior exposure to 30 nM–0.1 μM U46619 (*Student's t-test, P<0.05). The response to 1 μM Levcromakalim (LEV, n=4) and 40 μM NS1619 (NS) was unchanged (n=5).
U46619 but not PE depresses smooth muscle hyperpolarization and relaxation to ACh in mesenteric arteries
Ongoing stimulation of mesenteric arteries with U46619
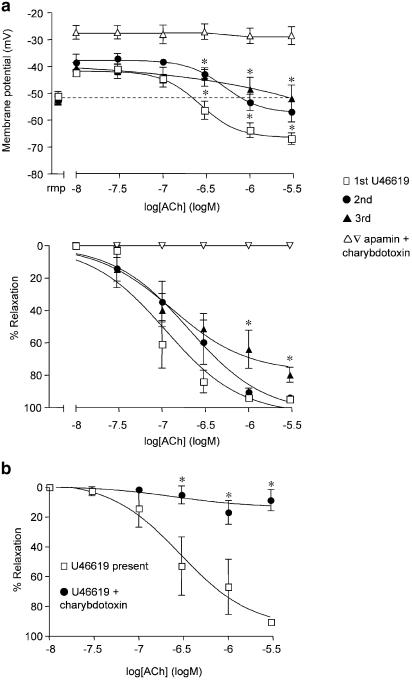
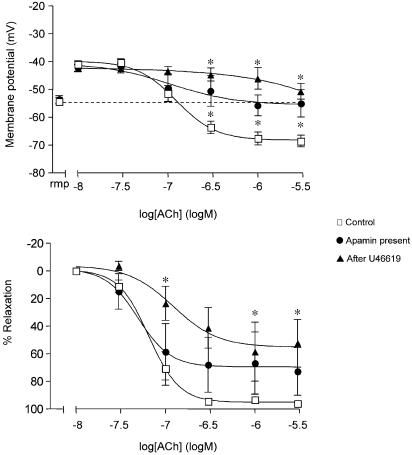
In this series of experiments, the resting membrane potential of the smooth muscle cells was −53±1 mV (n=5), which did not vary by more than 1–2 mV throughout each experiment. The application of 30 nM–0.1 μM U46619 stimulated a sustained smooth muscle depolarization (to −42±1 mV) and contraction (to 17±5.0 mN), both of which were reversed with ACh. ACh (10 nM–3 μM) increased the membrane potential beyond normal resting values, to −67±2 mV, and relaxed the artery by 95±1% (n=5). Hyperpolarization and relaxation to ACh were blocked in the presence of 50 nM apamin and 100 nM charybdotoxin in combination (n=5, see Figure 3a), but were unaffected in the presence of iberiotoxin (hyperpolarization to −60±4 mV, 97±1% relaxation, n=5, data not shown). Following the washout of U46619 and ACh, subsequent exposures to U46619 did not affect the depolarization and contraction of the arteries. However, the hyperpolarization and relaxation in response to cumulative addition of ACh was significantly depressed, so during a third exposure to U46619, ACh only repolarized the smooth muscle membrane potential to −52±5 mV, identical to the original resting potential of −52±2 mV. This effect was associated with a modest but significant decrease in overall relaxation (to 80±5%, n=5). In another series of experiments, this persistent relaxation to ACh was effectively abolished in the presence of 100 nM charybdotoxin, during a forth exposure to U46619 (9±7% relaxation, n=5), compared to the ACh control (91±2%, n=6) (Figure 3b). With higher concentrations of U46619 (3 μM), ACh only stimulated a transient repolarization from –40 mV to circa −49 mV, followed by an immediate depolarization to around −37 mV, changes associated with brief relaxation (4 mN) which rapidly reversed (back to 15–16 mN).
Figure 3.
