Abstract
Acute and chronic effects of the insulinotropic drug nateglinide upon insulin release were examined in the BRIN-BD11 cell line.
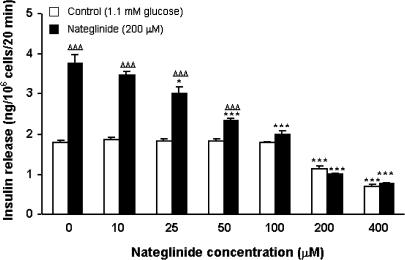
Nateglinide (10–400 μM) stimulated a concentration-dependent increase (P<0.05–P<0.001) in insulin release at a non-stimulatory (1.1 mM) glucose concentration. The insulinotropic response to 200 μM nateglinide was increased at 30 mM (P<0.01), but not 5.6–16.7 mM glucose concentrations.
In depolarized cells, nateglinide (50–200 μM) evoked KATP channel-independent insulin secretion (P<0.05–P<0.001) in the absence and presence of 5.6–30.0 mM glucose (P<0.001).
Exposure for 18 h to 100 μM nateglinide abolished the acute insulinotropic effects of 200 μM nateglinide, tolbutamide or glibenclamide, but had no effect upon the insulinotropic effect of 200 μM efaroxan.
While 18 h exposure to 100 μM nateglinide did not affect basal insulin release or insulin release in the presence of 16.7 mM glucose, 25 μM forskolin or 10 nM PMA, significant inhibition of the insulinotropic effects of 20 mM leucine and 20 mM arginine were observed.
These data show that nateglinide stimulates both KATP channel-dependent and-independent insulin secretion. The maintained insulinotropic effects of this drug with increasing glucose concentrations support the antihyperglycaemic actions of nateglinide in Type II diabetes.
Studies of the long-term effects of nateglinide indicate that nateglinide shares signalling pathways with sulphonylureas, but not the imidazoline efaroxan. This may be significant when considering a nateglinide treatment regimen, particularly in patients previously treated with sulphonylurea.
Keywords: Nateglinide, insulin release, clonal pancreatic beta cells, tolbutamide, glibenclamide, efaroxan
Introduction
Nateglinide, a D-phenylalanine derivative, is a novel insulinotropic drug which has recently been launched as a therapeutic agent for Type II diabetes (Dornhorst, 2001). Clinical studies have shown that nateglinide induces a rapid onset of insulin release (Gribble et al., 2001; Kalbag et al., 2001) synergistic with meal administration (Keilson et al., 2000), which effectively restores the early phase of insulin secretion (Hanefeld et al., 2000; Hollander et al., 2001). Since the loss of early insulin secretion is thought to play an important role in the development of glucose intolerance (Pratley & Weyer, 2001), the ability of nateglinide to promote early insulin release is potentially of considerable therapeutic benefit. In addition, its rapid onset and short duration (Gribble et al., 2001; Kalbag et al., 2001) reduces the risk of hypoglycaemic episodes due to the sustained unnecessary insulin exposure observed with longer-acting insulin secretagogues such as the well-established and widely used sulphonylureas (DeFronzo, 1998). A further desirable quality of nateglinide as a therapeutic tool is its proven ability to work well in combination therapy of Type II diabetes, as evidenced when combined with metformin (Horton et al., 2000; Marre et al., 2002) or troglitazone (Rosenstock et al., 2002).
In vitro studies have revealed that, like the sulphonylureas, the insulinotropic effects of nateglinide are primarily mediated via pancreatic B-cell KATP channels (Hu et al., 2000, 2003; Hansen et al., 2002; Hu, 2002). The B-cell KATP channel is a hetero-octameric complex of four inwardly rectifying potassium channel Kir6.2 subunits which form a tetrameric pore and four regulatory sulphonylurea (SUR1) subunits (Inagaki et al., 1997; Aguilar-Bryan et al., 1998). Binding of sulphonylureas or nateglinide to SUR1 results in closure of the Kir6.2 pore (Aguilar-Bryan et al., 1995; Hansen et al., 2002), which in turn evokes membrane depolarization, influx of extracellular calcium, and, ultimately, exocytosis of insulin (Ashcroft & Gribble, 1999). In addition to this mechanism, several studies have reported a direct effect of sulphonylureas upon exocytosis independent of KATP channel function (Flatt et al., 1994; Eliasson et al., 1996; Tian et al., 1998; Ball et al., 2000c). It is thought that these drugs may elicit insulin secretion in the absence of KATP channel function by targeting intracellular sites (McClenaghan & Flatt, 1999a; Renström et al., 2002). Interestingly, nateglinide has been shown to stimulate exocytosis in rat pancreatic A cells (Bokvist et al., 1999) and pituitary cells (Gromada et al., 2002), and one report has suggested a direct action of nateglinide on insulin release from rat pancreatic B cells (Bokvist et al., 1998). We have previously undertaken studies to examine the KATP channel-independent effects of sulphonylureas upon insulin secretion (Ball et al., 2000c). The present study reports that nateglinide can also stimulate insulin secretion when it is unable to exert its effects on KATP channels in the plasma membrane.
