Abstract
Voltage-gated Na+ channels are transmembrane proteins that are essential for the propagation of action potentials in excitable cells. Nav1.7 and Nav1.8 dorsal root ganglion Na+ channels exhibit different kinetics and sensitivities to tetrodotoxin (TTX). We investigated the properties of both channels in the presence of lidocaine, a local anesthetic (LA) and class I anti-arrhythmic drug.
Nav1.7 and Nav1.8 Na+ channels were coexpressed with the β1-subunit in Xenopus oocytes. Na+ currents were recorded using the two-microelectrode voltage-clamp technique.
Dose–response curves for both channels had different EC50 (dose producing 50% maximum current inhibition) (450 μM for Nav1.7 and 104 μM for Nav1.8). Lidocaine enhanced current decrease in a frequency-dependent manner. Steady-state inactivation of both channels was also affected by lidocaine, Nav1.7 being the most sensitive. Only the steady-state activation of Nav1.8 was affected while the entry of both channels into slow inactivation was affected by lidocaine, Nav1.8 being affected to a larger degree.
Although the channels share homology at DIV S6, the LA binding site, they differ in their sensitivity to lidocaine. Recent studies suggest that other residues on DI and DII known to influence lidocaine binding may explain the differences in affinities between Nav1.7 and Nav1.8 Na+ channels.
Understanding the properties of these channels and their pharmacology is of critical importance to developing drugs and finding effective therapies to treat chronic pain.
Keywords: Nav1.7, Nav1.8, sodium channels, lidocaine, local anesthetics, pain, peripheral nerve, dorsal root ganglion, Xenopus oocytes
Introduction
Voltage-gated sodium (Na+) channels are key in regulating neuronal excitability and the generation and propagation of action potentials (Hille, 2001). They thus play an important role in transmitting nociceptive information throughout the peripheral and central nervous systems. At least 10 different isoforms of Na+ channels have been identified in the brain, neurons and striated muscles. They differ in their gating properties, pharmacology (tetrodotoxin-sensitive (TTX-S) and tetrodotoxin-resistant (TTX-R)) and permeation (Goldin, 1999; Yu & Catterall, 2003). Dorsal root ganglion (DRG) neurons express at least six distinct isoforms of Na+ channels (Rush et al., 1998). While the roles of the individual isoforms are unclear, recent studies have suggested that the TTX-S channels in DRG neurons play an important role in the early part of the action potential, while TTX-R channels are thought to be crucial throughout the whole time course of the action potential (Blair & Bean, 2002).
The major component of the Na+ channels from different tissues is the 260 kDa α-subunit, which forms the pore of the channel (Goldin et al., 1986). The α-subunit is composed of four homologous domains (DI–DIV), each of which is composed of six transmembrane segments (S1–S6) (Catterall, 1992; Fozzard & Hanck, 1996). In vivo, most Na+ channels associate with auxiliary β-subunits (β1–β4), which have an average molecular weight of 30 kDa. β-Subunits modulate the level of expression and gating of these channels (Isom et al., 1992; Morgan et al., 2000; Yu et al., 2003). For instance, β1-subunits increase the expression of Nav1.8 and accelerate the time constant of inactivation and recovery from inactivation of Nav1.7 and Nav1.8 (Vijayaragavan et al., 2001).
Na+ channels are the target of multiple drugs that alter their activity (Chen et al., 2000; O'Leary & Chahine, 2002; Wang & Wang, 2003). For instance, a widely used local anesthetic (LA) and class I anti-arrhythmic, lidocaine, suppresses Na+ currents by binding not only to DIV–S6 but also to S6 of DI and DII, blocking the channels in a use-dependent (frequency-dependent) and voltage-dependent manner (Ragsdale et al., 1994; Kondratiev & Tomaselli, 2003). A major effect of LAs is to further decrease Na+ currents, thereby suppressing cellular excitability. It has been proposed that LA, in its charged form, preferentially binds inactivated channels (Hille, 1977; Hille, 2001). While most of the effect of lidocaine appears to develop gradually during depolarization, a significant proportion occurs rapidly. Lidocaine is thus believed to also block open channels (Matsubara et al., 1987).
The study presented here examined the effect of lidocaine on two DRG-specific Na+ channel isoforms, namely Nav1.7 and Nav1.8. Both channels appear to accumulate in painful human neuromas and are responsible for ectopic axonal hyper excitability, resulting in abnormal sensory phenomena such as pain and paresthesias (Kretschmer et al., 2002). The channels were expressed in Xenopus oocytes in the presence of the auxiliary β1-subunit. Their sensitivity to different concentrations of lidocaine as well as the effect on different gating properties were examined. We observed that Nav1.8 was more sensitive to blocking by lidocaine. Understanding the modulation of these channels by LAs is important to understanding LA mechanisms of action in anesthesia and pain management.
