Abstract
The aim of this study was to investigate the effect of Trolox on hepatic microsomal cytochrome P450 (CYP) activity and gene expression during ischemia and reperfusion (I/R).
Rats were subjected to 60 min of hepatic ischemia, and 5 h (acute phase) and 24 h (subacute phase) of reperfusion. Rats were treated intravenously with Trolox (2.5 mg kg−1) or vehicle, 5 min before reperfusion.
The serum alanine aminotransferase level and lipid peroxidation were increased as a result of I/R. These increases were attenuated by Trolox. Reduced glutathione concentration decreased in I/R group, and this decrease was inhibited by Trolox.
Both total hepatic CYP content and NADPH-cytochrome P450 reductase activity decreased after I/R, which were restored by Trolox.
CYP1A1 activity and its protein level decreased 24 h after reperfusion; decreases which were prevented by Trolox. Both the activity and mRNA expression of CYP1A2 decreased 24 h after reperfusion. The decrease in CYP1A2 mRNA was prevented by Trolox. CYP2B1 activity and mRNA expression decreased 5 h after reperfusion. The decrease in CYP2B1 activity was prevented by Trolox. In contrast, the CYP2E1 activity and its protein level increased 5 h after reperfusion and this increase was prevented by Trolox.
The expression of TNF-α and iNOS mRNAs increased after I/R. Trolox inhibited increase in iNOS mRNA expression.
Trolox ameliorates hepatic drug-metabolizing dysfunction, as indicated by abnormalities in CYP isoforms during I/R, and this protection is likely due to the scavenging of reactive oxygen species.
Keywords: Hepatic ischemia/reperfusion, cytochrome P450, oxidative stress, Trolox
Introduction
It is well accepted that a major proportion of ischemia and reperfusion (I/R) injury occurs during the period of reperfusion, when reactive oxygen species (ROS) are generated. During the acute phase of reperfusion, ROS can cause direct cellular damage through protein oxidation and degradation, lipid peroxidation, and DNA damage (Pardini, 1995). During the subacute phase of injury, cytokines produced by the initial acute phase activation of pro-inflammatory signal transduction cascades lead to the recruitment of neutrophils which amplify the redox burden in damaged tissue (Kurokawa et al., 1996).
Among the stress-regulated genes are the cytochrome P450 (CYP) monooxygenase enzymes. These CYP enzymes are largely responsible for the metabolism and elimination of drugs, and form a large but closely related superfamily of distinct gene products with diverse substrate specificities (Nelson et al., 1996). They are subject to regulation by a diverse array of xenobiotic chemicals, as well as by physiological factors, thus modifying the clinical and toxic effects of chemical agents in individuals (Jones et al., 1989). Indeed, I/R decreases the activities of ethylmorphine demethylation and pentoxyresorufin dealkylation exerted by the hepatic microsomes of 3-methylcholanthrene-treated rats, but not those exerted by phenobarbital-treated rats, indicating that I/R may degrade CYP molecule form specifically (Lindstrom et al., 1990). However, there is limited information available on the effect of I/R on individual CYP isoforms.
α-Tocopherol is considered as the most effective lipid-soluble antioxidant, protecting cell membranes from oxidative damage. Recently, α-tocopherol has been demonstrated to activate CYP gene expression via the pregnane X receptor, a nuclear receptor regulating a variety of drug-metabolizing enzymes (Landes et al., 2003). Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is a water-soluble analogue of the free radical scavenger α-tocopherol. A previous study suggested that α-tocopherol requires several days of pretreatment to exhibit antioxidative benefits, while Trolox, due to its enhanced water solubility, may function more rapidly during acute oxidative stress (Wu et al., 1991). However, the precise mechanism of the in vivo antioxidant effect of Trolox remains unclear.
Therefore, this study was designed to investigate the effects that Trolox has on post-ischemic injury, particularly on the profile of CYP isozyme activity and gene expression.
Methods
Hepatic ischemia procedure
Male Sprague-Dawley rats (260–300 g) were fasted overnight, but allowed access to water. All animals were treated humanely under Sungkyunkwan University Animal Care Committee Guidelines. Under sodium pentobarbital anesthesia (40 mg kg−1, i.p.), rats were laparotomized and liver ischemia was induced by clamping the pedicles of the left and median lobes for 60 min. At the end of ischemia, the clip around the left branches of the portal vein was removed and the branch to the right lobes was ligated. Control animals were prepared in the same manner except that the clip was not placed on the left and median lobes, but blood flow to the right lobes of the liver was occluded. At 5 and 24 h of reperfusion, blood was obtained from the abdominal aorta. The left and median lobes of the liver were isolated, immediately used for the preparation of microsome or RNA and stored at −75°C for later analysis.
