Abstract
The role of histamine H1, H2, H3 and H4 receptors in acute itch induced by histamine was investigated in female BalbC mice. Scratching was induced by intradermal injections of pruritogen into the back of the neck and ‘itch' assessed by quantifying the scratching evoked.
Histamine (0.03–80 μmol), histamine-trifluoromethyl-toluidine (HTMT, H1 agonist, 0.002–2 μmol), clobenpropit (H4 agonist, H3 antagonist, 0.002–0.6 μmol) and to a lesser extent imetit (H3/H4 agonist, 0.03–3 μmol) all induced dose-dependent scratching. Dimaprit (H2 agonist, 0.04–40 μmol) did not cause scratching.
Mepyramine (H1 antagonist, 20 mg kg−1, i.p.) reduced scratching evoked by histamine and HTMT, but not that caused by H3 or H4 agonists. Thioperamide (H3/H4 antagonist, 20 mg kg−1, i.p.) reduced scratching induced by histamine, H3 and H4 agonists, but not that caused by HTMT. The non-sedating H1 antagonist, terfenadine, also significantly reduced the scratching induced by the H1 agonist, HTMT. Cimetidine (H2 antagonist, 20 mg kg−1, i.p.) did not affect histamine-induced scratching.
These results indicate that activation of histamine H4 receptors causes itch in mice, in addition to the previously recognised role for H1 receptors in evoking itch. Histamine H4 receptor antagonists therefore merit investigation as antipruritic agents.
Keywords: Itch, pruritus, histamine, mouse, H1 receptor, H3 receptor, H4 receptor
Introduction
Itch (pruritus) is commonly defined in humans as an unpleasant sensation of the superficial layers of the skin (Shelley & Arthur, 1957) provoking the desire to scratch (Ekblom, 1995). It is a common clinical condition that can be associated with cutaneous (e.g. atopic eczema, contact dermatitis) or systemic (e.g. chronic renal failure) disease. Itch is difficult to study objectively in man and there are currently few reliable animal models of itch.
Histamine has long been recognised as a mediator of itch in humans (Lewis, 1927). Although traditional H1 receptor antagonists are effective at reducing histamine-induced itch (Hagermark et al., 1979) and are widely used clinically for treating pruritus (Greaves, 1997), they are not effective antipruritics in many clinical conditions, such as atopic eczema (Yosipovitch et al., 2003). It has been claimed that the effectiveness of H1 receptor antagonists in relieving itch results from central sedation rather than peripheral actions on sensory nerves, in conditions where histamine is not considered to be the primary mediator (Savin, 1980; Krause & Shuster, 1983; Savin et al., 1986). Histamine H2 receptor antagonists are also used clinically for treating itch (Monroe et al., 1981; Ring et al., 1999), but their ability to inhibit itching is not conclusive (Hagermark et al., 1979; Davies & Greaves, 1980). Overall the evidence suggests that mechanisms other than those involving H1/H2 receptors are involved in itch.
In murine studies, itch is estimated by measuring the scratching elicited by itch-provoking agents (Kuraishi et al., 1995). Although some reports have questioned the role of histamine as a pruritogen in mice (Kuraishi et al., 1995), others have shown that histamine H1 receptor antagonists are effective at reducing histamine-induced scratching (Sugimoto et al., 1998) in this species. In studies involving mice, it is necessary to appreciate that differences in strain can markedly influence scratching responses to drugs (Inagaki et al., 2001). Concerns have been raised that scratching in animal models of itch may reflect pain rather than pruritus (McMahon & Koltzenburg, 1992), but evidence shows that pain and itch do promote different behavioural responses in mice (Kuraishi et al., 1995; Laidlaw et al., 2002).
The present study was undertaken to investigate the role of H1, H2, H3 and H4 receptors in histamine-induced itch in BalbC mice based on the quantification of acutely induced scratching.
Methods
Animals
All experiments were performed under U.K. Home Office regulations. Female BalbC mice (Charles River, Margate, Kent, U.K.), 10-weeks old, were used in the experiments. Mice weighed 18–26 g and were housed under controlled light (07:00–19:00 h) and temperature (22°C) with food and water available ad libitum. Each mouse was used for only one experiment.
