Abstract
We investigated the effect of the active ingredients of Panax ginseng, ginsenosides, on store-operated Ca2+ entry (SOCE) using a two-electrode voltage clamp technique in Xenopus oocytes in which SOCE is monitored through Ca2+-activated Cl− currents.
Under hyperpolarizing voltage clamp conditions, treatment with ginsenosides produced a biphasic Ca2+-activated Cl− current consisting of a rapid transient inward current and a slowly developing secondary sustained inward current. The transient inward current was inactivated rapidly, whereas the sustained inward current persisted for nearly 10 min. The effect of ginsenosides on the biphasic current was dose-dependent and reversible. The EC50 was 42.8±11.6 and 46.6±7.1 μg ml−1 for the transient and sustained inward current, respectively.
In the absence of extracellular Ca2+ ginsenosides induced only a transient inward current but in the presence of extracellular Ca2+ ginsenosides induced the biphasic current. Magnitudes of the sustained currents were dependent on extracellular Ca2+ concentration. Sustained inward current induced by ginsenosides, but not transient inward current, and ginsenoside-induced store-operated Ca2+ (SOC) currents (ISOC) were blocked by La3+, a Ca2+ channel blocker, suggesting that the sustained inward current and ISOC was derived from an influx of extracellular Ca2+.
Treatment with 2-APB and heparin, which are IP3 receptor antagonists, inhibited the ginsenoside-induced biphasic current. Treatment with the PLC inhibitor, U73122, also inhibited the ginsenoside-induced biphasic current. Intraoocyte injection of ATP-γS, but not adenylyl AMP-PCP, induced a persistent activation of ginsenoside-induced sustained current but did not affect the transient current.
In rat hippocampal neurons, ginsenosides inhibited both carbachol-stimulated intracellular Ca2+ release and intracellular Ca2+ depletion-activated SOCE.
These results indicate that ginsenoside might act as a differential regulator of intracellular Ca2+ levels in neurons and Xenopus oocytes.
Keywords: Ginseng, ginsenosides, Ca2+-activated Cl− channels, SOCE, Xenopus oocytes
Introduction
Intracellular Ca2+ is a key molecule involved in many kinds of signal transduction pathways in a variety of cells. Increases in Ca2+ levels in cells regulate secretion, cell division, growth and differentiation, muscle contraction, and receptor internalization (Berridge et al., 1998). Free intracellular Ca2+ can be increased either by Ca2+ influx from extracellular fluid or through the release of stored Ca2+ from the intracellular compartment, endoplasmic reticulum (ER) (Parekh & Penner, 1997). In nonexcitable cells, an increase in cytoplasmic free Ca2+ is mediated via stimulation of receptors such as G protein coupled receptors (GPCRs), which are coupled to activation of the phospholipase C (PLC) pathway and production of inositol 1,4,5-triphosphate (IP3). IP3 then triggers an increase in the levels of cytosolic free Ca2+ from IP3-sensitive ER, resulting in depletion of intracellular calcium stores. Depletion of intracellular Ca2+ by IP3 causes Ca2+ entry through plasma membrane store-operated Ca2+ (SOC) channels via a process known as store-operated Ca2+ entry (SOCE) (Putney, 1992). This Ca2+ influx is important not only for refilling the depleted stores but also for modulating various Ca2+-dependent intracellular signals after GPCR agonist stimulation (Yao & Parker, 1994).
Xenopus oocytes, with their large size and easy handling, are a useful model system for investigating the machinery of membrane signal transduction. The intracellular injection of putative second messengers or the introduction and expression of foreign genes can substantially facilitate investigations into the intermediate steps of signaling pathways. Moreover, Xenopus oocytes have endogenous Ca2+-activated Cl− channels that are well understood (Barish, 1983; Miledi & Parker, 1984), and which have been used for the study of both intracellular Ca2+ release and the SOCE process (Lechleiter & Clapham, 1992; Callamaras & Parker, 1994; Parker & Yao, 1994). For example, stimulation of oocyte muscarinic receptors by ACh leads to intracellular Ca2+ mobilization and activation of Ca2+-activated Cl− channels, resulting in depletion of calcium stores and initiation of SOCE (Dascal et al., 1984; Berridge & Irvine, 1989; Lechleiter & Clapham, 1992).
