Abstract
Noladin ether has recently been reported to be an endocannabinoid, with selectivity for the cannabinoid (CB) CB1 receptor. In the present study, we investigated the effects of noladin ether in the rat isolated mesenteric arterial bed, cultured dorsal root ganglia (DRG) cells and human vanilloid (TRPV1)-receptor-expressing HEK293 cells (TRPV1-HEK293 cells).
Electrical field stimulation of the mesenteric bed evoked frequency-dependent vasorelaxation due to the action of calcitonin gene-related peptide (CGRP) released from sensory nerves. Noladin ether (0.1–3 μM) attenuated sensory neurogenic relaxation in a concentration-dependent manner. Noladin ether (1 μM) reduced vasorelaxation at a submaximal frequency (8 Hz), from 57.3±6.8 to 23.3±3.8% (P<0.05, n=4).
The inhibitory effects of noladin ether were unaffected by the CB1 antagonists SR141716A and LY320135, and the CB2 antagonist SR144528 (1 μM).
Noladin ether had no effect on vasorelaxation elicited by exogenous CGRP or capsaicin. These data suggest that noladin ether is acting at a prejunctional site and no interaction with TRPV1 is involved.
In mesenteric beds from pertussis toxin (PTX)-pretreated rats, the inhibitory actions of noladin ether on sensory neurotransmission were abolished, indicating the involvement of Gi/o protein-coupled receptors.
Noladin ether evoked a concentration-dependent increase in intracellular Ca2+ concentration in TRPV1-HEK293 cells at 10 μM (36.5±3.2% of maximal capsaicin-induced response), but it was a less potent agonist than both capsaicin and anandamide and at 1 μM it was essentially inactive. Noladin ether (1 μM) had no effect on capsaicin-evoked Ca2+ responses in DRG cells, and produced no response alone, indicating it neither modulates nor acts directly on TRPV1 receptors.
These data demonstrate that noladin ether attenuates sensory neurotransmission in rat mesenteric arteries via a non-CB1 non-CB2 PTX-sensitive prejunctional site, independently of TRPV1 receptors.
Keywords: Sensory neurotransmission, cannabinoids, rat mesenteric bed
Introduction
Anandamide and 2 arachidonoylglycerol (2AG) were the first endocannabinoids to be described; they bind to both CB1 and CB2 (CB: cannabinoid) receptors and exhibit cannabimimetic actions in vivo (Pertwee, 1999). Noladin ether, an ether-linked 2AG analogue, has recently been identified as another endocannabinoid; it was synthesised by Sugiura et al. (1999) and Mechoulam et al. (1998) and was simultaneously isolated from porcine brain and identified as an endogenous ligand at the CB1 receptor by Hanuš et al., 2001. Noladin ether binds only weakly to the CB2 receptor but exerts cannabimimetic effects in the mouse behavioural tetrad test, and is present in discreet brain regions; the highest levels are reported to be found in the hippocampus and thalamus (Fezza et al., 2002). However, more recent studies in various central nervous system (CNS) tissues indicate that noladin ether might not be present at detectable levels in the brain at least (Oka et al., 2003).
Anandamide has pronounced effects on the cardiovascular system, which are mediated by the activation of both CB1 and vanilloid (TRPV1) receptors; the latter are nonselective cation channels that are activated by noxious stimuli (Szallasi & Blumberg 1999; Zygmunt et al., 1999; Smart et al., 2000). Some vascular effects of CBs are mediated by sensory nerves. CB receptors are present in dorsal root ganglion (DRG) cells, and are transported to the peripheral terminals of the sensory neurones by axonal transport (Hohmann & Herkenham, 1999). Vanilloid receptors (TRPV1) are also present on capsaicin-sensitive sensory nerves and there is considerable overlap in the expression of TRPV1 receptors and CB1 receptors (Ahluwalia et al., 2000). Synthetic CB agonists such as WIN55,212, CP55,940 and HU210 act at CB1 and CB2 but have no activity at TRPV1 receptors. Similarly, the endocannabinoid 2AG has no activity at TRPV1 (Zygmunt et al., 1999).
The expression of functional CB1 receptors on perivascular sensory nerves has been shown in assays of the rat isolated mesenteric arterial bed, in which WIN55,212 and CP55,940 inhibited sensory neurotransmission and this could be reversed by the CB1 antagonists SR141716A and LY323501 (Duncan et al., 2001a,2001b). The inhibitory actions of Δ9-tetrahydrocannabinol (THC) and HU210 in the same preparations could not be reversed by antagonists of CB1 or CB2 receptors, indicating that inhibition of sensory neurotransmission by these compounds is via a prejunctional non-CB1/CB2 site (Ralevic & Kendall, 2001; Duncan et al., 2003a).
The aim of the present study was to determine whether the putative endocannabinoid noladin ether could mimic the actions of synthetic CBs on perivascular sensory neurotransmission in rat mesenteric arteries. To characterise the mode of action, the experiments were carried out in the presence of CB1 and CB2 receptor antagonists and in tissues from rats pretreated with pertussis toxin (PTX) to inactivate Gi/o protein-coupled receptors. To determine pre- or postjunctional actions, the effect of noladin ether on the vasorelaxant response to exogenous calcitonin gene-related peptide (CGRP) was investigated. There have been no reports to date of the action of noladin ether on vanilloid receptors. We have, therefore, also examined the effects of noladin ether on the vasorelaxant actions of capsaicin in the mesenteric bed and on the capsaicin-induced Ca2+ response in rat DRG cells in primary culture. A comparison was made between anandamide and noladin ether on the Ca2+ responses in HEK293 cells transfected with human TRPV1 receptors. Preliminary accounts of some of these results have been reported previously (Duncan et al., 2002,2003a,2003b,2003c).