Sulphonylurea therapy for type II diabetes tends to progressively fail (Grunberger, 1993; Matthews et al., 1998), an observation which has been attributed to a decline in the insulinotropic ability of these drugs due to a direct desensitization of the pancreatic B-cell to the actions of sulphonylureas (Rabuazzo et al., 1992; Kawaki et al., 1999). Recent data have suggested that nateglinide shares a common point of action on SUR1 with the sulphonylurea tolbutamide (Hansen et al., 2002), raising the possibility that a similar B-cell desensitization may occur following prolonged exposure to nateglinide. In vitro studies on the long-term effects of current and prospective therapeutic drugs have been greatly aided by the development of insulin-secreting cell lines, which retain secretory properties of the pancreatic B-cell over long periods of time in tissue culture (McClenaghan & Flatt, 1999b). Previous studies from our laboratory have successfully utilized the BRIN-BD11 cell line to examine the long-term actions and mechanisms of sulphonylureas (Ball et al., 2000a, c), imidazolines (Chapman et al., 1999; McClenaghan et al., 2001), and the novel insulinotropic drug BTS 67 582 (McClenaghan et al., 2000). This study utilizes this model system to examine the effects of prolonged exposure to nateglinide upon subsequent secretory responsiveness to a range of physiological and pharmacological modulators of insulin secretion.
Methods
Chemicals
Reagents of analytical grade and deionized water (Purite, Oxon, U.K.) were used. RPMI-1640 tissue culture medium, foetal bovine serum, and antibiotics were obtained from GibcoBRL (Paisley, Strathclyde, U.K.), rat insulin standard was from Novo-Nordisk (Bagsvaerd, Denmark), and [125I]-bovine insulin was from Lifescreen (Watford, U.K.). Nateglinide was a gift from Novartis Pharmaceuticals Corporation (Summit, New Jersey, U.S.A.). All other chemicals were from Sigma and BDH Chemicals Ltd (both of Poole, Dorset, U.K.).
Cell culture and measurement of insulin release
Clonal pancreatic BRIN-BD11 cells (passage numbers 20–30) were used for this study. The origin and characteristics of this glucose-responsive insulin-secreting cell line, including membrane potential and calcium ion channel properties, are described in detail elsewhere (McClenaghan et al., 1996; Chapman et al., 1999; Salgado et al., 2000). BRIN-BD11 cells were grown in RPMI-1640 tissue culture medium containing 11.1 mmol l−1 glucose and 0.3 g l−1 L-glutamine, and supplemented with 10% (v −1v) foetal calf serum, 100 IU ml−1 penicillin, and 0.1 g l−1 streptomycin at 37°C with 5% CO2 and 95% air. Tissue culture media were removed and replaced with fresh media every 24 h. Cells were washed with Hanks' balanced saline solution (HBSS) prior to detachment from tissue culture flasks with the aid of 0.025% (w −1v) trypsin containing 1 mM EDTA, and seeded at 1.5 × 105 cells per well into 24-multiwell plates. Monolayers of cells were then cultured for 18 h at 37°C. Culture medium was then replaced with 1 ml of a Krebs ringer bicarbonate (KRB) buffer, consisting of (in mM) 115 NaCl, 4.7 KCl, 1.2 MgSO4, 1.28 CaCl2, 1.2 KH2PO4, 25 HEPES and 8.4% (w−1v) NaHCO3 (pH 7.4) supplemented with 0.1% (w −1v) bovine serum albumin and 1.1 mmol −1l glucose. After 40 min preincubation at 37°C, the buffer was replaced with 1 ml of KRB test buffer containing glucose and test agents, as detailed in the legends to figures. After 20 min incubation at 37°C, aliquots of test buffer were removed and stored at −20°C for insulin radioimmunoassay (Flatt & Bailey, 1981).
Statistical analysis
Results are presented as mean±standard error of the mean (s.e.m.) for a given number of observations (n). Groups of data were compared using unpaired Student's t-test. Differences were considered significant if P<0.05.
Results
Insulinotropic responses to nateglinide in non-depolarized and depolarized cells
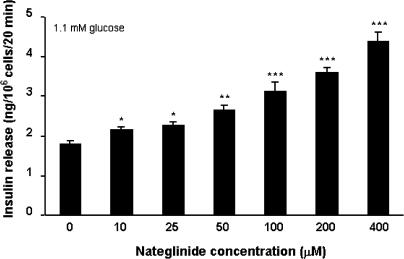
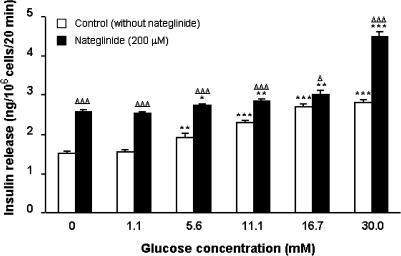
Acute static incubation with 10–400 μM nateglinide evoked a 1. 2–2.4-fold concentration-dependent increase (P<0.05–P<0.001) in insulin release at 1.1 mM glucose (Figure 1). In the absence of nateglinide, progressive increases in glucose concentration (5.6–30.0 mM) stimulated 1.3–1.9-fold increases (P<0.01–P<0.001) in insulin secretion, characteristic of BRIN-BD11 cells (Figure 2). In the presence of 200 μM nateglinide, these same increases in glucose concentration resulted in a 1.1–1.7-fold (P<0.05–P<0.001) potentiation of insulin release (Figure 2).