Methods
Molecular biology
The rat Nav1.7 α-subunit Na+ channel cloned into the pCDNA3a vector was provided by Gail Mandel (Department of Neurobiology, State University of New York, NY, U.S.A.). The rat Nav1.8 α-subunit was cloned from rat DRG neurons and inserted in the pSP64T vector (Vijayaragavan et al., 2001). The cRNA was prepared by the T7 (pCDNA3a) or SP6 (pSP64T) mMessage mMachine kit (Ambion, TX, U.S.A.).
Expression and electrophysiology
Xenopus laevis females were anesthetized by immersion in 1.5 mg ml−1 tricaine (Sigma, Oakville, ON, Canada), and two or three ovarian lobes were removed surgically under semisterile conditions. Follicular cells surrounding the oocytes were removed by incubation at 22°C for 2.5 h in calcium-free oocyte medium containing 82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 5 mM HEPES (pH 7.6) and 2 mg ml−1 collagenase (Sigma). The oocytes were washed first in calcium-free medium and then with 50% Leibovitz's L-15 medium (Life Technologies, Burlington, ON, Canada) supplemented with 15 mM HEPES (pH 7.6), 5 mM L-glutamine and 10 mg ml−1 gentamycin. The oocytes were stored in this medium until used. Stage IV–V oocytes were selected and microinjected with 50 nl of cRNA coding for the α-subunit of Nav1.7 and the β1-subunit or the Nav1.8 α-subunit and the β1-subunit (the β1 subunit was included in all experiments since it was previously shown to improve the expression levels of Nav1.8 and modulate its gating properties) (Vijayaragavan et al., 2001). The oocytes were stored at 18°C and used for experiments a few days later, depending on the level of expression of each channel type. The animals were treated in accordance with Canadian Institutes of Health Research guidelines. Whole-cell Na+ currents in cRNA-injected oocytes were measured using a two-microelectrode voltage clamp at room temperature (22°C). The oocytes were impaled with 2 MΩ electrodes filled with 3 M KCl and were voltage-clamped with an OC-725 oocyte clamp (Warner Instruments, Hamden, CT, U.S.A.). Currents were filtered at 1.5 kHz with an eight-pole Bessel filter and were sampled at 10 kHz. Data were acquired and analyzed with pClamp software v7 (Axon Instruments, Foster City, CA, U.S.A.). Oocytes were held at −100 mV and pClamp software was used to generate pulses that depended on the electrophysiological protocol.
Current activation curves of the channels were plotted using the following Boltzmann equation: GNa/GNa,max=1/(1+exp((V+V1/2)/kv)), for which the GNa (conductance) value for each clamped oocyte was determined by dividing the peak Na+ current by the driving force (Vm−ENa). The reversal potential (ENa) for each oocyte expressing either channel was estimated by extrapolating the linear ascending segment of the current voltage relationship (I/V) curve to the voltage axis between 0 and +20 mV for Nav1.7 and between +20 and +40 mV for Nav1.8. V is the voltage test, V1/2 is the voltage at which the channels are half-maximally activated and kv is the slope factor. Steady-state inactivation versus voltage was also plotted using a similar but decaying Boltzmann equation.
Solutions and reagents
The Ringer's bath solution contained 90 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2 and 5 mM HEPES (pH 7.6).
Lidocaine (Sigma) was diluted at room temperature in the Ringer's solution to produce a 5 mM stock solution. Different concentrations were then applied to the oocytes by continuous superfusion during the course of the experiments.
Statistical analysis
Results of representative measures are expressed as means±s.e.m. Data and graphs were analyzed using Sigmaplot 2001 for Windows version 7.0 (SPSS Inc., Chicago, IL, U.S.A.). The results were considered significant if P-values were <0.05. The fittings of the dose–response curves (Figure 1) were carried out with Sigmaplot 2001 for Windows version 7.0, using a four parameters Hill's equation: y=y0+a xb/(cb+xb).
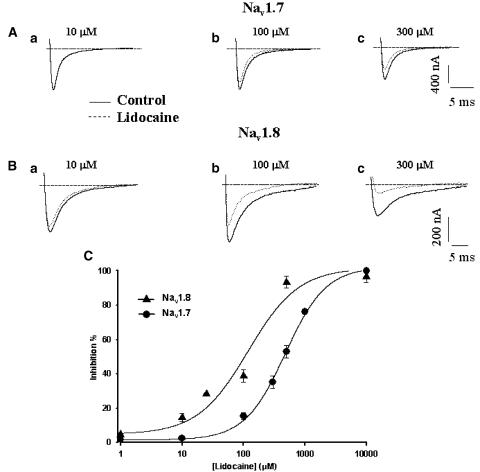
Figure 1.