Materials
Histamine diphosphate (Sigma, Poole, U.K. MW=307) was dissolved in saline (0.9% w v−1 aqueous NaCl solution). Histamine–trifluoromethyl–toluidine dimaleate (HTMT; H1 receptor agonist, MW=615), dimaprit dihydrochloride (H2 agonist, MW=234), imetit dihydrobromide (dual H3 and H4 agonist, MW=332), clobenpropit dihydrobromide (H4 agonist and H3 antagonist, MW=471), mepyramine maleate (sedating H1 antagonist, MW=401), terfenadine (nonsedating H1 antagonist, MW=472), cimetidine (H2 antagonist, MW=252) and thioperamide maleate (H3 and H4 antagonist, MW=413) (all Tocris, Avonmouth, U.K. unless specified) were dissolved in phosphate-buffered saline (PBS, pH=7.4). All drugs were injected in a volume of 100 μl.
Procedure
The itch-inducing properties of histamine and H1, H2, H3 and H4 agonists were studied by administering the drugs intradermally (i.d. ) into the back of the neck via a 26 G needle. Drugs were administered at 1-h intervals. Injection of PBS was used as a control. The injection site was chosen as it is only accessible by the animal's hind paws and therefore scratching behaviour can be separately identified from grooming, which is performed by the forelimbs.
In experiments involving antagonists, scratching was induced by a mid-range dose of histamine, which was repeated 30 and again 90 min after treatment with either an H1, H2 or H3 antagonist (20 mg kg−1, i.p.). This dose of antagonist was selected on the basis of its effectiveness in reducing scratching induced by H1, H2 or H3 antagonists.
The effects of antagonists on receptor-selective agonist-induced scratching were studied in separate groups of mice, as desensitisation to the agonists precluded repeated dosing. The response to agonist was recorded 30 min after antagonist treatment and compared to responses obtained from vehicle (saline)-treated mice. The dose of agonist was selected on the basis of its ability to elicit scratching in each mouse studied, and with the aim of eliciting approximately 50% of maximum scratching response.
Measurement of itch
Mice were filmed with a video camera (Vista, NCD 132) and the signal recorded on a VCR (Panasonic, NV-HD640). ‘Itch' was measured by counting the number of ‘bouts' of scratching in the 20-min period immediately following injection of histamine or selective H1, H2, H3 or H4 agonist. A bout of scratching was defined as three or more individual rapid scratch movements with the hind paws to the area around the injection site (i.e. the back of the neck).
Statistical analysis
All data were analysed using GraphPad Prism (v3.02) software. Log dose – response curves were plotted using the nonlinear regression (sigmoidal dose – response) function. The mean ‘apparent' ED50 was calculated from the pooled data and was the dose required to elicit half the apparent maximal response. Nonparametric statistical analysis was used and the null hypothesis was rejected at P<0.05.
The effects of antagonists on histamine-induced scratching were analysed using the Wilcoxon matched pairs test, comparing the response to a dose of histamine preantagonist with the response to the same dose 30 min after antagonist. A second postantagonist response to the dose of histamine was also recorded 90 min after antagonist, to give an indication of the antagonist's duration. The effect of antagonist on agonist-induced scratching was statistically analysed using the Mann – Whitney test.
Results
Histamine-induced scratching
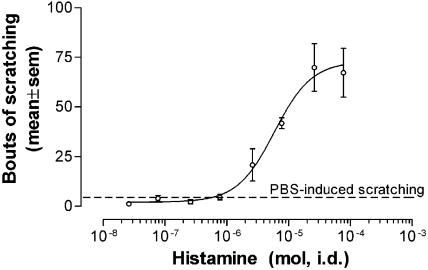
Histamine evoked dose-dependent scratching in all the mice studied, as summarised in Figure 1. Scratching was not obtained at lower doses (up to 0.8 μmol), but was consistently observed with higher doses, reaching a maximum at 26 μmol histamine. Above this dose there was no further increase, and often a decrease, in scratching. Histamine-induced scratching reached a maximum of 73±12 bouts (mean±s.e.m.), with a mean apparent ED50 of 5.8 μmol.
Figure 1.
