Abstract
Free radicals and other reactive species (RS) are thought to play an important role in many human diseases. Establishing their precise role requires the ability to measure them and the oxidative damage that they cause.
This article first reviews what is meant by the terms free radical, RS, antioxidant, oxidative damage and oxidative stress.
It then critically examines methods used to trap RS, including spin trapping and aromatic hydroxylation, with a particular emphasis on those methods applicable to human studies.
Methods used to measure oxidative damage to DNA, lipids and proteins and methods used to detect RS in cell culture, especially the various fluorescent ‘probes' of RS, are also critically reviewed.
The emphasis throughout is on the caution that is needed in applying these methods in view of possible errors and artifacts in interpreting the results.
Keywords: Cell culture, free radical, reactive species, antioxidant, oxidative stress, oxidative damage, fluorescent probe, lipid peroxidation, superoxide
Introduction
Free radicals and other ‘reactive oxygen (ROS)/nitrogen/chlorine species' (for an explanation of these terms see Table 1) are widely believed to contribute to the development of several age-related diseases, and perhaps, even to the aging process itself (Halliwell & Gutteridge, 1999; Sohal et al., 2002) by causing ‘oxidative stress' and ‘oxidative damage' (terms explained in Table 2). For example, many studies have shown increased oxidative damage to all the major classes of biomolecules in the brains of Alzheimer's patients (Halliwell, 2001; Butterfield, 2002; Liu et al., 2003). Other diseases in which oxidative damage has been implicated include cancer, atherosclerosis, other neurodegenerative diseases and diabetes (Hagen et al., 1994; Chowienczyk et al., 2000; Halliwell, 2000a, 2001, 2002a, 2002b; Parthasarathy et al., 2000). If oxidative damage contributes significantly to disease pathology (Table 3 lists the criteria needed to establish this), then actions that decrease it should be therapeutically beneficial (Halliwell, 2001; Lee et al., 2002a; Liu et al., 2003). If the oxidative damage is involved in the origin of a disease, then successful antioxidant treatment should delay or prevent the onset of that disease (Halliwell, 1991, 2002a, 2002b; Galli et al., 2002; Steinberg & Witztum, 2002). To establish the role of oxidative damage (Table 3), it is therefore essential to be able to measure it accurately. For example, the failure of interventions with antioxidants such as vitamin E, β-carotene or ascorbate to decrease disease incidence in several human intervention trials may have simply been due to the failure of these compounds to decrease oxidative damage in the subjects tested (Halliwell, 1999a, 2000c; Levine et al., 2001; Meagher et al., 2001). In this review, we will examine the methods available to measure reactive species (RS) and oxidative damage, with a particular emphasis on those applicable to human studies.
Table 1.
Nomenclature of reactive species
| Free radicalsa | Nonradicals |
|---|---|
| Reactive oxygen species (ROS) | |
| Superoxide, O2•− | Hydrogen peroxide, H2O2 |
| Hydroxyl, OH• | Hypobromous acid, HOBrb |
| Hydroperoxyl, HO2• | Hypochlorous acid, HOClc |
| Ozone O3 | |
| Peroxyl, RO2• | Singlet oxygen (O21Δg) |
| Alkoxyl, RO• | Organic peroxides, ROOH |
| Carbonate, CO3•− | Peroxynitrite, ONOO − d |
| Carbon dioxide, CO2•− | Peroxynitrous acid, ONOOHd |
| Reactive chlorine species (RCS) | |
| Atomic chlorine, Cl• | Hypochlorous acid, HOClc |
| Nitryl (nitronium) chloride, NO2Cle | |
| Chloramines | |
| Chlorine gas (Cl2) | |
| Reactive nitrogen species (RNS) | |
| Nitric oxide, NO• | Nitrous acid, HNO2 |
| Nitrogen dioxide, NO2• | Nitrosyl cation, NO+ |
| Nitroxyl anion, NO− | |
| Dinitrogen tetroxide, N2O4 | |
| Dinitrogen trioxide, N2O3 | |
| Peroxynitrite, ONOO−d | |
| Peroxynitrous acid, ONOOH | |
| Nitronium (nitryl) cation, NO2+ | |
| Alkyl peroxynitrites, ROONO | |
| Nitryl (nitronium) chloride, NO2Cle | |
ROS is a collective term that includes both oxygen radicals and certain nonradicals that are oxidizing agents and/or are easily converted into radicals (HOCl, HOBr, O3, ONOO−, 1O2, H2O2). In other words, all oxygen radicals are ROS, but not all ROS are oxygen radicals. Peroxynitrite and H2O2 are frequently erroneously described in the literature as free radicals, for example. RNS is also a collective term including nitric oxide and nitrogen dioxide radicals, as well as nonradicals such as HNO2 and N2O4. ‘Reactive' is not always an appropriate term: H2O2, NO• and O2•− react quickly with only a few molecules, whereas OH• reacts quickly with almost everything. RO2•, RO•, HOCl, HOBr, NO2•, ONOO−, NO2+ and O3 have intermediate reactivites.
A free radical is any species that contains one or more unpaired electrons, that is, electrons singly occupying an atomic or molecular orbital.
HOBr could also be regarded as a ‘reactive bromine species'.
HOCl is often included as an ROS.
ONOO− and ONOOH are often included as ROS.
NO2Cl is a chlorinating and nitrating species that can be formed by reaction of HOCl with NO2−.
Table 2.
Some key definitions
| Oxidative stress |
| The term oxidative stress is vaguely defined. In essence, it refers to a serious imbalance between production of reactive species and antioxidant defence. Sies (1991) defined it as a disturbance in the pro-oxidant–antioxidant balance in favor of the former, leading to potential damage. |
| Oxidative stress can result from: |
| 1. Diminished levels of antioxidants, for example, mutations affecting the activities of antioxidant defence enzymes such as CuZnSOD, or glutathione peroxidase, or toxins that deplete antioxidant defences. For example, many xenobiotics are metabolized by conjugation with GSH; high doses can deplete GSH and cause oxidative stress even if the xenobiotic is not itself a generator of reactive species. Deficiencies in dietary minerals (e.g. Zn2+, Mg2+, Fe2+, Cu2+, Se) and/or antioxidants can also cause oxidative stress. |
| And/Or |
| 2. Increased production of reactive species, for example, by exposure of cells or organisms to elevated levels of O2 or to other toxins that are themselves reactive species (e.g. NO2•) or are metabolized to generate reactive species (e.g. paraquat), or excessive activation of ‘natural' systems producing such species (e.g. inappropriate activation of phagocytic cells in chronic inflammatory diseases). |
| Oxidative damage |
| The biomolecular damage that can be caused by direct attack of reactive species during oxidative stress. |
| Consequences of oxidative stress can include: |
| 1. Adaptation of the cell or organism by upregulation of defence systems, which may (a) completely protect against damage; (b) protect against damage to some extent but not completely; or (c) ‘overprotect' (e.g. the cells is then resistant to higher levels of oxidative stress imposed subsequently). |
| 2. Cell injury: This involves damage (oxidative damage) to any or all molecular targets: lipids, DNA, protein, carbohydrate, etc. Oxidative damage can also occur during adaptation (point 1 above). Not all damage caused by oxidative stress is oxidative damage: damage to biomolecules can result from oxidative stress-related changes in ion levels (e.g. Ca2+) or activation of proteases, for example. |
| 3. Cell death: The cell may (a) recover from the oxidative damage by repairing it or replacing the damaged molecules, or (b) it may survive with persistent oxidative damage or (c) oxidative damage, especially to DNA, may trigger cell death, by apoptosis or necrosis. |
| Antioxidant |
| ‘Antioxidant' is a much-misused word. One can make almost any chemical exert antioxidant effects in vitro by choosing appropriate assay conditions. To reflect the fact that calling something an antioxidant means little without specifying the assay methodology used, Halliwell & Gutteridge (1999) defined an antioxidant as any substance that, when present at low concentrations compared with those of an oxidizable substrate, significantly delays or prevents oxidation of that substrate. The term ‘oxidizable substrate' includes every type of molecule found in vivo. This definition emphasizes the importance of the damage target studied and the source of reactive species used when antioxidant actions are examined. |
Table 3.
Criteria for implicating RS as a significant mechanism of tissue injury in human disease
| 1 The RS (or the oxidative damage it causes) should always be demonstrable at the site of injury. |
| 2 The time course of formation of the RS (or of the oxidative damage it causes) should be consistent with the time course of tissue injury, preceding or accompanying it. |
| 3 Direct application of the RS over a relevant time course (point 2 above) to the tissue at concentrations within the range found in vivo should reproduce most or all of the tissue injury and oxidative damage observed. |
| 4 Removing the RS or inhibiting its formation should diminish the tissue injury to an extent related to the degree of inhibition of the oxidative damage caused by the RS. |
Measuring RS in vivo: basic principles
Some fascinating techniques such as L-band electron spin resonance (ESR) with nitroxyl probes and magnetic resonance imaging spin trapping are under development to measure RS directly in whole animals (e.g. Berliner et al., 2001; Han et al., 2001; Utsumi & Yamada, 2003), but no probes are currently suitable for human use. Most RS persist for only a short time in vivo and cannot be measured directly. There are a few exceptions: examples include H2O2 (discussed below), and perhaps, NO•, in the sense that serum levels of NO2− have been claimed to measure vascular endothelial NO• synthesis (Kelm et al., 1999), despite the fact that NO2− is quickly oxidized to NO3− in vivo (Kelm et al., 1999; Oldreive & Rice-Evans, 2001). Essentially, there are two approaches to detecting transient RS:
attempting to trap these species and measure the levels of the trapped molecules and
measuring the levels of the damage done by RS, that is, the amount of oxidative damage.
Sometimes other approaches are used. They include measurements of erythrocyte antioxidant defences and of total antioxidant activity of body fluids; falls in these parameters are often taken as evidence of oxidative stress. Erythrocytes cannot synthesize proteins, however, and their antioxidant enzyme levels may drop as they ‘age' in the circulation (Denton et al., 1975). Thus changes in their levels are more likely to reflect changes in the rates of red blood cell turnover: if this slows down, the circulating erythrocytes will be older on average and so levels of antioxidant enzymes in them will appear to fall. Vice versa, if an intervention accelerates red cell removal or increases erythropoiesis, levels of antioxidants in red cells will seem to rise. Hence, such data should be interpreted with caution.
Depending on the method that is used to measure it, the plasma or serum ‘total antioxidant capacity' (TAC) usually involves major contributions from urate, ascorbate and sometimes albumin −SH groups (Benzie & Strain, 1996; Halliwell & Gutteridge, 1999; Prior & Cao, 1999; Rice-Evans, 2000; Bartosz, 2003), although different methods measure different things (Schlesier et al., 2002; Bartosz, 2003). Thus, for example, if plasma albumin levels fall, TAC will fall. If urate levels rise, TAC will rise. The multiple changes in blood chemistry that occur in sick people mean that TAC changes should be interpreted with caution. TAC is also influenced by diet, often because consumption of certain foods may produce changes in plasma ascorbate and/or urate levels (Halliwell, 2003b).
Trapping of RS
The only technique that can ‘see' free radicals directly and specifically is electron spin resonance (ESR), because it detects the presence of unpaired electrons. However, ESR detects only fairly unreactive radicals, since reactive ones do not accumulate to high-enough levels to be measured. One solution to this problem is to add ‘traps' or ‘probes', agents that intercept reactive radicals, reacting with them to form a stable radical that can be detected by ESR. Whole-body ESR techniques are being used on animals (Berliner et al., 2001; Han et al., 2001; Utsumi & Yamada, 2003), but are currently inapplicable to humans due to the lack of any human safety data on the probes. A wide range of traps is available for use in animals and cell culture systems, not only the tried and tested N-tert-butyl-∝-phenylnitrone (PBN) and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) (Khan et al., 2003) but also such relative newcomers as 1,1,3-trimethyl-isoindole N-oxide (TMINO) (Bottle et al., 2003), 5,5-diethylcarbonyl-1-pyrroline N-oxide (DECPO) (Karoui et al., 2004), N-2-(2-ethoxycarbonyl-propyl)-α-phenylnitrone (EPPN) (Stolze et al., 2003), 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO) (Liu et al., 1999; Khan et al., 2003) and 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide (BMPO) (Zhao et al., 2001a). Potential problems in their use are summarized by Rosen et al. (1999), Halliwell & Gutteridge (1999) and Khan et al. (2003). One generally underappreciated problem is that the reaction products giving the ESR signal can be rapidly removed in vivo and in cultured cells, both by enzymic metabolism and by direct reduction by such agents as ascorbate. For example, when DMPO is used to trap OH•, any ascorbate present can directly reduce the DMPO−OH• adduct to an ESR-silent species (Halliwell & Gutteridge, 1999). Many papers have described alleged antioxidant actions of various compounds evaluated in vitro by measuring decreases in the ESR signal that results when the compound is added to DMPO (or other spin trap) in the presence of radical-generating systems (frequently a Fenton system, H2O2+Fe2+). These decreases are attributed to scavenging of OH• by the added compound, in competition with DMPO for OH•. Rarely do these papers report the essential controls (Halliwell, 1995): does the added compound interfere with the radical-generating system (e.g. decomposing H2O2 or chelating iron in the Fe2+/H2O2 system) or does it interact directly with the trap spin adduct, reducing it to an ESR-silent species?
Rizzi et al (2003) have introduced triarylmethyl free radical, TAM OX063, as a probe for the detection of O2•− in aqueous solution. In this case, the O2•− reacts with the probe to cause the loss of the ESR signal. One advantage of TAM OX063 is that it is not subject to reduction by such agents as ascorbate or reduced glutathione (GSH).
Ex vivo trapping
Presently available spin traps cannot be administered to humans because of unknown toxicity at the high levels that would be required for radical trapping in vivo, in order for the trap to complete with endogenous molecules for a reactive free radical. However, traps can be used on body fluids and tissue samples. For example, DMPO was used to measure free radicals in human skin biopsies (Haywood et al., 1999). A hydroxylamine probe has been used to measure free radicals in liver biopsies (Valgimigli et al., 2002). PBN has been employed to demonstrate free radical production by adding it to coronary sinus blood drawn during coronary bypass surgery (Tarkka et al., 2000; Clermont et al., 2002). Of course, very highly reactive radicals such as OH•, and highly reactive nonradical species (such as peroxynitrous acid) that may be formed in vivo (Sun et al., 1993; Halliwell & Gutteridge, 1999; Ferdinandy & Schulz, 2003), will simply not survive to be detected in ex vivo material. For example, OH• reacts with whatever is in its immediate vicinity within microseconds (Halliwell & Gutteridge, 1999) and ONOOH breaks down in seconds (Radi et al., 2001). ESR of ex vivo samples to which traps have been added probably detects secondary radicals resulting from reaction of RS with biomolecules. These secondary radicals will include lipid-derived radicals (alkoxyl, peroxyl, etc.) and possibly also protein radicals (Pantke et al., 1999; Ostdal et al., 2002). Ascorbic acid (vitamin C) reacts with a wide range of free radicals and other RS (Buettner, 1993; Halliwell, 1999c) and one of its oxidation products, semidehydroascorbate radical, can easily be detected by ESR (Buettner & Jurkiewicz, 1993). Measurement of semidehydroascorbate has been used as an indication of free radical production in organs, blood plasma and skin (Buettner & Jurkiewicz, 1993; Sharma & Buettner, 1993; Sharma et al., 1994; Jurkiewicz & Buettner, 1996; Haywood et al., 1999). It is only semiquantitative, since ascorbate radicals quickly react with each other to generate ESR-silent species (ascorbate and dehydroascorbate) (Bielski et al., 1975; Kobayashi et al., 1991). The ascorbate radical is also reduced by enzymes in vivo (May et al., 2001).
Traps, both the ESR ‘spin traps' described above and other types of trap (see below), are likely to perturb the biological system under investigation. If traps scavenge RS, and these RS are important mediators of cell or tissue injury, it follows logically that the traps should protect against damage, provided, of course, that they are scavenging enough of the RS (Table 3). Indeed, some compounds derived from spin traps have entered clinical trials, for example, for stroke (Lapchak & Araujo, 2003). However, one must never assume that any beneficial effects that traps exert are due to free radical scavenging without direct evidence for this mechanism, since these compounds can exert other pharmacological effects (Pietri et al., 1998; Floyd et al., 1999; Kotake, 1999).
Aromatic traps
Potentially more useful than spin traps in vivo in humans are aromatic ‘free radical' traps known to be acceptable for human consumption, including salicylate and phenylalanine (Kaur et al., 1996; Grootveld & Halliwell, 1986; Kaur & Halliwell, 1994; Halliwell & Kaur, 1997; Coudray & Favier, 2000; Themann et al., 2001). Salicylate is hydroxylated by OH• to yield, among other products, 2-3 dihydroxybenzoate, which is not apparently produced enzymically in vivo (Ingelman-Sundberg et al., 1991; Dupont et al., 1999). Both L- and D-phenylalanine are hydroxylated by OH• to yield ortho- and meta-tyrosines, which again seem not to be enzymically produced in humans, although the evidence supporting this statement is weak and more studies are required (Kaur & Halliwell, 1994; Halliwell and Kaur, 1997; Li et al., 2003). Both salicylate and phenylalanine have been used to measure ex vivo radical formation in blood from patients with rheumatoid arthritis (Kaur et al., 1996) or in humans exposed to ozone (Frischer et al., 1997; Liu et al., 1997a). Phenylalanine was used to detect OH• formation in saliva (Nair et al., 1995). Several human studies have also used salicylate to detect OH• in vivo, with some success. Examples include studies on diabetes (Ghiselli et al., 1992), alcoholism (Thome et al., 1997) and myocardial infarction (Tubaro et al., 1992). Endogenous levels of free (Lubec et al., 1997; Ogihara et al., 2003) or protein-bound (Fu et al., 1998; Lamb et al., 1999; Pennathur et al., 2001) ortho- or meta-tyrosines have also been used to implicate OH• formation in vivo.
The success of aromatic compounds (or spin traps) in detecting OH• will depend on their concentration at sites of free radical generation in relation to that of other OH• scavengers (virtually all other biomolecules react fast with OH• and will compete), and so it is unlikely that any trap can intercept more than a small percentage of any OH• generated. 2,3-Dihydroxybenzoate can be metabolized in the liver, and possibly other organs, causing its loss (unpublished data). It follows that aromatic hydroxylation techniques are not quantitative measures of OH• generation, especially if the end products are measured in the plasma, where their origin is unclear. Specificity of the traps for OH• is uncertain: ONOO− can hydroxylate both salicylate and phenylalanine, perhaps, in part, by direct reaction with ONOOH (Kaur et al., 1997). Simultaneous determination of nitration products can distinguish the two (Kaur et al., 1997; Ferger et al., 2001), since OH• cannot nitrate aromatic compounds whereas ONOO− usually does. Myeloperoxidase can also hydroxylate aromatic compounds, although the favored product from salicylate is 2,5-dihydroxybenzoate (Kettle & Winterbourn, 1994; Kawakami et al., 1999). Hypochlorous acid produced by myeloperoxidase can, however, react with O2•− to produce OH• (Candeias et al., 1993).
Another problem is that high-performance liquid chromatography (HPLC) analysis of meta-tyrosine in human samples can be confused by the frequent presence of a coeluting peak of unknown identity (Reddy et al., 1999). Be suspicious if you see an apparent meta-tyrosine peak on HPLC much greater than that of ortho-tyrosine: this is suggestive of an artifact since both products should be formed in comparable amounts when OH• attacks phenylalanine. Mass spectrometry (MS) can be used to avoid this problem (Pennathur et al., 2001). As an additional probe, the compound antipyrene is hydroxylated by OH• into para- and ortho-hydroxylation products, the latter again not apparently formed enzymically in humans. Antipyrene has been used to measure OH• production during exercise (Meijer et al., 2001).
Urate as a trap
An endogenous molecule might also function as a trap (indeed, measurement of meta- and ortho-tyrosines in body proteins as ‘endogenous traps' would fit into this category), although it can be argued that measuring specific end products of the trapping of RS by endogenous molecules is the same as measuring ‘biomarkers' (see below). Ascorbate reaction with free radicals is one example, as discussed above. Another example is urate, which is readily oxidized by a range of RS (Kaur & Halliwell, 1990), including peroxynitrite (Whiteman et al., 2002b). Several groups have used urate as a ‘selective' scavenger of ONOO− in animal studies, neglecting the fact that it reacts with many species (Kaur & Halliwell, 1990). One of urate's oxidation products, allantoin, can be measured in human body fluids and its plasma levels are elevated in conditions associated with oxidative stress, such as chronic inflammation, diabetes, premature birth, iron overload, chronic heart failure and exercise (Grootveld & Halliwell, 1987; Moison et al., 1997; Ogihara et al., 1998; Benzie et al., 1999; Mikami et al., 2000; Doehner et al., 2002; Pavitt et al., 2002; Yardim-Akaydin et al., 2004). Allantoin can also be measured in urine (Mikami et al., 2000) and cerebral microdialysis fluid (Marklund et al., 2000). Levels of allantoin rise in the human muscle during exhaustive exercise, presumably due to oxidation of urate by RS generated during exercise (Hellsten et al., 2001). Allantoin measurement may be one of the more promising methods for human use, since human urate levels in vivo are high and urate reacts with a wide range of RS.
