Abstract
Intracerebroventricular (i.c.v.) effects of bradykinin (BK) B1 and B2 receptor agonists and antagonists were assessed on mean arterial blood pressure (MAP) and heart rate (HR) in awake unrestrained spontaneously hypertensive rats (SHR, aged of 8 and 16 weeks) and age-matched Wistar Kyoto rats (WKY). Quantitative in vitro autoradiographic studies were also performed on the brain of both strains with specific radioligands for B2 receptors [125I]HPP-Hoe 140 and B1 receptors [125I]HPP-des-Arg10 and Hoe140.
MAP increased linearly with doses of BK (81–8100 pmol) and the amplitudes were significantly greater in SHR, particularly at 16 weeks. While BK evoked a negative linear trend on HR (bradycardia) in WKY, a positive one (tachycardia) was observed in adult SHR. In both strains, BK-induced pressor response was blocked by equimolar doses of B2 receptor antagonist, D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-BK (Hoe 140), but not by B1 receptor antagonist, AcLys[D-βNal7, Ile8]des-Arg9-BK (R-715).
B1 receptor agonists (Sar-[D-Phe8]-des-Arg9-BK, des-Arg9-BK, des-Arg10-Kallidin) and antagonist (R-715 alone or with Hoe 140) had no or marginal effect on MAP and HR at doses up to 8100 pmol in SHR and WKY.
Higher densities of specific [125I]HPP-Hoe 140 labelling were found in discrete brain areas of SHR, especially in regions associated with cardiovascular function. Low levels of [125I]HPP-[des-Arg10]-Hoe140 binding sites were seen in WKY and SHR, yet densities were significantly greater in midbrain and cortical regions of SHR aged of 16 weeks. Contrary to SHR, ageing caused a downregulation of B2 and B1 receptor binding sites in specific brain nuclei in WKY.
It is concluded that the hypersensitivity of the pressor response to i.c.v. BK in SHR occurs during both the early and established phases of hypertension in parallel with the enhancement of B2 receptor binding sites in various cardiovascular brain centres. In contrast, brain B1 receptors do not seem to participate in the central pressor effects of kinins nor in the maintenance of hypertension in SHR.
Keywords: Kinins, bradykinin, B1 and B2 receptors, brain, blood pressure, hypertension, autoradiography
Introduction
Kinins are known as vasoactive peptides released by kallikrein from kininogens during tissue injury and noxious stimulation. This family includes bradykinin (BK), kallidin (KD) and their active kininase I metabolites (des-Arg9-BK and des-Arg10-KD) whose biological effects are mediated by two transmembrane G-protein-coupled receptors named B1 and B2 (Regoli & Barabé, 1980; Marceau et al., 1998; Couture et al., 2001). Unlike B2 receptors, which are constitutively expressed in most tissues, B1 receptors are generally underexpressed in healthy animals and humans but induced and functionally expressed by cytokines, bacterial endotoxins and following tissue injury (Marceau et al., 1998).
All components of the kallikrein–kinin system have been identified within the central nervous system, and activation of B2 receptors exerts modulatory effects on arterial blood pressure through neuronal autonomic pathways (Couture & Lindsey, 2000). In comparison to normotensive Wistar Kyoto rat or WKY, spontaneously hypertensive rat (SHR) showed increased sensitivity to the pressor action of BK when injected into the lateral brain ventricle (Buñag & Takahashi, 1981; Lindsey et al., 1988), fourth ventricle (Lindsey et al., 1988; Martins et al., 1991), rostral ventrolateral medulla (Privitera et al., 1994) and thoracic spinal cord (Cloutier et al., 2002). Although acute and chronic B2 receptor blockade with antagonists injected centrally failed to alter systemic arterial blood pressure in SHR (Lindsey et al., 1988; Madeddu et al., 1990; 1994; Martins et al., 1991), evidence for a central tonic influence of kinins on blood pressure may be found in this model. Inhibition of kininase II, the major metabolic pathway for kinins, by intracerebroventricular (i.c.v.) injection of captopril caused a rise in blood pressure that was blocked by a B2 receptor antagonist, suggesting a prominent role for kininase II and endogenous kinins in SHR (Madeddu et al., 1990).
Data with regard to the functional relevance of B1 receptors in the brain of SHR are conflicting. Martins et al. (1991) reported that neither the B1 receptor agonist des-Arg9-BK nor the B1 receptor antagonist des-Arg9-Leu8-BK injected into the fourth ventricle altered blood pressure in SHR or WKY. In contrast, Alvarez et al. (1992) reported that the same antagonist injected into the lateral ventricle caused a long-lasting reduction in blood pressure and heart rate (HR) in SHR but not in WKY. Using a more stable B1 agonist (Sar-[D-Phe8]-des-Arg9-BK), Emanueli et al. (1999) reported that the i.c.v. activation of B1 receptors in SHR and WKY evokes increases in blood pressure, and that the peptidase-resistant B1 antagonist AcLys[D-βNal7, Ile8]des-Arg9-BK (R-715) lowers blood pressure in SHR. Moreover, i.c.v. injection of antisense oligodeoxynucleotides targeted to B1 receptor mRNA produced a profound blood pressure reduction that persisted more than 48 h in SHR (Emanueli et al., 1999).
A recent pharmacological and autoradiographic study conducted in 16-week-old SHR and WKY showed that the cardiovascular changes induced by the spinal injection of kinins are mediated exclusively by B2 receptor activation (Cloutier et al., 2002). B2 receptor binding sites were found to increase from the age of 8–16 weeks in the thoracic spinal cord of SHR. Conversely, B1 receptor binding sites were present in young (8 weeks) but not in old (16 and 24 weeks) SHR and WKY (Ongali et al., 2003a). Other differences between young and old SHR have been described such as the concentration of kinins (Khan et al., 1995) and kininase II activity (Israel & Saavedra, 1987) in the cerebrospinal fluid, which are, respectively, reduced and increased in adult SHR. Thus, ageing has a profound influence on the kinin system and may account for some discrepancies in the literature. Housing conditions in a pathogen-free environment is also a prerequisite if one intends to study the inducible B1 receptor as it is expressed in animals diagnosed with an established infection (Siebeck et al., 1998).
The present study was undertaken to re-evaluate the relative contribution of B1 and B2 receptors in the cardiovascular responses to brain kinins in SHR and age-matched WKY. This was achieved with: (i) a pharmacological approach following the i.c.v. injection of stable selective B1 and B2 receptor agonists and antagonists in 8-week-old SHR (early phase of hypertension) and 16-week-old SHR (established hypertension) and age-matched WKY; and (ii) an extensive quantitative analysis of the anatomical distribution of binding sites for both kinin receptors in the brain of SHR and WKY using in vitro autoradiography.
Methods
Animal source and care
Male SHR (8 and 16 weeks old) (n=40) and age-matched WKY (n=31) were purchased pathogen free at least 1 week prior to the experiments from Charles River, St Constant, Québec, Canada and Harlan, Indianapolis, IN, U.S.A. Equal number of matched WKY and SHR were purchased from the same source at each occasion. They were housed individually in plastic cages under a 12 h light–dark cycle in a room with controlled temperature (23°C) and humidity (50%) with food (Charles River Rodent) and tap water available ad libitum. The care of animals and research protocols were in compliance with the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of our University.
Surgery
Rats were anaesthetised with an intraperitoneal (i.p. ) injection of 65 mg kg−1 sodium pentobarbitone (Somnotol; MTC Pharmaceuticals, Cambridge, Ontario, Canada). The head of the rat was fixed to a stereotaxic apparatus (David Kopf Instrumentation, Tujunga, CA, U.S.A.), and one midline incision was made on the scalp. The angle of the head was adjusted according to the horizontal plan with respect to both bregma and lambda reference points. A hole was drilled in the skull according to stereotaxic coordinates: 8–week-old rats – 1.0 mm caudal to the bregma, 1.5 mm lateral to the midline, 4.5 mm vertical from the skull surface; 16-week-old rats – 0.6 mm caudal to the bregma, 1.3 mm lateral to the midline, 5 mm vertical from the skull surface. An i.c.v. cannula (PE-20; Fisher Scientific, Ontario, Canada) was inserted with a guide into the right lateral ventricle (i.c.v.) and fixed to the skull with dental cement (Reliance Dental MFG Co., Worth, IL, U.S.A.). Thereafter, the rats were allowed to recover in individual plastic cages (40 cm × 23 cm × 20 cm) and housed in the same controlled conditions. The correct position of the i.c.v. catheter was verified by post-mortem examination at the end of experiment.