Figure 1.
Effects of 0–400 μM nateglinide on insulin release at 1.1 mM glucose. Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of nateglinide were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). *P<0.05, **P<0.01, ***P<0.001 compared with the respective effect in the absence of nateglinide.
Figure 2.
Effects of glucose on nateglinide-induced insulin secretion. Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of glucose (0–30.0 mM) in the absence or presence of 200 μM nateglinide were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). *P<0.05, **P<0.01, ***P<0.001 compared with the respective effect in the absence of glucose. ΔP<0.05, ΔΔΔP<0.001 compared with the respective effect in the absence of 200 μM nateglinide.
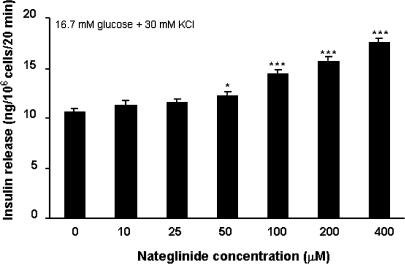
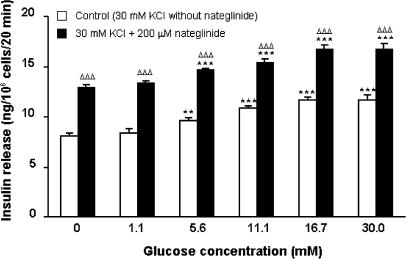
Figure 3 shows the effects of nateglinide upon insulin secretion under conditions sufficient to fully depolarize the plasma membrane and thus render KATP channels inactive (16.7 mM glucose and 30 mM KCl). Under these conditions, 50–400 μM nateglinide stimulated 1.2–1.7-fold insulin-secretory responses (P<0.05–P<0.001). In the presence of 30 mM KCl, glucose concentrations of 5.6–30.0 mM stimulated 1.2–1.4-fold increases (P<0.01–P<0.001) in insulin release, and resulted in an additive 1.1–1.3-fold potentiation (P<0.001) of nateglinide-induced insulin secretion (Figure 4).
Figure 3.
Effects of 0–400 μM nateglinide on insulin release in the presence of membrane-depolarizing conditions (16.7 mM glucose plus 30 mM KCl). Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of nateglinide were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). *P<0.05, ***P<0.001 compared with the respective effect in the absence of nateglinide.
Figure 4.
Effects of glucose on nateglinide-induced insulin secretion in the presence of membrane-depolarizing conditions (30 mM KCl). Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of glucose (0–30.0 mM) in the absence or presence of 200 μM nateglinide were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). **P<0.01, ***P<0.001 compared with the respective effect in the absence of glucose. ΔΔΔP<0.001 compared with the respective effect in the absence of 200 μM nateglinide.
Acute secretory responsiveness following prolonged exposure to nateglinide
BRIN-BD11 cells were cultured for 18 h in the presence of 0–400 μM nateglinide (Figure 5). A dose-dependent decline in acute nateglinide-induced insulin secretion (39–100%; P<0.05–P<0.001) was noted following 18 h exposure to 25–400 μM nateglinide, with 100–400 μM abolishing acute nateglinide-stimulated insulin release. Cell viability and insulin content were not affected by concentrations of nateglinide up to 200 μM (data not shown).
Figure 5.
Effects of 18 h culture with a range of nateglinide concentrations on subsequent acute responsiveness to nateglinide. BRIN-BD11 cells were cultured for 18 h in either standard tissue culture medium, or in tissue culture medium supplemented with 10–400 μM nateglinide. Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of 200 μM nateglinide were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). *P<0.05, ***P<0.001 compared with the respective effect in the absence of nateglinide during 18 h culture. ΔΔΔP<0.001 compared with the respective effect in the absence of nateglinide during acute incubation.
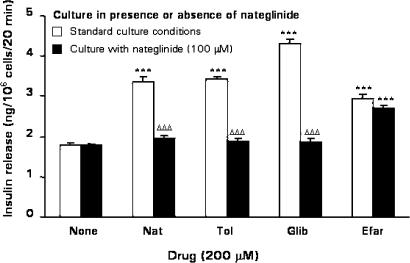
As shown in Figure 6, acute stimulation by nateglinide, the sulphonylureas tolbutamide and glibenclamide, and the imidazoline compound efaroxan (each at 200 μM) elicited respective 1.9-, 1.9-, 2.4-, and 1.6-fold (P<0.001) insulin-secretory responses from BRIN-BD11 cells after culturing for 18 h in standard RPMI-1640 media. An 18 h culture with 100 μM nateglinide abolished subsequent acute secretory responses to tolbutamide and glibenclamide, as well as nateglinide. However, the acute secretory response to efaroxan was unaffected (Figure 6).
