Abstract
Monocytes and macrophages provide key targets for the action of novel anti-inflammatory therapeutics targeted at inhibition of PDE4 cAMP-specific phosphodiesterases.
PDE4 enzymes provide the dominant cAMP phosphodiesterase activity in U937 human monocytic cells.
Differentiation of U937 monocytic cells to a macrophage-like phenotype causes a marked reduction in total cellular PDE4 activity.
Monocytic U937 cells express the long PDE4A4, PDE4D5 and PDE4D3 isoforms plus the short PDE4B2 isoform. Differentiation of U937 cells to a macrophage-like phenotype causes a marked downregulation of PDE4D3 and PDE4D5, elicits a marked upregulation of PDE4B2 and induces the novel PDE4A10 long isoform. Comparable patterns are found in human peripheral blood monocytes and macrophages differentiated from them.
Immunopurification of PDE4 subfamilies identifies long PDE4D isoforms as providing the major PDE4 activity in U937 monocytic cells.
In U937 macrophage-like cells, the activity of the short PDE4B2 isoform predominates.
No indication of either the expression or induction of PDE4C was evident.
Activation of ERK exerts an inhibitory effect on total PDE4 activity in monocytic U937 cells, where the activity of long PDE4 isoforms predominates.
The effect of ERK activation is switched to one of overall stimulation of total PDE4 activity in macrophage U937 cells, where the activity of the short PDE4B2 isoform predominates.
The profound differentiation-induced changes in PDE4 isoform profile identified here suggests that the development of inhibitors specific for particular PDE4 isoforms may allow for selective effects on monocytes and macrophages to be achieved.
Keywords: PDE4 cAMP-specific phosphodiesterase, rolipram, ERK, monocyte, macrophage, U937
Introduction
Tissue macrophages share a common precursor, namely the circulating monocyte (Mazzarella et al., 1998). These cells play a key role in cellular defence systems and in the process of inflammation (Gordon, 1998). Indeed, alveolar macrophages are considered to play a key role in eliciting the tissue injury seen in inflammatory diseases of the lung, such as chronic obstructive pulmonary disease (COPD) and asthma (Arm & Lee, 1992; Barnette & Underwood, 2000; Giembycz, 2002; Barnes, 2003). It is now well recognised that increases in the intracellular second messenger cyclic AMP in these cells have a potent anti-inflammatory action (Spina et al., 1998a; Barnette & Underwood, 2000; Huang et al., 2001; Giembycz, 2002; Barnes, 2003). Such functional attenuation of these cells can be achieved by the use of inhibitors selective for PDE4 cAMP phosphodiesterases (Houslay et al., 1998; Conti & Jin, 1999; Houslay, 2001; Conti et al., 2003; Houslay & Adams, 2003). Indeed, there is currently much interest in such compounds as potential therapeutic agents for treating conditions such as COPD and asthma (see, for example, Dent et al., 1998; Spina et al., 1998a, 1998b; Schudt et al., 1999; Barnette & Underwood, 2000; Burnouf & Pruniaux, 2002; Giembycz, 2002).
PDE4 phosphodiesterases specifically hydrolyse cAMP to 5′ AMP and provide one of a large family of enzymes able to break down this key second messenger (Manganiello & Degerman, 1999; Barnette & Underwood, 2000; Francis et al., 2001; Houslay, 2001; Beavo & Brunton, 2002; Conti et al., 2003; Maurice et al., 2003). The four PDE4 subfamilies are encoded by separate genes (A, B, C, D) that generate a myriad of isoforms through the use of alternative mRNA splicing and distinct promoters (Houslay et al., 1998; Conti & Jin, 1999; Houslay, 2001; Conti et al., 2003; Houslay & Adams, 2003). Isoforms generated by the four PDE4 subfamilies are each individually characterized by unique N-terminal regions. However, they can be divided into the so-called long forms, which possess both the Upstream Conserved Region 1 (UCR1) and Upstream Conserved Region (UCR2) regulatory modules, while the short isoforms lack UCR1 and the super-short isoforms not only lack UCR1 but also have a truncated UCR2 (Bolger et al., 1993). One key factor that distinguishes these various isoforms is their susceptibility to regulation by phosphorylation (Conti et al., 2003; Houslay & Adams, 2003). This was first shown for the PDE4D3 isoform (Sette et al., 1994), where PKA causes activation through the phosphorylation of a single serine residue in UCR1 (Sette et al., 1996; Hoffmann et al., 1998). Indeed, such phosphorylation and accompanying activation are characteristic of long isoforms from all the four PDE4 subfamilies as they exhibit a conserved PKA consensus phosphorylation site in UCR1 (MacKenzie et al., 2002). This module is lacking in the short and super-short isoforms, which do not provide targets for stimulatory PKA phosphorylation. Subsequently, it was shown that the catalytic units of all PDE4 subfamilies, save that of PDE4A, have a consensus site allowing phosphorylation by the MAP kinase ERK2 (Hoffmann et al., 1999; Baillie et al., 2000; MacKenzie et al., 2000). The functional outcome of this modification is orchestrated by the regulatory UCR1/2 modules, which direct long isoforms to be inhibited and short forms to be activated upon phosphorylation of their catalytic units by ERK2 (Baillie et al., 2000; MacKenzie et al., 2000).
The majority of PDE4 selective inhibitors reported on to date serve to inhibit PDE4 isoforms from all the four subfamilies with either little or no PDE4 subfamily selectivity (Barnette & Underwood, 2000; Huang et al., 2001; Burnouf & Pruniaux, 2002; Giembycz, 2002). However, future approaches are likely to be directed towards those subfamilies and isoforms present in key cellular targets. Such an approach can be expected to allow novel therapeutics to be developed for disease states that are associated primarily with specific cell types and also may help to widen the effective therapeutic window of PDE4 selective inhibitors, which has been compromised by side effects such as nausea (Barnette & Underwood, 2000; Huang et al., 2001; Burnouf & Pruniaux, 2002; Giembycz, 2002). Monocytes and macrophages provide key targets for the anti-inflammatory action of PDE4-selective inhibitors (Spina et al., 1998a, 1998b; Barnette & Underwood, 2000; Burnouf & Pruniaux, 2002; Giembycz, 2002). However, little is known about the expression pattern and regulation of PDE4 isoforms in these cells, save for the key findings that the PDE4B gene is crucial for the pro-inflammatory action of macrophages, as derived from targeted gene knockout studies (Jin & Conti, 2002), and also that the in vitro differentiation of cultured peripheral blood monocytes to a macrophage-like phenotype leads to a downregulation of PDE4 activity together with an upregulation of PDE3 and PDE1 activities (Tenor et al., 1995; Gantner et al., 1997). As monocyte to macrophage differentiation causes a marked reduction in total PDE4 activity, it is clearly important to establish if this is either simply due to an equivalent reduction in the expression of all PDE4 isoforms or whether differentiation leads to a remodelling of the PDE4 isoform profile, thus providing potential for selective therapeutic intervention. Analyses of blood and airway derived monocytic and macrophage cells are hampered by subject variability and availability of material as well as the small numbers of cells that can be derived, making it difficult to perform identification of low abundance signalling proteins such as PDE4 isoforms. We thus set out here to analyse a model system, namely the human U937 monocytic cell line, which has been shown to be capable of PMA-induced differentiation to adopt a macrophage-like phenotype (Hass et al., 1989; Twomey et al., 1993; Kuroda et al., 1997; Fukunaga & Tsuruda, 2001; Matheson et al., 2002).
Methods
Materials
U937 cells were from NCBI. RPMI 1640 cell culture medium, foetal bovine serum and trypsin EDTA were from GibCo Life Technologies Paisley, U.K. Rolipram, cilostamide and phorbol-12-myristate 13-acetate (PMA) were from Sigma Aldrich, U.K. UO126 and SB203580 were from Promega, U.K. All other chemicals used in this work were from Sigma Aldrich, U.K., and were of analytical grade. Rolipram, cilostamide, PMA, SB203580 and UO126 were dissolved in DMSO at 100 times their working concentrations used in the assay. Such levels of DMSO did not affect the activity of PDE4 subfamily enzymes. All other solutions were prepared in distilled water. Antisera specific for each of the four PDE4 subfamilies (MacKenzie & Houslay, 2000) as well as for the specific isoforms PDE4A4 (Huston et al., 1996; McPhee et al., 1999), PDE4A10 (Rena et al., 2001) and PDE4D5 (Yarwood et al., 1999) were all as described previously by us. Antisera for ERK2 and P-ERK2 were from Cell Signalling Technologies (MA, U.S.A.), for COX-2 from Upstate Biotech (NY, U.S.A.), for PKC-β from Transduction Labs (KY, U.S.A.) and for CD11b from Santa Cruz Biotech (CA, U.S.A.).
