Abstract
Smooth muscle membrane potential and tension measurements were made in isolated mesenteric resistance arteries from rats exposed to bacterial endotoxin (lipopolysaccharide, LPS; 10 mg kg−1, i.p.) for 3 h to mimic septic shock syndrome.
Over this period, rats developed an endotoxaemic response, assessed in vivo as a 41±4 mmHg drop in mean blood pressure, vascular hyporeactivity to noradrenaline (1 μg kg−1, i.v.) and a significant increase in core body temperature.
In mesenteric small resistance arteries from these rats (o.d. 180 – 240 μm), phenylephrine (0.01–3 μM)-evoked contraction was not altered when compared with arteries from sham-operated animals, but the concentration–relaxation curve to acetylcholine (ACh; 0.01 – 3 μM) displayed a small, but significant, shift to the right.
The smooth muscle resting membrane potential (−70.3±1.6 mV) in arteries from LPS-treated rats was significantly greater than in control arteries (−55.4±1.2 mV), but in both cases the smooth muscle was depolarized to a similar potential by the application of Nω-nitro-L-arginine methyl ester (L-NAME; 0.3 mM; −54.1±2.3 vs −52.4±2.5 mV) or glibenclamide (10 μM; −55.0±2.1 vs −50.4±2.0 mV).
ACh (1 μM) elicited a maximal hyperpolarization, which ranged from −14.7±3.2 mV (in arteries from LPS-treated rats) to –20.6±2.4 mV (in arteries from sham-operated rats), and was not altered by the presence of L-NAME. Levcromakalim (1 μM) increased the smooth muscle membrane potential by around −24 mV in arteries from both sets of experimental animals.
These results indicate that at the level of the resistance vasculature, endotoxaemia is associated with pronounced smooth muscle hyperpolarization reflecting the action of NO on KATP channels. These changes were not associated with vascular hyporeactivity or depressed endothelial cell function in vitro, suggesting that mesenteric resistance arteries may not contribute to equivalent changes in vivo.
Keywords: Endotoxin, NO, KATP channels, rat mesenteric resistance artery
Introduction
The disease state of septic shock reflects an overwhelming and widespread level of infection, leading to circulatory failure, which is associated with marked disturbances in tissue vascular perfusion. As Gram-negative infections are responsible for approximately 50% of cases of septic shock, the use of bacterial lipopolysaccharide (by injection of LPS) provides an effective means to induce a state of sepsis, which closely mimics the disease in man (Thiemermann, 1997; Deitch, 1998). Septic shock is characterized by a decrease in vascular systemic resistance and a dramatic hyporeactivity to vasoconstrictor agents. As vasoconstrictor agents are used routinely in attempts to reverse hypotension during septic shock, the hyporeactivity presents a very significant clinical problem.
The hyporeactivity to endogenous and to exogenous vasoconstrictor agents during septic shock appears to reflect an enhanced formation of nitric oxide (NO), principally by the calcium-independent, inducible isoform of NO synthase (Thiemermann, 1994). The evidence for this statement derives from the fact that NO synthase inhibitors can reverse most of the cardiovascular changes associated with endotoxic shock in a number of in vivo and in vitro animal models (Rees et al., 1990; Fleming et al., 1991; Gray et al., 1991; Wu et al., 1995a, 1995b). The majority of the in vitro studies to investigate the mechanism responsible for vascular hyporeactivity have used aortic tissue from rodents (Julou-Schaeffer et al., 1990; Wu et al., 1995a, 1995b; Hall et al., 1996; Scott et al., 1996; Wu et al., 1998; Chen et al., 1999; 2000; 2003). From a physiological standpoint, the aorta is of course a large conduit vessel that only makes a small relative contribution to systemic vascular resistance. Studies in smaller rodent resistance arteries suggest that it is much more difficult to induce vascular hyporeactivity in these arteries. For instance, Mitchell et al. (1993) used the isolated and superfused mesenteric arterial bed from endotoxaemic rats (ex vivo) and Glembot et al. (1995) superfused skeletal muscle arterioles with endotoxin. In both cases, no reduction in the responses to vasoconstrictor agents was observed. However, hyporeactivity could be demonstrated in small mesenteric and femoral vessels, ex vivo or in vitro, if the bathing medium contained the precursor of NO L-arginine, or the vessels were in culture medium containing bovine fetal serum (Scheider et al., 1992; 1994; Mitolo-Chieppa et al., 1996; O'Brien et al., 2001).