Figure 6.
Effects of 18 h culture with nateglinide on nateglinide-, tolbutamide-, glibenclamide- or efaroxan-stimulated insulin secretion. BRIN-BD11 cells were cultured for 18 h in either standard tissue culture medium, or in tissue culture medium supplemented with 100 μM nateglinide. Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of 200 μM nateglinide, tolbutamide, glibenclamide or efaroxan were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). ***P<0.001 compared with the respective effect in the absence of drug. ΔΔΔP<0.001 compared with the respective effect in the absence of nateglinide during 18 h culture.
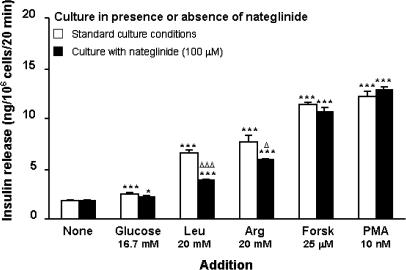
Insulinotropic responses to stimulatory (16.7 mM) glucose and 20 mM leucine or arginine were examined after 18 h culture in the absence or presence of 100 μM nateglinide (Figure 7). Raising the glucose concentration from 1.1 to 16.7 mM stimulated a 1.4-fold increase in insulin secretion, which remained unaffected following 18 h nateglinide culture. Leucine stimulated a 3.7-fold (P<0.001) insulin-secretory response, which was inhibited (57%, P<0.05) by nateglinide culture. Likewise, arginine stimulated a 4.3-fold (P<0.001) insulin-secretory response which was impaired (14% decrease; P<0.05) following culture with nateglinide. However, both leucine and arginine retained stimulatory actions (2.2- and 3.3-fold, respectively; P<0.05) upon insulin secretion following 18 h nateglinide exposure (Figure 7).
Figure 7.
Effects of 18 h culture with nateglinide on glucose-, leucine-, arginine-, forskolin- or phorbol-12-myristate 13-acetate-stimulated insulin secretion. BRIN-BD11 cells were cultured for 18 h in either standard tissue culture medium, or in tissue culture medium supplemented with 100 μM nateglinide. Following 40 min preincubation with a buffer containing 1.1 mM glucose, effects of 16.7 mM glucose, 20 mM leucine, 20 mM arginine, 25 μM forskolin or 10 nM phorbol-12-myristate 13-acetate were tested during a 20 min incubation period. Values are mean±s.e.m. (n=6). *P<0.05, ***P<0.001 compared with the respective effect in the absence of addition. ΔP<0.05, ΔΔΔP<0.001 compared with respective effect in absence of nateglinide during 18 h culture.
In addition, insulin-secretory responsiveness to forskolin (25 μM) or phorbol 12-myristate 13-acetate (PMA; 10 nM) were assessed after 18 h culture in the absence or presence of 100 μM nateglinide (Figure 7). The 6.4- and 6.9-fold insulin-secretory responses to forskolin and PMA, respectively, were unaffected following nateglinide culture (Figure 7), indicating that adenylate cyclase, protein kinase A- (PKA), and protein kinase C (PKC)-mediated signalling pathways remained intact following prolonged exposure to nateglinide.
Discussion
Insulinotropic drugs have been used with considerable success in the treatment of type II diabetes for many years, with sulphonylureas being the traditional drugs of choice (DeFronzo, 1998). However, the use of sulphonylureas has associated complications, such as the incidence of hypoglycaemic episodes as a result of their long duration of action (DeFronzo, 1998), and the tendency of long-term sulphonylurea treatment to progressively fail (Grunberger, 1993; Matthews et al., 1998). Thus, there has been an ongoing search for improved insulinotropic drugs. Nateglinide is one such drug. It has been recently launched, and reported to exhibit many favourable characteristics, such as a rapid onset of insulin secretion (Gribble et al., 2001) synergistic with food intake (Keilson et al., 2000), and a short duration of action (Kalbag et al., 2001). Plasma concentrations after single therapeutic 120 mg dose of nateglinide can be expected in the 50–100 μM range (Niemi et al., 2003). This study aimed at further characterizing the acute and long-term effects of nateglinide upon insulin secretion using the BRIN-BD11 cell line, which has been well characterized as a model for elucidating the mechanisms of action of insulinotropic drugs (Ball et al., 2000a, 2000c; McClenaghan et al., 2000, 2001).
Nateglinide was observed to elicit insulin-secretory responses in a concentration-dependent manner over the range 10–400 μM. In addition to this ability to initiate insulin secretion, nateglinide stimulated insulin release in the presence of physiological concentrations of glucose. Secretory responsiveness was enhanced only at very high glucose concentration (30 mM), making the effects of nateglinide very similar to other insulinotropic drugs such as sulphonylureas and repaglinide (Hu et al., 2001). These insulinotropic effects of nateglinide were mediated in non-depolarized cells, and reflect the interaction of the drug with B-cell KATP channels (Hansen et al., 2002; Hu, 2002), producing a secretory response which likely underlies the restoration of early-phase insulin secretion associated with nateglinide (Hollander et al., 2001; Uto et al., 2002).
