Abstract
We examined whether edaravone (Eda), a clinically available radical scavenger, directly protects cardiomyocytes from ischemia/reperfusion (I/R) injury, and whether the timing of its application is critical for protection.
Cardioprotective effects of edaravone were tested in the modified cell-pelleting model of ischemia and under exogenous oxidative stress (hydrogen peroxide: H2O2) in isolated adult rabbit ventricular cells. Cell death and reactive oxygen species (ROS) generation were detected using propidium iodide (PI) and DCFH-DA, respectively. These parameters were evaluated objectively using flow cytometory.
Hypoxia and reoxygenation aggravated the proportion of dead cells from 32.2±1.8% (Baseline) to 51.3±2.7% (Control). When 15 μM edaravone was applied either throughout the entire experiment (Through) or only at reoxygenation (Reox), cell death was significantly reduced to 39.9±1.8% (P<0.01 vs Control) and 43.3±2.5% (P<0.05 vs Control), respectively. In contrast, when edaravone was applied 10 min after reoxygenation, its protective effect disappeared. Cardioprotection by edaravone was more remarkable than that afforded by other free radical scavengers, such as ascorbate and superoxide dismutase (SOD). There is a positive correlation between the cardioprotective effect of edaravone and the extent of ROS reduction.
Edaravone blunted the H2O2-induced changes in electrical properties, and significantly prolonged the time to contracture induced by H2O2 in single ventricular myocytes.
Taken together, edaravone directly protects cardiomyocytes from I/R injury by attenuating ROS production, even when applied at the time of reoxygenation, suggesting that edaravone could be a potent cardioprotective therapeutic agent against hypoxia–reoxygenation injury.
Keywords: Ischemia, cardioprotection, radical scavenger, flow cytometry
Introduction
Treatment of acute myocardial infarction (AMI) has advanced considerably in terms of early reperfusion therapy, due to the development of catheter interventions and thrombolytic procedures. On the other hand, reperfusion therapy after brief ischemia not only triggers lethal arrhythmias and myocardial stunning but also paradoxically aggravates the area of infarction (Hearse, 1991). A number of reports have indicated that reactive oxygen species (ROS) play an important role in reperfusion injury. ROS generation increases not so much during ischemia but more substantially upon reperfusion (Bolli et al., 1988; Takemura et al., 1993; Courtois et al., 1998). With regard to this point, many different radical scavengers have been used both in vivo and in vitro to target this critical moment of ROS burst upon reperfusion (Hearse & Tosaki, 1986; 1987; Kuzuya et al., 1993; McDonald et al., 1999; Hangaishi et al., 2001). However, the cardioprotective effects of antioxidants against ischemia–reperfusion injury are not consistent. One reason for this discrepancy may be the differences among the drugs regarding the accessibility to myocytes during the window of protection. Furthermore, it has been reported that a small redox-dependent signal, derived from ROS preceding ischemia, is cardioprotective, but that large bursts of oxidants contribute to cell death during ischemia and reperfusion (Pain et al., 2000). This paradoxical involvement of ROS may complicate the elucidation of the roles of ROS and radical scavengers in cardioprotection.
Recently, 3-methyl-1-1phenyl-2-pyrazolin-5-one (edaravone), a newly developed free radical scavenger, has been applied clinically for cerebral infarction, and its neuroprotective effects are indisputable, even when applied after the onset of cerebral ischemia (Watanabe et al., 1994; Yoneda et al., 2003). With regard to the cardioprotective effects of this drug, Wu et al. (2002) demonstrated that, in an in vivo rabbit model, a single bolus injection of edaravone applied upon reperfusion, after a prolonged period of ischemia, reduced infarct size without significant hemodynamic changes. These findings indicate that edaravone combined with early reperfusion therapy would be beneficial for treatment of AMI. However, the precise mechanism of cardioprotection afforded by edaravon remains unknown. Therefore, in the present study, we attempted to clarify whether edaravone directly protects cardiomyocytes from ischemia/reperfusion (I/R) injury by attenuating intracellular ROS generation, and whether the timing of its application is critical for protection. Furthermore, we determined whether edaravone induces cell-protection from exogenous oxidative stress-induced injury.
Methods
All procedures were performed in accordance with the Tottori University animal care guidelines, which confirm to the Guide for the Care and Use of Laboratory Animal (NIH publication No. 85-23, revised 1985). Male New Zealand white rabbits (1.3–1.6 kg) were anesthetized by intravenous injection of pentobarbitone (30 mg kg−1). After confirming the absence of a corneal reflex, hearts were rapidly excised and mounted on a Langendorff apparatus. Ventricular myocytes were isolated by conventional enzymatic dissociation, as described previously (Sasaki et al., 2000; 2003; Sato et al., 2000). Collected cells were suspended with M-199 buffer (Nissui, Tokyo, Japan) supplemented with antibiotics (penicillin–streptomycin 0.01 mg ml−1) and 5% fetal bovine serum. Cells were then filtered through nylon mesh and washed several times with M-199. Tyrode's solution contains (in mM) NaCl 140, KCl 4, MgCl2 1.0, HEPES 10, CaCl2 1.0, and glucose 11.0 (adjusted to pH 7.4 with NaOH).
