Abstract
The objective of this study was to characterize pharmacologically bradykinin (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg, BK) receptors in the canine prostate.
Primary cultures of canine prostate stromal (PS) and epithelial cells (PE) were established and then characterized using cell-specific antibodies (actin, vimentin and cytokeratin). Cultured cells were assayed for BK receptors using fluorometric imaging plate reader assays. In addition, isolated strips of the canine prostate were studied for BK-induced isometric contraction.
PS cells were labeled only with anti-actin and -vimentin antibodies, while the anti-cytokeratin antibodies labeled only the PE cells.
In cultured prostate cells, the BK receptor 2 (B2)-preferring agonist BK induced mobilization of intracellular Ca2+ in a concentration-dependent manner with potencies (log[EC50]∣PE, pEC50) of 8.72±0.12 in PS and 8.75±0.06 in PE cells. In contrast, the BK receptor 1 (B1)-selective agonist [des-Arg9]BK (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe) did not elicit any significant effect (pEC50<5) on Ca2+ responses.
BK agonism (10 nM) was inhibited by HOE-140 (D-arginyl-L-arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahhydro-3-isoquinolinecarbonyl-L-(2a,3b,7ab)-octahydro-1H-indole-2-carbonyl-L-arginine), a B2-selective antagonist, with a log[IC50] (pIC50) of 8.11±0.19 and 9.23±0.20 in PS and PE cells, respectively. [des-Arg10]HOE-140 (D-arginyl-L-arginlyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-L-(2a, 3b,7ab)-octahydro-1H-indole-2-carbonyl), a B1-selective antagonist, displayed weak antagonism with pIC50 values of 4.87±0.23 and 6.38±0.16 in PS and PE cells, respectively.
Isolated tissue strips of the canine prostate contracted to BK (10 μM) but not to [des-Arg9]BK (10 μM). BK-induced contractility was attenuated by HOE-140 (1 μM).
In conclusion, canine prostates express functional B2 receptors, with no apparent B1 receptor subtypes.
Keywords: Benign prostate hyperplasia, bradykinin, bradykinin receptor 2, dog, fluorometric imaging plate reader, immunohistochemistry, isolated prostate, prostate stromal cells, prostate epithelial cells
Introduction
Bradykinin ([Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg], BK), a product of the kallikrein–kinin system, is a nine amino acid biologically active peptide that modulates pain and inflammatory pathways (Campbell, 2001; Howl & Payne, 2003). BK signaling is mediated by its cognate BK receptors. Two subtypes of BK receptors, namely, BK receptor 1 (B1) and BK receptor 2 (B2), have been cloned and characterized (Hess et al., 1992;). These receptors are members of the seven transmembrane G-protein-coupled receptor superfamily and preferentially couple via Gαq type of G-proteins (Blaukat, 2003). BK is the selective agonist for the B2 receptor, while the B1 receptor exhibits greater selectivity for agonism by the cleaved product of BK, [des-Arg9]BK (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe) (Simpson et al., 2000). Both peptide and nonpeptide antagonists, with high selectivity and affinity, for either B1 or B2, have been discovered (Altamura et al., 1999; Wood et al., 2003). HOE-140 (D-arginyl-L-arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahhydro-3-isoquinolinecarbonyl-L-(2a,3b,7ab)-octahydro-1H-indole-2-carbonyl-L-arginine) and [des-Arg10] HOE-140 (D-arginyl-L-arginlyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-L-(2a, 3b,7ab)-octahydro-1H-indole-2-carbonyl) are potent and selective peptide antagonists for the B2 and B1 receptors, respectively, and have been used, in this study, to characterize the BK receptors in canine prostate.
The role of BK in the lower urinary tract is not yet fully understood, although BK contracted both the canine prostate (Steidle et al., 1990) and the human urinary bladder (Meini et al., 2000). The kallikrein–kinin system is functional in the prostate and in male seminal secretions (Schill & Miska, 1992; Charlesworth et al., 1999; Walden et al., 1999). Immunoreactive kinins were detected in the transition zone of prostates from benign prostate hyperplasia (BPH) patients (Walden et al., 1999) and kallikreins are being evaluated as biomarkers of BPH (Scorilas et al., 2003). Prostatic cultures from BPH tissues were shown to express higher levels of B2 and B1 receptors in stromal and epithelial compartments, respectively (Walden et al., 1999). In diseased prostates, stromal B2 receptors were involved in cell growth and were speculated to contribute to the pathophysiology of BPH (Walden et al., 1999). Therefore, BK may activate cell growth and contribute to increased tone of the prostate leading to hyperplasia with increased outlet resistance around the prostatic urethra. BK was also implicated in the mitogenesis of human prostate cancer cell lines (Barki-Harrington & Daaka, 2001).
Rats or dogs may be considered useful models to study the role of BK in prostate pathophysiology, as BK contracted the isolated prostate from both species (Steidle et al., 1990; Watts & Cohen, 1991). Dogs offer a better in vivo model to study prostatic cancer since dogs, but not rats, develop spontaneous prostate cancers with clinical and biological outcomes identical to that observed in man (Andrawiss et al., 1999). Further, dogs also often develop age-related BPH-like pathology, and canine prostate mimics a human prostate as the prostate from both species is encapsulated (Waters et al., 1998). Therefore, canine prostates may be more directly relevant experimental model to study the pathophysiology of BPH.
The main objective of this study was to characterize BK receptor subtypes in primary cultures of the canine prostate. We demonstrated the presence of functional B2 receptors in both canine prostate stromal (PS) and prostate epithelial (PE) cell types. In addition, our data also indicate that the B2 receptors mediated contraction of isolated tissue strips from the canine prostate. Therefore, the canine prostate may be an excellent surrogate model to study the role of B2 receptors in development or progression of BPH and/or prostate cancer.
