Abstract
The effects of purinoceptor ligands for P2X1 and/or P2X3 receptors (α,β-meATP, IP5I, TNP-ATP, MRS 2179, PPADS, Phenol red and RO116-6446/008; i.v., n=4–5) and for P2Y1 receptors (PPADS, MRS 2179 and MRS 2269; i.v., n=3–5) were investigated on the distension-evoked ‘micturition reflex' in the urethane-anaesthetized female rat.
α,β-meATP (180 nmol kg−1 min−1), IP5I (10, 30 and 100 nmol kg−1), TNP-ATP (1 μmol kg−1), MRS 2179 (1 μmol kg−1) and PPADS (17 μmol kg−1) each caused maintained bladder contractions to occur during the infusion of saline into the bladder. PPADS (17 μmol kg−1 min−1) had a similar effect when infused intravesicularly. Regular bladder contractions were not observed until the infusion of saline was halted. For IP5I, TNP-ATP, MRS 2179 and PPADS, the magnitude of postinfusion isovolumetric contractions was significantly reduced and, for IP5I, this action was also associated with a significant reduction in urethral relaxation. Additionally, TNP-ATP caused a significant increase in the pressure and volume thresholds required to initiate a reflex.
Phenol red (a P2X1/P2X3 antagonist; 0.1 and 1 μmol kg−1) caused a significant increase in the pressure and volume thresholds required to initiate a reflex and, at the higher dose, also caused a reduction in postinfusion isovolumetric contractions.
RO116-6446/008 (a P2X1-selective antagonist; 1 and 10 μmol kg−1) only caused a reduction in postinfusion isovolumetric contractions.
It is concluded that P2X1 and P2X3 receptors play a fundamental role in the micturition reflex in urethane-anesthetized female rats. P2X3 receptor blockade raised the pressure and volume thresholds for the reflex, whereas P2X1 receptor blockade diminished motor activity associated with voiding. P2Y1 receptors may be involved in inhibition of rat detrusor tone.
Keywords: Purinoceptors; micturition; rats; P2X1 receptors; P2X3 receptors; P2Y1 receptors; α,β-methylene ATP; IP5I; TNP-ATP; Phenol red; RO116-6446/008; PPADS; MRS 2179; MRS 2269
Introduction
The neural control of urinary bladder emptying involves many different transmitters: acetylcholine, monoamines (noradrenaline, dopamine and 5-hydroxytryptamine), amino acids (glutamate and GABA), various peptides (e.g. enkephalins and neurokinins) and nitric oxide (NO) (see De Groat & Yoshimura, 2001). Interestingly, NO seems to be more involved with urethral function rather than with the bladder (Wibberley et al., 2002). Another transmitter system also identified as playing a role in urinary bladder contractions is the purinergic system, involving adenosine 5′-triphosphate (ATP) (Burnstock et al., 1978). Although there is extensive literature (see Burnstock, 2001) to indicate that many different types of P2 purinoceptors are present in the lower urinary tract, the physiological role of these receptors in micturition (or voiding) is still uncertain. In part, this uncertainty has been caused by a lack of P2 receptor subtype-selective agonists and antagonists to dissect the role of these receptors in the micturition reflex. To counteract the lack of selective receptor ligands for purinoceptors, gene knockout animal models have been used – particularly P2X1−/−, P2X2−/− and P2X3−/− gene deletion – in an attempt to identify the physiological roles of P2 receptor subtypes in the urinary bladder (Cockayne et al., 2000 2002; Vial & Evans, 2000; Vlaskovska et al., 2001).
In recent years, a new generation of selective antagonists for P2 receptors has been developed and such agents now provide the means to test and extend observations gleaned from knockout animal models. Thus, the present study was carried out to investigate further the physiological roles of P2X1, P2X3 and P2Y1 receptors in the micturition reflex in urethane-anaesthetized female rats using the archetypical smooth muscle P2X purinoceptor desensitizing agent α,β-methylene ATP (α,β-meATP; Kasakov & Burnstock, 1982), the P2X purinoceptor antagonist pyridoxal-5-phosphate-6-azophenyl 2′,4′-disulphonic acid (PPADS) (Lambrecht et al., 1992) and also a number of compounds with some selectivity towards various purinoceptors, such as for P2X1 receptors: diinosine pentaphosphate (IP5I, also a P2X3 receptor antagonist; King et al, 1999), trinitrophenyl adenosine 5′-triphosphate (TNP-ATP, also a P2X3 and P2X2/3 receptor antagonist; Virginio et al., 1998), RO116-6446/008 (a P2X1 receptor antagonist; A.P. Ford, personal communication), Phenol red (phenolsulphonephthalein sodium, also a P2X3 receptor antagonist; King et al., 2003); and for P2Y1 receptors: N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate (MRS 2179, also a P2X1 receptor antagonist; Boyer et al., 1998; Brown et al., 2000), MRS 2269 (Boyer et al, 1998) and PPADS (usually considered a P2X receptor antagonist; see above). The micturition reflex was evoked by bladder distension induced by intravesicular infusion of saline, and measurements were made of reflex-evoked bladder and urethral pressures, as well as of external urethral sphincter (EUS) electromyography (EMG). Preliminary accounts of some of these findings have been published (Knowles etal., 2000; 2001; 2003).
Methods
Experiments were carried out under the Animals (Scientific Procedures) Act, 1986. After completion of experiments, animals were killed by an overdose (administered i.v.) of pentobarbitone sodium.
General preparation
Experiments were carried out in 78 female, anaesthetized, spontaneously breathing Sprague–Dawley rats (250–350 g). Anaesthesia was induced and maintained during initial surgical procedures with isoflurane in oxygen (4%, reduced to 3%, as necessary). The left jugular vein, carotid artery and trachea were cannulated to permit intravenous injection of drugs, measurement of arterial blood pressure (and heart rate (HR)), and maintenance of a patent airway, respectively. Isoflurane administration was discontinued and anaesthesia was maintained for the remainder of each experiment with i.v. injections of urethane (25% solution, initial dose of 1.0–1.2 g kg−1; see Maggi et al., 1986). The depth of anaesthesia was assessed by the stability of blood pressure and HR, and by the absence of limb withdrawal in response to paw pinch. Supplementary doses of urethane (0.1 g kg−1) were given when necessary. Blood pressure was measured using a pressure transducer (Gould Statham P23Db), and the HR was derived electronically on-line from the blood pressure signal using AcqKnowledge version 3.5.3 software (Biopac Systems Inc., U.S.A.). Body core temperature was maintained between 36 and 38°C by feedback from a rectally placed thermosensor to a heated blanket. The animals were infused (6 ml kg−1 h−1, i.v.) with a solution comprising 10 ml plasma substitute (Gelofusine; Braun Medical, Sheffield, U.K.), 10 ml distilled water, 0.04 g glucose and 0.168 g sodium bicarbonate, to prevent the development of nonrespiratory acidosis and to maintain blood volume.