Choi et al. (2001b, 2001c) demonstrated that, in Xenopus oocytes, ginsenosides enhanced the endogenous Ca2+-activated Cl− current via a Gαq/11-PLC-β3 pathway coupled to IP3-mediated intracellular Ca2+ release. Thus, treatment of Xenopus oocytes with ginsenosides might initiate two events. Firstly, ginsenoside-induced intracellular Ca2+ release, which is mediated through IP3, coupled to activation of Ca2+-activated Cl− channels as reported in previous studies (Choi et al., 2001b, 2001c). Secondly, ginsenoside-induced intracellular Ca2+ release followed by intracellular Ca2+ depletion might be responsible for activation of the process of SOCE. However, it is not yet proven whether or not ginsenoside-induced activation of Ca2+-activated Cl− channels is also coupled to subsequent activation of the SOCE process. Moreover, little is known about the signal transduction component(s) involved in SOCE activation by ginsenoside. In the present study, we investigated the signal transduction pathway and the relationship between activation of Ca2+-activated Cl− channels and SOCE in the presence or absence of ginsenosides using Xenopus oocytes, a convenient in vitro model system (Dascal, 1987). In addition, we investigated the effect of ginsenosides on SOCE in neuronal cells, since it has not yet been determined whether ginsenosides might also regulate SOCE in neuronal cells.
Here, we demonstrate that ginsenoside-induced intracellular Ca2+ release in Xenopus oocytes is coupled to activation of SOCE via a PLC-IP3-extracellular Ca2+ influx pathway, in contrast to ginsenoside action in neurons whereby both receptor agonist-mediated intracellular Ca2+ release and depletion-activated SOCE are inhibited.
Methods
Drugs
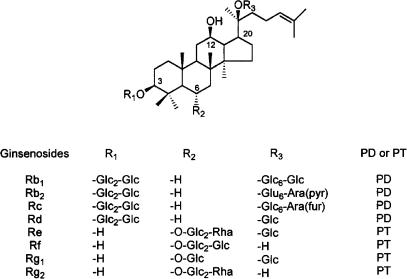
Figure 1 shows the structures of the eight representative ginsenosides. These ginsenosides (GTS) were kindly obtained from Korea Ginseng and Tobacco Research Institute (Taejon, Korea). GTS contained Rb1 (17.1%), Rb2 (9.07%), Rc (9.65%), Rd (8.26%), Re (9%), Rf (3%), Rg1 (6.4%), Rg2 (4.2%), Rg3 (3.8%), Ro (3.8%), Ra (2.91%) and other minor ginsenosides. GTS was diluted with bath medium ND96 before use. CNQX was purchased from Tocris (Ellisville, MO, USA). TTX was purchased from Alomone Labs (Jerusalem, Israel). 2-Aminoethxydiphenyl borate (2-APB), heparin, [adenosine 5-O-(3-Thiotriphosphate)] (ATP-[γS]), adenylyl 5′-(β,γ-methylene)-diphosphonate (AMP-PCP) and other chemicals were of analytical grade and purchased from Sigma (St Louis, MO, U.S.A.). The drugs used in this study were either bath-applied or injected into oocytes with a Nanoject Automatic Oocyte Injector (Drummond Scientific, PA, U.S.A.). The injection pipette was pulled from glass capillary tubing, and its tip was broken to an outer diameter of about 20 μm. In total, 23–50 nl of ATP-[γS], AMP-PCP, and heparin solutions were injected into oocytes to give a calculated intracellular concentration of about 100, 100, and 1 μg μl−1, respectively.
Figure 1.
Structures of the five representative ginsenosides. They differ at three side chains attached to the common steroid ring. Abbreviations for carbohydrates are as follows: Glc, glucopyranoside; Ara (pyr), arabinopyranoside; Rha, rhamnopyranoside. Superscripts indicate the carbon in the glucose ring that links the two carbohydrates.
