Abstract
In the rat isolated urinary bladder, NaHS (30 μM–3 mM) and capsaicin (10 nM–3 μM) produced concentration-dependent contractile responses (pEC50=3.5±0.02 and 7.1±0.02, respectively) undergoing dramatic tachyphylaxis. In preparations in which sensory nerves were rendered desensitized (defunctionalized) by high-capsaicin (10 μM for 15 min) pretreatment, neither capsaicin itself nor NaHS produced any motor effect. NaHS-induced contractile effects were totally prevented by the simultaneous incubation with tachykinin NK1 (GR 82334; 10 μM) and NK2 (nepadutant; 0.3 μM) receptor-selective antagonists. Tetrodotoxin (1 μM) only partially reduced the response to NaHS. These results provide pharmacological evidence that H2S stimulates capsaicin-sensitive primary afferent nerve terminals, from which tachykinins are released to produce the observed contraction by activating NK1 and NK2 receptors. While the molecular site of action of H2S remains to be investigated, our discovery may have important physiological significance since H2S concentrations capable of stimulating sensory nerves overlap those occurring in mammalian tissues under normal conditions.
Keywords: Hydrogen sulfide (H2S), capsaicin, sensory nerves, tachykinins, tachykinin receptors
Introduction
Hydrogen sulfide (H2S) is a well-known gas characterized by a peculiar rotten egg smell, representing a primary chemical hazard in natural gas production, in sewage treatment and in certain industrial manufacturings. While the toxic effects produced by high concentrations of H2S have been widely investigated (Guidotti, 1996), its biological activity at physiological concentrations has so far received lesser attention, despite that H2S is endogenously generated via nonenzymatic and enzymatic pathways in mammals. Cystathionine β-synthase in the CNS and cystathionine γ-lyase in the cardiovascular and gastrointestinal systems are two enzymes mostly responsible for H2S generation from cysteine (Hosoki et al., 1997; Kimura, 2002; Wang, 2003). Relatively high concentrations (50–160 μM) of H2S have been detected in rat, human and bovine brain, in rat and human blood (10–100 μM) and in various vascular tissues (Warenycia et al., 1989; Zhao et al., 2001; Wang, 2003, for review). The biological activities and the possible pathological relevance of H2S have recently been discussed by Moore et al. (2003). In particular, in the cardiovascular system, H2S relaxes isolated vessels and produces hypotension in vivo by opening ATP-sensitive K+channels (KATP) (Hosoki et al., 1997; Zhao et al., 2001). A smooth muscle relaxant activity of H2S has also been noted in gastrointestinal (Hosoki et al., 1997; Teague et al., 2002) and reproductive (Hayden et al., 1989; Teague et al., 2002) organs. However, unlike the inhibitory effects afforded by H2S in the rat aorta (Zhao et al., 2001), the relaxant effect produced by the gas in the guinea-pig ileum was unaffected by the K+ channel blocker glibenclamide, leading Teague et al. (2002) to conclude that a still unidentified mechanism – independent of KATP channels – is responsible for the intestinal effects of H2S. In the present study, we decided to investigate the effects produced by H2S in the detrusor muscle of the rat urinary bladder. Surprisingly, we noticed that H2S was not inhibitory, but produced contractile responses that underwent rapid and persistent tachyphylaxis: a pattern resembling the profile of action of capsaicin. Therefore, we challenged the hypothesis that H2S may stimulate capsaicin-sensitive primary afferent neurons in the rat urinary bladder and produce the observed excitatory motor effects by the release of tachykinins stored in the terminal endings of sensory neurons, in a capsaicin-like manner. Alike other investigators of H2S-induced biological effects (e.g. Hosoki et al., 1997; Zhao et al., 2001; Teague et al., 2002), as source of H2S in physiological solution we used NaHS, a salt that quickly dissociates into Na+ and HS− ions, which in turn react with water to give H2S and OH−.