Measurement of urinary bladder and urethral pressures and EUS-EMG
The ureters were isolated at the level of the kidney through retroperitoneal incisions. Each ureter was divided and the proximal end cannulated to drain the kidneys and prevent urine flow into the bladder. The rat's head was secured in a stereotaxic frame that was tilted at an angle of 10–20° so that the animal could lie in a supine position. This arrangement ensured that intravesical bladder pressure was not affected by the weight of the rat. The urinary bladder was exposed and two cannulae were inserted into the lumen of the bladder through an incision in the bladder dome. One cannula allowed the measurement of intraluminal pressure and the other permitted infusion of saline into the bladder. A third cannula was inserted through the bladder dome into the proximal urethra: for details of this method, plus a diagram, see Conley et al. (2001). The urethra was perfused at a constant rate (0.08 ml min−1), so that changes in urethral pressure were reflected as changes in resistance to the flow of saline. For the measurement of external urethral striated muscle sphincter (EUS) EMG, two fine copper wire electrodes (0.2 mm diameter) were used and inserted percutaneously approximately 0.5 cm lateral and 0.5 cm caudal on each side of the external urethral opening from which the EMG signal was measured. It should be noted that such a measurement cannot preclude that activity observed is not from the pelvic floor muscle as well. At the end of experiments, animals were given decamethonium (3 mg kg−1, i.v.) to confirm that the electrodes were in actuality recording from striated muscle EMG and also to determine the level of recording noise.
Experimental protocols
Surgical preparation was followed by a stabilization period of 30 min. During stabilization, inspired air was enriched with oxygen (0.05–0.10 L min−1) and the blood gases and pH were monitored and maintained at 90–130 mmHg pO2, 40–50 mmHg pCO2 and pH 7.3–7.4. Saline was then infused into the urinary bladder at 0.05 ml min−1 (the maximal physiological rate of diuresis in the cat; see Klevmark, 1974), which resulted in a gradual increase in bladder wall muscle tension and, eventually, the occurrence of spontaneous phasic bladder contractions. The saline infusion was discontinued after three consecutive bladder contractions of the same amplitude. Bladder and urethral activities were monitored for a further 5 min before the saline was drained from the bladder through the bladder infusion cannula. This first infusion was carried out to ‘test and prime' the system, and was followed by a rest period of 20 min. This priming procedure also ensured that the placement of the electrodes for recording the EMG signal was appropriate. A second infusion was carried out to evoke a ‘control' reflex. Again, the saline infusion was discontinued after three consecutive bladder contractions of the same amplitude. Bladder and urethral activities were monitored for a further 5 min before the saline was drained from the bladder. After 5 min, either saline, vehicle (DMSO) or a test drug was injected (i.v.) or α,β-meATP infusion (i.v.) was begun and 5 min later a distension-induced bladder reflex was evoked as described above. In the case of the intravesicular infusion of PPADS, this was carried out after the control reflex. PPADS (10 mg ml−1) was infused via the bladder cannula at a rate of 2 ml h−1 for 30 min. To prevent vesicular damage and the possibility of evoking micturition, the cannula measuring intravesicular pressure was open during this infusion period – thus allowing exposure of the bladder to PPADS and drainage of excess fluid. The bladder was then fully drained and 5 min later a distension-induced bladder reflex was evoked as described above.
Data capture and analysis
Arterial blood pressures, bladder and urethral pressures were continuously displayed on a chart recorder (Grass Instruments) and captured (1500 samples s−1) by an MP100WSW interface (Biopac Systems Inc., U.S.A.) to allow data to be acquired and analysed off-line using AcqKnowledge version 3.5.3 software (Biopac Systems Inc., U.S.A.). HRs were derived electronically on-line from the blood pressure signal using this software. The amplified EMG signal was captured (1500 samples s−1) and the input was integrated off-line, again using AcqKnowledge.
Analysis of reflex-evoked bladder and urethral responses and baseline values
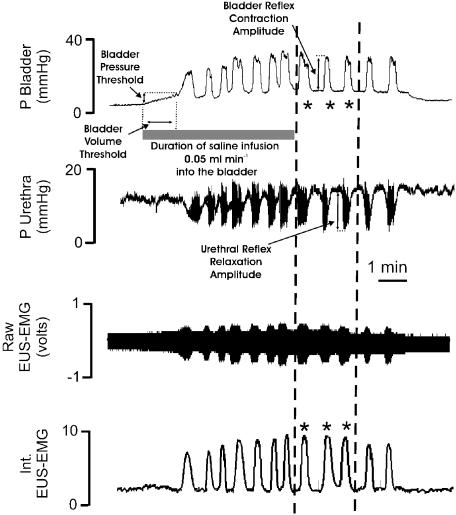
The micturition reflexes, evoked before and after the test solution (i.v.), were selected for analysis (Figure 1). Saline infusion into the bladder evoked large-amplitude, rhythmic bladder contractions, which have been assumed to represent a micturition reflex (Maggi et al., 1986). After saline infusion was discontinued, the mean amplitude (mmHg) and duration (s) of the next three bladder contractions were measured. These three bladder contractions were analysed because they represented isovolumetric reflex bladder contractions, that is, they occurred when the amount of saline in the bladder, and therefore resting bladder pressure, was constant. The mean amplitude (mmHg) and duration (s) of the three urethral relaxations accompanying isovolumetric bladder contractions were also measured. The mean area of the integrated EMG bursts associated with isovolumetric bladder contractions and urethral relaxations was measured as well. The micturition reflex pressure thresholds were taken as the bladder pressure (mmHg) at which the first reflex bladder contraction (i.e. the first fast rise in bladder pressure for those experiments in which a maintained contraction occurred) with concomitant reflex urethral activity (i.e. relaxation and high-frequency oscillations) occurred. The volume threshold (ml) was calculated from the time to evoke this first bladder contraction by the constant influx of saline infusion. All baseline values were the mean values over a 3 min period and were measured 2 min before reflexes and test agent responses. The following baseline measurements were made: urethral pressure and background activity (mean value measured over 3 min); mean arterial pressure (MAP) and HR (measured over 30 s, 2 min before the beginning of the infusion to evoke the control reflex). Changes in baseline variables caused by the test substances were measured 2 min before the test reflex and compared to the values obtained 2 min before the control reflex. It should be noted that the control reflex did not affect baseline values. Changes in these variables caused by either drugs or vehicle were measured as a percentage of the control values and compared using Student's unpaired t-test. Values of P<0.05 were considered significant. All values are expressed as mean±s.e.m. unless otherwise stated.
Figure 1.
Analysis of bladder variables recorded from an urethane-anaesthetized female rat: traces showing the effects evoked by infusing (0.5 ml min−1) saline (stippled horizontal bar) into the bladder on bladder and urethra pressure (mmHg) and on raw and integrated external urethral sphincter (EUS-EMG) activity and how these changes were measured. Once three similar bladder contractions (phasic increases in bladder pressure) were observed, the saline infusion was switched off and measurements were made on the height and duration of each of these contractions (*), that is, those falling between the two dashed lines as well as the associated urethral relaxations and integrated EUS-EMG. These values were then averaged to give the control reflex-evoked changes (see Table 1). In addition, the thresholds, pressure and volume to evoke this reflex were also calculated. The measurements were made from the beginning of the saline infusion to the beginning of a bladder contraction associated with urethral relaxation.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: Phenol red, urethane and α,β-meATP (lithium salt) from Sigma Aldrich Chemical Co., (Poole, Dorset); pentobarbitone sodium from Rhône Mérieux Ltd (Harlow, Essex); isoflurane from Abbott Labs (Queenborough, Kent); PPADS from Tocris Cookson Ltd (Bristol); TNP-ATP from Molecular Probes (Eugene, OR, U.S.A.); RO116-6446/008 (1H-benzoimidazole-2-carboxylic acid[(R)-1-((1S,2R,3S)-1-cyclo-hexylmethyl-3-cyclopropyl-2,3-dihydroxy-propylcarbamoyl)-2-thiazol-4-yl-ethyl]-amide) was synthesized by Dr F. Padilla at Roche (Palo Alto, CA, U.S.A.); 2′-deoxy-N6-methyladenosine 3′,5′-diphosphate diammonium (MRS 2179) and N6-methyl-1,5-anhydro(2-adenin-9-yl)-2,3-dideoxy-D-arabino-hexitol-4′,6′-bisphosphate (MRS 2269) were gifts from Dr K.A. Jacobson at NIH (Bethesda, U.S.A.); IP5I was a gift from Dr J. Pintor (University of Complutense, Madrid, Spain). Drugs were given i.v. as their respective salts. α,β-meATP, PPADS and IP5I were dissolved in saline, while RO116-6446/008, MRS 2179, MRS 2269 and Phenol red were dissolved in 10% DMSO to give a final volume of administration of 0.1 ml.