Methods
Mesenteric arterial bed preparation
Male Wistar rats (250–300 g) were killed by decapitation after exposure to CO2. Mesenteric beds were isolated and perfused via the superior mesenteric artery. The abdomen was opened and the superior mesenteric artery was exposed and cannulated with a blunted hypodermic needle. The superior mesenteric vein was cut, blood flushed from preparation with 0.5 ml Krebs' solution and the gut dissected away from the mesenteric vasculature. The preparations were mounted on stainless steel grids (7 × 5 cm) in a humid chamber and perfused at a constant flow rate of 5 ml min−1 using a perfusion pump (model: 7554-30, Cole Parmer Ltd, Chicago, IL, U.S.A.). The perfusate was Krebs' solution of the following composition (mM): NaCl 133, KCl 4.7, NaH2PO4 1.35, NaHCO3 16.3, MgSO4 0.61, CaCl2 2.52 and glucose 7.8 gassed with 95% O2–5% CO2 and maintained at 37°C. In order to ascertain that the electrical circuit was complete, electrical field stimulation (EFS) was applied comprising a brief (5 s) stimulus (20 Hz, 90 V, 0.1 ms) via the metal grid (negative electrode) and the hypodermic needle (positive electrode), to excite the sympathetic fibres thus eliciting a small vasoconstriction and a transient rise in perfusion pressure. Guanethidine (5 μM) was added to block sympathetic neurotransmission, and after 30 min methoxamine (5–100 μM) was added to preconstrict the preparation (30–80 mmHg above baseline). EFS (2–12 Hz, 0.1 ms, 60 V, 30 s) was applied with a Grass S9D stimulator. The resulting vasorelaxation response has been shown to be blocked by tetrodotoxin (1 μM) and capsaicin (1 μM) (Ralevic et al., 1991). Responses were measured as changes in perfusion pressure (mmHg) with a pressure transducer (model: P23XL, Viggo-Spectramed, Oxnard, CA, U.S.A.), situated on a side arm proximal to the preparation, and recorded using an A to D converter (PowerLab/400, ADI Instruments, Australia). Preparations were allowed to equilibrate for 30 min prior to experimentation.
Experimental protocol
Three consecutive relaxant response curves to EFS at 1–12 Hz, ‘EFS control', ‘EFS I' and ‘EFS II', were generated in the preconstricted mesenteric arterial beds. After each stimulus, the tone of the preparation was allowed to return to its preconstricted value before the next stimulus was applied. The first response curve acted as a control. The compound under investigation was then added to the perfusate, and after 15 min response curves EFS I and EFS II were generated. Antagonists were added at the start of the equilibration period. Only a single concentration of agonist was used per preparation. In a separate series of experiments, dose–response curves were constructed to CGRP (0.05 pmol–0.5 nmol) and, in separate preparations, capsaicin (0.05 pmol–5 nmol), by applying 50 μl bolus injections via norprene tubing proximal to the preparation.
PTX pretreatment
Animals were pretreated using 10 mg kg−1 i.p. PTX 48 h prior to experiment (Schultz et al., 1998). The mesenteric beds were isolated and perfused with Krebs' solution containing guanethidine as previously described. The protocol for the preparations from PTX pretreated rats had to be modified, as methoxamine was unable to maintain tone throughout the experiment. Endothelin (0.2–2 nM) was added to the perfusate after the preparations had equilibrated, which raised the tone to 30–40 mmHg above baseline. With further additions of methoxamine (1–10 μM), the tone was sustained at this level for the remainder of the experiment. The preparations were stimulated at two frequencies (8 and 12 Hz), noladin ether was then added for 40 min and the preparations were again stimulated at 8 and 12 Hz. Control experiments were carried out to determine if the Gi/o proteins had been successfully inactivated by the PTX pretreatment. In these experiments, the adenosine A1 agonist cyclopentyladenosine (CPA) was used in the place of noladin ether. CPA is known to attenuate capsaicin-sensitive sensory neurotransmission in the rat isolated mesenteric arterial bed via Gi/o-coupled A1 receptors (Rubino et al., 1993).
Data analysis – mesenteric arterial beds
Vasorelaxant responses of the mesenteric beds were expressed as a percentage relaxation of the methoxamine-induced increase in tone above baseline. Data were compared by Student's t-test and one-way/two-way analysis of variance (ANOVA) with Tukey's post hoc test. A value of P<0.05 was taken to indicate a statistically significant difference. The software package Prism GraphPad (3.0) was used to perform the analyses. RMAX indicates maximal relaxation.
DRG preparation
DRG were isolated from adult Wistar rats (300–350 g) and neurones cultured as described by Lindsay (1988) with minor modifications. Cells were grown on 13 mm glass coverslips for 24 h prior to incubation with Fura 2-AM (5 μM, 30 min, 37°C). The mean diameter of the cells sampled was 18.6±0.4 μm (n=241). Intracellular Ca2+ concentrations ([Ca2+]i) in individual neurones in fields of 241 cells were estimated as the ratios of peak fluorescence intensities (measured at 500 nm) at excitation wavelengths of 340 and 380 nm respectively (Bundey & Kendall, 1999), using an Improvisation imaging system with Ion Vision software.
DRG neurones were superfused (2 ml min−1) with capsaicin (100 nM) for 60 s followed by a 45 min washout. Noladin ether (1 μM) was then added for 4 min, and capsaicin (100 nM) in combination with noladin ether (1 μM) was then added for 60 s. After a washout period of 45 min, capsaicin (100 nM) alone was added. This protocol has previously been used to demonstrate HU210 inhibition of capsaicin-induced increase of [Ca2+]i in DRG cells (Millns et al., 2001).
Data analysis – DRG calcium responses
Data are expressed as ratios of 340 : 380 peak fluorescence as a percentage (means±s.e.) of the initial capsaicin response for each cell. Statistical analysis was performed using unpaired Student's t-test.
Cloning and expression of human vanilloid TRPV1 receptors in HEK293 cells
The cloning of the human vanilloid TRPV1 receptor was conducted as described previously (Hayes et al., 2000). Briefly, human vanilloid TRPV1 receptor cDNA was identified using the published rat vanilloid TRPV1 receptor sequence (GenBank accession AF029310) to search public nucleotide databases. Expressed sequence tag T48002 was identified and its sequence extended by rapid amplification of the cDNA ends using cDNA templates from a number of tissue sources. The full cDNA was amplified from brain cDNA, inserted into the expression vector pcDNA3.1, double strand sequenced and stably expressed in HEK293 cells.