Cell growth, differentiation and acute challenge with PMA
U937 cells were maintained in RPMI medium (Gibco Life Tech), supplemented with penicillin 1 unit ml−1, streptomycin 1 mg ml−1 and enriched with 10% v v−1 foetal bovine serum and 2 mM L-glutamine (complete RPMI). Cells were passaged at a density of approximately 2 × 106 cells ml−1. In experiments comparing differentiated, ‘macrophage-like' U937 cells to control, ‘monocyte-like' U937 cells, then cells from the same passage were used. U937 cell differentiation was achieved as described previously by others (Hass et al., 1989; Twomey et al., 1993; Kuroda et al., 1997; Fukunaga & Tsuruda, 2001; Matheson et al., 2002). Briefly, this was done by incubating 1 × 106 cells ml−1 with complete RPMI medium supplemented with 4 nM PMA for a total of 4 days. Medium was changed after 2 days and nonadherent cells were removed unless being assayed for adherence. After 4 days, cells were transferred to complete RPMI and measurements were made following 24 h in PMA free medium. Where cells were treated acutely with PMA (50 nM), they were maintained for the previous 16 h in serum-free medium and, in the case of the macrophage-like cells, medium that was free of PMA for 24 h. Signal transduction inhibitors, as specified in text and legends, were added 20 min before treatment with PMA (50 nM), and vehicle-only treatments were included as control. To ensure that PMA activated ERK in both forms of U937 cells, Western blot analysis was done using anti-serum raised against the phosphorylated residue of ‘active' ERK with total ERK identified by Western blot analysis to ensure equal loading of ERK in each lane. These experiments were repeated in the presence of the MEK inhibitor UO126 (10 μM).
Cell adherence
Cells were grown in 75 cm2 flasks containing glass coverslips of known surface area with either complete RPMI or RPMI containing 4 nM PMA. After 5 days of treatment with PMA (differentiation) nonadherent cells were aspirated, coverslips were removed and adherent cells were counted using a haemocytometer with viable cells identified by trypan blue exclusion. The remaining adherent cells were suspended by gentle incubation with trypsin, added to the nonadherent fraction and the total cell number was calculated. From these two quantities, the proportion of adherent cells (adherence index) was calculated.
Immunological characterization of U937 cells
Expression of CD-11b, COX-2, PKCβ and PDE4 isoforms were characterized using either Western blot or ELISA analysis, as indicated. For Western blotting, cell lysate protein was separated on SDS–acrylamide gels by electrophoresis and transferred to nitrocellulose membranes (Bio-RAD). Membranes were blocked in 5% milk protein in Tris-buffered saline containing 1% tween (TBSt) solution before overnight refrigerated incubation with anti-serum raised against the target protein. Membranes were washed the following day in TBSt and incubated at room temperature with the appropriate detection antibody conjugated to horseradish peroxidase (Sigma, U.K.). Proteins were visualized using the ECL method (Amersham, U.K.) and detected using an autoradiographic film (Kodak, U.K.). Where comparative immunoblots were prepared, the amounts of protein representing equal cell numbers were loaded. In this way, the measured differences in cellular protein content between monocytic and macrophage U937 cells were accounted for. Cell number was calculated by both DNA analysis and cytometry. Quantification of Western blotting was done as described previously by us under conditions where linear responses were obtained (Huston et al., 1996; McPhee et al., 1999).
Protein isolation and immunoprecipitation
This was done as described before by us (MacKenzie et al., 1998; MacKenzie & Houslay, 2000). Briefly, detergent soluble proteins were isolated from cells by disruption in lysis buffer (1% (v v−1) Triton X-100, 50 mM HEPES buffer pH 7.2, 10 mM EDTA, 100 mM NaH2PO4, 2H2O) containing complete protease inhibitor mixture (Roche Molecular Biochemicals) to 8% volume. Detergent insoluble proteins were removed by centrifugation at 10,000 × gav for 10 min and the soluble fraction was retained. Note that in cells studied here this procedure extracted and solubilised all of the immunoreactive PDE4 species as assessed by immunoblotting (>94%). To quantify the proportion of total PDE4 activity accounted for by the activity of individual PDE4 subfamilies, quantitative immunopurification was done using anti-sera developed against the C-terminal regions of PDE4A, PDE4B, PDE4C and PDE4D, as described before in some detail (MacKenzie et al., 1998; MacKenzie & Houslay, 2000). Briefly, equal volumes of cell lysate containing 500 μg protein were cleared by incubation with 30 μl pre-immune serum and 30 μl protein A slurry. The beads were then removed by centrifugation at 10,000 × gav for 10 min at 4°C and cleared lysate was incubated at 4°C for 2 h with constant agitation with a volume of antiserum previously determined to immunoprecipitate all of the specific PDE4 subfamily. Immunoglobulins were then isolated by incubation with protein-A-coated sepharose beads for 1 h before retrieval by refrigerated centrifugation at 10,000 × gav for 5 min. PDE4 isoform–immunoglobulin conjugates attached to the beads were then washed in phosphate-buffered saline (PBS) twice before two further washes in KHEM (50 mM KCl, 10 mM EGTA, 50 mM HEPES and 1.92 mM MgCl2 (pH 7.2) with added 0.001 mM dithiothreitol and complete protease inhibitor mixture to 8% volume prior to use) and one wash in PDE4 assay buffer (20 mM Tris, pH 7.4) prior to use. In doing this, we ensured that the complete and specific immunopurification of the entire pool of the indicated PDE4 subfamily was achieved by Western blotting the supernatant with antisera for the four PDE4 subfamilies. Immunopurified material thus acquired was used for either Western blot analysis or PDE4 assay.
Harvesting and lysis of cells for PDE assay
This was done essentially as described previously by us (MacKenzie & Houslay, 2000). Monocytic U937 cells were harvested by centrifugation for 3 min at 1000 × g at 4°C. The medium was removed and the cell pellet rinsed with PBS (137 mM NaCl, 3 mM KCl, 1 mM KH2PO4, 6mM Na2HPO4, pH 7.4) prior to its removal and subsequent addition of lysis buffer. For the adherent, differentiated macrophage-like U937 cells, the medium was first aspirated and the cells rinsed with ice-cold PBS, which was then aspirated before lysis buffer was added. The lysis buffer used to extract PDE activities for PDE assay was made from 25 mM HEPES, 2.5 mM EDTA, 50 mM NaCl, 50 mM NaF, 30 mM Na pyrophosphate, 10% glycerol, 1% Triton X-100 (pH 7.5) with added protease inhibitors (complete EDTA-free protease inhibitor cocktail, Roche diagnostics GmbH, Mannheim, Germany; one tablet to 50 ml buffer to give final concentrations of 40 μg ml−1 PMSF, 150 μg ml−1 benzamine, 1 μg ml−1 aprotinin, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin A and 1 μg ml−1 antipain). Lysis was allowed to progress on ice for 1 h before gentle agitation and detergent insoluble proteins were removed by centrifugation (12,000 × gav, 10 min, 4°C) and the supernatant retained for further analysis. Such a procedure released >96% of total cAMP PDE activity.
Phosphodiesterase assay
PDE activity was assayed using a modification of the Thompson and Appleman two-step procedure (Thompson & Appleman, 1971) as described previously by us (Marchmont & Houslay, 1980). Briefly, a 2 μM mixture of [3H] cAMP (Amersham Biosciences) and unlabelled cAMP in 20 mM Tris (pH 7.4), 10 mM MgCl2 assay buffer was mixed with PDE4 isoform immunoprecipitates or complete cell lysates to a final concentration of 1 μM cAMP. To determine the fraction of activity due to PDE3 and PDE4 families, PDE activity was measured when the selective inhibitors cilostamide 1 μM (Manganiello & Degerman, 1999) or rolipram 10 μM (Houslay & Adams, 2003), respectively, were included in the reaction mixture. PDE1 activity towards cAMP (1 μM substrate) was assessed as done before by us (Spence et al., 1997), namely as that activity increased by the addition of Ca2+ (5 mM) and calmodulin (10 U) in the presence of EGTA (2 mM). Reactions were then incubated at 30°C for 20 min with frequent agitation. Cyclic AMP hydrolysis was stopped by plunging the tubes into boiling water for 3 min and then placing on ice for 10 min. In all, 25 μl of venom from Crotalus attrox (Sigma) was added and the samples incubated at 30°C for a further 10 min. Dowex resin (Sigma 1X8-400) pH 3 stored as a 50 : 50 dowex/water mixture was prepared immediately prior to the assay by the addition of 1 volume of 100% ethanol to 2 volumes of the Dowex/water mixture to create a slurry. A measure of 400 μl of the Dowex slurry was added to each reaction, vortexed and placed on ice for 15 min. During this period, samples were frequently mixed by repeated inversion. Samples were then vortexed before being centrifuged at 13,000 × gav at 4°C for 3 min. Then, 150 μl of the supernatant fraction from each tube was placed in a corresponding scintillation vial containing 1 ml of scintillant and taken for counting on a beta counter.