Results
Initial reflex-evoked responses
Infusion of saline into the urinary bladder in 78 female rats caused distension of the bladder, which subsequently evoked a micturition reflex (Figure 1). This reflex was characterized by the appearance of rhythmic bladder contractions of 29±3 mmHg. The contractions had a mean duration of 28±5 s and occurred at a frequency of 1±0.2 contractions min−1. The mean bladder pressure threshold to evoke the micturition reflex was 8.2±1.8 mmHg, which was reached when 0.10±0.02 ml of saline had been infused into the bladder. In addition, each of the rhythmic bladder contractions was associated with a fall in urethral pressure of 16±4 mmHg, which continued for 29±3 s before returning to baseline levels. In 67 out of the 78 animals, high-frequency oscillations in urethral pressure occurred at the peak of each bladder contraction and these oscillations had a mean amplitude of 12±4 mmHg and duration of 11±3 s. In the remaining animals, high-frequency oscillations in urethral pressure were not observed. This was probably due to variations in the position of the urethral cannula. The fall in urethral pressure was accompanied by bursts of EUS-EMG activity. Each reflex bladder contraction was associated with an increase in MAP (6±2 mmHg) and HR (16±4 beats min−1). The mean data for the control reflex in each experimental group are shown in Table 1 . The combined mean baseline data for urethral pressures, MAP and HR were 20±4 mmHg, 108±2 mmHg and 391±6 beats min−1, respectively. The mean baseline data for individual experimental groups are shown in Table 2 .
Table 1.
Control reflex-evoked changes, caused by ‘initial' bladder distension, in bladder and urethral pressures and urethral striated muscle activity (EUS-EMG) for each experimental group in urethane-anaesthetized female rats
| Thresholds | Rhythmic bladder contractions | Urethral relaxations | EUS-EMG activity | |||||
|---|---|---|---|---|---|---|---|---|
| Experimental group | n | Bladder pressure (mmHg) | Saline volume (ml) | Amplitude (mmHg) | Duration (s) | Amplitude (mmHg) | Duration (s) | Total amount (area s−1) |
| Saline bolus i.v. | 5 | 8.2±1.8 | 0.10±0.02 | 29±3 | 28±5 | 16±4 | 29±3 | 4.3±0.5 |
| Saline infusion i.v. | 5 | 8.4±1.5 | 0.12±0.02 | 27±5 | 27±4 | 17±5 | 29±4 | 3.8±0.9 |
| DMSO | 5 | 7.9±1.4 | 0.12±0.04 | 28±4 | 29±5 | 17±3 | 31±2 | 4.4±0.7 |
| α,β-meATP | ||||||||
| 60 nmol kg−1 min−1, i.v. | 4 | 8.3±2.1 | 0.10±0.03 | 30±4 | 27±6 | 19±5 | 30±5 | 3.6±0.9 |
| 180 nmol kg−1 min−1, i.v. | 5 | 8.2±1.7 | 0.12±0.05 | 31±3 | 28±2 | 16±2 | 28±3 | 4.3±0.5 |
| PPADS | ||||||||
| 17 μmol kg−1, i.v. | 5 | 8.4±1.5 | 0.13±0.04 | 27±3 | 29±2 | 17±5 | 30±2 | 4.5±0.6 |
| 17 μmol kg−1 min−1 | 5 | 8.0±2.2 | 0.11± 0.03 | 28±2 | 28±4 | 16±3 | 29±1 | 3.3±0.8 |
| IP5I | ||||||||
| 10 nmol kg−1 | 3 | 7.7±1.4 | 0.11±0.05 | 30±2 | 27±3 | 18±2 | 27±4 | 3.6±1.2 |
| 30 nmol kg−1 | 5 | 8.6±1.1 | 0.15±0.01 | 27±5 | 29±4 | 16±2 | 30±3 | 3.9±0.7 |
| 100 nmol kg−1 | 5 | 8.2±1.9 | 0.13±0.03 | 28±2 | 26±5 | 18±4 | 28±1 | 4.1±0.9 |
| TNP-ATP | ||||||||
| 1 μmol kg−1 | 5 | 7.7±1.6 | 0.19±0.04 | 31±2 | 30±3 | 21±5 | 30±4 | 4.1±1.2 |
| MRS 2179 | ||||||||
| 0.1 μmol kg−1 | 3 | 8.7±1.5 | 0.13±0.04 | 30±6 | 26±2 | 18±2 | 29±6 | 2.7±1.1 |
| 1 μmol kg−1 | 5 | 9.1±1.3 | 0.12±0.05 | 32±3 | 28±5 | 20±3 | 29±3 | 3.6±0.8 |
| Phenol red | ||||||||
| 0.1 μmol kg−1 | 5 | 8.1±1.7 | 0.09±0.03 | 28±2 | 26±3 | 19±2 | 33±2 | 4.2±0.8 |
| 1 μmol kg−1 | 5 | 7.6±2.0 | 0.11±0.04 | 32±4 | 29±4 | 17±4 | 35±3 | 3.7±0.7 |
| RO116-6446/008 | ||||||||
| 1 μmol kg−1 | 5 | 7.8±1.5 | 0.09±0.03 | 30±1 | 26±5 | 17±3 | 27±2 | 3.2±0.4 |
| 10 μmol kg−1 | 5 | 7.6±1.3 | 0.08±0.02 | 30±4 | 24±2 | 19±2 | 25±1 | 3.8±0.8 |
| MRS 2269 | ||||||||
| 1 μmol kg−1 | 3 | 8.9±1.9 | 0.08±0.02 | 30±4 | 28±4 | 15±3 | 30±2 | 3.3±1.4 |
All values are mean±s.e.m.
Table 2.