Administration of Trolox
Trolox, dissolved in phosphate-buffered saline (pH 7.4, vehicle), was administered by intravenous injection at a dose of 2.5 mg kg−1 of body weight, 5 min before reperfusion. Four treatment groups were studied: (a) vehicle-treated control, (b) Trolox-treated control, (c) vehicle-treated ischemic, and (d) Trolox-treated ischemic. As there were no differences found in any of the parameter between Trolox- and vehicle-treated rats in the control group, the results of group (a) and (b) were pooled, and were referred to as controls.
Isolation of hepatic microsomal fraction
The excised liver was minced, then homogenized with a Teflon® pestle homogenizer in 4 volumes of ice-cold 1.15 M KCl for 1 g of the liver, and centrifuged at 9,000 × g for 60 min. The supernatant was collected and centrifuged at 105,000 × g for 60 min. Microsomal precipitates were resuspended with 4 volumes of 0.1 M phosphate buffer, pH 7.4, for 1 g of the liver microsome and stored at −75°C until assayed.
Analytical procedures
Serum alanine aminotransferase was determined by standard spectrophotometric procedures using Sigma diagnostics INFINITY™ kit 52-UV (Sigma Chemical Co., St Louis, MO, U.S.A.). Lipid peroxide was assayed by the method of Buege & Aust (1978), and 1,1,3,3-tetraethoxypropane (malondialdehyde (MDA) tetraethyl acetal) was used as the standard. Total glutathione was determined in liver homogenates after precipitation with 1% picric acid and using yeast-glutathione reductase, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), and NADPH, at 340 nm. Glutathione disulfide (GSSG) was determined by the same method in the presence of 2-vinylpyridine and reduced glutathione (GSH) calculated from the difference between total glutathione and GSSG (Anderson, 1985). CYP content was calculated by using the molar extinction coefficient of 91 mM−1 cm−1 for the absorbance difference between 450 and 480 nm measured with a differential spectrophotometer (Omura & Sato, 1964). The activity of NADPH-cytochrome P450 reductase was indirectly determined by its NADPH-cytochrome c reductase activity (Vermillion & Coon, 1978). Catalytic activities of CYP1A1, 1A2, and 2B1 in liver microsomal fraction were measured as 7-ethoxyresorufin O-deethylase (EROD), methoxyresorufin O-demethylase (MROD), and pentoxyresorufin O-dealkylase (PROD) activities, respectively, by the method of Burke et al. (1985). Microsomal CYP2E1 activity was determined by measuring 4-hydroxylation of aniline to p-aminophenol (Schenkman et al., 1967). Protein content was estimated by the dye binding assay of Bradford (1976).
Total RNA extraction and reverse transcription–polymerase chain reaction (RT–PCR)
Isolation of total RNA was carried out according to the method described by Chomczynski & Sacchi (1987). Reverse transcription of total RNA was performed to synthesize the first-strand cDNA using oligo(dT)12–18 primer and SuperScriptTM II RNase H- Reverse Transcriptase (Invitrogen Tech-Line™, Carlsbad, CA, U.S.A.). PCR reaction was performed with a diluted cDNA sample and amplified in each 20 μl reaction volume. The final reaction concentrations were: primers, 10 pmol; dNTP mix, 250 μM; 10 × PCR buffer; Ex Taq DNA polymerase, 0.5 U per reaction. The gene-specific primers are listed in Table 1 . All PCR reactions had an initial denaturation step at 94°C for 5 min, and a final extension at 72°C for 5 min using GeneAmp 2700 thermocycler (Applied Biosystems, Foster city, CA, U.S.A.). Each PCR amplification cycling condition was as follows: 94°C 30 s, 57°C 30 s, 72°C 60 s, 30 cycles for CYP1A1; 94°C 30 s, 60°C 30 s, 72°C 60 s, 23 cycles for CYP2E1; 94°C 30 s, 62°C 30 s, 72°C 60 s, 22 cycles for CYP1A2 and 23 cycles for CYP2B1; 94°C 45 s, 65°C, 45 s; 72°C, 60 s, 32 cycles for iNOS; 94°C 30 s, 54°C 30 s, 72°C 60 s, 26 cycles and 25 cycles for TNF-α and β-actin, respectively. Following RT–PCR, 10 μl samples of amplified products were resolved by electrophoresis in 1.5% agarose gel, and stained with ethidium bromide. The intensity of each PCR product was semiquantitatively evaluated using a digital camera (DC120, Eastman Kodak, New Haven, CT, U.S.A.) and a densitometric scanning analysis program (1D Main, Advanced American Biotechnology, Fullerton, CA, U.S.A.).
Table 1.