Dose-dependent block of Nav1.7 and Nav1.8 currents by different lidocaine concentrations. Whole-cell Na+ currents were evoked every 5 s by 40 ms pulses to −15 mV from a holding potential of −100 mV until current stabilized (Nav1.7). Solid lines represent control currents while dotted lines represent the current amplitude after superfusion with lidocaine to produce a steady-state lidocaine effect. For Nav1.8, whole-cell Na+ currents were evoked every 20 s by 40 ms pulses to +20 mV from a holding potential of −100 mV until current stability was obtained. Solid lines represent currents under control conditions while dotted lines represent currents after lidocaine superfusion to produce a steady-state lidocaine effect. (a) 10 μM , 100 μM (b) and 300 μM (c). Nav1.7 (A) and Nav 1.8 (B). (C) Dose–response curves for both Nav1.7 and Nav1.8 channels (n=5) were obtained from fits of a four parameters Hill's equation described in the Methods section. The values of the Hill coefficients for Nav1.7 were: a=100.79, b=1.31, c=477.10 and y0=1.52. For Nav1.8, the values were: a=96.98, b=1.06, c=118.31 and y0=4.78. Filled circles represent Nav1.7 and filled triangles represent Nav1.8. Nav1.8 exhibits greater sensitivity to lidocaine than Nav1.7 (∼4.4-fold), with EC50 values of 104 and 450 μM, respectively.
Results
Nav1.7 and Nav1.8 Na+ channels have different sensitivities to lidocaine
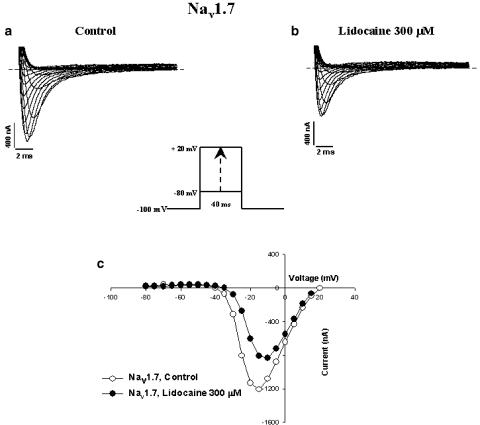
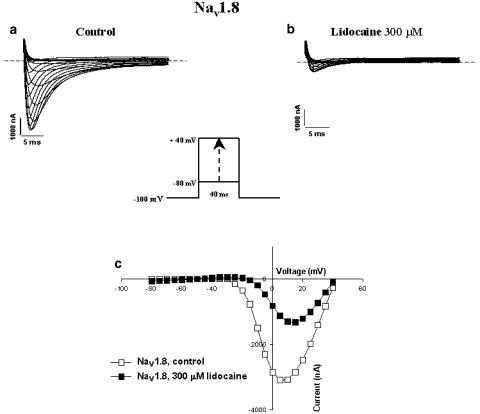
Nav1.7 and Nav1.8 exhibited different sensitivities to lidocaine. Figures 1A and B show representative current traces before and after superfusion of different concentrations of lidocaine for Nav1.7 (10, 100 and 300 μM, Figures 1Aa, Ab and Ac, respectively) and Nav1.8 (10, 100 and 300 μM, Figures 1Ba, Bb and Bc, respectively). The resulting dose–response curve (Figure 1C) shows a difference in sensitivity between the channels. Nav1.8 has an EC50 (concentration of drug necessary to inhibit 50% of Na+ currents) of 104 μM, while Nav1.7 has an EC50 of 450 μM. Figures 2a and b show representative whole-cell Na+ current traces recorded from oocytes expressing Nav1.7 under control conditions (a) and in the presence of 300 μM lidocaine (b). Inward Na+ currents were evoked by applying a series of depolarizing voltage steps between −80 to +20 mV in 5 mV increments (see figure inset for protocol). Figure 2C shows representative I/V curves for Nav1.7 with and without the drug. The channels activated at −40 mV and peaked at −10 mV in both the absence and presence of 300 μM lidocaine. Figures 3A and B show representative whole-cell traces of Na+ currents recorded from oocytes expressing Nav1.8, under control conditions (a) and in the presence of 300 μM lidocaine (b). Na+ currents were evoked by applying a series of depolarizing steps between −80 to +40 mV in 5 mV increments (see figure inset for protocol). Figure 3c shows representative I/V curves of Nav1.8 in the presence and absence of 300 μM lidocaine. Lidocaine (300 μM) added to the bath solution produced a ∼10 mV depolarized shift.
Figure 2.
Effect of lidocaine on Nav1.7 Na+ channels heterologously expressed in Xenopus oocytes. Whole-cell Na+ current traces of oocytes expressing Nav1.7 before (a) and after (b) superfusion with 300 μM of lidocaine. Also shown in (c) are the effects of lidocaine (300 μM) on the current–voltage relationship (I/V curves) in control conditions (open circles) and in the presence of the anesthetic (filled circles). Currents were elicited by depolarizing steps between −80 and +20 mV in 5 mV increments from a holding potential of −100 mV (see figure inset for protocol). Dashed lines are zero current. I/V curves were obtained by plotting the current amplitude versus the voltage for the currents shown in (a) and (b).