Methods
Male albino rats (Wistar strain, 275–350 g) were decapitated under ether anaesthesia. The whole urinary bladder, cleared of surrounding tissue, was excised and cut along its longitudinal axis into four parallel strips. The strips were placed in 5-ml organ baths filled with oxygenated normal Krebs–Henseleit solution added of atropine (1 μM) – to rule out the possible contribution of muscarinic receptors to the excitatory motor responses studied – and indomethacin (3 μM) – to prevent the generation of endogenous prostanoids having disturbing activity on the basal tone of the preparations. Motor activity of the strips was recorded isotonically (load 5 mN). After a 60-min equilibration period, the preparations were challenged with KCl (80 mM) to obtain the maximal contractile response of the tissue. The preparations were then exposed to electrical field stimulation (EFS; single pulses of stimuli of 0.1 Hz, 0.5 ms pulse width, supramaximal voltage) for 15 min, by means of two platinum wire electrodes placed at the top and the bottom of the organ bath and connected to a Grass S88 stimulator. Afterwards, some preparations received capsaicin (10 μM for 15 min), in order to achieve a complete in vitro desensitization (defunctionalization) of capsaicin-sensitive primary afferent nerve terminals (Patacchini et al., 1990), or [βAla8]NKA(4–10) – a tachykinin NK2 receptor-selective agonist – (1 μM) which produced a comparable contractile response, in control strips. After a 60-min recovery period, cumulative concentration–response curves to NaHS or capsaicin were constructed in control or capsaicin-pretreated preparations. In other experiments, the effect of NaHS was evaluated after pretreatment with tetrodotoxin (1 μM, 15 min before) or after the combined administration of the tachykinin NK1 (GR 82334; 10 μM) and NK2 (nepadutant; 0.3 μM) receptor-selective antagonists, 30 min before. In a different set of experiments, the ability of NaHS to produce smooth muscle relaxant effects was checked in carbachol (1 μM)-precontracted preparations. The maximal inhibition of tone was obtained by isoprenaline (10 μM). Neither atropine nor indomethacin was added to the Krebs–Henseleit solution used for these latter experiments. Each value in the text, tables and figures is the mean±s.e.m. Statistical analysis was performed by means of Student's t-test for unpaired data or by means of analysis of variance, when applicable. Ethical approval of the experimental protocol with animals was obtained from the local Ethics Committee.
Chemicals
Capsaicin, NaHS, indomethacin, carbachol, atropine sulphate and tetrodotoxin were from Sigma (St Louis, MO, U.S.A.); isoprenaline was from Serva (Heidelberg, Germany); GR 82334 was from Neosystem (Strasbourg, France). Nepadutant (or MEN 11420) and [βAla8]NKA(4–10) were synthesized at Menarini Ricerche (Florence, Italy) by the conventional solid-phase method.
Results
Excitatory and desensitizing effects produced by NaHS and capsaicin on rat detrusor muscle
EFS of preparations evoked a twitch contraction averaging 7.8±0.9% of KCl (80 mM)-induced response (n=32).
Cumulative additions of NaHS (30 μM–3 mM) in control preparations produced slowly developing tonic contractions, accompanied by increased phasic contractile activity of the detrusor muscle (Table 1; Figures 1, 2a). Assuming that dissociation of NaHS is about 30% at physiological pH of 7.4, as the vehicle we administered an equivalent concentration of OH− ions (NaOH, 1 mM), which proved to be devoid of any motor effect per se (n=4). After a thorough washout, the strips were allowed to recover their initial tone for 1 h. At this point, a further administration of NaHS was totally ineffective (n=4), whereas EFS-induced twitch was unchanged or slightly increased (not shown).
Table 1.
Potencies of NaHS and capsaicin in producing excitatory motor responses in control and drug-pretreated strips of rat detrusor muscle
| Compound | pEC50a | Emax % EFSb |
|---|---|---|
| NaHS | ||
| Control | 3.5±0.02 | 249±56 |
| Capsaicin (10 μM)c | IN | IN |
| NaHS (3 mM) d | IN | IN |
| Nepadutant (0.3 μM) plus GR 82334 (10 μM) | IN | IN |
| TTX (1 μM)e | 3.5±0.04 | 153±31* |
| Capsaicin | ||
| Control | 7.1±0.02 | 708±132 |
| Capsaicin (10 μM) | IN | IN |
| NAHS (3 mM) | 7.0±0.03 | 468±67 |
pEC50 (−log EC50).
% of the twitch response induced by electrical field stimulation.
The strips had received capsaicin 10 μM, 90 min before.
The strips had received NaHS 3 mM, 90 min before.