Oocyte preparation
Xenopus laevis frogs were obtained from Xenopus I (Ann Arbor, MI, U.S.A.). Before oocyte extraction, they were kept in a temperature-controlled aquarium (18±1°C) with a 12 : 12 h light–dark cycle, and food was given every 2 days. Oocytes were extracted under deep anesthesia, which was induced by immersing frogs in an aerated solution of 0.15% 3-amino benzoic acid ethyl ester. Following oocyte extraction, frogs were killed by anesthetic overdose. The extracted oocytes were separated by treatment with collagenase and agitation for 2 h in a Ca2+-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 U ml−1 penicillin and 100 μg μl−1 streptomycin. Stage V–VI oocytes were collected and stored in ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 0.5 mM theophylline and 50 μg μl−1 gentamicin. This oocyte-containing solution was maintained at 18°C with continuous gentle shaking and refreshed everyday.
Oocyte recording
A custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings. The chamber was constructed by milling two concentric wells into the chamber bottom (diameter/height for upper well: 8/3 mm, lower well: 6/5 mm) and gluing plastic meshes (∼0.4-mm grid diameter) onto the bottom of the upper well. The perfusion inlet (∼1-mm diameter) was formed through the wall of the lower well, and the suction tube was placed on the edge of the upper well. The oocyte was placed on the net that separates the upper and lower wells. The grids of the net served as dimples that kept the oocytes in place during electrophysiological recordings. The oocytes were impaled with two microelectrodes filled with 3 M KCl (0.2–0.7 MΩ) and voltage-clamped at −40 mV. In our experiments, the membrane potential was held at −40 mV, stepped up to +40 mV for 1 s, down to −140 mV for 1 s, and then back to +40 mV for 1 s before returning to the holding potential. These steps were repeated every 10 s. For ISOC current measurement, oocytes were held at −40 mV and voltage ramps were applied from −140 to +60 mV for 300 ms. For ISOC current measurement, the external solution was 30 mM CaCl2, 55 mM NaCl, and 10 mM HEPES (pH 7.5). The electrophysiological experiments were performed at room temperature with Oocyte Clamp (OC-725C, Warner Instrument, CT, USA) and both stimulation and data acquisition were controlled by pClamp 8 (Axon Instruments) (Choi et al., 2003a).
Hippocampal neuron preparation
Hippocampal cell cultures were prepared from neonatal (P1) Sprague–Dawley rats. The hippocampi were dissected and incubated with 0.05% papain in Leibovitz L-15 medium at 37°C for 20 min. Cells were then mechanically dissociated with fire-polished Pasteur pipettes by trituration and plated on poly-L-lysine coated coverslips in a 35-mm culture dish. Cells were maintained in Neurobasal/B27 medium containing 0.5 mM L-glutamine, 25 μM glutamate, 25 μM 2-mercaptoethanol, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Cultures were fed twice a week with the same medium without glutamate. Experiments were carried out on neurons after 7–15 days in culture (Kim et al., 2002).
Intracellular Ca2+ imaging
The acetoxymethyl-ester form of fura-2 (Fura-2/AM; Molecular probes, Eugene, OR, U.S.A.) was used as fluorescent Ca2+ indicator. Hippocampal neurons were incubated for 40–60 min at 37°C with 5 μM Fura-2/AM and 0.001% Pluronic F-127 in normal HEPES-buffered medium composed of (in mM): 150NaCl, 5KCl, 1MgCl2, 2CaCl2, 10 HEPES, 10 glucose, pH adjusted to 7.4 with NaOH. High K+ buffered medium contained (in mM): 105NaCl, 50KCl, 1MgCl2, 2CaCl2, 10 HEPES, 10 glucose, pH adjusted to 7.4 with NaOH. The cells were then washed with HEPES-buffered solution, placed on an inverted microscope (Olympus, Japan), and illuminated using a xenon arc lamp. Excitation wavelengths (340 and 380 nm) were selected by a computer-controlled filter wheel (Sutter Instruments, CA, USA). Data were recorded every 2 s but with a shutter in the light path between exposures to protect the cells from photo-toxicity. Emitter fluorescence light was reflected through a 515 nm long-pass filter to a frame transfer cooled CCD camera. Ratios of emitted fluorescence were calculated using a digital fluorescence analyzer and converted to indicate the intracellular free Ca2+ concentration ([Ca2+]i). All imaging data were collected and analyzed using Universal Imaging software (West Chester, PA, U.S.A.) (Kim et al., 2002).