Figure 3.
Effect of lidocaine on Nav1.8 Na+ channels heterologously expressed in Xenopus oocytes. Whole-cell Na+ current traces of oocytes expressing Nav1.8 before (a) and after (b) superfusion with 300 μM of lidocaine. Also shown in (c) are the effects of lidocaine (300 μM) on the current–voltage relationship (I/V curves) in control conditions (open circles) and in the presence of the anesthetic (filled circles). Currents were elicited by depolarizing steps between −80 and +40 mV in 5 mV increments from a holding potential of −100 mV (see figure inset for protocol). Dashed lines are zero current. I/V curves were obtained by plotting the current amplitude versus the voltage for the currents shown in (a) and (b).
Effect of lidocaine on the gating of Nav1.7 and Nav1.8
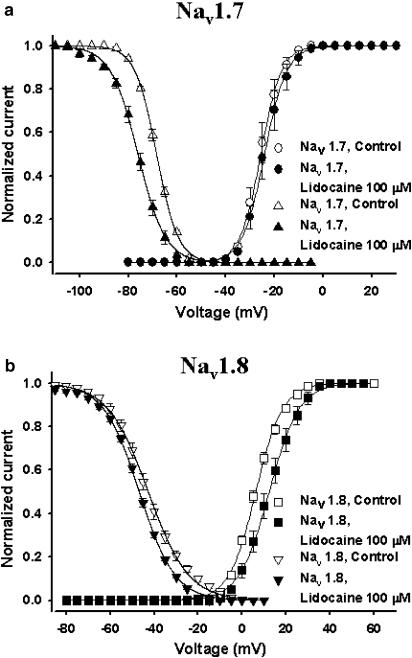
The availability of Na+ channels upon depolarization is dependent on a number of factors, one of them being the cell membrane resting potential. Fewer channels become available as the resting membrane potential progressively moves towards more depolarized voltages. This effect is due to the accumulation of channels in the nonconducting inactivated state. Experimentally, this phenomenon was measured using constant 500 ms conditioning pulses to voltages between −110 and +30 mV. The fraction of available current left was measured by standard test pulses (–10 mV for Nav1.7 and +15 mV for Nav1.8). The normalized currents were then plotted against the conditioning voltage (Figure 4). Lidocaine (100 μM) significantly shifted the V1/2 of inactivation for Nav1.7 by 10.6 mV (P<0.05) towards more hyperpolarized values but did not significantly shift the slope factor of the curve (Table 1 and Figure 4a). Figure 4b shows that lidocaine had less effect on Nav1.8. Lidocaine significantly shifted the V1/2 towards more hyperpolarized voltages by 4 mV (P<0.05) and had a nonsignificant effect on the slope factor (kv) (Table 1).
Figure 4.
Effect of lidocaine on the steady-state inactivation and steady-state activation curves of Nav1.7 (a) and Nav1.8 (b). Steady-state activation curves were derived from the same family of currents used for the I/V curves (Figures 2c and 3c) using the standard procedure (see Methods). Steady-state inactivation were determined using 500 ms conditioning pulses to voltages between −110 and +30 mV and a standard test pulse to −20 mV for Nav1.7 or +15 mV for Nav1.8. Test currents were normalized and plotted against the conditioning voltage. The steady-state properties for Nav1.7 (a, open circles and open triangles, respectively) and Nav1.8 (b, open squares and open reversed triangles, respectively) in the absence of lidocaine are shown on the same graph as in the presence of lidocaine 100 μM (filled circles and filled triangles (a) and filled squares and filled reversed triangles (b)). The smooth curves are Boltzmann fits (the equations are shown in Methods). See Table 1 for V1/2 and kv values for both activation and inactivation.
Table 1.
Effects of lidocaine on fast activation, inactivation and slow inactivation parameters for Nav 1.7 and Nav 1.8
| Activation | Inactivation | Slow inactivation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1/2 (mV) | kv | n | V1/2 (mV) | kv | n | τF (ms) | τI (ms) | τS (ms) | n | |
| Nav 1.7, control | −25.56±1.53 | −3.75±0.17 | 5 | −68.38±0.42 | 4.37±0.08 | 5 | 14.42±2.95 | 428.88±92.83 | 989.50±165.88 | 4 |
| Nav 1.7, lidocaine (100 μM) | −23.92±1.85 | −3.89±0.22 | 5 | −79.02±1.49* | 5.52±0.24* | 5 | 2.40±0.40* | 47.00±3.62* | 107.60±59.88* | 4 |
| Nav 1.8, control | 6.24±0.68 | −5.73±0.21 | 5 | −42.72±1.23 | 9.05±0.08 | 5 | 11.27±0.22 | 31.54±8.42 | 1656.34±278.81 | 5 |
| Nav 1.8, lidocaine (100 μM) | 12.32±1.78* | −6.63±0.26 | 5 | −46.81±0.58* | 8.07±0.20* | 5 | 6.52±0.85* | 6.65±0.73* | 6.49±0.86* | 5 |
τF=fast inactivation time constant; τI=intermediate inactivation time constant; τS=slow inactivation time constant; n=number of experiments.