Baseline values, before ‘initial' bladder distension-evoked reflexes, for urethral MAP and HR for each experimental group in urethane-anaesthetized female rats
| Experimental group | n | Bladder pressure (mmHg) | Urethral pressure (mmHg) | MAP (mmHg) | Heart (beat) |
|---|---|---|---|---|---|
| Saline bolus i.v. | 5 | 2.9±0.4 | 20.2±3.2 | 102±4 | 385±10 |
| Saline infusion i.v. | 5 | 2.4±0.5 | 18.1±2.7 | 110±3 | 376±12 |
| DMSO i.v. | 5 | 3.3±0.4 | 23.3±2.2 | 109±3 | 401±12 |
| α,β-meATP | |||||
| 60 nmol kg−1 min−1, i.v. | 4 | 2.3±0.8 | 19.6±2.8 | 112±5 | 390±8 |
| 180 nmol kg−1 min−1, i.v. | 5 | 3.7±0.6 | 21.5±4.6 | 108±2 | 395±15 |
| PPADS | |||||
| 17 μmol kg−1, i.v. | 5 | 2.7±0.6 | 18.6±3.2 | 115±5 | 380±18 |
| 17 μmol kg−1 min−1 | 5 | 2.4±0.5 | 21.1±4.6 | 112±2 | 389±13 |
| IP5I | |||||
| 10 nmol kg−1 | 3 | 3.2±0.9 | 19.6±5.3 | 108±4 | 374±14 |
| 30 nmol kg−1 | 5 | 2.8±0.5 | 17.3±2.4 | 107±2 | 393±9 |
| 100 nmol kg−1 | 5 | 3.5±0.6 | 19.1±4.3 | 115±3 | 386±12 |
| TNP-ATP | |||||
| 1 μmol kg−1 | 5 | 3.4±0.5 | 22.5±5.9 | 120±8 | 383±12 |
| MRS 2179 | |||||
| 0.1 μmol kg−1 | 3 | 3.8±0.9 | 17.8±5.6 | 104±6 | 393±14 |
| 1.0 μmol kg−1 | 5 | 2.7±0.7 | 20.9±2.6 | 118±3 | 375±10 |
| Phenol red | |||||
| 0.1 μmol kg−1 | 5 | 2.6±0.5 | 18.2±4.6 | 112±5 | 404±20 |
| 1 μmol kg−1 | 5 | 2.5±0.8 | 19.2±2.8 | 116±4 | 377±18 |
| RO116-6446/008 | |||||
| 1 μmol kg−1 | 5 | 2.2±0.6 | 18.3±4.4 | 106±3 | 387±8 |
| 10 μmol kg−1 | 5 | 2.5±0.5 | 16.4±3.5 | 101±5 | 382±14 |
| MRS 2269 | |||||
| 1 μmol kg−1 | 3 | 3.1±1.1 | 21.1±4.2 | 113±5 | 390±7 |
All values are mean±s.e.m.
Effects of α,β-meATP (60 and 180 nmol kg−1 min−1), PPADS (17 μmol kg−1), IP5I (10, 30 and 100 nmol kg−1), TNP-ATP (1 μmol kg−1) and MRS 2179 (0.1 and 1 μmol kg−1)
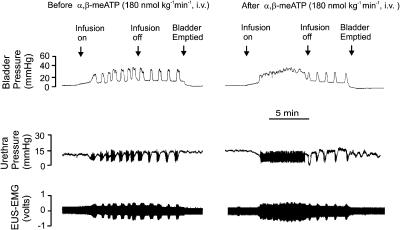
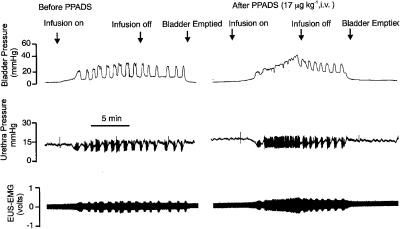
Over the period of continuous saline infusion into the bladder, the intravenous administration of each ligand caused the initial volume-evoked bladder contraction to be maintained, in contrast to the rhythmic contractions observed over the same time period in the control animals (Figures 2, 3, 4, 5 and 6). These maintained contractions were associated with a maintained fall in urethral pressure of between 7 and 10 mmHg and the appearance of sustained EUS-EMG activity. For α,β-meATP (n=5), these effects were only observed at the dose of 180 nmol kg−1 min−1 (Figure 2). The same effects were observed when PPADS (Figure 3) was administered either by intravenous injection (17 μmol kg−1, n=5) or by intravesicular infusion (17 μmol kg−1 min−1, n=5) and were also observed with Ip5I, TNP-ATP and MRS 2179 (i.v.).
Figure 2.
Urethane-anaesthetized female rat: traces showing the effects of i.v. infusion of α,β-meATP (180 nmol kg−1 min−1) for 5 min on bladder and urethral pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after infusion of α,β-meATP. Note that there were no rhythmic contractions during the infusion of saline and this was associated with a maintained relaxation of the urethra and an increase in EUS-EMG activity, but these rhythmic contractions of the bladder returned on switching off the saline infusion.
Figure 3.
Urethane-anaesthetized female rat: traces showing the effects of i.v. PPADS (17 μmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after administration i.v. of PPADS. Note that there were no rhythmic contractions during the infusion of saline and this was associated with a maintained relaxation of the urethra and increase in EUS-EMG activity but these rhythmic contractions of the bladder returned on switching off the saline infusion.
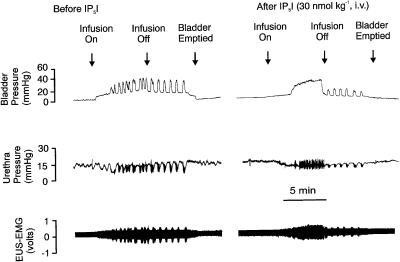
Figure 4.
Urethane-anaesthetized female rat: traces showing the effects of i.v. IP5I (30 nmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after administration i.v. of IP5I. Note that there were no rhythmic contractions during the infusion of saline and this was associated with a maintained relaxation of the urethra and increase in EUS-EMG activity. On turning off the saline infusion, these rhythmic bladder contractions returned although of reduced magnitude.
Figure 5.
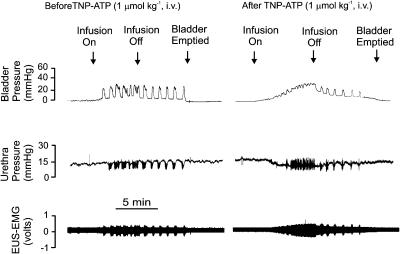
Urethane-anaesthetized female rat: traces showing the effects of i.v. TNP-ATP (1 μmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after administration i.v. of TNP-ATP. Note that there were no rhythmic contractions during the infusion of saline and this was associated with a maintained relaxation of the urethra and increase in EUS-EMG activity. On turning off the saline infusion, these rhythmic bladder contractions returned although of reduced magnitude.
Figure 6.
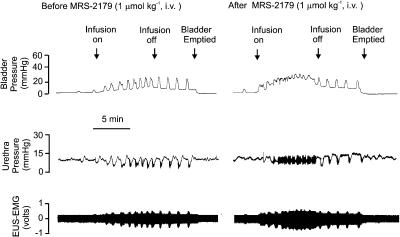
Urethane-anaesthetized female rat: traces showing the effects of i.v. MRS 2179 (1 μmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after administration i.v. of MRS 2179. Note that there were no rhythmic contractions during the infusion of saline and this was associated with a maintained relaxation of the urethra and increase in EUS-EMG activity. On turning off the saline infusion, these rhythmic bladder contractions returned although of reduced magnitude.
For the postinfusion period when the bladder still contained saline, the actions of PPADS, IP5I, TNP–ATP and MRS 2179 differed from α,β-meATP in that the magnitude of isovolumetric bladder contractions was significantly reduced by 21±5% for i.v. PPADS (but not for PPADS given by intravesicular infusion), by 20±5, 65±8 and 81±5%, at the doses of 10, 30 (Figure 4) and 100 nmol kg−1 for IP5I, by 10±2% for TNP-ATP (Figure 5) and by 20±5% for MRS 2179 (Figure 6) at the dose of 1 μmol kg−1, respectively. With IP5I, the reduction in isovolumetric bladder contractions also correlated with significant decreases in the associated urethral relaxations (56±11 and 79±12%) and increases in EUS-EMG activity (61±11 and 72±13%). However, this was not observed for TNP-ATP and MRS 2179. TNP-ATP caused an increase in volume and pressure threshold (Figure 5) of 10±2 and 12±2%, respectively. The combined data for all these ligands on reflex-evoked variables are shown in Table 3 . None of these drugs affected baseline bladder or urethral pressures. Furthermore, these drugs also had little effect on blood pressure and HR. However, α,β-meATP, at both doses, and PPADS evoked a transient rise in MAP and, in the case of α,β-meATP, the infusion of this drug was associated with a transient fall in HR.