Pooled data for histamine-induced scratching in female BalbC mice during the 20-min postinjection period (n=4–8). Histamine caused dose-dependent scratching. Dashed line represents the mean level of scratching induced by PBS, which served as a control (5±1 bouts of scratching, n=12).
Scratching induced by phosphate-buffered saline served as a control (5±1 bouts of scratching, n=12).
A painful stimulus (9 M HCl, 0.1 ml, i.d. n=4) did not induce scratching (0±0 bouts) and was associated with behaviour not seen with histamine (vocalisation upon injection and ‘hunching' after the injection).
Role of histamine H1 receptors in histamine agonist-induced scratching
The selective histamine H1 receptor agonist, HTMT, evoked dose-dependent scratching in all six mice studied (Figure 2a). Maximal scratching was 133±17 bouts, with a mean apparent ED50 for HTMT of 0.1 μmol.
Figure 2.

Pooled data illustrating scratching induced by (a) HTMT (H1 receptor agonist, n=6), (b) clobenpropit (H4 agonist, H3 antagonist, n=10), (c) imetit (H3/H4 agonist, n=6) and (d) dimaprit (H2 agonist, n=3) during the 20 min postinjection. HTMT, clobenpropit, and to a lesser extent imetit, caused dose-related scratching, whereas dimaprit had no effect (dashed line illustrates PBS-induced scratching).
Histamine-induced scratching (8 μmol, i.d.) was significantly reduced following mepyramine (20 mg kg−1 i.p.; P<0.05, n=6, Figure 3a). Saline (0.9% w v−1 aqueous NaCl solution), used as a control for the antagonist, did not significantly reduce histamine-induced scratching (P>0.05, n=5, Figure 3b).
Figure 3.
(a) Mepyramine (20 mg kg−1 i.p.) significantly reduced scratching induced by histamine (8 μmol, i.d.) in female BalbC mice, 30 min after treatment with the antagonist versus pretreatment value (*P<0.05, Wilcoxon matched pairs test, n=6). (b) Saline vehicle did not significantly reduce scratching induced by histamine (P>0.05, Wilcoxon matched pairs test, n=5). (c) Mepyramine (20 mg kg−1 i.p.) pretreated mice showed a significantly lower scratching response to HTMT (0.2 μmol i.d.) in comparison with vehicle-treated animals (*P<0.05, Mann–Whitney test, n=6 in each group). Mepyramine at lower doses (1, 3 or 10 mg kg−1) did not significantly reduce HTMT-induced scratching (P>0.05, Mann–Whitney test, n=6 in each group). (d) Terfenadine significantly reduced scratching induced by HTMT (0.2 μmol i.d.) in a dose-dependent manner (terfenadine 20 mg kg−1 versus control, *P<0.05, Mann–Whitney test, n=6 in each group) (V=vehicle for antagonist. Dashed line illustrates PBS-induced scratching).
HTMT-induced scratching (0.2 μmol i.d.) was significantly reduced in mice pretreated with mepyramine (20 mg kg−1 i.p.) in comparison with vehicle-treated mice (P<0.05, Figure 3c). Lower doses of mepyramine (1–10 mg kg−1) did not significantly reduce HTMT-induced scratching.
HTMT-induced scratching (0.2 μmol i.d.) was also significantly reduced by pretreatment with the nonsedating histamine H1 receptor antagonist, terfenadine (20 mg kg−1 i.p.) as compared with vehicle-treated mice (P<0.05, Figure 3d). Lower doses of terfenadine (1–10 mg kg−1) did not significantly reduce HTMT-induced scratching (P>0.05) but there was variability with responses, and the trend indicated that the antiscratch effect of terfenadine was dose related.
Role of histamine H2 receptors in histamine agonist-induced scratching
Dimaprit, a histamine H2 receptor agonist, did not induce dose-dependent scratching in mice (0.04–40 μmol, n=3, Figure 2d). The H2 antagonist cimetidine (20 mg kg−1 i.p.) did not significantly reduce histamine-induced scratching (P>0.05, n=6; data not shown).
Role of histamine H3 receptors in histamine agonist-induced scratching
Imetit, a histamine H3 and H4 receptor agonist, induced dose-dependent scratching in all six mice studied (Figure 2c). The maximal scratching was 69±23 bouts, with a mean apparent ED50 for imetit of 0.9 μmol.