Measuring stable species in body fluids: hydrogen peroxide
Several laboratories have demonstrated the presence of H2O2 in freshly voided human urine (Varma & Devamanoharan, 1990; Kuge et al., 1999; Long et al., 1999; Laborie et al., 2000; Long & Halliwell, 2000; Hiramoto et al., 2002; Kirschbaum, 2002; Yuen & Benzie, 2003). It has also been measured in rabbit urine (Pi et al., 2003). Since assay of urinary H2O2 can be performed rapidly and simply (Long et al., 1999), the question has been raised if its levels in urine (after creatinine standardization) can be related to rates of generation of RS in vivo. Caution must be exercised, since drinking coffee artifactually raises urinary H2O2 levels (Long & Halliwell, 2000; Hiramoto et al., 2002), apparently because hydroxyhydroquinone in coffee is absorbed into the body, excreted into the urine and autooxidizes therein to produce H2O2 (Hiramoto et al., 1998; Halliwell et al., 2004a). Patients with cancer showed elevated urinary H2O2 levels (Banerjee et al., 2003). The currently available data are insufficient to support the use of urinary H2O2 as a biomarker of oxidative stress and more work is required (Yuen & Benzie, 2003; Halliwell et al., 2004a).
Hydrogen peroxide can also be detected in exhaled air and breath condensate, and the levels appear to increase during inflammation. The origin of the H2O2 is unclear: release by respiratory tract cells and oral bacteria are possibilities (Jobsis et al., 2001; Geiszt et al., 2003; Rahman & Kelly, 2003).
Measuring changes in blood pressure and vascular tone
The free radical NO• plays a key role in vasodilatation. Its action can be antagonized by O2•−, which removes NO• by reacting fast with it to give ONOO− (reviewed by Beckman & Koppenol, 1996). In the healthy arterial wall, there is a balance between NO• and O2•−, but increased oxidative stress can diminish the bioactivity of NO• (Darley-Usmar et al., 1995; Kojda & Harrison, 1999). Sources of O2•− in and around the vessel wall include the vascular endothelium (especially in atherosclerotic lesions), fibroblasts, lymphocytes, phagocytes and the enzyme xanthine oxidase (Darley-Usmar & Halliwell, 1996; Reth, 2002; Rey & Pagano, 2002). Upregulation of O2•−-generating NAD(P)H oxidases may play a key role in causing impaired vascular tone and hypertension (Harrison et al., 2003; Lassegue & Clempus, 2003; Murphey et al., 2003).
Many papers have claimed that high doses of infused or orally administrated antioxidants (ascorbate being most commonly used) can scavenge ROS, restore NO• bioavailability and ameliorate human endothelial dysfunction (for examples see Plotnick et al., 1997; Duffy et al., 1999; Gokce et al., 1999; Wilkinson et al., 1999; May, 2000; Beckman et al., 2001; Duffy et al., 2001; Pleiner et al., 2002; Heiss et al., 2003), although negative reports are now accumulating (e.g. Darko et al., 2002; Singh et al., 2002; Svetkey & Loria, 2002; Widlansky et al., 2004; our unpublished date). The timing of antioxidant administration and the state of the vascular bed (healthy, mildly diseased or severely diseased) may be critical variables (Carroll & Schade, 2003). Hence it is possible that examination of short-term vascular effects, which is easily achievable in human studies by measurements of forearm blood flow, can gather evidence about localized scavenging of RS in blood vessel walls by antioxidants.
However, it must not be assumed that any beneficial effects of antioxidants on vascular function are necessarily due to free radical scavenging. For example, ascorbate may have a direct stimulating effect on eNOS activity by increasing endothelial tetrahydrobiopterin levels (Huang et al., 2000; d'Uscio et al., 2003).
Fingerprinting of RS
If a RS combines with a biological molecule to leave a unique chemical ‘fingerprint', then the presence of that fingerprint (which is of course an example of oxidative damage) can be used to infer that the RS has been formed (Halliwell & Gutteridge, 1999). Such ‘biomarkers' can then be used to investigate effects of dietary supplements or synthetic antioxidants on oxidative damage, hopefully leading to experimentally verifiable predictions of the likely effects of these compounds on oxidative stress-related diseases arising subsequently. Indeed, since methods currently available for the direct measurement of RS are of limited applicability to humans (see above), most clinical studies focus on the measurement of oxidative damage. This is to some extent logical, since it is the damage caused by RS that is important rather than the total amount of such species generated. For example, if highly reactive OH• radicals are generated within a cell, many of them may react with unimportant targets. The small fraction that reacts with DNA (to cause strand breakage and formation of mutagenic base or sugar oxidation products), with key proteins, or with lipids (initiating lipid peroxidation) may be the important fraction.
Table 4 summarizes the requirements of the ideal biomarker. No currently used biomarker has yet fulfilled the key criterion (A); the experiments have not been carried out. It is sad that biomarkers were never built into all the human intervention trials with antioxidants that have been carried out; they could have been of great value in interpreting the confusing and conflicting results (Halliwell, 1999a, 2000b, 2000c). No biomarker meets all parts of criterion (B), but some are better than others. We have already considered small molecules such as ascorbate and urate, so now will comment briefly on currently available biomarkers of oxidative damage to the three main classes of macromolecules: nucleic acids, proteins and lipids.
Table 4.
Criteria for the ideal biomarker of oxidative damage
| A. Core criterion |
| That the biomarker is predictive of the later development of disease. |
| B. Technical criteria |
| (i) The biomarker should detect a major part, or at least a fixed percentage, of total ongoing oxidative damage in vivo. |
| (ii) The coefficient of variation between different assays of the same sample should be small in comparison with the difference between subjects. |
| (iii) Its levels should not vary widely in the same subjects under the same conditions at different times. |
| (iv) It must employ chemically robust measurement technology. |
| (v) It must not be confounded by diet. |
| (vi) It should ideally be stable on storage, not being lost, or formed artifactually, in stored samples. |
Lipids
Lipids can be oxidized, chlorinated and nitrated by a range of RS (not including H2O2, NO• or O2•−, which are essentially unreactive with lipids; Halliwell & Gutteridge, 1999). Techniques for the measurement of chlorinated and nitrated lipids are being developed, and species such as nitrated linoleate have been detected in human blood plasma (Hazen et al., 1996; Lim et al., 2002; Lima et al., 2002, 2003; Thukkani et al., 2003). We await further studies on these compounds with interest.
Lipid peroxidation is a complex process, and a wide range of products is formed in variable amounts (Halliwell & Gutteridge, 1999). Lipid oxidation can be measured in many ways, but commonly used methods such as diene conjugation and thiobarbituric acid (TBA)-reactive material are of questionable validity (Halliwell & Gutteridge, 1999). In particular, the simple TBA test should be dismissed as unacceptable in modern research, simply because most TBA-reactive material in human body fluids is not related to lipid peroxidation. A significant improvement to the TBA test can be made by using HPLC to isolate the malondialdehyde (MDA)-TBA chromogen before analysis (Halliwell & Gutteridge, 1999). One can also assay MDA directly (e.g. Liu et al., 1997b), but MDA is only one of many aldehydes formed during lipid peroxidation, and MDA can also arise from free radical attack on sialic acid and deoxyribose (Halliwell & Gutteridge, 1999). Unsaturated aldehydes such as 4-hydroxynonenal and acrolein may cause considerably more cytotoxicity in vivo than MDA, so measuring them is perhaps more logical (Ong et al., 2000; Uchida, 1999, 2003). Lipid hydroperoxides and aldehydes can also be absorbed from the diet. For example, some foods contain MDA–amino-acid adducts that can be absorbed through the gut and then excreted in urine (Nelson et al., 1993; Richelle et al., 1999; Draper et al., 2000; Cohn, 2002; Indart et al., 2002; Wilson et al., 2002). It follows that measurements of urinary MDA can be confounded by diet and should not be used as an index of whole-body lipid peroxidation unless diet is controlled.
The isoprostanes
The best available biomarker of lipid peroxidation appears to be the isoprostanes, specific end products of the peroxidation of polyunsaturated fatty acids (Roberts & Morrow, 2002; Fam & Morrow, 2003). Most work has been carried out on the F2-isoprostanes, which arise from arachidonic acid (Roberts & Morrow, 2002), but some data are also available on isoprostanes from eicosapentaenoic (Nourooz-Zadeh et al., 1997) and docosahexaenoic acids. The latter are often called neuroprostanes or F4-isoprostanes (Nourooz-Zadeh et al., 1999; Reich et al., 2001; Roberts & Morrow, 2002). Isoprostanes are best measured by MS (Lawson et al., 1999; Roberts & Morrow, 2002). Although some immunoassay kits for F2-isoprostanes are commercially available, their reliability has been questioned (Proudfoot et al., 1999; Bessard et al., 2001; Roberts & Morrow, 2002). Although isoprostanes can be detected in foods, they do not appear to pass through the gut in sufficient quantities to affect plasma or urinary levels (Richelle et al., 1999; Gopaul et al., 2000). Reliable MS-based techniques have been established to detect isoprostanes and their metabolites in the plasma and urine (Lawson et al., 1999; Li et al., 1999; Fam & Morrow, 2003), although the ‘work-up' techniques prior to MS are tedious.
Even the isoprostanes do not qualify as ‘ideal' biomarkers. The products commonly measured (e.g. 8-iso-PGF2α) are often minor end products of peroxidation (Lawson et al., 1999), and the amounts formed can be influenced by such parameters as O2 concentration (Morrow et al., 1998; Fessel et al., 2002; Roberts & Morrow, 2002). Hence, much work is proceeding to identify new products arising from the isoprostane pathway (Roberts & Morrow, 2002; Fessel et al., 2002). Isoprostanes appear to be rapidly metabolized, turning over quickly (Roberts & Morrow, 2002; Basu, 2004). Thus a rise in plasma isoprostane levels could conceivably be due not only to increased formation by oxidative damage to lipids but also by slower metabolism. Measurement of isoprostanes in plasma and urine is, of course, a ‘whole-body' measurement; it is not necessarily clear from where the isoprostanes originated. They can also be measured in fluids drawn from specific sites, such as synovial fluid (Basu et al., 2001), pericardial fluid (Mallat et al., 1998), wound exudate (Sim and Stacey, 2003) and breath condensate (Montuschi et al., 1999).
Of especial current interest is the use of isoprostane measurements to study Alzheimer's disease (AD), a condition in which lipid peroxidation and other oxidative damage seem to play significant pathological roles (Montine et al., 1998, 1999; Pratico et al., 2000, 2001, 2002; Halliwell, 2001; Butterfield, 2002). Levels of F2- and F4-isoprostanes are elevated in CSF from AD patients (Montine et al., 1998, 2002; Pratico et al., 2000), and may even be elevated prior to the development of AD (Pratico et al., 2001, 2002), consistent with the view that peroxidation may be an important step in progressive neuronal injury leading to clinically manifested disease (Halliwell, 2001; Pratico et al., 2001, 2002). It has been claimed that urinary and plasma levels of isoprostanes are also elevated in AD patients to an extent correlated with the degree of cognitive impairment (Pratico et al., 2000, 2002), although this was not confirmed in other studies (Montine et al., 2002; Bohnstedt et al., 2003). It would be of great advantage in researching neuroprotective antioxidants to have a peripheral biomarker that could report on oxidative events in the brain, and so more work is needed to investigate urinary and plasma isoprostanes in this context and resolve the conflicting data. However, there does seem to be agreement that there are elevated CSF isoprostane levels in AD (Pratico et al., 2000, 2002; Montine et al., 2002).
Increased levels of isoprostanes have been observed in many animal (reviewed by Roberts & Morrow, 2002; Basu, 2004) and human conditions associated with oxidative stress, including cardiopulmonary bypass (Ulus et al., 2003), angioplasty (Guan et al., 1999, 2003; Iuliano et al., 2001) and diabetes (Gopaul et al., 2001; Jialal et al., 2002; Sampson et al., 2002). Hyperglycemia causes oxidative stress (Brownlee, 2001; Gopaul et al., 2001; Jialal et al., 2002), and plasma isoprostane levels in diabetes may be determined to a considerable extent by plasma glucose levels (Sampson et al., 2002).
Isoprostanes have also been used to study effects of dietary antioxidant supplementation on lipid peroxidation. Plasma and urinary isoprostane levels in humans have responded to antioxidant supplementation in some studies (reviewed by Halliwell, 2000a; Roberts & Morrow, 2002), but in general responses are limited or absent in healthy well-nourished subjects, indicating that lipid peroxidation is little affected by supplements. Supplementation of healthy volunteers with vitamins C or E, for example, usually decreases isoprostane levels only slightly, if at all (Levine et al., 2001; Meagher et al., 2001), which might help to explain why these vitamins have had little effect on disease outcomes in many human intervention trials (Halliwell, 2000c). However, some positive effects have been reported, for example, supplementation with vitamins E and C did decrease urinary isoprostanes in one study upon healthy American nonsmoking subjects (Huang et al., 2002). Isoprostane measurements also suggest that obesity and hypercholesterolemia (known risk factors for cardiovascular disease) are associated with elevated rates of lipid peroxidation. This is consistent with the view that lipid peroxidation is an important contributor to cardiovascular disease and can help explain why weight loss and statins decrease cardiovascular events (Roberts et al., 2002; Keaney et al., 2003; Morrow, 2003; Russell et al., 2003; Samuelsson et al., 2003). There is also increasing interest in the link between hypercholesterolemia, oxidative stress and AD (Refolo et al., 2001; Leonarduzzi et al., 2002; Puglielli et al., 2003; Wolfrum et al., 2003; Ong & Halliwell, 2004).
An important question for any biomarker is the coefficient of variation between assays of the same sample and between samples taken from the same subjects at different times. There have been claims that isoprostane levels vary with time of day and from day to day (Helmersson & Basu, 2001; Kanabrocki et al., 2002), possibly because oxidative stress also varies (reviewed by Halliwell et al., 2004a). If there is such a variation, it is essential to take it into account in human studies.
Exhaled hydrocarbons
Exhaled air contains not only isoprostanes (Rahman & Kelly, 2003; Montuschi et al., 1999) and aldehydes but also a range of hydrocarbons, including ethane and pentane (Andreoni et al., 1999; Phillips et al., 2000; Dale et al., 2003). The available data suggest that ethane may be a biomarker of lipid peroxidation, pentane perhaps less so. However, these hydrocarbons are minor end products of lipid peroxidation, formed to variable extents depending on the exact environment of the oxidizing lipids (Halliwell & Gutteridge, 1999). Exhaled hydrocarbons are also difficult to measure routinely in human studies, requiring cumbersome equipment, as compared to, for example, measuring isoprostanes in blood and urine samples.
DNA
Oxidative DNA damage seems to relate to an increased risk of cancer development later in life (reviewed by Halliwell, 2002b). DNA subjected to attack by hydroxyl radical generates a huge range of base and sugar modification products (Dizdaroglu et al., 2002). Such products can be measured by HPLC, gas chromatography (GC)–MS, liquid chromatography (LC)–MS and antibody-based techniques, and arguments rage over which is best (Halliwell, 1999b, 2000c; Dizdaroglu et al., 2002; Kasai, 2002; Collins et al., 2004). None of them is the ‘gold standard' we all seek. Initial products of free radical attack upon purines, pyrimidines and deoxyribose undergo transformation into stable end products, whose relative amounts depend on reaction conditions (Dizdaroglu, 1992; Alam et al., 1997; Halliwell, 1999b; Dizdaroglu et al., 2002). Thus it is intrinsically unreliable to measure any single reaction product as an index of oxidative DNA damage, but that is what is usually done, as 8-hydroxy-2′-deoxyguanosine (8OHdG) (Collins et al., 2004). The advantages and artifacts of measuring 8OHdG in cellular DNA have been recently reviewed (Halliwell, 2000c, 2002b; Collins et al., 2004), so they need not be reiterated here. The major problems arise from artifactual oxidative damage to DNA and consequent 8OHdG formation during isolation of DNA, its preparation for analysis and the analysis itself. In general, there is no agreement even on basal levels of 8OHdG in cellular DNA, nor does an agreement seem close (Collins et al., 2004). In particular, the suggestion almost 10 years ago that mitochondrial DNA (mtDNA) is more oxidized than nuclear DNA does not rest on a firm experimental foundation (Beckman & Ames, 1999). Our own studies (unpublished) indicate similar or lower (for some base damage products) levels of oxidative DNA damage in rat liver mtDNA as compared with nuclear DNA. Several RS formed at sites of inflammation, such as HOCl and ONOO−, can destroy 8OHdG in DNA (Whiteman et al., 1997, 2002a). Nevertheless, significant increases in levels of 8OHdG and other DNA base damage products have been observed in many studies on diseased human material (reviewed by Halliwell, 2000c; Evans et al., 2003), although (as with the isoprostanes) effects of dietary antioxidant supplementation on levels of 8OHdG or other base damage products in vivo seem limited (reviewed by Halliwell, 2000c; Moller & Loft, 2002). These limited effects may explain why antioxidant administration has generally not decreased cancer incidence in human intervention trials in Western countries.
One approach would be to bypass these problems of artifactual DNA oxidation during DNA isolation and analysis by measuring oxidative DNA damage in the intact cell. Antibody methods have been developed for 8OHdG (e.g. Toyokuni et al., 1997) and are useful for visualization of damage, but seem likely to be semiquantitative (Shimoi et al., 2002; Yoshida et al., 2002). The comet assay (Duthie et al., 1996) can be applied directly to cells and measures DNA strand breaks. If a digestion step with DNA repair enzymes is included in the protocol, the increased numbers of DNA strand breaks can be used to estimate the level of oxidized DNA bases in the cell. Studies with the comet assay seem more likely than other methods to show positive effects of antioxidant interventions in human volunteers, and the reasons for this need to be elucidated (Duthie et al., 1996; Toyokuni et al., 1997; Collins & Horvathova, 2001; Moller & Loft, 2002). Is it that the comet assay is more reliable because the baseline is lower, artifactual DNA oxidation having been minimized? Or does the comet assay simply just generate a different kind of artifact, in underestimating damage? It seems very unlikely that all the oxidized bases in compact DNA can be recognized by exogenously applied enzymes. Strand breaks, which are what the comet assay measures, arise not only during oxidative DNA damage but also during repair of that damage (Spencer et al., 1996a). Enzymic DNA cleavage during apoptosis may also generate comets, which can lead to false conclusions about the genotoxicity of apoptosis-inducing agents. An antioxidant that suppressed apoptosis could be misinterpreted to be protecting directly against DNA damage (Choucroun et al., 2001).
None of the analytical methods mentioned above identifies where the oxidative DNA damage is located: is it in expressed genes, inactive genes, merely in ‘junk DNA' or even in telomeres (Oikawa & Kawanishi, 1999)? Attempts are underway to address this problem (e.g. Rodriguez et al., 2000; Choi et al., 2003; Sawyer et al., 2003). Another problem in studying damage to DNA by RS is the limited availability of human tissues from which to obtain DNA. Most studies are performed on DNA isolated from lymphocytes or total white cells from human blood, and it is assumed (possibly erroneously: reviewed by Halliwell, 2002b) that changes here are reflected in other tissues. Sperm, buccal cells, placenta, and biopsies of muscle, skin, colon and other tissues are other potential sources of DNA.
Urinary measurements
Measurement of 8OHdG in urine has been used to assess rates of ‘whole-body' oxidative DNA damage. This can be achieved by HPLC and MS techniques (Bogdanov et al., 1999; Gackowski et al., 2001; Kasai, 2002; Pilger et al., 2002; Lin et al., 2004), but ELISA techniques have also been described (Shimoi et al., 2002; Yoshida et al., 2002). The presence of interfering peaks in certain urine samples is a major bugbear, and sample ‘cleanup' techniques are critical (Bogdanov et al., 1999; Lin et al., 2004). The assays seem reliable (except that ELISA can give high levels sometimes) and data fairly comparable between laboratories (Bogdanov et al., 1999; Gackowski et al., 2001; Cooke et al., 2002; Kasai, 2002; Pilger et al., 2002; Shimoi et al., 2002; Yoshida et al., 2002; Lin et al., 2004), unlike what is seen with the measurement of 8OHdG in isolated DNA (Collins et al., 2004). No urinary 8OHdG appears to originate from diet (Gackowski et al., 2001). However, 8OHdG can arise from degradation of oxidized dGTP in the DNA precursor pool, not just from removal of oxidized guanine residues from DNA by repair processes (Cooke et al., 2002). Hence urinary levels may not truly reflect rates of oxidative damage to DNA. Another reason why one cannot equate 8OHdG in urine with oxidative DNA damage is that there are many other products of this process (Dizdaroglu, 1992; Dizdaroglu et al., 2002). Hence, urinary 8OHdG is a partial measure of damage to guanine residues in DNA and its nucleotide precursor pool. Nevertheless, 8OHdG is the best urinary measure we have at the moment. Other oxidized bases can be measured in urine (e.g. Ravanat et al., 1999), but a confounding by diet cannot yet be ruled out. DNA in foods can be oxidized during storage and cooking, and it is possible that oxidized bases can be absorbed and reexcreted, just as it happens with MDA. This is not a problem with 8OHdG (Gackowski et al., 2001).