Table 3.
Changes (Δ) in % from control reflex-evoked increases in bladder pressure, urethral relaxation, increase in urethral striated muscle activity (EUS-EMG) and the pressure threshold to initiate these reflex-evoked effects caused by test substances in urethane-anaesthetized female rats
| Experimental group | n | Δ Reflex-evoked bladder contraction (%) | Δ Reflex-evoked urethral relaxations (%) | Δ Reflex-evoked EUS-EMG (%) | Δ Reflex pressure threshold (%) |
|---|---|---|---|---|---|
| Saline bolus i.v. | 5 | −3±2 | −4±7 | −4±4 | −6±5 |
| Saline infusion i.v. | 5 | −9±3 | −4±3 | −3±2 | −7±3 |
| DMSO i.v. | 5 | −2±2 | −6±3 | 3±6 | −6±4 |
| α,β-meATP | |||||
| 60 nmol kg−1, i.v. | 4 | −4±2 | −8±2 | −4±3 | −1±2 |
| 180 nmol kg−1 min−1, i.v. | 5 | −8±5 | −2±3 | −5±3 | −5±3 |
| PPADS | |||||
| 17 μmol kg−1, i.v. | 5 | −21±5* | 6±8 | −3±4 | −4±3 |
| 17 μmol kg−1 min−1 | 5 | −9±5 | 5±3 | 5±2 | −4±2 |
| IP5I | |||||
| 10 nmol kg−1 | 3 | −20±5* | −7±3 | −13±9 | −3±5 |
| 30 nmol kg−1 | 5 | −65±8** | −56±3** | −61±11** | −8±3 |
| 100 nmol kg−1 | 5 | −81±5** | −79±11** | −72±13** | −5±5 |
| TNP-ATP | |||||
| 1 μmol kg−1 | 5 | −10±2* | −2±2 | −2±2 | 12±2* |
| MRS 2179 | |||||
| 0.1 μmol kg−1 | 3 | −5±2 | −3±3 | 2±2 | −4±2 |
| 1.0 μmol kg−1 | 5 | −20±5* | 7±3 | −13±9 | −5±5 |
| Phenol red | |||||
| 0.1 μmol kg−1 | 5 | −5±3 | −4±2 | −10±4 | 84±9** |
| 1 μmol kg−1 | 5 | −15±9* | −6±3 | −8±4 | 60±3** |
| RO116-6446/008 | |||||
| 1 μmol kg−1 | 5 | −18±3* | 18.3±4.4 | −7±4 | 2±2 |
| 10 μmol kg−1 | 5 | −26±8** | 16.4±±3.5 | −10±4 | 4±6 |
| MRS 2269 | |||||
| 1 μmol kg−1 | 3 | −5±2 | −5±3 | −11±4 | −3±5 |
All values are mean±s.e.m.
P<0.05 compared with the appropriate vehicle control using Student's unpaired t-test.
P<0.001 compared with the appropriate vehicle control using Student's unpaired t-test.
Effects of Phenol red (0.1 and 1 μmol kg−1) on reflex-evoked responses
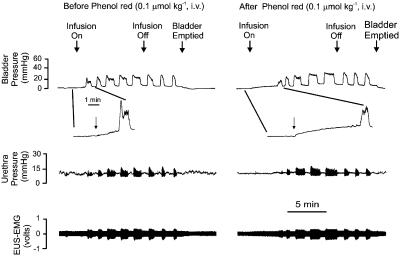
The P2X1/P2X3 antagonist Phenol red caused a significant increase in the pressure and volume thresholds required to evoke micturition compared with vehicle (see Table 3). A trace of one of the low-dose experiments is shown in Figure 7. At the higher dose, there was a significant reduction in the magnitude of isovolumetric bladder contractions by 15±9%, although neither dose affected the accompanying urethral relaxations and associated increase in EUS-EMG activity. Administration (i.v.) of Phenol red (n=5) had no significant effect on the baseline bladder and urethral pressures, MAP and HR.
Figure 7.
Urethane-anaesthetized female rat: traces showing the effects of i.v. Phenol red (0.1 μmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. There is also an inset showing an expanded time base of the period it took for the saline infusion to initiate a bladder contraction. The second panel shows the same scenario 5 min after administration i.v. of the low dose of phenol red. It should be noted that the time to initiate the first bladder contraction by the saline infusion was delayed. This is better illustrated by comparing the expanded time base inserted with that in the control panel.
Effects of RO116-6446/008 (1 and 10 μmol kg−1) on reflex-evoked responses
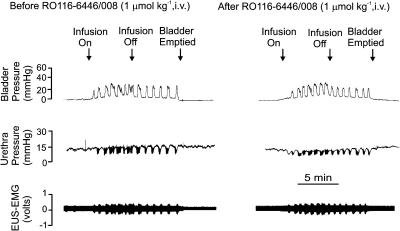
The P2X1-selective antagonist RO116-6446/008 (i.v.) did not have any effect on the initial infusion-evoked reflex responses. However, the magnitude of isovolumetric bladder contractions was significantly reduced at both doses when compared to vehicle control (see Table 3). The associated reduction in urethral pressure and increase in EUS-EMG activity was similar to that seen with other P2X1 antagonists (Figure 8), except at the higher dose where there was a small but significant inhibition of the increase in EUS-EMG activity. The volume and pressure thresholds required to evoke the micturition reflex were unaffected. RO116-6446/008 had no significant effect on the baseline bladder and urethral pressures, MAP and HR.
Figure 8.
Urethane-anaesthetized female rat: traces showing the effects of i.v. RO116-6446/008 (1 μmol kg−1) on bladder and urethra pressure (mmHg) and on raw external urethral sphincter (EUS-EMG) activity during the micturition reflex evoked by distension of the urinary bladder by saline (0.05 ml min−1). The first panel shows the control effects of infusion of saline into the bladder (infusion on) to evoke contractions (increases in bladder pressure); after three similar contractions the infusion was stopped (infusion off) and the bladder allowed to contract isovolumetrically. At 5 min after the saline infusion had been switched off, the bladder was emptied. The second panel shows the same scenario 5 min after administration i.v. of RO116-6446/008.
MRS 2269 (1 μmol kg−1) on reflex-evoked responses
Administration of the P2Y1-selective antagonist MRS 2269 (i.v., n=3) had no effect on the reflex (see Table 3) or baseline bladder and urethral pressures, MAP and HR.
Effects of vehicles on reflex-evoked responses and baseline values
Administration of the vehicles saline (bolus i.v. or slow infusion i.v.) or 10% DMSO (i.v.) had no significant effect on reflex-evoked changes in bladder and urethral pressures and EUS-EMG activity in all experimental groups (see Table 3). In addition, neither of the vehicles had any effect on baseline bladder and urethral pressures, blood pressure and HR.