Under depolarizing conditions (16.7 mM glucose and 30 mM KCl) sufficient to render KATP channels inactive (Flatt et al., 1994; Eliasson et al., 1996), nateglinide retained an insulin-secretory response over the concentrations 50–400 μM. Such KATP channel-independent stimulation of insulin secretion has previously been reported for sulphonylureas (Tian et al., 1998; Ball et al., 2000c), and attributed to actions of these drugs at intracellular sites in the B-cell (McClenaghan & Flatt, 1999a; Renström et al., 2002). It seems likely that nateglinide shares KATP channel-independent intracellular signalling pathways with sulphonylureas. This idea is lent support by the recent finding that a binding site for nateglinide resides on the intracellular side of SUR1 and shares at least one point of interaction with the binding site for the sulphonylurea tolbutamide (Hansen et al., 2002).
In the presence of depolarizing concentrations of KCl, glucose is known to retain an insulinotropic action in the pancreatic B-cell (Gembal et al., 1992). This KATP channel-independent effect of glucose is increasingly thought to play an important role in the regulation of phase 2 insulin secretion (Bratanova-Tochkova et al., 2002; Straub & Sharp, 2002). We observed that the KATP channel-independent insulinotropic effects of 5.6–30.0 mM glucose were significantly potentiated by 200 μM nateglinide. These data suggest that nateglinide may prove valuable in modulating phase 2 insulin secretion, which could provide considerable benefits in the therapy of type II diabetes.
Previous studies from our laboratory (Ball et al., 2000a, c; McClenaghan et al., 2000, 2001) have utilized a system of prolonged culture of BRIN-BD11 cells with a range of insulinotropic drugs to elucidate their mechanisms of action and long-term effects. This study found that 18 h culture in the presence of 25–400 μM nateglinide reduced its subsequent acute responsiveness to nateglinide. These effects were not accompanied by appreciable decline in cell viability or cellular insulin content. We have previously reported a similar desensitization of insulin secretion following prolonged administration of tolbutamide (Ball et al., 2000c), glibenclamide (Ball et al., 2000a), efaroxan (McClenaghan et al., 2001), and the novel guanidine derivative BTS 67 582 (McClenaghan et al., 2000). Given that the progressive failure of sulphonylurea therapy over time has led to a call to consider intermittent rather than continuous sulphonylurea administration (Grunberger, 1993), these findings may be important when devising appropriate therapeutic regimens for nateglinide.
Prolonged culture with nateglinide also induced desensitization to the acute insulinotropic effects of tolbutamide and glibenclamide. These data are suggestive of common sites of action between nateglinide and the sulphonylureas. Indeed, it is interesting to note that a recent study reported a shared binding site between nateglinide and the sulphonylureas (Hansen et al., 2002). In contrast to its effects on sulphonylurea-induced insulin release, nateglinide was unable to desensitize efaroxan-induced insulin secretion, indicative of different mechanisms of action of this imidazoline and nateglinide. A partial explanation for these findings may lie in differential interactions with KATP channel subunits. The effects of nateglinide, tolbutamide, and glibenclamide are all known to be mediated via the SUR1 subunits, whereas the imidazoline-binding site has been reported to lie on the pore-forming Kir6.2 subunits (Proks & Ashcroft, 1997) of the KATP channel. However, it may be over-simplistic to attribute nateglinide-induced desensitization simply to an effect on SUR1, given that other authors (Hu et al., 2001) have reported that rat islets exposed overnight to glibenclamide or tolbutamide showed attenuated insulin response to sulphonylureas, but not nateglinide.
Further studies showed that glucose-stimulated insulin secretion was largely retained following prolonged exposure to nateglinide, although the significance value of this effect was diminished from P<0.001 to P<0.05. This finding corresponds to results in a previous in vivo study (Laghmich et al., 1999). It should be noted that another study using MIN-6 cells (Sunaga et al., 2001) showed a decline in glucose-stimulated insulin release following nateglinide exposure. However, this difference may reflect differences in methodology, as MIN-6 cells were exposed to nateglinide for 2 weeks. Interestingly, nateglinide culture reduced the acute secretory effects of leucine and arginine. These actions may reflect a change in B-cell function relating to the regulation of B-cell electrical activity by these amino acids, as leucine and arginine, despite contrasting mechanisms of action, each stimulate insulin secretion as a result of membrane depolarization and calcium influx (Yada, 1994). In contrast, the insulinotropic actions of forskolin and PMA were unaffected following nateglinide culture, indicating intact PKA- and PKC-mediated signalling pathways. In a previous study (McClenaghan et al., 2001), prolonged exposure to tolbutamide resulted in decreased secretory responsiveness to forskolin, suggesting a difference in the mechanisms of action of nateglinide and tolbutamide, with a greater role for the PKA/cAMP signalling pathway in mediating the insulinotropic effects of the sulphonylurea.