Measurement of 8OHdG in urine gives no information about its tissue origin, although it is sometimes possible to sample from specific sites, such as cerebrospinal fluid (Rozalski et al., 2003). The possibility of diurnal variations in urinary 8OHdG levels has been raised, so spot measurements should be interpreted with caution (Kanabrocki et al., 2002). However, our laboratory has not found this to be a major problem (Lin et al., 2004). Products of reaction of DNA bases with end products of lipid peroxidation such as MDA can also be detected in human urine (Hanaoka et al., 2002; Otteneder et al., 2003), but a confounding effect of diet is possible since these adducts may be present in foods, for example, after cooking. DNA base–aldehyde adducts can also be measured in DNA isolated from cells and tissues (Bartsch & Nair, 2000; Marnett et al., 2003), but one must again be cautious because tissue homogenates can undergo rapid peroxidation in vitro (Halliwell & Gutteridge, 1999), leading to possible modification of DNA by aldehydes generated artifactually during the isolation process.
Reactive nitrogen and chlorine species
DNA can also be damaged by reactive nitrogen species, undergoing mainly nitration and deamination of purines (Yermilov et al., 1995; Spencer et al., 1996b; Zhao et al., 2001b; Lee et al., 2002b). It was initially thought that 8-nitroguanine was a specific product of reaction of ONOO− with DNA, but this does not seem to be the case (Byun et al., 1999). Methods for the measurement of DNA base nitration and deamination products have been developed, but may need more refinement before they can be routinely applied to human material. However, cigarette smoking was observed to raise the level of 8-nitroguanine in peripheral lymphocytes of humans (Hsieh et al., 2002). Antibody-based methods have been used to demonstrate rises in 8-nitroguanine during inflammation in animals (Akaike et al., 2003; Pinlaor et al., 2003). Assays to identify chlorinated DNA bases have also been developed (Whiteman et al. 1997; Henderson et al., 2003).
Ultraviolet light
Ultraviolet light produces a range of lesions in DNA, including thymine dimers (Douki et al., 2000), which can be measured in human urine. Levels increase after exposure to sunlight (Cooke et al., 2001; Le Curieux & Hemminki, 2001) and in psoriasis (Ahmad et al., 1999). Urinary thymine dimers might thus be a useful biomarker to assess effects of antioxidants on sunlight-induced DNA damage.
Proteins
Oxidative damage to proteins may be important in vivo both in its own right (affecting the function of receptors, enzymes, transport proteins, etc., and perhaps, generating new antigens that can provoke immune responses) (Halliwell, 1978; Casciola-Rosen et al., 1997) and because it can contribute to secondary damage to other biomolecules, for example, inactivation of DNA repair enzymes and loss of fidelity of damaged DNA polymerases in replicating DNA (Wiseman & Halliwell, 1996). However, analyzing protein oxidative damage products is an order of magnitude more complex than dealing with DNA: rather than four bases and one sugar there are 20 different amino acids, each of which can be attacked by RS in multiple ways (Davies et al., 1999; Headlam & Davies, 2003). Free radical attack on proteins can generate amino-acid radicals, which may crosslink or react with O2 to give peroxyl radicals. These may abstract H•, triggering more free radicals and forming protein peroxides, which can decompose in complex ways, accelerated by transition metal ions, to generate yet more radicals (Headlam & Davies, 2003). Proteins can also be oxidized during food cooking, meaning that oxidized amino acids could conceivably be absorbed from the diet, which could confound measurements of them in body fluids as putative biomarkers of oxidative damage. Individual amino-acid oxidation products that have been measured in various human tissues include kynurenines (from tryptophan), bityrosine (which appears to be metabolically stable and can be detected in urine), valine and leucine hydroxides, L-dihydroxyphenylalanine (L-DOPA), ortho-tyrosine, 2-oxo-histidine, glutamate semialdehyde and adipic semialdehyde (Uchida & Kawakishi, 1993; Morin et al., 1998; Leeuwenburgh et al., 1999a, 1999b; Giulivi & Davies, 2001; Harth et al., 2001; Headlam & Davies, 2003). Bityrosine, easily detectable in human and other animal urines, formed by free radical attack on a wide range of proteins, and apparently not metabolized, might be a biomarker worth further development for human use (Davies et al., 1999; Leeuwenburgh et al., 1999a, 1999b; Giulivi & Davies, 2001). Patients with sepsis were reported to have increased urinary excretion of bityrosine (Bhattacharjee et al., 2001), as were children with kwashiorkor (Manary et al., 2000), a disease which is believed to involve increased oxidative stress (Golden & Ramdath, 1987; Fechner et al., 2001). An immunohistochemical method to detect bityrosine in human brain has been described (Kato et al., 1998). Sakharov et al. (2003) described the use of an acetyltyramine–fluorescein probe to detect formation of tyrosine radicals in proteins in cells subjected to oxidative stress. If both the tyramine probe and tyrosine residues are oxidized, the probe can crosslink to the protein tyrosyl residues, generating a stable crosslink similar to that in bityrosine. The fluorescein-labeled proteins can then be visualized and identified by proteomic methods.
Theoretically also useful might be the measurement of methionine sulfoxide, since methionine residues appear to be a ‘radical sink' in proteins (Levine et al., 1999), that is, oxidative attack at different sites on a protein may eventually appear as methionine sulfoxide. However, methionine sulfoxide residues are usually quickly removed by methionine sulfoxide reductase enzymes (Levine et al., 1999; Sharov et al., 1999), and this amino-acid does not appear to have been used as a biomarker in human studies.
Proteins can also be attacked by reactive chlorine, bromine and nitrogen species (defined in Table 1), giving products such as 3-chlorotyrosine, para-hydroxyphenylacetaldehyde, 3,5-dichlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine (Hazen & Heinecke, 1997; Wu et al., 1999, 2000; Winterbourn & Kettle, 2000; Greenacre & Ischiropoulos, 2001; Himmelfarb et al., 2001; Aldridge et al., 2002; Gaut et al., 2002a). 3-Nitrotyrosine is often thought to be a specific marker of attack of ONOO− upon proteins, but in fact it can be formed from tyrosine by a range of reactive nitrogen species and its production in vivo is often dependent on the production of such species as nitrogen dioxide radical (NO2•) by myeloperoxidase or other peroxidases (Halliwell et al., 1999; Greenacre & Ischiropoulos, 2001; MacPherson et al., 2001; Gaut et al., 2002b). 3-Nitrotyrosine has been frequently measured by immunostaining, HPLC and MS in human tissues, but we have been unable to detect it in human urine (unpublished data). Nitrotyrosine is metabolized to 3-nitro-4-hydroxyphenylacetate (NHPA), which some groups have measured in urine as an estimate of nitrotyrosine production in vivo (Ohshima et al., 1990). However, some (and perhaps most) urinary NHPA arises by other mechanisms (Mani et al., 2003), making the assay questionable. Simple HPLC-based assays for 3-nitrotyrosine are prone to artifact (Kaur et al., 1998), and so MS-based techniques are preferred.
Another problem is that exposure of body fluids or tissues to acid during staining or protein hydrolysis (frequently HCl digestion is used to liberate protein-bound nitrotyrosine) can cause artifactual nitration of tyrosine by species derived from nitrous acid (HNO2), generated when nitrite in the sample is exposed to acid (Frost et al., 2000). Even freezing phosphate- and nitrite-containing samples can cause artifactual nitration (Daiber et al., 2003). In general, the more accurate the measurement methods that are used, the lower the levels of 3-nitrotyrosine detected in human material (Frost et al., 2000; Gaut et al., 2002a; Duncan, 2003; Morton et al., 2003). Nitrotyrosine can be destroyed by hypochlorous acid, generated by activated neutrophils (Whiteman & Halliwell, 1999). Immunostaining for nitrotyrosine is prone to artifacts (Halliwell et al., 1999; Ogino et al., 2002; Ichimori et al., 2003) but, when carried out with appropriate controls, has proved useful in qualitative demonstration of increased formation of reactive nitrogen species in tissues (e.g. MacMillan-Crow et al., 1996; Kooy et al., 1997; Greenacre & Ischiropoulos, 2001). Provided that sensitive and accurate MS-based assays are used, measurements of nitro-, chloro- and ortho-tyrosines can be useful biomarkers of protein damage by RS and how such damage can be modulated by antioxidant or other interventions (Hazen & Heinecke, 1997; Buss et al., 2003). For example, statin treatment decreased levels of these products in the plasma of hypercholesterolemia patients, consistent with the view (discussed above) that hyperchloesterolemia leads to oxidative stress (Shishehbor et al., 2003). Proteomic methods can be used to separate proteins, followed by recognition of modified protein by blotting, and this technique was used to identify targets of nitration in muscle, for example (Kanski et al., 2003). Protein nitrosylation, a reversible modification involving attachment of NO• to a metal site or a cysteine residue, can also be measured in human material (Mannick & Schonhoff, 2004).
The carbonyl assay
The most frequently used biomarker of protein damage is the carbonyl assay, measurement of protein carbonyl groups (Chevion et al., 2000; Levine, 2002; Dalle-Donne et al., 2003). Carbonyls can arise as a result of protein glycation by sugars, by the binding of aldehyes (including many of those formed during lipid peroxidation) to proteins and by the direct oxidation of amino-acid side chains by RS to generate such products as glutamate and aminoadipic semialdehydes (Reznick et al., 1992; Calingasan et al., 1999; Chevion et al., 2000; Adams et al., 2001; Requena et al., 2001; Levine, 2002; Dalle-Donne et al., 2003). Carbonyls can be readily measured spectrophotometrically and by ELISA techniques (Buss & Winterbourn, 2002; Levine, 2002), and tissue or plasma levels have been shown to be elevated in many human diseases (reviewed by Dalle-Donne et al., 2003). For example, serum levels were increased during cardiopulmonary bypass (Pantke et al., 1999). Carbonyls can also be measured in other body fluids and in tissues (Chapman et al., 1989; Buss et al., 1997; Chevion et al., 2000; Frank et al., 2000; Schock et al., 2001; Levine, 2002; Tanaka et al., 2003). Of course, carbonyls are not specific as markers of oxidative damage because bound aldehydes and glycated protein are also measured. Indeed, immunochemical assays for protein-bound aldehydes such as acrolein and 4-HNE are widely used (Uchida, 1999, 2003). Data from their use have shown that both aldehydes may be important neurotoxic agents in neurodegenerative diseases (Calingasan et al., 1999; Ong et al., 2000).
The carbonyl assay as applied to tissues and body fluids measures the ‘average' extent of protein modification. It is informative to use proteomic techniques to identify the specific proteins damaged. Often only a small selection of proteins is oxidized. For example, in human plasma subjected to oxidative stress, carbonyls appear to reside mostly on fibrinogen (Shacter et al., 1994). In the brains of subjects who died with AD, several specific proteins including enolase, UCH-L1 and DRP-2 seemed to be oxidatively modified (Butterfield et al., 2003).
Direct measurements of glutamate and aminoadipate semialdehyde (major contributors to total protein carbonyl residues) in human plasma proteins have also been used to assess the effects of alterations in dietary antioxidant intake on plasma protein oxidation, but no decreases in their levels were observed after increased intake of flavonoid-rich foods (Nielsen et al., 1999; Young et al., 2000a, 2000b). Whether this is due to the insensitivity of this biomarker or due to a lack of effect of flavonoids on oxidative damage in the human body (Dragsted, 2003; Halliwell et al., 2004b) is uncertain as yet. The possible confounding effect of uptake of oxidized amino acids from the diet also needs to be addressed.
Is there a single biomarker of oxidative stress or oxidative damage?
No, there is not (England et al., 2000), although (as might be expected) biomarkers of damage to several different molecules frequently rise in parallel in cells subjected to severe oxidative stress. Why is there no such correlation in healthy tissues? Levels of 8OHdG in DNA are a steady-state balance between rate of oxidative DNA damage and rate of removal of lesions by DNA repair mechanisms. Isoprostanes, once formed, are metabolized quickly. Oxidized proteins are degraded, mostly by the proteasome system, and appear to turn over more slowly. Thus even if all biomolecules are damaged, the extents and time courses of the biomarkers of such damage can be very different. Hence, no correlation was observed between levels of plasma isoprostanes and oxidative DNA damage products in healthy human subjects (England et al., 2000).
Measurement of RS in cells
In addition to those of us trying to study oxidative stress in humans, many groups are engaged in the apparently simpler task of trying to measure oxidative stress in cells in culture. Many methods allegedly measuring this have become widely used, but precise information on what they really measure is surprisingly sparse, as discussed below. An important first consideration is that the cell culture process itself imposes oxidative stress, both by facilitating generation of RS and by hindering adaptive upregulation of cellular antioxidants (reviewed by Halliwell, 2003a). For example, the ‘Hayflick limit' in fibroblasts (replicative senescence after a certain number of cell divisions) may be largely an artifact of oxidative stress during cell culture (Wright & Shay, 2002). In addition, results can be confounded by free radical reactions taking place in the culture media (Grzelak et al., 2000; Roques et al., 2002; Halliwell, 2003a; Wee et al., 2003). For example, many reports of effects of ascorbate and polyphenolic compounds (e.g. flavonoids) on cells in culture are artifacts caused by the oxidation of these compounds in the culture media (Long et al., 2000; Clement et al., 2001; Long & Halliwell, 2001). It is essential to examine what the medium alone might do when you add compounds to it; the results can be surprising (e.g. Clement et al., 2001, 2002). Sometimes, indeed, the presence of cells suppresses free radical reactions occurring in the medium (Halliwell, 2003a).
Some simple principles can be used as guidelines in understanding oxidative stress/oxidative damage in cell culture. Hydrogen peroxide generally crosses cell membranes readily, probably through the aquaporins (Henzler & Steudle, 2000). Thus catalase added outside cells can exert both intracellular and extracellular effects on H2O2 level, the former by ‘draining' H2O2 out of the cell by removing extracellular H2O2 and thus establishing a concentration gradient (Halliwell, 2003a). In contrast, O2•− does not generally cross cell membranes readily (Lynch & Fridovich, 1978; Marla et al., 1997). Thus if externally added superoxide dismutase is protective against an event in cell culture, be wary of what this means; it could be indicative of extracellular O2•−−generating reactions. Similarly, neither the iron-chelating agent deferoxamine (which suppresses most, but not all, iron-dependent free radical reactions) nor the thiol antioxidant GSH enter cells easily, so again be wary if they have protective effects in short-term experiments: this is suggestive of extracellular effects (Meister & Anderson, 1983; Halliwell, 1989; Marla et al., 1997). As an example, Clement et al. (2002) showed that GSH protects against the cytotoxicity of dopamine simply because it reacts with dopamine oxidation products generated in the cell culture medium. Given long enough, however, everything can enter cells, including deferoxamine, superoxide dismutase (SOD) and GSH (Doulias et al., 2003; Rius et al., 2003).
Studies of RS production in cells can, of course, be achieved by using aromatic compounds or spin traps, described above, and oxidative damage can be measured by assaying such end products as oxidized DNA bases, protein carbonyls, isoprostanes and 3-nitrotyrosine. Spin traps have been used successfully in many cell studies, since a wider range of traps at higher concentrations can be used than could ever be employed in vivo. Again, one must be aware of the possibility of rapid reduction of free radical–spin trap adducts to ESR-silent species by nonenzymic antioxidants (such as ascorbate) and cellular enzymatic reducing systems. An interesting combination is 5-((2-carboxy)phenyl)-5-hydroxy-1-(2,2,5,5-tetramethyl-1-oxypyrrolidin-3-yl)methyl-3-phenyl-2-pyrrolin-4-one sodium salt, a nitroxide that is nonfluorescent. When it combines with a RS, the nitroxide is removed, the ESR signal is lost and the fluorescence is restored (Pou et al., 1993).
Let us now comment on the use of probes, usually fluorescence based, that are alleged to detect cellular production of RS.
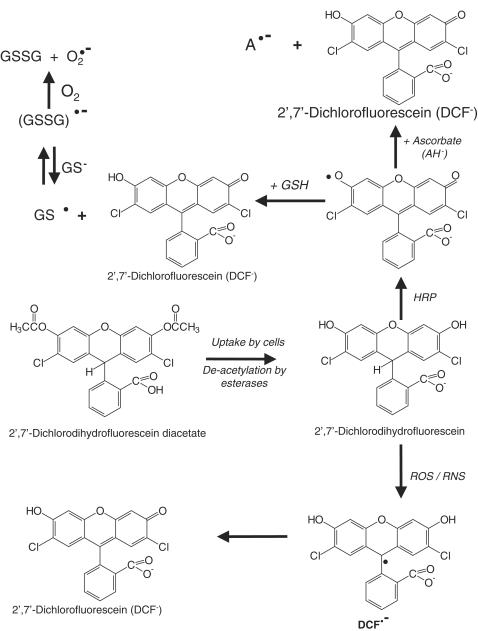
Dichlorofluorescin diacetate (DCFDA)
DCFDA is the most popular of these probes, frequently being used to detect ‘cellular peroxides'. In fact, it is unlikely to do so because it reacts only slowly with H2O2 or lipid peroxides (LeBel et al., 1992; Ischiropoulos et al., 1999; Bilski et al., 2002; Ohashi et al., 2002; Wardman et al., 2002) (Table 5). DCFDA enters cells and accumulates mostly in the cytosol. To avoid any cytotoxicity, cells should be loaded with DCFDA at low concentrations. With a variety of cell types, we have found loading at 1–10 μM for 45 min–1 h is adequate. Serum-free media must be used since serum will contain endogenous esterase activity and de-esterified dichlorofluorescein (DCF) is less cell permeable and will generate inconsistent data. Higher levels of DCFDA or high light intensities can also result in an artifactual photochemical oxidation to fluorescent products that can be mistaken for ROS generation (Marchesi et al., 1999; Afzal et al., 2003; Bindokas et al., 2003) (Table 5).
Table 5.
Sensitivity of various probes to different reactive species
| Reactive species | HPF | APF | DCFH |
|---|---|---|---|
| OHa | 730 | 1200 | 7400 |
| ONOO−b | 120 | 560 | 6600 |
| Hypochloritec | 6 | 3600 | 86 |
| Singlet O2d | 5 | 9 | 26 |
| Superoxidee | 8 | 6 | 67 |
| H2O2f | 3 | <1 | 190 |
| NO•g | 6 | <1 | 150 |
| ROO•h | 17 | 2 | 710 |
| Autoxidationi of the probe | <1 | <1 | 2000 |
Fluorescence probe reagents were added to sodium phosphate buffer (0.1 M, pH 7.4) (final 10 μM; 0.1% DMF as a cosolvent). The fluorescence intensities of HPF, APF and DCFH were measured at excitation wavelength of 490, 490 and 500 nm and fluorescence emission wavelength of 515, 515 and 520 nm, respectively.
100 μM of ferrous perchlorate (ll) and 1 mM of H2O2 were added.
3 μM (final) of ONOO− was added.
3 μM (final) of NaOCI was added.
100 μM of 3-(1,4-dihydro-1,4-epidioxy-1-naphthyl)propionic acid was added.
100 μM of KO2 was added.
100 μM of H2O2 was added.
100 μM of 1-hydroxy-2-oxo-3-(3-aminopropyl)-3-methyl-1-triazene was added.
100 μM of 2,2′-azobis(2-amidinopropane)dihydrochloride was added.
Fluorescence probe reagents solutions were placed under a fluorescent lamp for 2.5 h.
Adapted from Setsukinai et al. (2003).
We thank Professor Tetsuo Nagano and the American Society of Biochemistry and Molecular Biology for granting permission to reproduce this table.