In the presence of tetrodotoxin 1 μM, 30 min before.
IN=inactive up to 3 μM (capsaicin) or up to 3 mM (NaHS).
Significantly different from control response; P<0.05. All data are the mean±s.e.m. of 4–12 experiments.
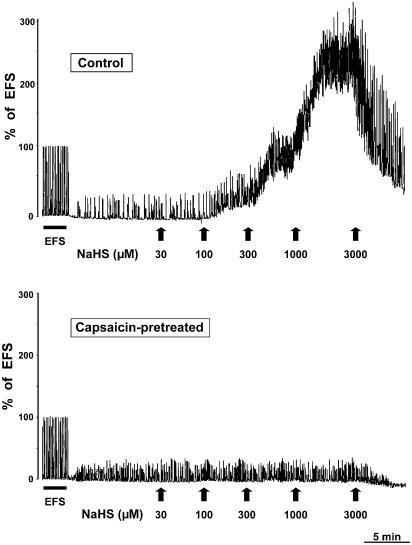
Figure 1.
Typical tracings showing NaHS-induced contractions of rat isolated urinary bladder in control (upper panel) or capsaicin-pretreated (10 μM for 15 min; lower panel) strips.
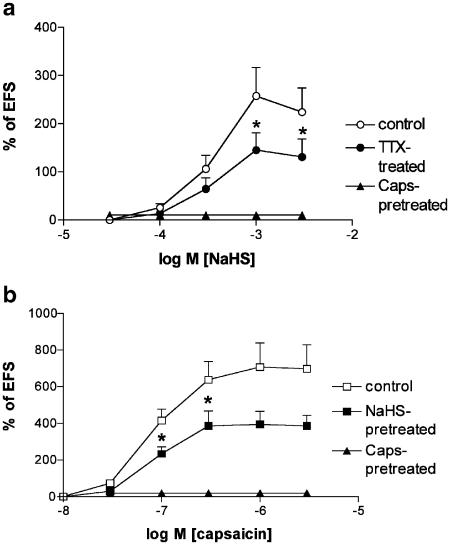
Figure 2.
(a) NaHS-induced contractions of rat isolated urinary bladder in control strips, or in the presence of tetrodotoxin (TTX, 1 μM, 30 min before) or in strips which had received capsaicin (Caps., 10 μM, for 15 min) 90 min before. (b) Capsaicin-induced contractions of rat isolated urinary bladder in control strips, or in strips which had received NaHS (3 mM, for 15 min) or capsaicin itself (Caps., 10 μM, for 15 min), 90 min before. (*) Significantly different from the control matched value; P<0.05. Each value is the mean±s.e.m. of 4–12 experiments.
In other preparations, capsaicin (10 nM–3 μM) administered cumulatively produced contractile responses (Figure 2b), whose maximum was about two-fold higher than that produced by NaHS (cf. Table 1). The administration of a supramaximal concentration of capsaicin (10 μM for 15 min) at the beginning of the experiments afforded a complete desensitization of sensory fibres, so that the detrusor muscle became totally unresponsive to further administration of capsaicin itself (Figure 2b; Table 1). Also, NaHS was totally ineffective in preparations undergoing high capsaicin pretreatment (Table 1; Figures 1, 2a), whereas responsiveness to other stimuli of capsaicin-pretreated tissues was unchanged (e.g. EFS: 4.2±0.8% and 4.7±1.0% of KCl, before and after high capsaicin pretreatment, respectively; n=4). In preparations which had received NaHS (30 μM–3 mM) 90 min before, capsaicin produced weaker contractile effects as compared to those observed in control preparations (Figure 2b), although its potency was not significantly changed (Table 1).
Effect of tachykinin receptor antagonists and tetrodotoxin on NaHS-induced contractions of the rat detrusor muscle
The combined administration of the tachykinin NK1 (GR 82334; 10 μM) and NK2 (nepadutant; 0.3 μM) receptor-selective antagonists completely prevented the contractile response to NaHS up to 3 mM (n=4; Table 1), whereas EFS-induced twitch contraction was not modified (6.0±0.9 vs 4.8±1.1% of KCl before and after administration of the two antagonists; n=4). Tetrodotoxin (1 μM) completely prevented the response to EFS (n=4) and significantly reduced the Emax of NaHS in the detrusor muscle (Figure 2a), although it failed to reduce NaHS potency (EC50) (cf. Table 1).