In summary, this study provides further support for nateglinide as a therapeutic agent in type II diabetes. It is a potent insulin secretagogue with the ability to stimulate KATP channel-dependent and -independent insulin secretion. Additionally, nateglinide potentiates initiation and augmentation pathways for glucose-induced insulin release, suggesting likely effectiveness on both first- and second-phase insulin secretion.
Acknowledgments
These studies were supported in part by the Research and Development Office of the Northern Ireland Department of Personal Health and Social Services. We thank Dr S. Hu and Ms H. Eckhardt of Novartis for their advice and assistance.
Abbreviations
- cAMP
3′:5′-cyclic monophosphate
- KATP channel
ATP-sensitive K+ channel
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
References
- AGUILAR-BRYAN L., CLEMENT J.P., GONZALEZ G., KUNJILWAR K., BABENKO A., BRYAN J. Toward understanding the assembly and structure of KATP channels. Physiol. Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- AGUILAR-BRYAN L., NICHOLS C.G., WECHSLER S.W., CLEMENT J.P., BOYD A.E., GONZÁLEZ G., HERRERA-SOSA H., NGUY K., BRYAN J., NELSON D.A. Cloning of the β-cell high-affinity sulphonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–425. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- BALL A.J., FLATT P.R., MCCLENAGHAN N.H. Desensitization of sulphonylurea-and nutrient-induced insulin secretion following prolonged treatment with glibenclamide. Eur. J. Pharmacol. 2000a;408:327–333. doi: 10.1016/s0014-2999(00)00782-2. [DOI] [PubMed] [Google Scholar]
- BALL A.J., FLATT P.R., MCCLENAGHAN N.H. Stimulation of insulin secretion in clonal BRIN-BD11 cells by the imidazoline derivatives KU14R and RX801080. Pharmacol. Res. 2000b;42:575–579. doi: 10.1006/phrs.2000.0739. [DOI] [PubMed] [Google Scholar]
- BALL A.J., MCCLUSKEY J.T., FLATT P.R., MCCLENAGHAN N.H. Drug-induced desensitization of insulinotropic actions of sulfonylureas. Biochem. Biophys. Res. Commun. 2000c;271:234–239. doi: 10.1006/bbrc.2000.2609. [DOI] [PubMed] [Google Scholar]
- BOKVIST K., HOY M., BUSCHARD K., HOLST J.J., THOMSEN M.K., GROMADA J. Selectivity of prandial glucose regulators: nateglinide, but not repaglinide, accelerates exocytosis in rat pancreatic A-cells. Eur. J. Pharmacol. 1999;386:105–111. doi: 10.1016/s0014-2999(99)00754-2. [DOI] [PubMed] [Google Scholar]
- BOKVIST K., HØY M., POULSEN C.R., BUSCHARD K., GROMADA J. A4166, but not repaglinide, stimulate Ca2+-evoked KATP-channel independent secretion in rat pancreatic α- and β-cells. Diabetologia. 1998;41:A139. [Google Scholar]
- BRATANOVA-TOCHKOVA T.K., CHENG H., DANIEL S., GUNAWARDANA S., LIU Y.J., MULVANEY-MUSA J., SCHERMERHORN T., STRAUB S.G., YAJIMA H., SHARP G.W. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes. 2002;51:S83–S90. doi: 10.2337/diabetes.51.2007.s83. [DOI] [PubMed] [Google Scholar]
- CHAPMAN J.C., MCCLENAGHAN N.H., COSGROVE K.E., HASHMI M.N., SHEPHERD R.M., GIESBERTS A.N., WHITE S.J., AMMALA C., FLATT P.R., DUNNE M.J. ATP-sensitive potassium channels and efaroxan-induced insulin release in the electrofusion-derived BRIN-BD11 beta-cell line. Diabetes. 1999;48:2349–2357. doi: 10.2337/diabetes.48.12.2349. [DOI] [PubMed] [Google Scholar]
- DEFRONZO R.A. Current Therapy of Diabetes Mellitus 1998St Louis: Mosby; ed [Google Scholar]
- DORNHORST A. Insulinotropic meglitinide analogues. Lancet. 2001;358:1709–1716. doi: 10.1016/S0140-6736(01)06715-0. [DOI] [PubMed] [Google Scholar]
- ELIASSON L., RENSTRÖM E., ÄMMÄLÄ C., BERGGREN P.-O., BERTORELLO A.M., BOKVIST K., CHIBALIN A., DEENEY J.T., FLATT P.R., GÄBEL J., GROMADA J., LARSSON O., LINDSTRÖM P., RHODES CJ, RORSMAN P. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic β-cells. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- FLATT P.R., BAILEY C.J. Abnormal plasma glucose and insulin responses in heterozygous (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- FLATT P.R., SHIBIER O., SZECOWKA J., BERGGREN P.-O. New perspectives on the actions of sulphonylureas and hyperglycamic sulphonamides on the pancreatic B-cell. Diab. Metabol. 1994;20:157–162. [PubMed] [Google Scholar]