Modified cell pelleting model of ischemia
We modified a conventional cell pelleting model reported by Vander Heide et al. (1990) to assess hypoxia- and reoxygenation-induced cell death. In brief, an aliquot of each cell suspension in M-199 (0.5 ml) was placed into a microcentrifuge tube and centrifuged at 80 × g for 60 s into a pellet. An aliquot (0.2 ml) of the supernatant was removed to leave a thin layer above the pellet, and mineral oil (0.2 ml) was layered on top of the pellet to prevent gaseous diffusion. After incubation of the pellet for 60 min at 37°C, cells were fully reoxygenated by gently pipetting with normoxic Tyrode's solution including propidium iodide (PI, 1 μg ml−1). Cells stained by PI (‘PI positive') were determined to be dead cells and were expressed as a percentage of the total cells, as assessed by flow cytometry (EPICS XL, Beckman Coulter, USA) (>30,000 cells for each sample) (Vanden Hoek et al., 2000; Akao et al., 2003). In a subset of experiments to study ROS generation, cells were loaded with 10 μM 2′ 7′-dichlorofluorescin diacetate (DCFH-DA) for 15 min before pelleting at room temperature in the dark. To eliminate the influence of intracellular accumulation of DCF during pelleting, cells were washed twice with Tyrode's solution prior to incubation with M-199. PI and DCF fluorescence (FR) were detected using conventional flow cytometry. Adult rabbit cardiomyocytes would appear to be too large for flow cytometry, but we were able to overcome the problems of stuck cells in the flow circuit by filtering cell suspension several times, especially after changing to a Ca2+-containing solution. For quantification of the generation of ROS, DCF FR was evaluated only in the PI-negative cells, as the DCF dye leaks from cells if the plasma membrane is not intact. Flow cytometry data were analyzed using WinMDI software.
Experimental protocol for the pelleting model and exogenous oxidant stress
Four groups were mainly studied in the pelleting experiments. All cells were subjected to 60 min of pelleting followed by 30 min of reoxygenation. In the control group, cells were pelleted, reoxygenated, and then sampled. In the edaravone-throughout group (Through), edaravone (1.5–1000 μM) was applied 15 min prior to pelleting and was maintained throughout the experiment. In the edaravone-reoxygenation group (Reox), edaravone (1.5–1000 μM) was applied upon reoxygenation. In the edaravone-late group (Reox-late), 15 μM edaravone was applied 10 min after reoxygenation. In separate experiments, additional well-known radical scavengers, ascorbic acid (100 μM) and SOD (80 U ml−1) (Mickle et al., 1990), were also applied as reference drugs, either throughout the experiment or only upon reoxygenation.
To examine the cardioprotective effects against exogenous oxidants, cell viability and intracellular ROS levels were assessed after exposure to hydrogen peroxide (H2O2; 20–1000 μM) for 60 min at 37°C in the absence or presence of edaravone. Cell death and ROS were evaluated using flow cytometry, as described above.
Electrophysiological experiment
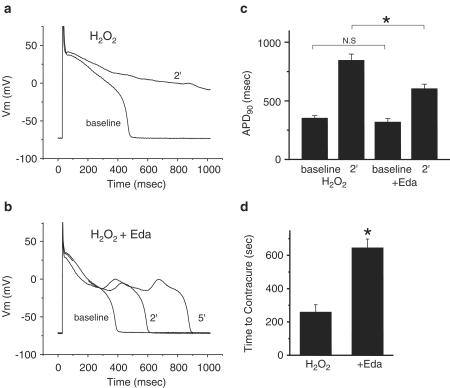
Ac tion potentials of single ventricular cells stimulated at 1 Hz were recorded under the current clamp condition (pCLAMP 9, Axon Instruments, USA) during exposure to H2O2 in the absence or presence of 100 μM edaravone. Exposure to H2O2 induced action potential duration (APD) prolongation, eventually resulting in cell contracture. The extent of the APD prolongation, 2 min after exposure to H2O2, was used as an index of oxidant-induced changes in electrical property (Figure 6a–c). The morphological changes of the ventricular cells were determined microscopically, and the time to contracture after exposure to H2O2 was measured as an index of oxidant-induced cell injury (Figure 6d). To eliminate the influence of intracellular dialysis with the pipette solution, we employed a perforated patch clamp technique with amphotericin B (600 μg ml−1) to record action potentials (Sasaki et al., 1999). The pipette solution contained (in mM) K-glutamate 120, KCl 25, MgCl2 1, EGTA 10, HEPES 10 (pH 7.2 with KOH).
