Abstract
The influence of sodium ion substitutes on the 5-hydroxytryptamine (5-HT)-induced flux of the organic cation [14C]guanidinium through the ion channel of the mouse 5-HT3 receptor and on the competition of 5-HT with the selective 5-HT3 receptor antagonist [3H]GR 65630 was studied, unless stated otherwise, in mouse neuroblastoma N1E-115 cells.
Under physiological conditions (135 mM sodium), 5-HT induced a concentration-dependent [14C]guanidinium influx with an EC50 (1.3 μM) similar to that in electrophysiological studies.
The stepwise replacement of sodium by increasing concentrations of the organic cation hydroxyethyl trimethylammonium (choline) concentration dependently caused both a rightward shift of the 5-HT concentration–response curve and an increase in the maximum effect of 5-HT. Complete replacement of sodium resulted in a 34-fold lower potency of 5-HT and an almost two times higher maximal response. A low potency of 5-HT in choline buffer was also observed in other 5-HT3 receptor-expressing rodent cell lines (NG 108-15 or NCB 20).
Replacement of Na+ by Li+ left the potency and maximal effects of 5-HT almost unchanged. Replacement by tris (hydroxymethyl) methylamine (Tris), tetramethylammonium (TMA) or N-methyl-D-glucamine (NMDG) caused an increase in maximal response to 5-HT similar to that caused by choline. The potency of 5-HT was only slightly reduced by Tris, to a high degree decreased by TMA (comparable to the decrease by choline), but not influenced by NMDG.
The potency of 5-HT in inhibiting [3H]GR65630 binding to intact cells was 35-fold lower when sodium was completely replaced by choline, but remained unchanged after replacement by NMDG.
The results are compatible with the suggestion that choline competes with 5-HT for the 5-HT3 receptor; the increase in maximal response may be partly due to a choline-mediated delay of the 5-HT-induced desensitization. For studies of 5-HT-evoked [14C]guanidinium flux through 5-HT3 receptor channels, NMDG appears to be an ‘ideal' sodium substituent since it increases the signal-to-noise ratio without interfering with 5-HT binding.
Keywords: Mouse 5-HT3 receptor, tracer flux, [3H]GR65630 binding, sodium substitutes, [14C]guanidinium
Introduction
The 5-HT3 receptor channel is the only 5-hydroxytryptamine (5-HT) receptor subtype that belongs to the superfamily of ligand-gated ion channels (Derkach et al., 1989; Kilpatrick et al., 1990; Maricq et al., 1991). It is permeable to sodium, potassium and calcium, and it is closely related to the nicotinic acetylcholine receptor. A characteristic feature of the 5-HT3 receptor is its rapid desensitization (Jackson & Yakel, 1995; Reeves & Lummis, 2002). Physiologically, 5-HT3 receptors are involved in emesis and pain (Aapro, 1991; Mitchelson, 1992; Karim et al., 1996; Voog et al., 2000; Simpson et al., 2000). Several classes of drugs, including general anaesthetics (Barann et al., 2000), alcohols (Lovinger and Zhou, 1994; Barann et al., 1995) and cannabinoids (Fan, 1995; Barann et al., 2002), are assumed to modulate directly 5-HT3 receptor function.
Several cell lines are available for the study of native 5-HT3 receptors, including mouse neuroblastoma cells of the clone N1E-115 (Barann et al., 1993), NCB 20 mouse neuroblastoma × chinese hamster hybrid cells (Hellevuo et al., 1991) and NG108-15 rat neuroblastoma × glioma cells (Reiser & Hamprecht, 1989). In addition, in the mouse cells, a mixture of the long and short, mouse-specific 5-HT3A receptor splice variants may occur (Werner et al., 1994).
As an alternative to electrophysiological techniques, measurement of the flux of the radioactively labelled organic cation [14C]guanidinium, which behaves like a sodium ion (Reith, 1990), is a useful method to investigate the effects of drugs on channel function. Various groups have unanimously shown that this method is suitable to characterize the 5-HT3 receptor-mediated cation influx in neural cell lines (Reiser & Hamprecht, 1989; Emerit et al., 1993; Bönisch et al., 1993). However, the potencies of 5-HT in inducing influx were markedly different; thus, the EC50 value of 5-HT reported by Bönisch et al. (1993) was about 10 times higher than the values reported by the other groups. This discrepancy appears to be related to the fact that Bönisch et al. (1993), in contrast to others, used hydroxyethyl trimethylammonium (choline) to replace sodium. This buffer condition, under which the 5-HT-mediated flux was sensitive to the selective 5-HT3 receptor antagonist ondansetron but not to tetrodotoxin was applied for the study of drug effects on 5-HT3 receptors and sodium channels (stimulation with veratridine), because it resulted in larger specific ion fluxes, thus facilitating the analysis of the drug effects (Barann et al., 1993).
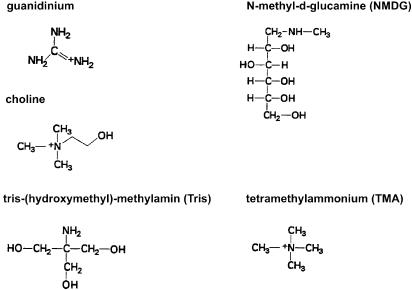
The mechanism by which the cation influx through the 5-HT3 receptor channel is increased, when sodium is replaced by the quarternary organic cation choline, is still unknown. Therefore, the main aim of the present study was to elucidate the mechanism underlying the increase in maximum 5-HT-induced cation influx and the reduction in the potency of 5-HT under the choline buffer condition. In this context, we examined whether other sodium substitutes such as lithium, tris(hydroxyethyl)methylamine (Tris), tetramethyl ammonium (TMA) and N-methyl-D-glucamine (NMDG) differ from choline in this respect. The chemical structure of all cations applied in this study is shown in Figure 1 (in case of guanidinium, the unlabelled compound is shown).