Protein determination
Protein concentration was determined using the Bradford method (Bradford, 1976).
Preparation of monocyte-derived macrophages
This was done as described by others previously in some detail (Gantner et al., 1997). Briefly, 50 ml of blood was drawn from normal subjects and cells were sedimented by centrifugation at 220 × gav. The cell pellets were mixed with 10 ml RPMI medium, layered over an equal volume of Histopaque-1077 (Sigma) and subjected to centrifugation at 400 × gav for 30 min. The mononuclear cell layer was aspirated from the interphase and then gently mixed with 10 ml PBS and the harvested cells sedimented by centrifugation at 250 × gav for 10 min. Monocytic cells were taken for analysis at this point as detailed above for experiments done on U937 cells. However, in order to induce macrophage differentiation of the isolated mononuclear cells, they were resuspended in RPMI containing 5% foetal calf serum, pooled and then left to adhere in cell culture flasks. They were incubated at 37°C and 5% CO2 for 1 h, after which nonadherent cells were washed off and fresh medium added. Differentiation to a monocyte phenotype, through adherence, was achieved by continuing incubations for a period of 6 days in total (Gantner et al., 1997), at which point adhering cells were taken for various experiments done on U937 cells, as detailed above.
Results
Differentiation of U937 monocytic cells
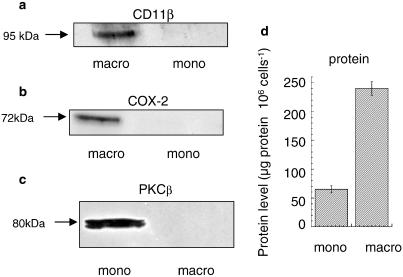
U937 cells are a human monocytic-like cell line that grows in suspension culture. It has been shown previously by various investigators (Hass et al., 1989; Twomey et al., 1993; Kuroda et al., 1997; Fukunaga & Tsuruda, 2001; Matheson et al., 2002) that chronic treatment with PMA over a period of several days causes them to differentiate to a macrophage-like phenotype. Following the protocol of these investigators, we treated U937 cells for 4 days with PMA (4 nM) to generate macrophage-like cells. One indicator of such differentiation is an ability of macrophage-like cells to adhere to surfaces (Hass et al., 1989). We observed here that while the untreated monocytic U937 cells are unable to adhere to glass surfaces (1–3% adherence; range n=3), the macrophage-like cells were able to adhere (75–95% adherence; range n=3). A further indicator of differentiation to a macrophage-like phenotype is expression of the β2 integrin CD11b (Hass et al., 1989, 1990; Prudovsky et al., 2002), which is clearly evident in PMA-differentiated cells but not in untreated, monocyte cells (Figure 1a). Induction of COX2 has also been reported as a feature of the differentiation of U937 cells to a macrophage-like phenotype (Koehler et al., 1990) and is characteristic of alveolar macrophages (Ermert et al., 2000). Indeed, here we see that while no immunoreactive COX2 is evident in U937 monocytic cells it is clearly expressed in cells chronically treated with PMA to elicit differentiation (Figure 1b). It has also been reported that levels of the PKCβ isoform are significantly reduced in alveolar macrophages compared to monocytes (Monick et al., 1998). Indeed, we find here that immunoreactive PKCβ is indeed present in U937 monocytic cells but not in the differentiated cells (Figure 1c). However, as differentiation of U937 cells was elicited by PMA, the lack of PKCβ might well be considered to be due to its downregulation caused by chronic exposure to PMA rather than, necessarily, to downregulation consequent on the differentiation process itself. Notwithstanding this, it is clear that, as with alveolar macrophages, the PMA-differentiated U937 cells lack the PKCβ isoform. It has been noted that differentiation of monocytes to macrophages is accompanied by a large increase in total protein per cell (Gantner et al., 1997), and we observe a similar marked change here in the differentiation of U937 monocytic cells (Figure 1d). Owing to this, all the comparative expression and activity analyses done in this study were analysed on a ‘per cell' basis rather than by comparing specific activities, which would necessarily be compromised by the altered protein per cell ratio occurring upon differentiation.
Figure 1.
Differentiation of U937 monocytic cells. Shows Western blots comparing expression of (a) CD11b, (b) COX2 and (c) PKCβ doublet in nondifferentiated, monocyte-like (mono) and differentiated, macrophage-like (macro) U937 cells. In each case, the protein-loading amounts used reflected equal cell numbers. These data are typical of experiments done at least three times. In (d) is shown the protein content per cells for nondifferentiated, monocyte-like (mono) and differentiated, macrophage-like (macro) U937 cells.
The various indicators and changes reported here (Figure 1 and above) are consistent with the U937 cells undergoing differentiation, whereby they change from a monocyte-like phenotype to a macrophage-like phenotype, consequent upon chronic treatment with PMA.
Changes in PDE4 isoform expression upon differentiation of U937 monocytic cells
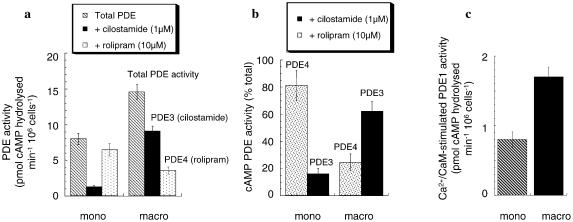
Shown in Figure 2 is the cAMP PDE activity, assayed at 1 μM cAMP substrate, for whole-cell lysates of human U937 monocytic cells. The PDE4 selective inhibitor rolipram (10 μM) and the PDE3 selective inhibitor cilostamide (1 μM) are used to determine the activity of these enzyme families in lysates from U937 cells. Doing this allows us to determine that the majority of the cAMP PDE activity in U937 monocytic cells is provided by the action of enzymes of these two PDE families. Furthermore, the activity of PDE4 clearly predominates, providing around 80% of the total cAMP PDE activity (Figure 2). These data are consistent with those of others (Torphy et al., 1992) who used a chromatographic procedure to separate PDEs from U937 monocytic cells, and showed that around 70–90% of total cAMP PDE activity was due to that of PDE4 enzymes, whilst the remainder was due to PDE3.
Figure 2.
Altered cAMP PDE activity upon differentiation of U937 monocytic cells. Shown for nondifferentiated, monocyte-like (mono) and differentiated, macrophage-like (macro) U937 cells is (a) the total cAMP PDE activity, the PDE4 rolipram-inhibited (10 μM) PDE activity and the PDE3 cilostamide-inhibited (1 μM) PDE activity, assayed at 1 μM cAMP substrate. The activity is given as pmol cAMP hydrolysed min−1 106 cells−1. (b) The % age of total PDE activity that is provided by PDE4 and by PDE3, through selective inhibition using rolipram (10 μM) and cilostamide (1 μM), respectively. (c) PDE1 activity towards cAMP is given here as that activity increased by the addition of Ca2+ (5 mM) and calmodulin (10 U) in the presence of EGTA (2 mM). We did note, however, that similar levels of activity were found when adding Ca2+ and calmodulin alone. These data are for experiments done three times with means±s.d., n=3.
The PMA-mediated differentiation of U937 cells to a macrophage-like phenotype elicits a profound increase in PDE3 activity, coupled to a decrease in that of PDE4, such that PDE4 activity provides only around 25% of the total cAMP PDE activity in macrophage-like U937 cells (Figure 2a, b). Ca2+/calmodulin-stimulated PDE1 activity appears to provide a minor fraction of the total cAMP PDE in monocytic U937 cells (Figure 2c). However, it has been shown that when human peripheral blood monocytes are differentiated to macrophages this causes an increase in PDE1 activity (Gantner et al., 1997; Bender et al., 2004). Consistent with differentiation affecting PDE1, we observed that Ca2+/calmodulin-activated PDE1 activity roughly doubled upon differentiation of U937 cells to a macrophage-like phenotype (Figure 2c).