Figure 6.
H2O2-induced changes in electrical property and morphological changes in a single beating ventricular myocyte. Representative traces recorded 2 and 5 min after exposure to H2O2 are indicated in the absence (a) or presence of edaravone (b). Summarized data for APD90 is shown in panel (c). In panel (d), summarized data for the time to contracture in the absence (left) or presence of edaravone (right).
Chemicals
Edaravone was kindly provided by Mitsubishi Pharma Corporation (Tokyo, Japan), ascorbate, SOD, and H2O2 were obtained from Sigma Chemical (MO, USA), and PI and DCFH-DA were from Molecular Probes Inc. (OR, USA). M-199 cell culture media were purchased from GIBCO (CA, USA). Stock solutions of edaravone at 100 mM were prepared by dissolving in DMSO. The final concentration of DMSO was <0.3%. Amphotericin B was purchased from Wako Chemical (Tokyo, Japan) and dissolved in DMSO to make a stock solution (60 mg ml−1) and was adjusted to the final concentration before use.
Data analysis
Pooled data are presented as mean±s.e.m., and the number of cells or experiments is shown as N. Statistical comparisons were evaluated by one-way ANOVA or paired student t-test, with a value of P<0.05 considered significant.
Results
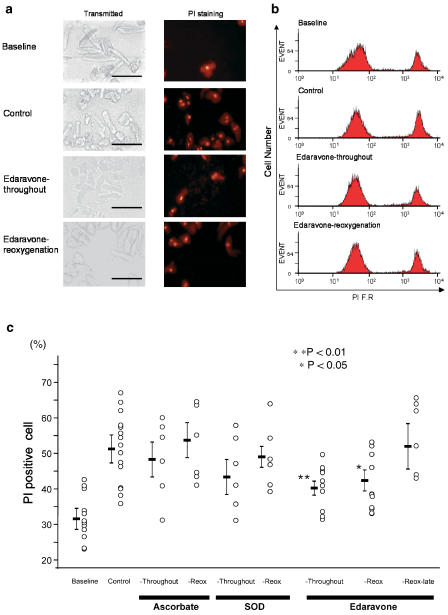
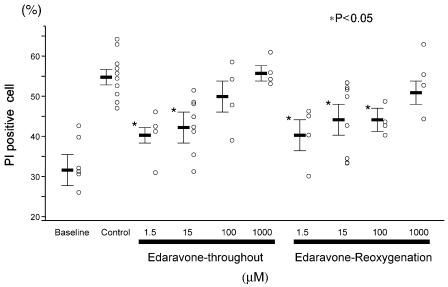
Figure 1a presents representative data indicating the morphological changes induced by the modified pelleting model in transmitted image (left) and PI staining (right). At baseline, some cells were ‘PI-positive' without pelleting due to the cell isolation procedure; the mean value for % PI-negative cells was 67.8%. Representative distributions of the red FR intensity after PI staining are shown in Figure 1b. In the control cells, pelleting increased cell death from 28.3 to 39.7%. Edaravone applied either throughout the experiment (Throughout) or at the time of reoxygenation (Reox) attenuated cell death to 31.0 and 33.4%, respectively. As shown in the summarized data (Figure 1c), the well-known radical scavengers (Mickle et al., 1990), ascorbate (Throughout: 47.9±5.1%, Reox: 53.4±4.8% vs Control: 51.3±2.7%) and SOD (Throughout: 43.2±4.8%, Reox: 48.8±3.0%), tended to reduce the cell death but did not show any statistically significant differences. On the other hand, 15 μM edaravone applied either throughout the experiment or upon reoxygenation significantly attenuated the cell injury (39.9±1.8%, P<0.01 vs Control, and 43.3±2.5%, P<0.05 vs Control, respectively). However, the cardioprotective effects of edaravone did not occur when it was applied 10 min after reoxygenation. In the next series of experiments, the dose–response relationships for the cardioprotective effects of edaravone were examined. As shown in Figure 2, 1.5, 15, and 100 μM edaravone applied at the time of reoxygenation exhibit cardioprotective effects, but edaravone applied throughout the experiment at relatively high concentrations (100 and 1000 μM) was not cardioprotective. Although the cause of the disappearance of the cardioprotective effect of edaravone at high concentration is unknown, we confirmed that 100 and 1000 μM edaravone applied alone, under normoxic conditions, without pelleting, did not change cell viability (data not shown). These results indicate that edaravone is a potent cardioprotective agent against hypoxia– reoxygenation injury.
Figure 1.