Figure 1.
Chemical structures of the organic cations used in this study to replace sodium. NMDG (pka=9.5) and Tris (pka=8.3) are positively charged at physiological pH. The ion diameters (Angstrom units, three dimensions) are as follows: sodium 1.924, 1.924 and 1.924; choline 7.487, 5.020 and 4.488; guanidinium 6.498, 4.952 and 3.103; NMDG 13.056, 5.582 and 5.332; TEA 8.431, 6.358 and 5.841; TMA 5.291, 4.824 and 4.556; and Tris 7.644, 5.712 and 4.491. The values for lithium are 1.198, 1.198 and 1.198.
Methods
Cell culture
Mouse neuroblastoma cells of the clone N1E-115 (Amano et al., 1972; passage numbers 40–50) were grown in Dulbecco's modified Eagle's medium (DMEM) with HEPES (7.6 mM) and sodium bicarbonate (30 mM). The growth medium was supplemented (as described by Hoyer & Neijt, 1988) with the antibiotics penicillin (100 IU ml−1) and streptomycin (100 μg ml−1), 10% fetal calf serum (Gibco BRL) and the following amino-acid mix (mM): L-cysteine hydrochloride (0.3), L-alanine (0.4), L-asparagine (0.45), L-aspartic acid (0.4), L-proline (0.4) and L-glutamic acid (0.4). Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C in 750 ml vent flasks (Nunc) and fed every 3 day. About 3 days before starting experiments, cells were subcultured in 24-well cell culture cluster plates (Falcon). At the beginning of the experiment, cells were confluent (the protein content per well was about 0.2 mg). Mouse neuroblastoma X Chinese hamster hybrid cells of the clone NCB20 were cultured as described above except for the addition of 10% newborn calf serum and the omission of the amino-acid mix. For culture of rat neuroblastoma X glioma cells of the clone NG108-15, the medium contained hypoxanthine (10 mM), thymidine (16 mM) and aminopterin (0.5 mM), but no amino-acid mix.
[14C]guanidinium influx measurements
After removal of the growth medium, cells were washed and preincubated (for 20 min, 1.5 ml per well) with buffer (36°C) containing (in mM): 135 NaCl, 25 HEPES/Tris (pH 7.4), 5.4 KCl, 0.98 MgSO4 and 5.5 D-glucose or with buffer in which NaCl was replaced by either of choline chloride, TMA chloride, Tris or NMDG (under the latter two conditions, the pH was adjusted to 7.4 with HCl). Subsequently, the cells were incubated for 2 min with buffer containing 5 μM [14C]guanidinium chloride (specific activity 59 mCi mmol−1) and the 5-HT3 receptor agonist 5-HT. The flux was terminated by the removal of the incubation medium and by rapidly washing the cells three times with ice-cold incubation buffer. Thereafter, the cells were dissolved and lysed in 0.5 ml 0.1% Triton X-100, and the [14C]guanidinium content of this solution was determined by scintillation counting. An aliquot of the cell lysate was used for the determination of the protein content according to Lowry et al. (1951).
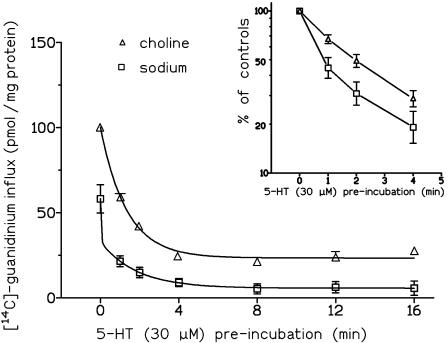
In each experiment, the [14C]guanidinium flux was also determined in the absence of 5-HT. The resulting basal flux was subtracted from the total influx (measured in the presence of 5-HT) in order to calculate the specific flux. The absolute values of [14C]guanidinium influx (in pmol × mg protein−1) of one experimental series are shown in Figure 4. Experiments were carried out in duplicate or triplicate.
Figure 4.
Effect of preincubation with 30 μM 5-HT on the [14C]guanidinium influx above basal influx in N1E-115 cells evoked by 2-min exposure to 30 μM 5-HT. Experiments were carried out using either sodium buffer (squares) or choline buffer (triangles). The responses are expressed as absolute values of [14C]guanidinium (pmol mg−1protein) and were fitted to a monoexponential (choline) and biexponential (sodium) decay. (Inset) Normalization of values to the respective maximum signal (no preincubation with 5-HT) showing that a rate difference between both conditions developed within the first minute of 5-HT preincubation. Shown are means ±s.e.m. of n=3–6 experiments.
Radioligand binding studies
The buffer conditions for radioligand binding on intact cells or on cell membranes were identical to those applied for tracer flux measurements. For studies on intact cells, N1E-115 cells were seeded on 24-well cell culture cluster plates. On days 3–4, when cells were confluent, the medium was removed and the wells were washed with 750 μl of the respective incubation buffer (see above). Thereafter, the cells were incubated for 60 min with 500 μl of incubation buffer (36°C) containing 1 nM [3H] 3-(5-methyl-1H-imidazol-4-yl)-1-(1-methyl-1H-indol-3-yl)-1-propanone (GR65630), a selective 5-HT3 receptor antagonist (Kilpatrick et al., 1989) and the unlabelled physiological ligand 5-HT at the concentration under study. [3H]GR65630 binding remaining in the presence of 10 mM 5-HT was regarded as non-specific. The experiment was stopped after the removal of the incubation buffer by washing the cells three times with ice-cold incubation buffer. Finally, the cells were lysed in 500 μl 0.1% Triton X-100 and the [3H]GR65630 content of this solution was determined by liquid scintillation counting. An aliquot of the cell lysate was used for the determination of the protein content according to Lowry et al. (1951). Experiments were carried out in duplicate or triplicate.