In addition to variability between experimental regimes, both the pattern of the LPS-induced changes in vascular reactivity and the contribution of inducible NO synthase to the impaired vascular constriction differ between vascular beds (Piepot et al., 2002). Thus, at the level of resistance arteries, it remains unclear how significant hyporeactivity is, and the mechanism(s) responsible. Smooth muscle relaxation to NO is known to be mediated in a number of ways including (i) increasing the intracellular cyclic guanylyl 3',5'-monophosphate (cyclic GMP) level (see Butler et al., 1995), (ii) hyperpolarizing the smooth muscle cell membrane directly and/or indirectly by activating K+ channels (Tare et al., 1990; Garland & McPherson, 1992; Bolotina et al., 1994; Mistry & Garland, 1998), (iii) altering the Na+-K+ pump activity in motor nerves in animals treated with LPS (Liu et al., 1995; Chen et al., 1999; 2000; 2003) and (iv) decreasing the intracellular Ca2+ level (Weisbrod et al., 1998). In the present study, we provide the first investigation of vascular hyporeactivity using myographic and electrophysiological recording techniques in isolated mesenteric small resistance arteries taken from rats in which endotoxic shock had been shown to develop.
Methods
In vivo experiments
Experiments were performed using male Wistar rats (250–280 g, Charles River, UK) using procedures approved by the University of Bath Ethical Review Committee and performed under Home Office Licence Authority (Animals Scientific Procedure Act 1986). All surgery was performed under anaesthesia induced with intraperitoneal injection of urethane (1.2 g kg−1), with no recovery. The trachea was cannulated to facilitate respiration followed by the right carotid artery to measure phasic and mean arterial blood pressure (MAP). The left jugular vein was cannulated to enable the administration of drugs. Following surgery, cardiovascular parameters were allowed to stabilize for 15 min, then noradrenaline (NA, 1 μg kg−1 i.v.) and 10 min later either vehicle (saline) or Escherichia coli LPS (10 mg kg−1 i.v.) was administered, and cardiovascular system parameters and body temperature were monitored for the next 3 h. The pressor responses to NA were reassessed prior to vehicle or LPS injection (i.e. at time 0) and then every hour.
In vitro experiments
At 3 h after the injection of vehicle or LPS, rats were killed by exsanguination and the mesentery quickly removed and placed in Krebs buffer at room temperature. A segment (2 mm in length) of a third-order branch of the superior mesenteric artery was carefully cleared of adherent tissue and mounted in Krebs buffer at 37°C in a small vessel myograph (model 400A, Danish Myotechnology, Denmark), at a tension equivalent to that generated at 0.9 times the diameter of the vessel at 100 mmHg (Garland & McPherson, 1992). The functional viability of endothelial cells was assessed by the ability of 1 μM acetylcholine (ACh) to induce over 95 and 80% relaxation in preparations from sham-operated rats and LPS-treated animals, respectively, following contraction of the arterial segments with a submaximal concentration of phenylephrine (PE, 3 μM). The artery was superfused at 3–4 ml min−1 with oxygenated Krebs buffer at 37°C, except during experimental recording when flow was stopped. Drugs were applied under static conditions and mixed by continuous gassing with 5% CO2 in 95% O2 at 37°C. Individual smooth muscle cells were impaled with sharp glass electrodes (backfilled with 2 M KCl, tip resistances approximately 80–100 MΩ), to enable measurement of smooth muscle membrane potential (Garland & McPherson, 1992). An impalement was considered to be successful only if it was maintained continuously before, during and after the drug application. In addition, at the end of every protocol, the potential of electrode withdrawal almost went back to 0 mV, if not, we had to recalculate its shift in the potential.
Before any intracellular recording, PE (0.01–3 μM) and ACh (0.01–3 μM) were used to evaluate the in vitro vascular reactivity in the mesenteric arteries. Smooth muscle cells were then impaled and hyperpolarization to either ACh (1 μM) or levcromakalim (LV; 1 μM) measured in arteries from sham-operated and LPS-treated rats. In order to clarify the role of NO and ATP-sensitive K+ channels (KATP channels) in endotoxin-evoked hyporeactivity, inhibitors of NO synthase (i.e. Nω-nitro-L-arginine methyl ester, L-NAME; 0.3 mM) and KATP channels (i.e. glibenclamide; 10 μM) were also used in these preparations.