DCFDA is deacetylated by esterases to dichlorofluorescin (DCFH). This nonfluorescent product is converted by RS into DCF, which can easily be visualized by strong fluorescence at around 525 nm when excited at around 488 nm. Many papers have confused dichlorofluorescin and dichlorofluorescein, so it may be best to stick to correct chemical nomenclature (Figure 1). The chemistry of the conversion is complex (Figure 1). Washing cells after loading will allow unreacted DCFDA to diffuse out again. DCFH and DCF can also diffuse out and undergo extracellular reactions (Ohashi et al., 2002; Subramaniam et al., 2002). Thus be careful if you use a plate reader: you may be measuring light emission from the medium as well as from the cells. Neither H2O2 nor O2•− can oxidize DCFH, but peroxyl, alkoxyl, NO2•, carbonate (CO3•−) and OH• radicals can, as can peroxynitrite (LeBel et al., 1992; Ischiropoulos et al., 1999; Bilski et al., 2002; Ohashi et al., 2002; Wardman et al., 2002; Myhre et al., 2003) (Table 5). DCFDA can only detect cellular peroxides efficiently if they are decomposed to radicals, for example, by transition metal ions. For example, in bovine aortic endothelial cells, the generation of a signal from DCFDA upon addition of H2O2 required the uptake of extracellular iron from the medium (Tampo et al., 2003). Horseradish peroxidase, myeloperoxidase and other heme proteins can also oxidize DCFH in the presence of H2O2 (indeed, DCFDA was first used in biology as a detector for H2O2 by adding horseradish peroxidase; Keston & Brandt, 1965; LeBel et al., 1992). Hence cellular peroxidase level and heme protein content are other variables to consider when interpreting studies with this probe (LeBel et al., 1992; Ohashi et al., 2002). For example, Ohashi et al (2002) showed that manipulating the heme content of cells by adding hemin or δ-aminolevulinic acid affected rates of DCFH oxidation.
Figure 1.
It follows that DCF fluorescence is an assay of generalized oxidative stress rather than of any particular RS, and is not a direct assay of H2O2, NO•, lipid peroxides, singlet O2 or O2•−. One-electron oxidation of DCFH by various radicals and heme proteins is likely to produce intermediate radicals (Figure 1), including phenoxyl radicals, that can interact with such cellular antioxidants as GSH and ascorbate and with NADH to create more free radicals (Rota et al., 1999). Lawrence et al. (2003) pointed out that cytochrome c is a powerful catalyst of DCFH oxidation, and so use of DCFDA to probe oxidative stress during apoptosis should be approached with caution, since a rise in cytosolic cytochrome c levels could result in a bigger ‘signal' without any change in cellular peroxide levels. Chromium (V), pyocyanin, mitoxantrone and ametantrone can directly oxidize DCFH and cause an artifactual signal, and the possibility of such direct oxidations must always be checked before using DCFDA to measure oxidative stress in cells exposed to various toxins (Martin et al., 1998; O'Malley et al., 2004). Variation in cellular esterase content could also conceivably affect the use of DCFDA as a probe, but this issue has not been explored in the literature.
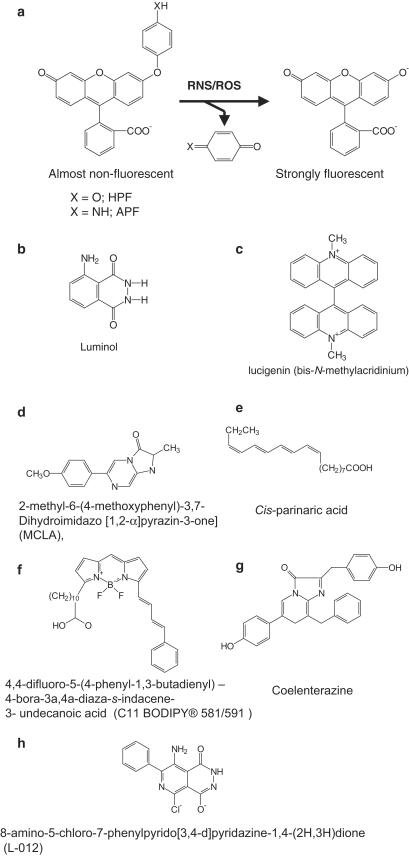
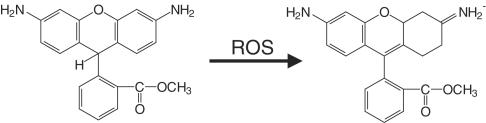
Hempel et al. (1999) have stated that dihydrofluorescein diacetate has certain advantages over DCFDA in some cellular systems, but again it detects a range of RS. 2-[6-4′hydroxy]phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (HPF) and 2-[6-(4′amino)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (APF) (Figure 2) have been introduced as probes of ROS. Both fluoresce after reaction with OH•, ONOO− and peroxidase-derived species, but only APF emits light after exposure to HOCl. Both appear more stable in cell systems than DCFDA (Setsukinai et al., 2003). Neither responds to 1O2, O2•−, H2O2, NO• or peroxyl radicals and both are more stable to photochemical events than DCFDA (Table 5).
Figure 2.
Structures of some common probes used for the detection of RS: (a) HPF and APF, (b) luminol, (c) LC and (d) MCLA. (e) Cis-parinaric acid, (f) 4,4-difluoro-5-(4-phenyl-1,3,-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY 581/591), (g) coelenterazine and (h) 8-amino-5-chloro-7-phenylpyridazo [3,4-d] pyridazine-1,4-(2H, 3H) dione (L-O12).
Dihydrorhodamine 123 (DHR)
DHR is a probe widely used to detect several RS (OH•, ONOO−, NO2•, peroxidase-derived species), but is poorly responsive to O2•−, H2O2 or NO• (Buxser et al., 1999). DHR is oxidized to rhodamine 123, which is highly fluorescent around 536 nm when excited at about 500 nm (Figure 3). Rhodamine 123 is lipophilic and positively charged, and tends to accumulate in mitochondria, held there by the membrane potential. As a result, once it is formed, little rhodamine 123 leaks out of cells. DHR is also more sensitive at detecting HOCl than DCFDA (Buxser et al., 1999; Ischiropoulos et al., 1999). Rhodamine 123 and ethidium (see below) can be ejected from cells by drug conjugate efflux pumps, so the presence and activity of these membrane transport systems in the cells being studied is a factor that needs to be considered (Buxser et al., 1999).
Figure 3.
Conversion of dihydrorhodamine 123 to rhodamine 123.
At high levels, rhodamine 123 can sensitize singlet O2 formation in mitochondria and cause NAD(P)H oxidation (Petrat et al., 2003). Palomba et al. (2000) found that exposure of PC12 cells to the synthetic hydroperoxide tert-butylhydroperoxide caused light emission from dihydrorhodamine. This appeared to be due to increased formation of NO• leading to ONOO− formation, which in turn activated the enzyme phospholipase A2 to raise cellular arachidonic acid levels. Arachidonic acid metabolism to RS was suggested to be the source of the DHR response.
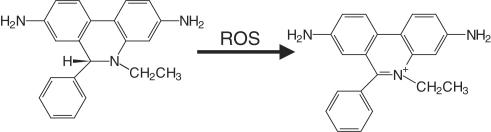
Dihydroethidium (dihydroethidine) (DHE)
This is frequently used as a probe for O2•−, being oxidized to a fluorescent product. This is usually thought to be ethidium (Figure 4), which tends to intercalate into nuclear DNA. Ethidium fluoresces strongly at around 600 nm when excited at 500–530 nm. Recent work, however, suggests that the product is not ethidium (Zhao et al., 2003). Although somewhat specific for O2•− (Buxser et al., 1999; Zhao et al., 2003), DHE is readily spontaneously oxidized and often gives a high background (Buxser et al., 1999). It can also undergo a direct redox reaction with ferricytochrome c (Benov et al., 1998).
Figure 4.
Conversion of DHE to ethidium.
Luminol and lucigenin (LC)
These two compounds (Figure 2) are often used to detect the production of RS by activated phagocytes, although they have also been used in other cell types (Faulkner & Fridovich, 1993). A luminol analogue L-012 (Figure 2) was reported to be more sensitive than luminol for the detection of O2•− and ONOO− or than dihydroethidine for O2•− detection (Daiber et al., 2004).The use of luminol to detect O2•− is a problematic area. It does not react directly with O2•− but must first be oxidized in a one-electron step (e.g. by OH•, ONOO− or peroxidase plus H2O2). The resulting luminol radical reacts with O2•− to generate a light-emitting product. Unfortunately, the luminol radical can also reduce O2 to generate O2•−, that is, the presence of luminol plus an oxidizing agent can lead to artifactual O2•− generation (Faulkner & Fridovich, 1993). Hence luminol is an unreliable probe; any oxidizing agent that can oxidize luminol by one electron will cause light emission inhibitable by SOD and the luminol is both the source and the detector of the O2•−. LC is often said to be more specific for the detection of O2•− than luminol, but again it does not react directly with O2•− (Faulkner & Fridovich, 1993; Spasojevic et al., 2000). It must first be reduced to LC cation radical (LC•+), which then reacts with O2•− to give the fluorescent product. Conversion of LC to LC•+ cannot be achieved rapidly by O2•−, and requires other cellular reducing systems (e.g. xanthine oxidase, the mitochondrial electron transport chain or the phagocyte NADPH oxidase), introducing an obvious complexity in interpreting results. LC•+ can also reduce O2 to O2•−, that is, addition of LC can artifactully generate more O2•− (Tarpey et al., 1999; Sohn et al., 2000; Spasojevic et al., 2000). The extent to which this artifact can interfere with accurate measurement of O2•− by LC continues to be debated (Munzel et al., 2002), but appears to be significant in some cell systems (Tarpey et al., 1999; Sohn et al., 2000; Spasojevic et al., 2000; Wardman et al., 2002). Alternative probes that might be more specific include coelenterazine (Tarpey et al., 1999; Munzel et al., 2002) and the luciferin analogue 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-∝]pyrazin-3-one (MCLA) (Figure 2). However, MCLA may also react with peroxyl radicals (Ukamoto and Yonaha, 1998) and coelenterazine with ONOO− (Tarpey et al., 1999). Both these compounds are highly light sensitive.
Probes of lipid peroxidation/membrane RS
Several membrane-partitioning probes have been introduced to measure lipid peroxidation or RS within membranes. One is cis-parinaric acid (Figure 2). When incorporated into lipids undergoing peroxidation, it is rapidly oxidized, losing its characteristic fluorescence (emission at 413 nm, excitation 324 nm) (Ritov et al., 1996; Drummen et al., 1999). Parinaric acid needs careful handling, because its polyunsaturated structure (Figure 2) makes it highly susceptible to nonspecific oxidation, and so it should be stored in the dark under a N2 atmosphere. We have found it to work well, although the need to use the UV range is one disadvantage. For example, the required wavelengths are not always available on microplate readers or confocal/flow cytometry systems. It can be added as the free acid or incorporated into specific phospholipids (parinaroyl lipids) and used to study their relative susceptibilities to oxidative damage within membranes (Ritov et al., 1996; Shvedova et al., 2002).
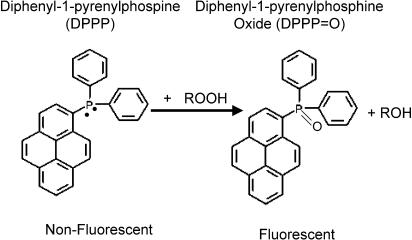
Another probe is diphenyl-1-pyrenylphosphine (Takahashi et al., 2001), reported to react with peroxides to generate a product that fluoresces at 380 nm when excited at 351 nm (Figure 5).We have found it to be light and air sensitive and difficult to obtain reproducible results using it under our laboratory conditions. Another fatty acid probe becoming widely used is C-11-BODIPY581/591, which upon oxidation shifts its fluorescence excitation and emission from red to green, so that the green/(red+green) ratio can be used as an estimate of membrane oxidation. Use of the ratio helps to decrease variations caused by heterogeneous probe distributions (cell thickness, uneven dye loading, compartmentalization, etc.) (Drummen et al., 2002). C-11-BODIPY581/591 responds to a range of RS (OH•, peroxyl, alkoxyl, ONOO−) but not to O2•−, NO•, H2O2, singlet O2 or hydroperoxides. Hence it can be used to determine RS within membranes (Drummen et al., 2002; Yoshida et al., 2003). When added to cells, C-11-BODIPY581/591 seems to enter most membranes, with no preference for any organelle (Drummen et al., 2002). Again, the presence of these various probes can be expected to alter the rate, and perhaps, the mechanistic pathway, of lipid peroxidation. Serum proteins, especially albumin, avidly bind fatty acids and can deter loading of probes into cells, and may also cause slow leaching out when loaded cells are incubated in the cell culture media with added serum. Yet, serum-deprived cells suffer oxidative stress, which can elevate background production of RS (Lee et al., 2001).
Figure 5.
Conversion of diphenyl-1-pyrenylphosphine to fluorescent product by peroxides.
How should we measure the output of these probes?
The simplest technique is the fluorescence microplate reader, where data are presented as increases or decreases in relative fluorescence. However, the quality and sensitivity of the machines commercially available vary tremendously, and the requirement for additional excitation and emission filters often makes them expensive. Newer models are available that do not require expensive filters but act on a dual monochromator principle and offer greater flexibility. Simultaneous dual wavelength excitation and emission measurements are particularly useful in determining the fluorescence ratio changes required for C-11-BODIPY581/591 or the product of reaction of O2•− with DHE. It is important to check if the machine being used is a top-reading fluorescence machine, since this requires the cells to be in suspension. Bottom-reading machines have the advantage that the cells can be measured in situ without the need for trypsinization or cell scraping, processes that themselves generate cellular oxidative stress (Halliwell, 2003a) and result in artifactual changes in fluorescence. Plate readers measure total fluorescence, that is, they do not distinguish between intracellular and extracellular fluorescence from chemical reactions in the culture medium. We have already alluded to this problem in the case of DCFDA.
Flow cytometry offers the advantage of being able to measure the intracellular fluorescence of cells in the culture media. Quantitative data on the numbers of cells emitting fluorescence can be obtained rather than just relative fluorescence units. However, cells are required to be in suspension and require either scraping or trypsinization, which induce oxidative stress. Control experiments to optimize assay conditions must always be conducted to limit this. An additional disadvantage of flow cytometry is that often the cytometer is at room temperature rather than 37°C, which can give variable data with certain cells such as murine primary cortical neurones (Dr Nam Sang Cheung, unpublished observation).
Confocal microscopy is a powerful tool; cells can be loaded with fluorescent dyes and viewed in real time in situ in culture chambers at 37°C. The intracellular location of RS can be visualized, and the role of mitochondrial, endoplasmic reticulum or lysosomal events in oxidative stress may be visualized using counter stains such as MitoTracker, ER Tracker or Lysotracker dyes coloaded with dyes that detect RS. However, caution must be excercised to ensure that the signal from the organelle Tracker stain does not interfere with the measurement of the RS-sensitive dye and vice versa. It should also be stressed that simple overlapping of the Tracker stains with the signal from the RS-sensitive dyes only suggests involvement of a particular organelle, but does not rigorously prove it.
Effects of RS on other probes
A wide range of probes is available to study many cellular events, such as pH changes and ion movements and to identify various cellular organelles (as mentioned above). For example, rises in Ca2+ frequently accompany oxidative stress (Halliwell & Gutteridge, 1999). Several papers have indicated that ion probes can be affected by the generation of RS (e.g. the Ca2+ probe Fura-2 can be degraded; Sarvazyan et al., 1998) or can themselves contribute to RS formation, such as calcein (Beghetto et al., 2000). MitoTracker Red, a fluorescent probe used to identify mitochondria, can stimulate mitochondrial ROS generation (Minamikawa et al., 1999), for example.
Conclusions
Whatever method you use to trap RS or measure oxidative damage, it is necessary to think carefully about how the method works, what is likely to confound it and how quantitative it can be (how, what and how much). With careful attention to understanding these points, erroneous interpretations can be minimized and the vibrant field of free radical research can continue to move forward.
Acknowledgments
We are grateful to the Biomedical Research Council (Grant 03/1/21/18/213), the NUS Office of Life Sciences (Grant R183000603712) and the National Medical Research Council (Grants 0600/2001, 0474/2000, 0481/2000, 0635/2002) for their generous research support.