For studies on cell membranes, N1E-115 cells were grown in cell culture flasks (750 ml). Confluent cells were harvested in 50 mM HEPES-Na+ (pH 7.4) buffer at 4°C and centrifuged at 300 × g (5 min, 4°C). The cell pellet was washed by resuspension and centrifugation as decribed before, and cells were then homogenized at 4°C by usage of an IKA® Ultra-Turrax homogenizer (maximum velocity, 2 × 5 s). Cell homogenate was centrifuged for 20 min at 40,000 × g (4°C). The pellet was resuspended (in 50 mM HEPES/NaOH buffer) and re-centrifuged (as described above), and the final pellet was diluted (in 50 mM HEPES-Na+ pH 7.4) to obtain a concentration of 0.5 mg ml−1 protein, determined according to Lowry et al. (1951). Binding assays were performed at ambient temperature with membranes 10-fold diluted in sodium incubation buffer (final volume 500 μl) as described above for intact cells. However, specific binding of [3H]GR65630 was determined not only by 5-HT (10 mM) but also by MDL 72222 (3 μM). The reaction was stopped by rapid vacuum filtration with a Brandel® cell harvester through GF/B glass fiber filters, followed by two rapid washings of the filters with ice-cold buffer. The radioactivity retained in the filters was determined by liquid scintillation counting.
Data analysis
Calculations of EC50 values, Emax and IC50 values were performed and the concentration–response curves were fitted according to a commercially available software (Graphpad Inplot and Graphpad Prism 3.0; sigmoidal concentration–response curve, variable slope). Calculations of Ki values were performed using the equation of Cheng and Prusoff (1973).
Calculations of single-site and two-site competition of radioligand binding were performed according to Graphpad Prism 3.0 software, using the following Equations: (1) one- site: 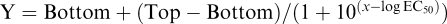 and (2) two site: Y=Bottom+Part a+Part b. (Part a=Span*Fraction
and (2) two site: Y=Bottom+Part a+Part b. (Part a=Span*Fraction 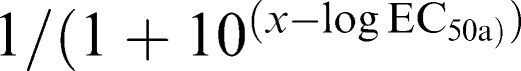 Part b=Span*(1−Fraction 1)/
Part b=Span*(1−Fraction 1)/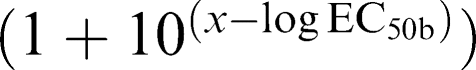 .
.
Materials
Constituents of cell culture media were obtained from Gibco BRL (Karlsruhe, Germany) or from Sigma (Munich, Germany). The following compounds were used in the experiments (for chemical structures, see Figure 1): 5-hydroxytryptamine creatinine sulphate from Sigma, Munich, Germany; [14C]guanidinium chloride (specific activity=59 mCi mmol−1) from CEA (Biotrend, Cologne, Germany); [3H]GR65630, (specific activity 64.8 Ci mmol−1) from NEN DuPont (Dreieich, Germany); choline chloride, TMA chloride, Tris and NMDG were all from Sigma (Munich, Germany).
Results
[14C]guanidinium influx
Basal [14C]guanidinium influx: In N1E-115 cells incubated in buffer containing 135 mM sodium, basal influx of [14C]guanidinium, that is, influx in the absence of 5-HT, amounted to 16.0±0.5 pmol × mg protein−1 (n=19). When sodium was completely replaced by other inorganic or organic cations, the following basal influx values (in pmol × mg protein−1) were obtained: lithium (n=6), 12.5±1.3; NMDG (n=8), 15.7±2.9; Tris (n=12), 10.3±2.2; choline (n=39), 7.8±0.4; TMA (n=10), 4.1±0.9. The basal fluxes were subtracted from the corresponding total guanidinium influxes during exposure to the various 5-HT concentrations to give the 5-HT-induced guanidinium influxes.
5-HT-induced [14C]guanidinium influx in N1E-115 cells: The 5-HT (100 μM)-induced influx above basal influx was insensitive to tetrodotoxin (10 μM) and sensitive to ondansetron (100% inhibition at 0.3 μM) irrespective of whether this effect was studied in buffer containing 135 mM sodium or 135 mM choline (n=4 under each control, buffer and drug condition; effects not shown).
5-HT produced a concentration-dependent influx of [14C]guanidinium above basal influx, irrespective of whether the buffer contained the physiological sodium concentration of 135 mM or whether sodium was partly or completely substituted by other inorganic or organic cations (Figures 2 and 3). In N1E-115 cells incubated in buffer containing 135 mM sodium, the mean EC50 of 5-HT was 1.3 μM (Table 1 ) and the mean maximum 5-HT-evoked influx above basal influx amounted to 30 pmol × mg protein−1; this value corresponded to 58.3% of the reference response (i.e. influx elicited by 100 μM 5-HT in buffer containing 135 mM choline but no sodium; Table 1); the EC50 of 5-HT was in the same range as in previous patch-clamp experiments in excised patches of N1E-115 cells (3.8 μM; Barann et al., 1997).
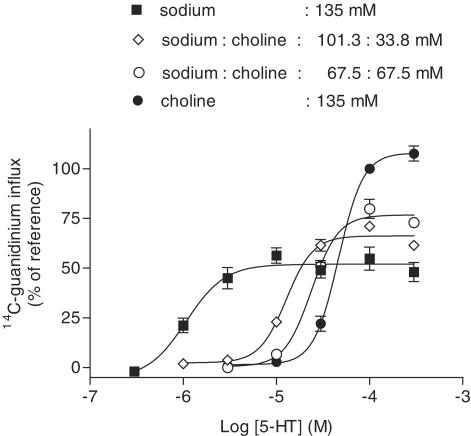
Figure 2.
Influence of gradual replacement of sodium by choline on the 5-HT (2-min exposure)-evoked [14C]guanidinium influx above basal influx in N1E-115 cells. The responses are expressed as percentages of the effect elicited by 100 μM 5-HT using choline (135 mM) buffer (=reference); 100 % corresponds to 51 pmol × mg protein−1. Shown are means±s.e.m. of n=5–12 experiments.