Smooth muscle-relaxing effects of NaHS on precontracted preparations
The ability of NaHS to produce smooth muscle relaxant effects was checked in preparations precontracted by carbachol (1 μM), which produced a stable tonic contraction averaging 77±1% of KCl (80 mM) (n=8). NaHS produced similar smooth muscle inhibitory responses in both control or high-capsaicin (10 μM for 15 min, 90 min before NaHS) pretreated preparations. The inhibition afforded by NaHS in control preparations averaged 1±0.1, 8±0.3 and 76±4% (n=4), and in capsaicin-pretreated preparations it was 4±0.5, 14±2 and 71±6% (n=4) of maximal relaxation produced by isoprenaline (10 μM) at 0.3, 1 and 3 mM, respectively.
Discussion
Capsaicin, the pungent ingredient of chilli pepper, possesses the ability to stimulate a subset of primary afferent neurons by directly activating their transient receptor potential vanilloid receptor (1) (TRPVR1) receptors (Caterina et al., 1997). The ability of capsaicin to produce smooth muscle excitatory and/or inhibitory effects by activating the local efferent function of capsaicin-sensitive sensory nerves has widely been investigated by our and other groups (Holzer, 1991; Maggi, 1995, for reviews). In particular, in the rat urinary bladder capsaicin produces excitatory motor responses that are mediated by tachykinins (mainly substance P, and neurokinin A) released from the activated sensory nerve terminals (Maggi et al., 1991). Tachykinins, in turn, stimulate tachykinin NK1 and NK2 receptors present on detrusor smooth muscle cells to produce the observed contractile response to capsaicin (Maggi et al., 1991). One peculiar feature of capsaicin action is that the excitatory effects produced by this drug are followed by desensitization of sensory nerves, which become progressively less responsive to capsaicin itself and other activating stimuli (Maggi, 1995). In particular, the in vitro exposure of rat detrusor smooth muscle to capsaicin (10 μM) for 15 min renders the preparations totally unresponsive to further capsaicin administration (Patacchini et al., 1990).
Our present data provide functional evidence that H2S produces excitatory motor responses in the rat urinary bladder by activating capsaicin-sensitive primary afferent neurons, which in turn release tachykinins activating postjunctional NK2 and NK1 receptors. This conclusion is supported by the following observations: (1) H2S-induced contractile effects are completely prevented by in vitro desensitization of sensory neurons achieved by high-capsaicin pretreatment. H2S itself undergoes a complete tachyphylaxis, a second administration of this drug being totally ineffective; (2) preparations pretreated with H2S (30 μM–3 mM) become less responsive to capsaicin, showing that a (partial) cross-desensitization phenomenon occurs between the two drugs; (3) a combination of tachykinin NK2 (nepadutant) and NK1 (GR 82334) receptor-selective antagonists, administered at concentrations producing strong, albeit selective, inhibition of responses mediated by these two receptors (Meini et al., 1994), completely prevents H2S-induced contractile effects. In a previous study (Maggi et al., 1991), we had shown that less potent and selective NK1 and NK2 antagonists (i.e. spantide and L 659877, respectively) are capable of strongly reducing the response to capsaicin in the rat urinary bladder. Furthermore, our experiments show that the response to H2S is mostly resistant to tetrodotoxin, as is the effect of capsaicin in this organ (Maggi, 1995; Maggi & Meli, 1988, for reviews). We interpret this result as evidence that H2S directly activates the sensory neuron terminal, through a mechanism independent of axonal conduction via fast sodium channels. A (minor) component of the response to H2S, however, is probably due to activation of an axon reflex arrangement, by which afferent sensory stimuli, triggered by stimulation of the nerve terminals, invade axon collaterals and provoke a local release of their neuropeptide content in a tetrodotoxin-sensitive manner. We had previously collected evidence that the above-described mode of action of H2S at sensory neuron terminals is shared by several (weaker) congeners of capsaicin, like piperine and others (Patacchini et al., 1990). The possibility that TRPVR1 receptors, as for capsaicin and other (patho)physiological stimuli, mediate the excitatory effects of H2S on primary afferent neurons remains to be investigated.