Smooth muscle hyperpolarization and relaxation to ACh during depolarization and contraction to U46619. (a) Simultaneous measurement of changes in smooth muscle membrane potential (upper panel) and relaxation (lower panel) in mesenteric arteries during U46619 (10 nM–0.1 μM) stimulation. Increases in the membrane potential and relaxation with ACh, showed progressive reduction during the second and third stimulation with U46619. By the third exposure, membrane potential did not overshoot the original resting value, *P<0.05, ANOVA with Bonferroni multiple comparisons test, n=5. Resting membrane potential (rmp) for each group is shown in the left segment of the graph. A small but significant reduction in relaxation was observed during the third stimulation with U46619. The ACh hyperpolarization and relaxation was completely blocked in the presence of 50 nM apamin and 100 nM charybdotoxin combined. (b) Cumulative % relaxation to ACh in U46619 stimulated mesenteric arteries. Membrane potential was not recorded during these experiments. Relaxation to ACh during a forth stimulation with U46619 (n=6) was completely blocked with charybdotoxin alone, without a requirement for apamin (*P<0.05, n=5, Bonferroni multiple comparisons test).
Ongoing stimulation of mesenteric arteries with PE
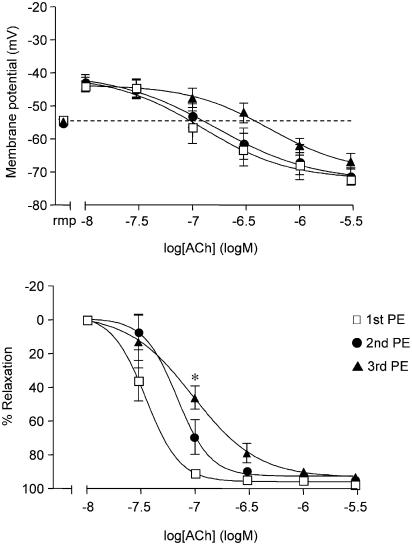
In this series, the resting potential of the smooth muscle cells was −54±1 mV (n=5), and again did not change by more than 1–2 mV throughout each experiment. PE (0.6 μM) evoked sustained smooth muscle depolarization (to −43±2 mV) and contraction (to 14±1 mN). ACh (10 nM–3 μM) increased the membrane potential to −72±1 mV and relaxed the artery by 98±1% (n=5). Neither the depolarization nor the hyperpolarization to ACh changed significantly overall in the presence of a second or third exposure to PE. Moreover, ACh still caused hyperpolarization to −67±2 mV and relaxed the arteries by 93±1% (n=5) during the third exposure to PE, (Figure 4).
Figure 4.
Effect of repeated PE stimulation on hyperpolarization and relaxation to ACh. Simultaneous measurement of changes in smooth muscle membrane potential (upper panel) and relaxation (lower panel) in mesenteric arteries during stimulation with 0.6 μM PE. Mesenteric arteries hyperpolarized and relaxed to ACh to a similar extent during three successive stimulations with PE (n=5). Resting membrane potential (rmp) for each group is shown in the left segment of the graph. Relaxation shifted slightly at intermediate concentrations of ACh during consecutive stimulation with PE, reaching significance with 0.1 μM ACh (*P<0.05, Bonferroni multiple comparisons test).
Effect of prior exposure to U46619 on the subsequent hyperpolarization and relaxation to ACh during stimulation with PE
PE (1 μM) reduced the smooth muscle resting potential from −54±1 to −40±1 mV, and evoked contraction of 17±1 mN (n=9). ACh then hyperpolarized the arteries to −69±2 mV with complete relaxation (96±1%, n=9). Three subsequent exposures, followed by washout, to U46619 (up to 0.1 μM) did not modify the subsequent depolarization to PE, although the contraction was progressively reduced (to 11±3 mN, n=6; P<0.05). If these arteries were then stimulated with PE, subsequent responses to ACh were depressed by a similar extent to arteries stimulated with U46619 alone. The membrane potential only repolarized to −51±3 mV, and maximum relaxation at 52±17% (n=6; P<0.05) was also reduced. Exposure to 50 nM apamin caused a similar reduction in ACh-evoked hyperpolarization and relaxation to arteries stimulated with U46619. In arteries depolarized and contracted with PE, ACh reversed the membrane potential to −55±5 mV with relaxation of 73±17% (n=4) Figure 5.