PCR primers used in the study
| Gene (Accession number) | Primer sequences (5′ → 3′) | Product length (bp) |
|---|---|---|
| CYP1A1 | sense : CTGGTTCTGGATACCCAGCTG | 331 |
| (X00469) | anti-sense : CCTAGGGTTGGTTACCAGG | |
| CYP1A2 | sense : CAGTCACAACAGCCATCTTC | 302 |
| (X01031) | anti-sense : CCACTGCTTCTCATCATGGT | |
| CYP2B1 | sense : TTGTTTGGTGCTGGGACAGAG | 443 |
| (XM_342078) | anti-sense : GGCTAGGCCCTCTCCTGCACA | |
| CYP2E1 | sense : AAACTTCATGAAGAAATTGAC | 311 |
| (M20131) | anti-sense : TCTCCAACACACACACGCTTTCC | |
| TNF-α | sense : GTAGCCCACGTCGTAGCAAA | 346 |
| (X66539) | anti-sense : CCCTTCTCCAGCTGGAAGAC | |
| iNOS | sense : TTCTTTGCTTCTGTGCTTAATGCG | 1061 |
| (D44591) | anti-sense : GTTGTTGCTGAACTTCCAATCGT | |
| β-actin | sense : TTGTAACCAACTGGGACGATATGG | 764 |
| (BC063166) | anti-sense : GATCTTGATCTTCATGGTGCTAG |
Western blot immunoassay
Microsomal proteins (10 μg per well) were separated by 10% SDS–PAGE and were transferred to nitrocellulose membrane using semi-dry transfer process. Bands were immunologically detected using polyclonal antibodies against rat CYP1A1, 2B1, and 2E1 (Gentest, Woburn, MA, U.S.A.) and visualized with an alkaline phosphatase-conjugated monoclonal rabbit anti-goat IgG secondary antibody using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate as the substrate. The intensity of the immunoreactive bands was determined by densitometric analysis program.
Statistical analysis
Overall significance was tested by two-way ANOVA. Differences between groups at specific time points were considered significant at P<0.05 with the appropriate Bonferronic correction made for multiple comparisons. All results are presented as means±s.e.m.
Results
Serum alanine aminotransferase (ALT) and lipid peroxidation
The serum level of ALT in the control rats was 445±148 i. u. l−1 5 h after reperfusion and 69±20 i.u. l−1 24 h after reperfusion. In the ischemic group, the serum ALT level increased to 11.4-fold 5 h after reperfusion and 9.1-fold of control 24 h after reperfusion (P<0.01), respectively. This increase was significantly suppressed by Trolox. In the control animals, the liver MDA level remained constant at approximately 1.03 nmol mg−1 protein throughout the experiment. At 5 and 24 h after reperfusion, the MDA level increased to 1.9- and 1.4-fold of control (P<0.01), respectively. This elevation was attenuated by Trolox (Table 2 ).
Table 2.
Effect of Trolox on ALT activity and lipid peroxidation in rat liver after ischemia and reperfusion
| ALT (i.u. l−1) | MDA (nmol mg−1 protein) | |||
|---|---|---|---|---|
| Groups | 5 h | 24 h | 5 h | 24 h |
| Control | 445.0±148.4 | 68.5±20.3 | 0.95±0.06 | 1.12±0.07 |
| Ischemia/reperfusion | 5067.4±704.7** | 623.9±126.0** | 1.82±0.09** | 1.61±0.10** |
| Trolox+ischemia/reperfusion | 1131.5±174.0**,++ | 231.9±30.1**,+ | 1.17±0.04++ | 1.08±0.03++ |
Each value is the mean±s.e.m. of 7–10 animals per group.
Significantly different (**P<0.01) from controls.
Significantly different (+P<0.05, ++P< 0.01) from ischemia and reperfusion.
Hepatic glutathione
The hepatic concentrations of GSH in controls were 4.06±0.34 and 5.68±0.14 μmol g−1 liver 5 and 24 h after reperfusion, respectively. GSH concentration significantly decreased 5 and 24 h after reperfusion. This decrease was attenuated by Trolox. Hepatic GSSG concentration was unchanged among any of the experimental groups. The ratio of GSH to GSSG (an indicator of the hepatocellular redox state) markedly declined during reperfusion. The decrease in the ratio of GSH to GSSG was attenuated by Trolox (Table 3).
Table 3.
Effect of Trolox on concentrations of GSH, GSSG and GSH/GSSG ratio in rat liver after ischemia and reperfusion
| GSH (μmol g−1 liver) | GSSG (μmol g−1 liver) | GSH/GSSG ratio | ||||
|---|---|---|---|---|---|---|
| Groups | 5 h | 2 4h | 5 h | 24 h | 5 h | 24 h |
| Control | 4.06±0.34 | 5.68±0.14 | 0.26±0.03 | 0.26±0.01 | 15.64±0.75 | 22.35±1.27 |
| Ischemia/reperfusion | 3.02±0.12* | 4.32±0.29* | 0.32±0.03 | 0.34±0.05 | 9.44±1.10** | 13.90±2.60** |
| Trolox+ischemia/reperfusion | 3.82±0.23+ | 6.40±0.54+ | 0.27±0.05 | 0.30±0.02 | 14.15±1.47+ | 21.36±1.54+ |
Each value is the mean±s.e.m. of 7–10 animals per group.