Methods
Isolation and establishment of canine prostate-derived primary cultures
Whole prostate tissues (with distal urethra intact) from 18- to 24-month-old dogs (six dogs) were obtained from Marshall Farms U.S.A., Inc., (North Rose, NY, U.S.A.). The capsule was removed using sterile scalpels, and tissues (urethra excluded) were cut into small pieces and placed into separate Petri dishes. Tissues were then chopped into fine pieces and transferred to 50 ml conical tubes. Tissue pieces were then washed three times with phosphate-buffered saline (PBS) containing an antibiotic mixture with a 5 min centrifugation step (1700 × g) between each wash. PBS was then aspirated and then the pellets were resuspended in either stromal cell medium (RPMI-1640 with 10% serum, 25 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) and 1 × antibiotic–antimycotic cocktail) or epithelial cell medium (keratinocyte–SFM liquid with L-glutamine, 2.5 μg epidermal growth factor, 25 mg bovine pituitary extract and 1 × antibiotic–antimycotic cocktail). The stromal and epithelial cell media were chosen to culture selectively the stromal or epithelial cells, respectively, as described by Walden et al. (1999). Tissues in either the stromal or epithelial media were centrifuged, media were aspirated and the tissue pellets were resuspended in a collagenase solution (600U ml−1). The pellets were incubated in the collagenase solution for 3–4 h at 37°C with gentle shaking. After digestion with collagenase, cells were washed three times with PBS and one time with either the epithelial cell media or the stromal cell media. Cells were then resuspended in appropriate media (stromal or epithelial) for selection of PS and PE cells. PS cells were grown on cell culture treated T-75 cm2 flasks, while the PE cells were grown on collagen-coated T-75 cm2 flasks. Both PS and PE cells were maintained as monolayers in 95% CO2/5% O2 at 37°C. Cells were passaged every 3–4 days and the highest passage number used was 5.
Cell culture of hB2-CHO cells
hB2-CHO cells stably expressing the hB2 receptors were generated as described previously (Jarnagin et al., 1996). The cells were cultured in Ham F-12 media supplemented with 10% serum containing antibiotic/antimycotic cocktail. Cells were passaged every 3–4 days and the highest passage number used was 30.
Immunohistochemical characterization
PS and PE cells were grown on six-well dishes containing uncoated or collagen-coated sterile cover slips, respectively. Cells were fixed at −20°C for 10 min, in a 7 : 3 mixture of methanol : acetone. Nonspecific binding sites were blocked using 5% bovine serum albumin (BSA) for 30 min at 37°C. Cells were then incubated with antibodies (mouse-monoclonal) against smooth muscle actin (SMA), vimentin or cytokeratin (1 : 500 dilution in 5% BSA) for 1 h at room temperature. Cells were then washed six times with PBS containing 0.05% Triton-X with the last wash in the well for 30 min at 37°C. Cells were then incubated with a goat-anti-mouse secondary antibody conjugated to Alexa-488 nm (1 : 1000 dilution, 5% BSA) for 1 h at room temperature, followed by repeated PBS washes. The coverslip was then mounted onto slides using vectastain mounting media with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) and sealed with cytoseal. To evaluate the specificity of the staining, secondary only control conditions were performed for each experiment. The labeling was viewed with a Nikon Microphot 5A microscope and images acquired using an attached digital camera (Diagnostic Imaging Inc., Sterling Heights, MI, U.S.A.).
Measurement of intracellular [Ca2+] using the fluorometric imaging plate reader (FLIPR)
Intracellular calcium flux was measured by FLIPR as described by Sullivan et al. (1999). hB2-CHO cells and PS cells were seeded into black-walled clear-bottom 96-well plates (Corning, NY, U.S.A.) at a density of 25,000 cells well−1. PE cells were seeded into collagen-1-coated black-walled clear-bottom 96-well plates (BD Biosciences, Bedford, MA, U.S.A.) at a density of 25,000 cells well−1. All the cells were grown in appropriate supplemented media as described previously and cultured overnight. The cells were then washed with FLIPR buffer (Hanks-buffered saline solution with 10 mM HEPES, 2.5 mM probenecid, 2 mM CaCl2 and 0.1% BSA). FLIPR buffer containing Fluo-3 AM (4 μM; Teflabs, Austin, TX, U.S.A.) was then added to the cells. The cells were loaded with the dye for 1 h at 37°C in 5% CO2. Cells were then washed in FLIPR buffer, before being incubated for 30 min at 25°C with either buffer alone (vehicle) or buffer containing various antagonists. The plates were then placed in the FLIPR device (Molecular Devices, CA, U.S.A.) and relative changes in fluorescence upon addition of various agonists were monitored (λex=488 nm, λem=540 nm).
Isolated canine prostate contraction assay
Isometric contraction was studied in canine prostate strips as described previously (Normandin & Lodge, 1996). Briefly, prostates were removed and dissected in Krebs' buffer. After removal of adherent connective tissue and capsular layer, transverse strips of prostate around the urethra were dissected out. Tissue strips (10–15 mm long, 3 mm wide, 3 mm thick) were mounted in thermostatically controlled (37°C) organ baths (10 ml) containing Krebs' buffer, gassed continuously with 95% O2 and 5% CO2. The composition of the buffer was (mM): NaCl 118.2, KCl 4.6, CaCl2·2H2O 1.6, KH2PO4 1.2, MgSO4 1.2, dextrose 10 and NaHCO3 24.8. Prostate strips were equilibrated at a resting tension of 5 mN for 1 h with 10 min interval washing. Tissues were primed with BK (10 μM), contraction measured over 5 min, to determine if the tissues were viable and responded to BK. Tissues were then washed three times, in 10 min intervals, and incubated with either HOE-140 (1 μM) or vehicle for 30 min. BK (10 μM) was then added to all baths. Some tissues were restimulated with [des-Arg9]BK (10 μM) or vehicle (H2O). Changes in isometric force were measured from Grass FTO3c transducers and digitized using IOX data-acquisition software (EMKA Tech., France).