Solutions and drugs
All experiments used Krebs buffer of the following composition (mM): NaCl 118.0, NaHCO3 25.0, KCl 3.6, MgSO4·7H2O 1.2, KH2PO4 1.2, glucose 11.0, CaCl2 2.5. Drugs used were all from Sigma. LV (0.01 M) and glibenclamide (10 mM) were dissolved initially in dimethylsulphoxide (DMSO). Control experiments indicated that DMSO had no direct action in the final concentrations applied. All other stock solutions were prepared in distilled water.
Statistical analysis
All values in the figures and text are expressed as mean±s.e.m. of n observations, where n represents the number of animals studied. Statistical evaluation was performed using analysis of variance (ANOVA) followed by a multiple comparison test (Scheffe's test), except for the determination of membrane potential changes, which were analysed by unpaired Student's t-test. A P-value of less than 0.05 was considered to be statistically significant.
Results
Endotoxin evoked changes in vivo
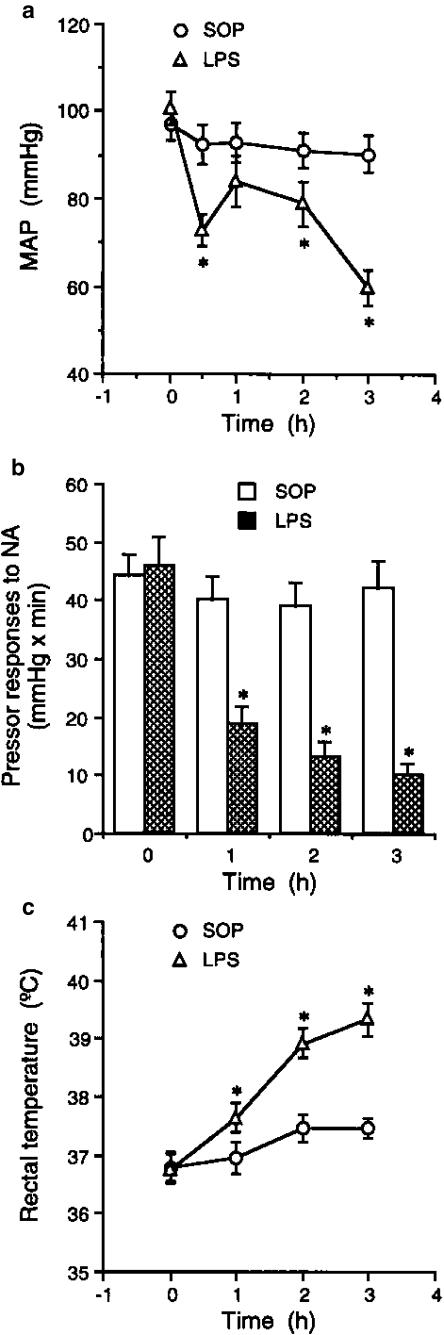
The baseline values for MAP, pressor responses to NA (1 μg kg−1 i.v.) and rectal temperature did not differ significantly between sham-operated and LPS-treated rats studied (Figure 1a–c). Following the injection of LPS (10 mg kg−1 i.v.), a significant fall in MAP developed over the 3 h period, reflecting the development of endotoxaemia (Figure 1a). This fall was also associated with a marked vascular hyporeactivity to NA (Figure 1b) and a significant increase in rectal temperature (Figure 1c).
Figure 1.
Effects of endotoxin on (a) MAP, (b) pressor responses to NA and (c) rectal temperature in the anaesthetized rat. Depicted are the changes in MAP, pressor responses to NA (1 μg kg−1 i.v.) and rectal temperature during the experimental period in animals, which received injection of vehicle (SOP; n=7) or E. coli LPS (10 mg kg−1; n=9) for 3 h. Data are expressed as mean±s.e.m. *P<0.05 represents significant differences when compared with the SOP group.