After 5 days, rats were reanaesthetised with sodium pentobarbitone (65 mg kg−1, i.p.) and an intravascular siliconised (Sigmacote) PE-50 catheter, filled with physiological saline containing 100 IU ml−1 heparin sodium salt, was inserted into the abdominal aorta through the femoral artery for direct blood pressure recording and exteriorised at the back of the neck. Before both surgeries, the animals received the antibiotics trimethoprime and sulphadiazine (Tribissen 24%, 30 mg kg−1, s.c., Schering Canada Inc., Pointe Claire, Québec, Canada). Ketoprophen, an anti-inflammatory and analgesic drug, was given during the first surgery only (Anafen, 5 mg kg−1, s.c., Merial Canada Inc., Baie d'Urfé, Québec, Canada). Recovery from anaesthesia was monitored closely under a warming lamp to maintain the body temperature of animals. Thereafter, rats were housed individually in polyethylene cages with a top grid and returned to their resident room. Experimental protocols were initiated 24 h later, in awake and unrestrained rats.
Measurement of cardiovascular parameters
Blood pressure and HR were measured, respectively, with a Statham pressure Transducer (P23ID) and a cardiac tachometer (model 7P4) (triggered by the arterial blood pressure pulse) coupled to a Grass polygraph (model 79; Grass Instruments Co., Quincy, MA, U.S.A.). The cardiovascular response was measured 1 h after the rats were transported to the testing room. They remained in their resident cage, but the top grid was removed and they had no more access to the food and water during experiments (5–6 h). When resting blood pressure and HR were stable, rats received an i.c.v. injection of 1 μl artificial cerebrospinal fluid (aCSF).
Experimental protocols
Dose–response curves to i.c.v. agonists
SHR (8 and 16 weeks old) (n=7–9) and age-matched WKY (n=7–10) were used on 3 consecutive days. On days 1 and 2, rats initially received an i.c.v. injection of aCSF (1 μl) followed 15 min later by increasing doses of either BK (81, 202, 405, 810 and 8100 pmol) or the metabolically stable B1 receptor agonist Sar-[D-Phe8[-des-Arg9-BK (81, 810 and 8100 pmol) (Regoli et al., 1998) to construct a complete dose–response curve. Rats received only one of the two agonists at random on the same day. The two prototypic B1 receptor agonists des-Arg9-BK (8100 pmol) and des-Arg10-KD (8100 pmol) (Regoli et al., 1998) were injected following the highest dose of Sar-[D-Phe8]-des-Arg9-BK. On the third day, the highly selective and metabolically stable B1 receptor antagonist AcLys[D-βNal7, Ile8]des-Arg9-BK (R-715, 81, 810 and 8100 pmol) (Regoli et al., 1998) was administered. Increasing doses of B1 agonists and R-715 were given at 40–60 min intervals, while 60–90 min intervals were left between increasing doses of BK. No desensitisation of the cardiovascular response to BK was seen under these conditions in pilot experiments. Peptides were administered in a volume of 1 μl of vehicle followed by 4 μl volume of aCSF, which corresponds to the void volume of the catheter. Each dose was calculated per rat in 1 μl solution.
Effects of i.c.v. kinin receptor antagonists
SHR (8 and 16 weeks old) (n=6–7) and age-matched WKY (n=6–7) initially received 405 or 810 pmol BK, and 1 h after were given i.c.v. an equimolar dose of B2 receptor antagonist, D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-BK (Hoe 140), and then 3 min later was given the same dose of BK. The agonist was reinjected alone 24 h later to assess the reversibility of any blockade observed with the antagonist on the preceding day.
In another group of 16-week-old SHR (n=6), the B1 antagonist R-715 (8100 pmol) was tested i.c.v. 3 min prior to BK (405 pmol). The same group received 24 h later, R-715 (8100 pmol) and Hoe 140 (8100 pmol) together, and the effect on mean arterial blood pressure (MAP) and HR was continuously recorded for up to 24 h postinjection.
Tissue preparation for autoradiography
Autoradiographic studies were performed according to the procedures described previously (Cloutier et al., 2002; Ongali et al., 2003b). For this purpose, two groups of four WKY and two groups of four SHR (8 and 16 weeks old) not submitted to any surgery were used. After killing under carbon dioxide inhalation and decapitation, whole brains were immediately removed, frozen in 2-methyl butane cooled at −45 to −55°C with liquid nitrogen and then stored at −80°C until use. Matched whole brains of the same strain were mounted together (two brains per gelatine bloc) and serially cut into 20 μm thick coronal sections with a cryostat at temperature varying between −10 and −12°C. Adjacent sections were taken for experiments using B1 and B2 receptor ligands and alternatively thaw-mounted on 0.2% gelatine/0.033% chromium potassium sulphate-coated slides, and stored at −80°C. Sets of three slides were used for total binding and two sets for nonspecific binding. About 400 slides × three sections per slide were obtained per bloc × two blocs giving a total of 2400 sections per group of four WKY or SHR.
In vitro receptor autoradiography
On the day of experiments, sections were thawed at room temperature and preincubated for 30 s in 25 mM piperazine-N,N′-bis[2-ethanesulphonic-acid] (PIPES) buffer (pH 7.4; 4°C). Thereafter, slides were incubated for 90 min at room temperature in 25 mM PIPES buffer containing: 1 mM 1,10-phenanthroline, 1 mM dithiothreitol (DTT), 0.014% bacitracin, 0.1 mM captopril, 0.2% bovine serum albumin (BSA) (protease free) and 7.5 mM magnesium chloride in the presence of 150 pM [125I]HPP-desArg10-Hoe 140 (B1 receptor ligand) or 200 pM [125I]HPP-Hoe 140 (B2 receptor ligand). Peptides were iodinated by means of the chloramine T method (Hunter & Greenwood, 1962), and the specific activity of both ligands was calculated to be approximately 2000 c.p.m. fmol−1 or 1212 Ci mmol−1. The nonspecific binding was determined in the presence of 1 μM of unlabelled ligands (HPP-desArg10-Hoe 140 for B1 receptor and HPP-Hoe 140 for B2 receptor). To ascertain the specificity of the labelled B2 radioligand, the same concentration of unlabelled B1 ligand was added to the solution. Likewise, the same concentration of the unlabelled B2 ligand was added to the labelled B1 ligand solution. At the end of the incubation period, slides were transferred sequentially through four rinses of 4 min each in 25 mM PIPES (pH 7.4; 4°C), dipped for 15 s in distilled water (4°C) to remove the excess of salts and air-dried. Kodak Scientific Imaging Films BIOMAX™ MS® were juxtaposed onto the slides in the presence of [125I]-microscales and exposed at room temperature for 3 days (B1 ligand) or 2 days (B2 ligand). The films were developed in D-19 (Kodak developer) and fixed in Kodak Ektaflo. Densitometric readings were performed with an image analysis system (MCID™, Imaging Research Inc., Ontario, Canada). A standard curve from [125I]-microscales was used to convert density levels into fentomoles per milligram of tissue (fmol mg−1 tissue).