Materials
BK, [des-Arg9]BK, HOE-140, [des-Arg10]HOE 140, probenecid, N-(α-rhamnopyranosyloxyhydroxyphosphinyl)-Leu-Trp disodium salt (phosphoramidon), N-[(S)-3-mercapto-2-methylpropionyl]-L-proline (captopril) and BSA were from Sigma-Aldrich Corp., (St Louis, MO, U.S.A.). Antibodies against SMA, vimentin, high-molecular-weight cytokeratin were from DAKO Corp. (Carpenteria, CA, U.S.A.), while the Alexa-488 nm anti-mouse secondary antibody was from Molecular Probes (Eugene, OR, U.S.A.). Vectastain mounting media with DAPI (Vector Labs, Burlingame, CA, U.S.A.), cytoseal (Richard-Allan Scientific, Kalamazoo, MI, U.S.A.), Triton X-100 (VWR Scientific, West Chester, PA, U.S.A.) and Collagenase-1 (Atlanta Biologicals, Norcross, GA, U.S.A.) were also used. All other cell culture media and reagents were from Invitrogen Corp. (Carlsbad, CA, U.S.A.).
Data analysis
Data obtained from the FLIPR assay were collected as maximum fluorescence intensity (spatial uniformity correction was on to normalize for cell number). The data were then expressed as a percentage of appropriate concentration of BK. Nonlinear regression analysis was performed on concentration–response curves and inhibition curves (Graph Pad Software Inc., San Diego, CA, U.S.A.). Affinity estimates were calculated using the Cheng–Prusoff equation from log[IC50] (pIC50) values (Cheng & Prusoff, 1973). The data from the tissue contraction studies were normalized to prime (first addition) of individual tissues with 10 μM BK. Statistical significance, at 95% confidence, was determined using Student's t-test.
Results
Immunohistochemical characterization of canine prostate-derived primary cultures
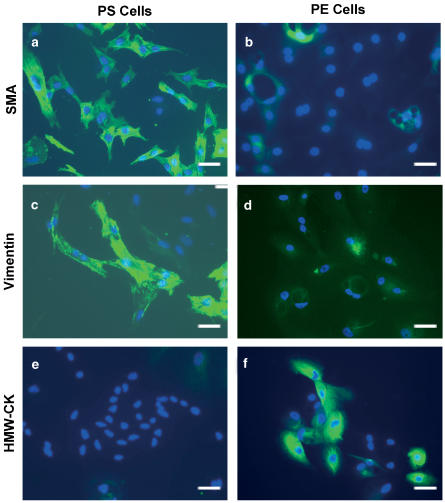
Cells cultured from canine prostates were first characterized immunohistochemically to validate our selection criteria for PS and PE cells (Figure 1). Antibodies against SMA, vimentin or high-molecular-weight cytokeratin (HMW-CK) were used as SMA or vimentin are expressed in smooth muscle cells or fibroblasts, while cytokeratins are specific to epithelial cells (Kooistra et al., 1995). PS cells were specifically labeled with SMA (Figure 1a) and vimentin (Figure 1c). In contrast, PE cells did not stain with antibodies to actin and vimentin, although nuclear staining with DAPI (blue) clearly indicated the presence of cells in the field of view (Figure 1b and d). These results suggest that PS cells in culture were predominantly fibromuscular cells. PE cells, but not PS cells, labeled for HMW-CK (Figure 1e and f) and were therefore predominantly epithelial cells.
Figure 1.
Primary cultures of canine PS and PE cells. PS (a, c, e) and PE (b, d, f) cells were labeled with SMA, vimentin or HMW-CK. Nuclear staining is indicated by the blue DAPI labeling. Data shown were taken at × 20 magnification and is representative of three independent experiments. The horizontal bar represents 5 μm.
BK elicited concentration-dependent Ca2+ mobilization
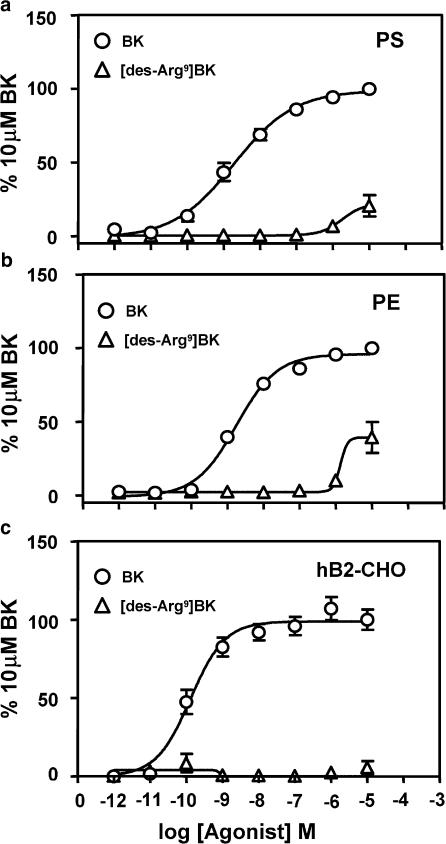
The next study was established to address functional presence of BK receptors in canine PS and PE cells. Although BK activates many intracellular signaling cascades, BK is classically known to activate the inositolphosphate pathway, and thereby mobilize intracellular Ca2+ (Blaukat, 2003). We therefore assessed BK-receptor-mediated activation of the inositolphosphate pathway using the FLIPR assay (see Methods). BK mobilized intracellular Ca2+ in a concentration-dependent manner in both PS and PE cells (Figure 2). Potency (log[EC50]∣PE, pEC50) of BK in the PS and PE cells was 8.72±0.12 and 8.75±0.06, respectively (Figure 1a and b; Table 1). The B1-selective agonist, [des-Arg9]BK did not elicit concentration-dependent responses in either cell type except at high concentrations (10 μM). These findings are consistent with that seen in the hB2-CHO cells as BK potently (9.88±0.10) mobilized Ca2+, while [des-Arg9]BK failed to elicit any significant effect (Figure 1c). These results indicate the presence of functional B2 receptors, and not the B1 subtype, in canine PS and PE cells.