Cell culture – TRPV1-HEK293 cells
Human vanilloid TRPV1 receptor-expressing HEK293 cells were routinely grown as monolayers in minimum essential medium supplemented with non-essential amino acids, 10% foetal calf serum and 0.2 mM L-glutamine, and maintained under 95%/5% air/CO2 at 37°C. Cells were passaged every 3–4 days and the highest passage number used was 28.
Measurement of intracellular Ca2+ concentrations using the FLIPR™
Intracellular Ca2+ concentrations were monitored using FLIPR™ (Molecular Devices, U.K.) as described previously (Smart et al., 2001). Briefly, human vanilloid TRPV1 receptor-expressing HEK293 cells, seeded into 96-well plates (25,000 cells per well), were incubated with culture medium containing the cytoplasmic Ca2+ indicator, Fluo-3 (4 μM; Teflabs, Austin, TX, U.S.A.), at 25°C for 120 min. The cells were then washed four times with Tyrode's medium containing 0.1% BSA, before being incubated for 30 min at 25°C in the presence or absence of various antagonists. The plates were then placed into a FLIPR™ to monitor cell fluorescence (λEX=488 nm, λEM=540 nm) (Wood & Smart, 2001) before and after the addition of various agonists.
Data analysis – recombinant vanilloid TRPV1-expressing cells
Responses were measured as peak minus basal fluorescence intensity, and where appropriate were expressed as a percentage of a maximum capsaicin-induced response. Data are expressed as mean±s.e.m. unless otherwise stated. Curve-fitting and parameter estimation were carried out using Graph Pad Prism 3.0 (GraphPad Software Inc., California, U.S.A.). Statistical comparisons were made where appropriate using Student's t-test.
Drugs
Noladin ether was a kind gift from Prof. T. Sugiura (Teikyo University, Japan), or was purchased from Tocris U.K. PTX was from Calbiochem, U.K. SR141716A (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide) and SR144528 (N-[1S)-endo-1,3,3,-trimethyl bicyclo [2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were gifts from Sanofi (Montpellier, France). LY320135 [6-methoxy-2-(4-methoxyphenyl) benzo[b]-thien-3-yl][4-cyanophenyl] methanone) was a gift from Eli Lilly. CGRP and capsaicin were from Sigma (Poole, Dorset, U.K.). Guanethidine (Ismelin) was from Alliance Pharmaceuticals (Chippenham, Wiltshire, U.K.).
All CBs and capsaicin were dissolved in ethanol at a stock concentration of 10−2 M. CBs were added directly to the perfusate reservoir. Further dilutions of capsaicin were made in distilled H2O. CGRP was dissolved in distilled water.
In the DRG experiments, drug dilutions were made in superfusion buffer of the following composition: (mM) NaCl 145; KCl 5; CaCl2 2; MgSO4 1; HEPES 10; glucose 10; pH 7.4.
Results
Effect of noladin ether on vasorelaxant responses to EFS
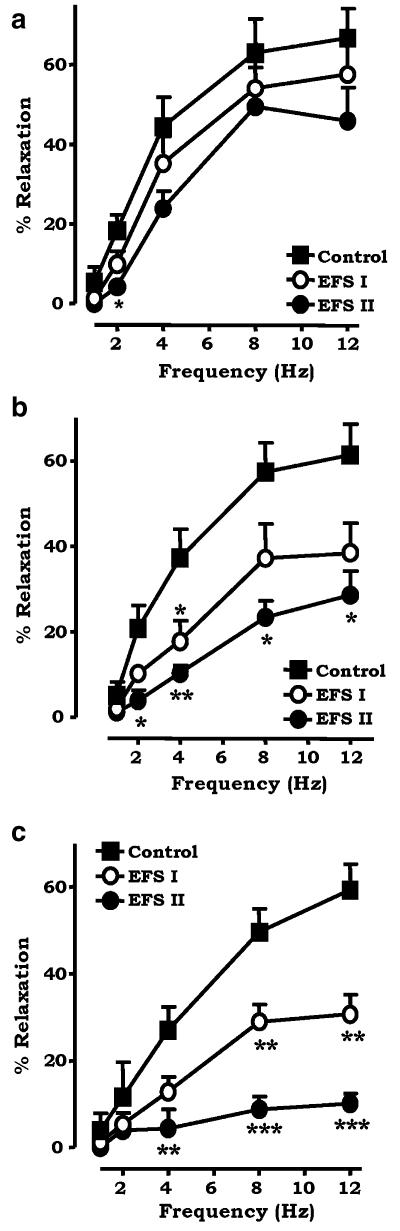
EFS (1–12 Hz, 0.1 ms, 60 V, 30 s) produced frequency-dependent relaxation of the rat isolated mesenteric arterial bed. Three frequency–response curves were constructed, EFS control, EFS I and EFS II, and were reproducible under control conditions. The equivalent concentration of the drug vehicle (0.01% ethanol) had no effect on the vasorelaxant response (data not shown). The first curve acted as a control. Noladin ether attenuated EFS I and EFS II in a concentration- and time-dependent manner. At the lowest concentration (0.1 μM, n=6), there was a small inhibition of the relaxation response at 2 Hz (P<0.05) but no significant difference in RMAX (value obtained at 12 Hz) (Figure 1a). At concentrations of 1 and 3 μM noladin ether, the relaxation response was significantly reduced: at a submaximal frequency of 8 Hz, 1 μM reduced EFS control 57.3±6.8% to EFS II 23.3±3.8% (P<0.05, n=4) and 3 μM reduced EFS control 49.6±5.4% to EFS II 8.7±2.9% (P<0.001, n=5) (Figure 1b and c). RMAX was significantly reduced at both concentrations: at 1 μM, noladin ether reduced EFS control from 61.4±7.2% to EFS II 28.5±5.6% (P<0.05); at 3 μM, 59.3±6.0% to EFS II 10.1±2.2% (P<0.001). Noladin ether reduced the tone of the preparation, which was restored and maintained using methoxamine.