(a) Representative data showing the morphological changes induced by pelleting in transmitted images (left) and FR images (right), respectively. Inserted bars indicate 100 μm, respectively. (b) Distribution of red FR (610 nm) as detected by flow cytometry in each group. (c) Summarized data for cardioprotection afforded by ascorbate, SOD, and each edaravone group in the cell pelleting model, respectively.
Figure 2.
Summarized data for the relationships between the doses of edaravone and cell death induced by hypoxia and reoxygenation.
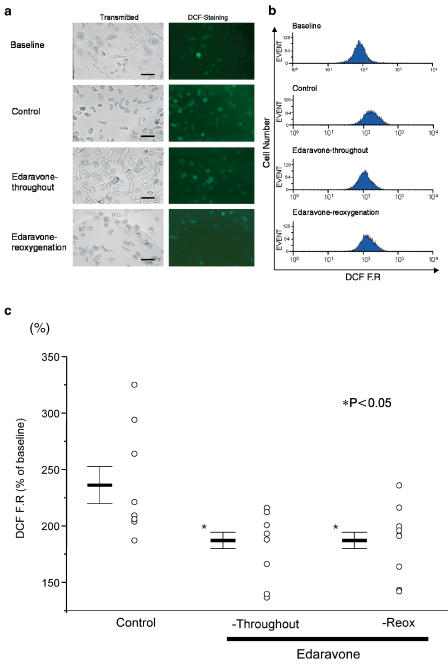
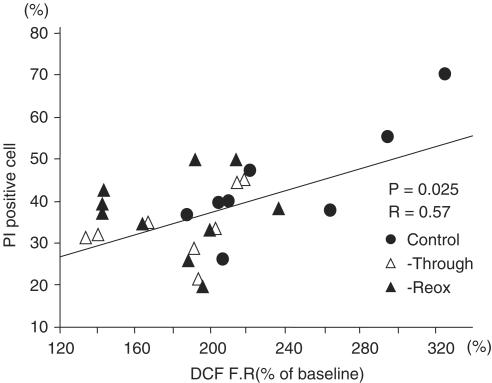
We subsequently examined whether the cardioprotective effects of edaravone are actually mediated by attenuating ROS generation. Figure 3a demonstrates that some cells exhibit green FR at baseline, but that pelleting remarkably increased the FR intensity in the majority of cells. Edaravone (15 μM) applied either throughout the experiment or upon reoxygenation blunted the morphological changes induced by pelleting–reoxygenation, and apparently decreased DCF FR. As shown in Figure 3b, the pelleting-induced right shift of DCF distribution was attenuated by edaravone when applied either throughout or at the time of reoxygenation. The summarized data presented in Figure 3c indicates that edaravone significantly decreased the ROS generation induced by hypoxia–reoxygenation. However, either SOD or ascorbate tended to reduce ROS generation, but did not show statistically significant differences (data not shown). Figure 4 presents the relationships between the proportion of PI positive cells and the increase in DCF FR (% of baseline) in the three groups: pelleting, Eda-Throughout, and Eda-Reox. There is a positive correlation between these parameters (R=0.57, P<0.01), suggesting that the elevation of ROS increased cell death independently of the experimental groups. We then tried to determine whether the cardioprotective effects of edaravone were actually related to the decreased production of ROS. For this purpose, each parameter was calculated by the following formula:
Figure 3.
Representative data for pelleting-induced production of ROS, as detected by DCF FR using flow cytometry. Transmitted images and FR images are indicated in panels (a) and (b), respectively. Inserted bars indicate 100 μm. Note that edaravone attenuated the morphological changes recognized in transmitted images, and blunted the enhancement of green FR. In panel (c), the summarized data showing that edaravone applied either throughout or at the time of reoxygenation, attenuates ROS generation. DCF FR=mean DCF fluorescence in each sample/mean DCF FR at baseline × 100.
Figure 4.
Relationship between ROS generation and proportion of PI positive cells in control and both edaravone groups.
There is also positive correlation between Cell protection and ROS reduction in both edaravone-treated groups (Eda-Through: R=0.65, P<0.05, N=7; Eda-Reox: R=0.91, P<0.05, N=5), indicating that the cardioprotective effects of edaravone against hypoxia–reoxygenation injury are mediated by decreasing the generation of ROS.
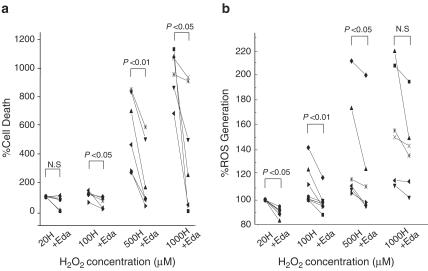
We subsequently examined whether edaravone protects cardiomyocytes from exogenous oxidant stress. In preliminary experiments (data not shown), both 1.5 and 15 μM edaravone failed to prevent H2O2 (0.5 and 1 mM)-induced cell injury, while 100 μM edaravone exerted protective effects. Therefore, 100 μM edaravone was used in this series of experiments. Figure 5a presents the H2O2-induced cell death at various concentrations from 20 to 1000 μM in the absence or presence of 100 μM edaravone. Exposure (60 min) to H2O2 resulted in cell death in a dose-dependent manner, while co-application of edaravone significantly attenuated the H2O2-induced cell death. Figure 5b presents the H2O2-induced increase in DCF FR in the absence or presence of edaravone. Edaravone significantly blunted the increase in DCF FR induced by H2O2, indicating that edaravone attenuated the exogenous oxidant-induced cell injury.