- GEMBAL M., GILON P., HENQUIN J.-C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Invest. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBBLE F.M., MANLEY S.E., LEVY J.C. Randomized dose ranging study of the reduction of fasting and postprandial glucose in type 2 diabetes by nateglinide (A-4166) Diabetes Care. 2001;24:1221–1225. doi: 10.2337/diacare.24.7.1221. [DOI] [PubMed] [Google Scholar]
- GROMADA J., BOKVIST K., HOY M., OLSEN H.L., LINDSTROM P., HANSEN B.S., GOTFREDSEN C.F., RORSMAN P., THOMSEN M.K. Nateglinide, but not repaglinide, stimulates growth hormone release in rat pituitary cells by inhibition of K channels and stimulation of cyclic AMP-dependent exocytosis. Eur. J. Endocrinol. 2002;147:133–142. doi: 10.1530/eje.0.1470133. [DOI] [PubMed] [Google Scholar]
- GRUNBERGER G. Continuous versus intermittent sulphonylurea therapy in non-insulin-dependent diabetes mellitus. Drug. Saf. 1993;9:249–253. doi: 10.2165/00002018-199309040-00002. [DOI] [PubMed] [Google Scholar]
- HANEFELD M., BOUTER K.P., DICKINSON S., GUITARD C. Rapid and short-acting mealtime insulin secretion with nateglinide controls both prandial and mean glycemia. Diab. Care. 2000;23:202–207. doi: 10.2337/diacare.23.2.202. [DOI] [PubMed] [Google Scholar]
- HANSEN A.M., CHRISTENSEN I.T., HANSEN J.B., CARR R.D., ASHCROFT F.M., WAHL P. Differential interactions of nateglinide and repaglinide on the human beta-cell sulphonylurea receptor 1. Diabetes. 2002;51:2789–2795. doi: 10.2337/diabetes.51.9.2789. [DOI] [PubMed] [Google Scholar]
- HOLLANDER P.A., SCHWARTZ S.L., GATLIN M.R., HAAS S.J., ZHENG H., FOLEY J.E., DUNNING B.E. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet-treated patients with type 2 diabetes. Diab. Care. 2001;24:983–988. doi: 10.2337/diacare.24.6.983. [DOI] [PubMed] [Google Scholar]
- HORTON E.S., CLINKINGBEARD C., GATLIN M., FOLEY J., MALLOWS S., SHEN S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23:1660–1665. doi: 10.2337/diacare.23.11.1660. [DOI] [PubMed] [Google Scholar]
- HU S. Interaction of nateglinide with K(ATP) channel in beta-cells underlies its unique insulinotropic action. Eur. J. Pharmacol. 2002;442:163–171. doi: 10.1016/s0014-2999(02)01499-1. [DOI] [PubMed] [Google Scholar]
- HU S., BOETTCHER B.R., DUNNING B.E. The mechanisms underlying the unique pharmacodynamics of nateglinide. Diabetologia. 2003;46:M37–M43. doi: 10.1007/s00125-002-0935-1. [DOI] [PubMed] [Google Scholar]
- HU S., WANG S., DUNNING B.E. Glucose-dependent and glucose-sensitizing insulinotropic effect of nateglinide: comparison to sulfonylureas and repaglinide. Int. J. Exp. Diabetes Res. 2001;2:63–72. doi: 10.1155/EDR.2001.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU S., WANG S., FANELLI B., BELL P.A., DUNNING B.E., GEISSE S., SCHMITZ R., BOETTCHER B.R. Pancreatic beta-cell K(ATP) channel activity and membrane-binding studies with nateglinide: A comparison with sulfonylureas and repaglinide. J. Pharmacol. Exp. Ther. 2000;293:444–452. [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., SEINO S. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:715–720. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- KALBAG J.B., WALTER Y.H., NEDELMAN J.R., MCLEOD J.F. Mealtime glucose regulation with nateglinide in healthy volunteers: comparison with repaglinide and placebo. Diab. Care. 2001;24:73–77. doi: 10.2337/diacare.24.1.73. [DOI] [PubMed] [Google Scholar]
- KAWAKI J., NAGASHIMA K., TANAKA J., MIKI T., MIYAZAKI M., GONOI T., MITSUHASHI N., NAKAJIMA N., Iwanaga T., YANO H., SEINO S. Unresponsiveness to glibenclamide during chronic treatment induced by reduction of ATP-sensitive K+ channel activity. Diabetes. 1999;48:2001–2006. doi: 10.2337/diabetes.48.10.2001. [DOI] [PubMed] [Google Scholar]
- KEILSON L., MATHER S., WALTER Y.H., SUBRAMANIAN S., MCLEOD J.F. Synergistic effects of nateglinide and meal administration on insulin secretion in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2000;85:1081–1086. doi: 10.1210/jcem.85.3.6446. [DOI] [PubMed] [Google Scholar]
- LAGHMICH A., LADRIERE L., MALAISSE-LAGAE F., MALAISSE W.J. Long-term effects of glibenclamide and nateglinide upon pancreatic islet function in normal and diabetic rats. Pharmacol. Res. 1999;40:475–482. doi: 10.1006/phrs.1999.0551. [DOI] [PubMed] [Google Scholar]