Figure 1.
Effects of (a) 0.1 μM (n=6), (b) 1 μM (n=4) and (c) 3 μM noladin ether (n=5) on frequency-dependent vasorelaxation to EFS in the rat isolated mesenteric arterial bed. Three consecutive curves were constructed to EFS: the first acted as a control, noladin ether was added to the perfusate, then EFS I and EFS II were constructed. Data are presented as means±s.e.m. *P<0.05 with respect to control, **P<0.01 with respect to control, ***P<0.001 with respect to control.
Effect of SR141716A and LY320135 on the inhibitory actions of noladin ether on the vasorelaxant response to EFS
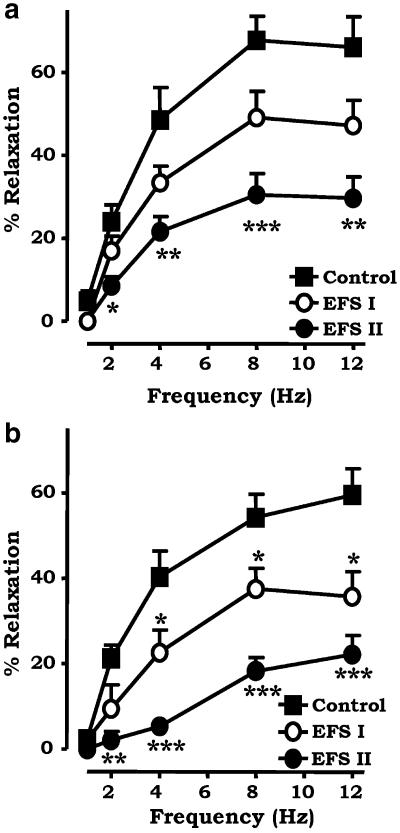
In order to determine if the inhibitory actions exerted by noladin ether were mediated by CB1 receptors, the experiments in the mesenteric beds were repeated in the presence of 1 μM of the CB1 selective antagonists SR141716A and LY320135 (Figure 2). The concentration of 1 μM SR141716A was used to minimise nonselective effects, which have been reported at higher concentrations (Ralevic, 2003). SR141716A had no effect on the inhibitory actions of noladin ether on sensory neurogenic vasorelaxation (Figure 2a). At a submaximal frequency of 8 Hz, there was significant inhibition: EFS control 67.8±5.8% to EFS II 30.5±5.0%; n=6, P<0.001. LY320135 (1 μM) also failed to block the inhibitory actions of noladin ether on sensory neurogenic vasorelaxation. At 8 Hz, there was a significant inhibition of neurogenic relaxations by noladin ether: EFS control 54.2±5.5% to EFS II 18.2±3.1%; n=8, P<0.001 (Figure 2b). SR141716A has previously been reported to augment sensory neurogenic relaxations to EFS in the rat isolated arterial mesenteric bed (Ralevic & Kendall, 2001); however, in control experiments, it had no effect in the present study (data not shown).
Figure 2.
Effect of the CB1 selective antagonists (a) SR141716A (1 μM, n=6) and (b) LY320135 (1 μM, n=8) on the inhibitory actions of 1 μM noladin ether on frequency-dependent vasorelaxation to EFS in the rat isolated mesenteric arterial bed. Three consecutive curves were constructed to EFS: the first acted as a control, noladin ether was added to the perfusate, then EFS I and EFS II were constructed. Data are presented as means±s.e.m. *P<0.05 with respect to control, **P<0.01 with respect to control, ***P<0.001 with respect to control.
Effect of SR144528 on the inhibitory actions of noladin ether on the vasorelaxant response to EFS
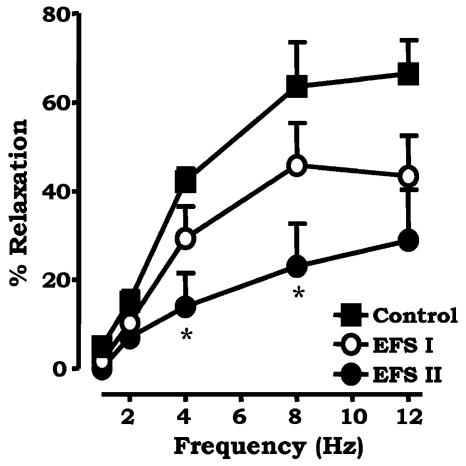
In order to investigate the possibility that noladin ether was acting at CB2 receptors, we repeated the experiment in the mesenteric beds in the presence of the CB2 selective antagonist SR144528 (1 μM). SR144528 had no effect on the inhibitory actions of noladin ether (1 μM) on sensory neurogenic vasorelaxation (Figure 3). At a submaximal frequency of 8 Hz, there was significant inhibition due to noladin ether of EFS control 68.0±8.9% to EFS II 23.9±7.5%; n=5, P<0.05.
Figure 3.
Effect of the CB2 selective antagonist SR144528 (1 μM, n=5) on the inhibitory actions of 1 μM noladin ether on frequency-dependent vasorelaxation to EFS in the rat isolated mesenteric arterial bed. Three consecutive curves were constructed to EFS: the first acted as a control, noladin ether was added to the perfusate, then EFS I and EFS II were constructed. Data are presented as means±s.e.m. *P<0.05 with respect to control.
Effect of noladin ether on the vasorelaxant response to exogenous CGRP
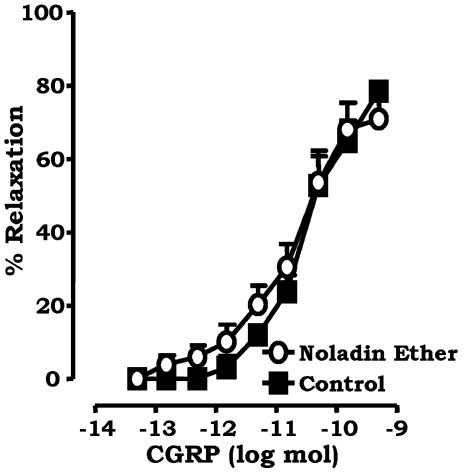
To determine if the inhibitory actions on sensory neurotransmission by the endocannabinoid noladin ether are via a pre- or postjunctional site, dose–response curves to CGRP, the principal motor neuropeptide involved in sensory neurogenic relaxation, were constructed in the mesenteric beds, in the absence and presence of 1 μM noladin ether (Figure 4). The pD2 value was unaltered: 10.7±0.2 and 10.5±0.1 in the presence and absence, respectively, of 1 μM noladin ether (P>0.05, unpaired t-test).