Figure 5.
(a) H2O2-induced cell death in the absence or presence of 100 μM edaravone. Each value is expressed as a % and is normalized by assuming that the extent of 20 μM H2O2-induce cell death is 100%. % cell death=(% of PI negative cells at baseline−% of PI negative cells in each sample)/(% of PI negative cells at baseline−% of PI negative cells in 20 μM H2O2) × 100. In panel (b), H2O2-induced intracellular ROS levels detected by DCF, in the absence or presence of 100 μM edaravone. Each value is expressed as a %, assuming that the 20 μM H2O2-induced increase in DCF FR is 100%. H: H2O2, +Eda: with edaravone.
We then attempted to examine the protective effects of edaravone against exogenous oxidant stress in beating myocytes. As previously reported by Cerbai et al. (1991), initial exposure to H2O2 caused action potential prolongation with early after-depolarization. Contraction was augmented regardless of the presence of edaravone, but eventually cells went into a state of contracture. Some cells became unresponsive due to activation of IK, ATP before contracture (Cerbai et al., 1991). As shown in Figure 6a,b, H2O2 prolonged the APD90 with prolongation of the plateau phase. The extent of APD prolongation 2 min after exposure to H2O2 was used as an index of the oxidative stress-induced changes in the electrical properties (Figure 6c). H2O2 prolonged the APD90 from 351±24 to 850±51 ms, while co-application of edaravone significantly inhibited the H2O2-induced prolongation of APD90 (from 321±29 to 606±42 ms, P<0.01). The time to contracture after exposure to H2O2 was used as an index of oxidant-induced cell injury. As shown in Figure 6d, co-application of edaravone significantly prolonged the time to contracture. These results indicate that edaravone protects beating cells from exogenous oxidative stress, as previously observed in quiescent cells.
Discussion
The present study demonstrated that edaravone, a recently developed potent radical scavenger, exerted direct cardioprotection from I/R injury, even when administered at the time of reoxygenation. We modified the cell-pelleting model of ischemia, originally proposed by Vander Heide et al. (1990), to examine the cardioprotective effects of edaravone. These investigators demonstrated that cardiomyocytes exposed to hypoxia in the pelleting tubes were similar to those observed in other well-documented models of in vivo and in vitro ischemia, in terms of cellular metabolism and morphology. The cardioprotective effects of edaravone were more remarkable than that of other well-known antioxidants, such as ascorbate and SOD, in our pelleting model, and was mediated by reducing intracellular generation of ROS. Edaravone also alleviated the cell injury induced by exogenous oxidative stress (H2O2). These findings suggest that the cardioprotective effects of edaravone are consistent with the results in an in vivo rabbit model (Wu et al., 2002) and eliminate concerns regarding species differences (Minhaz et al., 1996). Regarding the generation of ROS upon reperfusion, Blasig et al. (1990) reported that the formation of ROS reached a maximum level about 3 min after reperfusion in the isolated perfused heart model using an ESR spin trapping procedure, and that the initial amount of ROS showed an inverse correlation with the degree of restoration of heart function within 30 min of reperfusion. Kuzuya et al. (1993) demonstrated that the degree of ROS generation correlated positively with the percentage of dead cells in canine cardiac myocytes cultured under hypoxia–reoxygenation conditions, and that the application of radical scavengers during reoxygenation attenuated both cell death and ROS generation. In the present study, when edaravone was applied 10 min after reoxygenation, the cardioprotective effects disappeared. These findings support the concept that significant production of ROS at a critical moment during reoxygenation plays an important role in I/R injury (Hearse & Tosaki, 1986; Hearse, 1991; Pain et al., 2000; Vanden Hoek et al., 2000). Therefore, delayed application of edaravone might abrogate its cardioprotective effects.