Data analysis
To obtain a concentration–response curve of the effect of GTS on the transient or sustained Ca2+-activated Cl− current, the observed peak amplitudes were measured, plotted, and then fitted to the Hill equation below using Origin software (Northampton, MA, U.S.A.): y/ymax=[A]n/([A]n+[EC50]n), where y is the transient or sustained Ca2+-activated Cl− current at a given concentration of GTS; ymax the maximal peak transient or sustained Cl− current; EC50 is the concentration of GTS producing half-maximum effect of the control response to GTS; and [A] is the concentration of GTS and n is the interaction coefficient. All values are presented as mean±s.e.m. The differences between control and treatment data means were analyzed using an unpaired t-test, with P<0.05 considered as significant.
Results
Effect of ginsenosides on two types of Ca2+-activated Cl− current in oocytes held in voltage clamp and subjected to multistep voltage cycles
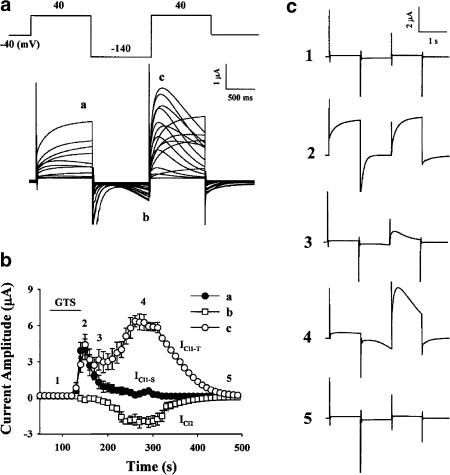
In previous reports, Kuruma & Hartzell (1999) demonstrated fully that intraoocyte injection of IP3 induced two distinct Ca2+-activated Cl− currents in Xenopus oocytes using multisteps in a voltage clamp. Using this protocol they could distinguish Cl− currents that are activated by intracellular Ca2+ release from Cl− currents that are activated by extracellular Ca2+ influx (SOCE). Our group earlier reported that in Xenopus oocytes, ginsenosides enhanced endogenous Ca2+-activated Cl− currents evoked by depolarizing voltage clamps (Choi et al., 2001b, 2001c). Here, we first tested whether treatment with ginsenosides also induces those distinct Ca2+-activated Cl− currents following the same protocol. For this, oocytes were held at −40 mV and stepped to +40 mV for 1 s, to −140 mV for 1 s, then back to +40 mV for 1 s before return to the holding potential (Figure 2a). The oocytes recorded in this study had a resting membrane potential (Vr) of about −20 to −30 mV. As shown in Figure 2b, ginsenoside treatment induced two distinct Ca2+-activated Cl− currents over different time periods during this voltage protocol, similar to those found by Kuruma & Hartzell (1999) in IP3 injected oocytes. The ICl1-S (activated by release of Ca2+ from intracellular Ca2+ stores) current first appeared at the end of the first 1 s step to +40 mV. The outward ICl1-T (activated by external Ca2+ entry) current was measured as the peak time-dependent outward current during the second 1 s +40 mV step, which was preceded by a −140 mV pulse where the driving force for Ca2+ influx is higher than −40 mV. This preceding −140 mV step, before the +40 mV step up, also induced the inward ICl2 (also activated by external Ca2+ entry). Thus, the ICl1-S current (a) activated by intracellular Ca2+ release was recorded first and this was then followed by ICl1-T (c) and ICl2 (b), activated by external Ca2+ influx (Figure 2a). This suggests that treatment of oocytes with ginsenosides induced not only intracellular Ca2+ release but also Ca2+ influx from external media (Figures 2b and c).
Figure 2.