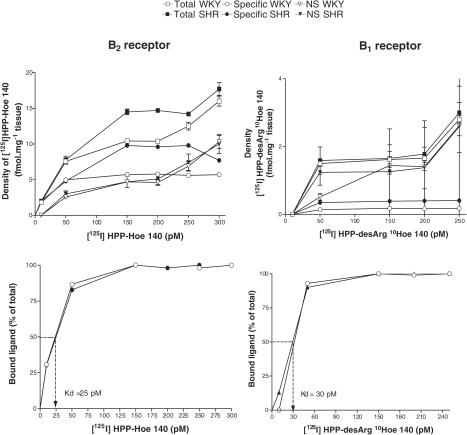
Concentrations of radioligands were chosen on the basis of previous studies (Murone et al., 1997; Cloutier et al., 2002; Ongali et al., 2003b) and correspond to maximal specific binding on the saturation curves (Bmax). The dissociation constant (Kd) was similar in SHR and WKY for B1 receptor (30 pM) and B2 receptor (25 pM) radioligands as shown in the spinal trigeminal nucleus (Figure 1). Hence, increases in the density of kinin receptor binding sites in SHR are likely due to increases in the number of receptors and not due to changes in receptor affinity. Moreover, the absolute nonspecific B1 and B2 receptor binding in SHR and WKY was the same, suggesting that the increase in specific binding (determined by subtracting nonspecific labelling from total binding) measured in SHR was due to a true increase of kinin receptor protein (Figure 1). Similar results were obtained in the other selected regions of the brain. Nonspecific binding defined in the presence of 1 μM unlabelled ligands accounted for about 50 and 40% of total binding for B1 and B2 receptors, respectively, and no differences in nonspecific binding were found between-rat groups in both strains and ages. The number of replicates analysed for each brain region was a minimum of three per animal. The anatomical structures and their nomenclature were taken from the atlas of Paxinos & Watson (1998).
Figure 1.
Amount of the B2 receptor radioligand [125I]HPP-Hoe 140 (left panels) and B1 receptor radioligand [125I]HPP-[des-Arg10]-Hoe140 (right panels) bound to the spinal trigeminal nucleus (SP5) of 8-week-old SHR (n=4) and age-matched WKY (n=4) as a function of their concentrations. Specific binding is calculated as the mathematical difference between total and nonspecific (NS) binding, which persists in the presence of 1 μM of unlabelled ligand (upper panels). The affinity of the binding, which is expressed as a dissociation constant (Kd), was calculated as the concentration of B2 or B1 radioligand that results in 50% of maximal specific binding (lower panels).
Drugs and solutions
The composition of aCSF was (in mM): NaCl 128.6, KCl 2.6, MgCl2 2.0 and CaCl2 1.4; pH adjusted to 7.2. BK (MW: 1060.3) and des-Arg9-BK (MW: 904.1) were purchased from Peninsula laboratories (San Carlos, CA, U.S.A.). Des-Arg10-KD (MW: 1032.2) was purchased from Bachem Bioscience Inc. (King of Prussia, PA, U.S.A.). Hoe 140 (1305.7), R-715 (1140.5) and Sar-[D-Phe8]-des-Arg9-BK (975.2) were obtained from Dr D. Regoli of Sherbrooke University (Sherbrooke, Québec, Canada). HPP-desArg10-Hoe 140 (3-4 hydroxyphenyl-propionyl-desArg9-D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK) and HPP-Hoe 140 (3-4 hydroxyphenyl-propionyl-D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK) were developed from the selective B1 receptor antagonist desArg10-Hoe 140 (Wirth et al., 1991) and the B2 receptor antagonist Hoe 140 or Icatibant (Hock et al., 1991), respectively. Bacitracin, BSA (protease free), captopril, DTT, heparin sodium salt (porcine, grade 1-A), magnesium chloride, PIPES, 1,10-phenanthroline and Sigmacote were purchased from Sigma-Aldrich Canada Ltd (Oakville, Ontario, Canada). Autoradiographic [125I]-microscales (20 μm) and Kodak Scientific imaging film BIOMAX™ MS (double coated, 24 cm × 30 cm) were purchased from Amersham Pharmacia Biotech Canada. Antagonists and agonists were dissolved directly in aCSF. The stock solutions (8100 pmol μl−1) of agonists and antagonists were stored in aliquots of 10 μl at −20°C until use.
Statistical analysis of data
Results are expressed as means±s.e.m. Statistical significance of differences were evaluated with Student's t-test on unpaired (between groups) or paired (within the same group) samples. Multiple comparisons were analysed with the analysis of variance (ANOVA). Data from MAP and HR were transformed in two variables: maximal values and area under the curve (AUC). These two variables were analysed for significance using a 2 × 2 × 4 factorial ANOVA with strains at two levels, age at two levels and doses at four levels. This analysis allows the between-rats evaluation of strain, age and their interaction, as well as the within-rats evaluation of BK kinetic effects and the mixed interactions of strain and age with BK kinetics. Data from autoradiographic receptor binding were analysed for significance using a 2 × 2 factorial ANOVA with strains at two levels and age at two levels. Differences in the number of rats per group were weighted using orthogonalised coefficients (Draper & Smith, 1981). For all variables, the critical level of significance was set at 5%.
Results
In vivo studies
Baseline MAP values in SHR were significantly higher at the age of 8 and 16 weeks, and particularly at 16 weeks old, when compared to age-matched WKY. However, baseline HR values did not differ significantly between strains or age. Moreover, the body weight was not significantly different between WKY and SHR at either age (Table 1).
Table 1.
Baseline values for MAP, HR and body weight in 8- and 16-week-old WKY and SHR
| Basal MAP (mm Hg) | Basal HR (beats min−1) | Body weight (g) | n | |
|---|---|---|---|---|
| 8 week-old | ||||
| WKY | 103±4 | 335±10 | 211±5 | 10 |
| SHR | 154±5*** | 354±14 | 190±7 | 14 |
| 16 week-old | ||||
| WKY | 116±5 | 298±17 | 321±5 | 13 |
| SHR | 166±4***,† | 342±8 | 315±4 | 18 |
Data are means±s.e.m. of n rats. Statistical comparison between SHR and age-matched WKY (*) or between 8- and 16-week-old SHR (†) is indicated by †P<0.05; ***P<0.001.
Effects of BK on MAP and HR in SHR and WKY
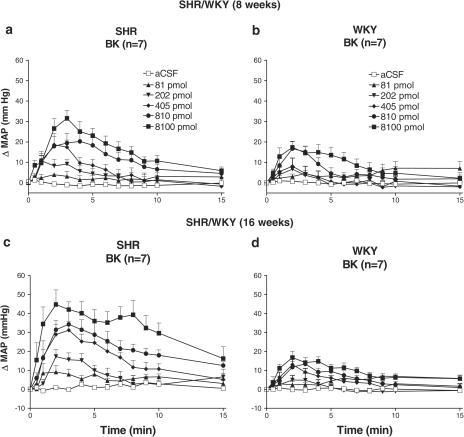
The effects of five increasing doses of BK on MAP in 8- and 16-week-old SHR and WKY are shown in Figure 2. BK (81–8100 pmol) evidenced dose- and time-dependent increases in MAP that peaked at 2–3 min postinjection. The ANOVA shows a significant effect of BK on maximal changes in MAP when compared to aCSF values for both WKY (F1,54=25.06) and SHR (F1,54=144.76) (Figure 3a). Maximal changes in MAP differed significantly between strains (F1,18=25.94) and ages (F1,18=8.51) and revealed strain by age interaction (F1,18=5.46). Whereas no age effect was found in WKY (F1,18=0.004), an age effect was found in SHR (F1,18=13.95). In WKY, maximal changes in MAP increased linearly with doses (F1,54=8.54) but much less than in SHR (F1,54=143.07). The 16-week-old SHR were more sensitive to BK than the 8-week-old SHR (F1,18=8.51). In WKY, no quadratic trend was observed (F1,54=0.09) but a weak quadratic trend was seen in SHR (F1,54=5.73). These qualitative differences between SHR and WKY in BK pressor responses led to a significant strain by dose interaction (F3,72=13.52).
Figure 2.
Time-course effects on changes in mean arterial blood pressure (ΔMAP) evoked by five increasing doses of BK injected i.c.v. in 8- and 16-week-old SHR (a and c) and age-matched WKY (b and d). Each point represents means±s.e.m. of seven rats.
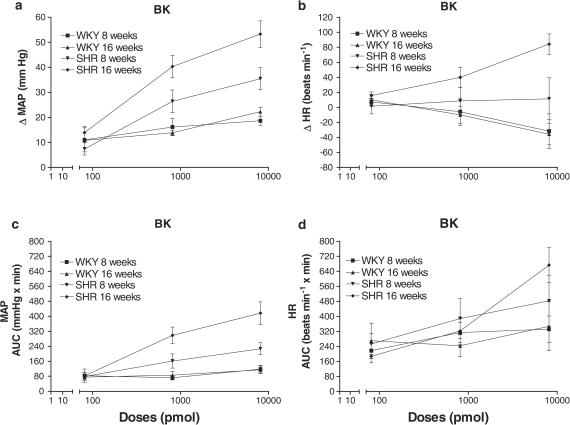
Figure 3.