Figure 2.
BK-induced increases in intracellular calcium in canine PS, canine PE and hB2-CHO cells. BK increased intracellular calcium in PS, PE and hB2-CHO cells (a, b and c, respectively) in a concentration-dependent manner. Responses were measured as changes in fluorescence and expressed relative to 10 μM BK. Points represent mean values and vertical lines indicate standard errors of the mean from three to seven independent experiments.
Table 1.
Potency and affinity values of BK ligands in canine PS and PE cells (FLIPR assay)
| PS cells | PE cells | |||||
|---|---|---|---|---|---|---|
| pEC50 | pIC50 | pKi | pEC50 | pIC50 | pKi | |
| BK | 8.72±0.12 | ND | ND | 8.75±0.06 | ND | ND |
| [des-Arg9]BK | <5.0 | ND | ND | <5.0 | ND | ND |
| HOE-140 | ND | 8.11±0.19 | ∼8.9* | ND | 9.23±0.2 | ∼10.0* |
| [des-Arg10]HOE-140 | ND | 4.87±0.23 | ∼5.6* | ND | 6.38±0.16 | ∼7.2* |
ND: not determined;
: nonequilibrium condition.
Pharmacological characterization of the BK receptor subtype in PS and PE cells using selective B2 and B1 antagonists
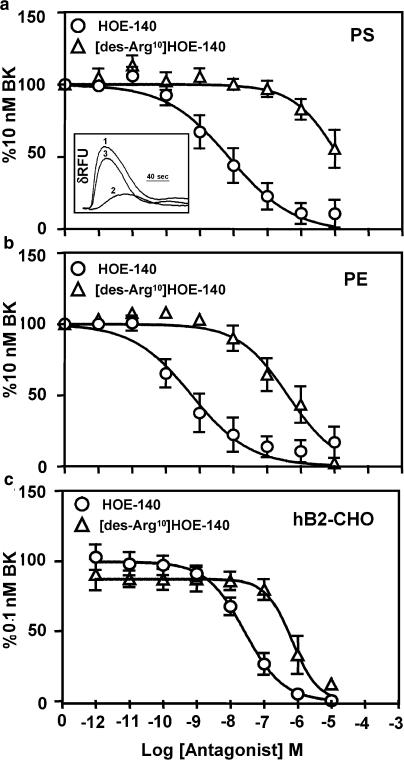
In order to confirm the expression of functional B2 receptors and absence of B1 receptors in PS and PE cells, we used increasing concentrations of the relatively selective B2 and B1 peptide antagonists, HOE-140 and [des-Arg10]HOE-140, to inhibit BK (10 nM)-induced calcium fluxes (Figure 3a and b). Representative FLIPR traces displaying the kinetics of intracellular Ca2+ changes are depicted as an inset to Figure 3a. The trace indicates changes in relative fluorescence units (δRFU) over time when PS cells were treated with 10 nM BK either in the presence of vehicle (1), or in the presence of 10 nM HOE-140 (2) or in the presence of 10 nM [des-Arg10]HOE-140 (3). Similar raw traces were obtained with PE and hB2-CHO cells (data not shown).
Figure 3.
Antagonism of BK-induced calcium mobilization in canine PS, canine PE and hB2-CHO cells. Representative FLIPR traces, from PS cells (similar for PE and hB2-CHO cells), displaying the kinetics of BK (10 nM)-induced intracellular Ca2+ mobilization measured as changes in δRFU (trace 1) in the presence of 10 nM HOE-140 (trace 2) or 10 nM [des-Arg10]HOE-140 (trace 3) are shown as an inset to Figure 3a. Increasing concentrations of HOE-140 and [des-Arg10]HOE-140 were used to antagonize BK-induced FLIPR responses in canine PS (a), canine PE (b) and hB2-CHO (c) cells. Responses were normalized to 10 nM BK (a and b) or 0.1 nM BK (c). Points represent mean values and vertical lines indicate standard errors of the mean from four to six independent experiments.
In PS cells, increasing concentrations of HOE-140 inhibited BK-induced calcium mobilization with a potency (pIC50) of 8.11±0.19. In contrast, the B1-selective antagonist [des-Arg10]HOE-140 was 2000 times less potent (pIC50∼4.87±0.23) than the B2-selective HOE-140 in PS cells (Figure 3a; Table 1). Likewise, in the PE cells, HOE-140 and [des-Arg10]HOE-140 inhibited BK-induced agonism, consistent with B2 receptor pharmacology (Figure 3b). Potency (pIC50) of HOE-140 and [des-Arg10]HOE-140 in PE cells was 9.23±0.20 and 6.38±0.16, respectively. Interestingly, the selectivity of HOE-140 in canine prostate was significantly more pronounced than that observed in a recombinant system overexpressing the human B2 receptor (Figure 3c; Table 2). The B2 antagonist HOE-140 displayed weak inhibition (pIC50∼7.57±0.13) of BK-induced Ca2+ changes at the human B2 receptor (Figure 3c). Interestingly, [des-Arg10] HOE-140 also inhibited BK (0.1 nM) agonism with a significant affinity estimate at the human B2 receptor (Table 2).