=P<0.05.
The effect of lidocaine on the steady-state activation of the two channels was also investigated. The activation curves were derived from the I/V curves (see Methods). The activation curves of Nav1.7 and Nav1.8 in the absence and presence of lidocaine 100 μM were plotted against voltage (Figures 4a and b). For Nav1.7, lidocaine did not shift the midpoint of steady-state activation or the slope factor significantly. For Nav1.8, lidocaine caused a significant 6.1 mV depolarized shift of the midpoint of steady-sate activation (P<0.05). Lidocaine did not change significantly the slope factor.
Overall, lidocaine caused a significant hyperpolarizing shift in the steady-state inactivation of Nav1.7 channels and affected the voltage-dependence of Nav1.8 inactivation to a lesser extent. However, lidocaine significantly shifted the steady-state activation curve of Nav1.8 toward more depolarized potentials but did not affect the voltage-dependent activation of Nav1.7.
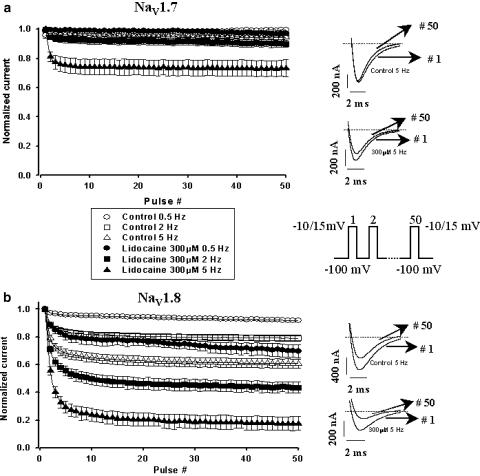
Lidocaine produces a use-dependent inhibition of both Nav1.7 and Nav1.8 channels
During step depolarization, Na+ channels are induced to cycle through activated, inactivated and resting states. However, when they are subjected to a train of depolarizing pulses, the number of channels available to open is reduced and they progressively accumulate in the inactivated state. This phenomenon is referred to as use-dependence or ‘frequency-dependent' blocking. In the presence of a LA, the further decrease in currents could be attributed to the accumulation of channels in a drug-modified state. This effect of rapid pulsing on Nav1.7 and Nav1.8 was tested by applying a series of 50 short 8 ms depolarizing pulses (−10 mV for Nav1.7 and +15 mV for Nav1.8). We observed a dramatic difference in the sensitivities of both Nav1.7 and Nav1.8 at different frequencies when they were superfused with the same concentrations of lidocaine (300 μM, Figure 5). As shown in Figure 5a, there was little change in Nav1.7 channel availability when they were stimulated at frequencies between 0.5 to 5 Hz since the currents remained above 90% of their initial value. However, in the presence of 300 μM lidocaine, Nav1.7 channel availability was reduced to slightly less than 80% of their maximal peak when stimulated at 5 Hz (Figure 5a). On the other hand, Nav1.8 was more sensitive to the frequencies used when superfused with the same concentration of lidocaine (300 μM in Figure 5b). While little reduction in current was observed when the channels were pulsed at 0.5 Hz under control conditions, superfusion with lidocaine induced a 20% reduction in Na+ currents. Pulsing under control conditions at 2 Hz also led to a decrease of approximately 20%, but, in the presence of lidocaine, the currents were reduced to 50% of their initial amplitude. At 5 Hz without the drug, a decrease to 70% of the initial current value was observed, while, in the presence of lidocaine, the decrease stabilized at 20% of the normalized initial current. The reduction in currents observed for Nav1.8 increased with the frequency used.
Figure 5.
Frequency-dependent inhibition of Nav1.7 (a) and Nav1.8 (b) Na+ currents in the presence and absence of lidocaine 300 μM. Oocytes were held at −100 mV and a train of fifty 8 ms pulses was applied to −10 mV (Nav1.7) or +15 mV (Nav1.8) at three different frequencies (0.5, 2 and 5 Hz), with the interpulse potential also set at −100 mV. The peak currents elicited by each pulse were normalized to the current of the first pulse (Pn−P1, where n=1–50) and were then plotted versus pulse number. Different open symbols represent control conditions while filled symbols represent the protocol in the presence of 300 μM lidocaine for the different frequencies (circles represent 0.5 Hz, squares represent 2 Hz and triangles represent 5 Hz). Examples of current traces at the 1st and 50th pulse of the protocol for each channel in the presence and absence of lidocaine are shown in the right panel. The central panel shows a schematic representation of the electrical protocol used.