Significantly different (*P<0.05, **P<0.01) from controls.
Significantly different (P<0.05) from ischemia and reperfusion.
Total hepatic CYP content and NADPH-cytochrome P450 reductase activity
In the vehicle-treated ischemic group, the hepatic microsomal CYP content was found to significantly decrease after 5 h of reperfusion (P<0.01). This decrease persisted at least until 24 h of reperfusion. The decrease in CYP content was attenuated by Trolox. Similar to CYP content, the hepatic microsomal NADPH-cytochrome P450 reductase activity significantly decreased both 5 and 24 h after reperfusion. This decrease was prevented by Trolox (Table 4).
Table 4.
Effect of Trolox on total hepatic cytochrome P450 content and NADPH-cytochrome P450 reductase activity after ischemia and reperfusion
| Cytochrome P450 content | NADPH-cytochrome P450 reductase activity | |||
|---|---|---|---|---|
| (nmol min−1 mg−1 protein) | (nmol min−1 mg−1 protein) | |||
| Groups | 5 h | 24 h | 5 h | 24 h |
| Control | 0.41±0.03 | 0.39±0.03 | 86.0±5.4 | 88.1±2.9 |
| Ischemia/reperfusion | 0.26±0.03** | 0.23±0.03** | 69.3±2.0** | 66.0±3.7** |
| Trolox+ischemia/reperfusion | 0.36±0.02+ | 0.29±0.04* | 80.6±1.6++ | 75.7±2.9* |
Each value is the mean±s.e.m. of 7–10 animals per group.
Significantly different (*P<0.05, **P<0.01) from controls.
Significantly different (+P<0.05, ++P<0.01) from ischemia and reperfusion.
Hepatic microsomal CYP isozyme activities
The results of CYP isozyme activities are summarized in Table 5 . No significant effects on CYP1A1 activity were observed in the vehicle-treated ischemic group 5 h after reperfusion. At 24 h of reperfusion, however, CYP1A1 activity was reduced in the vehicle-treated ischemic group to levels about 59% of those seen in microsomes from control (P<0.05). This decrease was ameliorated by Trolox. Although there were no changes until 5 h after reperfusion, the activity of CYP1A2 in the ischemic rats significantly decreased 24 h after reperfusion. Trolox had little effect on the decrease in CYP1A2 activity. CYP2B1 activity significantly decreased 5 h after reperfusion, a decrease which was attenuated by Trolox. CYP2B1 activity was unchanged among any of the experimental groups 24 h after reperfusion. In contrast to CYP1A1, 1A2, and 2B1, the CYP2E1 activity markedly increased 3.4 times (5 h after reperfusion) and 1.5 times (24 h after reperfusion) the control values in I/R rats, respectively. This increase was prevented by Trolox.
Table 5.
Effect of Trolox on hepatic microsomal cytochrome P450 isozyme activities after ischemia and reperfusion
| Ethoxyresorufin O-deethylase | Methoxyresorufin O-demethylase | Penthoxyresorufin O-dealkylase | Aniline p-hydroxylase | |||||
|---|---|---|---|---|---|---|---|---|
| (pmol min−1 mg−1 protein) | (pmol min−1 mg−1 protein) | (pmol min−1 mg−1 protein) | (nmol min−1 mg−1 protein) | |||||
| Groups | 5 h | 24 h | 5 h | 24 h | 5 h | 24 h | 5 h | 24 h |
| Control | 50.9±4.2 | 51.3±6.6 | 24.8±1.4 | 16.4±0.7 | 31.2±2.6 | 44.3±2.3 | 0.23±0.02 | 0.16±0.01 |
| Ischemia/reperfusion | 34.2±4.4 | 30.2±2.5* | 27.0±2.0 | 7.2±0.7** | 15.4±2.4** | 35.9±3.0 | 0.78±0.09** | 0.24±0.02* |
| Trolox+ischemia/reperfusion | 43.2±3.0 | 46.7±7.6+ | 26.4±2.1 | 8.3±0.4** | 23.7±1.4+ | 41.9±4.2 | 0.31±0.05++ | 0.17±0.01+ |
Each value is the mean±s.e.m. of 7–10 animals per group.
Significantly different (*P<0.05, **P<0.01) from controls.
Significantly different (+P<0.05, ++P<0.01) from ischemia and reperfusion.
CYP isozyme protein expression
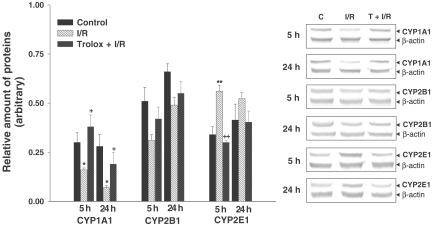
The amount of CYP1A1 protein in microsome significantly decreased both 5 and 24 h after reperfusion, a decrease which was significantly attenuated by Trolox. The amount of CYP2B1 protein was unchanged among any of the experimental groups. The amount of CYP2E1 protein significantly increased 5 h after reperfusion. This increase in CYP2E1 protein was prevented by Trolox (Figure 1).