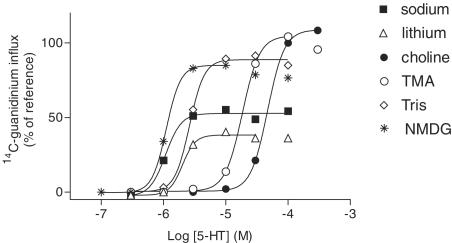
Figure 3.
Influence of complete replacement of sodium by different cations on the 5-HT (2-min exposure)-evoked [14C]-guanidinium influx above basal influx in N1E-115 cells. The responses are expressed as percentages of the effect elicited by 100 μM 5-HT in choline buffer (=reference effect); 100% corresponds to 51 pmol × mg protein−1). Shown are means of n=5–12 experiments. Error bars were omitted for the sake of clarity; they were in the range of values shown in Figure 2.
Table 1.
Influence of sodium substitutes on 5-HT-induced [14C]guanidinium influx in N1E-115 cells
| Response to 5-HT | |||
|---|---|---|---|
| n | pEC50±s.e.m. | Emax±s.e.m. | |
| (mean EC50; μM) | (% of reference) | ||
| Sodium (135 mM) | 12 | 5.89±0.09 (1.3) | 58.3±6.2 |
| Sodium : choline | 5 | 5.22±0.02* (6.0) | 60.4±1.4 |
| (118.3 : 16.9 mM) | |||
| Sodium:choline | 5 | 4.89±0.07* (12.3) | 66.5±2.0 |
| (101.3 : 33.8 mM) | |||
| Sodium : choline | 5 | 4.62±0.07* (24.5) | 79.9±4.5* |
| (67.5 : 67.5 mM) | |||
| Choline (135 mM) | 9 | 4.33±0.01* (44.7) | 110.2±3.1* |
| Lithium (135 mM) | 4 | 5.69±0.09 (2.3) | 40.6±1.9* |
| TMA (135 mM) | 7 | 4.73±0.01* (19.1) | 108.8±5.4* |
| Tris (135 mM) | 7 | 5.58±0.02* (3.6) | 97.7±6.2* |
| NMDG (135 mM) | 8 | 5.95±0.01 (1.3) | 93.4±7.8* |
The pEC50 and Emax values of 5-HT (2 min exposure to each concentration) under the cation conditions indicated were calculated from the concentration–response curves shown in Figures 2 and 3. The respective maximal responses (Emax) are expressed as percentages of the effect elicited by 100 μM 5-HT using choline (135 mM) buffer (=reference effect). Shown are means±s.e.m.
Significantly different from pEC50 and Emax at 135 mM sodium.
When sodium was stepwise replaced by choline, there was not only a stepwise decrease in the potency (EC50) of 5-HT in stimulating [14C]guanidinium influx (as reflected by the concentration-dependent parallel rightward shift of the 5-HT concentration–response curve), but also a stepwise increase in the maximum response (Figure 2 and Table 1). Complete replacement of sodium by 135 mM choline resulted in an EC50 of 5-HT of 44.7 μM which is 34 times higher than in the presence of buffer containing 135 mM sodium and in an about 2-fold higher maximum response (Figure 2 and Table 1). Low potencies (EC50=69±10 and 47.9±9 μM) and high maximal effects (Emax=81±2.7 and 123±9.1% of reference) of 5-HT were also observed in NCB20 and NG108-15 cells, respectively, incubated with buffer containing 135 mM choline instead of sodium (n=3–5; not illustrated).
Replacement of sodium by 135 mM lithium in the incubation buffer of N1E-115 cells closely resembled the 135 mM sodium condition, in that the potency and maximal effect of 5-HT were in the same range under both conditions (only slightly lower than in the presence of sodium; Figure 3, Table 1). When sodium was replaced by 135 mM TMA, a similar increase in the maximum 5-HT effect occurred as under the 135 mM choline condition, but the potency of 5-HT was still decreased by a factor of about 15 (Figure 3, Table 1). In the presence of 135 mM Tris, the maximal effect of 5-HT was also almost doubled compared to the 135 sodium condition, but the potency of 5-HT was still slightly lower than in the presence of sodium (Figure 3, Table 1). NMDG proved to be ideal as a complete subtitute of sodium (135 mM), in that it doubled the maximal effect of 5-HT without changing the potency of 5-HT (Figure 3, Table 1).
Since all organic sodium substitutes except NMDG produced parallel rightward shifts of the 5-HT concentration–response curve (slopes of concentration–response curves virtually identical under all conditions) as an indicator of competitive inhibition, the equation of Cheng & Prusoff (1973) could be applied for calculation of the apparent inhibition constants [Ki(sodium substitute)]. These calculations were based on the EC50 values of 5-HT in the presence of 135 mM sodium on the one hand and of sodium plus substitute (together 135 mM) or of 135 mM substitute alone on the other. When in N1E-115 cells 16.3, 33.8, 67.5 or 135 mM choline partially or totally replaced sodium, the Ki(choline) values were 4.6, 3.9, 3.8 and 4.0 mM, respectively, yielding a mean Ki (choline) of 4.07 mM. Complete replacement of sodium by 135 mM TMA or Tris in the incubation buffer revealed Ki(TMA) and Ki(Tris) values of 9.8 and 77 mM, respectively.