Dose–response curves to BK on MAP (a and c) and HR (b and d). Shown are maximal changes (a and b) and AUC for a period of 15 min (c and d). Values represent means±s.e.m. of seven rats. aCSF values are: ΔMAP (mmHg): 2.0±1.2 (WKY, 8 weeks), 1.7±1.2 (WKY, 16 weeks), 1.8±0.9 (SHR, 8 weeks), 1.4±0.7 (SHR, 16 weeks); AUC MAP (mmHg × min): 42.1±18.7 (WKY, 8 weeks), 20.7±8.5 (WKY, 16 weeks), 25.5±12.5 (SHR, 8 weeks), 29.6±13.1 (SHR, 16 weeks); ΔHR (beats min−1): 4.3±5.3 (WKY, 8 weeks), −4.3±5.2 (WKY, 16 weeks), 4.3±9.7 (SHR, 8 weeks), −1.4±8.8 (SHR, 16 weeks); AUC HR (beats min−1 × min): 152.0±42.3 (WKY, 8 weeks), 121.2±46.3 (WKY, 16 weeks), 203.0±34.8 (SHR, 8 weeks), 190.6±55.0 (SHR, 16 weeks).
When MAP responses were expressed as AUC (Figure 3c), differences in strains (F1,18=22.16) and ages (F1,18=8.84) were observed with a significant strain by age interaction (F1,18=5.43).This interaction is explained by an absence of age effect in WKY and a significant one in SHR (F1,18=0.011; F1,18=14.25, respectively). Both a strain by dose (F3,72=9.78) and age per dose (F3,72=4.56) interactions were found. Pressor effects to BK were significant in both WKY (F1,54=6.66) and SHR (F1,54=68.77). However, the BK effect did not vary significantly from 81 to 8100 pmol in WKY (Flinear,54=2.86 and Fquad,54=0.92), while it increased dose dependently in SHR (Flinear,54=78.30 and Fquad,54=1.38).
As illustrated in Figure 3b, the maximal changes in HR induced by BK (81–8100 pmol) show a strain by age interaction (F1,24=6.09) since no changes were found between 8 and 16 weeks old in WKY (F1,24=0.13) but in SHR (F1,24=9.82), and because the differences between the two strains were observed only in the 16-week-old rat (F1,24=24.57). The maximal changes in HR induced by BK differed between the two strains leading to significant interactions with the comparison of BK to aCSF values (F1,96=5.45) and with the linear trend of BK (F1,96=18.25). Indeed, the pharmacological effect of BK was observed only in SHR (F1,72=5.27). A negative linear trend was observed in WKY (F1,72=9.93), while a positive linear trend was observed in SHR (F1,72=8.35).
When HR responses to BK were expressed as AUC (Figure 3d), SHR evidenced a significant increase in comparison to WKY (F1,24=7.77). BK induced in both strains a significant augmentation of the AUC (F1,72=12.73). However, this augmentation was dose linear dependent only in SHR (F1,72=18.98) leading to a strain by linear trend interaction (F1,96=5.10).
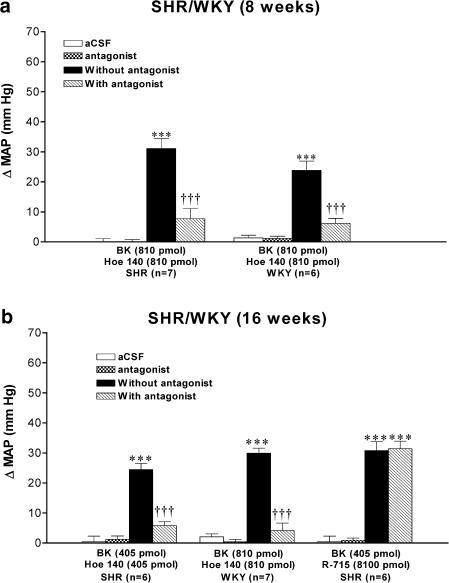
Effects of BK under B2 receptor blockade in SHR and WKY
The B2 receptor antagonist Hoe 140 was tested against the maximal MAP increase induced by 405 or 810 pmol BK in SHR and WKY. The MAP response evoked by BK was blocked by the prior i.c.v. injection of Hoe 140 (at equimolar dose with BK, 3 min earlier) in both strains at the age of 8 weeks (Figure 4a) and 16 weeks (Figure 4b). In all groups, the pressor response to BK was completely back to preantagonist values when the agonist was reinjected alone 24 h later (data not shown). Hoe 140 was devoid of any direct effect on MAP (Figure 4) and HR (data not shown).
Figure 4.
Maximal changes in mean arterial blood pressure (ΔMAP) induced by 405 or 810 pmol BK injected i.c.v. in 8-week-old (a) and 16-week-old (b) SHR and age-matched WKY without or with kinin antagonists (Hoe 140 or R-715). Data are means±s.e.m. of (n) rats. Statistical comparison to aCSF values (*) or to the agonist without Hoe 140 (†) is indicated by ***,††† P<0.001.
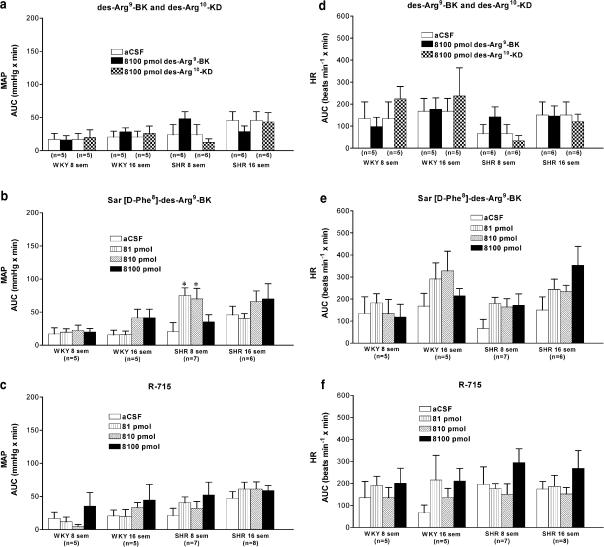
Lack of effect of B1 receptor antagonist in SHR and WKY
The pressor response to BK (405 pmol) was not affected by the prior i.c.v. injection of R-715 (8100 pmol) in 16-week-old SHR (Figure 4b). Moreover, i.c.v. injection of R-715 (81–8100 pmol) failed to alter MAP (Figure 5c) and HR (Figure 5f) for a period up to 24 h postinjection when compared to aCSF values in both strains and ages. Coadministration of Hoe 140 and R-715 (8100 pmol each) did not alter blood pressure for a period up to 24 h postinjection in SHR (data not shown).
Figure 5.
I.c.v. effects of kinin B1 receptor agonists (des-Arg9-BK, des-Arg10-KD, Sar-[D-Phe8]-des-Arg9-BK) and antagonist (R-715) on MAP (a–c) and HR (d–f) in 8- and 16-week-old SHR and age-matched WKY. Data represent means±s.e.m. of the AUC for a period of 15 min in (n) rats. Statistical comparison to aCSF (*) values is indicated by *P<0.05.
Lack of effect of B1 receptor agonists on MAP and HR in SHR and WKY
The cardiovascular effects of three B1 receptor agonists in 8- and 16-week-old SHR and WKY are shown in Figure 5. I.c.v. injection of des-Arg9-BK (8100 pmol) or des-Arg10-KD (8100 pmol) did not significantly alter MAP and HR when compared to aCSF values in SHR and WKY at both ages (Figure 5a and d). Whereas Sar-[D-Phe8]-des-Arg9-BK (81–8100 pmol) was inactive on MAP and HR in young and adult WKY, it evoked a small but significant effect on MAP at the two lowest doses in 8-week-old SHR. However, this agonist had no significant pressor effect in 16-week-old SHR (Figure 5b). Moreover, Sar-[D-Phe8]-des-Arg9-BK also failed to alter significantly HR in SHR at 8 and 16 weeks (Figure 5e).