Discussion
All the P2 purinoceptor ligands examined, with the exception of MRS 2269, affected the micturition reflex, indicating that P2 purinoceptors do play a physiological role in the control of micturition, at least in the rat. These effects were mainly concerned with bladder regulation – rather than urethral regulation – and could be divided into three phenomena: increasing the volume and pressure thresholds for initiation of the reflex bladder contractions; attenuation of the height of the isovolumetric contractions after saline infusion was terminated; replacement of rhythmic contractions with maintained contractions during the distension period. TNP-ATP and Phenol red were the only ligands found to increase significantly the volume and pressure thresholds to elicit micturition. In the following potency order (based on the threshold concentration to cause inhibition) IP5I, TNP-ATP, RO116-6446/008, Phenol red, MRS 2179 and PPADS caused a significant attenuation of the height of the isovolumetric contractions. Curiously, the slow infusion (i.v.) of α,β-meATP did not affect the height of postinfusion isovolumetric bladder contractions. Lastly, all the tested ligands (except RO116-6446/008 and Phenol red) caused maintained contractions of the bladder during the distension period. The potency order (based on the threshold concentration to elicit tonic contraction) was IP5I, TNP-ATP, MRS 2179, followed by PPADS; α,β-meATP also caused maintained contractions although it acts primarily as a desensitizing agonist/antagonist (Kasakov & Burnstock, 1982). Thus, the question arose, by what mechanisms did these P2 ligands bring about the above three phenomena?
Increases in volume and pressure thresholds
For the first of these phenomena (increase in the micturition threshold), it is suggested that the key to the observed changes can be found in the nature of the neurogenic reflex evoked by saline infusion into the urinary bladder. The steady infusion of saline and subsequent increase in wall smooth muscle tension will activate the sensory nerves of the urinary bladder (Maggi et al., 1986). The suburothelial sensory nerve endings of the rat bladder are heavily invested with P2X3 receptors (Cockayne et al., 2000; Elneil et al., 2001; Vlaskovska et al., 2001) and the cell bodies of afferent nerves passing through rat bladder smooth muscle also possess P2X3 receptors as well as heteromeric P2X2/3 receptors (Zhong et al., 2003). The urothelium itself releases ATP in proportion to the intravesicular pressure and thereby activates P2X receptors on bladder sensory nerves (Ferguson et al., 1997; Vlaskovska et al., 2001). Deletion of the P2X3 gene (P2X3−/−) results in a marked urinary bladder hyporeflexia, in terms of increased voiding volume (Cockayne et al., 2000) and markedly decreased bladder afferent sensitivity in response to increasing intravesicular pressure (Vlaskovska et al., 2001). Furthermore, deletion of the P2X2 gene (P2X2−/−), and loss of heteromeric P2X2/3 and homomeric P2X2 receptors, also results in a marked urinary bladder hyporeflexia, in terms of increased thresholds to contraction on bladder filling (Ford et al., 2002).
From the above observations, it could be predicted that those P2 purinoceptor ligands showing blocking activity at homomeric P2X3 receptors and heteromeric P2X2/3 receptors (see Table 4 ) would significantly alter the volume and pressure thresholds of the micturition reflex. In this respect, TNP-ATP did alter the volume and pressure thresholds in our experiments (Figure 5). This drug is a potent inhibitor of cloned rat P2X3 and P2X2/3 receptors (Virginio et al., 1998; Liu et al., 2001) and native rat P2X3 and P2X2/3 receptors in isolated DRG neurons (Burgard et al., 1999). It is also active under in vivo conditions and, in comparison to morphine, blocked nociceptive behaviour to the same extent in mice (Honore et al., 2002). The actions of TNP-ATP on the volume and pressure thresholds were mimicked by Phenol red (Figure 7) – a newly identified P2 purinoceptor ligand (King et al., 2003) – which is the least potent of the tested drugs at recombinant P2X3 receptors and is quite inactive at P2X2/3 receptors (Table 4). Phenol red mimicry of TNP-ATP, yet its inactivity at P2X2/3 receptors, suggested that TNP-ATP could not exert its actions via P2X2/3 receptors. Instead, TNP-ATP and phenol red may have acted solely on P2X3 receptors. The higher potency in vivo of non-nucleotidic phenol red compared to other tested P2X3 receptor antagonists (including PPADS) might be explained by its resistance to ectoATPase breakdown (as opposed to the nucleotide-based antagonists used) and that it does not possess a chemically unstable azo bridge (in contrast to PPADS). Accordingly, its relative potency may well be higher in vivo than so far noted in vitro. Thus, the weight of evidence suggests that only those drugs blocking P2X3 receptors significantly altered the threshold of the micturition reflex, a conclusion supported by the observed disruption of urinary bladder reflexes in P2X3−/− gene-deficient mice (Cockayne et al., 2000; Vlaskovska et al., 2001).
Table 4.
Potency of P2 purinoceptor ligands at recombinant P2X1, P2X2/3, P2X3 (rat (r) isoforms) and P2Y1 (turkey (t) isoform) receptors
| rP2X3 | rP2X2/3 | rP2X1 | tP2Y1 | |
|---|---|---|---|---|
| receptora,b,c,d | receptora,c | receptorb,e,f | receptorf,g | |
| *EC50/IC50 (μM) | *EC50/IC50 (μM) | *EC50/IC50 (μM) | IC50/Kb § (μM) | |
| α,β-meATPa,e,g | *1.9±0.3 | *8.6±1.2 | *3.2±0.2 | Inactive |
| TNP-ATPa,e | 0.29±0.04 (× 10−3) | 11.2±2.5 (× 10−3) | 1.1±0.04 (× 10−3) | ND |
| PPADSa,e,g | 0.22±0.05 | 2.3±0.4 | 0.12±0.07 | 1.58§ |
| IP5Ia,e | 2.8±0.7 | Inactive | 3.1±0.4 (× 10−3) | ND |
| MRS 2179b,f | 12.9±0.1 | ND | 1.15±0.21 | 0.33±0.06 |
| Phenol redc | 25.2±0.3 | Inactive | 3.3±0.3 | ND |
| RO116-6446/008d | Inactive at 1 μM | ND | 0.97±0.07 (× 10−3) | ND |
| MRS 2269b,f | Inactive at 10 μM | ND | Inactive at 10 μM | 1.64±0.43 |
EC50 (*: for α,β-meATP) and either IC50 or Kb (§) values for other drugs acting at recombinant P2 receptors. Data expressed as mean±s.e.m. and taken from:
Liu et al. (2001).
Brown et al.(2000).
King et al. (2003).
B.F. King, J. Gever & A.P. Ford (unpublished data).
Nandanan et al. (1999).
Attenuation of isovolumetric contractions
The second major action of the drugs tested was the attenuation of the height of the isovolumetric phasic contractions in the bladder after saline infusion was terminated. The motor innervation of the rat detrusor muscle is complex and involves both cholinergic and purinergic components (see Burnstock, 2001). It has been shown that atropine-resistant (non-cholinergic) electrical events (EJPs) underpinning phasic contractions are purinergic in rat detrusor muscle (Hoyle & Burnstock, 1985). Also, arterial injections of ATP and α,β-meATP close to the bladder immediately initiate phasic contractions in the unanaesthetized rat (Igawa et al., 1993). Furthermore, the P2X receptor antagonist PPADS inhibited purinergic motor responses in the pithed rat (Hegde et al., 1998), and the P2 receptor antagonists suramin and PPADS displaced high-affinity [3H]-α,β-meATP binding from rat detrusor muscle preparations (Bo et al., 1994). Additionally, there is dense P2X1-like immunoreactivity (Hansen et al., 1998; Lee et al., 2000; Elneil et al., 2001) in close apposition to motor nerve varicosities in the rat detrusor (Hansen et al., 1998), and there is a significant reduction in neurogenic motor activity in the bladder of P2X1−/− gene-deficient mice (Vial & Evans, 2000).