In an additional series of tracerflux experiments, the time course of desensitization of the 5-HT (30 μM)-induced cation influx caused by 1–16 min preincubation of the cells with 30 μM 5-HT was studied under both sodium and choline buffer conditions (Figure 4). Under both conditions preincubation with 5-HT (30 μM) resulted in a reduction of the 5-HT (2 min)-induced [14C]-guanidinium influx, which was dependent on the preincubation time. Desensitization was faster in the presence of sodium since 50% desensitization of the response was observed after less than 1 min of 5-HT preincubation, whereas in choline buffer 50% desensitization was seen after about 2 min (Figure 4). In the presence of sodium, the time course of preincubation-induced decay fitted to a biexponential function, yielding an initial fast time constant (k=29.1 min−1) and a slow decay component (k=0.544 min−1; Figure 4). In the presence of choline, this decay could be better fitted to a monoexponential curve (with a k value of 0.749 min−1); this k value was in the same range as the value for the slow decay component in the presence of sodium (Figure 4).
[3H]GR65630 binding
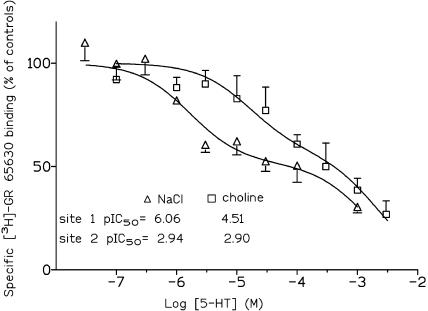
Binding of the potent and selective 5-HT3 receptor antagonist [3H]GR65630 (Kilpatrick et al., 1987; 1989) to intact N1E-115 cells was determined using three alternative buffer conditions, which were identical to the sodium, choline and NMDG buffer used for the [14C]guanidinium influx measurements described above. Specific binding of 1 nM [3H]GR65630 in buffer containing sodium, choline or NMDG amounted to 361±27, 283±15 and 407±32 fmol × mg protein−1 (n=4 each), respectively. This binding of [3H]GR65630 was concentration-dependently inhibited by 5-HT (Figure 5). Under all three buffer conditions, a biphasic displacement of [3H]GR65630 binding by 5-HT was observed, which could be resolved into two binding components, a high-affinity component (site 1) and a low-affinity component (site 2; Table 2 ). Since the displacement by 5-HT was almost identical under sodium and NMDG buffer conditions, only results obtained with sodium and choline buffer are shown in Figure 5. In the presence of sodium and NMDG, about 50% of the binding displaceable by 10 mM 5-HT was to the high-affinity site characterized by an IC50 for 5-HT of about 1 μM (Table 2). In choline buffer, this binding component to site 1 was smaller (32.5% of total binding); the IC50 value for 5-HT amounted to 31 μM, suggesting competition of choline with 5-HT for binding to this site. Using the equation of Cheng & Prusoff (1973), a Ki value for choline of 3.91 mM could be calculated. The low-affinity binding site for 5-HT was virtually identical under all three buffer conditions with an IC50 value for 5-HT as high as about 120 μM (Table 2).
Figure 5.
Influence of complete replacement of sodium (triangles) by choline (squares) on the inhibition of [3H]GR65630 binding to intact N1E-115 cells by 5-HT. The 5-HT effects are expressed as percentages of [3H]GR65630 binding (=control) after 60 min exposure to 1 nM of the radioligand in the absence of 5-HT. Under both buffer conditions, two components with two different IC50 values could be calculated. Shown are means±s.e.m. of n=4 experiments.
Table 2.
(a) Effect of complete substitution of sodium by choline or NMDG on the potency (pIC50, IC50) of 5-HT in inhibiting the binding of [3H]GR65630 to intact N1E-115 cells and (b) displacement of [3H]GR65630 by 5-HT under sodium buffer condition using membranes from N1E-115 cells
| Sodium substituent | Site 1 (%) | site 2 (%) |
|---|---|---|
| pIC50±s.e.m | pIC50±s.e.m | |
| (mean IC50; μM) | (mean IC50; μM) | |
| (a) | ||
| Sodium | [52.5%] | [47.5%] |
| 6.06±0.14 | 2.94±0.06 | |
| (0.87) | (115) | |
| Choline | [32.5%] | [67.5%] |
| 4.51±0.47 | 2.90±0.17 | |
| (30.9) | (126) | |
| NMDG | [49.7%] | [50.3%] |
| 5.86±0.14 | 2.93±0.03 | |
| (1.38) | (117) | |
| (b) | ||
| Sodium | [73.3%] | [26.7%] |
| 6.80±0.14 | 2.58±0.02 | |
| (0.16) | (263) | |
Specific binding was defined as binding sensitive to inhibition by 10 mM 5-HT. From the biphasic displacement curves (shown, for example, for sodium and choline buffer in Figure 5) two binding components, a high affinity (site 1) and a low-affinity (site 2), could be calculated. Shown are means (±s.e.m.) of the pIC50 values of n=4 experiments.
Biphasic displacement of [3H]GR65630 binding by 5-HT was also observed in N1E-115 cell membranes in sodium buffer. At cell membranes, the high-affinity 5-HT binding site (IC50=160 nM, Table 2) amounted to about 73% and the low-affinity site (IC50=260 μM, Table 2) to 27%. However, if specific binding was determined as MDL 72222-sensitive [3H]GR65630 binding, it was only 72.0±6.9% of the 5-HT-sensitive binding and no low-affinity site was detectable. This indicates that low-affinity, 5-HT-sensitive [3H]GR65630 binding does not occur at a MDL 72222-sensitive site. [3H]GR65630 binding to the MDL 72222-sensitive site was characterized by a pIC50 value for 5-HT of 6.75±0.06 (n=4); this value is very similar to the pIC50 values determined for [3H]GR65630 binding to the high-affinity 5-HT-sensitive site shown in Table 2.
If in the binding studies on intact cells only that part of [3H]GR65630 binding was examined, which was sensitive to 100 μM 5-HT, displacement was characterized by a single component, which showed nearly the same pIC50 values as the high-affinity component (Table 2), namely, 5.77±0.04, 4.78±0.35 and 5.76±0.04 in the presence of sodium, choline or NMDG, respectively. Binding of 5-HT to the high-affinity binding site in sodium and NMDG buffer (with IC50 values of about 1 μM) and in choline buffer (with an about 30-fold higher IC50) is compatible with the high potency of 5-HT in the [14C]guanidinium influx experiments in the presence of sodium or NMDG and the lower potency in choline buffer.