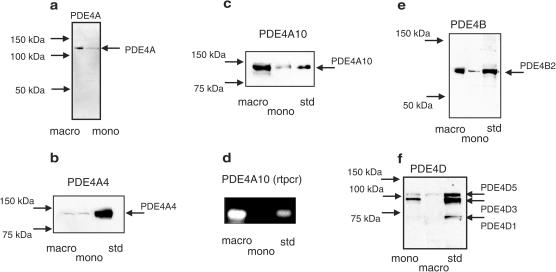
As four genes, encoding a number of isoforms, provide PDE4 activity in cells (Houslay, 2001; Conti et al., 2003; Houslay & Adams, 2003), we set out to evaluate whether this differentiation-induced remodelling of cAMP hydrolysis in U937 cells extended to alterations in PDE4 isoform expression. All active isoforms within a specific PDE4 subfamily have identical C-terminal regions (Swinnen et al., 1989; Bolger et al., 1993; Houslay et al., 1998; Conti et al., 2003). However, the sequence of such regions is different in each of the four PDE4 subfamilies. Antisera specific for the extreme C-terminal portion of each PDE4 subfamily have been generated and characterised previously by us (Bolger et al., 1997; MacKenzie et al., 1998; McPhee et al., 1999; MacKenzie & Houslay, 2000). These are able to detect all active isoforms within any particular PDE4 subfamily by virtue of their common conserved C-terminal regions. Immunoblotting with PDE4A-specific antisera identifies an immunoreactive species of around 121 kDa in lysates from differentiated U937 cells (Figure 3) and a weak, similarly migrating species in lysates from U937 monocytic cells. This species migrates at the size similar to that of both the novel PDE4A isoform PDE4A10 (121 kDa) (Rena et al., 2001), and the PDE4A4 isoform (124 kDa) (Huston et al., 1996); two species that are not able to be effectively resolved on SDS–PAGE because of their similar size (Rena et al., 2001). Thus, in order to discriminate between these two species, we used isoform-specific antisera, targeted to the unique N-terminal regions that characterise these two isoforms. Such an approach clearly shows an induction of the novel PDE4A10 isoform upon differentiation (Figures 3, 4), with no change in the low levels of expression of the PDE4A4 isoform (Figures 3, 4). To provide additional support for the induction of PDE4A10, we undertook a transcript analysis using RT–PCR. These data clearly showed transcripts for PDE4A10 in the macrophage-like, but not the monocyte-like U937 cells (Figure 3), which is consistent with the immunoblot data.
Figure 3.
Altered PDE4 isoform expression upon differentiation of U937 monocytic cells. Comparative analyses done for nondifferentiated, monocyte-like (mono) and differentiated, macrophage-like (macro) U937 cells. (a) Western blot using antisera specific for PDE4A isoforms; (b) Western blot using antisera specific for the PDE4A4 isoform; (c) Western blot using antisera specific for the PDE4A10 isoform; (d) RT–PCR for PDE4A10 transcripts; (e) Western blot using antisera specific for PDE4B isoforms and (e) Western blot using antisera specific for PDE4D isoforms. In all cases, comparisons were done using loadings that equated to equal numbers of cells. This, for western blots, was protein from 0.75 × 106 cells per track. These data are typical of experiments done at least three times.
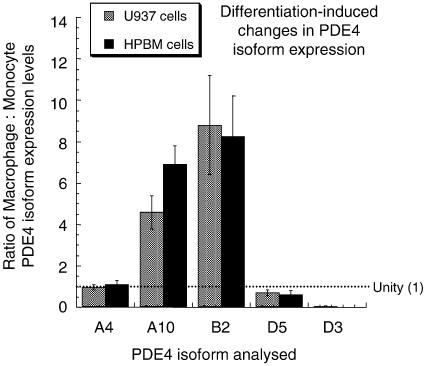
Figure 4.
Altered PDE4 isoform expression upon monocyte-macrophage differentiation in U937 cells and human peripheral blood monocytes. Densitometric scanning of immunoblots similar to and including those shown in Figure 3 was carried out to compare the relative levels of expression of the indicated PDE4 isoforms. The isoforms detected and analysed were the long PDE4A4 (A4), PDE4A10 (A10), PDE4D3 (D3) and PDE4D% (D5) isoforms, as well as the short PDE4B2 (B2) isoform. Results are shown as ratios to indicate relative changes, and are done by comparing expression on a per cell basis, where equal expression is a value of 1 (unity). Data are shown for both the differentiation of U937 monocytic cells to macrophages and also primary human peripheral blood monocytes plated out and allowed to differentiate to macrophages, as in Methods. Data are shown as means±s.d. for n=3 separate experiments.
Immunoblotting with PDE4B antisera identifies a single immunoreactive species of 80 kDa in macrophage-like cells (Figure 3), which is consistent with the presence of the PDE4B2 short form (Huston et al., 1997). The presence of transcripts for PDE4B2 was confirmed by RT–PCR using specific primers (data not shown). However, while monocytic U937 cells show a similarly migrating, single immunoreactive species, its levels are clearly very much lower than those seen in the macrophage-like cells (Figures 3, 4). Thus, differentiation to a macrophage-like phenotype increases the level of expression of the short PDE4B2 isoform (Figures 3, 4).
Immunoblotting with PDE4D-specific antisera identifies two discrete immunoreactive species in monocyte-like U937 cells of 95 and 105 kDa, which indicate (Sette & Conti, 1996; Bolger et al., 1997) the presence of the PDE4D3 and PDE4D5 long isoforms, respectively (Figure 3). In these cells, PDE4D3 clearly predominated over PDE4D5 (Figures 3, 4). The presence of these two isoforms was confirmed by RT–PCR using specific primers and for PDE4D5 using an isoform-specific antiserum (data not shown). However, differentiation of U937 cells to a macrophage-like phenotype clearly causes a profound decrease in the expression levels of PDE4D3, which is accompanied by a marked reduction in levels of PDE4D5 (Figures 3, 4).
We failed to identify any PDE4C immunoreactive species by immunoblotting and failed to immunopurify PDE4 activity, using PDE4C-specific antisera, from both monocyte and differentiated forms of U937 cells (data not shown). Indeed, both Barnette, Torphy and co-workers (Torphy et al., 1992) and Wang and co-workers (Ma et al., 1999; Wang et al., 1999) failed to identify transcripts for the PDE4C subfamily in human monocytes, while finding transcripts identifying the other three PDE4 subfamilies.
Immunoblotting of both primary human peripheral blood monocytes and the macrophages that can be induced to differentiate from them yielded identical patterns of PDE4 isoform expression to those seen here for the U937 model system (data not shown). Thus, we noted comparable changes in expression levels of PDE4 isoforms occurring upon differentiation to macrophages (Figure 4). This suggests that the monocyte and macrophage states of U937 cells provide an effective model for analysing PDE4 isoform expression and functioning of human monocytes and macrophages. The one observable difference, however, was in the ratio of expression of the long PDE4D5 and PDE4D3 isoforms in monocytes, where densitometric scanning indicated a PDE4D5 : PDE4D3 expression ratio of 0.32±0.03 in U937 monocytes and 3.1±0.5 in human peripheral blood monocytes (means±s.d.; n=3).
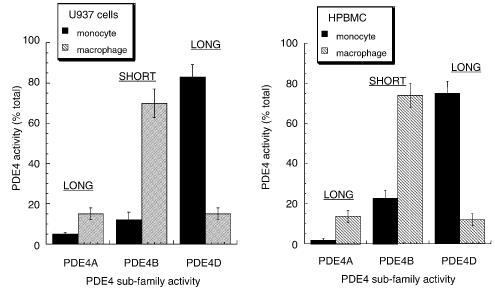
We have shown before that it is possible to assess the activity provided by each of the four PDE4 subfamilies by selective immunopurification using appropriate antisera directed at the unique C-terminal region that characterises each of the subfamilies (see, for example, MacKenzie et al., 1998; MacKenzie & Houslay, 2000). In doing this, we see that the activity of the PDE4D subfamily provides the major fraction of total PDE4 activity in monocytic U937 cells (Figure 5). Thus, PDE4D3 and PDE4D5 (Figures 3, 4), which are both long isoforms (Bolger et al., 1997; Houslay et al., 1998; Conti et al., 2003), provide the dominant PDE4 activity in U937 monocytic cells (Figure 5). In marked contrast to this, differentiation of U937 cells to a macrophage-like phenotype profoundly reduces the importance of PDE4D isoforms to total PDE activity, where they now contribute less than 20% of the total (Figure 4). In these differentiated macrophage-like cells, it is now the PDE4B2 short isoform, the sole PDE4B species expressed in both forms of U937 cells, that provides the dominant PDE4 activity (around 70%) (Figure 5). Analysis of PDE4A activity, by immunopurification using a PDE4A-specific antiserum, shows that this subfamily provides around 4% of the total PDE4 activity in U937 cells (Figure 5), which is due to the activity of PDE4A4, the sole identifiable species present in these cells (Figure 3). However, differentiation to a macrophage-like phenotype induces PDE4A10 expression (Figure 3) and this leads to around a four-fold increase in PDE4A activity, such that the PDE4A subfamily now provides some 16% of the total PDE activity. Similar data were obtained using human peripheral blood monocytes and the macrophages differentiated from them (Figure 5). In each case, we noted that the fold increase in PDE4B2 protein (Figure 4) was somewhat greater than the apparent increase in activity (Figures 4, 5). This may indicate that in one or other of these cell types PDE4B2 is modified in some way so as to alter its activity. This could be through post-translational effects or by association with another protein. In the latter regard, the inhibitory association of PDE4A5, the rodent homologue of PDE4A4, with the immunophilin XAP2 stands as a precedent (Bolger et al., 2003).
Figure 5.