Assessment of reactivity in isolated mesenteric arteries
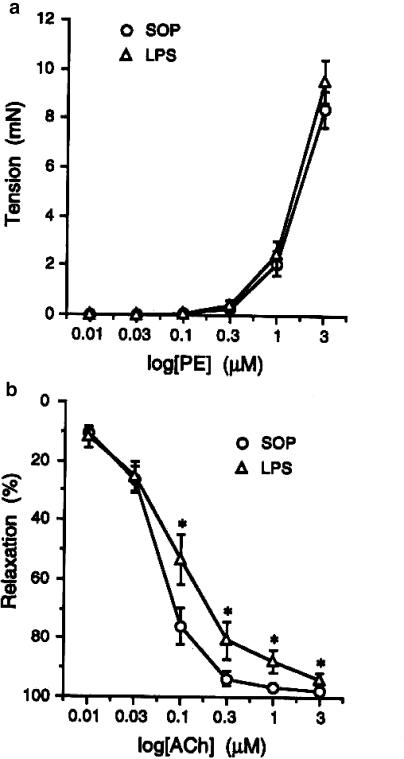
In isolated mesenteric arteries, there was no change in the ability of cumulative applications of PE (0.01–3 μM). to evoke contraction in arteries from sham-operated and LPS-treated rats (Figure 2a). In contrast, endothelium-dependent relaxation to ACh (0.01–3 μM) was slightly attenuated in arteries from the LPS-treated rats (Figure 2b).
Figure 2.
Effects of endotoxin on (a) the contraction induced by PE and (b) the relaxation induced by ACh ex vivo. Depicted are the changes in the contraction induced by PE (0.01–3 μM) and in the relaxation induced by ACh (0.01–3 μM) in mesenteric small resistance arterial rings obtained from animals that received injection of vehicle (saline; sham-operated, SOP; n=7) or E. coli LPS (10 mg kg−1; n=9) for 3 h. Data are expressed as mean±s.e.m. *P<0.05 represents significant differences when compared with the SOP group.
Influence of LPS on smooth muscle membrane potential and effect of L-NAME and glibenclamide
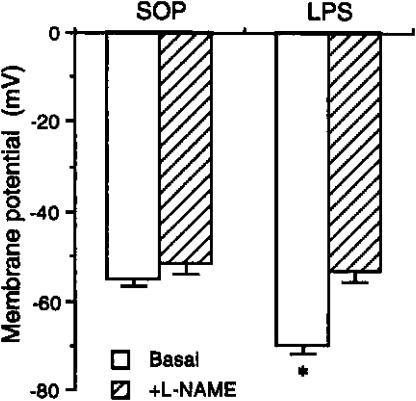
The resting membrane potential of smooth muscle cells in arteries from LPS-treated rats (−70.3±1.6 mV, n=9), was significantly increased compared to cells in arteries from sham-operated animals (−55.4±1.2 mV, n=11) (Figure 3). This difference in resting potential was abolished in the presence of L-NAME (0.3 mM) (−54.1±2.3 mV in the LPS-treated and −52.4±2.5 mV in the sham-operated rat, respectively).
Figure 3.
Effects of L-NAME on the basal membrane potential changes in mesenteric small resistance arteries from rats treated with saline or endotoxin. Depicted are the changes in the membrane potential of basal (n=7) and when challenged with L-NAME (0.3 mM, n=5–7) in mesenteric small resistance arterial rings obtained from animals that received injection of vehicle (saline; sham-operated, SOP) or E. coli LPS (10 mg kg−1) for 3 h. Data are expressed as mean±s.e.m. *P<0.05 represents significant differences when compared with the SOP group.
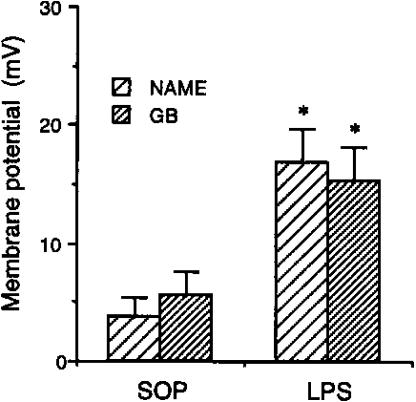
An equivalent reduction in the resting membrane potential to L-NAME was obtained with the KATP channel inhibitor glibenclamide (10 μM) (Figure 4). The resting potential in the presence of glibenclamide was reduced to a similar value, −50.4±2.0 and −55.0±2.1 mV in arteries from sham-operated and LPS-treated rats, respectively. This meant that the reduction was greater in the arteries from LPS-treated rats, reflecting the larger resting potential (Figure 4).