Discussion
The aim of this study was to resolve the discrepancy in potencies of 5-HT at 5-HT3 receptors of N1E-115 mouse neuroblastoma cells when using [14C]guanidinium flux measurements and buffers in which sodium was replaced by choline because choline improved the signal-to-noise ratio of the 5-HT-induced [14C]guanidinium influx. Thus, with this technique, we (Bönisch et al., 1993; Barann et al., 1993) had reported EC50 values of 5-HT at the 5-HT3 receptor of N1E-115 cells, which were more than 10-fold higher than those published in patch-clamp experiments on the same cells (Barann et al., 1997) or in [14C]guanidinium flux experiments in which sodium was the main cation (Reiser & Hamprecht, 1989; Emerit et al., 1993). In agreement with a previous study (Figure 2 in Bönisch et al., 1993), we excluded in the present investigation that this increased maximal signal is mediated by additional influx via voltage-gated sodium channels since the 5-HT3-evoked influx was insensitive to tetrodotoxin.
We could also confirm our earlier results that an about two-fold higher maximum of the 5-HT-induced [14C]guanidinium influx was observed if the physiological main cation sodium was replaced by the organic cation choline. When sodium was substituted by Tris, TMA or NMDG, a similar maximum of the 5-HT-induced [14C]guanidinium influx was observed as with choline, whereas this flux was even slightly lower as in the presence of sodium if sodium was replaced by lithium. A major reason for the lower maximum of the 5-HT-induced [14C] guanidinium influx in the presence of either sodium or lithium might be the more efficient competition of these smaller inorganic than the larger organic cations with the influx of the sodium-imitating guanidinium through the cation channel of the 5-HT3 receptor channel. It is known that the 5-HT3 receptor channel shows the same high conductance for lithium as for sodium (Yang, 1990; Furukawa et al., 1992), whereas the organic cations show low permeability (rank order: choline⩾Tris>NMDG) (Yakel et al., 1990; Yang, 1990; Robertson & Bevan, 1991). Thus, the high flux traffic of sodium (or lithium) cations through the 5-HT3 receptor channel probably impairs the simultaneous flux of the guanidinium cation, whereas the relatively impermeable organic cations (such as choline) allow the guanidinium cation to permeate more easily through the channel.
A further reason for the lower 5-HT-induced [14C]guanidinium influx in the presence of sodium (or lithium) compared to choline (or the other organic cations) may be the degree of cell depolarization, since depolarization should reduce the driving force for the permeant [14C]guanidinium cation: the highly permeable cations sodium and lithium should cause a more rapid and more pronounced depolarization than the less permeable organic cations. Another reason contributing to the lower maximum effect of 5-HT in sodium buffer (compared to choline buffer) seems to be the very fast initial component of desensitisation, which was not seen in choline buffer (see Figure 4), since this time period (initial 1–2 min) is most relevant for the standard (2 min) [14C]guanidinium influx experiments. Thus, in the presence of buffers containing organic cations such as choline, in which no fast component of desensitization of the 5-HT receptor channel occurs, 5-HT should induce a larger [14C]guanidinium influx than in the presence of buffer containing sodium. The relatively lower rate of desensitization induced by choline (and presumably also by the other organic cations) could be a consequence of the relatively low permeability of these organic cations through the 5-HT3 receptor channel (see above).
The desensitization kinetics (within minutes) reported here are slower than those reported for 5-HT3 receptors using electrophysiological techniques (e.g. within milliseconds, Barann et al., 1997). In this context, it should be considered that in the present system the agonist 5-HT is applied simultaneously to one cell dish containing thousands of cells with different states of receptor sensitivity at any time of the two min exposure, whereas in a typical patch-clamp experiment only one cell or even an excised patch only is superfused with agonist-containing solutions within milliseconds. This difference may also be involved in the slow onset of the signal within 2-min using [14C]guanidinium influx. Thus, any absolute value of time constants determined for 5-HT3 receptors in single cells or membrane patches cannot be compared to the absolute values found in the present study.
In the presence of the physiological cation sodium, the potency of 5-HT (1.3 μM) in inducing influx of [14C]guanidinium through the 5-HT3 receptor channels of N1E-115 cells was approximately identical to the EC50 values of 5-HT reported in patch-clamp studies (Barann et al., 1997). Choline, Tris and TMA not only increased the maximum 5-HT-induced [14C]guanidinium influx but they also reduced the potency of 5-HT. The reduction in 5-HT potency induced by the replacement of sodium by choline is in agreement with previous studies (Barann et al., 1993; Bönisch et al., 1993). The reduction of 5-HT potency, reflecting the rightward shift of the concentration response curve, was not restricted to the mouse 5-HT3 receptor, but it was also seen at the rat 5-HT3 receptor of NG108-15 cells and in cells expressing the homomeric human 5-HT3A receptor (Brüss et al., 2000). This reduction suggests a competitive interaction of these organic cations with the binding of 5-HT to its receptor binding site. The rank order of potency of these organic cations in reducing the potency of 5-HT was choline>TMA>Tris≫NMDG. Interestingly, this rank order agrees with the rank order of permeability of these organic cations through 5-HT3 receptor channels (Yakel et al., 1990). At least for choline a competitive interaction can be postulated since the rightward shift of the concentration-response curve of 5-HT was clearly concentration–dependent. In agreement with this, choline also reduced the potency of 5-HT as a competitor for the binding of [3H]GR65630 to the 5-HT3 receptor on whole cells. In these experiments, we observed a second (low-affinity) binding component, which had also been observed in a previous study on intact N1E-115 cells (Barann et al., 1995). In addition, [3H]GR65630 binding to a low-affinity site was also seen in experiments on isolated cell membranes if specific binding was defined as sensitive to the natural agonist 5-HT (10 mM; see Table 2). However, if specific [3H]GR65630 binding on cell membranes was defined as sensitive to the antagonist MDL 72222, the low-affinity site was not detectable. These results indicate that low-affinity binding of [3H]GR65630 occurs not to a cytosolic recognition site but to a cell membrane-associated site, which is either not identical to the 5-HT3 receptor protein or at least not identical to the MDL 72222 recognition site of the 5-HT3 receptor protein. This low-affinity component was not affected by the buffer condition and, in view of its low sensitivity to 5-HT, might have no (or at best limited) physiological relevance.