Figure 4.
Effect of 1 μM noladin ether (n=6) on vasorelaxant responses of rat isolated mesenteric arterial beds to CGRP (0.05 pmol–0.5 nmol). Data are presented as means±s.e.m.
Effect of noladin ether on the vasorelaxant response to capsaicin
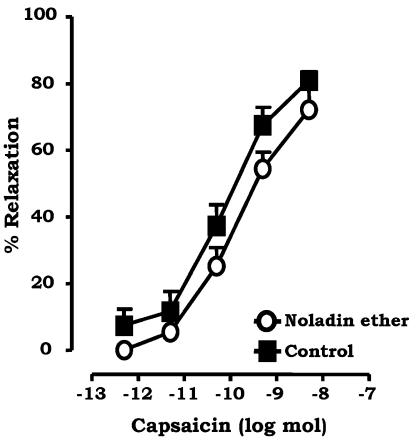
Vanilloid receptors are abundant in perivascular sensory nerves in the rat mesenteric arterial bed, and their activation by capsaicin results in CGRP release and vasodilatation. To determine if noladin ether is acting at TRPV1 receptors or modulating the TRPV1 response, dose–response curves to the TRPV1 agonist capsaicin were constructed, in the presence of 1 μM noladin ether or vehicle (ethanol 0.01%) (Figure 5). The pD2 value was unaltered: 9.8±0.2 and 10.1±0.2 in the presence and absence, respectively, of 1 μM noladin ether (P>0.05, unpaired t-test).
Figure 5.
Effect of 1 μM noladin ether (n=8) on vasorelaxant responses of rat isolated mesenteric arterial beds to capsaicin (0.05 pmol–5 nmol). Data are presented as means±s.e.m.
Effects of PTX in vivo pretreatment on the inhibitory actions of noladin ether
Both CB receptors that have been isolated to date (CB1 and CB2) are Gi/o protein-linked receptors. In order to determine if the inhibitory actions of noladin ether are Gi/o protein-mediated, rats were pretreated with PTX (10 mg kg−1; Schultz et al., 1998) 48 h prior to the experiment. Mesenteric beds were isolated and perfused and the tone was raised to 30–40 mmHg above baseline using endothelin and methoxamine (see Methods). Electrical stimulation at 8 and 12 Hz was then applied. Noladin ether (1 μM) was added to the perfusate and left for 40 min and the stimulation was then repeated.
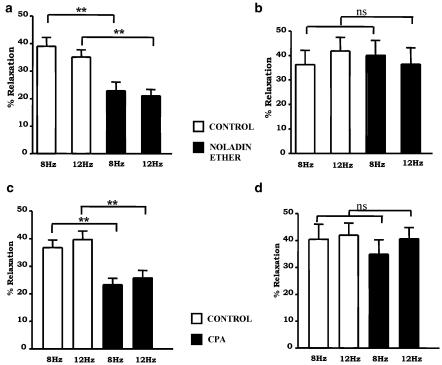
In the control animals, 1 μM noladin ether significantly attenuated the sensory neurogenic vasorelaxant response at both frequencies (Figure 6a). The neurogenic response to 8 Hz was reduced from 39.0±4.4 to 22.9±6.1% (P<0.01, n=10) and at 12 Hz the response was reduced from 35.1±4.9 to 21.0±3.1% (P<0.01, n=10). In the tissues from PTX-pretreated animals, there was no significant difference in the sensory neurogenic relaxant response in the presence of 1 μM noladin ether (Figure 6b). At 8 Hz, the response was unaffected, 36.2±5.9 to 41.8±5.6% (n=8, P>0.05), and at 12 Hz, 40.1±6.1 to 36.5±6.8% (n=8, P>0.05)
Figure 6.
Effect of 1 μM noladin ether on sensory neurogenic relaxation to EFS in mesenteric beds from (a) control (n=10) and (b) PTX-pretreated rats (n=8) and the effect of 3 nM CPA on sensory neurogenic relaxation to EFS in mesenteric beds from (c) control (n=10) and (d) PTX-pretreated rats (n=7). Two frequencies of EFS, 8 and 12 Hz, were applied. Noladin ether and CPA were added to the perfusate for 40 min, and then stimulation was repeated. Data are presented as means±s.e.m. **P<0.01 with respect to control; ns=not significant.
The control compound CPA, an adenosine A1 receptor agonist, significantly reduced sensory neurogenic relaxations in control tissues (Figure 6c): at 8 Hz the response was reduced from 36.8±2.7 to 23.3±2.3% (P<0.01), and at 12 Hz the response was reduced from 39.7±2.3 to 25.8±2.7% (n=10, P<0.01). In the tissues from PTX-pretreated animals, the inhibitory actions of CPA were abolished (Figure 6d). The response at 8 Hz was unaffected, 40.0±7.0 to 34.9±6.9% (P>0.05), and at 12 Hz, 42.0±5.9 to 40.7±4.0% (n=7, P>0.05).