The above observations on the motor innervation of the bladder are consistent with the involvement of P2X receptors, most likely the P2X1 receptor subtype. In the present study, the observed potency order for P2 purinoceptor ligands attenuating phasic bladder contractions, in vivo, was in good agreement with their potency order for blockade of cloned rat P2X1 receptors, in vitro (see Table 4). This list of P2X1-active drugs included PPADS and MRS 2179, each of which is also known to block P2Y1 receptors (Table 4). Since this G-protein-coupled P2Y1 receptor has been located in rat detrusor (Obara et al., 1998), the selective P2Y1 receptor antagonist MRS 2269 (Nanadanan et al., 1999; Brown et al., 2000) was tested but was found to be inactive. Thus, again the weight of evidence suggested that the observed reduction in isovolumetric phasic contractions was due to an effective blockade of P2X1 receptors in rat detrusor muscle, and that the residual phasic contractions after administration of P2X1 receptor antagonists were in all probability cholinergic in origin.
Maintained urinary bladder contraction
The third major action of the tested compounds – but not of Phenol red and RO116-6446/008 at the doses given – was the maintained bladder contraction after the threshold for the micturition reflex had been reached. The inactivity of Phenol red and RO116-6446/008, which are P2X1 receptor antagonists, suggested that maintained bladder contraction could not be mediated by the P2X1 subtype. Of the other tested drugs, the most potent was IP5I followed by TNP-ATP, MRS 2179 and PPADS in this order. Curiously, the desensitizing agonist/antagonist α,β-meATP also mimicked the above blocking agents. No single P2X or P2Y receptor is blocked by this range of antagonists apart from the P2X1 subtype, but, even then, it is difficult to imagine how blockade of excitatory P2X1 receptors could lead to maintained contraction of the bladder. Thus, it is proposed that these maintained contractions may involve the blockade of other types of P2 purinoceptors distinct from P2X1.
The ability of MRS 2179 (and PPADS) to cause maintained contraction implies an involvement of P2Y1 receptors, which are known to be present in the rat detrusor (Obara et al., 1998). In carbachol-precontracted rat and mouse detrusor muscle, muscle tone is inhibited by ATP (Boland et al., 1993; Bolego et al., 1995) through a G-protein-coupled P2Y1-like receptor that is unaffected by adenosine receptor antagonists and adenosine uptake inhibitors (Bolego et al., 1995). An inhibitory G-protein-coupled P2Y1-like receptor has also been found in marmoset detrusor, where inhibition was evoked by ATP=2-MeSATP⩾ADP (α,β-meATP inactive) and was unaffected by adenosine receptor antagonists (McMurray et al., 1998). If inhibitory P2Y1 receptors occur in rat detrusor, as the above relaxant actions of ATP, 2-MeSATP and ADP suggest, the maintained contractions caused by MRS 2179 (and PPADS) might be due to the blockade of P2Y1 receptors excited by ATP released from the distension-stimulated urothelium. The inactivity of the P2Y1-selective antagonist MRS 2269 ran counter to a proposal for inhibitory P2Y1 receptors, unless the dose used was too low to cause an effective blockade. MRS 2269 is five-fold less potent than MRS 2179 at turkey P2Y1 receptors (Table 4), and could not be used at a five-fold higher dose in our experiments because only a small sample of this development compound was available.
The role of IP5I and TNP-ATP (and α,β-meATP) in also causing maintained contraction remains unclear. None of these drugs is known to block P2Y1 receptors and, therefore, they must have exerted their effect in another way. IP5I and TNP-ATP are antagonists of the P2X3 receptors on sensory nerve endings beneath the urothelium. TNP-ATP, but not IP5I, blocks heteromeric P2X2/3 receptors and, therefore, IP5I mimicry of TNP-ATP suggested that P2X2/3 receptors were not involved. Therefore, it is possible that blockade of P2X3 receptors by TNP-ATP and IP5I (and by the desensitizing agent α,β-meATP) might prevent local axon reflexes from releasing ATP to activate inhibitory P2Y1 receptors. Certainly, sensory nerve endings are known to release ATP (Holton, 1959) and local axon reflexes are also known to occur within the detrusor muscle (Lecci et al., 1999). Thus, this further proposal of local axon reflexes, combined with the presence of inhibitory P2Y1 receptors, could possibly explain the complex pharmacology of P2 purinoceptor antagonists in causing maintained contraction of the bladder.
Conclusion
It should be pointed out that the above interpretations of these purionoceptor ligands have considered that the main site of action is in the periphery, at the level of bladder. However, it is possible that some of the differences in the actions between these ligands could be due to these drugs having the ability to access different sites at which purinoceptors are involved in micturition, for instance the central nervous system. Little is known about central purinergic pathways in the control of micturition, if it is involved at all, and the present experiments with ionically charged antagonists that might not easily pass through the blood–brain barrier do tend to favour a peripheral site of action for the effects of these ligands. In this latter case, the ability to cause a maintained bladder contraction after the threshold for the micturition reflex has been reached seems to be peripheral, at the level of bladder itself, as PPADS also causes this effect when applied intravesically. Another surprising observation was that when isovolumetric bladder contractions were attenuated, as in the case of PPADS, TNP-ATP and MRS 2179, there was no reduction in the associated urethral relaxation. Such relaxations were only observed for IP5I, and the mechanism behind this observation remains to be determined.
Thus, in summary, P2 purinoceptor ligands interacting with P2X3 receptors appeared to alter the initiation of the micturition reflex, whereas P2 ligands interacting with P2X1 receptors altered the phasic contractions associated with voiding. Additionally, P2Y1 receptor blockade may remove an accommodatory, inhibitory drive to rat detrusor muscle. These in vivo experiments demonstrate the importance of purinergic signalling in the urinary bladder, at least in the rat, and further indicate that the development of drugs selective to each P2 purinoceptor subtype may be influential in correcting urinary bladder instability in its many clinical forms.
Acknowledgments
We thank Drs A.P. Ford and F. Padilla (Roche, Palo Alto, U.S.A.) for the provision of RO116-6446/008 and Roche (Palo Alto, U.S.A.) for financial support. B.F.K. was also funded by BBSRC.