Autoradiographic studies
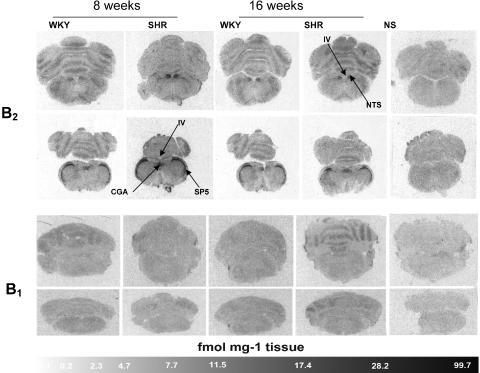
Autoradiographic distribution and density values of B1 and B2 receptors in WKY and SHR aged of 8 and 16 weeks are depicted in Figure 6 and in Tables 2 and 3, and are described herein.
Figure 6.
Autoradiographic distribution of kinin B1 and B2 receptors in the brain of 8- and 16-week-old WKY and SHR. Shown are autoradiograms representing total binding of [125I]HPP-desArg10-Hoe 140 (B1 receptor ligand) or [125I]HPP-Hoe 140 (B2 receptor ligand). Nonspecific (NS) binding in the presence of 1 μM of HPP-Hoe 140 (B2 receptors) and HPP-[des-Arg10]-Hoe 140 (B1 receptors) are also shown. Pictures are presented as obtained with the image analysis system. CGA, central gray (alpha part); SP5, spinal trigeminal tract; NTS, nucleus tractus solitarius; IV, fourth ventricle.
Table 2.
Densities of specific B2 receptor binding sites measured in the rat brain of 8- and 16-week-old WKY and SHR
| 8 week-old | 16 week-old | ||||
|---|---|---|---|---|---|
| WKY | SHR | WKY | SHR | ||
| Brain area | F1,12 | ||||
| Midbrain | |||||
| Thalamus | 2.95±0.29 | 2.06±0.25 | 1.48±0.39† | 2.97±0.64* | 7.92c |
| LDDM | 4.01±0.46 | 3.03±0.11 | 2.29±0.74 | 2.99±0.56 | 2.61 |
| LDVL | 3.94±0.64 | 3.09±0.22 | 2.20±0.76 | 3.15±0.79 | 1.93 |
| VPM | 3.31±0.39 | 2.43±0.22 | 1.71±0.38† | 3.05±0.75 | 5.45c |
| VPL | 3.05±0.46 | 2.58±0.11 | 1.94±0.34 | 3.04±0.78 | 2.58 |
| Hypothalamus | 3.01±0.25 | 2.78±0.27 | 1.95±0.61 | 3.63±0.40 * | 5.56c |
| DA | 3.07±0.34 | 2.26±0.11 | 1.86±0.58 | 4.60±0.62***,†† | 14.72c |
| VMH | 3.29±0.21 | 2.91±0.27 | 2.09±0.68 | 4.55±0.62**,† | 8.45c |
| Amygdalaa,b | 1.14±0.02 | 1.24±0.06 | 0.87±0.06 | 0.98±0.42 | 0.02 |
| Hippocampus | 1.01±0.03 | 1.65±0.12*** | 0.94±0.10 | 0.89±0.15††† | 10.14c |
| Hindbrain | |||||
| Colliculus | 2.09±0.12 | 2.43±0.21 | 0.67±0.38 | 3.29±0.98** | 4.48c |
| Subiculum | 2.53±0.14 | 2.61±0.26 | 1.29±0.49 | 2.62±0.91 | 1.37 |
| Pna,b | 1.91±0.13 | 2.98±0.34 | 0.45±0.26 | 1.04±0.46 | 0.56 |
| PDTga | 5.23±0.97 | 11.30±1.08 | 5.95±1.07 | 10.78±0.85 | 0.39 |
| CGAa | 3.33±0.41 | 4.88±0.58 | 3.88±1.10 | 7.71±0.93 | 2.01 |
| SCP | 4.67±1.07 | 3.74±0.94 | 2.24±0.88 | 6.56±0.43**,† | 9.26c |
| SP5a | 5.72±0.42 | 12.94±0.53 | 5.69±0.36 | 10.68±1.11 | 2.71 |
| PA5a,b | 4.23±0.47 | 9.21±0.64 | 1.78±0.54 | 5.23±0.99 | 1.22 |
| NTS | 3.24±0.30 | 3.60±0.72 | 0.79±0.24† | 4.17±1.14** | 4.66c |
| Pyt | 1.75±0.30 | 2.01±0.15 | 0.26±0.16† | 2.14±0.39*** | 9.26c |
| Amb | 2.09±0.39 | 1.72±0.91 | 0.22±0.19† | 2.94±0.52** | 7.45c |
Data are means±s.e.m. of four rats. Values represent densities measured in fmol mg−1 tissue.
Significant difference between WKY and SHR but not dependent on age.
Significant difference between 8 weeks and 16 weeks but not dependent on strains.
Significant differences between both strains and ages.
F-values showing strain and age interaction. In that case, significant differences are between SHR and age-matched WKY (*P< 0.05; **P< 0.01; ***P< 0.001) or between 8 and 16 weeks in each strain (†P<0.05; ††P<0.01; †††P<0.001).
Table 3.
Densities of specific B1 receptor binding sites measured in the rat brain of 8 and 16 week-old WKY and SHR
| 8 week-old | 16 week-old | ||||
|---|---|---|---|---|---|
| WKY | SHR | WKY | SHR | ||
| Brain area | F1,12 | ||||
| Cortical regions | |||||
| Parietal cortex | 0.41±0.21 | 0.52±0.19 | 0.07±0.02 | 1.35±0.33**,† | 7.29c |
| Occipital | 0.81±0.16 | 0.88±0.26 | 0.22±0.02† | 1.88±0.15**,††† | 21.25c |
| Perihinal/entorhinal | 0.33±0.19 | 0.64±0.22 | 0.16±0.01 | 1.57±0.22***,†† | 8.89c |
| Midbrain | |||||
| Thalamus | 0.37±0.16 | 0.44±0.17 | 0.08±0.02 | 0.87±0.17** | 5.98c |
| LDDM | 0.45±0.06 | 0.45±0.16 | 0.08±0.03 | 1.10±0.21***,†† | 14.02c |
| LDVL | 0.46±0.10 | 0.43±0.23 | 0.09±0.02 | 1.04±0.25**,† | 7.69c |
| VPMa | 0.44±0.19 | 0.56±0.21 | 0.08±0.02 | 0.76±0.17 | 2.89 |
| VPL | 0.44±0.19 | 0.41±0.07 | 0.14±0.07 | 0.82±0.17**,† | 7.41c |
| Hypothalamusa | 0.39±0.15 | 0.54±0.18 | 0.10±0.01 | 0.80±0.20 | 3.33 |
| DAa | 0.44±0.18 | 0.50±0.26 | 0.09±0.01 | 0.86±0.17 | 3.96 |
| VMHa | 0.30±0.08 | 0.44±0.18 | 0.13±0.02 | 0.83±0.20 | 4.08 |
| Amygdala | 0.52±0.20 | 0.45±0.16 | 0.14±0.01 | 1.90±0.30***,††† | 21.41c |
| Hippocampus | 0.34±0.10 | 0.54±0.16 | 0.17±0.04 | 1.35±0.29***,†† | 7.87c |
| Hindbrain | |||||
| Colliculus | 0.72±0.13 | 0.66±0.13 | 0.12±0.03†† | 0.65±0.15** | 5.93c |
| Subiculum | 0.73±0.07 | 0.76±0.12 | 0.15±0.01††† | 0.85±0.13*** | 12.31c |
| Pn | 0.65±0.15 | 0.26±0.11* | 0.02±0.01††† | 0.07±0.07 | 5.07c |
| PDTga,b | 0.29±0.02 | 0.41±0.09 | 0.13±0.01††† | 0.26±0.08 | 0.02 |
| CGA | 0.13±0.09 | 0.30±0.06 | 0.12±0.02 | 0.10±0.03 | 3.12 |
| SCP | 0.14±0.10 | 0.38±0.12 | 0.10±0.02 | 0.18±0.06 | 0.85 |
| SP5 | 0.17±0.11 | 0.39±0.16 | 0.18±0.03 | 0.22±0.12 | 0.57 |
| PA5 | 0.28±0.15 | 0.25±0.15 | 0.16±0.03 | 0.13±0.06 | 0.00 |
| NTS | 0.20±0.13 | 0.42±0.20 | 0.17±0.04 | 0.19±0.12 | 0.53 |
| Pyt | 0.18±0.16 | 0.12±0.09 | 0.08±0.02 | 0.10±0.02 | 0.17 |
| Amb | 0.08±0.06 | 0.30±0.18 | 0.17±0.04 | 0.18±0.14 | 0.74 |
Data are means±s.e.m. of four rats. Values represent densities measured in fmol mg−1 tissue.