Effect of ginsenosides (GTS) on noninactivating outward Cl− current (ICl1-S), slow inward Cl− current (ICl2), and transient outward Cl− (ICl1-T). Oocytes were voltage-clamped with two microelectrodes and GTS (50 μg ml−1) was added to the bath solution following the voltage pulse every 10 s. (a) Current amplitudes recorded at every 10 s for 50 trials with a three-step pulse command were overlapped. Peak current values of three Cl− current components were measured at the points a (ICl1-S), b (ICl2), and c (ICl1-T) indicated in the figure. (b) Time course of development of ICl1-S (a), ICl2 (b), and ICl1-T (c) in response to GTS treatment of an oocyte bathed in ND96 (mean±s.e.m; n=8 oocytes). Steady-state outward current at the end of the first +40 mV pulse is ICl1-S, inward current during −140 mV pulse is ICl2, and transient outward current during second +40 mV pulse is ICl1-T. Current amplitudes recorded at points a, b, and c were illustrated as a function of time. The bar represents continuous application of GTS. (c) Five representative current traces of development and decay of ICl1-S, ICl2, and ICl1-T at different times 1, 2, 3, 4, and 5 during the experiment. Times of traces are indicated by 1–5 in (b).
Ginsenoside treatment induces biphasic inward currents following hyperpolarizing voltage steps in Xenopus oocytes
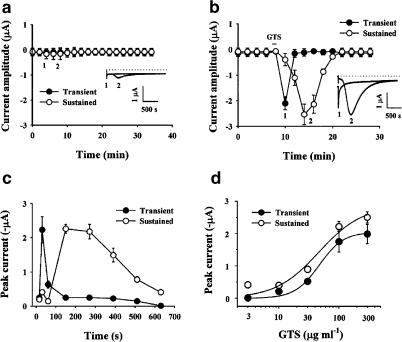
Parekh (1995) and Hartzell (1996) demonstrated, using a hyperpolarizing voltage step, that addition of 5-HT to oocytes that are heterologously expressing 5-HT2C receptor, or intraoocyte injection of IP3, both evoked a biphasic inward Cl− current. This biphasic current consists of a rapid transient inward current, which is activated by release of Ca2+ from intracellular Ca2+ stores, followed by a slowly developing secondary sustained inward current, which is activated by external Ca2+ entry. We tested whether ginsenoside treatment induced a biphasic Cl− current under hyperpolarizing voltage conditions. The oocytes were held (Vh) at −40 mV and stepped to −140 mV for 5 s with 2 min intervals. As shown in Figure 3a, in the absence of ginsenosides we could observe only a slight sustained current but not a transient current (Figure 3a). In contrast, we could observe a biphasic current following application of ginsenosides to the bath (50 μg ml−1) for 60 s. The first phase was a rapid transient current and the second was a sustained inward current (Figure 3b, inset). The first transient phase of inward Cl− current was rapidly inactivated and the second phase was persistent for about 10 min, even after removal of the ginsenosides by washing (Figure 3b). To further characterize the two kinds of Cl− currents evoked by ginsenosides, we studied the currents over a time course. Figure 3c shows the time course for activation and inactivation of the biphasic current recorded after 15, 30, 60, 150, 270, 390, 510, and 630 s in the presence of ginsenosides. The transient inward current appeared 15 s after bath application of ginsenosides and was inactivated by 60 s. The sustained current appeared around 150 s after ginsenoside addition and persisted for more than 10 min. The effect of ginsenosides on both transient and sustained currents was concentration-dependent. The EC50 was 42.8±11.6 and 46.6±7.1 μg ml−1 for the transient and sustained inward Cl− currents, respectively (Figure 3d).
Figure 3.
Treatment with GTS induces a biphasic inward Cl− current under hyperpolarizing conditions. Oocytes were voltage-clamped at −40 mV and stepped to −140 mV for 5 s. The amplitude of the inward current evoked every 2 min is plotted against time in the absence (a) or presence of GTS (50 μg ml−1). Inset: Traces of current recorded in the absence (a) or presence of GTS (b) with transient and sustained currents superimposed. The time points of the recording are indicated in the graph as 1 and 2. The graph is representative of data obtained from six oocytes from three different frogs. (c) Time course of appearance of biphasic currents. The inward current was recorded at 15, 30, 60, 150, 270, 390, 510 and 630 s after GTS (50 μg ml−1) application. The peak current amplitude induced by GTS is plotted against time. These currents were generated by stepping to −140 mV from a holding potential of −40 mV, for 5 s. (d) Concentration-dependent effect of GTS on the transient and sustained currents. The illustrated histograms shows peak current response recorded during the treatment of GTS (3, 10, 30, 100, or 300 μg ml−1). These data are shown as mean±s.e.m. (n=10–12 oocytes each).