Figure 1.
Effect of Trolox on CYP1A1, CYP 2B1 and 2E1 protein expression after hepatic ischemia and reperfusion. Values are means±s.e.m. for 7–10 rats per group. *,**Significantly different (P<0.05, P<0.01) from controls. +,++Significantly different (P<0.05, P<0.01) from ischemia/reperfusion. C, control; I/R, ischemia/reperfusion; T, Trolox.
CYP isozyme mRNA expression
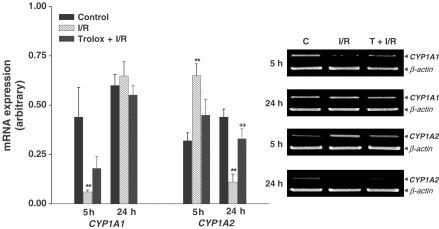
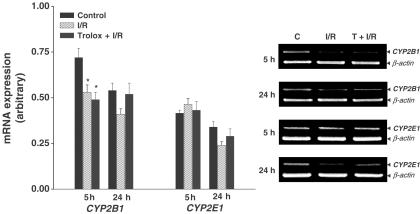
As shown in Figure 2, the level of CYP1A1 mRNA significantly decreased 5 h after reperfusion. Trolox had little effect on the decrease in CYP1A1 mRNA expression. The level of CYP1A2 mRNA expression significantly decreased 24 h after reperfusion, a decrease which was attenuated by Trolox. As shown in Figure 3, the level of CYP2B1 mRNA significantly decreased 5 h after reperfusion. Trolox did not affect the level of CYP2B1 mRNA. The level of CYP2E1 mRNA expression was unchanged among any of the experimental groups.
Figure 2.
Effect of Trolox on CYP1A1 and CYP1A2 mRNA expression after hepatic ischemia and reperfusion. Values are means±s.e.m. for 7–10 rats per group. **Significantly different (P<0.01) from controls. ++Significantly different (P<0.01) from ischemia/reperfusion. C, control; I/R, ischemia/reperfusion; T, Trolox.
Figure 3.
Effect of Trolox on CYP2B1 and CYP2E1 mRNA expression after hepatic ischemia and reperfusion. Values are means±s.e.m. for 7–10 rats per group. *Significantly different (P<0.05) from controls. C, control; I/R, ischemia/reperfusion; T, Trolox.
TNF-α and iNOS mRNA expression
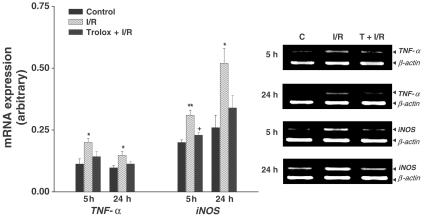
TNF-α and iNOS have been demonstrated to act as mediators of tissue damage during I/R. To examine if the increased expression of TNF-α and iNOS mRNA after hepatic I/R is related to the production of ROS, we tested the effect of Trolox on their activation. As shown in Figure 4, there was a low level of TNF-α mRNA in the control group. The level of TNF-α mRNA expression significantly increased after reperfusion; an increase that was not prevented by Trolox. The level of iNOS mRNA in I/R group significantly increased; Trolox significantly inhibited this increase.
Figure 4.
Effect of Trolox on TNF-α and iNOS mRNA expression after hepatic ischemia and reperfusion. Values are means±s.e.m. for seven rats per group. *,**Significantly different (P<0.05, P<0.01) from controls. +Significantly different (P<0.05) from ischemia/reperfusion. C, control; I/R, ischemia/reperfusion; T, Trolox.
Discussion
There is now a substantial body of evidence supporting the notion that many of the pathophysiological events triggered by I/R injury are mediated through the production of ROS. I/R injury in the liver has been demonstrated to occur in a biphasic pattern, consisting of both acute- and subacute-phase responses. The acute phase, characterized by hepatocellular injury after 3–6 h of reperfusion, is associated with free radical generation with T-lymphocyte and Kupffer cell activation. Numerous studies suggest that the burst of ROS generated after reperfusion may contribute to the initiation of post-ischemic liver injury, and to the subsequent inflammatory infiltration (Zwacka et al., 1997). The subacute-phase response following liver I/R is characterized by massive neutrophil infiltration, peaking after 18–24 h of reperfusion. Pro-inflammatory cytokines secreted by neutrophils during the subacute phase serve to perpetuate the organ damage and the generation of intracellular ROS in the damaged tissue through receptor-mediated pathways (Kurokawa et al., 1996).
Trolox was reported to scavenge peroxyl radicals from artificial systems better than its parent compound. Wu et al. (1990) observed that Trolox protects human myocytes and hepatocytes against in situ generated oxyradicals. Furthermore, Trolox reduced hypoxia/reoxygenation-induced hepatic injury in the isolated perfused rat liver (Lee & Cho, 1997). However, few studies have rigorously determined whether Trolox exhibits antioxidant activity in vivo animal model of hepatic I/R.