Most in vitro studies have demonstrated that antioxidants given at the time of reoxygenation is sufficient to afford cardioprotection against hypoxia–reoxygenation injury (Qian et al., 1997; Vanden Hoek et al., 2000; Sorescu & Griendling, 2002). By contrast, the desirable effects of antioxidants on ischemia–reperfusion injury in vivo remain controversial (Tamura et al., 1988; Tanaka et al., 1990). It has been reported that superoxide dismutase (SOD) can protect cultured rat cardiac myocytes against hypoxia–reoxygenation injury (Qian et al., 1997), but that SOD could not limit myocardial infarct size in an in vivo ischemia–reperfusion model in dogs (Tanaka et al., 1990). Furthermore, in a clinical trial, recombinant human SOD administered before successful percutaneous transluminal coronary angioplasty, failed to improve recovery of ventricular function in patients with AMI (Flaherty et al., 1994). Although the precise reason remains unknown, this discrepancy can be explained by the mechanism by which any delay in the delivery of the drug to the site of oxidant generation will likely attenuate the extent of protection, by allowing unchecked oxidant stress to begin (Vanden Hoek et al., 2000; Kloner & Jennings, 2001). Regarding the site of intracellular ROS generation upon reoxygenation, the mitochondrial electron-transport chain is considered to be a primary source (Vanden Hoek et al., 1997; 2000; Sorescu & Griendling, 2002). Intracellular ROS levels increase 10-fold during reoxygenation, and the extent of this increase correlates closely with cell death (Sorescu & Griendling, 2002). Therefore, it is likely that SOD is unable to reach the intracellular site of ROS generation during the narrow window for protection, when applied upon reperfusion in vivo, due to its low membrane permeability; thus, resulting in the discrepancy between in vivo and in vitro model. McDonald et al. (1999) demonstrated that the membrane-permeable radical scavenger tempol reduced myocardial infarct size in rats and rabbits. These mixed findings among studies indicate that accessibility of a drug to the site of oxidant generation, before the onset of significant oxidative stress, appears to be essential for salvaging cardiomyocytes from I/R injury.
Edaravone, which is lipophilic and is rapidly accessible to intracellular space (Watanabe et al., 1994), has been used clinically to treat patients with cerebral infarction in Japan (Yoneda et al., 2003). The primary mechanism of this drug for cell protection was considered to be through direct scavenging of OH· and through the inhibition of lipoxygenase activity and 15-HPETE, which have been reported to play an important role in cardiac ischemia–reperfusion injury (Kuzuya et al., 1993). Edaravone also directly neutralizes peroxy radicals (LOO−), but does not scavenge O2−·(Watanabe et al., 1994). However, strong suppression of the arachidonate cascade eventually causes O2− reduction and diminishes iron-dependent lipid peroxidation. A number of experimental and clinical studies have shown a protective effect of edaravone against ischemic injury in brain and liver even when administered after the onset of ischemia (Okatani et al., 2003). Okatani et al. (2003) reported that, in their hepatic ischemia reperfusion model, edaravone protected against the I/R induced-impairment of cellular function, including inhibition of mitochondrial respiration, swelling, and lipid peroxidation. Rajesh et al. (2003) demonstrated that the PT pore inhibiting property of edaravone was specific for the attenuation of cytochrome c release, thereby pathological apoptosis was decreased in rat hearts in vivo. These findings suggest that the cardioprotective effects of edaravone are possibly due to protection of the mitochondria, the main source of energy production and ROS generation (Vanden Hoek et al., 1997; 2000; Sorescu & Griendling, 2002). In our model, the superiority of edaravone seemed to depend upon the ability to reduce ROS generation and the improved membrane permeability compared to the other reference antioxidants. It remains possible that edaravone exerted its cardioprotective effects by targeting the mitochondoria at the time of reoxygenation. However, further studies are necessary to evaluate this mechanism.
Radical scavengers that provide cardioprotection are not presently in clinical use. Edaravone, which is a clinically available radical scavenger, has previously demonstrated a virtual absence of serious toxicity in many studies (Otomo et al., 1998; Shibata et al., 1998). Plasma concentrations of edaravone were reported to be ∼6 μM in a phase I clinical trial. Our in vitro findings suggest that clinically relevant doses of edaravone could be helpful for salvaging myocytes from ischemia reperfusion injury, if the drug reaches the myocytes during the window of protection.
In conclusion, edaravone is a potent cardioprotective agent against ischemia reperfusion injury in both in vitro and in vivo animal models. These findings indicate that edaravone might be helpful as an adjuvant therapy for early reperfusion procedure in AMI.
Acknowledgments
We thank Ms Noriko Kamei, Ms Yoshiko Oda, and Ms Maki Kameda for their secretarial and technical support.