Effects of extracellular Ca2+ concentration and Ca2+ channel blocker on ginsenoside-evoked biphasic inward Cl− current
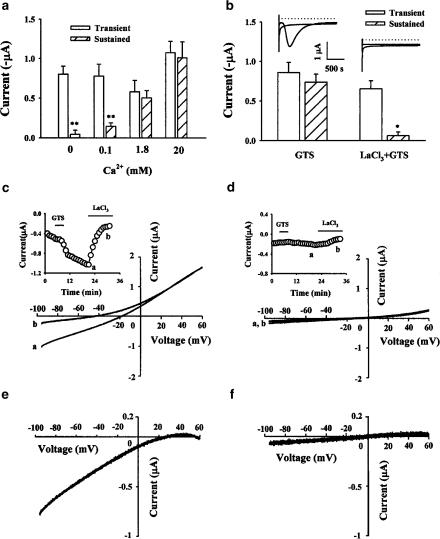
The above results prompted us to formulate a working hypothesis that treatment with ginsenosides might activate the SOCE process following initial Ca2+ depletion from intracellular Ca2+ stores in Xenopus oocytes. This follows since the time course of appearance of the sustained inward Cl− current was much slower than the transient inward Cl− current. It suggests that the transient inward Cl− current therefore reflects Ca2+ release from internal stores, whereas the sustained inward Cl− current is derived from extracellular Ca2+ influx via a pathway activated by IP3-mediated internal store depletion as proven in previous reports (Choi et al., 2001b). As the first step toward testing this hypothesis, we investigated the effect of ginsenosides on changes in the biphasic Cl− current at various extracellular Ca2+ concentrations. Thus, we recorded the ginsenoside-evoked biphasic current in ND96 plus various extracellular Ca2+ concentrations (0, 0.1, 1.8, and 20 mM). As shown in Figure 4a, the transient inward Cl− current was not much changed in the range of external Ca2+ concentrations tested. However, the sustained inward Cl− currents were abolished or significantly reduced when external Ca2+ was absent or lowered to 0.1 mM as shown in a previous report (Parekh, 1995). Moreover, when we raised external Ca2+ from 0.1 to 1.8 mM, we observed the sustained inward Cl− currents (from 0.14±0.04 to 0.51±0.09 μA, **P<0.01). We also tested whether or not the sustained inward Cl− current evoked by ginsenoside treatment is affected by external La3+ treatment, a Ca2+ channel blocker. As shown in Figure 4b, La3+ treatment (100 μM) slightly but not significantly reduced the transient inward Cl− current (from 0.86±0.12 to 0.72±0.10 μA). However, treatment with La3+ abolished the sustained inward Cl− current significantly (from 0.73±0.1 to 0.06±0.04 μA, *P<0.01).
Figure 4.
GTS-induced sustained inward Cl− current and GTS-induced ISOC current are dependent on extracellular Ca2+. (a) Histograms illustrate the mean peak amplitude of GTS-induced transient and sustained inward Cl− currents (mean±s.e.m; n=8–10 oocytes each). Oocytes were bathed in Ca2+-free ND96 with various extracellular Ca2+ concentrations (0, 0.1, 1.8, or 20 mM). The other experimental procedures were performed as described in Figure 1. (b) Treatment with LaCl3 (100 μM) blocks GTS-induced sustained inward Cl− current. The histogram represents the peak mean amplitude of GTS-evoked inward Cl− currents recorded in the presence or absence of LaCl3 (mean±s.e.m; n=16–18 oocytes each). These current amplitudes were recorded by a voltage step to −140 mV from a holding potential of −40 mV for 5 s. Inset: Traces depicting the currents measured during the transient and sustained currents in the presence of GTS (50 μg ml−1) or LaCl3+GTS. (c and d) Oocytes were preinjected with BAPTA to a final concentration of 20 mM and voltage clamped using the ramp shown in the inset once every 30 s in the absence (d) or presence (c) of extracellular Ca2+. The external solution was 30 mM CaCl2, 55 mM NaCl, 10 mM HEPES, pH 7.2. GTS treatment induced an inward current at −140 mV. To measure ISOC current, 100 μM La3+ was added at the end of the experiment. ISOC current was measured as the La3+-inhibited current following GTS treatment. Current ramps before (a) and after (b) La3+ addition are shown. ISOC current or current–voltage relationship was obtained as the difference between the currents before and after La3+ addition. Thus, GTS induced ISOC current in the presence of extracellular Ca2+ (d) but not in the absence of extracellular Ca2+ (e). The graph is representative of data obtained from six oocytes from three different frogs.