Abbreviations
- 8OHdG
8-hydroxy-2′-deoxyguanosine
- APF
2-[6-(4′amino)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid
- BMPO
5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide
- CuZnSOD
copper and zinc-containing superoxide dismutase
- DCFDA
dichlorofluorescin diacetate
- DCF
dichlorofluorescein
- DCFH
dichlorofluorescin
- DECPO
5,5-diethylcarbonyl-1-pyrroline N-oxide
- DEPMPO
5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- EMPO
5-ethoxycarbonyl-5-methyl-1-pyrroline N-oxide
- EPPN
N-2-(2-ethoxycarbonyl-propyl)-α-phenylnitrone
- ESR
electron spin resonance
- GC
gas chromatography
- GSH
reduced glutathione
- HPF
2-[6-4′hydroxy]phenoxy-3H-xanthen-3-on-9-yl]benzoic acid
- HPLC
high-performance liquid chromatography
- LC
liquid chromatography
- L-DOPA
L-dihydroxyphenylalanine
- MCLA
2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-∝]pyrazin-3-one
- MDA
malondialdehyde
- MnSOD
manganese-containing superoxide dismutase
- MRI
magnetic resonance imaging
- MS
mass spectrometry
- PBN
N-tert-butyl-∝-phenylnitrone
- TBA
thiobarbituric acid
- TMINO
1,1,3-trimethyl-isoindole N-oxide
References
- ADAMS S., GREEN P., CLAXTON R., SIMCOX S., WILLIAMS M.V., WALSH K., LEEUWENBURGH C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front. Biosci. 2001;6:A17–24. doi: 10.2741/adams. [DOI] [PubMed] [Google Scholar]
- AFZAL M., MATSUGO S., SASAI M., XU B., AOYAMA K., TAKEUCHI T. Method to overcome photoreaction, a serious drawback to the use of dichlorofluorescin in evaluation of reactive oxygen species. Biochem. Biophys. Res. Commun. 2003;304:619–624. doi: 10.1016/s0006-291x(03)00641-7. [DOI] [PubMed] [Google Scholar]
- AHMAD J., COOKE M.S., HUSSIENI A., EVANS M.D., PATEL K., BURD R.M., BLEIKER T.O., HUTCHINSON P.E., LUNEC J. Urinary thymine dimers and 8-oxo-2′-deoxyguanosine in psoriasis. FEBS Lett. 1999;460:549–553. doi: 10.1016/s0014-5793(99)01402-7. [DOI] [PubMed] [Google Scholar]
- AKAIKE T., OKAMOTO S., SAWA T., YOSHITAKE J., TAMURA F., ICHIMORI K., MIYAZAKI K., SASAMOTO K., MAEDA H. 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:685–690. doi: 10.1073/pnas.0235623100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAM Z.I., JENNER A., DANIEL S.E., LEES A.J., CAIRNS N., MARSDEN C.D., JENNER P., HALLIWELL B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- ALDRIDGE R.E., CHAN T., VAN DALEN C.J., SENTHILMOHAN R., WINN M., VENGE P., TOWN G.I., KETTLE A.J. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic. Biol. Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
- ANDREONI K.A., KAZUI M., CAMERON D.E., NYHAN D., SEHNERT S.S., ROHDE C.A., BULKLEY G.B., RISBY T.H. Ethane: a marker of lipid peroxidation during cardiopulmonary bypass in humans. Free Radic. Biol. Med. 1999;26:439–445. doi: 10.1016/s0891-5849(98)00220-2. [DOI] [PubMed] [Google Scholar]
- BANERJEE D., MADHUSOODANAN U.K., NAYAK S., JACOB J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin. Chim. Acta. 2003;334:205–209. doi: 10.1016/s0009-8981(03)00236-5. [DOI] [PubMed] [Google Scholar]
- BARTOSZ G. Total antioxidant capacity. Adv. Clin. Chem. 2003;37:219–292. doi: 10.1016/s0065-2423(03)37010-6. [DOI] [PubMed] [Google Scholar]
- BARTSCH H., NAIR J. New DNA-based biomarkers for oxidative stress and cancer chemoprevention studies. Eur. J. Cancer. 2000;36:1229–1234. doi: 10.1016/s0959-8049(00)00095-2. [DOI] [PubMed] [Google Scholar]
- BASU S. Isoprostanes: novel bioactive products of lipid peroxidation – an overview. Free Radic. Res. 2004;38:105–122. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- BASU S., WHITEMAN M., MATTEY D.L., HALLIWELL B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann. Rheum. Dis. 2001;60:627–631. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.A., GOLDFINE A.B., GORDON M.B., CREAGER M.A. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103:1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- BECKMAN K.B., AMES B.N. Endogenous oxidative damage of mtDNA. Mutat. Res. 1999;424:51–58. doi: 10.1016/s0027-5107(99)00007-x. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BEGHETTO C., RENKEN C., ERIKSSON O., JORI G., BERNARDI P., RICCHELLI F. Implications of the generation of reactive oxygen species by photoactivated calcein for mitochondrial studies. Eur. J. Biochem. 2000;267:5585–5592. doi: 10.1046/j.1432-1327.2000.01625.x. [DOI] [PubMed] [Google Scholar]
- BENOV L., SZTEJNBERG L., FRIDOVICH I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic. Biol. Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- BENZIE I.F., CHUNG W., TOMLINSON B. Simultaneous measurement of allantoin and urate in plasma: analytical evaluation and potential clinical application in oxidant:antioxidant balance studies. Clin. Chem. 1999;45:901–904. [PubMed] [Google Scholar]
- BENZIE I.F., STRAIN J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power': the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- BERLINER L.J., KHRAMTSOV V., FUJII H., CLANTON T.L. Unique in vivo applications of spin traps. Free Radic. Biol. Med. 2001;30:489–499. doi: 10.1016/s0891-5849(00)00491-3. [DOI] [PubMed] [Google Scholar]
- BESSARD J., CRACOWSKI J.L., STANKE-LABESQUE F., BESSARD G. Determination of isoprostaglandin F2alpha type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J. Chromatogr. B. 2001;754:333–343. doi: 10.1016/s0378-4347(00)00621-6. [DOI] [PubMed] [Google Scholar]
- BHATTACHARJEE S., PENNATHUR S., BYUN J., CROWLEY J., MUELLER D., GISCHLER J., HOTCHKISS R.S., HEINECKE J.W. NADPH oxidase of neutrophils elevates o,o′-dityrosine cross-links in proteins and urine during inflammation. Arch. Biochem. Biophys. 2001;395:69–77. doi: 10.1006/abbi.2001.2557. [DOI] [PubMed] [Google Scholar]
- BIELSKI B.H., RICHTER H.W., CHAN P.C. Some properties of the ascorbate free radical. Ann. NY Acad. Sci. 1975;258:231–237. doi: 10.1111/j.1749-6632.1975.tb29283.x. [DOI] [PubMed] [Google Scholar]
- BILSKI P., BELANGER A.G., CHIGNELL C.F. Photosensitized oxidation of 2′,7′-dichlorofluorescin: singlet oxygen does not contribute to the formation of fluorescent oxidation product 2′,7′-dichlorofluorescein. Free Radic. Biol. Med. 2002;33:938–946. doi: 10.1016/s0891-5849(02)00982-6. [DOI] [PubMed] [Google Scholar]
- BINDOKAS V.P., KUZNETSOV A., SREENAN S., POLONSKY K.S., ROE M.W., PHILIPSON L.H. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J. Biol. Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- BOGDANOV M.B., BEAL M.F., MCCABE D.R., GRIFFIN R.M., MATSON W.R. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radic. Biol. Med. 1999;27:647–666. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- BOHNSTEDT K.C., KARLBERG B., WAHLUND L.O., JÖNHAGEN M.E., BASUN H., SCHMIDT S. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2003;796:11–19. doi: 10.1016/s1570-0232(03)00600-7. [DOI] [PubMed] [Google Scholar]
- BOTTLE S.E., HANSON G.R., MICALLEF A.S. Application of the new EPR spin trap 1,1,3-trimethylisoindole N-oxide (TMINO) in trapping HO and related biologically important radicals. Org. Biomol. Chem. 2003;1:2585–2589. doi: 10.1039/b300644a. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- BUETTNER G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- BUETTNER G.R., JURKIEWICZ B.A. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic. Biol. Med. 1993;14:49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- BUSS H., CHAN T.P., SLUIS K.B., DOMIGAN N.M., WINTERBOURN C.C. Protein carbonyl measurement by a sensitive ELISA method. Free Radic. Biol. Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- BUSS I.H., SENTHILMOHAN R., DARLOW B.A., MOGRIDGE N., KETTLE A.J., WINTERBOURN C.C. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: association with adverse respiratory outcome. Pediatr. Res. 2003;53:455–462. doi: 10.1203/01.PDR.0000050655.25689.CE. [DOI] [PubMed] [Google Scholar]
- BUSS I.H., WINTERBOURN C.C. Protein carbonyl measurement by ELISA. Methods Mol. Biol. 2002;186:123–128. doi: 10.1385/1-59259-173-6:123. [DOI] [PubMed] [Google Scholar]
- BUTTERFIELD D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic. Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- BUTTERFIELD D.A., BOYD-KIMBALL D., CASTEGNA A. Proteomics in Alzheimer's disease: insights into potential mechanisms of neurodegeneration. J. Neurochem. 2003;86:1313–1327. doi: 10.1046/j.1471-4159.2003.01948.x. [DOI] [PubMed] [Google Scholar]
- BUXSER S.E., SAWADA G., RAUB T.J. Analytical and numerical techniques for evalution of free radical damage in cultured cells using imaging cytometry and fluorescent indicators. Methods Enzymol. 1999;300:256–275. doi: 10.1016/s0076-6879(99)00133-0. [DOI] [PubMed] [Google Scholar]
- BYUN J., HENDERSON J.P., MUELLER D.M., HEINECKE J.W. 8-Nitro-2′-deoxyguanosine, a specific marker of oxidation by reactive nitrogen species, is generated by the myeloperoxidase-hydrogen peroxide-nitrite system of activated human phagocytes. Biochemistry. 1999;38:2590–2600. doi: 10.1021/bi9822980. [DOI] [PubMed] [Google Scholar]
- CALINGASAN N.Y., UCHIDA K., GIBSON G.E. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer's disease. J. Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- CANDEIAS L.P., PATEL K.B., STRATFORD M.R., WARDMAN P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett. 1993;333:151–153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- CARROLL M.F., SCHADE D.S. Timing of antioxidant vitamin ingestion alters postprandial proatherogenic serum markers. Circulation. 2003;108:24–31. doi: 10.1161/01.CIR.0000074221.68903.77. [DOI] [PubMed] [Google Scholar]
- CASCIOLA-ROSEN L., WIGLEY F., ROSEN A. Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis. J. Exp. Med. 1997;185:71–79. doi: 10.1084/jem.185.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN M.L., RUBIN B.R., GRACY R.W. Increased carbonyl content of proteins in synovial fluid from patients with rheumatoid arthritis. J. Rheumatol. 1989;16:15–18. [PubMed] [Google Scholar]
- CHEVION M., BERENSHTEIN E., STADTMAN E.R. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic. Res. 2000;33 Suppl:S99–S108. [PubMed] [Google Scholar]
- CHOI J., KIM D.Y., HYUN J.W., YOON S.H., CHOI E.M., HAHM K.B., RHEE K.H., CHUNG M.H. Measurement of oxidative damage at individual gene levels by quantitative PCR using 8-hydroxyguanine glycosylase (OGG1) Mutat. Res. 2003;523–524:225–235. doi: 10.1016/s0027-5107(02)00339-1. [DOI] [PubMed] [Google Scholar]
- CHOUCROUN P., GILLET D., DORANGE G., SAWICKI B., DEWITTE J.D. Comet assay and early apoptosis. Mutat. Res. 2001;478:89–96. doi: 10.1016/s0027-5107(01)00123-3. [DOI] [PubMed] [Google Scholar]
- CHOWIENCZYK P.J., BRETT S.E., GOPAUL N.K., MEEKING D., MARCHETTI M., RUSSELL-JONES D.L., ANGGARD E.E., RITTER J.M. Oral treatment with an antioxidant (raxofelast) reduces oxidative stress and improves endothelial function in men with type II diabetes. Diabetologia. 2000;43:974–977. doi: 10.1007/s001250051478. [DOI] [PubMed] [Google Scholar]
- CLEMENT M.V., LONG L.H., RAMALINGAM J., HALLIWELL B. The cytotoxicity of dopamine may be an artefact of cell culture. J. Neurochem. 2002;81:414–421. doi: 10.1046/j.1471-4159.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- CLEMENT M.V., RAMALINGAM J., LONG L.H., HALLIWELL B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid. Redox Signal. 2001;3:157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- CLERMONT G., VERGELY C., JAZAYERI S., LAHET J.J., GOUDEAU J.J., LECOUR S., DAVID M., ROCHETTE L., GIRARD C. Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology. 2002;96:80–87. doi: 10.1097/00000542-200201000-00019. [DOI] [PubMed] [Google Scholar]
- COHN J.S. Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease. Curr. Opin. Lipidol. 2002;13:19–24. doi: 10.1097/00041433-200202000-00004. [DOI] [PubMed] [Google Scholar]
- COLLINS A.R., CADET J., MOLLER L., POULSEN H.E., VINA J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells. Arch. Biochem. Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- COLLINS A.R., HORVATHOVA E. Oxidative DNA damage, antioxidants and DNA repair: applications of the comet assay. Biochem. Soc. Trans. 2001;29:337–341. doi: 10.1042/0300-5127:0290337. [DOI] [PubMed] [Google Scholar]
- COOKE M.S., EVANS M.D., BURD R.M., PATEL K., BARNARD A., LUNEC J., HUTCHINSON P.E. Induction and excretion of ultraviolet-induced 8-oxo-2′-deoxyguanosine and thymine dimers in vivo: implications for PUVA. J. Invest. Dermatol. 2001;116:281–285. doi: 10.1046/j.1523-1747.2001.01251.x. [DOI] [PubMed] [Google Scholar]
- COOKE M.S., EVANS M.D., LUNEC J. DNA repair: insights from urinary lesion analysis. Free Radic. Res. 2002;36:929–932. doi: 10.1080/1071576021000006635. [DOI] [PubMed] [Google Scholar]
- COUDRAY C., FAVIER A. Determination of salicylate hydroxylation products as an in vivo oxidative stress marker. Free Radic. Biol. Med. 2000;29:1064–1070. doi: 10.1016/s0891-5849(00)00403-2. [DOI] [PubMed] [Google Scholar]
- DAIBER A., BACHSCHMID M., KAVAKLÍ C., FREIN D., WENDT M., ULLRICH V., MUNZEL T. A new pitfall in detecting biological end products of nitric oxide – nitration, nitros(yl)ation and nitrite/nitrate artefacts during freezing. Nitric Oxide Biol. Chem. 2003;9:44–52. doi: 10.1016/j.niox.2003.08.002. [DOI] [PubMed] [Google Scholar]
- DAIBER A., OELZE M., AUGUST M., WENDT M., SYDOW K., WIEBOLDT H., KLESCHYOV A.L., MUNZEL T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic. Res. 2004;38:259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- DALE O., BERGUM H., LUND T., NILSEN T., AADAHL P., STENSETH R. A validated method for rapid analysis of ethane in breath and its application in kinetic studies in human volunteers. Free Radic. Res. 2003;37:815–821. doi: 10.1080/1071576031000107353. [DOI] [PubMed] [Google Scholar]
- DALLE-DONNE I., GIUSTARINI D., COLOMBO R., ROSSI R., MILZANI A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- DARKO D., DORNHORST A., KELLY F.J., RITTER J.M., CHOWIENCZYK P.J. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin. Sci. 2002;103:339–344. doi: 10.1042/cs1030339. [DOI] [PubMed] [Google Scholar]
- DARLEY-USMAR V., HALLIWELL B. Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm. Res. 1996;13:649–662. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- DARLEY-USMAR V., WISEMAN H., HALLIWELL B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- DAVIES M.J., FU S., WANG H., DEAN R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999;27:1151–1163. doi: 10.1016/s0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- DENTON M.J., SPENCER N., ARNSTEIN H.R. Biochemical and enzymic changes during erythrocyte differentiation. The significance of the final cell division. Biochem. J. 1975;146:205–211. doi: 10.1042/bj1460205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIZDAROGLU M. Oxidative damage to DNA in mammalian chromatin. Mutat. Res. 1992;275:331–342. doi: 10.1016/0921-8734(92)90036-o. [DOI] [PubMed] [Google Scholar]
- DIZDAROGLU M., JARUGA P., BIRINCIOGLU M., RODRIGUEZ H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- DOEHNER W., SCHOENE N., RAUCHHAUS M., LEYVA-LEON F., PAVITT D.V., REAVELEY D.A., SCHULER G., COATS A.J., ANKER S.D., HAMBRECHT R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- DOUKI T., COURT M., SAUVAIGO S., ODIN F., CADET J. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography–tandem mass spectrometry. J. Biol. Chem. 2000;275:11678–11685. doi: 10.1074/jbc.275.16.11678. [DOI] [PubMed] [Google Scholar]
- DOULIAS P.T., CHRISTOFORIDIS S., BRUNK U.T., GALARIS D. Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic. Biol. Med. 2003;35:719–728. doi: 10.1016/s0891-5849(03)00396-4. [DOI] [PubMed] [Google Scholar]
- DRAGSTED L.O. Antioxidant actions of polyphenols in humans. Int. J. Vitam. Nutr. Res. 2003;73:112–119. doi: 10.1024/0300-9831.73.2.112. [DOI] [PubMed] [Google Scholar]
- DRAPER H.H., CSALLANY A.S., HADLEY M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic. Biol. Med. 2000;29:1071–1077. doi: 10.1016/s0891-5849(00)00367-1. [DOI] [PubMed] [Google Scholar]
- DRUMMEN G.P., OP DEN KAMP J.A., POST J.A. Validation of the peroxidative indicators, cis-parinaric acid and parinaroyl-phospholipids, in a model system and cultured cardiac myocytes. Biochim. Biophys. Acta. 1999;1436:370–382. doi: 10.1016/s0005-2760(98)00142-8. [DOI] [PubMed] [Google Scholar]
- DRUMMEN G.P., VAN LIEBERGEN L.C., OP DEN KAMP J.A., POST J.A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- DUFFY S.J., GOKCE N., HOLBROOK M., HUANG A., FREI B., KEANEY J.F., JR, VITA J.A. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- DUFFY S.J., KEANEY J.F., JR, HOLBROOK M., GOKCE N., SWERDLOFF P.L., FREI B., VITA J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- DUNCAN M.W. A review of approaches to the analysis of 3-nitrotyrosine. Amino Acids. 2003;25:351–361. doi: 10.1007/s00726-003-0022-z. [DOI] [PubMed] [Google Scholar]
- DUPONT I., BERTHOU F., BODENEZ P., BARDOU L., GUIRRIEC C., STEPHAN N., DREANO Y., LUCAS D. Involvement of cytochromes P-450 2E1 and 3A4 in the 5-hydroxylation of salicylate in humans. Drug Metab. Dispos. 1999;27:322–326. [PubMed] [Google Scholar]
- D'USCIO L.V., MILSTIEN S., RICHARDSON D., SMITH L., KATUSIC Z.S. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ. Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- DUTHIE S.J., MA A., ROSS M.A., COLLINS A.R. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996;56:1291–1295. [PubMed] [Google Scholar]
- ENGLAND T., BEATTY E., REHMAN A., NOUROOZ-ZADEH J., PEREIRA P., O'REILLY J., WISEMAN H., GEISSLER C., HALLIWELL B. The steady-state levels of oxidative DNA damage and of lipid peroxidation (F2-isoprostanes) are not correlated in healthy human subjects. Free Radic. Res. 2000;32:355–362. doi: 10.1080/10715760000300351. [DOI] [PubMed] [Google Scholar]
- EVANS M.D., DIZDAROGLU M., COOKE M.S. Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. 2003;7780:1–62. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- FAM S.S., MORROW J.D. The isoprostanes: unique products of arachidonic acid oxidation – a review. Curr. Med. Chem. 2003;10:1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- FAULKNER K., FRIDOVICH I. Luminol and lucigenin as detectors for O2−. Free Radic. Biol. Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- FECHNER A., BOHME C., GROMER S., FUNK M., SCHIRMER R., BECKER K. Antioxidant status and nitric oxide in the malnutrition syndrome kwashiorkor. Pediatr. Res. 2001;49:237–243. doi: 10.1203/00006450-200102000-00018. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGER B., THEMANN C., ROSE S., HALLIWELL B., JENNER P. 6-hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. J. Neurochem. 2001;78:509–514. doi: 10.1046/j.1471-4159.2001.00429.x. [DOI] [PubMed] [Google Scholar]
- FESSEL J.P., PORTER N.A., MOORE K.P., SHELLER J.R., ROBERTS L.J., II Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOYD R.A., HENSLEY K., JAFFERY F., MAIDT L., ROBINSON K., PYE Q., STEWART C. Increased oxidative stress brought on by pro-inflammatory cytokines in neurodegenerative processes and the protective role of nitrone-based free radical traps. Life Sci. 1999;65:1893–1899. doi: 10.1016/s0024-3205(99)00443-9. [DOI] [PubMed] [Google Scholar]
- FRANK J., POMPELLA A., BIESALSKI H.K. Histochemical visualization of oxidant stress. Free Radic. Biol. Med. 2000;29:1096–1105. doi: 10.1016/s0891-5849(00)00395-6. [DOI] [PubMed] [Google Scholar]
- FRISCHER T., PULLWITT A., KUHR J., MEINERT R., HASCHKE N., STUDNICKA M., LUBEC G. Aromatic hydroxylation in nasal lavage fluid following ambient ozone exposure. Free Radic. Biol. Med. 1997;22:201–207. doi: 10.1016/s0891-5849(96)00292-4. [DOI] [PubMed] [Google Scholar]
- FROST M.T., HALLIWELL B., MOORE K.P. Analysis of free and protein-bound nitrotyrosine in human plasma by a gas chromatography/mass spectrometry method that avoids nitration artifacts. Biochem. J. 2000;345:453–458. [PMC free article] [PubMed] [Google Scholar]