Abbreviations
- AMI
acute myocardial infarction
- APD
action potential duration
- DCFH-DA
2′ 7′-dichlorofluorescin diacetate
- Eda
Edaravone
- FR
fluorescence
- H2O2
hydrogen peroxide
- I/R
ischemia/reperfusion
- ROS
reactive oxygen species
- Reox
edaravone-reoxygenation group
- SOD
superoxide dismutase
- Through
edaravone-throughout group
References
- AKAO M., O'ROURKE B., TESHIMA Y., SEHARASEYON J., MARBÁN E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ. Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- BLASIG E.I., EBERT B., HENNIG C., PALI T., TOSAKI A. Inverse relationship between ESR spin trapping of oxyradicals and degree of functional recovery during myocardial reperfusion in isolated working rat heart. Cardiovasc Res. 1990;24:263–270. doi: 10.1093/cvr/24.4.263. [DOI] [PubMed] [Google Scholar]
- BOLLI R., PATEL B.S., JEROUDI M.O., LAI E.K., MCCAY P.B. Demostration of free radical generation in ‘stunned'myocardium of intact dogs with the use of the spin trap(-phenylN-tertbuty nitrone) J. Clin. Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERBAI E., AMBROSIO G., PORCIATTI F., CHIARIELLO M., GIOTTI A., MUGELLI A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- COURTOIS M., MAUPOIL V., FANTINI E., DUROT I., JAVOUHEY-DONZEL A., ATHIAS P., GRYNBERG A., ROCHETTE L. Correlation between direct ESR spectroscopic measurements and electromechanical and biochemical assessment of exogenous free radical injury in isolated rat cardiac myocytes. Free Radical Biol Med. 1998;24:121–131. doi: 10.1016/s0891-5849(97)00167-6. [DOI] [PubMed] [Google Scholar]
- FLAHERTY J.T., PITT B., GRUBER J.W., HEUSER R.R., ROTHBAUM D.A., BURWELL L.R., GEORGE B.S., KEREIAKES D.J., DEITCHMAN D., GUSTAFSON N. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- HANGAISHI M., NAKAJIMA H., TAGUCHI J., IGARASHI R., HOSHINO J., KUROKAWA K., KIMURA S., NAGAI R., OHNO M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia–reperfusion injury in rat in vivo. Biochem Biophys Res Commun. 2001;285:1220–1225. doi: 10.1006/bbrc.2001.5319. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J. Reperfusion-induced injury: a possible role for oxidant stress and its manipulation. Cardiovasc Drugs Therapy. 1991;5:225–236. doi: 10.1007/BF00054745. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., TOSAKI A. Free radical and reperfusion-induced arrhythmias: protection by spin trap agent PBN in the rat heart. Circ. Res. 1986;60:375–383. doi: 10.1161/01.res.60.3.375. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., TOSAKI A. Reperfusion-induced arrhythmias and free radicals: studies in the rat heart with DMPO. J Cardiovasc Pharmacol. 1987;9:641–650. doi: 10.1097/00005344-198706000-00002. [DOI] [PubMed] [Google Scholar]
- KLONER R.A., JENNINGS R.B. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- KUZUYA T., HOSHIDA S., KIM Y., OE H., HORI M., KAMADA T., TADA M. Free radical generation coupled with arachidonate lipoxygenase reaction relates to reoxygenation induced myocardial cell injury. Cardiovasc. Res. 1993;27:1056–1060. doi: 10.1093/cvr/27.6.1056. [DOI] [PubMed] [Google Scholar]
- MCDONALD M.C., ZACHAROWSKI K., BOWES J., CUZZOCREA S., THIEMERMANN C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radical Biol. Med. 1999;27:493–503. doi: 10.1016/s0891-5849(99)00100-8. [DOI] [PubMed] [Google Scholar]
- MICKLE D.A.G., LI R., WEISEL R.D., TUMIATI L.C., WU TW. Water-soluble antioxidant specificity against free radical injury using cultured human ventricular myocytes and fibroblasts and saphenous vein endothelial cells. J. Mol. Cell Cardiol. 1990;22:1297–1304. doi: 10.1016/0022-2828(90)90065-a. [DOI] [PubMed] [Google Scholar]
- MINHAZ U., TANAKA M., TSUKAMOTO H., WATANABE K., KOIDE S., SHOHTSU A., NAKAZAWA H. Effect of MCI-186 on postischemic reperfusion injury in isolated rat heart. Free Radical Res. 1996;24:361–367. doi: 10.3109/10715769609088034. [DOI] [PubMed] [Google Scholar]
- OTOMO E., TOHGI H., KOGURE K., HIRAI S., TERASHI A., GOTOH F., SZABO I., ITO E., SAWADA T., KOBAYASHI S., FUJISHIMA M., NAKASHIMA M. Clinical efficacy of a free radical scavenger, MCI-186 on acute cerebral infarction-earlyb phase II clinical trial. Ther. Res. 1998;19:1311–1331. [Google Scholar]