Figure 5.
ACh hyperpolarization and relaxation in PE-stimulated mesenteric arteries are depressed by prior U46619 exposures or by apamin. Simultaneous measurement of smooth muscle membrane potential change (upper panel) and relaxation (lower panel) in mesenteric arteries during stimulation with 1 μM PE. ACh hyperpolarization during the first stimulation with PE (n=9) was reduced to a similar extent by the presence of apamin (n=4), as it was in arteries following three previous exposures (and washout) to U46619 (n=6). The loss of membrane potential overshoot was associated with reduced relaxation, which was more extensive in the arteries previously exposed to U46619 (*P<0.05, ANOVA with Bonferroni multiple comparisons test). Resting membrane potential (rmp) for each group is shown in the left segment of the graph.
Unlike the inhibition of the EDHF response, hyperpolarization to the IKCa channel opener 1-EBIO (300 μM) was not depressed by prior stimulation with U46619. Relaxation of around 90% was obtained in response to 1-EBIO during each of three exposures to U46619, and then in the presence of a subsequent additional contraction to PE. 1-EBIO, which did not hyperpolarize unstimulated arteries by more than a few mV, evoked repolarization, from −38±2 to −48±2 mV (n=4). During subsequent depolarization in the presence of 1 μM PE, the increase in membrane potential in response to 1-EBIO was additionally associated with slight hyperpolarization. The membrane potential in the presence of PE was −37±5 mV, and increased to −57±1 mV with 1-EBIO (n=3; original resting membrane potential −51±2 mV).
Discussion
The main findings in this study are that the stimulation of TXA2 receptors in isolated mesenteric resistance arteries results in a progressive loss of smooth muscle hyperpolarization due to EDHF. An early component of this effect appears to reflect the inactivation of the SKCa channels which are localized on the endothelium. These data are likely to be of physiological relevance in situations where the local concentration of TXA2 is elevated, for example, during platelet activation, and in the interpretation of experimental studies of EDHF where TXA2 is used as the background constrictor agonist.
Activation of both SKCa and IKCa channels in the arterial endothelium is a key step leading to smooth muscle hyperpolarization and relaxation, independently of both NO and prostacyclin. This effect is ascribed to EDHF (Bolz et al., 1999; Busse et al., 2002). In a previous study in rat mesenteric resistance arteries, smooth muscle contraction and depolarization in response to the TXA2 mimetic U46619 was shown to cause complete loss of EDHF evoked hyperpolarization and relaxation, while relaxation to endothelium-derived NO was not altered. In that study, high (0.5–3 μM) concentrations of U46619 were used (Garland & McPherson, 1992). We now show that a more selective and progressive block can be evoked on repeated stimulation with lower concentrations of U46619.
Our present data show that the first apparent consequence of vascular stimulation with U46619, is a loss of ACh's ability to evoke EDHF-induced hyperpolarization in uncontracted arteries, and of the overshooting hyperpolarization normally obtained during EDHF activation in depolarized and contracted arteries. This indicates an action against SKCa channels, which underpin this effect in mesenteric resistance arteries (Crane et al., 2003). This conclusion is supported by the fact that in the present study apamin alone abolished ACh hyperpolarization in both quiescent arteries and in arteries stimulated with PE (the overshoot of resting membrane potential). The fact that similar hyperpolarization was also stimulated in the mesenteric artery by the SKCa activator riluzole, and that this hyperpolarization was lost following prior exposure to U46619, is also consistent with an action of the thromboxane–mimetic against SKCa. Riluzole is an opener of SKCa channels, although it has other effects, such as inhibition of Na+ channels and twin-pore domain K channels (Taylor & Meldrum, 1995; Song et al., 1997; Duprat et al., 2000). The SKCa channels are restricted to the endothelium, as they are in other arteries (Marchenko & Sage, 1996; Edwards et al., 1998; Mistry & Garland, 1998a, 1998b; Burnham et al., 2002; Eichler et al., 2003).