- FU S., DAVIES M.J., STOCKER R., DEAN R.T. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem. J. 1998;333:519–525. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GACKOWSKI D., ROZALSKI R., ROSZKOWSKI K., JAWIEN A., FOKSINSKI M., OLINSKI R. 8-Oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine levels in human urine do not depend on diet. Free Radic. Res. 2001;35:825–832. doi: 10.1080/10715760100301321. [DOI] [PubMed] [Google Scholar]
- GALLI R.L., SHUKITT-HALE B., YOUDIM K.A., JOSEPH J.A. Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Ann. NY Acad. Sci. 2002;959:128–132. doi: 10.1111/j.1749-6632.2002.tb02089.x. [DOI] [PubMed] [Google Scholar]
- GAUT J.P., BYUN J., TRAN H.D., HEINECKE J.W. Artifact-free quantification of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography–electrospray ionization tandem mass spectrometry. Anal. Biochem. 2002a;300:252–259. doi: 10.1006/abio.2001.5469. [DOI] [PubMed] [Google Scholar]
- GAUT J.P., BYUN J., TRAN H.D., LAUBER W.M., CARROLL J.A., HOTCHKISS R.S., BELAAOUAJ A., HEINECKE J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Invest. 2002b;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEISZT M., WITTA J., BAFFI J., LEKSTROM K., LETO T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- GHISELLI A., LAURENTI O., DE MATTIA G., MAIANI G., FERRO-LUZZI A. Salicylate hydroxylation as an early marker of in vivo oxidative stress in diabetic patients. Free Radic. Biol. Med. 1992;13:621–626. doi: 10.1016/0891-5849(92)90036-g. [DOI] [PubMed] [Google Scholar]
- GIULIVI C., DAVIES K.J. Mechanism of the formation and proteolytic release of H2O2-induced dityrosine and tyrosine oxidation products in hemoglobin and red blood cells. J. Biol. Chem. 2001;276:24129–24136. doi: 10.1074/jbc.M010697200. [DOI] [PubMed] [Google Scholar]
- GOKCE N., KEANEY J.F., JR, FREI B., HOLBROOK M., OLESIAK M., ZACHARIAH B.J., LEEUWENBURGH C., HEINECKE J.W., VITA J.A. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- GOLDEN M.H., RAMDATH D. Free radicals in the pathogenesis of kwashiorkor. Proc. Nutr. Soc. 1987;46:53–68. doi: 10.1079/pns19870008. [DOI] [PubMed] [Google Scholar]
- GOPAUL N.K., HALLIWELL B., ANGGARD E.E. Measurement of plasma F2-isoprostanes as an index of lipid peroxidation does not appear to be confounded by diet. Free Radic. Res. 2000;33:115–127. doi: 10.1080/10715760000300671. [DOI] [PubMed] [Google Scholar]
- GOPAUL N.K., MANRAJ M.D., HEBE A., LEE KWAI YAN S., JOHNSTON A., CARRIER M.J., ANGGARD E.E. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia. 2001;44:706–712. doi: 10.1007/s001250051679. [DOI] [PubMed] [Google Scholar]
- GREENACRE S.A., ISCHIROPOULOS H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- GROOTVELD M., HALLIWELL B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem. J. 1986;237:499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROOTVELD M., HALLIWELL B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo. Biochem. J. 1987;243:803–808. doi: 10.1042/bj2430803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRZELAK A., RYCHLIK B., BARTOSZ G. Reactive oxygen species are formed in cell culture media. Acta Biochim. Pol. 2000;47:1197–1198. [PubMed] [Google Scholar]
- GUAN W., OSANAI T., KAMADA T., HANADA H., ISHIZAKA H., ONODERA H., IWASA A., FUJITA N., KUDO S., OHKUBO T., OKUMURA K. Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J. Cardiovasc. Pharmacol. 2003;41:699–705. doi: 10.1097/00005344-200305000-00005. [DOI] [PubMed] [Google Scholar]
- GUAN W., OSANAI T., KAMADA T., ISHIZAKA H., HANADA H., OKUMURA K. Time course of free radical production after primary coronary angioplasty for acute myocardial infarction and the effect of vitamin C. Jpn. Circ. J. 1999;63:924–928. doi: 10.1253/jcj.63.924. [DOI] [PubMed] [Google Scholar]
- HAGEN T.M., HUANG S., CURNUTTE J., FOWLER P., MARTINEZ V., WEHR C.M., AMES B.N., CHISARI F.V. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol. Int. Rep. 1978;2:113–128. doi: 10.1016/0309-1651(78)90032-2. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action. Free Radic. Biol. Med. 1989;7:645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Drug antioxidant effects. A basis for drug selection. Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL B. Antioxidant characterization. Methodology and mechanism. Biochem. Pharmacol. 1995;49:1341–1348. doi: 10.1016/0006-2952(95)00088-h. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Establishing the significance and optimal intake of dietary antioxidants: the biomarker concept. Nutr. Rev. 1999a;57:104–113. doi: 10.1111/j.1753-4887.1999.tb06933.x. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat. Res. 1999b;443:37–52. doi: 10.1016/s1383-5742(99)00009-5. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Vitamin C: poison, prophylactic or panacea. Trends Biochem. Sci. 1999c;24:255–259. doi: 10.1016/s0968-0004(99)01418-8. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward. Cardiovasc. Res. 2000a;47:410–418. doi: 10.1016/s0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. The antioxidant paradox. Lancet. 2000b;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come. Am. J. Clin. Nutr. 2000c;72:1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Vitamin E and the treatment and prevention of diabetes: a case for a controlled clinical trial. Singapore Med. J. 2002a;43:479–484. [PubMed] [Google Scholar]
- HALLIWELL B. Effect of diet on cancer development: is oxidative DNA damage a biomarker. Free Radic. Biol. Med. 2002b;32:968–974. doi: 10.1016/s0891-5849(02)00808-0. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Oxidative stress in cell culture: an under-appreciated problem. FEBS Lett. 2003a;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B. Health benefits of eating chocolate. Nature. 2003b;426:787. doi: 10.1038/426787a. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., CLEMENT M.V., LONG L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M. Free Radicals in Biology and Medicine. Oxford, U.K.: Oxford University Press; 1999. [Google Scholar]
- HALLIWELL B., KAUR H. Hydroxylation of salicylate and phenylalanine as assays for hydroxyl radicals: a cautionary note visited for the third time. Free Radic. Res. 1997;27:239–244. doi: 10.3109/10715769709065762. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., LONG L.H., YEE T.P., LIM S., KELLY R.Establishing biomarkers of oxidative stress. The measurement of hydrogen peroxide in human urine Curr. Med. Chem. 2004a(in press) [DOI] [PubMed]
- HALLIWELL B., RAFTER J., JENNER A.Health promotion by flavonoids, tocopherols, tocotrienols and other phenols. Direct or indirect effects? Antioxidant or not Am. J. Clin. Nutr. 2004b(in press) [DOI] [PubMed]
- HALLIWELL B., ZHAO K., WHITEMAN M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: a personal view of recent controversies. Free Radic. Res. 1999;31:651–669. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]
- HAN J.Y., TAKESHITA K., UTSUMI H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic. Biol. Med. 2001;30:516–525. doi: 10.1016/s0891-5849(00)00501-3. [DOI] [PubMed] [Google Scholar]
- HANAOKA T., NAIR J., TAKAHASHI Y., SASAKI S., BARTSCH H., TSUGANE S. Urinary level of 1,N(6)-ethenodeoxyadenosine, a marker of oxidative stress, is associated with salt excretion and omega 6-polyunsaturated fatty acid intake in postmenopausal Japanese women. Int. J. Cancer. 2002;100:71–75. doi: 10.1002/ijc.10437. [DOI] [PubMed] [Google Scholar]
- HARRISON D.G., CAI H., LANDMESSER U., GRIENDLING K.K. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- HARTH R., GERLACH M., RIEDERER P., GOTZ M.E. A highly sensitive method for the determination of protein bound 3,4-dihydroxyphenylalanine as a marker for post-translational protein hydroxylation in human tissues ex vivo. Free Radic. Res. 2001;35:167–174. doi: 10.1080/10715760100300711. [DOI] [PubMed] [Google Scholar]
- HAYWOOD R.M., WARDMAN P., GAULT D.T., LINGE C. Ruby laser irradiation (694 nm) of human skin biopsies: assessment by electron spin resonance spectroscopy of free radical production and oxidative stress during laser depilation. Photochem. Photobiol. 1999;70:348–352. [PubMed] [Google Scholar]
- HAZEN S.L., HEINECKE J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAZEN S.L., HSU F.F., DUFFIN K., HEINECKE J.W. Molecular chlorine generated by the myeloperoxidase–hydrogen peroxide–chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J. Biol. Chem. 1996;271:23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- HEADLAM H.A., DAVIES M.J. Cell-mediated reduction of protein and peptide hydroperoxides to reactive free radicals. Free Radic. Biol. Med. 2003;34:44–55. doi: 10.1016/s0891-5849(02)01181-4. [DOI] [PubMed] [Google Scholar]
- HEISS C., DEJAM A., KLEINBONGARD P., SCHEWE T., SIES H., KELM M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- HELLSTEN Y., SVENSSON M., SJODIN B., SMITH S., CHRISTENSEN A., RICHTER E.A., BANGSBO J. Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free Radic. Biol. Med. 2001;31:1313–1322. doi: 10.1016/s0891-5849(01)00631-1. [DOI] [PubMed] [Google Scholar]
- HELMERSSON J., BASU S. F2-isoprostane and prostaglandin F2∝ metabolite excretion rate and day to day variation in healthy humans. Prostaglandin Leukot. Essent. Fatty Acid. 2001;65:99–102. doi: 10.1054/plef.2001.0295. [DOI] [PubMed] [Google Scholar]
- HEMPEL S.L., BUETTNER G.R., O'MALLEY Y.Q., WESSELS D.A., FLAHERTY D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- HENDERSON J.P., BYUN J., TAKESHITA J., HEINECKE J.W. Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue. J. Biol. Chem. 2003;278:23522–23528. doi: 10.1074/jbc.M303928200. [DOI] [PubMed] [Google Scholar]
- HENZLER T., STEUDLE E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H(2)O(2) across water channels. J. Exp. Bot. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- HIMMELFARB J., MCMENAMIN M.E., LOSETO G., HEINECKE J.W. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic. Biol. Med. 2001;31:1163–1169. doi: 10.1016/s0891-5849(01)00697-9. [DOI] [PubMed] [Google Scholar]
- HIRAMOTO K., KIDA T., KIKUGAWA K. Increased urinary hydrogen peroxide levels caused by coffee drinking. Biol. Pharm. Bull. 2002;25:1467–1471. doi: 10.1248/bpb.25.1467. [DOI] [PubMed] [Google Scholar]
- HIRAMOTO K., LI X., MAKIMOTO M., KATO T., KIKUGAWA K. Identification of hydroxy-hydroquinone in coffee as a generator of reactive oxygen species that break DNA single strands. Mutat. Res. 1998;419:43–51. doi: 10.1016/s1383-5718(98)00123-5. [DOI] [PubMed] [Google Scholar]
- HSIEH Y.S., CHEN B.C., SHIOW S.J., WANG H.C., HSU J.D., WANG C.J. Formation of 8-nitroguanine in tobacco cigarette smokers and in tobacco smoke-exposed Wistar rats. Chem. Biol. Interact. 2002;140:67–80. doi: 10.1016/s0009-2797(02)00018-2. [DOI] [PubMed] [Google Scholar]
- HUANG A., VITA J.A., VENEMA R.C., KEANEY J.F., JR Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- HUANG H.Y., APPEL L.J., CROFT K.D., MILLER E.R., III, MORI T.A., PUDDEY I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am. J. Clin. Nutr. 2002;76:549–555. doi: 10.1093/ajcn/76.3.549. [DOI] [PubMed] [Google Scholar]
- ICHIMORI K., FUKUYAMA N., NAKAZAWA H., ARATANI Y., KOYAMA H., TAKIZAWA S., KAMEOKA Y., ISHIDA-OKAWARA A., KOHI F., SUZUKI K. Myeloperoxidase has directly-opposed effects on nitration reaction – study on myeloperoxidase-deficient patient and myeloperoxidase-knockout mice. Free Radic. Res. 2003;37:481–489. doi: 10.1080/1071576031000099830. [DOI] [PubMed] [Google Scholar]
- INDART A., VIANA M., GROOTVELD M.C., SILWOOD C.J., SANCHEZ-VERA I., BONET B. Teratogenic actions of thermally-stressed culinary oils in rats. Free Radic. Res. 2002;36:1051–1058. doi: 10.1080/1071576021000006716. [DOI] [PubMed] [Google Scholar]
- INGELMAN-SUNDBERG M., KAUR H., TERELIUS Y., PERSSON J.O., HALLIWELL B. Hydroxylation of salicylate by microsomal fractions and cytochrome P-450. Lack of production of 2,3-dihydroxybenzoate unless hydroxyl radical formation is permitted. Biochem. J. 1991;276:753–757. doi: 10.1042/bj2760753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., GOW A., THOM S.R., KOOY N.W., ROYALL J.A., CROW J.P. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 1999;301:367–373. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- IULIANO L., PRATICO D., GRECO C., MANGIERI E., SCIBILIA G., FITZGERALD G.A., VIOLI F. Angioplasty increases coronary sinus F2-isoprostane formation: evidence for in vivo oxidative stress during PTCA. J. Am. Coll. Cardiol. 2001;37:76–80. doi: 10.1016/s0735-1097(00)01040-8. [DOI] [PubMed] [Google Scholar]
- JIALAL I., DEVARAJ S., VENUGOPAL S.K. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic. Res. 2002;36:1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- JOBSIS R.Q., SCHELLEKENS S.L., FAKKEL-KROESBERGEN A., RAATGEEP R.H., DE JONGSTE J.C. Hydrogen peroxide in breath condensate during a common cold. Mediators Inflamm. 2001;10:351–354. doi: 10.1080/09629350120102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURKIEWICZ B.A., BUETTNER G.R. EPR detection of free radicals in UV-irradiated skin: mouse versus human. Photochem. Photobiol. 1996;64:918–922. doi: 10.1111/j.1751-1097.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
- KANABROCKI E.L., MURRAY D., HERMIDA R.C., SCOTT G.S., BREMNER W.F., RYAN M.D., AYALA D.E., THIRD J.L., SHIRAZI P., NEMCHAUSKY B.A., HOOPER D.C. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol. Int. 2002;19:423–439. doi: 10.1081/cbi-120002914. [DOI] [PubMed] [Google Scholar]
- KANSKI J., ALTERMAN M.A., SCHONEICH C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic. Biol. Med. 2003;35:1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- KAROUI H., CLÉMENT J.L., ROCKENBAUER A., SIRI D., TORDO P. Synthesis and structure of 5,5-diethoxycarbonyl-1-pyrroline N-oxide (DECPO). Application to superoxide radical trapping. Tetrah. Lett. 2004;45:149–152. [Google Scholar]
- KASAI H. Chemistry-based studies on oxidative DNA damage: formation, repair, and mutagenesis. Free Radic. Biol. Med. 2002;33:450–456. [PubMed] [Google Scholar]
- KATO Y., MARUYAMA W., NAOI M., HASHIZUME Y., OSAWA T. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998;439:231–234. doi: 10.1016/s0014-5793(98)01372-6. [DOI] [PubMed] [Google Scholar]
- KAUR H., EDMONDS S.E., BLAKE D.R., HALLIWELL B. Hydroxyl radical generation by rheumatoid blood and knee joint synovial fluid. Ann. Rheum. Dis. 1996;55:915–920. doi: 10.1136/ard.55.12.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR H., HALLIWELL B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem. Biol. Interact. 1990;73:235–247. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- KAUR H., HALLIWELL B. Detection of hydroxyl radicals by aromatic hydroxylation. Methods Enzymol. 1994;233:67–82. doi: 10.1016/s0076-6879(94)33009-3. [DOI] [PubMed] [Google Scholar]
- KAUR H., LYRAS L., JENNER P., HALLIWELL B. Artefacts in HPLC detection of 3-nitrotyrosine in human brain tissue. J. Neurochem. 1998;70:2220–2223. doi: 10.1046/j.1471-4159.1998.70052220.x. [DOI] [PubMed] [Google Scholar]
- KAUR H., WHITEMAN M., HALLIWELL B. Peroxynitrite-dependent aromatic hydroxylation and nitration of salicylate and phenylalanine. Is hydroxyl radical involved. Free Radic. Res. 1997;26:71–82. doi: 10.3109/10715769709097786. [DOI] [PubMed] [Google Scholar]
- KAWAKAMI N., HAYAKAWA T., SHIMOHAMA S., FUJIMOTO S. The hydroxyl radical formation system in polymorphonuclear leukocytes. Biol. Pharm. Bull. 1999;22:1034–1037. doi: 10.1248/bpb.22.1034. [DOI] [PubMed] [Google Scholar]
- KEANEY J.F., JR, LARSON M.G., VASAN R.S., WILSON P.W., LIPINSKA I., COREY D., MASSARO J.M., SUTHERLAND P., VITA J.A., BENJAMIN E.J. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- KELM M., PREIK-STEINHOFF H., PREIK M., STRAUER B.E. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: evidence for biochemical assessment of the endothelial L-arginine–NO pathway. Cardiovasc. Res. 1999;41:765–772. doi: 10.1016/s0008-6363(98)00259-4. [DOI] [PubMed] [Google Scholar]
- KESTON A.S., BRANDT R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal. Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- KETTLE A.J., WINTERBOURN C.C. Superoxide-dependent hydroxylation by myeloperoxidase. J. Biol. Chem. 1994;269:17146–17151. [PubMed] [Google Scholar]
- KHAN N., WILMOT C.M., ROSEN G.M., DEMIDENKO E., SUN J., JOSEPH J., O'HARA J., KALYANARAMAN B., SWARTZ H.M. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic. Biol. Med. 2003;34:1473–1481. doi: 10.1016/s0891-5849(03)00182-5. [DOI] [PubMed] [Google Scholar]
- KIRSCHBAUM B. Correlative studies of urine fluorescence and free radical indicators. Clin. Nephrol. 2002;58:344–349. doi: 10.5414/cnp58344. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI K., HARADA Y., HAYASHI K. Kinetic behavior of the monodehydroascorbate radical studied by pulse radiolysis. Biochemistry. 1991;30:8310–8315. doi: 10.1021/bi00098a005. [DOI] [PubMed] [Google Scholar]
- KOJDA G., HARRISON D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., LEWIS S.J., ROYALL J.A., YE Y.Z., KELLY D.R., BECKMAN J.S. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit. Care Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- KOTAKE Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid. Redox Signal. 1999;1:481–499. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- KUGE N., KOHZUKI M., SATO T. Relation between natriuresis and urinary excretion of hydrogen peroxide. Free Radic. Res. 1999;30:119–123. doi: 10.1080/10715769900300121. [DOI] [PubMed] [Google Scholar]
- LABORIE S., LAVOIE J.C., CHESSEX P. Increased urinary peroxides in newborn infants receiving parenteral nutrition exposed to light. J. Pediatr. 2000;136:628–632. doi: 10.1067/mpd.2000.105131. [DOI] [PubMed] [Google Scholar]
- LAMB N.J., GUTTERIDGE J.M., BAKER C., EVANS T.W., QUINLAN G.J. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 1999;27:1738–1744. doi: 10.1097/00003246-199909000-00007. [DOI] [PubMed] [Google Scholar]
- LAPCHAK P.A., ARAUJO D.M. Development of the nitrone-based spin trap agent NXY-059 to treat acute ischemic stroke. CNS Drug Rev. 2003;9:253–262. doi: 10.1111/j.1527-3458.2003.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSEGUE B., CLEMPUS R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- LAWRENCE A., JONES C.M., WARDMAN P., BURKITT M.J. Evidence for the role of a peroxidase compound I-type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2. Implications for oxidative stress during apoptosis. J. Biol. Chem. 2003;278:29410–29419. doi: 10.1074/jbc.M300054200. [DOI] [PubMed] [Google Scholar]
- LAWSON J.A., ROKACH J., FITZGERALD G.A. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J. Biol. Chem. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- LE CURIEUX F., HEMMINKI K. Cyclobutane thymidine dimers are present in human urine following sun exposure: quantitation using 32P-postlabeling and high-performance liquid chromatography. J. Invest. Dermatol. 2001;117:263–268. doi: 10.1046/j.1523-1747.2001.01416.x. [DOI] [PubMed] [Google Scholar]
- LEBEL C.P., ISCHIROPOULOS H., BONDY S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- LEE C.K., ALLISON D.B., BRAND J., WEINDRUCH R., PROLLA T.A. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc. Natl. Acad. Sci. U.S.A. 2002a;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J.M., NILES J.C., WISHNOK J.S., TANNENBAUM S.R. Peroxynitrite reacts with 8-nitropurines to yield 8-oxopurines. Chem. Res. Toxicol. 2002b;15:7–14. doi: 10.1021/tx010093d. [DOI] [PubMed] [Google Scholar]
- LEE M., HYUN D.H., HALLIWELL B., JENNER P. Effect of overexpression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative stress and cell death induced by hydrogen peroxide, 4-hydroxynonenal or serum deprivation: potentiation of injury by ALS-related mutant superoxide dismutases and protection by Bcl-2. J. Neurochem. 2001;78:209–220. doi: 10.1046/j.1471-4159.2001.00417.x. [DOI] [PubMed] [Google Scholar]
- LEEUWENBURGH C., HANSEN P.A., HOLLOSZY J.O., HEINECKE J.W. Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic. Biol. Med. 1999a;27:186–192. doi: 10.1016/s0891-5849(99)00071-4. [DOI] [PubMed] [Google Scholar]