Altered PDE4 subfamily activity upon differentiation of U937 monocytic cells. (a) Shown for nondifferentiated, monocyte-like and differentiated, macrophage-like U937 cells is the relative contribution (%) of cAMP PDE activity supplied by each of the PDE4 subfamilies, as determined by selective immunopurification using specific antisera. In each case, a sufficient amount of antiserum was added to ensure complete immunopurification of that specific PDE4 subfamily, while at the same time not affecting any of the other PDE4 subfamilies, as determined by Western blotting of the remaining lysates. The total PDE4 activity immunoprecipitated using these antisera accounted for >90% of the total input lysate PDE4 activity, as determined by inhibition using 10 μM rolipram. No detectable activity was found using the PDE4C-specific antiserum (<2% total). (b) shows similar studies done on human peripheral blood monocytes and the macrophage form differentiating from them. These data are for experiments done three times with means±s.d., n=3.
Activation of ERK by acute PMA challenge of U937 monocyte- and macrophage-like cells on cellular PDE4 activity
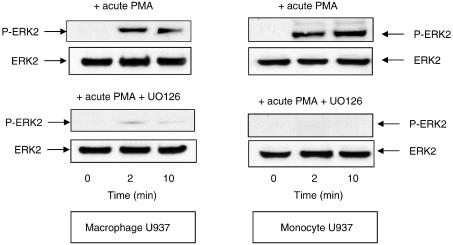
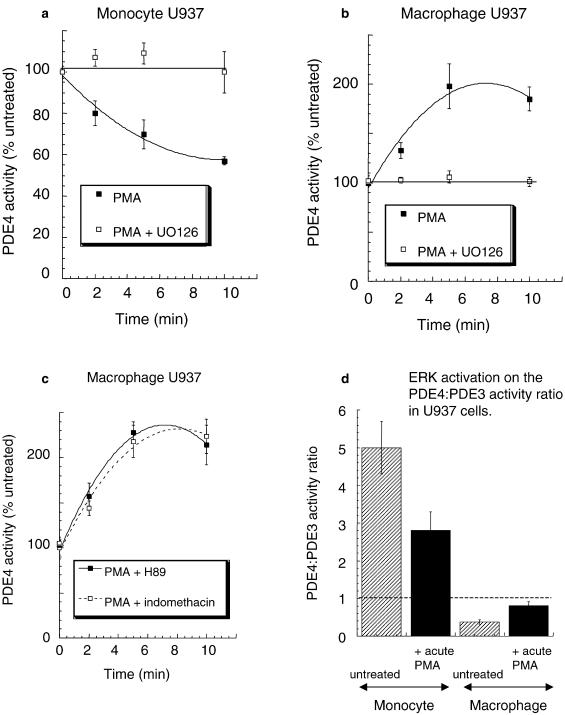
The endotoxin-mediated activation of monocytes to generate pro-inflammatory cytokines is critically dependent on activation of the ERK MAP kinase pathway (van der Bruggen et al., 1999), as is the ability of macrophages to be activated, for example, in generating GMCSF (Koch et al., 2003). Various investigators (see, for example, Franklin et al., 1993; Biggs et al., 1998; Ragg et al., 1998) have shown that the acute challenge of U937 cells with PMA causes the activation of ERK. We show here that acute challenge with PMA causes the accumulation of P-ERK2 in both U937 monocyte- and macrophage-like cells (Figure 6). It has been shown that the ERK2-mediated phosphorylation of the catalytic unit of both the PDE4D3 and PDE4D5 long isoforms leads to their inhibition (Hoffmann et al., 1999; Baillie et al., 2000; MacKenzie et al., 2000). In contrast to this, ERK2-mediated phosphorylation of the PDE4B2 short isoform by ERK leads to its activation, while the catalytic unit of PDE4A isoforms is not phosphorylated by ERK due to a lack of consensus motif (Baillie et al., 2000). We show here that acute challenge of U937 monocytic cells with PMA leads to the clear time-dependent inhibition of total PDE4 activity in these cells (Figure 7a). In profound contrast to this, acute challenge of U937 macrophage-like cells with PMA causes a marked, time-dependent increase in total PDE4 activity over a matter of minutes (Figure 7b). These distinctive PMA-induced changes in PDE4 activity are, in both instances, ablated by the ERK inhibitor UO126 (Figures 7a, b). This is consistent with the involvement of ERK in causing these diametrically opposed alterations in overall cellular PDE4 activity.
Figure 6.
ERK2 phosphorylation upon acute challenge with PMA. Shown are Western blots for both (a) nondifferentiated, monocyte-like and (b) differentiated, macrophage-like U937 cells. Identification was done using antisera specific for activated phospho ERK2 (P-ERK2) and also for ERK2 itself to indicate equal loading of lanes. These data are for a time course of cells treated acutely with PMA (50 nM) for the indicated time. Evaluation was done using loadings that equated to equal numbers of cells. In this instance, protein was from 0.75 × 106 cells per track. These data are typical of experiments done at least three times.
Figure 7.
Altered ERK regulation of PDE4 activity upon differentiation of U937 monocytic cells. Shown is the rolipram-inhibited cAMP PDE4 activity of lysates from both nondifferentiated, monocyte-like (a) and differentiated, macrophage-like (b, c) U937 cells challenged for the indicated time with PMA (50 nM). In some instances, cells were also treated with (a, b) the ERK inhibitor, UO126 (10 μM), (c) the PKA inhibitor H89 (1 μM) and (c) the COX inhibitor indomethacin (100 nM). In (d) is shown the ratio of PDE4/PDE3 activity in U937 monocytic and macrophage cells challenged acutely with PMA (50 nM) for 10 min prior to harvesting for PDE assay. PDE4 and PDE3 activities were determined here as those components inhibited by 10 μM rolipram and 1 μM cilostamide, respectively, added to assays done with 1 μM cAMP as substrate. All experiments were done three times with means±s.d., n=3. Activity data for PDE3 and PDE4 in monocyte-like and differentiated, macrophage-like U937 cells are as shown in Figure 2.
It has been noted, in aortic smooth muscle cells, that activation of ERK can lead to the activation, rather than inhibition, of long isoforms (Liu & Maurice, 1999; Baillie et al., 2001) via a process involving their phosphorylation by PKA (Baillie et al., 2001). This is because ERK activation can lead to the generation of PGE2 production through a process involving phospholipase A2 and COX2 (Baillie et al., 2001). Thus, an autocrine effect is established whereby PGE2 serves to stimulate adenylyl cyclase to generate cAMP, which then activates PKA. Such activated PKA will phosphorylate PDE4 long isoforms, at a conserved serine within a PKA consensus motif in UCR1, which not only causes PDE4 activation but also serves to nullify the inhibitory effect caused by ERK phosphorylation of the PDE4 catalytic unit (Hoffmann et al., 1999; Baillie et al., 2000, 2001; MacKenzie et al., 2000). However, we see here that the COX inhibitor indomethacin had no effect (Figure 7c) on the ability of PMA to increase PDE4 activity in U937 macrophage-like cells, consistent with activation being due to stimulatory ERK phosphorylation of the predominant PDE4B2 short form. Indeed, PDE4B2, which provides the dominant activity in the macrophage-like cells, is not a target for PKA phosphorylation as it lacks UCR1, the region containing the PKA consensus site. Additionally, the PKA inhibitor H89 did not alter the ability of acute PMA challenge to increase PDE4 activity in U937 macrophage-like cells (Figure 7c). Indeed, the combined activities of PDE4A and PDE4D, long isoforms that could provide potential targets for PKA action, provide only around 30% of the total PDE4 activity in macrophage-like cells (Figure 5). The effect on the activity of phosphorylation of these various isoforms by PKA has been determined previously using recombinant species (Sette & Conti, 1996; Baillie et al., 2000). Based upon this, one can calculate that full phosphorylation of these isoforms by PKA in U937 macrophage-like cells would be expected to lead to around a 15–20% increase in total PDE4 activity, compared to the doubling of activity caused by PMA action (Figure 7). Thus, it seems most likely that PMA mediates activation of total PDE4 activity in these macrophage-like cells through the ERK-mediated phosphorylation of the PDE4B2 short form. Such acute treatment with PMA did not alter PDE3 activity found in either the monocyte or the macrophage forms of U937 cells (<10% change; data not shown). Thus, acute activation of ERK serves to decrease the PDE4/PDE3 activity ratio in the monocyte form of U937 cells, while it increases the PDE4/PDE3 activity ratio in the macrophage form of U937 cells (Figure 7d).
Discussion
PDE4 selective inhibitors are currently being developed to treat the major respiratory diseases COPD and asthma (Torphy, 1998; Spina et al., 1998a; Schudt et al., 1999; Barnette & Underwood, 2000; Huang et al., 2001; Burnouf & Pruniaux, 2002; Giembycz, 2002; Spina, 2003). The deployment of such agents has been delayed, however, due to the association of certain side effects, predominantly nausea, with first-generation PDE4 inhibitors. The current generation of PDE4 inhibitors shows a much-reduced propensity in this regard. However, one way that might aid in further widening the effective therapeutic window of future therapeutics would be to target inhibitors to either particular PDE4 isoforms or PDE4 subfamilies expressed in those cell types associated with the pathological state. As a prerequisite for this, an analysis of the PDE4 expression pattern in such key cell types is required.