The inhibitory effects of choline were characterized by Ki values of about 4 mM in both the [14C]guanidinium influx and the [3H]GR65630 binding experiments. Since the monoamine 5-HT is positively charged at physiological pH and thus represents an organic cation, and since the ligand recognition site at the extracellular N-terminus of the 5-HT3 receptor has been proposed to consist of a site interacting with cations (Gozlan & Langlois, 1992) one may speculate that the organic cation choline interacts with 5-HT at this site. The decrease in potency of 5-HT evoked by Tris or TMA may also be due to a competition of these organic cations with the binding of 5-HT to this site of the 5-HT3 receptor. The calculated Ki of 77 mM for Tris and 9.8 mM for TMA suggests that these organic cations exhibit lower affinity than choline for the cation recognition domain of the ligand binding site. That TMA might competitively interact with 5-HT is supported by the fact that TEA, an analogue of TMA, exhibits such an interaction in electrophysiological as well as in [3H]GR65630 binding studies (Kooyman et al., 1993); this antagonism was supposed to take place at the cation recognition site of the 5-HT3 receptor pharmacophore (Kamato et al., 1995).
NMDG was the only sodium substitute that (like choline) increased the maximum effect of 5-HT but that (unlike choline and the other organic cations) did not reduce the potency of 5-HT. NMDG showed no affinity for the 5-HT3 receptor, neither in [14C]guanidinium flux experiments nor in [3H]GR65630 binding studies. Accordingly, the potency of 5-HT was identical in sodium and NMDG buffer. Nevertheless, the maximum 5-HT-induced [14C]guanidinium flux was about two times higher in NMDG than in sodium buffer. This enhancing effect might again be due to the fact that NMDG penetrates much slower than sodium through the 5-HT3 receptor channel (Yakel et al., 1990; Robertson & Bevan, 1991), and thus may also produce less desensitization (within the first 2 min) than sodium. The favourable properties of NMDG compared to those of sodium were also observed for the calcium ion flux through the 5HT3 receptor pore (Hargreaves et al., 1994). Thus, NMDG seems to be an ‘ideal' sodium substitute to increase the signal-to-noise ratio in 5-HT-mediated [14C]guanidinium flux studies of 5-HT3 receptors.
Acknowledgments
We thank Professor M. Wiese for calculating three-dimensional diameters of inorganic and organic cations. We thank Ms Zita Dorner for expert technical support and Mr Jan Walkembach for performing some experiments. In addition, we thank Dr James P. Dilger for critical and valuable comments to this paper. This study was supported by the DFG.
Abbreviations
- Choline
hydroxyethyl trimethylammonium
- GR65630
3-(5-methyl-1H-imidazol-4-yl)-1-(1-methyl-1H-indol-3-yl)-1-propanone
- 5-HT
5-hydroxytryptamine
- NMDG
N-methyl-D-glucamine
- TMA
tetramethyl-ammonium
- Tris
(tris hydroxymethyl)methylamin
References
- AAPRO M.S. 5-HT3 receptor antagonists. An overview of their present status and future potential in cancer therapy-induced emesis. Drugs. 1991;42:551–568. doi: 10.2165/00003495-199142040-00002. [DOI] [PubMed] [Google Scholar]
- AMANO T., RICHELSON E., NIRENBERG P.G. Neurotransmitter synthesis by neuroblastoma clones. Proc. Natl. Acad. Sci. U.S.A. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARANN M., DILGER J.P., BÖNISCH H., GÖTHERT M., DYBEK A., URBAN B.W. Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology. 2000;39:1064–1074. doi: 10.1016/s0028-3908(99)00205-1. [DOI] [PubMed] [Google Scholar]
- BARANN M., GÖTHERT M., BÖNISCH H., DYBEK A., URBAN B. 5-HT3 receptors in outside-out patches of N1E-115 neuroblastoma cells: Basic properties and effects of pentobarbital. J. Neuropharmacol. 1997;36:655–664. doi: 10.1016/s0028-3908(97)00059-2. [DOI] [PubMed] [Google Scholar]
- BARANN M., GÖTHERT M., FINK K., BÖNISCH H. Inhibition by anesthetics of 14C-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:125–132. doi: 10.1007/BF00169256. [DOI] [PubMed] [Google Scholar]
- BARANN M., MOLDERINGS G., BRÜSS M., BÖNISCH H., URBAN B.W., GÖTHERT M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARANN M., RUPPERT K., GÖTHERT M., BÖNISCH H. Increasing effect of ethanol on 5-HT3 receptor-mediated 14C-guanidinium influx inN1E-115 neuroblastoma cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:149–156. doi: 10.1007/BF00176768. [DOI] [PubMed] [Google Scholar]
- BÖNISCH H., BARANN M., GRAUPNER J., GÖTHERT M. Characterization of 5-HT3 receptors of N1E-115 neuroblastoma cells by use of the influx of the organic cation [14C]-guanidinium. Br. J. Pharmacol. 1993;108:436–442. doi: 10.1111/j.1476-5381.1993.tb12822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRÜSS M., BARANN M., HAYER-ZILLGEN M., EUCKER T., GÖTHERT M., BÖNISCH H. Modified 5-HT3A receptor function by co-expression of alternatively spliced human 5-HT3A receptor isoforms. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;362:392–401. doi: 10.1007/s002100000342. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DERKACH V., SUPRENANT A., NORTH R.A. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- EMERIT M.B., RIAD M., FATTACCINI C.M., HAMON M. Characteristics of [14C]-guanidinium accumulation in NG 108-15 cells exposed to serotonin 5-HT3 receptor ligands and substance P. J. Neurochem. 1993;60:2059–2067. doi: 10.1111/j.1471-4159.1993.tb03490.x. [DOI] [PubMed] [Google Scholar]