Figure 4.
Changes of membrane potential caused by L-NAME and glibenclamide (GB) in mesenteric small resistance arteries from rats treated with saline or endotoxin. Depicted are the changes in the membrane potential at the presence of L-NAME (n=8–11) and GB (n=3–4) in mesenteric small resistance arterial rings obtained from animals that received injection of vehicle (saline; sham-operated, SOP) or E. coli LPS (10 mg kg−1) for 3 h. Data are expressed as mean±s.e.m. *P<0.05 represents significant differences when compared with the SOP group.
In addition, in two further series of experiments, either glibenclamide or L-NAME was added after the other, to assess the effect on resting membrane potential in arteries from sham-operated rats. Glibenclamide applied after L-NAME did not stimulate any further depolarization (−49.4±0.4 mV vs −51.1± 2.7 mV, n=3; P>0.05), while the addition of L-NAME on the top of glibenclamide also failed to depolarize further the membrane potential (−52.7±4.5 mV vs −53.4±2.6 mV, n=4; P>0.05).
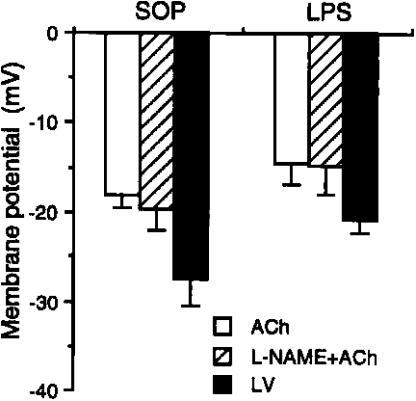
Hyperpolarization to ACh and LV
The maximal hyperpolarization evoked with ACh (1 μM), −18.1±1.5 and −14.7±2.3 mV, was not different in arteries from sham-operated and LPS-treated rats. With LV (1 μM), the situation was similar, with values of −27.0±3.0 and −21.1±1.3 mV (Figure 5). With ACh, the hyperpolarization in arteries from both LPS and SOP animals was not altered by the additional presence of L-NAME (Figure 5).
Figure 5.
Changes of membrane potential caused by ACh, L-NAME plus ACh, and LV in mesenteric small resistance arteries from rats treated with saline or endotoxin. Depicted are the changes in the membrane potential in the presence of ACh (n=7–8), L-NAME plus ACh (1 μM, n=4–5), and LV (n=3–5) in mesenteric small resistance arterial rings obtained from animals that received injection of vehicle (saline; sham-operated, SOP) or E. coli LPS (10 mg kg−1) for 3 h. Data are expressed as mean±s.e.m.
Discussion
This study provides the first demonstration that sepsis is associated with a pronounced hyperpolarization of the smooth muscle cells in a resistance artery, and that this is most likely due to the increased synthesis of NO serving to activate KATP channels. Furthermore, these data show that hypotension and vascular hyporeactivity in vivo do not reflect any significant functional damage to the endothelium nor a depression in the reactivity of isolated mesenteric resistance arteries.
Although LPS-treated animal models have been used to investigate the mechanisms responsible for vascular hyporeactivity during sepsis, the majority of studies in vitro have involved large conduit arteries such as the aorta (Julou-Schaeffer et al., 1990; Wu et al., 1995a, 1995b; 1998; Hall et al., 1996; Scott et al., 1996; Chen et al., 1999; 2000; 2003). The reactivity of these large arteries cannot be extrapolated directly to the small arteries and arterioles, which represent the major source of systemic vascular resistance. Therefore, it is clearly essential to assess the effect of sepsis at the level of the resistance vasculature. Studies using small arteries are very limited in number, and present a conflicting picture with regard to reactivity to constrictor agents (Scheider et al., 1992; 1994; Mitchell et al., 1993; Glembot et al., 1995; Mitolo-Chieppa et al., 1996; O'Brien et al., 2001). There are a number of possible explanations for these discrepancies, such as the species used, the origin of specific vessels and/or variation in experimental conditions. For example, using vessels harvested from endotoxaemic animals (ex vivo) or vessels superfused with endotoxin in vitro, Mitchell et al. (1993) and Glembot et al. (1995) failed to observe any significant reduction in constrictor responses, whereas hyporeactivity was apparent in small mesenteric and femoral vessels ex vivo or in vitro if incubated with either L-arginine or with serum (Scheider et al., 1992; 1994; Mitolo-Chieppa et al., 1996; O'Brien et al., 2001). These differences may indicate a role for some contributory factor in the sepsis-associated redistribution of cardiac output, and/or possibly a central action due to inducible NO synthase.