Significant difference between WKY and SHR but not dependent on age.
Significant difference between 8 weeks and 16 weeks but not dependent on strains.
Significant differences between both strains and ages.
F-values showing strain and age interaction. In that case, significant differences are between SHR and age-matched WKY (*P< 0.05; **P< 0.01; ***P< 0.001) or between 8 and 16 weeks in each strain (†P<0.05; ††P<0.01; †††P<0.001).
B2 receptor distribution
Data show moderate levels of B2 receptor binding sites in several distinct brain regions of WKY. At the midbrain level, B2 receptor labelling (ranging between 1.0±0.0 and 4.0±0.5 fmol mg−1 tissue) was found in thalamic- and hypothalamic-related areas, such as laterodorsal thalamic nuclei (LDDM and LDVL), ventral posteromedial thalamic nuclei (VPM and VPL), whole hypothalamus, dorsal hypothalamic area (DA), ventromedial hypothalamic nucleus (VMH) as well as in amygdala and hippocampus. These values were significantly decreased in the thalamus, VPM and amygdala of 16-week-old WKY (Table 2). Greater density values (5.3±1.0 to 5.9±1.0 fmol mg−1 tissue) were seen in the hindbrain, including the posterodorsal tegmental nucleus (PDTg) and the spinal trigeminal tract (SP5) of 8- and 16-week-old WKY (Table 2). In some hindbrain areas such as the pontine nucleus (Pn), paratrigeminal nucleus (PA5), nucleus tractus solitarius (NTS), pyramidal tract (Pyt) and ambiguus nucleus (Amb), density values measured in WKY (8 weeks) were also significantly reduced in 16-week-old WKY (Table 2).
In SHR, the pattern of B2 receptor brain distribution was quite similar than that observed in WKY, although values in several structures were significantly higher in SHR. B2 receptor-specific labelling in SHR was markedly increased in several regions, including the amygdala, Pn, PDTg, central gray (alpha part) (CGA), SP5 and PA5 indistinctly of the age (Table 2). When compared to age-matched WKY, the density of B2 receptor binding sites was significantly augmented in the hippocampus of SHR at 8 weeks, and in thalamus, hypothalamus, DA, VMH, superior and inferior colliculus, superior cerebellar peduncle (SCP), NTS, Pyt and Amb of SHR at 16 weeks (Table 2). Moreover, adult SHR exhibited significant increase of B2 receptor density values in DA, VMH and SCP when compared to young SHR. In contrast, B2 receptor-specific labelling measured in hippocampus was decreased in older SHR (Table 2).
B1 receptor distribution
With respect to B1 receptors, low levels of specific binding sites were seen in both WKY and SHR of 8 and 16 weeks of age (ranging between 0.02±0. 01 and 1.90±0.30 fmol mg−1 tissue). In 8-week-old WKY, B1 receptor densities were notably higher than those measured in 16-week-old rats; significant differences were seen in structures including occipital cortex, colliculus, subiculum, Pn and PDTg (Figure 6, Table 3). B1 receptor densities differed significantly between WKY and SHR indistinctly of the age, and were higher in VPM, hypothalamus, DA, VMH and PDTg of SHR. When compared to age-matched WKY, B1 receptor densities were significantly enhanced (6–19-fold) in 16 weeks SHR in all cortical regions, thalamus, LDDM, LDVL, VPL, amygdala, hippocampus, colliculus and subiculum (Table 3). B1 receptor densities were also significantly greater in all cortical regions, LDDM, LDVL, VPL, amygdala and hippocampus of 16-week-old SHR when compared to 8 weeks SHR.
Discussion
In vivo studies
Our results are consistent with a number of studies which have reported increased pressor responses to BK administered into the lateral or fourth cerebral ventricles or in specific cardiovascular centres of the brain stem and thoracic spinal cord in SHR (Buñag & Takahashi, 1981; Lindsey et al., 1988; Martins et al., 1991; Privitera et al., 1994; Couture & Lindsey, 2000; Cloutier et al., 2002). However, the present study shows for the first time that the hypersensitivity to BK occurs during the early phase of hypertension and continues to develop thereafter. Our data with selective antagonists do not support, however, a contribution of endogenous kinins on cerebral B2 and B1 receptors in the maintenance of arterial hypertension in SHR nor in the tonic control of blood pressure in WKY.
Sympathetic hyperactivity has been consistently demonstrated in SHR (Takeda & Buñag, 1978; Buñag & Takeda, 1979; de Champlain, 1990) and seems to increase in older animals (Judy et al., 1979). As pressor effects induced by i.c.v. BK are partly mediated by the activation of the sympathetic nervous system (Buñag & Takahashi, 1981; Takahashi & Buñag, 1981; Qadri et al., 1999), it is likely that the greater BK-induced pressor responses derive at least partly from an exaggerated sympathetic tone in SHR. Changes in kininase II activity cannot provide an explanation for the hypersensitivity to BK since it was found higher in the CSF of adult SHR, suggesting a higher metabolic activity for kinins (Israel & Saavedra, 1987).
The present pharmacological study confirms earlier reports suggesting that central administration of BK and related peptides causes cardiovascular changes through the activation of B2 receptors in normotensive and hypertensive rats (for a review see Couture & Lindsey, 2000), although other studies suggested that B1 receptors may also play a role (Alvarez et al., 1992; Emanueli et al., 1999). Using the same pharmacological B1 receptor agonists and antagonists (and from the same supplier), we failed to reproduce the results of Emanueli et al. (1999), which showed pressor effects with B1 receptor agonists (including the stable agonist Sar-[D-Phe8]-des-Arg9-BK) and antihypertensive effect with the B1 receptor antagonists (R-715). This discrepancy cannot be explained by the age or the gender of rats as in both studies male were used and we took into consideration the early and established phases of hypertension in this model. However, the inducible behaviour of B1 receptors may explain these conflicting results. Indeed, this receptor is highly inducible by a wide range of externally applied stressors, including inflammatory cytokines (TNFα, IL-1β) and bacterial products (Marceau et al., 1998). It is worth mentioning that instrumentation with i.c.v. cannula induces localised and robust expression of TNFα and IL-1β mRNA in the tissue surrounding the lesion (Zhang & Rivest, 2001). Also, brain injury is known to activate the transcriptional nuclear factor-κB (NF-κB) (Nonaka et al., 1999; Pennypacker et al., 2000), which is involved in the induction of B1 receptors (Marceau et al., 1998; Couture et al., 2001). One should also pay attention to the housing condition of animals in a pathogen-controlled environment, as B1 receptors are associated with pre-existing infection (Siebeck et al., 1998). In our study, antibiotics and an anti-inflammatory analgesic (ketoprophen) were used before surgery to reduce infections and inflammation following trauma caused by surgery. Thus, utilisation of antibiotics and ketoprophen may have prevented the induction of B1 receptors by cytokines and bacterial agents in our colony of SHR and WKY, which in addition were housed in our animal facilities under controlled and well-identified viral contaminants.
Autoradiographic studies
Autoradiographic results demonstrate that B2 receptors are widely distributed throughout the brain of WKY. This widespread distribution of B2 receptors is consistent with other studies performed in the rat brain (Chen et al., 2000; Couture & Lindsey, 2000; Ongali et al., 2003b) and other animal species, including guinea-pig (Fujiwara et al., 1988; 1989; Privitera et al., 1991), sheep (Murone et al., 1997) and human (Buck et al., 2002). Taken together, these data reinforce the idea suggesting a physiological role for B2 receptors in the CNS. Interestingly, our results also indicate that brain B2 receptors are subjected to downregulation in specific brain nuclei of 16 weeks old WKY in comparison to 8 weeks old rats. This suggests an age- and tissue-dependent regulation of central B2 receptors in WKY.