Effect of ginsenosides on store-operated Ca2+ (ISOC) current
To see whether or not ISOC can be directly measured when oocytes are treated with ginsenosides, we first preinjected oocytes with bis (o-aminophenoxy) ethane-N,N,N′,N′-tetracetic acid (BAPTA) (20 mM final) in order to inhibit endogenous Ca2+-activated Cl− current, as described by Yao & Tsien (1997) and Machaca & Haun (2000). ISOC current was measured using a ramp voltage from −140 to +60 mV applied every 30 s to oocytes preinjected with BAPTA. As shown in Figure 4c, when we treated oocytes with ginsenosides in the presence of external 30 mM Ca2+, we observed an inward current at −140 mV. As described in previous reports, ISOC current is measured as the La3+-inhibited current induced in response to ginsenosides. The ISOC current–voltage relationship showed the typical inward rectifying character (Figure 4e) (Machaca & Haun, 2000). However, in the absence of external Ca2+, we could not observe any ginsenoside-induced inward ISOC current (Figure 4d and f), indicating that treatment with ginsenosides induces La3+-sensitive inward ISOC current in the presence of external Ca2+.
Effects of IP3 receptor antagonists on ginsenoside-evoked biphasic inward current
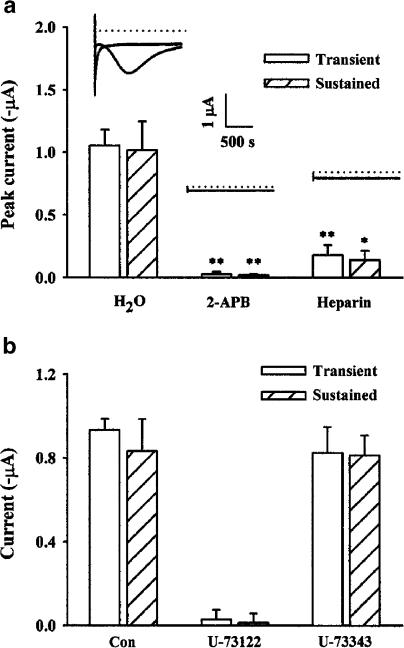
As a next step, we examined whether or not intracellular Ca2+ release via IP3 receptors was responsible for ginsenoside-evoked biphasic inward currents. For this, we tested the effects of IP3 receptor antagonists, such as membrane permeable 2-APB and heparin, on ginsenoside-evoked biphasic current (Parekh, 1995; Broad et al., 2001; Dobrydneva & Blackmore, 2001). As shown in Figure 5a, addition of 2-APB almost completely abolished both transient and sustained Cl− currents induced by ginsenosides (for transient current from 1.05±0.12 to 0.03±0.01 μA and for sustained current from 1.01±0.23 to 0.02±0.07 μA; *P<0.01, **P<0.001). Intraoocyte injection of another IP3 receptor antagonist, heparin (1 μg final), also attenuated both ginsenoside-induced phases of Cl− current but a control H2O injection did not significantly alter the oocyte response to ginsenosides (Figure 5a).
Figure 5.
Effects of the IP3 receptor antagonists, 2-aminoethoxydiphenyl borate (2-APB) and heparin, and U73122 (active PLC inhibitor) or U73343 (inactive PLC inhibitor) on GTS action. (a) Oocytes were injected with H2O (as control) or heparin (1 μg, final concentration), or treated with 2-APB (100 μM) and incubated for 20 min. Histograms show the peak inward transient and sustained Cl− currents (mean±s.e.m; n=10–12 oocytes each) evoked every 2 min by a voltage step of −140 mV from −40 mV holding potential. Inset: Traces showing the peak inward transient and sustained Cl− currents. (b) Oocytes were treated with ND96 for control (Con) or ND96 plus U73122 or U73343 (1 μM) for 20 min. The peak inward currents are shown (mean±s.e.m; n=10–12 oocytes each) from experiments following the protocol as described in (a).