It is well recognised that alveolar macrophages and their precursors, circulating monocytes, play a key role in airway inflammatory diseases (Arm & Lee, 1992; Spina et al., 1998a; Barnette & Underwood, 2000; Jeffery, 2000; Giembycz, 2002; Barnes, 2003) and we have set out here using the human U937 cell line to define the PDE4 isoforms expressed in both their monocytic and macrophage-like states (Hass et al., 1989; Twomey et al., 1993; Kuroda et al., 1997; Fukunaga & Tsuruda, 2001; Matheson et al., 2002). Intriguingly, it has been shown (Tenor et al., 1995; Gantner et al., 1997; Bender et al., 2004) that the in vitro differentiation of both peripheral blood-derived and alveolar monocytes to macrophages leads to a marked downregulation of PDE4 activity, concomitant with an upregulation of PDE3 and PDE1 activities. However, in the case of PDE4, it is not known whether this change was due to a simple downregulation in the level of expression of those PDE4 isoforms found in monocytes or whether some degree of remodelling of the pattern of PDE4 isoform expression had occurred. Here we show that differentiation of the U937 human monocyte-like cell line to a macrophage-like phenotype similarly leads to the upregulation of PDE3 activity and downregulation of PDE4 activity (Figure 2), suggesting that this is indeed an appropriate model system. However, by using immunological reagents to identify members of PDE4 subfamilies, we are able to demonstrate here that differentiation of these cells leads to an extensive remodelling of the pattern of PDE4 isoform expression. One particularly striking observation is that one isoform, namely the novel PDE4A10 long form, while absent from monocytes is induced upon differentiation to a macrophage-like phenotype (Figure 3). Furthermore, while differentiation causes a marked increase in the expression levels of the short PDE4B2 isoform, it elicits a profound decrease in the expression of the long PDE4D3 and PDE4D5 isoforms (Figure 3). Similar results were seen using primary human monocytes and the macrophages induced to differentiate from them (Figure 4), indicating that U937 cells do indeed provide a good model system for analysing the role of PDE4 isoforms. Thus, the seemingly ‘simple' fall in PDE4 activity seen upon differentiation masks a change of considerable complexity, with the expression of certain PDE4 isoforms actually being increased. We obtained further insight into this by using specific antisera for immunopurification in order to assess the contribution of each PDE4 subfamily to total PDE4 activity (Figure 4). Doing this, we can appreciate now that the magnitude of the increase in PDE4 activity, consequent upon the induction of PDE4A10 and PDE4B2 isoforms upon differentiation, is completely dwarfed by the dramatic fall in PDE4 activity caused by downregulation of the expression of PDE4D isoforms, whose activity is seen to clearly dominate that of all other PDE4 subfamilies in monocytic cells (Figures 3, 4).
The differentiation of these monocytic cells to a macrophage-like phenotype is underpinned by a complex change in the PDE4 isoform profile. Given that specific PDE4 isoforms have been shown to be differentially regulated by phosphorylation and can interact with other proteins that serve anchoring, scaffold and regulatory roles (Conti & Jin, 1999; Conti et al., 2003; Houslay, 2001; Houslay & Adams, 2003), this altered pattern of expression is likely to be of functional significance. Appreciation of the particular roles of individual PDE4 isoforms in specific cell types is poorly understood and such investigations pose a considerable challenge. However, one evident facet of the remodelling in PDE4 isoform expression seen here is that whereas the predominant PDE4 activity in monocytic U937 cells is supplied by long PDE4D isoforms, in particular PDE4D3, this situation is profoundly altered in macrophage-like U937 cells, where the activity of the short PDE4B2 form predominates (Figure 5). It well established that activation of both monocytes and macrophages is critically dependent on activation of the ERK MAP kinase pathway (see, for example, van der Bruggen et al., 1999; Guha et al., 2001; Mancuso et al., 2002; Giri et al., 2003; Guerra et al., 2003; Koch et al., 2003). Interestingly, it has also been shown (Hoffmann et al., 1999; Baillie et al., 2000; MacKenzie et al., 2000) that the catalytic unit of various PDE4 isoforms can be phosphorylated by ERK action with the functional outcome depending upon whether they are long or short isoforms. Thus, for example, the PDE4D3/5 isoforms will become inhibited and the PDE4B2 isoform will become activated upon their phosphorylation by ERK, while the PDE4A isoforms are unaffected as their catalytic units are not subject to ERK phosphorylation (Hoffmann et al., 1999; Baillie et al., 2000; MacKenzie et al., 2000). Indeed, we show here that acute activation of ERK2 causes diametrically opposed actions on total PDE4 activity in U937 monocytic cells compared to cells in the differentiated macrophage-like state (Figure 7). Thus, acute ERK activation causes an inhibitory effect on total PDE4 activity in the monocyte-like cells (Figure 7a), whereas a clear activation of total PDE4 activity is evident in the macrophage-like cells (Figure 7b). Such a functional outcome is consistent with the activity of long isoforms, capable of being inhibited by ERK, predominating in monocytic cells, while the activity of the PDE4B2 short form, which is capable of being activated by ERK, predominating in macrophage-like cells. Thus monocyte/macrophage differentiation appears to lead to a functional re-programming of the effect of ERK activation on total PDE4 activity. Given that PDE4 inhibitors attenuate the activation of both monocytes and macrophages, it seems likely that the activity of at least certain PDE4 isoforms may actually aid in regulating the activation of these cells. As activators of both these cell types cause the phosphorylation and activation of ERK, it may be that in monocytes the consequential inhibition of PDE4 long forms may serve a feedback role in attenuating cell activation akin to the transient application of a PDE4 inhibitor. In contrast to this, the ERK-mediated activation of PDE4B2 in macrophages may serve to have the converse effect and actually aid activation. Indeed, targeted gene knockout studies have identified products of the PDE4B, but not the PDE4D, gene as being essential for alveolar macrophage function (Jin & Conti, 2002). We show here that the upregulated PDE4B2 is the only PDE4B isoform expressed in U937 macrophage-like cells, suggesting it may be this specific, ERK-activated short isoform that provides a key role in facilitating macrophage activation. Such an ERK-mediated increase in PDE4B2 activity upon cell activation might also help explain why at inflammatory sites macrophages continue to produce high levels of TNF despite being exposed to high concentrations of the adenylyl cyclase activator PGE2. That PDE3 activity is, seemingly, unaffected by ERK activation (this study and see Ahmad et al., 1999), which means that the ratio of PDE4/PDE3 activities is differently affected in monocytes compared to macrophages (Figure 7d). Thus, ERK activation will serve to decrease the activity of PDE4 relative to PDE3 in monocytes and increase it relative to PDE3 in macrophages (Figure 7d), which may have relevance to the development and action of selective therapeutics.
The differentiation of U937 monocytic cells to macrophage-like cells leads to a profound change in the PDE4 isoform profile. If inhibitors selective for different PDE4 subfamilies and isoforms can be generated, they would appear to offer the possibility of differentially regulating the functioning of macrophages and monocytes.
Acknowledgments
M.D.H. thanks the Medical Research Council (U.K.) G8604010 and the European Union (QLG2-CT-2001-02278; QLK3-CT-2002-02149) for support funding. M.C.S thanks the Medical Research Council (U.K.) for a Research Training Fellowship.