In ischemic rats, ALT markedly increased after 5 h of reperfusion. After 24 h of reperfusion, the elevation in ALT activity declined, but still remained increased as compared with that in the control rats. Moreover, treatment with Trolox markedly attenuated ALT release after both 5 and 24 h of reperfusion. The present results demonstrate that treatment with Trolox improves hepatic function after ischemia and reperfusion. Accumulating evidences indicate that ROS play a major role in producing the microvascular and parenchymal cell damage associated with the reperfusion of ischemic tissues. ROS attack on biological membrane can lead to the oxidative destruction of the membrane polyunsaturated fatty acids by lipid peroxidation. It has been shown that the levels of lipid peroxidation products are lowered when GSH is added to organ storage fluids (Bryan et al., 1994). GSH plays an important role as a free radical scavenger (Nakano et al., 1995). In ischemic rats, hepatic GSH significantly decreased 5 and 24 h after reperfusion. In contrast, hepatic lipid peroxidation significantly increased after both 5 and 24 h of reperfusion. Thus, our results suggest that ROS, produced in post-ischemic livers, cause direct cell damage through thiol oxidation and subsequent lipid peroxidation. Moreover, treatment with Trolox attenuated a decrease in hepatic GSH content and lipid peroxidation, which suggest that it increases the hepatic pool of GSH and reduces oxidative stress.
Our previous study suggested that abnormalities in the microsomal drug-metabolizing function associated with lipid peroxidation occur following hepatic I/R in vivo (Lee et al., 2000). In ischemic rats, CYP concentrations significantly decreased after both 5 and 24 h of reperfusion, which coincides with the results of lipid peroxidation. This decrease in CYP concentration was prevented by Trolox. Such a decrease in the total concentration of CYP suggests that the overall activity of CYP-dependent oxidations is likely to be similarly decreased and that this results from injury to the endoplasmic via disruption of the membrane lipid environment, as well as the specific downregulation of specific CYP isoforms. CYP requires NADPH-cytochrome P450 reductase for electron transfer. After 5 and 24 h of reperfusion, the activity of NADPH-cytochrome P450 reductase decreased, as did that of CYP, and this decrease at 5 h of reperfusion was prevented by Trolox. This suggests that ROS may damage the CYP protein (heme protein), as well as the NADPH-cytochrome P450 reductase protein (non-heme protein) in the acute phase of reperfusion.
In the present study, both CYP1A1 and CYP1A2 activities significantly decreased in the late phase of reperfusion. Trolox attenuated the decrease in CYP1A1 activity, but not in CYP1A2 activity. Western blot analysis revealed that the levels of CYP1A1 protein significantly decreased after both 5 and 24 h of reperfusion; this decrease was prevented by Trolox. This suggests that ROS enhance the degradation of CYP1A1 protein and/or suppress CYP1A1 synthesis. Unfortunately, we could not identify any band equivalent to CYP1A2 enzyme size (59.3 kDa; data not shown).
Isozymes belonging to the CYP2B subfamily have been studied extensively in many species. In the rat, CYP2B1/2B2 is known as the major phenobarbital-inducible CYP isozyme (Guengerich et al., 1982). In the present study, the activity of CYP2B1 decreased after 5 h of reperfusion. The decrease in the activity of CYP2B1 occurred earlier than the decrease in the activities of CYP1A1 and CYP1A2. This decrease in the activity of CYP2B1 was prevented by the Trolox. However, there was no appreciable decrease in the level of the CYP2B1 protein. Thus, these results indicate that the decrease in CYP2B1 activity is ROS-dependent, but is not explained by translational regulation. We may infer that ROS mediate their effects through a post-translational mechanism in which the enzyme protein is not active but is still recognized by the antibodies. There is evidence that ROS may have reduced the activity of selected isoforms of the CYP indirectly by inducing the phosphorylation of the isoforms. Effectively, activation of kinase can phosphorylate CYP, resulting in its inactivation (Rhee, 1999). CYP2E1 is significant for its adaptive response to high blood ethanol level, with a corresponding acceleration of ethanol metabolism, and is inducible by small organic molecules and pathophysiological states (Hong et al., 1987). However, the 80% suppression of CYP2E1 mRNA caused by the injection of endotoxin has also been reported (Morgan, 1993). The CYP2E1 has been shown to be loosely coupled and produces ROS in high amounts relative to other CYP isoforms (Ekstrom & Ingelman-Sundberg, 1989), as CYP2E1 is rapidly reduced even in the absence of substrate (Guengerich & Johnson, 1997). In contrast to other CYP isozymes, the activity of CYP2E1 markedly increased, with a concomitant increase in its protein level, after 5 h of reperfusion, and this increase was prevented by Trolox. These results suggest that ROS is involved in the I/R-induced upregulation of CYP2E1 at the translational level.