Table 2.
Comparative pharmacology of canine and human BK receptors (FLIPR assay)
| HOE-140 | [des-Arg10]HOE-140 | |||||
|---|---|---|---|---|---|---|
| ∼pIC50 | ∼pKi | Ref. | ∼pIC50 | ∼pKi | Ref. | |
| Canine PS cells | 8.1 | 8.9 | Figure 3 | 4.9 | 5.6 | Figure 3 |
| Canine PE cells | 9.2 | 10.0 | Figure 3 | 6.4 | 7.2 | Figure 3 |
| Canine B1 clone | ND | ND | 7.0 | 7.7 | * | |
| Canine B2 clone | 8.2 | 8.8 | * | <6.0 | ND | * |
| Human B1 clone | 5.5 | 5.8 | ** | 6.7 | 7.0 | ** |
| Human B2 clone | 7.1 | 7.5 | ** | <5.5 | ND | ** |
| Human B2 clone | 7.6 | 7.8 | Figure 3 | 6.2 | 6.4 | Figure 3 |
ND: not determined;
: Hess et al. (2001);
: Simpson et al. (2000).
B2 receptor mediated smooth muscle contraction of the canine prostate
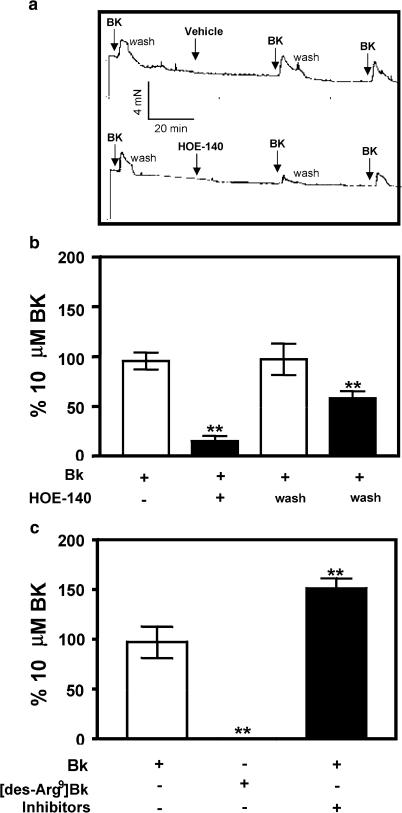
We measured isometric contraction of canine prostate strips to BK (10 μM) in the absence or presence of 1 μM B2 antagonist, HOE-140 (Figure 4a and b). Shown in Figure 4a is a representative trace obtained while studying the effects of HOE-140 on inhibiting BK-induced contraction of a canine prostatic strip. There was no tachyphylaxis with 10 μM BK, as the vehicle control did not show any significant differences in BK-induced contraction 1 h after the priming contraction (Figure 4a and b). HOE-140 (1 μM) inhibited BK-mediated contraction of the tissues. The effect of the B2 antagonist was reversible upon washout (wash), as BK induced significant prostate contraction, although the function was not recovered completely as compared to the time control (black and white bars; Figure 4b). On the contrary, the B1 receptor agonist, [des-Arg9]BK (10 μM) did not elicit any significant contraction (Figure 4c), indicating that the receptor mediating the effect in the canine prostate was B2 and not B1, consistent with our data on PS and PE cells in culture. We also incubated the prostate strips that failed to contract to [des-Arg9]BK with phosphoramidon and captopril (10 μM), protease inhibitor and angiotensin-converting enzyme inhibitor, respectively, for 15 min and then challenged the tissues with BK (10 μM). As depicted in Figure 4c, BK-mediated contraction was potentiated, indicating (a) the presence of endogenous proteases or angiotensin-converting enzyme, in the canine prostate, which may modulate BK levels and subsequent signaling; (b) failure of the tissues to contract to [des-Arg9]BK was not due to a unviable prostate strip.
Figure 4.
B2 receptor-mediated contraction of the isolated canine prostate. Representative traces of BK (10 μM)-induced tissue contraction in the presence of vehicle or 1 μM HOE-140 (a). The first addition of BK is the priming of tissues followed by a second addition of BK in the presence or absence of HOE-140, and then the tissues were challenged again with BK after washout (third addition) to study reversibility of the antagonist (a). BK (10 μM)-induced contraction was blocked with pretreatment with HOE-140 (black bar) that was partially recovered after washout (wash) (b). The B1 agonist (10 μM) did not contract the prostate strips, while inhibition of proteases resulted in a stronger BK-induced contraction (c). Responses were normalized to 10 μM BK. The bars represent the mean values and the vertical lines indicate standard errors of the mean of 3 (c) or 4 (b) independent experiments. Data shown in (b) are pooled from 13 tissue strips, while all the data shown in (b) are pooled from 11 tissue strips. **Significant statistical difference (P<0.001) of the values represented, by the corresponding bar, when compared to BK-induced contraction in the same tissue, pre- and post-treatment.
Discussion and conclusions
In this study, primary cultures of dog PS and PE cells as well as prostate tissue were used to characterize pharmacologically the BK receptors present. Canine prostate cells in culture immunohistochemically and morphologically resembled the human prostate stromal and epithelial cells (Peehl & Sellers, 2000). Our data strongly suggest that the canine PS and PE cells express B2 receptors that, upon stimulation by BK, mobilize intracellular Ca2+. Further, the stimulation of B2 receptors by BK mediates smooth muscle contraction of the canine prostate.