Densities of B2 receptor binding sites were seen markedly enhanced in some brain areas of both 8- and 16-week-old SHR, in comparison with their normotensive controls. Thus, the increased number of B2 receptors may represent an important contributory factor to the enhanced responsiveness to BK in SHR. B2 receptors were upregulated mostly in the hindbrain nuclei, which are thought to be the most relevant sites of action of i.c.v. kinins (Couture & Lindsey, 2000). Likewise, the hypersensitivity of the pressor response to intrathecal BK in SHR was correlated with increased level of B2 receptor binding sites in the thoracic spinal cord (Cloutier et al., 2002). This is in keeping with the age-related increase of B2 receptor binding sites in the spinal cord of SHR (Ongali et al., 2003a) and of B2 receptor mRNA levels in the hypothalamus of SHR (Qadri et al., 2002). Evidence was also provided that B2 receptors are upregulated in several medullary cardiovascular centres of post-mortem human donors afflicted by arterial hypertension (Buck et al., 2002). Whether or not the observed changes of central kinin receptors reflect a secondary effect of hypertension rather that an underlying mechanism has been addressed in a recent study (Ongali et al., 2003a). In the latter study, it was concluded that the greater density of B2 receptor binding sites occurring in the thoracic spinal cord of SHR is unlikely secondary to arterial hypertension because it was further increased in adult SHR, which had undergone a chronic antihypertensive therapy from the age of 4 weeks with angiotensin-1-converting enzyme inhibitors or with an angiotensin AT1 receptor antagonist. Thus, alterations of central kinin receptors may reflect a genetic trait associated to hypertension. It is however unknown at the present time whether or not these alterations contribute to the development of arterial hypertension. Nevertheless, all these latter studies including ours contrast with a recent autoradiographic study, which failed to detect significant difference in B2 receptor binding sites in the nucleus of the solitary tract, area postrema, dorsal motor nucleus of the vagus and caudal subnucleus of the spinal trigeminal nucleus of adult WKY and SHR using [125I-Tyr0]-BK as radioligand (Privitera et al., 2003). Discrepancies may result from differences in methodology or the radioligand used; for instance, an agonist (e.g. [Tyr0]-BK) may cause internalisation of the receptor that does not occur with an antagonist such as HPP-Hoe 140 (Marceau et al., 1998).
In addition to the pharmacological study performed with i.c.v. injections of selective B1 receptor agonists and antagonist, our autoradiographic study does not support a role for B1 receptors in the central action of kinins nor in the maintenance of hypertension in SHR. A similar conclusion for the B1 receptor was reached at the level of the spinal cord in SHR (Cloutier et al., 2002; Ongali et al., 2003a). Densities of B1 receptor binding sites were very low in all examined brain structures in young SHR, and only small differences were found between SHR and WKY at the age of 8 weeks despite the occurrence of hypersensitivity to i.c.v. BK. B1 receptor binding sites increased in some brain regions of 16-week-old SHR, while B1 receptor density values declined with ageing in WKY. The exact meaning of this opposite regulation of B1 receptors in mature SHR and WKY is still unknown. Most elevated densities of B1 receptors in SHR were seen in cortical areas, thalamic regions, amygdala and hippocampus. B1 receptors were also upregulated in some pressor-related hypothalamic areas of 16-week-old SHR, such as DA and VMH (Wardener, 2001); however, it is uncertain whether or not these areas can be stimulated by i.c.v. kinins. The latter data are congruent with the increased B1 receptor mRNA expression in the hypothalamus of 12- to 13-week-old SHR (Qadri et al., 2002). Thus one cannot exclude a putative role for B1 receptors in hypothalamic functions unrelated to the cardiovascular control in SHR.
Conclusion
Our study shows that the hypersensitivity of the pressor response to i.c.v. BK in SHR occurs not only during the established phase of hypertension but also during its early phase in parallel with the enhancement of B2 receptor binding sites in various cardiovascular areas of the brain. On the other hand, pharmacological and autoradiographic results do not support a primary role for brain B1 receptors neither in the central pressor effect of kinins nor in the maintenance of hypertension in SHR.
Acknowledgments
We acknowledge Dr R. Elie (Université de Montréal) for his invaluable help and expertise in the statistical analysis of the data and Dr D. Regoli (Sherbrooke Université, Sherbrooke, Canada) for the donation of kinin B1 and B2 receptor agonists and antagonists. This work was supported by a grant-in-aid to R.C. from the Canadian Institutes of Health Research (MT-14379). F. Cloutier and B. Ongali were the holders of PhD studentships from the Fonds de la Recherche en Santé du Québec and the Republic of Gabon, respectively. M.M. Campos was awarded a postdoctoral Fellowship from The Research Group on Autonomic Nervous System at Université de Montréal.
Abbreviations
- Amb
ambiguus nucleus
- aCSF
artificial cerebrospinal fluid
- BSA
bovine serum albumin
- CGA
central gray (alpha part)
- DA
dorsal hypothalamic area
- DTT
dithiothreitol
- HR
heart rate
- i.c.v.
intracerebroventricular
- LDDM
laterodorsal thalamic nucleus (dorsomedial)
- LDVL
laterodorsal thalamic nucleus (ventrolateral)
- MAP
mean arterial blood pressure
- NTS
nucleus tractus solitarius
- PA5
paratrigeminal nucleus
- PDTg
posterodorsal tegmental nucleus
- PIPES
piperazine-N,N′-bis[2-ethanesulphonic-acid]
- Pn
pontine nucleus
- Pyt
pyramidal tract
- SHR
spontaneously hypertensive rat
- SCP
superior cerebellar peduncle
- SP5
spinal trigeminal tract
- VMH
ventromedial hypothalamic nucleus
- VPL
ventral posterolateral thalamic nucleus
- VPM
ventral posteromedial thalamic nucleus
- WKY
Wistar Kyoto rat
References
- ALVAREZ A.L., DELORENZI A., SANTAJULIANA D., FINKIELMAN S., NAHMOD V.E., PIROLA C.J. Central bradykininergic system in normotensive and hypertensive rats. Clin. Sci. 1992;82:513–519. doi: 10.1042/cs0820513. [DOI] [PubMed] [Google Scholar]
- BUCK H.S., ONGALI B., THIBAULT G., LINDSEY C.J., COUTURE R. Autoradiographic detection of kinin receptors in the human medulla of control, hypertensive, and diabetic donors. Can. J. Physiol. Pharmacol. 2002;80:249–257. doi: 10.1139/y02-050. [DOI] [PubMed] [Google Scholar]
- BUÑAG R.D., TAKAHASHI H. Exaggerated sympathetic responses to bradykinin in spontaneously hypertensive rats. Hypertension. 1981;3:433–440. doi: 10.1161/01.hyp.3.4.433. [DOI] [PubMed] [Google Scholar]
- BUÑAG R.D., TAKEDA K. Sympathetic hyperresponsiveness to hypothalamic stimulation in young hypertensive rats. Am. J. Physiol. 1979;237:R39–R44. doi: 10.1152/ajpregu.1979.237.1.R39. [DOI] [PubMed] [Google Scholar]
- CHEN E.Y., EMERICH D.F., BARTUS R.T., KORDOWER J.H. B2 bradykinin receptor immunoreactivity in rat brain. J. Comp. Neurol. 2000;427:1–18. [PubMed] [Google Scholar]
- CLOUTIER F., BUCK H.S., ONGALI B., COUTURE R. Pharmacologic and autoradiographic evidence for an up-regulation of kinin B2 receptors in the spinal cord of spontaneously hypertensive rats. Br. J. Pharmacol. 2002;135:1641–1654. doi: 10.1038/sj.bjp.0704632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- COUTURE R., LINDSEY C.J.Brain kallikrein–kinin system: from receptors to neuronal pathways and physiological functions Handbook of Chemical Neuroanatomy 2000241–300.ed. Quirion, R., Björklund, A. & Hökfelt, T. Vol. 16: Peptide Receptors, Part I