Effects of noladin ether on capsaicin-evoked calcium response in cultured DRG cells
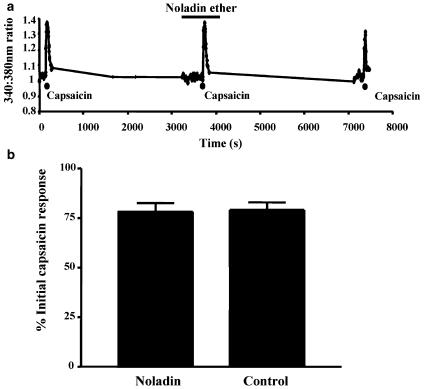
HU210 has been reported to inhibit the capsaicin-induced Ca2+ response in DRG neurones via a functional CB receptor sensitive to SR141716A (Millns et al., 2001). We investigated whether noladin ether also acts at CB1 receptors in DRG cells. A total of 48% of the cells examined (mean diameter 18.5±0.5 μm, n=155) responded to capsaicin. The initial capsaicin response (100 nM, 60 s) was taken as control (100%) response. The responses were then calculated as a percentage of the initial capsaicin response. In untreated cells, the mean 340 : 380 ratio (basal [Ca2+]i) was 1.1±0.01 (n=52). The capsaicin-evoked calcium response in the presence of 1 μM noladin ether was 78.3±4.3% (n=52), compared to 79.1±3.8% (n=35) in the separate control experiments (Figure 7). Noladin ether alone had no effect: 6.4±1.35% of the initial capsaicin response.
Figure 7.
(a) A representative trace showing the 340 : 380 nm ratios in a single DRG neurone, in response to capsaicin in the presence of 1 μM noladin ether. The cell is initially exposed to capsaicin (100 nM) for 60 s; after a 45 min washout period, noladin ether was applied alone for 4 min; a combination of capsaicin and noladin ether was then applied for 60 s. After a further 45 min, the cell was challenged with capsaicin alone. (b) Effect of 1 μM noladin ether on capsaicin-induced calcium response in individual DRG cells (n=52), compared with the response to capsaicin alone in separate control experiments (n=35). Results are expressed as percentages of the responses to 100 nM capsaicin alone.
Effects of noladin ether on intracellular Ca2+ concentrations in hTRPV1-HEK293 cells
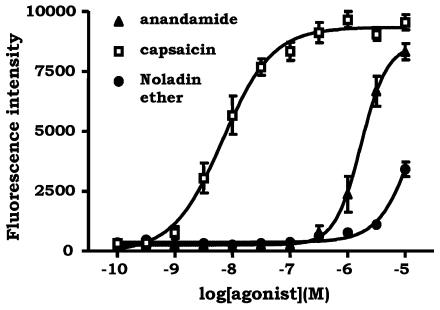
Capsaicin and anandamide (100 pM–10 μM) evoked concentration-dependent Ca2+ responses in hTRPV1-HEK293 cells (Figure 8), with pEC50 values of 8.16±0.05 and 5.75±0.02 respectively, consistent with previous reports (Smart et al., 2000). Noladin ether (1–10 μM) also evoked a concentration-related Ca2+ response in hTRPV1-HEK293 cells (Figure 8), but was a much weaker agonist (36.5±3.2% of a maximal capsaicin-induced response at 10 μM). At 1 μM noladin ether, the concentration used in the vasorelaxation studies in the mesenteric beds, there was no significant response. The responses to all three ligands in hTRPV1-HEK293 were blocked by capsazepine (1 μM), and were absent in wild-type HEK293 cells (data not shown), confirming that all three were acting as TRPV1 agonists.
Figure 8.
Noladin ether is a weak agonist at hTRPV1. Intracellular Ca2+ concentration was monitored using Fluo-3 in human vanilloid TRPV1 receptor-expressing HEK293 cells before and after the addition of capsaicin (100 pM–30 μM), anandamide (100 pM–10 μM) or noladin ether (100 pM–10 μM). Responses were measured as peak increase in fluorescence minus basal and are given as mean±s.e.m. n=3.
Discussion
The data presented clearly show that noladin ether attenuates capsaicin-sensitive sensory neurotransmission in the rat isolated mesenteric arterial bed via a PTX-sensitive prejunctional site. However, the inhibitory actions could not be attenuated by selective antagonists for CB1 and CB2 receptors at concentrations previously shown to block the actions of synthetic CBs (Duncan et al., 2001a,2001b). Postjunctional actions and modulation of TRPV1 receptors were ruled out as potential mechanisms of action of noladin ether by the finding that dose–response curves to CGRP and capsaicin in the mesenteric bed were unaltered in the presence of noladin ether.
The inhibitory actions of noladin ether on the sensory neurogenic vasorelaxant response in the rat isolated mesenteric arterial bed are comparable to our previously published findings for HU210 and THC (Ralevic & Kendall, 2001; Duncan et al., 2003a). Neurogenic vasorelaxation mediated by the actions of CGRP release from sensory nerves was inhibited by HU210 (0.1–3 μM) and these responses were unaffected by selective antagonists for CB1 and CB2 receptors. Likewise, HU210 had no effect on the relaxation response to CGRP, indicating a prejunctional site of action (Ralevic & Kendall, 2001). The inhibitory actions of THC are also resistant to CB1 and CB2 antagonists, and have no effect on the relaxation response to CGRP (Duncan et al., 2003a). It is therefore conceivable that the actions of HU210, THC and noladin ether are mediated by a non-CB1 CB2 receptor on sensory nerves in rat mesenteric arteries.
There have been several proposed novel CB receptors, although to date only two putative novel CB receptors have been characterised in the vascular system. One of these is an endothelial ‘anandamide receptor' (Di Marzo et al., 2002), through which anandamide mediates vasodilatory effects in mesenteric artery preparations; abnormal cannabidiol (abn-CBD) also activates this novel receptor (Járai et al., 1999). The actions of anandamide, releasing endothelium-derived hyperpolarising factor (EDHF) to the underlying smooth muscle, at this novel receptor are blocked by PTX pretreatment (Offertaler et al., 2003). Since the actions of both anandamide and abn-CBD are antagonised by SR141716A, it is therefore unlikely that the SR141716A-insensitive noladin ether responses that we have observed could be mediated by the endothelial anandamide receptor. Moreover, the endothelial anandamide receptor studies were performed in perfused rat mesenteric beds, where SR141716A was maximally effective at 0.5 μM (Wagner et al., 1999). Therefore, the concentration of SR141716A used in these experiments would rule out the novel anandamide receptor involvement. The effects of noladin ether do appear to be mediated by a G protein-coupled receptor since they were absent in preparations from animals treated with the Gi/o protein inactivator PTX.