Whatever the precise explanation for these apparent discrepancies, in the present study, vascular hyporesponsiveness was shown to be present in vivo before smooth muscle reactivity was assessed directly in a resistance artery in vitro. As a result, we confirmed that rats treated with endotoxin display a similar hypotension (MAP=60±4 mmHg at 3 h after LPS injection) and vascular hyporeactivity (pressor responses to NA were only 20±3% at 3 h after LPS injection, compared to sham-operated rats) to patients with clinically defined septic shock. However, although the animals developed a vascular hyporeactivity similar to that in human septic shock, this reduction in reactivity did not reflect an inherent change at the level of the smooth muscle or a significant loss of endothelial cell function. In the latter case, although there was a reduction in endothelium-dependent relaxation, the reduction was only small and the ability of ACh to evoke an endothelium-derived hyperpolarizing factor (EDHF)-mediated hyperpolarization was not attenuated, indicating that overall endothelial cell function was fairly well maintained. Certainly, the evidence does not suggest significant endothelial cell damage as reported previously (Arditi et al., 1993).
The absence of any hyporeactivity to the α1-agonist PE in isolated mesenteric arteries from LPS-treated animals is similar to data reported by Mitchell et al. (1993) using superfused mesenteric arteries from endotoxin-treated animals. However, both these studies contrast with our previous work with the aorta (Chen et al, 2000). Using a similar approach to the present study, we exposed rats to LPS in vivo, allowed an endotoxaemic response to develop and then removed the aorta for in vitro measurement of reactivity. In contrast to the mesenteric resistance arteries, constrictor responses to PE in the aorta were significantly depressed. But as in the present study, the resting potential of the vascular smooth muscle cells was significantly increased, and by slightly more than that in the mesenteric arteries. In the aorta, the raised resting membrane potential appeared to reflect an action of NO on both KATP and BKCa channels (Chen et al, 2000). This is slightly different from the mesenteric vessels, where hyperpolarization could be explained solely by an overproduction of NO activating KATP, as normal resting potentials were obtained in the presence of glibenclamide. Furthermore, the specific BKCa blocker iberiotoxin did not reverse the increased membrane potential in the mesenteric artery (−68.4±2.1 vs −70.1±2.4 mV, n=3).
The increased resting potential in mesenteric arteries from LPS-treated rats appeared simply to reflect the action of the increased synthesis of NO, presumably by iNOS. The NO synthase inhibitor L-NAME evoked an effective depolarization in arteries from both sham-operated and LPS-treated animals, but this was far less in the former although a similar final potential was achieved. Furthermore, the sulphonylurea glibenclamide reduced the smooth muscle membrane potential by an almost identical amount in both cases. Overall, the evidence clearly points to activation of KATP channels as the underlying cause of the altered resting potential. NO has been shown to activate acutely vascular smooth muscle KATP channels, causing hyperpolarization, which is consistent with this suggestion (Garland & McPherson, 1992; Butler et al., 1995; Brayden, 1996). This was also seen in this study showing that either L-NAME or glibenclamide caused a small depolarization, but the subsequent addition of either glibenclamide or L-NAME had no further effect on membrane potential.
These observations may be of relevance to septic shock syndrome in a number of ways. They clearly indicate that hyporeactivity in vivo is not necessarily reflected at the level of resistance arteries, at least in the mesenteric vascular bed. They also indicate that at a time when endotoxic changes are established within the cardiovascular system, that endothelial cell function is maintained, at least in terms of an ability to generate an EDHF response. Therefore, as a change in membrane potential of just a few mV can result in a substantial change in artery diameter (see Nelson & Quayle, 1995), presumably the high membrane potential, reflecting enhanced NO production, and capability to generate an EDHF-linked hyperpolarization and thus smooth muscle relaxation will together serve to limit the drive to constriction that will follow increased sympathetic nerve activity during endotoxaemia. Overall, this will limit reduced tissue perfusion and the development of ischaemia, until the drive to vasoconstriction becomes predominant, reflecting the progression of the disease state. Interestingly, ischaemia in the gastric vascular bed in sepsis is believed to be an early marker for the development of multiple organ failure, which may indicate that this mechanism is indeed widespread in the resistance vessels.