Scratching induced by histamine (3 μmol i.d.) was significantly reduced after treatment with the H3/H4 antagonist thioperamide (20 mg kg−1 i.p.; P<0.05, n=6, Figure 4a). Saline vehicle (i.p.) did not significantly reduce histamine-induced scratching (P>0.05, n=6, Figure 4b).
Figure 4.
(a) Thioperamide (20 mg kg−1 i.p.) significantly reduced scratching induced by histamine (3 μmol, *P<0.05, Wilcoxon matched pairs test, n=6). (b) Saline vehicle did not significantly reduce scratching induced by histamine (P>0.05, Wilcoxon matched pairs test, n=6). (c) Thioperamide (20 mg kg−1 i.p.) pretreated mice showed a significantly lower scratching response to imetit (3 μmol, i.d.) in comparison with vehicle treated animals (*P<0.05, Mann–Whitney test, n=5 in each group). Thioperamide at lower doses (1, 3 or 10 mg kg−1) did not significantly reduce imetit-induced scratching (P>0.05, Mann–Whitney test, n=4 in each group) (V=vehicle for antagonist. Dashed line illustrates PBS-induced scratching).
Imetit-induced scratching (3 μmol i.d.) was significantly reduced in mice pretreated with thioperamide (20 mg kg−1 i.p.) in comparison with vehicle-treated animals (P<0.05, Figure 4c). Lower doses of thioperamide (1–10 mg kg−1) reduce the mean level of imetit-induced scratching, but due to variability in responses, the difference between means (versus saline) was not statistically significant (P>0.05) (Figure 5).
Figure 5.
Thioperamide (20 and 10 mg kg−1 i.p.) pretreated mice showed a significantly lower scratching response to clobenpropit (0.06 μmol, i.d.) in comparison with vehicle-treated animals (*P<0.05, Mann–Whitney test, n=6 in each group). Thioperamide at lower doses (1, or 3 mg kg−1) did not significantly reduce imetitinduced scratching (P>0.05, Mann–Whitney test, n=6 in each group) (V=vehicle for antagonist. Dashed line illustrates PBS induced scratching).
Role of histamine H4 receptors in histamine agonist-induced scratching
Clobenpropit, an H4 agonist and H3 antagonist, induced dose-dependent scratching in all 10 mice studied (Figure 2b). The maximal level of scratching was 144±20 bouts, with a mean apparent ED50 for clobenpropit of 0.05 μmol.
Clobenpropit-induced scratching (0.06 μmol, i.d.) was significantly lower in mice pretreated with the highest doses of thioperamide used (10 and 20 mg kg−1, i.p.) when compared to vehicle-treated mice (P<0.05, Figure 6).
Figure 6.
(a) Thioperamide (20 mg kg−1 i.p.) did not significantly reduce HTMT-induced scratching (0.2 μmol i.d.) in comparison to vehicle treated mice (P>0.05, Mann–Whitney test, n=6 in each group), whereas the H1 antagonist, terfenadine (20 mg kg−1 i.p.) did reduce HTMT-induced scratching (*P<0.05, Mann–Whitney test, n=6 in each group). (b) Mepyramine and terfenadine, sedating and nonsedating H1 receptor antagonists, respectively (20 mg kg−1 i.p.) did not significantly reduce clobenpropit-induced scratching (0.06 μmol i.d.) in comparison to vehicle-treated mice (P>0.05, Mann–Whitney test, n=6 in each group), whereas the H3/4 antagonist, thioperamide (20 mg kg−1 i.p.) did reduce clobenpropit-induced scratching (*P<0.05, Mann–Whitney test, n=6 in each group). Dashed line illustrates PBS-induced scratching.
H1versus H3/H4 receptor involvement in itch
The H1 agonist, HTMT (0.2 μmol. i.d.), induced scratching that was not significantly reduced by the H3/H4 receptor antagonist, thioperamide (20 mg kg−1, Figure 6a, P>0.05 versus vehicle), whereas it was by the H1 receptor antagonists, terfenadine and mepyramine (as described above).