Abbreviations
- MAP
mean arterial pressure
- HR
heart rate
- ATP
adenosine 5′-triphosphate
- α,β-meATP
α,β-methylene ATP
- PPADS
pyridoxal-5-phosphate-6-azophenyl 2′,4′-disulphonic acid
- IP5I
diinosine pentaphosphate
- EMG
electromyogram
- EUS
external urethral sphincter
- TP
threshold pressure
References
- BO X., FISCHER B., MAILLARD M., JACOBSON K.A., BURNSTOCK G. Comparative studies on the affinities of ATP derivatives for P2X-purinoceptors in rat urinary bladder. Br. J. Pharmacol. 1994;112:1151–1159. doi: 10.1111/j.1476-5381.1994.tb13204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLAND B., HIMPENS B., PAQUES C., CASTEELS R., GILLIS J.M. ATP induced-relaxation in the mouse bladder smooth muscle. Br. J. Pharmacol. 1993;108:749–753. doi: 10.1111/j.1476-5381.1993.tb12872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLEGO C., PINNA C., ABBRACCHIO M.P., CATTABENI F., PUGLISI L. The biphasic response of rat vesical smooth muscle to ATP. Br. J. Pharmacol. 1995;114:1557–1562. doi: 10.1111/j.1476-5381.1995.tb14939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br. J. Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN S.G., KING B.F., KIM Y.C., JANG S.Y., BURNSTOCK G., JACOBSON K.A. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev. Res. 2000;49:253–259. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGARD C., NIFORATOS W., VAN BIESEN T., LYNCH K.J., TOUMA E., METZGER R.E., KOWALUK E.A., JARVIS M.F. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J. Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.Purinergic signalling in the lower urinary tract Purinergic and Pyrimidinergic Signalling I: Molecular, Nervous and Urinogenitary System Function 2001151Heidelberg, Berlin: Springer; 423–515.Handbook of Experimental Pharmacology. ed. Abbracchio, M.P. & Williams, M. VolChap 15, pp [Google Scholar]
- BURNSTOCK G., COCKS T., CROWE R., KASAKOV L. Purinergic innervation of the guinea-pig urinary bladder. Br. J. Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKAYNE D.A., DUNN P.M., BURNSTOCK G., FORD A.P.D.Generation and electrophysiological characterization of P2X2 and P2X2/P2X3 knockout (KO) mice Soc. Neurosci. Abstr. 20022852.12 [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G., ZHU Q.M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- CONLEY R.K., WILLIAMS T.J., FORD A.P., RAMAGE A.G. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br. J. Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GROAT W.C., YOSHIMURA N. Pharmacology of the lower urinary tract. Annu. Rev. Pharmacol. Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- DUNN P.M., LIU M., ZHONG Y., KING B.F., BURNSTOCK G. Diinosine pentaphosphate: an antagonist which discriminates between recombinant P2X3 and P2X2/3 receptors and between slowly- and rapidly-desensitising purinergic responses in sensory neurones. Br. J. Pharmacol. 2000;130:1378–1384. doi: 10.1038/sj.bjp.0703404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELNEIL S., SKEPPER J.N., KIDD E.J., WILLIAMSON J.G., FERGUSON D.R. Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- FERGUSON D.R., KENNEDY I., BURTON T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism. J. Physiol. (Lond.) 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD A.P.D., ZHU Q.M., ZHONG Y., RONG W., YIP P., HAMILTON S.G., MCMAHON S.B., BURNSTOCK G., COCKAYNE D.P2X receptors and ATP regulation of urinary bladder function Soc. Neurosci. Abstr. 200228633.1 [Google Scholar]
- HANSEN M.A., BALCAR V.J., BARDEN J.A., BENNETT M.R. The distribution of single P2X1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J. Neurocytol. 1998;27:529–539. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., MANDEL D.A., WILFORD M.R., BRIAUD S., FORD A.P., EGLEN R.M. Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur. J. Pharmacol. 1998;349:75–82. doi: 10.1016/s0014-2999(98)00173-3. [DOI] [PubMed] [Google Scholar]
- HOLTON P. The liberation of ATP on antidromic stimulation of sensory nerves. J. Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONORE P., MIKUSA J., BIANCHI B., MCDONALD H., CARTMELL J., FALTYNEK C., JARVIS M.F. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H., BURNSTOCK G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene ATP. Eur. J. Pharmacol. 1985;114:239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., MATTIASSON A., ANDERSSON K.E. Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br. J. Pharmacol. 1993;109:473–479. doi: 10.1111/j.1476-5381.1993.tb13593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASAKOV L., BURNSTOCK G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1982;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- KING B.F., LIU M., BROWN S.G., KNIGHT G., TOWNSEND-NICHOLSON A., DUNN P.M., CUNNANE T.C., FORD A.P., BURNSTOCK G. P2X receptor blockade by the pH indicator dye, Phenol Red. J. Physiol. (Lond.) 2003;547P:C69. [Google Scholar]
- KING B.F., LIU M., PINTOR J., GUALIX J., MIRAS-PORTUGAL M.T., BURNSTOCK G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br. J. Pharmacol. 1999;128:981–988. doi: 10.1038/sj.bjp.0702876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEVMARK B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta. Physiol. Scand. 1974;90:565–577. doi: 10.1111/j.1748-1716.1974.tb05621.x. [DOI] [PubMed] [Google Scholar]
- KNOWLES I.D., BURNSTOCK G., RAMAGE A.G. Investigation of the role of P2X receptors in the micturition reflex anaesthetized female rats. J. Physiol. 2000;526:65P. [Google Scholar]
- KNOWLES I.D., KING B.F., BURNSTOCK G., RAMAGE A.G. Investigation of the role of P2X1 and P2Y1 receptors in the micturition reflex of anaesthetized female rats. J. Physiol. (Lond.) 2001;533P:136P. [Google Scholar]
- KNOWLES I.D., KING B.F., RAMAGE A.G. Investigation of the effects of phenol red P2X1 and P2X3 receptor antagonist on the micturition reflex in anaesthetized female rats. Br. J. Pharmacol. 2003;138:99P. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBRECHT G., FRIEBE T., GRIMM U., WINDSCHEIF U., BUNGARDT E., HILDEBRANDT C., BAUMERT H.G., SPATZ-KUMBEL G., MUTSCHLER E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur. J. Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- LECCI A., MEINI S., TRAMONTANA M., GIULINANI S., CRISCOLI M., MAGGI C.A. Kinin B1 receptor-mediated motor responses in normal and inflamed rat urinary bladder in vivo. Regul. Peptides. 1999;80:41–47. doi: 10.1016/s0167-0115(99)00009-9. [DOI] [PubMed] [Google Scholar]
- LEE H.Y., BARDINI M., BURNSTOCK G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J. Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- LIU M., KING B.F., DUNN P.M., RONG N.W., TOWNSEND-NICHOLSON A., BURNSTOCK G. Co-expression of P2X3 and P2X2 receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J. Pharmacol. Exp. Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., MELI A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J. Pharmacol. Methods. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- MCMURRAY G., DASS N., BARADING A. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br. J. Pharmacol. 1998;123:1579–1586. doi: 10.1038/sj.bjp.0701774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANDANAN E., CAMAIONI E., JANG S.Y., KIM Y.C., CRISTALLI G., HERDEWIJN P., SECRIST J.A., TIWARI K.N., MOHANRAM A., HARDEN T.K., BOYER J.L., JACOBSON K.A. Structure–activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J. Med. Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBARA K., LEPOR H., WALDEN P.D. Localization of P2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J. Urol. 1998;160:587–591. [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G., SURPRENANT A., NORTH R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- VLASKOVSKA M., KASAKOV L., RONG W., BODIN P., BARDINI M., COCKAYNE D.A., FORD A.P., BURNSTOCK G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBBERLEY A., NUNN P.A., NAYLOR A.M., RAMAGE A.G. An investigation of the effects of zaprinast, a PDE inhibitor, on the nitrergic control of the urethra in anaesthetized female rats. Br. J. Pharmacol. 2002;136:399–414. doi: 10.1038/sj.bjp.0704735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., RAHMAN M., NOEL C.A., CHURCHILL L., BURNSTOCK G., UNWIN R.J., KING B.F. Sensitization by extracellular Ca2+ of rat P2X5 receptor and its pharmacological properties compared with rat P2X1. Mol. Pharmacol. 2002;62:957–966. doi: 10.1124/mol.62.4.957. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., BANNING A.S., COCKAYNE DA, FORD A.P.D.W., BURNSTOCK G., MCMAHON S.B. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]