- DE CHAMPLAIN J. Pre- and post-synaptic adrenergic dysfunctions in hypertension. J. Hypertens. 1990;8 Suppl 7:S77–S85. [PubMed] [Google Scholar]
- DRAPER N.R., SMITH H. Wiley Series in Probability and Mathematical Statistics. New York: John Wiley & Sons Inc; 1981. Applied regression analysis; pp. 275–278. [Google Scholar]
- EMANUELI C., CHAO J., REGOLI D., CHAO L., NI A., MADEDDU P. The bradykinin B1 receptor and the central regulation of blood pressure in spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1769–1776. doi: 10.1038/sj.bjp.0702527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIWARA Y., MANTIONE C.R., VAVREK R.J., STEWART J.M., YAMAMURA H.I. Characterization of [3H]bradykinin binding sites in guinea-pig central nervous system: possible existence of B2 subtypes. Life Sci. 1989;44:1645–1653. doi: 10.1016/0024-3205(89)90481-5. [DOI] [PubMed] [Google Scholar]
- FUJIWARA Y., MANTIONE C.R., YAMAMURA H.I. Identification of B2 bradykinin binding sites in guinea-pig brain. Eur. J. Pharmacol. 1988;147:487–488. doi: 10.1016/0014-2999(88)90187-2. [DOI] [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE ST., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W.M., GREENWOOD F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- ISRAEL A., SAAVEDRA J.M. High angiotensin converting enzyme (kininase II) activity in the cerebrospinal fluid of spontaneously hypertensive adult rats. J. Hypertens. 1987;5:355–357. doi: 10.1097/00004872-198706000-00015. [DOI] [PubMed] [Google Scholar]
- JUDY W.V., WATANABE A.M., MURPHYW R., APRISON B.S., YU P.L. Sympathetic nerve activity and blood pressure in normotensive backcross rats genetically related to the spontaneously hypertensive rat. Hypertension. 1979;6:598–604. doi: 10.1161/01.hyp.1.6.598. [DOI] [PubMed] [Google Scholar]
- KHAN I.M., MILLER D.H., STRICKLAND J., MARGOLIUS H.S., PRIVITERA P.J.Brain kallikrein–kinin system abnormalities in spontaneously hypertensive rats Hypertension 199525524–530.(Part 1) [DOI] [PubMed] [Google Scholar]
- LINDSEY C.J., FUJITA K., MARTINS T.O. The central pressor effect of bradykinin in normotensive and hypertensive rats. Hypertension. 1988;11 Suppl 1:126–129. doi: 10.1161/01.hyp.11.2_pt_2.i126. [DOI] [PubMed] [Google Scholar]
- MADEDDU P., GLORIOSO N., SORO A., TONOLO G., MANUNTA P., TROFFA C., DEMONTIS M.P., VARONI M.V., ANANIA V. Brain kinins are responsible for the pressor effect of intracerebroventricular captopril in spontaneously hypertensive rats. Hypertension. 1990;15:407–412. doi: 10.1161/01.hyp.15.4.407. [DOI] [PubMed] [Google Scholar]
- MADEDDU P., GLORIOSO N., VARONI M.V., DEMONTIS M.P., FATTACCIO M.C., ANANIA V. Cardiovascular effects of brain kinin receptor blockade in spontaneously hypertensive rats. Hypertension. 1994;23 Suppl 1:I189–I292. doi: 10.1161/01.hyp.23.1_suppl.i189. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARTINS D.T.O., FIOR D.R., NAKAIE C.R., LINDSEY C.J. Kinin receptors of the central nervous system of spontaneously hypertensive rats related to the pressor response to bradykinin. Br. J. Pharmacol. 1991;103:1851–1856. doi: 10.1111/j.1476-5381.1991.tb12341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURONE C., PAXINOS G., McKINLEY M.J., OLDFIELD B.J., MÜLLER-ESTERL W., MENDELSOHN F.A., CHAI S.Y. Distribution of bradykinin B2 receptors in sheep brain and spinal cord visualized by in vitro autoradiography. J. Comp. Neurol. 1997;381:203–218. doi: 10.1002/(sici)1096-9861(19970505)381:2<203::aid-cne7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- NONAKA M., CHEN X.H., PIERCE J.E., LEONI M.J., MCINTOSH T.K., WOLF J.A., SMITH D.H. Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- ONGALI B., BUCK H.S., CLOUTIER F., LEGAULT F., REGOLI D., LAMBERT C., THIBAULT G., COUTURE R. Chronic effects of angiotensin-converting enzyme inhibition on kinin receptor binding sites in the rat spinal cord. Am. J. Physiol. (Heart Circ. Physiol.) 2003a;284:H1949–H1958. doi: 10.1152/ajpheart.01113.2002. [DOI] [PubMed] [Google Scholar]
- ONGALI B., CAMPOS M.M., BREGOLA G., RODI D., REGOLI D., THIBAULT G., SIMONATO M., COUTURE R. Autoradiographic analysis of rat brain kinin B1 and B2 receptors: normal distribution and alterations induced by epilepsy. J. Comp. Neurol. 2003b;461:506–519. doi: 10.1002/cne.10706. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1998San Diego, CA: Academic Press; 4th edn [Google Scholar]
- PENNYPACKER K.R., KASSED C.A., EIDIZADEH S., O'CALLAGHAN J.P. Brain injury: prolonged induction of transcription factors. Acta Neurobiol. Exp. 2000;60:515–530. doi: 10.55782/ane-2000-1373. [DOI] [PubMed] [Google Scholar]
- PRIVITERA P.J., BECKSTEAD R.M., YATES P., WALGREN R. Autoradiographic localization of [125I-Tyr0]bradykinin binding sites in brains of Wistar–Kyoto and spontaneously hypertensive rats. Cell. Mol. Neurobiol. 2003;23:805–815. doi: 10.1023/A:1025061205355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIVITERA P.J., THIBODEAUX H., YATES P. Rostral ventrolateral medulla as a site for the central hypertensive action of kinins. Hypertension. 1994;23:52–58. doi: 10.1161/01.hyp.23.1.52. [DOI] [PubMed] [Google Scholar]
- PRIVITERA P.J., DAUM P.R., HILL D.R., HILEY C.R. Autoradiographic visualization and characteristics of [125I]bradykinin binding sites in guinea-pig brain. Brain Res. 1991;577:73–79. doi: 10.1016/0006-8993(92)90539-l. [DOI] [PubMed] [Google Scholar]
- QADRI F., HAUSER W., JOHREN O., DOMINIAK P. Kinin B1 and B2 receptor mRNA expression in the hypothalamus of spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2002;80:258–263. doi: 10.1139/y02-051. [DOI] [PubMed] [Google Scholar]
- QADRI F., BÄURLE L., HÄUSER W., RASCHER W., DOMINIAK P. Centrally bradykinin B2-receptor-induced hypertensive and positive chronotropic effects are mediated via activation of the sympathetic nervous system. J. Hypertens. 1999;17:1265–1271. doi: 10.1097/00004872-199917090-00005. [DOI] [PubMed] [Google Scholar]
- REGOLI D., NSA ALLOGHO S., RIZZI A., GOBEIL F. Bradykinin receptors and their antagonists. Eur. J. Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- SIEBECK M., SCHORR M., SPANNAGL E., LEHNER M., FRITZ H., CHERONIS J.C., WHALLEY E.T. B1 kinin receptor activity in pigs is associated with pre-existing infection. Immunopharmacology. 1998;40:49–55. doi: 10.1016/s0162-3109(98)00035-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI H., BUÑAG R.D. Centrally induced cardiovascular and sympathetic nerve responses to bradykinin in rats. J. Pharmacol. Exp. Ther. 1981;216:192–197. [PubMed] [Google Scholar]
- TAKEDA K., BUÑAG R.D. Sympathetic hyperactivity during hypothalamic stimulation in spontaneously hypertensive rats. J. Clin. Invest. 1978;62:642–648. doi: 10.1172/JCI109171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDENER H.E. The hypothalamus and hypertension. Physiol. Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- WIRTH K., BREIPOHL G., STECHL J., KNOLLE J., HENKE S., SCHÖLKENS B.A. Des-Arg9-D-Arg-[Hyp3,Thi5,D-Tic7,Oic8]-BK (des-Arg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur. J. Pharmacol. 1991;205:217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- ZHANG J., RIVEST S. Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J. Neurochem. 2001;76:855–864. doi: 10.1046/j.1471-4159.2001.00080.x. [DOI] [PubMed] [Google Scholar]