- LEEUWENBURGH C., HANSEN P.A., HOLLOSZY J.O., HEINECKE J.W. Oxidized amino acids in the urine of aging rats: potential markers for assessing oxidative stress in vivo. Am. J. Physiol. 1999b;276:R128–135. doi: 10.1152/ajpregu.1999.276.1.R128. [DOI] [PubMed] [Google Scholar]
- LEONARDUZZI G., SOTTERO B., POLI G. Oxidized products of cholesterol: dietary and metabolic origin, and proatherosclerotic effects (review) J. Nutr. Biochem. 2002;13:700–710. doi: 10.1016/s0955-2863(02)00222-x. [DOI] [PubMed] [Google Scholar]
- LEVINE M., WANG Y., PADAYATTY S.J., MORROW J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R.L. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic. Biol. Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- LEVINE R.L., BERLETT B.S., MOSKOVITZ J., MOSONI L., STADTMAN E.R. Methionine residues may protect proteins from critical oxidative damage. Mech. Ageing Dev. 1999;107:323–332. doi: 10.1016/s0047-6374(98)00152-3. [DOI] [PubMed] [Google Scholar]
- LI H., LAWSON J.A., REILLY M., ADIYAMAN M., HWANG S.W., ROKACH J., FITZGERALD G.A. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI M., CARLSON S., KINZER J.A., PERPALL H.J. HPLC and LC–MS studies of hydroxylation of phenylalanine as an assay for hydroxyl radicals generated from Udenfriend's reagent. Biochem. Biophys. Res. Commun. 2003;312:316–322. doi: 10.1016/j.bbrc.2003.10.116. [DOI] [PubMed] [Google Scholar]
- LIM D.G., SWEENEY S., BLOODSWORTH A., WHITE C.R., CHUMLEY P.H., KRISHNA N.R., SCHOPFER F., O'DONNELL V.B., EISERICH J.P., FREEMAN B.A. Nitrolinoleate, a nitric oxide-derived mediator of cell function: synthesis, characterization, and vasomotor activity. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15941–15946. doi: 10.1073/pnas.232409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMA E.S., DI MASCIO P., ABDALLA D.S. Cholesteryl nitrolinoleate, a nitrated lipid present in human blood plasma and lipoproteins. J. Lipid. Res. 2003;44:1660–1666. doi: 10.1194/jlr.M200467-JLR200. [DOI] [PubMed] [Google Scholar]
- LIMA E.S., DI MASCIO P., RUBBO H., ABDALLA D.S. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry. 2002;41:10717–10722. doi: 10.1021/bi025504j. [DOI] [PubMed] [Google Scholar]
- LIN H.S., JENNER A., ONG C.N., HUANG S.H., WHITEMAN M., HALLIWELL B.High-throughput and sensitive methodology for the quantitation of urinary 8OHdG; measurement with GC–MS after single solid phase extraction Biochem. J. 2004(in press) [DOI] [PMC free article] [PubMed]
- LIU J., YEO H.C., DONIGER S.J., AMES B.N. Assay of aldehydes from lipid peroxidation: gas chromatography–mass spectrometry compared to thiobarbituric acid. Anal. Biochem. 1997b;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- LIU K.J., MIYAKE M., PANZ T., SWARTZ H. Evaluation of DEPMPO as a spin trapping agent in biological systems. Free Radic. Biol. Med. 1999;26:714–721. doi: 10.1016/s0891-5849(98)00251-2. [DOI] [PubMed] [Google Scholar]
- LIU L., LEECH J.A., URCH R.B., SILVERMAN F.S. In vivo salicylate hydroxylation: a potential biomarker for assessing acute ozone exposure and effects in humans. Am. J. Respir. Crit. Care Med. 1997a;156:1405–1412. doi: 10.1164/ajrccm.156.5.9610105. [DOI] [PubMed] [Google Scholar]
- LIU Q., RAINA A.K., SMITH M.A., SAYRE L.M., PERRY G. Hydroxynonenal, toxic carbonyls, and Alzheimer disease. Mol. Aspects Med. 2003;24:305–313. doi: 10.1016/s0098-2997(03)00025-6. [DOI] [PubMed] [Google Scholar]
- LONG L.H., CLEMENT M.V., HALLIWELL B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- LONG L.H., EVANS P.J., HALLIWELL B. Hydrogen peroxide in human urine: implications for antioxidant defense and redox regulation. Biochem. Biophys. Res. Commun. 1999;262:605–609. doi: 10.1006/bbrc.1999.1263. [DOI] [PubMed] [Google Scholar]
- LONG L.H., HALLIWELL B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human volunteers. Free Radic. Res. 2000;32:463–467. doi: 10.1080/10715760000300461. [DOI] [PubMed] [Google Scholar]
- LONG L.H., HALLIWELL B. Oxidation and generation of hydrogen peroxide by thiol compounds in commonly used cell culture media. Biochem. Biophys. Res. Commun. 2001;286:991–994. doi: 10.1006/bbrc.2001.5514. [DOI] [PubMed] [Google Scholar]
- LUBEC G., WIDNESS J.A., HAYDE M., MENZEL D., POLLAK A. Hydroxyl radical generation in oxygen-treated infants. Pediatrics. 1997;100:700–704. doi: 10.1542/peds.100.4.700. [DOI] [PubMed] [Google Scholar]
- LYNCH R.E., FRIDOVICH I. Permeation of the erythrocyte stroma by superoxide radical. J. Biol. Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- MACMILLAN-CROW L.A., CROW J.P., KERBY J.D., BECKMAN J.S., THOMPSON J.A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON J.C., COMHAIR S.A., ERZURUM S.C., KLEIN D.F., LIPSCOMB M.F., KAVURU M.S., SAMOSZUK M.K., HAZEN S.L. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J. Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., PHILIP I., LEBRET M., CHATEL D., MACLOUF J., TEDGUI A. Elevated levels of 8-iso-prostaglandin F2∝ in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- MANARY M.J., LEEUWENBURGH C., HEINECKE J.W. Increased oxidative stress in kwashiorkor. J. Pediatr. 2000;137:421–424. doi: 10.1067/mpd.2000.107512. [DOI] [PubMed] [Google Scholar]
- MANI A.R., PANNALA A.S., ORIE N.N., OLLOSSON R., HARRY D., RICE-EVANS C.A., MOORE K.P. Nitration of endogenous para-hydroxyphenylacetic acid and the metabolism of nitrotyrosine. Biochem. J. 2003;374:521–527. doi: 10.1042/BJ20030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNICK J.B., SCHONHOFF C. NO means no and yes: regulation of cell signaling by protein nitrosylation. Free Radic. Res. 2004;38:1–7. doi: 10.1080/10715760310001629065. [DOI] [PubMed] [Google Scholar]
- MARCHESI E., ROTA C., FANN Y.C., CHIGNELL C.F., MASON R.P. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 1999;26:148–161. doi: 10.1016/s0891-5849(98)00174-9. [DOI] [PubMed] [Google Scholar]
- MARKLUND N., OSTMAN B., NALMO L., PERSSON L., HILLERED L. Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions. Description of a HPLC method and human brain microdialysis data. Acta Neurochir. 2000;142:1135–1142. doi: 10.1007/s007010070042. [DOI] [PubMed] [Google Scholar]
- MARLA S.S., LEE J., GROVES J.T. Peroxynitrite rapidly permeates phospholipid membranes. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARNETT L.J., RIGGINS J.N., WEST J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN B.D., SCHOENHARD J.A., SUGDEN K.D. Hypervalent chromium mimics reactive oxygen species as measured by the oxidant-sensitive dyes 2′,7′-dichlorofluorescin and dihydrorhodamine. Chem. Res. Toxicol. 1998;11:1402–1410. doi: 10.1021/tx9801559. [DOI] [PubMed] [Google Scholar]
- MAY J.M. How does ascorbic acid prevent endothelial dysfunction. Free Radic. Biol. Med. 2000;28:1421–1429. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- MAY J.M., QU Z., COBB C.E. Recycling of the ascorbate free radical by human erythrocyte membranes. Free Radic. Biol. Med. 2001;31:117–124. doi: 10.1016/s0891-5849(01)00566-4. [DOI] [PubMed] [Google Scholar]
- MEAGHER E.A., BARRY O.P., LAWSON J.A., ROKACH J., FITZGERALD G.A. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- MEIJER E.P., COOLEN S.A., BAST A., WESTERTERP K.R. Exercise training and oxidative stress in the elderly as measured by antipyrine hydroxylation products. Free Radic. Res. 2001;35:435–443. doi: 10.1080/10715760100300951. [DOI] [PubMed] [Google Scholar]
- MEISTER A., ANDERSON M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- MIKAMI T., KITA K., TOMITA S., QU G.J., TASAKI Y., ITO A. Is allantoin in serum and urine a useful indicator of exercise-induced oxidative stress in humans. Free Radic. Res. 2000;32:235–244. doi: 10.1080/10715760000300241. [DOI] [PubMed] [Google Scholar]
- MINAMIKAWA T., SRIRATANA A., WILLIAMS D.A., BOWSER D.N., HILL J.S., NAGLEY P. Chloromethyl-X-rosamine (MitoTracker Red) photosensitises mitochondria and induces apoptosis in intact human cells. J. Cell Sci. 1999;112:2419–2430. doi: 10.1242/jcs.112.14.2419. [DOI] [PubMed] [Google Scholar]
- MOISON R.M., DE BEAUFORT A.J., HAASNOOT A.A., DUBBELMAN T.M., VAN ZOEREN-GROBBEN D., BERGER H.M. Uric acid and ascorbic acid redox ratios in plasma and tracheal aspirate of preterm babies with acute and chronic lung disease. Free Radic. Biol. Med. 1997;23:226–234. doi: 10.1016/s0891-5849(97)00033-6. [DOI] [PubMed] [Google Scholar]
- MOLLER P., LOFT S. Oxidative DNA damage in human white blood cells in dietary antioxidant intervention studies. Am. J. Clin. Nutr. 2002;76:303–310. doi: 10.1093/ajcn/76.2.303. [DOI] [PubMed] [Google Scholar]
- MONTINE T.J., MARKESBERY W.R., MORROW J.D., ROBERTS L.J., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer's disease. Ann. Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- MONTINE T.J., MARKESBERY W.R., ZACKERT W., SANCHEZ S.C., ROBERTS L.J., II, MORROW J.D. The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer's disease patients. Am. J. Pathol. 1999;155:863–868. doi: 10.1016/S0002-9440(10)65185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTINE T.J., QUINN J.F., MILATOVIC D., SILBERT L.C., DANG T., SANCHEZ S., TERRY E., ROBERTS L.J., II, KAYE J.A., MORROW J.D. Peripheral F2-isoprostanes and F4-neuroprostanes are not increased in Alzheimer's disease. Ann. Neurol. 2002;52:175–179. doi: 10.1002/ana.10272. [DOI] [PubMed] [Google Scholar]
- MONTUSCHI P., CORRADI M., CIABATTONI G., NIGHTINGALE J., KHARITONOV S.A., BARNES P.J. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am. J. Respir. Crit. Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- MORIN B., BUBB W.A., DAVIES M.J., DEAN R.T., FU S. 3-Hydroxylysine, a potential marker for studying radical-induced protein oxidation. Chem. Res. Toxicol. 1998;11:1265–1273. doi: 10.1021/tx980118h. [DOI] [PubMed] [Google Scholar]
- MORROW J.D. Is oxidant stress a connection between obesity and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23:368–370. doi: 10.1161/01.ATV.0000063107.86298.FD. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., ROBERTS L.J., DANIEL V.C., AWAD J.A., MIROCHNITCHENKO O., SWIFT L.L., BURK R.F. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo – effects of oxygen tension and glutathione. Arch. Biochem. Biophys. 1998;353:160–171. doi: 10.1006/abbi.1998.0645. [DOI] [PubMed] [Google Scholar]
- MORTON L.W., PUDDEY I.B., CROFT K.D. Comparison of nitration and oxidation of tyrosine in advanced human carotid plaque proteins. Biochem. J. 2003;370:339–344. doi: 10.1042/BJ20020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNZEL T., AFANAS'EV I.B., KLESCHYOV A.L., HARRISON D.G. Detection of superoxide in vascular tissue. Arterioscler. Thromb. Vasc. Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- MURPHEY L.J., MORROW J.D., SAWATHIPARNICH P., WILLIAMS G.H., VAUGHAN D.E., BROWN N.J. Acute angiotensin II increases plasma F2-isoprostanes in salt-replete human hypertensives. Free Radic. Biol. Med. 2003;35:711–718. doi: 10.1016/s0891-5849(03)00395-2. [DOI] [PubMed] [Google Scholar]
- MYHRE O., ANDERSEN J.M., AARNES H., FONNUM F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- NAIR U.J., NAIR J., FRIESEN M.D., BARTSCH H., OHSHIMA H. Ortho- and meta-tyrosine formation from phenylalanine in human saliva as a marker of hydroxyl radical generation during betel quid chewing. Carcinogenesis. 1995;16:1195–1198. doi: 10.1093/carcin/16.5.1195. [DOI] [PubMed] [Google Scholar]
- NELSON G.J., MORRIS V.C., SCHMIDT P.C., LEVANDER O. The urinary excretion of thiobarbituric acid reactive substances and malondialdehyde by normal adult males after consuming a diet containing salmon. Lipids. 1993;28:757–761. doi: 10.1007/BF02536000. [DOI] [PubMed] [Google Scholar]
- NIELSEN S.E., YOUNG J.F., DANESHVAR B., LAURIDSEN S.T., KNUTHSEN P., SANDSTROM B., DRAGSTED L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999;81:447–455. doi: 10.1017/s000711459900080x. [DOI] [PubMed] [Google Scholar]
- NOUROOZ-ZADEH J., HALLIWELL B., ANGGARD E.E. Evidence for the formation of F3-isoprostanes during peroxidation of eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1997;236:467–472. doi: 10.1006/bbrc.1997.6869. [DOI] [PubMed] [Google Scholar]
- NOUROOZ-ZADEH J., LIU E.H., YHLEN B., ANGGARD E.E., HALLIWELL B. F4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer's disease. J. Neurochem. 1999;72:734–740. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- OGIHARA T., HIRANO K., OGIHARA H., MISAKI K., HIROI M., MORINOBU T., KIM H.S., OGAWA S., BAN R., HASEGAWA M., TAMAI H. Non-protein-bound transition metals and hydroxyl radical generation in cerebrospinal fluid of newborn infants with hypoxic ischemic encephalopathy. Pediatr. Res. 2003;53:594–599. doi: 10.1203/01.PDR.0000054685.87405.59. [DOI] [PubMed] [Google Scholar]
- OGIHARA T., KIM H.S., HIRANO K., IMANISHI M., OGIHARA H., TAMAI H., OKAMOTO R., MINO M. Oxidation products of uric acid and ascorbic acid in preterm infants with chronic lung disease. Biol. Neonate. 1998;73:24–33. doi: 10.1159/000013956. [DOI] [PubMed] [Google Scholar]
- OGINO K., NAKAJIMA M., KODAMA N., KUBO M., KIMURA S., NAGASE H., NAKAMURA H. Immunohistochemical artifact for nitrotyrosine in eosinophils or eosinophil containing tissue. Free Radic. Res. 2002;36:1163–1170. doi: 10.1080/1071576021000016427. [DOI] [PubMed] [Google Scholar]
- OHASHI T., MIZUTANI A., MURAKAMI A., KOJO S., ISHII T., TAKETANI S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- OHSHIMA H., FRIESEN M., BROUET I., BARTSCH H. Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem. Toxicol. 1990;28:647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- OIKAWA S., KAWANISHI S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- OLDREIVE C., RICE-EVANS C. The mechanisms for nitration and nitrotyrosine formation in vitro and in vivo: impact of diet. Free Radic. Res. 2001;35:215–231. doi: 10.1080/10715760100300761. [DOI] [PubMed] [Google Scholar]
- O'MALLEY Y.Q., RESZKA K.J., BRITIGAN B.E. Direct oxidation of 2′,7′-dichlorodihydrofluorescein by pyocyanin and other redox-active compounds independent of reactive oxygen species production. Free Radic. Biol. Med. 2004;36:90–100. doi: 10.1016/j.freeradbiomed.2003.09.021. [DOI] [PubMed] [Google Scholar]
- ONG W.Y., HALLIWELL B.Iron, atherosclerosis and neurodegeneration. A key role for cholesterol in promoting iron-dependent oxidative damage Ann. NY Acad. Sci. 2004(in press) [DOI] [PubMed]
- ONG W.Y., LU X.R., HU C.Y., HALLIWELL B. Distribution of hydroxynonenal-modified proteins in the kainate-lesioned rat hippocampus: evidence that hydroxynonenal formation precedes neuronal cell death. Free Radic. Biol. Med. 2000;28:1214–1221. doi: 10.1016/s0891-5849(00)00238-0. [DOI] [PubMed] [Google Scholar]
- OSTDAL H., DAVIES M.J., ANDERSEN H.J. Reaction between protein radicals and other biomolecules. Free Radic. Biol. Med. 2002;33:201–209. doi: 10.1016/s0891-5849(02)00785-2. [DOI] [PubMed] [Google Scholar]
- OTTENEDER M., SCOTT DANIELS J., VOEHLER M., MARNETT L.J. Development of a method for determination of the malondialdehyde–deoxyguanosine adduct in urine using liquid chromatography–tandem mass spectrometry. Anal. Biochem. 2003;315:147–151. doi: 10.1016/s0003-2697(02)00697-8. [DOI] [PubMed] [Google Scholar]
- PALOMBA L., SESTILI P., GUIDARELLI A., SCIORATI C., CLEMENTI E., FIORANI M., CANTONI O. Products of the phospholipase A2 pathway mediate the dihydrorhodamine fluorescence response evoked by endogenous and exogenous peroxynitrite in PC12 cells. Free Radic. Biol. Med. 2000;29:783–789. doi: 10.1016/s0891-5849(00)00381-6. [DOI] [PubMed] [Google Scholar]
- PANTKE U., VOLK T., SCHMUTZLER M., KOX W.J., SITTE N., GRUNE T. Oxidized proteins as a marker of oxidative stress during coronary heart surgery. Free Radic. Biol. Med. 1999;27:1080–1086. doi: 10.1016/s0891-5849(99)00144-6. [DOI] [PubMed] [Google Scholar]
- PARTHASARATHY S., SANTANAM N., RAMACHANDRAN S., MEILHAC O. Potential role of oxidized lipids and lipoproteins in antioxidant defense. Free Radic. Res. 2000;33:197–215. doi: 10.1080/10715760000301381. [DOI] [PubMed] [Google Scholar]
- PAVITT D.V., DE FONSEKA S., AL-KHALAF N., CAM J.M., REAVELEY D.A. Assay of serum allantoin in humans by gas chromatography–mass spectrometry. Clin. Chim. Acta. 2002;318:63–70. doi: 10.1016/s0009-8981(01)00805-1. [DOI] [PubMed] [Google Scholar]
- PENNATHUR S., WAGNER J.D., LEEUWENBURGH C., LITWAK K.N., HEINECKE J.W. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J. Clin. Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRAT F., PINDIUR S., KIRSCH M., GROOT H. NAD(P)H, a primary target of 1O2 in mitochondria of intact cells. J. Biol. Chem. 2003;278:3298–3307. doi: 10.1074/jbc.M204230200. [DOI] [PubMed] [Google Scholar]
- PHILLIPS M., GREENBERG J., CATANEO R.N. Effect of age on the profile of alkanes in normal human breath. Free Radic. Res. 2000;33:57–63. doi: 10.1080/10715760000300611. [DOI] [PubMed] [Google Scholar]
- PI J., HORIGUCHI S., SUN Y., NIKAIDO M., SHIMOJO N., HAYASHI T., YAMAUCHI H., ITOH K., YAMAMOTO M., SUN G., WAALKES M.P., KUMAGAI Y. A potential mechanism for the impairment of nitric oxide formation caused by prolonged oral exposure to arsenate in rabbits. Free Radic. Biol. Med. 2003;35:102–113. doi: 10.1016/s0891-5849(03)00269-7. [DOI] [PubMed] [Google Scholar]
- PIETRI S., LIEBGOTT T., FREJAVILLE C., TORDO P., CULCASI M. Nitrone spin traps and their pyrrolidine analogs in myocardial reperfusion injury: hemodynamic and ESR implications – evidence for a cardioprotective phosphonate effect for 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide in rat hearts. Eur. J. Biochem. 1998;254:256–265. doi: 10.1046/j.1432-1327.1998.2540256.x. [DOI] [PubMed] [Google Scholar]
- PILGER A., IVANCSITS S., GERMADNIK D., RUDIGER H.W. Urinary excretion of 8-hydroxy-2′-deoxyguanosine measured by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:393–401. doi: 10.1016/s0378-4347(01)00449-2. [DOI] [PubMed] [Google Scholar]
- PINLAOR S., YONGVANIT P., HIRAKU Y., MA N., SEMBA R., OIKAWA S., MURATA M., SRIPA B., SITHITHAWORN P., KAWANISHI S. 8-nitroguanine formation in the liver of hamsters infected with Opisthorchis viverrini. Biochem. Biophys. Res. Commun. 2003;309:567–571. doi: 10.1016/j.bbrc.2003.08.039. [DOI] [PubMed] [Google Scholar]
- PLEINER J., MITTERMAYER F., SCHALLER G., MACALLISTER R.J., WOLZT M. High doses of vitamin C reverse Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm. Circulation. 2002;106:1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- PLOTNICK G.D., CORRETTI M.C., VOGEL R.A. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278:1682–1686. [PubMed] [Google Scholar]
- POU S., HUANG Y.I., BHAN A., BHADTI V.S., HOSMANE R.S., WU S.Y., CAO G.L., ROSEN G.M. A fluorophore-containing nitroxide as a probe to detect superoxide and hydroxyl radical generated by stimulated neutrophils. Anal. Biochem. 1993;212:85–90. doi: 10.1006/abio.1993.1295. [DOI] [PubMed] [Google Scholar]
- PRATICO D., CLARK C.M., LEE V.M., TROJANOWSKI J.Q., ROKACH J., FITZGERALD G.A. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- PRATICO D., CLARK C.M., LIUN F., ROKACH J., LEE V.Y., TROJANOWSKI J.Q. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- PRATICO D., URYU K., LEIGHT S., TROJANOSWKI J.Q., LEE V.M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR R.L., CAO G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic. Biol. Med. 1999;27:1173–1181. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- PROUDFOOT J., BARDEN A., MORI T.A., BURKE V., CROFT K.D., BEILIN L.J., PUDDEY I.B. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation – a comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal. Biochem. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- PUGLIELLI L., TANZI R.E., KOVACS D.M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- RADI R., PELUFFO G., ALVAREZ M.N., NAVILIAT M., CAYOTA A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- RAHMAN I., KELLY F. Biomarkers in breath condensate: a promising new non-invasive techique in free radical research. Free Radic. Res. 2003;37:1253–1266. doi: 10.1080/10715760310001623331. [DOI] [PubMed] [Google Scholar]