The H4 agonist, clobenpropit (0.06 μmol i.d.), induced scratching that was not significantly reduced by either mepyramine (sedating), or terfenadine (nonsedating) H1 receptor antagonists (20 mg kg−1, Figure 6b, P>0.05 versus vehicle), whereas it was by the H3/H4 receptor antagonist thioperamide (see above).
Discussion
Results from this study indicate the involvement of histamine H4 receptors in the scratching (itch) evoked by histamine, in mice. The lack of effectiveness of classical H1 receptor antihistamines in alleviating many chronic pruritic conditions (Greaves, 1997) led to the focus of research for antipruritic drugs being shifted from histamine to other putative mediators. The present study suggests that histamine can generate itching in mice via H4 and possibly H3 receptors.
We confirmed that acutely administered histamine induces dose-dependent scratching when injected intradermally in BalbC mice (Inagaki et al., 2001), as it does in humans (Keele & Armstrong, 1964). It has previously been shown in mice that the dose of histamine required to induce scratching behaviour in this species is substantially higher (approximately 100 times) than that which evokes itch in humans (Kuraishi et al., 1995; Maekawa et al., 2000; Inagaki et al., 2001). This probably reflects species differences in the properties of histamine receptors, as has been shown to be the case for mice and man (Liu et al., 2001), but caution is needed when interpreting results from functional studies undertaken in mouse or man. The H1 antagonist, mepyramine, reduced histamine-induced scratching, as previously established in mice (Sugimoto et al., 1998) and humans (Hagermark et al., 1979). Some authors have suggested this may, at least in part, be due to the sedating properties of H1 antagonists rather than a direct peripheral action at pruritoceptors (Krause & Shuster, 1983). We have shown that i.d. injection of the histamine H1 receptor agonist HTMT induces acute scratching in mice, strongly suggesting the involvement of peripheral H1 receptors in itch. This action of HTMT was mediated via H1 receptors because mepyramine reduced agonist induced scratching. We also showed that a nonsedating H1 receptor antagonist, terfenadine, significantly reduced HTMT-induced scratching, thus suggesting that sedation is not responsible for the reduction in scratching observed following H1 receptor antagonists.
Further studies investigating the mechanism of histamine-induced scratching in other strains of mouse and in other species, including humans, would be desirable. In preliminary studies using ICR mice, we found that they were more sensitive by a factor of approximately 30 to histamine, in comparison with BalbC mice. Variability in individual responses meant that we were unable to obtain clear evidence from the ICR strain concerning the effects of agonists and antagonists selective for different histamine receptor subtypes.
We found no evidence for involvement of H2 receptors in the scratching evoked by histamine in the mice studied. The H2 receptor agonist, dimaprit, did not evoke scratching, and the H2 receptor antagonist, cimetidine, had no significant effect on histamine-induced scratching. This is consistent with evidence in the literature: although a combination of H1 and H2 histamine antagonists is sometimes used clinically for treating itch (Drake et al., 1994; Ring et al., 1999), H2 receptors are not thought to be crucial in histamine-evoked itch (Hagermark et al., 1979; Davies & Greaves, 1980).
The discovery of histamine H3 (Haaksma et al., 1990) and, more recently, H4 (Oda et al., 2000) receptors, has implications for histamine as a mediator of itch (Repka-Ramirez, 2003). The tissue distribution of both receptor subtypes indicates a potential role for them in the periphery: the H3 receptor affects histamine-mediated axonal transport in mouse dorsal root ganglia (Amano et al., 2001), whereas the H4 receptor is associated with immune responses, is preferentially expressed on leukocytes (Oda et al., 2000), and plays a role in mast cell chemotaxis (Hofstra et al., 2003). We found that the dual H3 and H4 receptor antagonist thioperamide significantly reduced histamine-induced scratching in mice. The nonselective H3 and H4 receptor agonist, imetit, evoked scratching that was reduced by thioperamide. In order to differentiate between histamine H3 and H4 mechanisms, we used clobenpropit, which is an H3 antagonist but an H4 agonist (Oda et al., 2000). Clobenpropit induced dose-dependent scratching in mice, which was antagonised by thioperamide, a finding that implicates a peripheral H4 receptor in scratching induced by i.d. histamine.