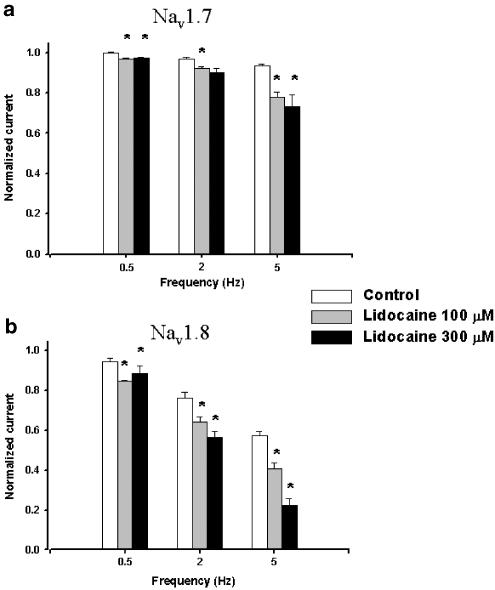
Nav1.8's decrease in currents during use-dependent block was more pronounced in the presence of lidocaine with a higher frequency causing a more important blocking effect (Figure 6). This again pointed to a difference in channel sensitivity and shows that Nav1.8 was more affected by the presence of lidocaine. Figure 6 shows a comparison of the current amplitudes at the 50th pulse of the use-dependent protocol at different concentrations of lidocaine (100 and 300 μM). No obvious differences were noted between the two concentrations for Nav1.7, while a drastic decrease in the amplitudes of Nav1.8 currents occurred with the increase in drug concentration when current amplitudes at the same frequencies were compared.
Figure 6.
Bar plot representation of the relative amplitudes at the 50th sweep of the frequency dependence protocol used in Figure 5 for each frequency. The amplitudes of the last step were normalized versus the first step of the protocol. White columns are control currents, gray columns are currents' amplitudes after perfusion with 100 μM lidocaine and black columns are currents' amplitudes in the presence of 300 μM lidocaine. (*=P<0.05).
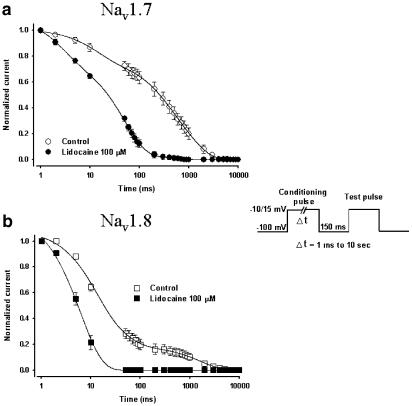
Development of slow inactivation for Nav1.7 and Nav1.8 in the presence of lidocaine
Na+ channels have different inactivation states: fast, intermediate and slow. Nav1.7 and Nav1.8 expressed with the β1-subunit exhibit different slow inactivation development kinetics (Vijayaragavan et al., 2001). It therefore seemed appropriate to determine whether lidocaine affects the slow inactivation development of both channels. We therefore decided to compare the effects of lidocaine on Nav1.7 and Nav1.8. The onset of slow inactivation was measured experimentally by depolarizing the oocytes to either −20 mV (Nav1.7) or +20 mV (Nav1.8) for an interval that varied from 0 ms to 10 s to induce channel inactivation. After the depolarization step, the voltage was returned to −100 mV for 150 ms to allow recovery of fast-inactivated channels, before a standard 8 ms test pulse was applied to measure the amount of available Na+ currents (Vijayaragavan et al., 2001). The amplitudes of the Na+ currents measured by the test pulse were then normalized versus the control currents and plotted against the duration of the conditioning-pulse interval. Using this protocol, the progressive decrease in currents with the increase of the prepulse duration was representative of channel entry into the slow-inactivated state from which channels do not recover during the short depolarization that precedes the test pulse (100 mV for 20 ms) (Figure 7 inset). The onset of slow inactivation for Nav1.7 was best fitted with the sum of three exponentials (See Table 1 and Figure 7a). For Nav1.8, the onset of slow inactivation was also best fitted with the sum of three exponentials (See Table 1 and Figure 7b). The time constants of slow inactivation of Nav1.7 and Nav1.8 are different in control conditions (Vijayaragavan et al., 2001). We showed that lidocaine enhanced entry into slow inactivation. The time constants in the presence of 100 μM lidocaine were significantly accelerated by lidocaine based on their response to slow inactivation, Nav1.8 was the most affected (Table 1).
Figure 7.
Development of slow inactivation by both Nav1.7 (a) and Nav1.8 (b) channels with and without lidocaine. Control conditions are open circles (Nav1.7) and open squares (Nav1.8) while experiments with the drug are represented by filled circles and squares. The entry into slow inactivation was measured using a double-pulse protocol consisting of a conditioning pulse of variable duration (1 ms to 10 s) to −10 mV (Nav1.7) or 15 mV (Nav1.8) to inactivate the channels. A 150 ms pulse to −100 mV was then applied to allow rapid recovery and a standard test pulse was used to measure the amount of available channels (see inset). The measured currents were then normalized and plotted against the duration of the conditioning pulse. The decrease in currents was best fitted in all cases with the sum of three exponentials (solid lines). See Table 1 for the time-constant values.