Another, different, non-CB1/CB2 receptor also appears to be present in perivascular sensory nerves (Zygmunt et al., 2002); it is activated by THC and cannabidiol, resulting in an endothelium-independent release of CGRP. This effect is resistant to the TRPV1 antagonist capsazepine, and is blocked by the CGRP antagonist 8-37 CGRP and the nonselective channel blocker ruthenium red. The effect is also observed in TRPV1 knockout mice, and the ability of ruthenium red to block the action implies that an ion channel is involved. However, as the inhibitory actions of noladin ether on sensory neurotransmission were found to be resistant to ruthenium red (Duncan et al., 2003b), this suggests that it is unlikely that noladin ether is acting at the putative sensory nerve ionotropic receptor.
Other novel CB receptors have been described in the brain and central nervous system. A receptor activated by WIN55,212 and anandamide has been described in the mouse brain; both of these compounds cause enhanced [35S]GTPγS binding in CB1 knockout (CB1−/−) mice but this effect is not produced by THC, HU210 or CP55,940. High levels of binding were reported in the cerebral cortex, midbrain, hippocampus and brain stem (Di Marzo et al., 2000; Breivogel et al., 2001). A presynaptic CB receptor has been described on central glutamate terminals, activated by WIN55,212 in CB−/− mice, in electrophysiological experiments using mouse hippocampal slices (Hájos et al., 2001). This is, however, SR141716A-sensitive, again indicating that it is unlikely that this is same as the putative receptor activated by noladin ether.
Capsaicin excites initially, via the ionotropic receptor TRPV1, and then attenuates the action of sensory nerves in the rat mesenteric arterial bed. Thus, the ability of noladin ether to induce Ca2+ responses in hTRPV1-HEK293 cells was studied as a possible mechanism involved in its inhibitory action on sensory neurotransmission. Noladin ether was a weak agonist at TRPV1 receptors in hTRPV1-HEK293 cells. At a concentration of 10 μM, noladin ether only produced 36.5±3.2% of the maximal capsaicin-induced response compared with approximately 80% for anandamide, and at 1 μM it was essentially inactive at TRPV1. Noladin ether (30 nM) has been reported to induce rapid transient [Ca2+]i fluxes in NG 108-15 (neuroblastoma × glioma hybrid cells), but this action was reversed by 1 μM SR141716A (Sugiura et al., 1999), indicating CB1 involvement. In addition to its agonist actions at TRPV1 (Zygmunt et al., 1999; Smart et al., 2000), anandamide (1 μM) evokes an increase in intracellular Ca2+ concentrations in DRG cells, which is unaffected by capsazepine but blocked by 1 μM SR141716A (Millns et al., 2002). However, in the cultured DRG cells, noladin ether (1 μM) had no effect alone, and so does not appear to interact with CB1 or TRPV1 receptors.
HU210 has been reported to inhibit the capsaicin-evoked Ca2+ response via a CB1-like receptor in cultured DRG cells (Millns et al., 2001); to determine if noladin ether modulates TRPV1 in a similar manner to HU210, the effects of noladin ether on the capsaicin-evoked Ca2+ response in DRG cells were studied. Noladin ether, however, had no significant effect on the capsaicin-evoked calcium response. Noladin ether also had no significant effect on TRPV1-mediated relaxation to capsaicin in the mesenteric bed. Thus, while noladin ether mimics the antagonist-resistant actions of HU210 on capsaicin-sensitive sensory nerves in the mesenteric bed, it does not interact with the HU210-sensitive CB receptor in DRG cells.
Noladin ether-activated [35S]GTPγS binding in rat brain membranes indicated the presence of CB receptors in the hippocampus, globus pallidus, substantia nigra and the molecular layer of the cerebellum (Savinainen et al., 2001). Binding in all brain regions was reversible by SR141716A, indicating that, in the brain, noladin ether acts at CB1 rather than novel CB receptors (Savinainen et al., 2001). Noladin ether has been reported to occur naturally in the pig brain (0.6 nmol g−1 brain; Hanuš et al., 2001) and rat brain (25.4 pmol g−1 brain; Fezza et al., 2002). However, Oka et al. (2003) recently reported that no appreciable amounts of noladin ether (<0.2 pmol g−1 brain) could be detected in the rat, mouse, hamster, guinea pig and porcine brain, casting doubt on its role as an endocannabinoid. Levels of noladin ether in the periphery have not been measured, but the presence of the noladin ether-sensitive CB-like receptors that we have pharmacologically characterised in perivascular capsaicin-sensitive sensory nerves in the rat mesentery indicates a possible role in physiological or pathophysiological conditions. However, a more definitive characterisation of noladin ether as a member of the endocannabinoid messenger family must await analytical confirmation of its presence in the peripheral and/or central nervous system.
Our previous studies have demonstrated that WIN55,212 and CP55,940 inhibit sensory neurotransmission in the rat isolated mesenteric arterial bed, in an SR141716A- and LY320135-sensitive manner (Duncan et al., 2001a,2001b), indicating the presence of a CB1-like receptor. Noladin ether, THC and HU210 also act at CB1 receptors, and there is molecular and pharmacological evidence for CB1 receptors in sensory nerves; however, the evidence suggests that these CBs are acting at a novel receptor present in the sensory nerves. The data from the present study suggest that noladin ether exerts inhibitory actions via a non-CB1/CB2, PTX-sensitive prejunctional receptor in the rat mesenteric bed.