Abbreviations
- ERK
extracellular signal regulated kinase
- PDE
cyclic 3′,5′ AMP phosphodiesterase
- PDE4
rolipram-inhibited, cAMP specific PDE
- rolipram
4-{3-(cyclopentoxyl)-4-methoxyphenyl}-2-pyrrolidone
- PKA
protein kinase A
References
- AHMAD F., GAO G., WANG L.M., LANDSTROM T.R., DEGERMAN E., PIERCE J.H., MANGANIELLO V.C. IL-3 and IL-4 activate cyclic nucleotide phosphodiesterases 3 (PDE3) and 4 (PDE4) by different mechanisms in FDCP2 myeloid cells. J. Immunol. 1999;162:4864–4875. [PubMed] [Google Scholar]
- ARM J.P., LEE T.H. The pathobiology of bronchial asthma. Adv. Immunol. 1992;51:323–382. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- BAILLIE G., MACKENZIE S.J., HOUSLAY M.D. Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK) Mol. Pharmacol. 2001;60:1100–1111. doi: 10.1124/mol.60.5.1100. [DOI] [PubMed] [Google Scholar]
- BAILLIE G.S., MACKENZIE S.J., MCPHEE I., HOUSLAY M.D. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br. J. Pharmacol. 2000;131:811–819. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J. New concepts in chronic obstructive pulmonary disease. Annu. Rev. Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- BARNETTE M.S., UNDERWOOD D.C. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Curr. Opin. Pulmon. Med. 2000;6:164–169. doi: 10.1097/00063198-200003000-00014. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A., BRUNTON L.L. Cyclic nucleotide research – still expanding after half a century. Nat. Rev. Mol. Cell. Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- BENDER A.T., OSTENSON C.L., GIORDANO D., BEAVO J.A. Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell Signal. 2004;16:365–374. doi: 10.1016/j.cellsig.2003.08.009. [DOI] [PubMed] [Google Scholar]
- BIGGS J.R., AHN N.G., KRAFT A.S. Activation of the mitogen-activated protein kinase pathway in U937 leukemic cells induces phosphorylation of the amino terminus of the TATA-binding protein. Cell Growth Differ. 1998;9:667–676. [PubMed] [Google Scholar]
- BOLGER G., MICHAELI T., MARTINS T., ST JOHN T., STEINER B., RODGERS L., RIGGS M., WIGLER M., FERGUSON K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol. Cell. Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLGER G.B., ERDOGAN S., JONES R.E., LOUGHNEY K., SCOTLAND G., HOFFMANN R., WILKINSON I., FARRELL C., HOUSLAY M.D.Characterization of five different proteins produced by alternatively spliced mRNAs from the human cAMP-specific phosphodiesterase PDE4D gene Biochem. J. 1997328539–548.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLGER G.B., PEDEN A.H., STEELE M.R., MACKENZIE C., MCEWAN D.G., WALLACE D.A., HUSTON E., BAILLIE G.S., HOUSLAY M.D. Attenuation of the activity of the cAMP-specific phosphodiesterase PDE4A5 by interaction with the immunophilin XAP2. J. Biol. Chem. 2003;278:33351–33363. doi: 10.1074/jbc.M303269200. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BURNOUF C., PRUNIAUX M.P. Recent advances in PDE4 inhibitors as immunoregulators and anti-inflammatory drugs. Curr. Pharm. Des. 2002;8:1255–1296. doi: 10.2174/1381612023394665. [DOI] [PubMed] [Google Scholar]
- CONTI M., JIN S.L. The molecular biology of cyclic nucleotide phosphodiesterases. Prog. Nucleic Acid Res. Mol. Biol. 1999;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- CONTI M., RICHTER W., MEHATS C., LIVERA G., PARK J.Y., JIN C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- DENT G., WHITE S.R., TENOR H., BODTKE K., SCHUDT C., LEFF A.R., MAGNUSSEN H., RABE K.F. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitors. Pulmon. Pharmacol. Ther. 1998;11:47–56. doi: 10.1006/pupt.1998.0115. [DOI] [PubMed] [Google Scholar]
- ERMERT L., ERMERT M., MERKLE M., GOPPELT-STRUEBE M., DUNCKER H.R., GRIMMINGER F., SEEGER W. Rat pulmonary cyclooxygenase-2 expression in response to endotoxin challenge: differential regulation in the various types of cells in the lung. Am. J. Pathol. 2000;156:1275–1287. doi: 10.1016/S0002-9440(10)64998-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS S.H., TURKO I.V., CORBIN J.D. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- FRANKLIN C.C., UNLAP T., ADLER V., KRAFT A.S. Multiple signal transduction pathways mediate c-Jun protein phosphorylation. Cell Growth Differ. 1993;4:377–385. [PubMed] [Google Scholar]
- FUKUNAGA M., TSURUDA K. Actinobacillus actinomycetemcomitans induces lethal effects on the macrophage-like human cell line U937. Oral. Microbiol. Immunol. 2001;16:284–289. doi: 10.1034/j.1399-302x.2001.016005284.x. [DOI] [PubMed] [Google Scholar]
- GANTNER F., KUPFERSCHMIDT R., SCHUDT C., WENDEL A., HATZELMANN A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-alpha release by PDE inhibitors. Br. J. Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A. Development status of second generation PDE4 inhibitors for asthma and COPD: the story so far. Monaldi Arch. Chest Dis. 2002;57:48–64. [PubMed] [Google Scholar]
- GIRI R.K., SELVARAJ S.K., KALRA V.K. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- GORDON S. The role of the macrophage in immune regulation. Res. Immunol. 1998;149:685–688. doi: 10.1016/s0923-2494(99)80039-x. [DOI] [PubMed] [Google Scholar]
- GUERRA A.N., FISETTE P.L., PFEIFFER Z.A., QUINCHIA-RIOS B.H., PRABHU U., AGA M., DENLINGER L.C., GUADARRAMA A.G., ABOZEID S., SOMMER J.A., PROCTOR R.A., BERTICS P.J. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J. Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- GUHA M., O'CONNELL M.A., PAWLINSKI R., HOLLIS A., MCGOVERN P., YAN S.F., STERN D., MACKMAN N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- HASS R., BARTELS H., TOPLEY N., HADAM M., KOHLER L., GOPPELT-STRUBE M., RESCH K. TPA-induced differentiation and adhesion of U937 cells: changes in ultrastructure, cytoskeletal organization and expression of cell surface antigens. Eur. J. Cell Biol. 1989;48:282–293. [PubMed] [Google Scholar]
- HASS R., GIESE G., MEYER G., HARTMANN A., DORK T., KOHLER L., RESCH K., TRAUB P., GOPPELT-STRUBE M. Differentiation and retrodifferentiation of U937 cells: reversible induction and suppression of intermediate filament protein synthesis. Eur. J. Cell Biol. 1990;51:265–271. [PubMed] [Google Scholar]
- HOFFMANN R., BAILLIE G.S., MACKENZIE S.J., YARWOOD S.J., HOUSLAY M.D. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 1999;18:893–903. doi: 10.1093/emboj/18.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN R., WILKINSON I.R., MCCALLUM J.F., ENGELS P., HOUSLAY M.D. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: generation of a molecular model. Biochem. J. 1998;333 (Part 1):139–149. doi: 10.1042/bj3330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSLAY M.D. PDE4 cAMP-specific phosphodiesterases. Prog. Nucleic Acid Res. Mol. Biol. 2001;69:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- HOUSLAY M.D., ADAMS D.R. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSLAY M.D., SULLIVAN M., BOLGER G.B. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv. Pharmacol. 1998;44:225–342. doi: 10.1016/s1054-3589(08)60128-3. [DOI] [PubMed] [Google Scholar]
- HUANG Z., Ducharme Y., MACDONALD D., ROBICHAUD A. The next generation of PDE4 inhibitors. Curr. Opin. Chem. Biol. 2001;5:432–438. doi: 10.1016/s1367-5931(00)00224-6. [DOI] [PubMed] [Google Scholar]
- HUSTON E., LUMB S., RUSSELL A., CATTERALL C., ROSS A.H., STEELE M.R., BOLGER G.B., PERRY M., OWENS R., HOUSLAY M.D. Molecular cloning and transient expression in COS7 cells of a novel human PDE4B cyclic AMP specific phosphodiesterase, HSPDE4B3. Biochem. J. 1997;328:549–558. doi: 10.1042/bj3280549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSTON E., POOLEY L., JULIEN J., SCOTLAND G., MCPHEE I., SULLIVAN M., BOLGER G., HOUSLAY M.D. The human cyclic AMP-specific phosphodiesterase PDE-46 (HSPDE4A4B) expressed in transfected COS7 cells occurs as both particulate and cytosolic species which exhibit distinct kinetics of inhibition by the anti-depressant rolipram. J. Biol. Chem. 1996;271:31334–31344. doi: 10.1074/jbc.271.49.31334. [DOI] [PubMed] [Google Scholar]
- JEFFERY P.K. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 2000;117:251S–260S. doi: 10.1378/chest.117.5_suppl_1.251s. [DOI] [PubMed] [Google Scholar]
- JIN S.L., CONTI M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A., GIEMBYCZ M., ITO K., LIM S., JAZRAWI E., BARNES P.J., ADCOCK I., ERDMANN E., CHUNG K.F. MAP-kinase modulation of NF-{kappa}B-induced GM-CSF release from human alveolar macrophages. Am. J. Respir. Cell. Mol. Biol. 2003. [DOI] [PubMed]