Discussion
In this study, we examined the effects of the widely used LA, lidocaine, on Nav1.7 and Nav1.8 channels and showed that they had different sensitivities and were differentially affected by lidocaine. Nav1.8 was 4.4-fold more sensitive to lidocaine than Nav1.7. Similar findings have been observed with native DRG neurons, where the TTX-R currents is more sensitive to lidocaine than the TTX-S currents (Roy & Narahashi, 1992). The effect of LA on Na+ channels is generally characterized by a tonic block at low-frequency stimulations, a phasic block and a use-dependent block at high-frequency stimulations. Lidocaine decreased current in both tonic (0.5 Hz) and use-dependent blocking (2–5 Hz) of Nav1.7 and Nav1.8. However, during low-frequency stimulations, Nav1.8 was four-fold more sensitive to the same concentration of drug.
Voltage-gated Na+ channels cycle through several states but principally go through the resting, open and inactivated states. In this study, we observed that repetitive pulsing caused a major current reduction for Nav1.8 but had little effect on Nav1.7. Lidocaine produced an even greater decrease in Na+ currents for both channels (Figures 5 and 6). The enhancement of the inhibition of Na+ currents by lidocaine was more significant for Nav1.8 than for Nav1.7 channels. This suggests that the drug binds more effectively to Nav1.8 channels.
Lidocaine also differentially modified the gating properties of Nav1.7 and Nav1.8 channels. Lidocaine did not shift the steady-state activation curve significantly for Nav1.7, but did for Nav1.8. This may explain in part the greater affinity of lidocaine for Nav1.8. Lidocaine shifted the steady-state inactivation curve of Nav1.7 by 10.6 mV towards more hyperpolarized values. In contrast, lidocaine caused a hyperpolarizing shift of only 4.1 mV for Nav1.8. The 10.6 mV shift of steady-state inactivation observed with Nav1.7 could explain the decrease in Na+ currents observed at low-frequency stimulations. This suggests that different mechanisms are involved in the blocking of the two channels by lidocaine.
Since lidocaine is an open channel blocker that interacts with the channel pore and since previous studies have suggested that it induces Nav1.4 occupancy in the slow inactivated state, we investigated the effect of lidocaine on the slow inactivation of both channels. The onset of slow inactivation for Nav1.8 under control conditions was much faster than for Nav1.7. This tendency of Nav1.8 to enter the slow inactivated state more rapidly could explain the greater sensitivity of this channel to slow repetitive stimulations (Figures 5 and 6) since the channels, once in the slow inactivated state, do not recover in the short interval between the pulses of the use-dependence protocol. While lidocaine greatly affected the slow inactivation of both channels, Nav1.8 was affected the most. While Nav1.7 was affected, it tended to be much more resistant, which explains why we observed a larger decrease in Na+ currents during repetitive stimulations at the highest frequency and in the presence of lidocaine for Nav1.8 (Figures 5 and 6). The high-affinity binding of lidocaine to the slow inactivated state of Nav1.8 could be important in blocking nociceptor firing, as previously suggested (Blair & Bean, 2002).
Although Na+ channels' amino-acid sequences appear to be very conserved, some differences have been observed among the members of the Na+ channel family. While the DIV S6 segment is regarded by most as the high affinity-binding site for local anesthetics (the region conserved by both Nav1.7 and Nav1.8), mutations of residues on other S6 regions can affect the binding of LAs. For instance, three residues (I436, I782 and V787) on the S6 segments of DI and DII in Nav1.4 were suggested to play a role in the binding of LAs (Kondratiev & Tomaselli, 2003). Amino-acid sequence comparisons have shown that these three residues are not conserved between Nav1.7 and Nav1.8. The residue equivalent to I436 (DI) is conserved in Nav1.7, while it is a valine in Nav1.8. A mutation of the I436 residue in Nav1.4 increases channel sensitivity to lidocaine. Furthermore, I782 on DII of Nav1.4, which is not conserved in Nav1.8, affects use-dependent lidocaine blocking of the channel. When mutated, this residue causes the channel to become much more resistant to use-dependent blocking by lidocaine (Kondratiev & Tomaselli, 2003). A mutation of the V787 residue on the DII of Nav1.4 increases the affinity of the channel for lidocaine. The valine residue is present on Nav1.7 but the corresponding amino acid on Nav1.8 is a leucine. The residue is in position 831 in Nav1.8 but the corresponding amino acid is different in Nav1.7 (methionine). Changes to all these residues could explain the differential affinity of Nav1.8 and Nav1.7 for lidocaine. Further studies will be required to test the role of these residues in the differential modulation of Nav1.7 and Nav1.8 by lidocaine.