Whatever the relative cellular locations of SKCa and IKCa within the endothelial cells, it is clear that U46619-linked block of SKCa activity preceded that of the IKCa channels. The repolarization (and relaxation) remaining after three exposures to U46619 was sensitive to block with charybdotoxin and mimicked by the IKCa activator, 1-EBIO. The hyperpolarization associated with the action of 1-EBIO >100 μM appears to be due to the activation of IKCa (Walker et al., 2001). That block of endothelial cell SKCa channels, associated with U46619 stimulation, is selective is also supported by unaltered hyperpolarization to the BKCa channel activator NS1619 and the KATP channel activator levcromakalim after exposure to U46619. In the mesenteric artery, BKCa and KATP channels are restricted to the smooth muscle cells (Mistry & Garland, 1998a; White & Hiley, 2000), whereas the IKCa channels are on the endothelial not the smooth muscle cells (Walker et al., 2001).
How the stimulation of TXA2 receptors leads to a loss of endothelial K-channel activity, and as a consequence reduced vascular dilatation, is not clear. Neither is it clear if the receptors mediating this effect are on the smooth muscle or the endothelial cells, or both. Obviously, receptors for TXA2 are present on the smooth muscle to enable contraction (maximum contraction was not significantly changed in endothelium-denuded arteries, data not shown). In another vessel, the porcine coronary artery, the stimulation of dispersed smooth muscle cells with U46619 has been shown to block K channels, but the selectivity of this effect for K channels was not investigated. The channel block required the activation of TXA2 receptors and involved an alteration in the gating of what were apparently BKCa channels, but was not reversed by raising intracellular calcium (Scornik & Toro, 1992). In an intact artery, block of BKCa channels would be expected to increase the overall contraction to constrictor agents (Brayden & Nelson, 1992). As the contraction during repeated exposure to U46619 did not change in our study (range 15–17 mN, data not shown) block of BKCa channels seems unlikely.
Endothelial cells have been shown to contain TXA2 receptors, and their stimulation can cause detrimental cellular effects. In human endothelial cells, two isoforms have been reported, α and β, each linked to apoptosis (Gao et al., 2000). This effect seems to involve an inhibition of the phosphorylation of Akt kinase, which may involve activation of the protein kinase C isoform PKC-ς (Gao et al., 2000; Shizukuda & Buttrick, 2002). The TXA2 antagonist SQ29548 blocked endothelial cell apoptosis and also reduced the expression of intercellular adhesion molecule-1 (Zou et al., 2002). Endothelial cell death may also follow calcium overload, and this effect was used to explain the fact that both U46619 and another TXA2 agonist, I-BOP, caused time- and concentration-dependent cell death in neuroretinal endothelial cells (Beauchamp et al., 2001). The effect appeared to be selective, as associated smooth muscle and astroglial cells, and endothelial cells from nonneuronal tissue, were all unaffected. However, endothelial cell death per se, seems an unlikely explanation for our data. The first detrimental action of U46619 was a block of SKCa and at that time the endothelial cells were still able to cause significant smooth muscle relaxation. Even when U46619 had blocked EDHF-dependent relaxation, NO was able to sustain relaxation (Plane & Garland, 1996).
Other mechanisms to explain the loss of the EDHF response could involve gap junctions or the generation of reactive oxygen species. TXA2 receptor stimulation has been linked to alterations in the distribution of gap junctions in human endothelial cells, which might impair functional coupling (Ashton et al., 1999). This seems an unlikely explanation for the selective loss of SKCa-evoked hyperpolarization, unless these channels are located on separate endothelial cells to the IKCa channels, as IKCa-linked effects persisted. Furthermore, an essential requirement in this case would be that the SKCa endothelial cells are more susceptible to the effects of U46619, which also seems an unlikely scenario. Finally, TXA2 receptor stimulation can generate reactive oxygen species, at least under conditions of oxidant stress (Matsuo et al., 1996). In retinovascular endothelial cells, U46619 stimulated the formation of hydroperoxides and cell death was prevented by an antioxidant (Beauchamp et al., 2001). So reactive oxygen species might possibly underlie the loss of ion channel activity in the endothelium, with more prolonged and sustained periods of exposure progressively affecting other ion channels leading ultimately to endothelial cell death.