In summary, this is the first report to explore the role of NO in the vascular reactivity and membrane potential of small resistance artery during endotoxaemia. Our data indicate that an overproduction of NO and/or abnormal activation of KATP channels leads to a raised resting potential in the vascular smooth muscle. In contrast to the aorta, this is not linked with a reduction in vascular reactivity, both in terms of the arteries' ability to contract and to relax.
Acknowledgments
We gratefully acknowledge the technical assistance of Dr G.J. Crane, Dr K.A. Dora and Ms N.T. Gallagher. This work was supported by Grants NSC 89-2320-B-016-019 and NSC 90-2315-B-016-008 from the National Science Council, ROC, Taiwan, and by the Wellcome Trust, UK.
Abbreviations
- ACh
acetylcholine
- KATP channels
ATP-sensitive K+ channels
- L-NAME
Nω-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- LV
levcromakalim
- MAP
mean arterial blood pressure
- NA
noradrenaline
- NO
nitric oxide
- PE
phenylephrine
References
- ARDITI M., ZHOU J., DORIO R., RONG G.W., GOYERT S.M., KIM K.S. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect. Immun. 1993;60:3149–3156. doi: 10.1128/iai.61.8.3149-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E. Potassium channels in vascular smooth muscle. Clin. Exp. Pharmacol. Physiol. 1996;23:1069–1076. doi: 10.1111/j.1440-1681.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- BUTLER A.R., FLITNEY F.W., WILLIAMS D.L.H. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol. Sci. 1995;16:18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- CHEN S.J., CHEN K.H., WEBB R.C., YEN M.H., WU C.C. Abnormal activation of Na+–K+ pump in aortas from rats with endotoxaemia. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;368:57–62. doi: 10.1007/s00210-003-0762-z. [DOI] [PubMed] [Google Scholar]
- CHEN S.J., WU C.C., YANG S.N., LIN C.I., YEN M.H. Abnormal activation of K+ channels in aortic smooth muscle of rats with endotoxic shock: electrophysiological and functional evidence. Br. J. Pharmacol. 2000;131:213–222. doi: 10.1038/sj.bjp.0703564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S.J., WU C.C., YEN M.H. Role of nitric oxide and K+-channels in vascular hyporeactivity induced by endotoxin. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:493–499. doi: 10.1007/pl00005381. [DOI] [PubMed] [Google Scholar]
- DEITCH E.A. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- FLEMING I., JULOU-SCHAEFFER G., GRAY G.A., PARRATT J.R., STOCLET J.C. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br. J. Pharmacol. 1991;103:1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND C.J., MCPHERSON G.A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br. J. Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEMBOT T., BRITT L.D., HILL M.A. Lack of direct endotoxin-induced vasoactive effects on isolated skeletal muscle arterioles. Shock. 1995;3:216–223. doi: 10.1097/00024382-199503000-00010. [DOI] [PubMed] [Google Scholar]
- GRAY G.A., SCHOTT C., JULOU-SCHAEFFER G., FLEMING I., PARRATT J.R., STOCLET J.C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br. J. Pharmacol. 1991;103:1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL S., TURCATO S., CLAPP L. Abnormal activation of K+ channels underlies relaxation to bacterial lipopolysaccharide in rat aorta. Biochem. Biophys. Res. Commun. 1996;224:184–190. doi: 10.1006/bbrc.1996.1005. [DOI] [PubMed] [Google Scholar]