It is worth mentioning that at physiological pH it has been estimated that about one-third of H2S exists as undissociated gas, and the remaining two-thirds as HS− anion (Hosoki et al., 1997). Thus, assuming that H2S is the pharmacologically active moiety (although the contribution of HS− ions cannot be ruled out in principle), the presently reported potency of H2S as sensory fibre activator may be underestimated. This latter observation provides further support to the hypothesis that endogenous H2S (whose concentrations detected in cardiovascular, intestinal and central neuronal tissues largely overlap those found effective in the present study) is capable of stimulating capsaicin-sensitive primary afferent neurons under physiological conditions. The functional significance of such activity of H2S, as well as the possible pathological consequences linked to the variation of H2S production, are issues deserving further investigations. Also, the toxicological spectrum of activity of H2S (Guidotti, 1996) should be re-examined in light of the present results. Finally, it should be noted that the ability of H2S to stimulate sensory nerves occurs at concentrations overlapping those at which H2S activates other mechanisms affecting (reducing) smooth muscle tone (Moore et al., 2003).
Experiments in precontracted preparations have shown that H2S produces smooth muscle inhibitory effects even in the rat urinary bladder: this effect occurs at concentrations equal to or higher than those producing excitatory motor responses in this organ. Importantly, desensitization of sensory fibres by capsaicin pretreatment did not affect the smooth muscle inhibitory effect of H2S on the detrusor muscle, showing that this latter activity is totally independent of sensory neuron activation. The mechanism(s) underlying the inhibitory effects of H2S was not further investigated in the present study. In light of our present results, it will be interesting to ascertain whether the reported smooth muscle inhibitory effects of H2S in vascular, reproductive and intestinal organs originate from a sensory neuron-independent mechanism of action (as in the rat urinary bladder) or whether activation of primary afferent nerves by H2S, followed by local release of neuropeptides having smooth muscle inhibitory activity (e.g. CGRP), may contribute to the observed relaxations.
In conclusion, our results show a yet undiscovered mechanism of action through which H2S can affect smooth muscle tone in mammalian preparations, that is, activation of the efferent function of capsaicin-sensitive primary afferent neurons. Although the precise mechanism(s) through which H2S activates sensory nerves remains to be ascertained, our discovery may have important physiological significance, since H2S concentrations capable of stimulating sensory nerves overlap those occurring in mammalian tissues under normal conditions. The possibility that stimulation of sensory fibres by H2S contributes to the so far reported inhibitory activity of this gas in various smooth muscle tissues should be carefully considered in studies of this type.
Abbreviations
- EFS
electrical field stimulation
References
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI T.L. Hydrogen sulphide. Occup. Med. 1996;46:367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- HAYDEN L.J., FRANKLIN K.J., ROTH S.H., MOORE G.J. Inhibition of oxytocin-induced but not angiotensin-induced rat uterine contractions following exposure to sodium sulfide. Life Sci. 1989;45:2557–2560. doi: 10.1016/0024-3205(89)90239-7. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOSOKI R., MATSUKI N., KIMURA H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- KIMURA H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., SANTICIOLI P., GIULIANI S. Tachykinin antagonists and capsaicin-induced contraction of the rat isolated urinary bladder: evidence for tachykinin-mediated cotransmission. Br. J. Pharmacol. 1991;103:1535–1541. doi: 10.1111/j.1476-5381.1991.tb09823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEINI S., PATACCHINI R., MAGGI C.A. Tachykinin NK1 receptor subtypes in the rat urinary bladder. Br. J. Pharmacol. 1994;111:739–746. doi: 10.1111/j.1476-5381.1994.tb14800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., BHATIA M., MOOCHHALA S. Hydrogen sulphide: from the smell of the past to the mediator of the future. Trends Pharmacol. Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., MAGGI C.A., MELI A. Capsaicin-like activity of some natural pungent substances on peripheral endings of visceral primary afferents. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:72–77. doi: 10.1007/BF00178975. [DOI] [PubMed] [Google Scholar]
- TEAGUE B., ASIEDU S., MOORE P.K. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J. Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003;4:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- WARENYCIA M.W., GOODWIN L.R., BENISHIN C.G., REIFFENSTEIN R.J., FRANCOM D.M., TAYLOR J.D., DIEKEN F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- ZHAO W., ZHANG J., LU Y., WANG R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]