- OKATANI Y., WAKATSUKI A., ENZAN H., MIYAHARA Y. Edaravone protects against ischemia/reperfusion-induced oxidative damage to mitochondria in rat liver. Eur. J. Pharmacol. 2003;465:163–170. doi: 10.1016/s0014-2999(03)01463-8. [DOI] [PubMed] [Google Scholar]
- PAIN T., YANG X.M., CRITZ S.D., YUE Y., NAKANO A., LIU G.S., HEUSCH G., COHEN M.V., DOWNEY J.M. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- QIAN Z.M., XU M.F., TANG P.L. Superoxide dismutase does protect the cultured rat cardiac myocytes against hypoxia/reoxygenation injury. Free Radical Res. 1997;27:13–21. doi: 10.3109/10715769709097833. [DOI] [PubMed] [Google Scholar]
- RAJESH K.G., SASAGURI S., SUZUKI R., MAEDA H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and up regulates Bcl-2 expression. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2171–H2178. doi: 10.1152/ajpheart.00143.2003. [DOI] [PubMed] [Google Scholar]
- SASAKI N., MITSUIYE T., NOMA A., POWELL T. Sarcomere length during contraction of isolated guinea-pig ventricular myocytes. Pflugers. Arch. 1999;437:804–811. doi: 10.1007/s004240050849. [DOI] [PubMed] [Google Scholar]
- SASAKI N., MURATA M., GUO Y., JO S.H., OHLER A., AKAO M., O'ROURKE B., XIAO R.P., BOLLI R., MARBÁN E. MCC-134, a single pharmacophore, opens surface ATP-sensitive potassium channels, blocks mitochondrial ATP-sensitive potassium channels, and suppresses preconditioning. Circulation. 2003;107:1183–1188. doi: 10.1161/01.cir.0000051457.64240.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI N., SATO T., OHLER A., O'ROURKE B., MARBÁN E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- SATO T., SASAKI N., O'ROURKE B., MARBÁN E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning. Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- SHIBATA H., ARAI S., IZAWA M., MURASAKI M., TAKAMATSU Y., IZAWA O., TAKAHASHI C., TANAKA M. Phase I clinical study of MCI-186(Edaravone, 3-methyl-1-phenyl-2-pyrazolin-5-one)in healthy volunteers:safty and pharmacokinetics of single and multiple administrations. Jpn. J. Clin. Pharmacol. Ther. 1998;29:863–876. [Google Scholar]
- SORESCU D., GRIENDLING K.K. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Failure. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- TAKEMURA G., ONODERA T., MILLARD R.W., ASHRAF M. Demonstration of hydroxyl radical and its role in hydrogen peroxide-induced myocardial injury – hydroxyl radical dependent and independent mechanism. Free Radical Biol. Med. 1993;15:13–25. doi: 10.1016/0891-5849(93)90121-a. [DOI] [PubMed] [Google Scholar]
- TAMURA Y., CHI L.G., DRISCOLL E.M., JR., HOFF P.T., FREEMAN B.A., GALLAGHER K.P., LUCCHESI B.R. Superoxide dismutase conjugated to polyethylene glycol provides sustained protection against myocardial ischemia/reperfusion injury in canine heart. Circ. Res. 1988;63:944–959. doi: 10.1161/01.res.63.5.944. [DOI] [PubMed] [Google Scholar]
- TANAKA M., STOLER R.C., FITZHARRIS G.P., JENNINGS R.B., REIMER K.A. Evidence against the ‘early protection-delayed death' hypothesis of superoxide dismutase therapy in experimental myocardial infarction. Polyethylene glycol-superoxide dismutase plus catalase does not limit myocardial infarct size in dogs. Circ. Res. 1990;67:636–644. doi: 10.1161/01.res.67.3.636. [DOI] [PubMed] [Google Scholar]
- VANDEN HOEK T., BECKER L.B., SHAO Z.H., LI C.Q., SCHUMACKER P.T. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ. Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- VANDEN HOEK T.L., SHAO Z., LI C., SCHUMACKER P.T., BECKER L.B. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J. Mol. Cell Cardiol. 1997;29:2441–2450. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- VANDER HEIDE R.S., RIM D., HOHL C.M., GANOTE C.E. An in vitro model of myocardial ischemia utilizing isolated adult rat myocytes. J. Mol. Cell Cardiol. 1990;22:165–181. doi: 10.1016/0022-2828(90)91113-l. [DOI] [PubMed] [Google Scholar]
- WATANABE T., YUKI S., EGAWA M., NISHI H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994;268:1597–1604. [PubMed] [Google Scholar]
- WU T.W., ZENG L.H., WU J., FUNG K.P. Myocardial protection of MCI-186 in rabbit ischemia–reperfusion. Life Sci. 2002;71:2249–2255. doi: 10.1016/s0024-3205(02)01965-3. [DOI] [PubMed] [Google Scholar]
- YONEDA Y., UEHARA T., YAMASAKI H., KITA Y., TABUCHI M., MORI E. Hospital-based study of the care and cost of acute ischemic stroke in Japan. Stroke. 2003;34:718–724. doi: 10.1161/01.STR.0000056171.55342.FF. [DOI] [PubMed] [Google Scholar]