- RAVANAT J.L., GUICHERD P., TUCE Z., CADET J. Simultaneous determination of five oxidative DNA lesions in human urine. Chem. Res. Toxicol. 1999;12:802–808. doi: 10.1021/tx980194k. [DOI] [PubMed] [Google Scholar]
- REDDY S., HALLIWELL B., JONES A.D., LONGHURST J.C. The use of phenylalanine to detect hydroxyl radical production in vivo: a cautionary note. Free Radic. Biol. Med. 1999;27:1465. doi: 10.1016/s0891-5849(99)00165-3. [DOI] [PubMed] [Google Scholar]
- REFOLO L.M., PAPPOLLA M.A., LAFRANCOIS J., MALESTER B., SCHMIDT S.D., THOMAS-BRYANT T., TINT G.S., WANG R., MERCKEN M., PETANCESKA S.S., DUFF K.E. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- REICH E.E., MARKESBERY W.R., ROBERTS L.J., II, SWIFT L.L., MORROW J.D., MONTINE T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer's disease. Am. J. Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REQUENA J.R., CHAO C.C., LEVINE R.L., STADTMAN E.R. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. U.S.A. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RETH M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- REY F.E., PAGANO P.J. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler. Thromb. Vasc. Biol. 2002;22:1962–1971. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- REZNICK A.Z., CROSS C.E., HU M.L., SUZUKI Y.J., KHWAJA S., SAFADI A., MOTCHNIK P.A., PACKER L., HALLIWELL B. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem. J. 1992;286:607–611. doi: 10.1042/bj2860607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE-EVANS C.A. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic. Res. 2000;33 Suppl:S59–S66. [PubMed] [Google Scholar]
- RICHELLE M., TURINI M.E., GUIDOUX R., TAVAZZI I., METAIRON S., FAY L.B. Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS Lett. 1999;459:259–262. doi: 10.1016/s0014-5793(99)01259-4. [DOI] [PubMed] [Google Scholar]
- RITOV V.B., BANNI S., YALOWICH J.C., DAY B.W., CLAYCAMP H.G., CORONGIU F.P., KAGAN V.E. Non-random peroxidation of different classes of membrane phospholipids in live cells detected by metabolically integrated cis-parinaric acid. Biochim. Biophys. Acta. 1996;1283:127–140. doi: 10.1016/0005-2736(96)00083-1. [DOI] [PubMed] [Google Scholar]
- RIUS M., NIES A.T., HUMMEL-EISENBEISS J., JEDLITSCHKY G., KEPPLER D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38:374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- RIZZI C., SAMOUILOV A., KUTALA V.K., PARINANDI N.L., ZWEIER J.L., KUPPUSAMY P. Application of a trityl-based radical probe for measuring superoxide. Free Radic. Biol. Med. 2003;35:1608–1618. doi: 10.1016/j.freeradbiomed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- ROBERTS C.K., VAZIRI N.D., BARNARD R.J. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.J., II, MORROW J.D. Products of the isoprostane pathway: unique bioactive compounds and markers of lipid peroxidation. Cell. Mol. Life Sci. 2002;59:808–820. doi: 10.1007/s00018-002-8469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ H., AKMAN S.A., HOLMQUIST G.P., WILSON G.L., DRIGGERS W.J., LEDOUX S.P. Mapping oxidative DNA damage using ligation-mediated polymerase chain reaction technology. Methods. 2000;22:148–156. doi: 10.1006/meth.2000.1055. [DOI] [PubMed] [Google Scholar]
- ROQUES S.C., LANDRAULT N., TEISSEDRE P.L., LAURENT C., BESANCON P., ROUANE J.M., CAPORICCIO B. Hydrogen peroxide generation in caco-2 cell culture medium by addition of phenolic compounds: effect of ascorbic acid. Free Radic. Res. 2002;36:593–599. doi: 10.1080/10715760290025979. [DOI] [PubMed] [Google Scholar]
- ROSEN G.M., BRITIGAN B., HALPERN H., POU S. Free Radicals. Biology and Detection by Spin Trapping. Oxford, U.K.: Oxford University Press; 1999. [Google Scholar]
- ROTA C., FANN Y.C., MASON R.P. Phenoxyl free radical formation during the oxidation of the fluorescent dye 2′,7′-dichlorofluorescein by horseradish peroxidase. Possible consequences for oxidative stress measurements. J. Biol. Chem. 1999;274:28161–28168. doi: 10.1074/jbc.274.40.28161. [DOI] [PubMed] [Google Scholar]
- ROZALSKI R., WINKLER P., GACKOWSKI D., PACIOREK T., KASPRZAK H., OLINSKI R. High concentrations of excised oxidative DNA lesions in human cerebrospinal fluid. Clin. Chem. 2003;49:1218–1221. doi: 10.1373/49.7.1218. [DOI] [PubMed] [Google Scholar]
- RUSSELL A.P., GASTALDI G., BOBBIONI-HARSCH E., ARBOIT P., GOBELET C., DERIAZ O., GOLAY A., WITZTUM J.L., GIACOBINO J.P. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids. FEBS Lett. 2003;551:104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- SAKHAROV D.V., BUNSCHOTEN A., VAN WEELDEN H., WIRTZ K.W.A. Photodynamic treatment and H2O2-induced oxidative stress result in different patterns of cellular protein oxidation. Eur. J. Biochem. 2003;270:4859–4865. doi: 10.1046/j.1432-1033.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- SAMPSON M.J., GOPAUL N., DAVIES I.R., HUGHES D.A., CARRIER M.J. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON L., GOTTSATER A., LINDGARDE F. Decreasing levels of tumour necrosis factor alpha and interleukin 6 during lowering of body mass index with orlistat or placebo in obese subjects with cardiovascular risk factors. Diabetes Obes. Metab. 2003;5:195–201. doi: 10.1046/j.1463-1326.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- SARVAZYAN N., SWIFT L., MARTINEZ-ZAGUILAN R. Effects of oxidants on properties of fluorescent calcium indicators. Arch. Biochem. Biophys. 1998;350:132–136. doi: 10.1006/abbi.1997.0518. [DOI] [PubMed] [Google Scholar]
- SAWYER D.E., MERCER B.G., WIKLENDT A.M., AITKEN R.J. Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat. Res. 2003;529:21–34. doi: 10.1016/s0027-5107(03)00101-5. [DOI] [PubMed] [Google Scholar]
- SCHLESIER K., HARWAT M., BOHM V., BITSCH R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002;36:177–187. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- SCHOCK B.C., SWEET D.G., HALLIDAY H.L., YOUNG I.S., ENNIS M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1386–1391. doi: 10.1152/ajplung.2001.281.6.L1386. [DOI] [PubMed] [Google Scholar]
- SETSUKINAI K., URANO Y., KAKINUMA K., MAJIMA H.J., NAGANO T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- SHACTER E., WILLIAMS J.A., LIM M., LEVINE R.L. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic. Biol. Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- SHARMA M.K., BUETTNER G.R. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic. Biol. Med. 1993;14:649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- SHARMA M.K., BUETTNER G.R., SPENCER K.T., KERBER R.E. Ascorbyl free radical as a real-time marker of free radical generation in briefly ischemic and reperfused hearts. An electron paramagnetic resonance study. Circ. Res. 1994;74:650–658. doi: 10.1161/01.res.74.4.650. [DOI] [PubMed] [Google Scholar]
- SHAROV V.S., FERRINGTON D.A., SQUIER T.C., SCHONEICH C. Diastereoselective reduction of protein-bound methionine sulfoxide by methionine sulfoxide reductase. FEBS Lett. 1999;455:247–250. doi: 10.1016/s0014-5793(99)00888-1. [DOI] [PubMed] [Google Scholar]
- SHIMOI K., KASAI H., YOKOTA N., TOYOKUNI S., KINAE N. Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2′-deoxyguanosine in human urine. Cancer Epidemiol. Biomarkers Prev. 2002;11:767–770. [PubMed] [Google Scholar]
- SHISHEHBOR M.H., BRENNAN M.L., AVILES R.J., FU X., PENN M.S., SPRECHER D.L., HAZEN S.L. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- SHVEDOVA A.A., TYURINA J.Y., KAWAI K., TYURIN V.A., KOMMINENI C., CASTRANOVA V., FABISIAK J.P., KAGAN V.E. Selective peroxidation and externalization of phosphatidylserine in normal human epidermal keratinocytes during oxidative stress induced by cumene hydroperoxide. J. Invest. Dermatol. 2002;118:1008–1018. doi: 10.1046/j.1523-1747.2002.01759.x. [DOI] [PubMed] [Google Scholar]
- SIES H. Oxidative Stress: Oxidants and Antioxidants. New York: Academic Press; 1991. [DOI] [PubMed] [Google Scholar]
- SIM Y.E., STACEY M.C. Iron and 8-isoprostane levels in acute and chronic wounds. J. Invest. Dermatol. 2003;121:918–925. doi: 10.1046/j.1523-1747.2003.12471.x. [DOI] [PubMed] [Google Scholar]
- SINGH N., GRAVES J., TAYLOR P.D., MACALLISTER R.J., SINGER D.R. Effects of a ‘healthy' diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc. Res. 2002;56:118–125. doi: 10.1016/s0008-6363(02)00514-x. [DOI] [PubMed] [Google Scholar]
- SOHAL R.S., MOCKETT R.J., ORR W.C. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- SOHN H.Y., KELLER M., GLOE T., CRAUSE P., POHL U. Pitfalls of using lucigenin in endothelial cells: implications for NAD(P)H dependent superoxide formation. Free Radic. Res. 2000;32:265–272. doi: 10.1080/10715760000300271. [DOI] [PubMed] [Google Scholar]
- SPASOJEVIC I., LIOCHEV S.I., FRIDOVICH I. Lucigenin: redox potential in aqueous media and redox cycling with O2•− production. Arch. Biochem. Biophys. 2000;373:447–450. doi: 10.1006/abbi.1999.1579. [DOI] [PubMed] [Google Scholar]
- SPENCER J.P., JENNER A., ARUOMA O.I., CROSS C.E., WU R., HALLIWELL B. Oxidative DNA damage in human respiratory tract epithelial cells. Time course in relation to DNA strand breakage. Biochem. Biophys. Res. Commun. 1996a;224:17–22. doi: 10.1006/bbrc.1996.0977. [DOI] [PubMed] [Google Scholar]
- SPENCER J.P., WONG J., JENNER A., ARUOMA O.I., CROSS C.E., HALLIWELL B. Base modification and strand breakage in isolated calf thymus DNA and in DNA from human skin epidermal keratinocytes exposed to peroxynitrite or 3-morpholinosydnonimine. Chem. Res. Toxicol. 1996b;9:1152–1158. doi: 10.1021/tx960084i. [DOI] [PubMed] [Google Scholar]
- STEINBERG D., WITZTUM J.L. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis. Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- STOLZE K., UDILOVA N., ROSENAU T., HOFINGER A., NOHL H. Spin trapping of superoxide, alky- and lipid-derived radicals with derivatives of the spin trap EPPN. Biochem. Pharmacol. 2003;66:1717–1726. doi: 10.1016/s0006-2952(03)00479-9. [DOI] [PubMed] [Google Scholar]
- SUBRAMANIAM R., FAN X.J., SCIVITTARO V., YANG J., HA C.E., PETERSEN C.E., SUREWICZ W.K., BHAGAVAN N.V., WEISS M.F., MONNIER V.M. Cellular oxidant stress and advanced glycation endproducts of albumin: caveats of the dichlorofluorescein assay. Arch. Biochem. Biophys. 2002;400:15–25. doi: 10.1006/abbi.2002.2776. [DOI] [PubMed] [Google Scholar]
- SUN J.Z., KAUR H., HALLIWELL B., LI X.Y., BOLLI R. Use of aromatic hydroxylation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo. Direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ. Res. 1993;73:534–549. doi: 10.1161/01.res.73.3.534. [DOI] [PubMed] [Google Scholar]
- SVETKEY L.P., LORIA C.M. Blood pressure effects of vitamin C: what's the key question. Hypertension. 2002;40:789–791. doi: 10.1161/01.hyp.0000038340.95407.43. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI M., SHIBATA M., NIKI E. Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radic. Biol. Med. 2001;31:164–174. doi: 10.1016/s0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- TAMPO Y., KOTAMRAJU S., CHITAMBAR R.C., KALIVENDI S.V., KESZLER A., JOESPH J., KALYANARAMAN B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endotheliel cells. Role of transferrin receptor-dependent iron uptake in apoptois. Circ. Res. 2003;92:56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- TANAKA T., WADA K., YAMAMORI H., TANAKA S., KUDO T., TAKEDA M. Biological markers for Alzheimer disease. Seishin Shinkeigaku Zasshi. 2003;105:387–392. [PubMed] [Google Scholar]
- TARKKA M.R., VUOLLE M., KAUKINEN S., HOLM P., ELORANTA J., KAUKINEN U., SISTO T., KATAJA J. Effect of allopurinol on myocardial oxygen free radical production in coronary bypass surgery. Scand. Cardiovasc. J. 2000;34:593–596. doi: 10.1080/140174300750064549. [DOI] [PubMed] [Google Scholar]
- TARPEY M.M., WHITE C.R., SUAREZ E., RICHARDSON G., RADI R., FREEMAN B.A. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ. Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- THEMANN C., TEISMANN P., KUSCHINSKY K., FERGER B. Comparison of two independent aromatic hydroxylation assays in combination with intracerebral microdialysis to determine hydroxyl free radicals. J. Neurosci. Methods. 2001;108:57–64. doi: 10.1016/s0165-0270(01)00370-3. [DOI] [PubMed] [Google Scholar]
- THOME J., ZHANG J., DAVIDS E., FOLEY P., WEIJERS H.G., WIESBECK G.A., BONING J., RIEDERER P., GERLACH M. Evidence for increased oxidative stress in alcohol-dependent patients provided by quantification of in vivo salicylate hydroxylation products. Alcohol Clin. Exp. Res. 1997;21:82–85. [PubMed] [Google Scholar]
- THUKKANI A.K., MCHOWAT J., HSU F.F., BRENNAN M.L., HAZEN S.L., FORD D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic Lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- TOYOKUNI S., TANAKA T., HATTORI Y., NISHIYAMA Y., YOSHIDA A., UCHIDA K., HIAI H., OCHI H., OSAWA T. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab. Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- TUBARO M., CAVALLO G., PENSA V., CHESSA M.A., NATALE E., RICCI R., MILAZZOTTO F., TUBARO E. Demonstration of the formation of hydroxyl radicals in acute myocardial infarction in man using salicylate as probe. Cardiology. 1992;80:246–251. doi: 10.1159/000175009. [DOI] [PubMed] [Google Scholar]
- UCHIDA K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc. Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- UCHIDA K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- UCHIDA K., KAWAKISHI S. 2-Oxo-histidine as a novel biological marker for oxidatively modified proteins. FEBS Lett. 1993;332:208–210. doi: 10.1016/0014-5793(93)80632-5. [DOI] [PubMed] [Google Scholar]
- UKAMOTO M., YONAHA M. The antioxidant action of 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-alpha]pyra z in-3-one (MCLA), a chemiluminescence probe to detect superoxide anions. FEBS Lett. 1998;430:348–352. doi: 10.1016/s0014-5793(98)00689-9. [DOI] [PubMed] [Google Scholar]
- ULUS A.T., AKSOYEK A., OZKAN M., KATIRCIOGLU S.F., BASU S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic. Biol. Med. 2003;34:911–917. doi: 10.1016/s0891-5849(03)00030-3. [DOI] [PubMed] [Google Scholar]
- UTSUMI H., YAMADA K. In vivo electron spin resonance-computed tomography/nitroxyl probe technique for non-invasive analysis of oxidative injuries. Arch. Biochem. Biophys. 2003;416:1–8. doi: 10.1016/s0003-9861(03)00285-6. [DOI] [PubMed] [Google Scholar]
- VALGIMIGLI M., VALGIMIGLI L., TRERE D., GAIANI S., PEDULLI G.F., GRAMANTIERI L., BOLONDI L. Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and cell proliferation. Free Radic. Res. 2002;36:939–948. doi: 10.1080/107156021000006653. [DOI] [PubMed] [Google Scholar]
- VARMA S.D., DEVAMANOHARAN P.S. Excretion of hydrogen peroxide in human urine. Free Radic. Res. Commun. 1990;8:73–78. doi: 10.3109/10715769009087976. [DOI] [PubMed] [Google Scholar]
- WARDMAN P., BURKITT M.J., PATEL K.B., LAWRENCE A., JONES C.M., EVERETT S.A., VOJNOVIC B. Pitfalls in the use of common luminescent probes for oxidative and nitrosative stress. J. Fluores. 2002;12:65–68. [Google Scholar]
- WEE L.M., LONG L.H., WHITEMAN M., HALLIWELL B. Factors affecting the ascorbate- and phenolic-dependent generation of hydrogen peroxide in Dulbecco's modified Eagles medium. Free Radic. Res. 2003;37:1123–1130. doi: 10.1080/10715760310001607041. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., HALLIWELL B. Loss of 3-nitrotyrosine on exposure to hypochlorous acid: implications for the use of 3-nitrotyrosine as a bio-marker in vivo. Biochem. Biophys. Res. Commun. 1999;258:168–172. doi: 10.1006/bbrc.1999.0564. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., HONG H.S., JENNER A., HALLIWELL B. Loss of oxidized and chlorinated bases in DNA treated with reactive oxygen species: implications for assessment of oxidative damage in vivo. Biochem. Biophys. Res. Commun. 2002a;296:883–889. doi: 10.1016/s0006-291x(02)02018-1. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., JENNER A., HALLIWELL B. Hypochlorous acid-induced base modifications in isolated calf thymus DNA. Chem. Res. Toxicol. 1997;10:1240–1246. doi: 10.1021/tx970086i. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., KETSAWATSAKUL U., HALLIWELL B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. NY Acad. Sci. 2002b;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- WIDLANSKY M.E., BIEGELSEN E.S., HAMBURG N.M., DUFFY S.J., KEANEY J.F., JR, VITA J.A. Coronary endothelial dysfunction is not rapidly reversible with ascorbic acid. Free Radic. Biol. Med. 2004;36:123–130. doi: 10.1016/j.freeradbiomed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- WILKINSON I.B., MEGSON I.L., MACCALLUM H., SOGO N., COCKCROFT J.R., WEBB D.J. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J. Cardiovasc. Pharmacol. 1999;34:690–693. doi: 10.1097/00005344-199911000-00010. [DOI] [PubMed] [Google Scholar]
- WILSON R., LYALL K., SMYTH L., FERNIE C.E., RIEMERSMA R.A. Dietary hydroxy fatty acids are absorbed in humans: implications for the measurement of ‘oxidative stress' in vivo. Free Radic. Biol. Med. 2002;32:162–168. doi: 10.1016/s0891-5849(01)00780-8. [DOI] [PubMed] [Google Scholar]
- WINTERBOURN C.C., KETTLE A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- WISEMAN H., HALLIWELL B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFRUM S., JENSEN K.S., LIAO J.K. Endothelium-dependent effects of statins. Arterioscler. Thromb. Vasc. Biol. 2003;23:729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- WRIGHT W.E., SHAY J.W. Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- WU W., CHEN Y., D'AVIGNON A., HAZEN S.L. 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry. 1999;38:3538–3548. doi: 10.1021/bi982401l. [DOI] [PubMed] [Google Scholar]
- WU W., SAMOSZUK M.K., COMHAIR S.A., THOMASSEN M.J., FARVER C.F., DWEIK R.A., KAVURU M.S., ERZURUM S.C., HAZEN S.L. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARDIM-AKAYDIN S., SEPICI A., ÖZKAN Y., TORUN M., SIMSEK B., SEPICI V.Oxidation of uric acid in rheumatoid arthritis: is allantoin a marker of oxidative stress Free Radic. Res. 2004(in press) [DOI] [PubMed]
- YERMILOV V., RUBIO J., OHSHIMA H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- YOSHIDA R., OGAWA Y., KASAI H. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values measured by an ELISA correlated well with measurements by high-performance liquid chromatography with electrochemical detection. Cancer Epidemiol. Biomarkers Prev. 2002;11:1076–1081. [PubMed] [Google Scholar]
- YOSHIDA Y., SHIMAKAWA S., ITOH N., NIKI E. Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic. Res. 2003;37:861–872. doi: 10.1080/1071576031000148736. [DOI] [PubMed] [Google Scholar]
- YOUNG J.F., DRAGSTED L.O., DANESHVAR B., LAURIDSEN S.T., HANSEN M., SANDSTROM B. The effect of grape-skin extract on oxidative status. Br. J. Nutr. 2000a;84:505–513. [PubMed] [Google Scholar]
- YOUNG J.F., NIELSEN S.E., HARALDSDOTTIR J., DANESHVAR B., LAURIDSEN S.T., KNUTHSEN P., CROZIER A., SANDSTROM B., DRAGSTED L.O. Polyphenolic antioxidants in fruit juice. Urinary excretion and effects on biological markers for antioxidative status. Ugeskr. Laeg. 2000b;162:1388–1392. [PubMed] [Google Scholar]
- YUEN J.W.M., BENZIE I.F. Hydrogen peroxide in human urine as a potential biomarker of whole body oxidative stress. Free Radic. Res. 2003;37:1209–1213. doi: 10.1080/10715760310001616032. [DOI] [PubMed] [Google Scholar]
- ZHAO H., JOSEPH J., ZHANG H., KAROUI H., KALYANARAMAN B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001a;31:599–606. doi: 10.1016/s0891-5849(01)00619-0. [DOI] [PubMed] [Google Scholar]
- ZHAO H., KALIVENDI S., ZHANG H., JOSEPH J., NITHIPATIKOM K., VASQUEZ-VIVAR J., KALYANARAMAN B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- ZHAO K., WHITEMAN M., SPENCER J.P., HALLIWELL B. DNA damage by nitrite and peroxynitrite: protection by dietary phenols. Methods Enzymol. 2001b;335:296–307. doi: 10.1016/s0076-6879(01)35252-7. [DOI] [PubMed] [Google Scholar]