- MARRE M., VAN GAAL L., USADEL K.H., BALL M., WHATMOUGH I., GUITARD C. Nateglinide improves glycaemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diab. Obes. Metab. 2002;4:177–186. doi: 10.1046/j.1463-1326.2002.00196.x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS D.R., CULL C.A., STRATTON I.M., HOLMAN R.R., TURNER R.C. UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diab. Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BALL A.J., FLATT P.R. Induced desensitization of the insulinotropic effects of antidiabetic drugs, BTS 67 582 and tolbutamide. Br. J. Pharmacol. 2000;130:478–484. doi: 10.1038/sj.bjp.0703306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BALL A.J., FLATT P.R. Specific desensitization of sulfonylurea- but not imidazoline-induced insulin release after prolonged tolbutamide exposure. Biochem. Pharmacol. 2001;61:527–536. doi: 10.1016/s0006-2952(00)00579-7. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BARNETT C.R., AH-SING E., ABDEL-WAHAB Y.H.A., O'HARTE F.P.M., YOON T.-W., SWANSTON-FLATT S.K., FLATT P.R. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., FLATT P.R. Physiological and pharmalogical regulation of insulin release: insights offered through exploitation of insulin-secreting cell lines. Diab. Obes. Metab. 1999a;1:1–14. doi: 10.1046/j.1463-1326.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., FLATT P.R. Engineering cultured insulin-secreting pancreatic B-cell lines. J. Mol. Med. 1999b;77:235–243. doi: 10.1007/s001090050344. [DOI] [PubMed] [Google Scholar]
- NIEMI M., BACKMAN J.T., NEUVONEN M., NEUVONEN P.J. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. Br. J. Clin. Pharmacol. 2003;56:427–432. doi: 10.1046/j.1365-2125.2003.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATLEY R.E., WEYER C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia. 2001;44:929–945. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- PROKS P., ASHCROFT F.M. Phentolamine block of KATP channels is mediated by Kir6.2. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABUAZZO A.M., BUSCEMA M., VINCI C., CALTABIANO V., VETRI M., FORTE F., VIGNERI R., PURRELLO F. Glyburide and tolbutamide induce desensitization of insulin release in rat pancreatic islets by different mechanisms. Endocrinology. 1992;131:1815–1820. doi: 10.1210/endo.131.4.1396327. [DOI] [PubMed] [Google Scholar]
- RENSTRÖM E., BARG S., THEVENOD F., RORSMAN P. Sulfonylurea-mediated stimulation of insulin exocytosis via an ATP-sensitive K(+) channel- -independent action. Diabetes. 2002;51:S33–S36. doi: 10.2337/diabetes.51.2007.s33. [DOI] [PubMed] [Google Scholar]
- ROSENSTOCK J., SHEN S.G., GATLIN M.R., FOLEY J.E. Combination therapy with nateglinide and a thiazolidinedione improves glycemic control in type 2 diabetes. Diab. Care. 2002;25:1529–1533. doi: 10.2337/diacare.25.9.1529. [DOI] [PubMed] [Google Scholar]
- SALGADO A.P., SANTOS R.M., FERNANDES A.P., TOME A.R., FLATT P.R., ROSARIO L.M. Glucose-mediated Ca(2+) signalling in single clonal insulin-secreting cells: evidence for a mixed model of cellular activation. Int. J. Biochem. Cell Biol. 2000;32:557–569. doi: 10.1016/s1357-2725(99)00146-6. [DOI] [PubMed] [Google Scholar]
- STRAUB S.G., SHARP G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diab. Metab. Res. Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- SUNAGA Y., GONOI T., SHIBASAKI T., ICHIKAWA K., KUSAMA H., YANO H., SEINO S. The effects of mitiglinide (KAD-1229), a new anti-diabetic drug, on ATP-sensitive K+ channels and insulin secretion: comparison with the sulfonylureas and nateglinide. Eur. J. Pharmacol. 2001;431:119–125. doi: 10.1016/s0014-2999(01)01412-1. [DOI] [PubMed] [Google Scholar]
- TIAN Y.-M., JOHNSON G., ASHCROFT S.J.H. Sulfonylureas stimulate exoxytosis from pancreatic β-cells by a mechanism that does not involve direct activation of protein kinase C. Diabetes. 1998;47:1722–1726. doi: 10.2337/diabetes.47.11.1722. [DOI] [PubMed] [Google Scholar]
- UTO Y., TENO S., IWAMOTO Y., OMORI Y., TAKIZAWA T. Improvement of glucose tolerance by nateglinide occurs through enhancement of early phase insulin secretion. Metabolism. 2002;51:20–24. doi: 10.1053/meta.2002.29008. [DOI] [PubMed] [Google Scholar]
- YADA T.Action mechanisms of amino acids in pancreatic B-cells Frontiers of Insulin Secretion and Pancreatic B-Cell Research 1994London: Smith-Gordon; 129–135.ed. Flatt, P.R. & Lenzen, S. pp [Google Scholar]