Whatever the mechanism, progressive loss of the EDHF pathway during stimulation with U46619 could complicate the interpretation of experimental data obtained when background contraction is evoked by TXA2 receptor stimulation. In this regard, the use of U46619 is fairly widespread, as it causes stable contraction (Parkington et al., 1995; McNeish et al., 2001; Bauersachs et al., 2002). Interestingly, in the study by McNeish et al. (2001), only the IKCa blocker charybdotoxin was required to abolish EDHF dilatation in the bovine ciliary vascular bed. The apparent absence of SKCa-evoked hyperpolarization in these vessels might then reflect their selective loss due to stimulation with U46619. Also, in a recent study with rat mesenteric arteries depolarized and contracted to U46619, ACh and C-type natriuretic peptide each reversed the depolarization without any overshooting hyperpolarization (Chauhan et al., 2003). These data could again reflect SKCa channel block with U46619. They also suggest that C-type natriuretic peptide may be acting to cause smooth muscle relaxation, at least in part, through the endothelium.
So, in the rat mesenteric artery, SKCa channels underlie the smooth muscle hyperpolarization evoked with ACh and represent a key component in the EDHF response. Stimulation of TXA2 receptors, most likely present on the endothelium, is associated with a progressive loss of K-channel activity, starting with SKCa and ultimately leading to a loss of EDHF-linked changes in smooth muscle membrane potential and tension.
Acknowledgments
This work was supported by the Wellcome Trust, U.K.
Abbreviations
- ACh
acetylcholine
- BKCa
large-conductance calcium-activated potassium channels
- CPA
cyclopiazonic acid
- 1-EBIO
1-ethyl-2-bezimidazolinone
- EDHF
endothelium-derived hyperpolarizing factor
- IKCa
intermediate conductance calcium-activated potassium channels
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- PE
phenylephrine
- SERCA
sarcoplasmic endoplasmic reticulum calcium ATPase
- SKCa
small-conductance calcium-activated potassium channels
- TXA2
thromboxane A2
References
- ASHTON A.W., YOKOTA R., JOHN G., ZHAO S., SUADICANI S.O., SPRAY D.C., WARE J.A. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A2. J. Biol. Chem. 1999;274:35562–35570. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- BAUERSACHS J., CHRIST M., ERTL G., MICHAELIS U.R., FISSLTHALER B., BUSSE R., FLEMING I. Cytochrome P450 2C expression and EDHF-mediated relaxation in porcine coronary arteries is increased by cortisol. Cardiovasc. Res. 2002;54:669–675. doi: 10.1016/s0008-6363(02)00257-2. [DOI] [PubMed] [Google Scholar]
- BEAUCHAMP M.H., MARTINEZ-BERMUDEZ A.K., GOBEIL JR F., MARRACHE A.M., HOU X., SPERANZA G., ABRAN D., QUINIOU C., LACHAPELLE P., ROBERTS J., III, ALMAZAN G., VARMA D.R., CHEMTOB S. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. J. Appl. Physiol. 2001;90:2279–2288. doi: 10.1152/jappl.2001.90.6.2279. [DOI] [PubMed] [Google Scholar]
- BOLZ S.S., DE WIT C., POHL U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br. J. Pharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- BURNHAM M.P., BYCHKOV R., FELETOU M., RICHARDS G.R., VANHOUTTE P.M., WESTON A.H., EDWARDS G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CHAUHAN S.D., NILSSON H., AHLUWALIA A., HOBBS A.J. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE G.J., GALLAGHER N.T., DORA K.A., GARLAND C.J. Small and intermediate calcium-dependent K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J. Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE G.J., GARLAND C.J.Inhibition of endothelium-dependent hyperpolarization in rat isolated mesenteric artery with the thromboxane-mimetic, U46619 Proceedings of the Physiological Society, University College Meeting, London 2003. 547P, CIII