We conclude that distinct H1 and H4 receptor mechanisms are responsible for itching evoked by exogenous histamine, and this is supported by data showing that scratching induced by H1 agonist was not significantly reduced by the H3/H4 antagonist. Also, scratching evoked by an H4 agonist was not significantly reduced by sedating or nonsedating H1 receptor antagonists.
Although our data strongly suggests a role for H1 and H4 receptors in histamine-mediated scratching, we were unable to determine whether H3 receptors play a role. This is because imetit, thioperamide and clobenpropit act at multiple histamine receptor subtypes. The H3/H4 receptor agonist imetit induced less scratching than the H1 and H4 agonists, which could be because imetit has a low affinity for the murine H4 receptor, or it may be due to a combined H3 and H4 action. It has recently been reported that mepyramine binds to the H4 receptor; which may explain why H1 antihistamines can relieve itch in some clinical conditions, even if H1 receptors are not involved (Nguyen et al., 2001). Further studies using drugs with greater selectivity for histamine receptor subtypes, particularly selective H4 agonists and antagonists are required to distinguish between an H3 and/or an H4 mechanism, but based on the use of currently available drugs, we conclude that H4 as well as H1 receptors contribute to itch evoked by histamine in mice.
We cannot be certain that the responses to histamine receptor agonists that we observed result from direct actions on pruritoceptors (the most likely explanation), or indirect actions via mast cells, basophils, etc. (Ring & Thomas, 1989; Poli et al., 1994); electrophysiological studies on pruritoceptor afferents in mice and humans would help clarify this. Nevertheless, we believe the results from our functional studies provide sufficient evidence to warrant further studies on the involvement of H4 receptors in the physiology and pathophysiology of itch in both mouse and man.
Acknowledgments
We wish to thank the British Skin Foundation (PhD research studentship) and GlaxoSmithKline Consumer Healthcare for financial support.
Abbreviations
- HTMT
histamine-trifluoromethyl-toluidine
- H1
histamine H1 receptor
- H2
histamine H2 receptor
- H3
histamine H3 receptor
- H4
histamine H4 receptor
- i.d.
intradermal
- i.p.
intraperitoneal
- PBS
phosphate buffered saline
References
- AMANO R., HIRUMA H., NISHIDA S., KAWAKAMI T., SHIMIZU K. Inhibitory effect of histamine on axonal transport in cultured mouse dorsal root ganglion neurons. Neurosci. Res. 2001;41:201–206. doi: 10.1016/s0168-0102(01)00275-9. [DOI] [PubMed] [Google Scholar]
- DAVIES M.G., GREAVES M.W. Sensory responses of human skin to synthetic histamine analogues and histamine. Br. J. Clin. Pharmacol. 1980;9:461–465. [PMC free article] [PubMed] [Google Scholar]
- DRAKE L.A., FALLON J.D., SOBER A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. The Doxepin Study Group. J. Am. Acad. Dermatol. 1994;31:613–616. doi: 10.1016/s0190-9622(94)70225-x. [DOI] [PubMed] [Google Scholar]
- EKBLOM A. Some neurophysiological aspects of itch. Semin. Dermatol. 1995;14:262–270. doi: 10.1016/s1085-5629(05)80046-x. [DOI] [PubMed] [Google Scholar]
- GREAVES M.W. Anti-itch treatments: do they work. Skin Pharmacol. 1997;10:225–229. doi: 10.1159/000211509. [DOI] [PubMed] [Google Scholar]
- HAAKSMA E.E., LEURS R., TIMMERMAN H. Histamine receptors: subclasses and specific ligands. Pharmacol. Ther. 1990;47:73–104. doi: 10.1016/0163-7258(90)90046-5. [DOI] [PubMed] [Google Scholar]
- HAGERMARK O., STRANDBERG K., GRONNEBERG R. Effects of histamine receptor antagonists on histamine-induced responses in human skin. Acta Derm. Venereol. 1979;59:297–300. [PubMed] [Google Scholar]