Walden et al. (1999) characterized the BK receptor subtypes in primary cultures of the human prostate obtained from BPH patients. In their system, similar to the results we obtained in canine PS cells, the human PS cells expressed functional B2 receptors. Interestingly, Walden et al. (1999) demonstrated (binding) that in the membranes of human PE cells, B1 receptors predominated, although functional data were not obtained. Their observation on B1 expression in human PE cells is fundamentally different from our finding that canine PE cells functionally express only B2 receptors. It may be possible that canine PE cells, like the human PE cells, express B1 receptors that do not efficiently couple to elicit changes in intracellular Ca2+. The possibility of the growth media used for culturing PE (and PS) cells in influencing the expression of the B1 receptors also exists and needs to be explored.
The first line of evidence demonstrating the presence of B2 receptors in the canine prostate was obtained from FLIPR studies performed on PS and PE cells. The potency of BK in canine PS and PE cells was 8.72±0.12 and 8.75±0.06, respectively (Figure 2; Table 1). The B1-selective agonist, [des-Arg9]BK, had no significant effect (pEC50<5), resulting in at least 5000-fold differences in potency between BK and [des-Arg9]BK. In a recombinant system, the potency of BK for the canine B2 receptor was 0.05 nM and that for the canine B1 receptor was >1 μM (Hess et al., 2001). On the other hand, the potency of [des-Arg9]BK for the recombinant canine B1 receptor was 8.7 nM and that for the B2 receptor was >10 μM (Hess et al., 2001). Hence, based on the rank order of potency for BK and [des-Arg9]BK in canine PS and PE cells, we believe that the cells express functional B2 receptors, with similar pharmacological profile as that of the human B2 receptors (Simpson et al., 2000). In CHO cells overexpressing the recombinant human B2 receptor, we demonstrated the potency of BK as 0.16 nM (Figure 2). The difference in potency of BK for the human B2 receptor observed in our hands and that by Simpson et al. (2000) may reflect differences in receptor reserve of B2 receptors in the CHO cells.
We then confirmed the presence of B2 receptors in canine PS and PE cells by using B2- and B1-selective peptide antagonists to inhibit BK-induced agonism (Figure 3). We did not observe any agonism caused by HOE-140 (data not shown), which is contrary to that reported in a cloned cell line expressing the B2 (Hess et al., 2001). The B2-selective antagonist, HOE-140, was more potent than the B1-selective antagonist, [des-Arg10]HOE-140, in inhibiting BK-induced Ca2+ mobilization in both PS and PE cells (Figure 3; Table 1). Although the FLIPR assay is not studied under true equilibrium conditions for estimation of affinity from antagonist potency (pIC50), we obtained an affinity (pKi) estimate (Cheng & Prusoff, 1973) of HOE-140 for B2 in canine PS cells of ∼8.9 (Tables 1 and 2). Hess et al. (2001) demonstrated an affinity (pKi) of HOE-140 for the canine B2 receptor to be ∼8.8 using similar assay conditions (Table 2). Hence, the canine PS cells expressed functional B2 receptors. Similarly, in PE cells, the rank order of HOE-140 and [des-Arg10]HOE-140 indicated expression of a B2 receptor subtype (Figure 3). Selectivity of HOE-140 over [des-Arg10]HOE-140 was less at the human B2 receptor (Figure 3c). Affinity estimate of HOE-140 for the human B2 receptor (Table 2), although in agreement with Simpson et al. (2000), was significantly less than that for the canine B2 receptor. This difference in the affinity of HOE-140 for canine B2 and human B2 receptors may indicate differences in the binding pocket of HOE-140 between the two species. It may also indicate a high-affinity site of HOE-140 present at the dog B2 receptor that is absent or masked in their human counterpart.
The second line of evidence confirming the presence of functional B2, and not B1 receptors, was performed on isolated tissue strips from canine prostate (Figure 4). In Figure 4b, we have shown that the contraction of the prostate strips elicited by stimulation with 10 μM BK was significantly attenuated by HOE-140. The B1 receptor agonist ([des-Arg10]HOE-140), on the other hand, did not cause any contraction of the canine prostate (Figure 4c). The potentiating effect of phosphoramidon and captopril on BK-induced contraction may suggest the presence of proteases or angiotensin-converting enzyme that regulate catabolism of BK. The individual effect of proteases or angiotensin-converting enzyme in the catabolism of BK in the canine prostate remains to be determined. Thus, using two independent approaches (FLIPR and isolated tissue contraction), the functional presence of B2 receptors in the canine prostate was established.
A role for kinins in the genitourinary system has been speculated upon due to the presence of prostate-specific antigen and other kallikreins (Fichtner et al., 1996; Monsees et al., 2003). Differences in the amounts of kallikreins in the prostate are being proposed as a marker to distinguish between normal, cancerous and benign prostatic hyperplasia (Scorilas et al., 2003). Significantly, kallikreins (and other proteases) themselves are now thought to bind directly to and activate the B2 receptor (Hecquet et al., 2000; Houle et al., 2003). We believe that studying the actions of kallikreins on B2 receptors present in the canine PS and PE cells would offer crucial mechanistic insight into the possible contributions of kallikreins in regulating or promoting diseases of the prostate. Further, as mentioned before, Walden et al. (1999) have shown that in primary cultures of human PS cells BK induced cellular proliferation and may contribute to abnormal growth pathologies of the prostate. In PC-3 cells, a prostate cancer cell line, evidence for a role of kinin receptors in proliferation have also been established (Barki-Harrington & Daaka, 2001). Owing to the evidence available so far, it is necessary to investigate the putative pathophysiological role of B2 receptors in the prostate.