It has been reported that the intracellular production of ROS is implicated in the activation of signal transduction cascades and in the regulation of gene expression. Changes in the cellular redox-state or the formation of ROS have been shown to activate redox-sensitive transcription factors, such as nuclear factor (NF)-kB and activator protein (AP)-1 in various cell lines (Palmer & Paulson, 1997). In the present study, both CYP1A1 and CYP2B1 mRNA expression were significantly downregulated during I/R, but Trolox did not affect the downregulation of CYP1A1 or CYP2B1 gene expression. This suggests that factors other (produced indirectly by cytokines generated during I/R) than ROS may also be responsible for the downregulation of CYP1A1 and CYP2B1 mRNA expression. In vivo and in vitro studies have shown that the cytokines interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α can mimic the downregulation of CYP gene products seen during inflammation. Cytokines also induce inducible nitric oxide synthase (iNOS) and result in the production of NO in many cell types. Several studies have suggested that NO mediate the suppression of hepatic CYP expression and activity (Carlson & Billings, 1996). Contrary evidence existed that NO is not required for the suppression of some CYP during inflammation (Sewer & Morgan, 1998). In the present study, we observed that levels of iNOS and TNF-α mRNA expression significantly increased during I/R. However, Trolox attenuated the increase in iNOS mRNA expression but did not affect the increase in TNF-α mRNA expression. These data indicate that the extent of iNOS expression is redox modulated and the inhibition of iNOS mRNA expression is responsible for the reduction of I/R-induced hepatic injury. The mRNA expression of CYP1A2 decreased, with a concomitant decrease in its activity, after 24 h of reperfusion and this decrease was prevented by Trolox. This result suggests that I/R downregulates CYP1A2 mRNA expression through a ROS-dependent mechanism.
Even though the full complex regulatory mechanisms of these inconsistent alterations in drug-metabolizing systems have not been identified, the individual CYP isozymes seem to be differentially affected by I/R injury, and the production of ROS during I/R may damage these CYP isoforms. These changes would be expected to variably affect the intrinsic hepatic clearance of certain drugs in patients with severe hepatic I/R injury. Furthermore, Trolox differently regulates not only the expression of each form of CYP, among the various CYP subfamilies, but also that of each isozyme within the same subfamily. Our data indicate that Trolox improves ischemia-induced hepatic drug-metabolizing dysfunction, which prevents a microsomal oxidant stress and lipid peroxidation.
Acknowledgments
This work was supported by grant R04–2001–000–00011-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
Abbreviations
- ALT
alanine aminotransferase
- CYP
cytochrome P450
- EROD
7-ethoxyresorufin O-deethylase
- GSH
glutathione
- GSSG
glutathione disulfide
- iNOS
inducible nitric oxide synthase
- I/R
ischemia and reperfusion
- MDA
malondialdehyde
- MROD
methoxyresorufin O-demethylase
- NF-kB
nuclear factor-kB
- NO
nitric oxide
- PROD
pentoxyresorufin O-dealkylase
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
References
- ANDERSON M.Determination of glutathione and glutathione disulfide in biological samples Methods in Enzymology. 1985New York: Academic Press; 548–555.ed. Meister, A. Vol 113 [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BRYAN C.L., PATEFIELD A.J., COHEN D., NIELSEN J.L., EMANUAL B., CALHOON J.H. Assessment of injury in transplanted and non-transplanted lungs after 6 h of cold storage with glutathione. J. Appl. Physiol. 1994;67:1231–1241. doi: 10.1152/jappl.1994.76.3.1232. [DOI] [PubMed] [Google Scholar]
- BUEGE J.A., AUST S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- BURKE M.D., THOMPSON S., ELCOMBE C.R., HALPERT J., HAPARANTA T., MAYER R.T. Ethoxy-, pentoxy-, and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- CARLSON T.J., BILLINGS R.E. Role of nitric oxide in the cytokine-mediated regulation of cytochrome P-450. Mol. Pharmacol. 1996;49:796–801. [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenolchloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- EKSTROM G., INGELMAN-SUNDBERG M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem. Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- GUENGERICH F.P., DANNAN G.A., WRIGHT S.T., MARTIN M.V., KAMINSKY L.S. Purification and characterization of microsomal cytochrome P-450s. Xenobiotica. 1982;12:701–716. doi: 10.3109/00498258209038945. [DOI] [PubMed] [Google Scholar]