- DORA K.A., GARLAND C.J.Heterocellular calcium signalling: a novel stimulus for the release of EDHF Endothelium-Dependent Hyperpolarizations. 1998New York: Harwood Academic Publishers; 237–242.ed. Vanhoutte, P.M. pp [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- DUPRAT F., LESAGE F., PATEL A.J., FINK M., ROMEY G., LAZDUNSKI M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EICHLER I., WIBAWA J., GRGIC I., KNORR A., BRAKEMEIER S., PRIES A.R., HOYER J., KOHLER R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- GAO Y., YOKOTA R., TANG S., ASHTON A.W., WARE J.A. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A2. Circ. Res. 2000;87:739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., MCPHERSON G.A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br. J. Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. (Lond.) 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUO Y., KIHARA T., IKEDA M., NINOMIYA M., ONODERA H., KOGURE K. Role of platelet-activating factor and thromboxane A2 in radical production during ischemia and reperfusion of the rat brain. Brain Res. 1996;709:296–302. doi: 10.1016/0006-8993(95)01324-5. [DOI] [PubMed] [Google Scholar]
- MCNEISH A.J., WILSON W.S., MARTIN W. Dominant role of an endothelium-derived hyperpolarizing factor (EDHF)-like vasodilator in the ciliary vascular bed of the bovine isolated perfused eye. Br. J. Pharmacol. 2001;134:912–920. doi: 10.1038/sj.bjp.0704332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON G.A., ANGUS J.A. Evidence that acetylcholine-mediated hyperpolarization of the rat small mesenteric artery does not involve the K+ channel opened by cromakalim. Br. J. Pharmacol. 1991;103:1184–1190. doi: 10.1111/j.1476-5381.1991.tb12321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Characteristics of single, large-conductance calcium-dependent potassium channels (BKCa) from smooth muscle cells isolated from the rabbit mesenteric artery. J. Memb. Biol. 1998a;164:125–138. doi: 10.1007/s002329900399. [DOI] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998b;124:1131–1148. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKINGTON H.C., CHOW J.A., EVANS R.G., COLEMAN H.A., TARE M. Role for endothelium-derived hyperpolarizing factor in vascular tone in rat mesenteric and hindlimb circulations in vivo. J. Physiol. 2002;542:929–937. doi: 10.1113/jphysiol.2002.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKINGTON H.C., TONTA M.A., COLEMAN H.A., TARE M. Role of membrane potential in endothelium-dependent relaxation of guinea-pig coronary arterial smooth muscle. J. Physiol. 1995;484:469–480. doi: 10.1113/jphysiol.1995.sp020679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANE F., GARLAND C.J. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br. J. Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCORNIK F.S., TORO L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am. J. Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- SHIZUKUDA Y., BUTTRICK P.M. Oxygen free radicals and heart failure: new insight into an old question. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L237–L238. doi: 10.1152/ajplung.00111.2002. [DOI] [PubMed] [Google Scholar]
- SONG J.H., HUANG C.S., NAGATA K., YEH J.Z., NARAHASHI T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1997;282:707–714. [PubMed] [Google Scholar]
- TAYLOR C.P., MELDRUM B.S. Na+ channels as targets for neuroprotective drugs. Trends Pharmacol Sci. 1995;16:309–316. doi: 10.1016/s0165-6147(00)89060-4. [DOI] [PubMed] [Google Scholar]
- WALKER S.D., DORA K.A., INGS N.T., CRANE G.J., GARLAND C.J. Activation of endothelial cell IKCa with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br. J. Pharmacol. 2001;134:1548–1554. doi: 10.1038/sj.bjp.0704415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. Hyperpolarisation of rat mesenteric endothelial cells by ATP-sensitive K+ channel openers. Eur. J. Pharmacol. 2000;397:279–290. doi: 10.1016/s0014-2999(00)00271-5. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., KLEMM M.F., EDWARDS F.R., SUZUKI H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J. Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU M.H., SHI C., COHEN R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]