- KOEHLER L., HASS R., WESSEL K., DEWITT D.L., KAEVER V., RESCH K., GOPPELT-STRUEBE M. Altered arachidonic acid metabolism during differentiation of the human monoblastoid cell line U937. Biochim. Biophys. Acta. 1990;1042:395–403. doi: 10.1016/0005-2760(90)90170-3. [DOI] [PubMed] [Google Scholar]
- KURODA A., SUGIYAMA E., TAKI H., MINO T., KOBAYASHI M. Interleukin-4 inhibits the gene expression and biosyntheis of cytosolic phospholipase A2 in lipopolysaccharide stimulated U937 macrophage cell line and freshly prepared adherent rheumatoid synovial cells. Biochem. Biophys. Res. Commun. 1997;230:40–43. doi: 10.1006/bbrc.1996.5885. [DOI] [PubMed] [Google Scholar]
- LIU H., MAURICE D.H. Phosphorylation-mediated activation and translocation of the cyclic AMP-specific phosphodiesterase PDE4D3 by cyclic AMP-dependent protein kinase and mitogen-activated protein kinases. A potential mechanism allowing for the coordinated regulation of PDE4D activity and targeting. J. Biol. Chem. 1999;274:10557–10565. doi: 10.1074/jbc.274.15.10557. [DOI] [PubMed] [Google Scholar]
- MA D., WU P., EGAN R.W., BILLAH M.M., WANG P. Phosphodiesterase 4B gene transcription is activated by lipopolysaccharide and inhibited by interleukin-10 in human monocytes. Mol. Pharmacol. 1999;55:50–57. doi: 10.1124/mol.55.1.50. [DOI] [PubMed] [Google Scholar]
- MACKENZIE S.J., BAILLIE G.S., MCPHEE I., BOLGER G.B., HOUSLAY M.D. ERK2 MAP kinase binding, phosphorylation and regulation of PDE4D cAMP specific phosphodiesterases: the involvement of C-terminal docking sites and N-terminal UCR regions. J. Biol. Chem. 2000;275:16609–16617. doi: 10.1074/jbc.275.22.16609. [DOI] [PubMed] [Google Scholar]
- MACKENZIE S.J., BAILLIE G.S., MCPHEE I., MACKENZIE C., SEAMONS R., MCSORLEY T., MILLEN J., BEARD M.B., VAN HEEKE G., HOUSLAY M.D. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br. J. Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE S.J., HOUSLAY M.D. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem. J. 2000;347:571–578. doi: 10.1042/0264-6021:3470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE S.J., YARWOOD S.J., PEDEN A.H., BOLGER G.B., VERNON R.J., HOUSLAY M.D. Stimulation of p70S6 kinase via a growth hormone controlled PI-3 kinase pathway leads to the activation of a PDE4 cAMP specific phosphodiesterases in 3T3-F442A preadipocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3549–3554. doi: 10.1073/pnas.95.7.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCUSO G., MIDIRI A., BENINATI C., PIRAINO G., VALENTI A., NICOCIA G., TETI D., COOK J., TETI G. Mitogen-activated protein kinases and NF-kappa B are involved in TNF-alpha responses to group B streptococci. J. Immunol. 2002;169:1401–1409. doi: 10.4049/jimmunol.169.3.1401. [DOI] [PubMed] [Google Scholar]
- MANGANIELLO V.C., DEGERMAN E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb. Haemost. 1999;82:407–411. [PubMed] [Google Scholar]
- MARCHMONT R.J., HOUSLAY M.D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem. J. 1980;187:381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHESON L.A., LABOW R.S., SANTERRE J.P. Biodegradation of polycarbonate-based polyurethanes by the human monocytes-derived macrophage and U937 cell systems. J. Biomed. Mater. Res. 2002;61:505–513. doi: 10.1002/jbm.10286. [DOI] [PubMed] [Google Scholar]
- MAURICE D.H., PALMER D., TILLEY D.G., DUNKERLEY H.A., NETHERTON S.J., RAYMOND D.R., ELBATARNY H.S., JIMMO S.L. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol. Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- MAZZARELLA G., PETILLO O., MARGARUCCI S., CALABRESE C., PELUSO G. Role of monocyte/macrophage population in immune response. Monaldi Arch. Chest Dis. 1998;53:92–96. [PubMed] [Google Scholar]
- MCPHEE I., YARWOOD S.J., HUSTON E., SCOTLAND G., BEARD M.B., ROSS A.H., HOUSLAY E.S., HOUSLAY M.D. Association with the src family tyrosyl kinase lyn triggers a conformational change in the catalytic region of human cAMP-specific phosphodiesterase HSPDE4A4B: consequences for rolipram inhibition. J. Biol. Chem. 1999;274:11796–11810. doi: 10.1074/jbc.274.17.11796. [DOI] [PubMed] [Google Scholar]
- MONICK M.M., CARTER A.B., GUDMUNDSSON G., GEIST L.J., HUNNINGHAKE G.W. Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am. J. Physiol. 1998;275:L389–L397. doi: 10.1152/ajplung.1998.275.2.L389. [DOI] [PubMed] [Google Scholar]
- PRUDOVSKY I., POPOV K., AKIMOV S., SEROV S., ZELENIN A., MEINHARDT G., BAIER P., SOHN C., HASS R. Antisense CD11b integrin inhibits the development of a differentiated monocyte/macrophage phenotype in human leukemia cells. Eur. J. Cell Biol. 2002;81:36–42. doi: 10.1078/0171-9335-00219. [DOI] [PubMed] [Google Scholar]
- RAGG S.J., KAGA S., BERG K.A., OCHI A. The mitogen-activated protein kinase pathway inhibits ceramide-induced terminal differentiation of a human monoblastic leukemia cell line, U937. J. Immunol. 1998;161:1390–1398. [PubMed] [Google Scholar]
- RENA G., BEGG F., ROSS A., MACKENZIE C., MCPHEE I., CAMPBELL L., HUSTON E., SULLIVAN M., HOUSLAY M.D. Molecular cloning and characterization of the novel cAMP specific phosphodiesterase, PDE4A10. Mol. Pharmacol. 2001;59:996–1011. doi: 10.1124/mol.59.5.996. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., GANTNER F., TENORS H., HATZELMANN A. Therapeutic potential of selective PDE inhibitors in asthma. Pulmon. Pharmacol. Ther. 1999;12:123–129. doi: 10.1006/pupt.1999.0182. [DOI] [PubMed] [Google Scholar]
- SETTE C., CONTI M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- SETTE C., IONA S., CONTI M. The short-term activation of a Rolipram-sensitive, cAMP-specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J. Biol. Chem. 1994;269:9245–9252. [PubMed] [Google Scholar]
- SPENCE S., RENA G., SULLIVAN M., ERDOGAN S., HOUSLAY M.D. Receptor-mediated stimulation of lipid signalling pathways in CHO cells elicits the rapid transient induction of the PDE1B isoform of Ca2+/calmodulin-stimulated cAMP phosphodiesterase. Biochem. J. 1997;321 (Part 1):157–163. doi: 10.1042/bj3210157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINA D. Theophylline and PDE4 inhibitors in asthma. Curr. Opin. Pulmon. Med. 2003;9:57–64. doi: 10.1097/00063198-200301000-00010. [DOI] [PubMed] [Google Scholar]
- SPINA D., LANDELLS L.J., PAGE C.P. The role of phosphodiesterase enzymes in allergy and asthma. Adv. Pharmacol. 1998a;44:33–89. doi: 10.1016/s1054-3589(08)60125-8. [DOI] [PubMed] [Google Scholar]
- SPINA D., LANDELLS L.J., PAGE C.P. The role of theophylline and phosphodiesterase4 isoenzyme inhibitors as anti-inflammatory drugs. Clin. Exp. Allergy. 1998b;28 Suppl 3:24–34. [PubMed] [Google Scholar]
- SWINNEN J.V., JOSEPH D.R., CONTI M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENOR H., HATZELMANN A., KUPFERSCHMIDT R., STANCIU L., DJUKANOVIC R., SCHUDT C., WENDEL A., CHURCH M.K., SHUTE J.K. Cyclic nucleotide phosphodiesterase isoenzyme activities in human alveolar macrophages. Clin. Exp. Allergy. 1995;25:625–633. doi: 10.1111/j.1365-2222.1995.tb01110.x. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.J., APPLEMAN M.M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971;10:311–316. [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., ZHOU H.L., CIESLINSKI L.B. Stimulation of beta adrenoceptors in a human monocyte cell line (U937) up-regulates cyclic AMP-specific phosphodiesterase activity. J. Pharmacol. Exp. Ther. 1992;263:1195–1205. [PubMed] [Google Scholar]
- TWOMEY B.M., MCCALLUM S., ISENBERG D.A., LATCHMAN D.S. Elevation of heat shock protein synthesis and hsp gene transcription during monocyte to macrophage differentiation of U937 cells. Clin. Exp. Immunol. 1993;93:178–183. doi: 10.1111/j.1365-2249.1993.tb07962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER BRUGGEN T., NIJENHUIS S., VAN RAAIJ E., VERHOEF J., VAN ASBECK B.S. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immunol. 1999;67:3824–3829. doi: 10.1128/iai.67.8.3824-3829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG P., WU P., OHLETH K.M., EGAN R.W., BILLAH M.M. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol. Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- YARWOOD S.J., STEELE M.R., SCOTLAND G., HOUSLAY M.D., BOLGER G.B. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform. J. Biol. Chem. 1999;274:14909–14917. doi: 10.1074/jbc.274.21.14909. [DOI] [PubMed] [Google Scholar]