- HOFSTRA C.L., DESAI P.J., THURMOND R.L., FUNG-LEUNG W.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., NAGAO M., IGETA K., KAWASAKI H., KIM J.F., NAGAI H. Scratching behavior in various strains of mice. Skin Pharmacol. Appl. Skin Physiol. 2001;14:87–96. doi: 10.1159/000056338. [DOI] [PubMed] [Google Scholar]
- KEELE C.A., ARMSTRONG D. Substances Producing Pain and Itch. London: E. Arnold; 1964. [Google Scholar]
- KRAUSE L., SHUSTER S. Mechanism of action of antipruritic drugs. Br. Med. J. (Clin Res Ed) 1983;287:1199–1200. doi: 10.1136/bmj.287.6400.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURAISHI Y., NAGASAWA T., HAYASHI K., SATOH M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- LAIDLAW A., FLECKNELL P., REES J.L. Production of acute and chronic itch with histamine and contact sensitizers in the mouse and guinea pig. Exp. Dermatol. 2002;11:285–291. doi: 10.1034/j.1600-0625.2002.110401.x. [DOI] [PubMed] [Google Scholar]
- LEWIS T. The Blood Vessels of the Human Skin and Their Responses. London: Shaw; 1927. [Google Scholar]
- LIU C., WILSON S.J., KUEI C., LOVENBERG T.W. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J. Pharmacol. Exp. Ther. 2001;299:121–130. [PubMed] [Google Scholar]
- MAEKAWA T., NOJIMA H., KURAISHI Y. Itch-associated responses of afferent nerve innervating the murine skin: different effects of histamine and serotonin in ICR and ddY mice. Jpn. J. Pharmacol. 2000;84:462–466. doi: 10.1254/jjp.84.462. [DOI] [PubMed] [Google Scholar]
- MCMAHON S.B., KOLTZENBURG M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- MONROE E.W., COHEN S.H., KALBFLEISCH J., SCHULZ C.I. Combined H1 and H2 antihistamine therapy in chronic urticaria. Arch. Dermatol. 1981;117:404–407. [PubMed] [Google Scholar]
- NGUYEN T., SHAPIRO D.A., GEORGE S.R., SETOLA V., LEE D.K., CHENG R., RAUSER L., LEE S.P., LYNCH K.R., ROTH B.L., O'DOWD B.F. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- ODA T., MORIKAWA N., SAITO Y., MASUHO Y., MATSUMOTO S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- POLI E., TODOROV S., POZZOLI C., BERTACCINI G. Presynaptic histamine H2 receptors modulate the sympathetic nerve transmission in the isolated rat vas deferens; no role for H3-receptors. Agents Actions. 1994;42:95–100. doi: 10.1007/BF01983472. [DOI] [PubMed] [Google Scholar]
- REPKA-RAMIREZ M.S. New concepts of histamine receptors and actions. Curr. Allergy Asthma Rep. 2003;3:227–231. doi: 10.1007/s11882-003-0044-3. [DOI] [PubMed] [Google Scholar]
- RING J., BROCKOW K., OLLERT M., ENGST R. Antihistamines in urticaria. Clin. Exp. Allergy. 1999;29 Suppl 1:31–37. doi: 10.1046/j.1365-2222.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- RING J., THOMAS P. Histamine and atopic eczema. Acta Derm. Venereol. Suppl. (Stockh) 1989;144:70–77. doi: 10.2340/000155551447077. [DOI] [PubMed] [Google Scholar]
- SAVIN J.A. Do systemic antipruritic agents work. Br. J. Dermatol. 1980;103:113–118. doi: 10.1111/j.1365-2133.1980.tb15850.x. [DOI] [PubMed] [Google Scholar]
- SAVIN J.A., DOW R., HARLOW B.J., MASSEY H., YEE K.F. The effect of a new non-sedative H1-receptor antagonist (LN2974) on the itching and scratching of patients with atopic eczema. Clin. Exp. Dermatol. 1986;11:600–602. doi: 10.1111/j.1365-2230.1986.tb00515.x. [DOI] [PubMed] [Google Scholar]
- SHELLEY W.B., ARTHUR R.P. The neurohistology and neurophysiology of the itch sensation in man. Arch. Dermatol. 1957;76:296–323. doi: 10.1001/archderm.1957.01550210020004. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., UMAKOSHI K., NOJIRI N., KAMEI C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur. J. Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- YOSIPOVITCH G., GREAVES M.W., SCHMELZ M. Itch. Lancet. 2003;361:690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]