For studying the significance of B2 receptors, the dog prostate may offer significant advantages over other non-human models, as they spontaneously develop age-related pathologies of the prostate, such as seen in man. Although primary cultures of dog trachea and corneal epithelial cells have been cultured and shown to express functional B2 receptors (Luo et al., 1999; Huang et al., 2002), to our knowledge this is the first study characterizing the BK receptors in the dog prostate primary cultures. As the dogs used were 18–24 months old, the prostates were considered to be ‘normal'. Whether, there occurs an age-dependent change in expression and signaling of BK receptors in the PS and PE cells of dog prostates remains to be determined. In conclusion, the dog prostate offers a good surrogate model to study a role for B2 receptors in pathophysiologies associated with the prostate.
Acknowledgments
We acknowledge Dr Paul Walden and Dorene Marinese for their support during optimization of the canine prostate primary culture. We also thank Chinh Bach and Sharon Jiang for sequencing the recombinant human B2 receptor.
Abbreviations
- B1
bradykinin receptor 1
- B2
bradykinin receptor 2
- BK
bradykinin [Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg]
- BPH
benign prostate hyperplasia
- Ca2+
calcium
- DAPI
2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride
- [des-Arg9]BK
Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe
- [des-Arg10]HOE-140
D-arginyl-L-arginlyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-L-(2a, 3b,7ab)-octahydro-1H-indole-2-carbonyl
- FLIPR
fluorometric imaging plate reader
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
- HOE-140
D-arginyl-L-arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahhydro-3-isoquinolinecarbonyl-L-(2a,3b,7ab)-octahydro-1H-indole-2-carbonyl-L-arginine
- PBS
phosphate-buffered saline
- pEC50
-log[EC50]
- PE
prostate epithelium
- pIC50
-log[IC50]
- PS
prostate stroma
References
- ALTAMURA M., MEINI S., QUARTARA L., MAGGI C.A. Nonpeptide antagonists for kinin receptors. Regul. Peptides. 1999;80:13–26. doi: 10.1016/s0167-0115(99)00003-8. [DOI] [PubMed] [Google Scholar]
- ANDRAWISS M., OPOLON P., BENIHOUD K., DEVAUCHELLE P., DI FALCO N., VILLETTE J-M., KREMER E., SAULNIER P., BERTHON P., PERRICAUDET M., CUSSENOT O. Adenovirus-mediated gene transfer in dog prostate: a preclinical study of a relevant model system for gene therapy of human prostatic cancer. Prostate Cancer Prostatic Dis. 1999;2:25–35. doi: 10.1038/sj.pcan.4500278. [DOI] [PubMed] [Google Scholar]
- BARKI-HARRINGTON L., DAAKA Y. Bradykinin induced mitogenesis of androgen independent prostate cancer cells. J. Urol. 2001;165:2121–2125. doi: 10.1097/00005392-200106000-00081. [DOI] [PubMed] [Google Scholar]
- BLAUKAT A. Structure and signaling pathways of kinin receptors. Andrologia. 2003;35:17–23. doi: 10.1046/j.1439-0272.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- CAMPBELL D.J. The kallikrein–kinin system in humans. Clin. Exp. Pharmacol. Physiol. 2001;28:1060–1065. doi: 10.1046/j.1440-1681.2001.03564.x. [DOI] [PubMed] [Google Scholar]
- CHARLESWORTH C.M., YOUNG C.Y., MILLER V.A., TINDALL D.J. Kininogenase activity of prostate-derived human glandular kallikrein (hK2) purified from seminal fluid. J. Androl. 1999;20:220–229. [PubMed] [Google Scholar]
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of the inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- FICHTNER J., GRAVES H.C., THATCHER K., YEMOTO C., SHORTLIFFE L.M. Prostate specific antigen releases a kinin-like substance on proteolysis of seminal vesicle fluid that stimulates smooth muscle contraction. J. Urol. 1996;155:738–742. [PubMed] [Google Scholar]
- HECQUET C., TAN F., MARCIC B.M., ERDOS E.G. Human bradykinin B(2) receptor is activated by kallikrein and other serine proteases. Mol. Pharmacol. 2000;58:828–836. doi: 10.1124/mol.58.4.828. [DOI] [PubMed] [Google Scholar]
- HESS J.F., BORKOWSKI J.A., YOUNG G.S., STRADER C.D., RANSOM R.W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem. Biophys. Res. Commun. 1992;184:260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- HESS J.F., HEY P.J., CHEN T-B., O'BRIEN J., OMALLEY S.S., PETTIBONE D.J., CHANG R.S.L. Molecular cloning and pharmacological characterization of the canine B1 and B2 bradykinin receptors. Biol. Chem. 2001;382:123–129. doi: 10.1515/BC.2001.018. [DOI] [PubMed] [Google Scholar]
- HOULE S., MOLINARO G., ADAM A., MARCEAU F. Tissue kallikrein actions at the rabbit natural or recombinant kinin B2 receptors. Hypertension. 2003;41:611–617. doi: 10.1161/01.HYP.0000054971.03046.9B. [DOI] [PubMed] [Google Scholar]