Nav1.8, the TTX-R Na+ channel, plays a key role in neuropathic pain (Gold, 1999; Gold et al., 2003). Its high sensitivity to LA drugs observed in the present study makes it an ideal target for the development of drugs to treat neuropathic pain.
Acknowledgments
This study was supported by grants from the Heart and Stroke Foundation of Québec (HSFQ) and the Canadian Institutes of Health Research (CIHR) MOP-49502. M. Chahine is an Edwards Senior Investigator (Joseph C. Edwards Foundation). We thank Dr. Laimonis Gailis for his valuable comments on the manuscript.
Abbreviations
- D
domain
- DRG
dorsal root ganglions
- EC50
dose producing 50% maximum current inhibition
- LA
local anesthetics
- Na+
sodium
- S
transmembrane segment
- TTX
tetrodotoxin
- TTX-S
tetrodotoxin-sensitive
- TTX-R
tetrodotoxin-resistant
References
- BLAIR N.T., BEAN B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATTERALL W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992;72:S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- CHEN Z., ONG B.H., KAMBOURIS N.G., MARBÁN E., TOMASELLI G.F., BALSER J.R.Lidocaine induces a slow inactivated state in rat skeletal muscle sodium channels J. Physiol. 200052437–49.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOZZARD H.A., HANCK D.A. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol. Rev. 1996;76:887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- GOLD M.S. Tetrodotoxin-resistant Na+ currents and inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7645–7649. doi: 10.1073/pnas.96.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD M.S., WEINREICH D., KIM C.S., WANG R., TREANOR J., PORRECA F., LAI J. Redistribution of Nav1.8 in uninjured axons enables neuropathic pain. J. Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDIN A.L. Diversity of mammalian voltage-gated sodium channels. Ann. N.Y. Acad. Sci. 1999;868:38–50. doi: 10.1111/j.1749-6632.1999.tb11272.x. [DOI] [PubMed] [Google Scholar]
- GOLDIN A.L., SNUTCH T., LÜBBERT H., DOWSETT A., MARSHALL J., AULD V., DOWNEY W., FRITZ L.C., LESTER H.A., DUNN R., CATTERALL W.A., DAVIDSON N. Messenger RNA coding for only the α subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 1986;83:7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLE B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes 2001Sunderland, MA: Sinauer Associates, Inc.814pp3rd edn. pp [Google Scholar]
- ISOM L.L., DE JONGH K.S., PATTON D.E., REBER B.F., OFFORD J., CHARBONNEAU H., WALSH K., GOLDIN A.L., CATTERALL W.A. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- KONDRATIEV A., TOMASELLI G.F. Altered gating and local anesthetic block mediated by residues in the I-S6 and II-S6 transmembrane segments of voltage-dependent Na+ channels. Mol. Pharmacol. 2003;64:741–752. doi: 10.1124/mol.64.3.741. [DOI] [PubMed] [Google Scholar]
- KRETSCHMER T., HAPPEL L.T., ENGLAND J.D., NGUYEN D.H., TIEL R.L., BEUERMAN R.W., KLINE D.G. Accumulation of PN1 and PN3 sodium channels in painful human neuroma-evidence from immunocytochemistry. Acta Neurochir. 2002;144:803–810. doi: 10.1007/s00701-002-0970-1. [DOI] [PubMed] [Google Scholar]
- MATSUBARA T., CLARKSON C., HONDEGHEM L. Lidocaine blocks open and inactivated cardiac sodium channels. Naunyn Schmiedebergs Arch. Pharmacol. 1987;336:224–231. doi: 10.1007/BF00165809. [DOI] [PubMed] [Google Scholar]
- MORGAN K., STEVENS E.B., SHAH B., COX P.J., DIXON A.K., LEE K., PINNOCK R.D., HUGHES J., RICHARDSON P.J., MIZUGUCHI K., JACKSON A.P. 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2308–2313. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'LEARY M.E., CHAHINE M. Cocaine binds to a common site on open and inactivated human heart (Nav1.5) sodium channels. J. Physiol. 2002;541:701–716. doi: 10.1113/jphysiol.2001.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGSDALE D.S., MCPHEE J.C., SCHEUER T., CATTERALL W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- ROY M.L., NARAHASHI T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J. Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSH A.M., BRÄU M.E., ELLIOTT A.A., ELLIOTT J.R. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J. Physiol. 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIJAYARAGAVAN K., O'LEARY M.E., CHAHINE M. Gating properties of Nav1.7 and Nav1.8 peripheral nerve sodium channels. J. Neurosci. 2001;21:7909–7918. doi: 10.1523/JNEUROSCI.21-20-07909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S.Y., WANG G.K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- YU F.H., CATTERALL W.A. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU F.H., WESTENBROEK R.E., SILOS-SANTIAGO I., MCCORMICK K.A., LAWSON D., GE P., FERRIERA H., LILLY J., DISTEFANO P.S., CATTERALL W.A., SCHEUER T., CURTIS R. Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. J. Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]