- GUENGERICH F.P., JOHNSON W.W. Kinetics of ferric cytochrome P450 reduction by NADPH-cytochrome P450 reductase: rapid reduction in the absence of substrate and variations among cytochrome P450 systems. Biochemistry. 1997;36:14741–14750. doi: 10.1021/bi9719399. [DOI] [PubMed] [Google Scholar]
- HONG J.Y., PAN J.M., GONZALEZ F.J., GELBOIN H.V., YANG C.S. The induction of a specific form of P-450 (P-450j) by fasting. Biochem. Biophys. Res. Commun. 1987;142:1077–1083. doi: 10.1016/0006-291x(87)91525-7. [DOI] [PubMed] [Google Scholar]
- JONES D.P., AW T.Y., SHAN X.Q. Drug metabolism and toxicity during hypoxia. Drug Metab. Rev. 1989;20:247–260. doi: 10.3109/03602538909103540. [DOI] [PubMed] [Google Scholar]
- KUROKAWA T., NONAMI T., HARADA A., NAKAO A., TAKAGI H. Mechanism and prevention of ischemia–reperfusion injury of the liver. Semin. Surg. Oncol. 1996;12:179–182. doi: 10.1002/(SICI)1098-2388(199605/06)12:3<179::AID-SSU6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- LANDES N., PFLUGER P., KLUTH D., BIRRINGER M., RUHL R., BOL G.F., GLATT H., BRIGELIUS-FLOHE R. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 2003;65:269–273. doi: 10.1016/s0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- LEE S.M., CHO T.S. Effect of Trolox on hypoxia/reoxygenation-induced injury in isolated perfused rat liver. Arch. Pharm. Res. 1997;20:471–475. doi: 10.1007/BF02973942. [DOI] [PubMed] [Google Scholar]
- LEE S.M., PARK MJ CHO T.S., CLEMENS M.G. Hepatic injury and lipid peroxidation during ischemia and reperfusion. Shock. 2000;13:279–284. doi: 10.1097/00024382-200004000-00005. [DOI] [PubMed] [Google Scholar]
- LINDSTROM T.D., HANSSEN B.R., BENDELE A.M. Effects of hepatic ischemia–reperfusion injury on the hepatic mixed function oxidase system in rats. Mol. Pharmacol. 1990;38:829–835. [PubMed] [Google Scholar]
- MORGAN E.T. Down-regulation of multiple cytochrome P450 gene products by inflammatory mediators in vivo. Independence from the hypothalamo–pituitary axis. Biochem. Phamacol. 1993;45:415–419. doi: 10.1016/0006-2952(93)90078-b. [DOI] [PubMed] [Google Scholar]
- NAKANO H., BOUDJEMA K., ALEXANDRE E., IMBS P., CHENARD M.P., WOLF P. Protective effect of N-acetylcysteine on hypothermic ischemia–reperfusion injury of rat liver. Hepatology. 1995;22:539–545. [PubMed] [Google Scholar]
- NELSON D.R., KOYMANS L., KAMATAKI T., STEGEMAN J.J., FEYEREISEN R., WAXMAN D.J., WATERMAN M.R., GOTOH O., COON M.J., ESTABROOK R.W., GUNSALUS I.C., NEBERT D.W. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. The carbon monoxide binding pigment of liver microsomes. J. Biol. Chem. 1964;239:2370–2379. [PubMed] [Google Scholar]
- PALMER H.J., PAULSON K.E. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr. Rev. 1997;55:353–361. doi: 10.1111/j.1753-4887.1997.tb01561.x. [DOI] [PubMed] [Google Scholar]
- PARDINI R.S. Toxicity of oxygen from naturally occurring redox-active pro-oxidants. Arch. Insect Biochem. Physiol. 1995;29:101–118. doi: 10.1002/arch.940290203. [DOI] [PubMed] [Google Scholar]
- RHEE S.G. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- SCHENKMAN J.B., REMMER H., ESTABROOK R.W. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol. Pharmacol. 1967;3:113–123. [PubMed] [Google Scholar]
- SEWER M.B., MORGAN E.T. Down-regulation of the expression of theree major rat liver cytochrome P450s by endotoxin in vivo occurs independently of nitric oxide production. J. Pharmacol. Exp. Ther. 1998;287:352–358. [PubMed] [Google Scholar]
- VERMILLION J., COON M.J. Purified liver microsomal NADPH-cytochrome P450 reductase. J. Biol. Chem. 1978;253:8812–8819. [PubMed] [Google Scholar]
- WU T.W., HASHIMOTO N., AU J.X., WU J., MICKLE D.A., CAREY D. Trolox protects rat hepatocytes against oxyradical damage and the ischemic rat liver from reperfusion injury. Hepatology. 1991;13:575–580. [PubMed] [Google Scholar]
- WU T.W., HASHIMOTO N., WU J., CAVEY D., LI R.K., MICKLE D.A., WEISEL R.D. The cytoprotective effect of Trolox demonstrated with three types of human cells. Biochem. Cell Biol. 1990;68:1189–1194. doi: 10.1139/o90-176. [DOI] [PubMed] [Google Scholar]
- ZWACKA R.M., ZHANG Y., HALLDORSON J., SCHLOSSBERG H., DUDUS L., ENGELHARDT J.F. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J. Clin. Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]