- JULOU-SCHAEFFER G., GRAY G.A., FLEMING I., SCHOTT C., PARRATT J.R., STOCLET J.C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am. J. Physiol. 1990;259:H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- LIU S.H., SHEU T.J., LIN R.H., LIN-SHIAU S.Y. The in vivo effect of lipopolysaccharide on the spontaneous release of transmitter from motor nerve terminals. Br. J. Pharmacol. 1995;116:1757–1760. doi: 10.1111/j.1476-5381.1995.tb16659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., KOHLHAAS K.L., SORRENTINO R., WARNER T.D., MURAD F., VANE J.R. Induction by endotoxin of nitric oxide synthase in the rat mesentery: lack of effect of action of vasoconstrictors. Br. J. Pharmacol. 1993;109:265–270. doi: 10.1111/j.1476-5381.1993.tb13563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITOLO-CHIEPPA D., SERIO M., POTENZA MA.A., MONTAGNANI M., MANSI G., PECE S., JIRILLO E., STOCLET J.C. Hyporeactivity of mesenteric vascular bed in endotoxin-treated rats. Eur. J. Pharmacol. 1996;309:175–182. doi: 10.1016/0014-2999(96)00347-0. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- O'BRIEN A.J., WILSON A.J., SIBBALD R., SINGER M., CLAPP L.H. Temporal variation in endotoxin-induced vascular hyporeactivity in a rat mesenteric artery organ culture model. Br. J. Pharmacol. 2001;133:351–360. doi: 10.1038/sj.bjp.0704079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIEPOT H.A., GROENEVELD A.B., VAN LAMBALGEN A.A., SIPKEMA P. The role of inducible nitric oxide synthase in lipopolysaccharide-mediated hyporeactivity to vasoconstrictors differs among isolated rat arteries. Clin. Sci. 2002;102:297–305. [PubMed] [Google Scholar]
- REES D.D., CELLEK S., PALMER R.M.J., MONCADA S. Dexamethasone prevents the induction of a nitric oxide synthase and the associated effects on the vascular tone: an insight into endotoxic shock. Biochem. Biophys. Res. Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- SCHEIDER F., BUCHER B., SCHOTT C., ANDRE A., JULOU-SCHAEFFER G., STOCLET J.C. Effect of bacterial lipopolysaccharide on function of rat small femoral arteries. Am. J. Physiol. 1994;266:H191–H198. doi: 10.1152/ajpheart.1994.266.1.H191. [DOI] [PubMed] [Google Scholar]
- SCHEIDER F., SCHOTT J.C., STOCLET J.C., JULOU-SCHAEFFER G. L-Arginine induces relaxation of small mesenteric arteries from endotoxin-treated rats. Eur. J. Pharmacol. 1992;211:269–272. doi: 10.1016/0014-2999(92)90539-g. [DOI] [PubMed] [Google Scholar]
- SCOTT J.A., MACHOUN M., MCCORMACK D.G. Inducible nitric oxide synthase and vascular reactivity in rat thoracic aorta: effect of aminoguanidine. J. Appl. Physiol. 1996;80:271–277. doi: 10.1152/jappl.1996.80.1.271. [DOI] [PubMed] [Google Scholar]
- TARE M., PARKINGTON H.C., COLEMAN H.A., NEILD T.O., DUSTING G.J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990;346:69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- THIEMERMANN C. The role of the L-arginine: nitric oxide pathway in circulatory shock. Adv. Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- THIEMERMANN C. Nitric oxide and septic shock. Gen. Pharmacol. 1997;29:159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- WEISBROD R.M., GRISWOLD M.C., YAGHOUBI M., KOMALAVILAS P., LINCOLN T.M., COHEN R.A. Evidence that additional mechanisms to cyclic GMP mediate the decrease in intracellular calcium and relaxation of rabbit aortic smooth muscle to nitric oxide. Br. J. Pharmacol. 1998;125:1695–1707. doi: 10.1038/sj.bjp.0702233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., CHEN S.J., SZABO C., THIEMERMANN C., VANE J.R. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br. J. Pharmacol. 1995a;114:1666–1672. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., CHEN S.J., YEN M.H. Nitric oxide-independent activation of soluble guanylyl cyclase contributes to endotoxin shock in rats. Am. J. Physiol. 1998;275:H1148–H1157. doi: 10.1152/ajpheart.1998.275.4.H1148. [DOI] [PubMed] [Google Scholar]
- WU C.C., THIEMERMANN C., VANE J.R. Glibenclamide-induced inhibition of the expression of inducible nitric oxide synthase in cultured macrophages and in the anesthetized rat. Br. J. Pharmacol. 1995b;114:1273–1281. doi: 10.1111/j.1476-5381.1995.tb13343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]