- HOWL J., PAYNE S.J. Bradykinin receptors as a therapeutic target. Expert Opin. Ther. Targets. 2003;7:277–285. doi: 10.1517/14728222.7.2.277. [DOI] [PubMed] [Google Scholar]
- HUANG S.C.M., HSIAO L., CHIEN C., WANG C., CHIU C., TSAI R.J.F., YANG C. Characterization of bradykinin receptors in canine cultured corneal epithelial cells: pharmacological and functional studies. J. Biomed. Sci. 2002;9:213–222. doi: 10.1007/BF02256068. [DOI] [PubMed] [Google Scholar]
- JARNAGIN K., BHAKTA S., ZUPPAN P., YEE C., HO T., PHAN T., TAHILRAMANI R., PEASE J.H.B., MILLER A., FREEDMAN R. Mutations in the B2 bradykinin receptor reveal a different pattern of contacts for peptide agonists and peptide antagonists. J. Biol. Chem. 1996;271:28277–28286. doi: 10.1074/jbc.271.45.28277. [DOI] [PubMed] [Google Scholar]
- KOOISTRA A., ELISSEN N.M., KONIG J.J., VERMEY M., VAN DER KWAST T.H., ROMIJN J.C., SCHRODER F.H. Immunocytochemical characterization of explant cultures of human prostatic stromal cells. Prostate. 1995;27:42–49. doi: 10.1002/pros.2990270108. [DOI] [PubMed] [Google Scholar]
- LUO S.F., PAN S.L., WU W.B., WANG C.C., CHIU C.T., TSAI Y.J., YANG C.M. Bradykinin-induced phosphoinositide hydrolysis and Ca2+ mobilization in canine cultured tracheal epithelial cells. Br. J. Pharmacol. 1999;126:1341–1350. doi: 10.1038/sj.bjp.0702431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEINI S., PATACCHINI R., GIULIANI S., LAZZERI M., TURINI D., MAGGI C.A., LECCI A. Characterization of bradykinin B(2) receptor antagonists in human and rat urinary bladder. Eur. J. Pharmacol. 2000;388:177–182. doi: 10.1016/s0014-2999(99)00882-1. [DOI] [PubMed] [Google Scholar]
- MONSEES T.K., BLOCHER S., LODDO C., STEGER K., SCHILL W.B. Tissue kallikrein and bradykinin B2 receptors in the reproductive tract of the male rat. Andrologia. 2003;35:24–31. doi: 10.1046/j.1439-0272.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- NORMANDIN D.E., LODGE N.J. Pharmacological characterization of the isolated canine prostate. J. Urol. 1996;155:1758–1761. [PubMed] [Google Scholar]
- PEEHL D.M., SELLERS R.G. Cultured stromal cells: an in vitro model of prostate mesenchymal biology. Prostate. 2000;45:115–123. doi: 10.1002/1097-0045(20001001)45:2<115::aid-pros5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- SCHILL W.B., MISKA W. Possible effects of the kallikrein–kinin system on male reproductive functions. Andrologia. 1992;24:69–75. doi: 10.1111/j.1439-0272.1992.tb02613.x. [DOI] [PubMed] [Google Scholar]
- SCORILAS A., PLEBANI M., MAZZA S., BASSO D., SOOSAIPILLAI A.R., KATSAROS N., PAGANO F., DIAMANDIS E.P. Serum human glandular kallikrein (hK2) and insulin-like growth factor 1 (IGF-1) improve the discrimination between prostate cancer and benign prostatic hyperplasia in combination with total and %free PSA. Prostate. 2003;54:220–229. doi: 10.1002/pros.10186. [DOI] [PubMed] [Google Scholar]
- SIMPSON P.B., WOOLLACOTT A.J., HILL R.G., SEABROOK G.R. Functional characterization of bradykinin analogues on recombinant human bradykinin B1 and B2 receptors. Eur. J. Pharmacol. 2000;392:1–9. doi: 10.1016/s0014-2999(00)00046-7. [DOI] [PubMed] [Google Scholar]
- STEIDLE C.P., COHEN M.L., NEUBAUER B.L. Bradykinin-induced contractions of canine prostate and bladder: effect of angiotensin-converting enzyme inhibition. J. Urol. 1990;144:390–392. doi: 10.1016/s0022-5347(17)39467-3. [DOI] [PubMed] [Google Scholar]
- SULLIVAN E., TUCKER E.M., DALE I.L. Measurement of [Ca2+] using the fluorometric imaging plate reader (FLIPR) Methods Mol. Biol. 1999;114:125–133. doi: 10.1385/1-59259-250-3:125. [DOI] [PubMed] [Google Scholar]
- WALDEN P.D., LEFKOWITZ G.K., ITTMANN M., LEPOR H., MONACO M.E. Mitogenic activation of human prostate-derived fibromuscular stromal cells by bradykinin. Br. J. Pharmacol. 1999;127:220–226. doi: 10.1038/sj.bjp.0702492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERS D.J., SAKR W.A., HAYDEN D.W., LANG C.M., MCKINNEY L., MURPHY G.P., RADINSKY R., RAMONER R., RICHARDSON R.C., TINDALL D.J. Workgroup 4: spontaneous prostate carcinoma in dogs and nonhuman primates. Prostate. 1998;36:64–67. doi: 10.1002/(sici)1097-0045(19980615)36:1<64::aid-pros12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- WATTS S.W., COHEN M.L. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12:1057–1062. doi: 10.1016/0196-9781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- WOOD M.R., KIM J.J., HAN W., DORSEY B.D., HOMNICK C.F., DIPARDO T.M., MURPHY K.L., LIS E.V., RANSOM R.W., STUMP G.L., LYNCH J.J., O'MALLEY S.S., MILLER P.J., CHEN T.-B., HARRELL C.M., CHANG R.S.L., SANDHU P., ELLIS J.D., BONDISKEY P.J., BOCK M.G. Benzodiazepines as potent and selective bradykinin B1 antagonists. J. Med. Chem. 2003;46:1803–1806. doi: 10.1021/jm034020y. [DOI] [PubMed] [Google Scholar]