Abbreviations
- abn-CBD
abnormal cannabidiol
- ANOVA
analysis of variance
- CB
cannabinoid
- CGRP
calcitonin gene-related peptide
- CPA
cyclopentyladenosine
- DRG
dorsal root ganglia
- EDHF
endothelium-derived hyperpolarising factor
- EFS
electrical field stimulation
- THC
Δ9-tetrahydrocannabinol
- TRPV1
vanilloid receptor
References
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein coupled cannabinoid receptor in the mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- BUNDEY R.A., KENDALL D.A. Inhibition of receptor-mediated calcium responses by corticotrophin-releasing hormone in the CATH.a cell line. Neuropharmacology. 1999;38:39–47. doi: 10.1016/s0028-3908(98)00173-7. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C.S., TAO Q., BRIDGEN D.T., RAZDAN R.K., ZIMMER A.M., ZIMMER A., MARTIN B.R. Levels, metabolism and pharmacological activity of anandamide in CB1 cannabinoid receptor knockout mice: evidence for non CB1, non CB2 receptor-mediated actions of anandamide in the mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., DE PETROCELLIS L., FEZZA F., LIGRESTI A., BISOGNO T. Anandamide receptors. Prostaglandins Leukotrienes Essential fatty acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- DUNCAN M., KENDALL D.A., RALEVIC V. Effect of WIN55,212, a cannabinoid receptor agonist, on sensory neurotransmission in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001a;135:65P. [Google Scholar]
- DUNCAN M., KENDALL D.A., RALEVIC V. CP55,940, a cannabinoid receptor agonist, attenuates sensory neurotransmission via CB1-like receptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001b;135:285P. [Google Scholar]
- DUNCAN M., KENDALL D.A., RALEVIC V. The endocannabinoid noladin ether attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2002;136:13P. doi: 10.1038/sj.bjp.0705789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCAN M., KENDALL D.A., RALEVIC V. Δ9-Tetrahydrocannabinol attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2003a;138:214p. doi: 10.1038/sj.bjp.0705789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCAN M., KENDALL D.A., RALEVIC V.The effects of ruthenium red on the inhibitory actions of noladin ether, THC & HU210 on sensory neurotransmission in the rat isolated mesenteric arterial bed 2003b. First European Workshop on Cannabinoid Research
- DUNCAN M., MILLNS P., KENDALL D.A., RALEVIC V.Noladin ether attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a novel Gi/o linked cannabinoid receptor 2003c. 13th Symposium on Cannabinoids, International Cannabinoid Research Society
- FEZZA F., BISOGNO T., MINASSI A., APPENDINO G., MECHOULAM R., DI MARZO V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., LEDENT C., FREUND T.F. Novel cannabinoid-sensitive receptor mediates inhibition of glutamateric synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- HANUŠ L., SALEH A., FRIDE E., BREUER A., VOGEL Z., SHALEV D.E., KUSTANOVICH I., MECHOULAM R. 2-Arachidonyl glyceryl ether, an endogenous agonist at the cannabinoid receptor. Proc Natl Acad Sci U.S.A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARRIES M.H., DUCKWORTH M.D., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J.L., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:207–212. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- JÁRAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSAY R.M. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., BEN-SHABAT S., MEIRI U., HOROWITZ M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur. J. Pharmacol. 1998;362:R1–R3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- MILLNS P., CHAPMAN V., KENDALL D.A. Cannabinoid inhibition of the capsaicin-induced calcium response in rat dorsal root ganglion neurones. Br. J. Pharmacol. 2001;132:969–971. doi: 10.1038/sj.bjp.0703919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLNS P., CHAPMAN V., KENDALL D.A. Anandamide-evoked [Ca2+]i responses in adult dorsal root ganglion neurones. Br. J. Pharmacol. 2002;135:256P. doi: 10.1038/sj.bjp.0703919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFERTALER L., MO F.M., BATKAI S., LIU J., BEGG M., RAZDAN R.K., MARTIN B.R., BUKOSKI R.D., KUNOS G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- OKA S., TSUCHIE A., TOKUMURA A., MURAMASTU M., SUHARA Y., TAKAYAMA H., WAKU K., SUGIURA T. Ether-linked analogue of 2-arachidonylglycerol (noladin ether) was not detected in the brains of various mammalian species. J. Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- RALEVIC V. Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur. J. Pharmacol. 2003;472:1–21. doi: 10.1016/s0014-2999(03)01813-2. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., ABERDEEN J.A., BURNSTOCK G. Acrylamide-induced autonomic neuropathy of rat mesenteric vessels: histological and pharmacological studies. J. Auton. Nerv. Syst. 1991;34:77–88. doi: 10.1016/0165-1838(91)90010-z. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in the rat mesenteric arterial bed. Eur. J. Pharmacol. 2001;418:117–125. doi: 10.1016/s0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. The P1-purinoceptors that mediate the prejunctional inhibitory affect of adenosine on capsaicin-sensitive nonadrenergic noncholinergic neurotransmission in the rat mesenteric arterial bed are of the A1 subtype. J. Pharmacol. Exp. Ther. 1993;267:1100–1104. [PubMed] [Google Scholar]
- SAVINAINEN J.R., JÄRVINEN T., LAINE K., LAITINEN J.T. Despite substantial degradation, 2-arachidonylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependant G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ J.O.E.L.J., HSU A.K., BARBIERI J.T., LI P., GROSS G.J. Pertussis toxin abolishes the cardioprotective effect of ischemic preconditioning in intact rat heart. Am. J. Physiol. 1998;275:H495–H500. doi: 10.1152/ajpheart.1998.275.2.H495. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., GUNTHORPE M.J., BROUGH S.J., RANSON J., CAIRNS W., HAYES P.D., RANDALL A.D., DAVIS J.B. Characterisation using FLIPR of human vanilloid receptor (VR1) pharmacology. Eur. J. Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., NAKANE S., MIYASHITA T., KONDO S., SUHARA Y., TAKAYAMA H., WAKU K., SEKI C., BABA N., ISHIMA Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., JARAI Z., KUNOS G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- WOOD M.D., SMART D.Real time receptor function in vitro: microphysiometry and FLIPR Receptors: A Practical Approach 2001Oxford University Press: Oxford; 175–191.ed. Stanford, C.S. & Horton, R.W [Google Scholar]
- ZYGMUNT P.M., ANDERSSON D.A., HÖGESTÄTT E.D. Δ9-Tetrahydrocannabinol and cannabidiol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SØRGARD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–456. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]