- FAN P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurones. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- FURUKAWA K., AKAIKE N., ONODERA H., KOGURE K. Expression of 5-HT3 receptors in PC12 cells treated with NGF and 8-Br-cAMP. J. Neurophysiol. 1992;67:812–819. doi: 10.1152/jn.1992.67.4.812. [DOI] [PubMed] [Google Scholar]
- GOZLAN H., LANGLOIS X.Structural analysis of receptor-ligand interactions Central and Peripheral 5-HT3 Receptors 1992London: Academic Press; 59–88.ed. Hamon, M [Google Scholar]
- HARGREAVES A.C., LUMMIS S.R.C., TAYLOR C.W. Ca2+ permeability of cloned and native 5-hydroxytryptamine receptors. Mol. Pharmacol. 1994;46:1120–1128. [PubMed] [Google Scholar]
- HELLEVUO K., HOFFMAN P.L., TABAKOFF B. Ethanol fails to modify [3H]-GR65630 binding to 5-HT3 receptors in NCB 20 cells and in rat cerebral membranes. Alcoholism: Clin. Exp. Res. 1991;15:775–778. doi: 10.1111/j.1530-0277.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- HOYER D., NEIJT H.C. Identification of serotin3 5-HT3 recognition sites in membranes of N1E-115 neuroblastoma cells by radioligand binding. Mol. Pharmacol. 1988;33:303–309. [PubMed] [Google Scholar]
- JACKSON M.B., YAKEL J.L. The 5-HT3 receptor channel. Annu. Rev. Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- KAMATO T., ITO H., SUZUKI T., MIYATA K., HONDA K. Studies on serotonin (5-HT)3 receptor antagonist effects of enantiomers of 4,5,6,7-tetrahydro-1H-benzimidazole derivatives. Jpn. J. Pharmacol. 1995;67:185–194. doi: 10.1254/jjp.67.185. [DOI] [PubMed] [Google Scholar]
- KARIM F., ROERIG S.C., SAPHIER D. Role of 5-hydroxytryptamine3 (5-HT3) antagonists in the prevention of emesis caused by anticancer therapy. Biochemical. Pharmacol. 1996;52:685–692. doi: 10.1016/0006-2952(96)00346-2. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., BUNCE K.T., TYERS M.B. 5-HT3 receptors. Med. Res. Rev. 1990;10:441–475. doi: 10.1002/med.2610100404. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., JONES B.J., TYERS M.B. The identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., JONES B.J., TYERS M.B. Binding of the 5-HT3 ligand , 3H-GR65630, to rat area postrema, vagus nerve and the brains of several species. Eur. J. Pharmacol. 1989;159:157–164. doi: 10.1016/0014-2999(89)90700-0. [DOI] [PubMed] [Google Scholar]
- KOOYMAN A.R., ZWART R., VIJVERBERG H.P. Tetraethylammonium ions block 5-HT3 receptor-mediated ion current at the agonist recognition site and prevent desensitization in cultured mouse neuroblastoma cells. Eur. J. Pharmacol. 1993;15:247–254. doi: 10.1016/0922-4106(93)90038-b. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., ZHOU Q. Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology. 1994;33:1567–1572. doi: 10.1016/0028-3908(94)90131-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARICQ A.V., PETERSON A.S., BRAKE A.J., MYERS R.M., JULIUS D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- MITCHELSON F. Pharmacollogical agents affecting emesis. A review (Part I). Drugs. 1992;43:295–315. doi: 10.2165/00003495-199243030-00002. [DOI] [PubMed] [Google Scholar]
- REEVES D.C., LUMMIS S.C.R. The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel. Molec. Membr. Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- REISER G., HAMPRECHT B. Substance P and serotonin act synergistically to activate a cation permeability in a neuronal cell line. Brain Res. 1989;479:40–48. doi: 10.1016/0006-8993(89)91333-4. [DOI] [PubMed] [Google Scholar]
- REITH M.E.A. 14C-Guanidinium ion influx into sodium channel preparations from mouse cerebral cortex. Eur. J. Pharmacol. 1990;172:195–198. doi: 10.1016/0922-4106(90)90245-s. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B., BEVAN S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. Br. J. Pharmacol. 1991;102:272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON K., SPENCER C.M., MCCLELLAN K.J. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2000;59:1297–1315. doi: 10.2165/00003495-200059060-00008. [DOI] [PubMed] [Google Scholar]
- VOOG O., ALSTERGEN P., LEIBUR E., KALLIKORM R., KOPP S. Immediate effects of the serotonin antagonist granisetron on temporomandibular joint pain in patients with systemic inflammatory disorders. Life Sci. 2000;68:591–602. doi: 10.1016/s0024-3205(00)00965-6. [DOI] [PubMed] [Google Scholar]
- WERNER P., KAWASHIMA E., REID J., HUSSY N., LUNDSTROM K., BUELL G., HUMBERT Y., JONES K.A. Organization of the mouse 5-HT3 receptor gene and functional expression of two splice variants. Brain Res. Mol. Brain Res. 1994;26:233–241. doi: 10.1016/0169-328x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- YAKEL J.L., SHAO X.M., JACKSON M.B. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 1990;533:46–52. doi: 10.1016/0006-8993(90)91793-g. [DOI] [PubMed] [Google Scholar]
- YANG J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J. Gen. Physiol. 1990;96:1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]