Effect of PLC inhibitor on ginsenoside-evoked biphasic inward current
We examined the effects of PLC inhibitors on ginsenoside-evoked biphasic inward current, since the ginsenoside effect on the biphasic inward current involves the participation of IP3, which is produced via PLC activation. To test this, the effects of the active PLC inhibitor U-73122 and its inactive analog U-73343 (Thompson et al., 1991) on ginsensoside action were examined. Bath application of U-73122 significantly depressed the action of ginsenosides (for transient current from 0.93±0.05 to 0.03±0.04 μA and for sustained current from 0.83±0.15 to 0.01±0.04 μA, respectively), but U-73343 had no effect (Figure 5b). These results indicate that the active PLC inhibitor blocked the effect of ginsenoside on the biphasic inward Cl− current.
Intraoocyte injection of ATP-γS but not AMP-PCP prolongs ginsenoside-evoked sustained Cl− currents
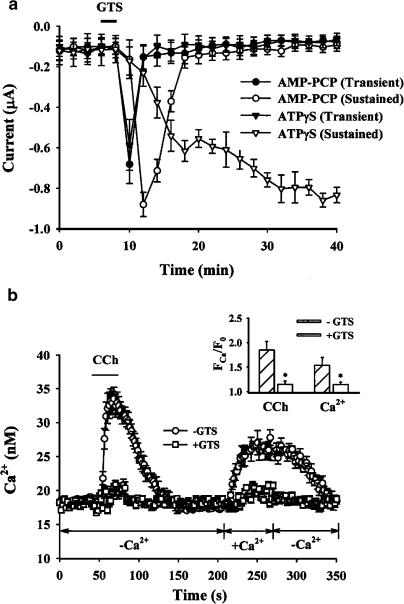
ATP-γS is an ATP analogue that can be used by protein kinases, and AMP-PCP is another ATP analogue that cannot be utilized by kinases. In a previous study, it was suggested that protein phosphorylation regulates capacitative Ca2+ influx following receptor stimulation (Parekh & Terlau, 1996). Hence, to examine whether the ginsensoside-induced biphasic current is affected by protein phosphorylation, we injected ATP-γS (100 μM, final concentration) or AMP-PCP (100 μM, final concentration) into Xenopus oocytes. As shown in Figure 6a, ATP-γS did not alter the ginsenoside-induced transient inward Cl− current compared to controls, but the ginsenoside-induced sustained inward Cl− current was not inactivated but was prolonged for more than 40 min. However, intraoocyte injection of AMP-PCP did not affect either phase of ginsenoside-induced of inward Cl− current.
Figure 6.
Effects of ATP-γS and AMP-PCP on GTS-induced biphasic Cl− currents and effects of GTS on receptor agonist-induced intracellular Ca2+ release and SOCE in hippocampal neurons. (a) Oocytes were injected with ATP-γS (100 μM, final concentration) or AMP-PCP (100 μM, final concentration) and incubated for 20 min. Oocytes were voltage clamped at −40 mV and stepped to −140 mV for 5 s. The current amplitudes were cycled every 2 min and plotted against time. The graph is representative of data obtained from 10 oocytes from three different frogs. (b) Cytosolic Ca2+ changes [Ca2+]i were measured by the Ca2+ indicator Fura-2/AM in the cell bodies of hippocampal neurons with well-defined neurites. The graph shows the [Ca2+]i changes over time in the presence or absence of GTS (100 μM ml−1) (mean±s.e.m. from 10 to 15 neurons). The medium contained 0.4 μM TTX and 10 μM CNQX with or without 10 mM Ca2+. TTX and CNQX were used to prevent the action potential-dependent entry of Ca2+ and the spontaneously occurring excitatory synaptic input, respectively. A measure of 200 μM carbachol (CCh) was added as indicated by the horizontal bar on the graph. Since we showed both 50 mM K+-induced intracellular Ca2+ increase and the effect of GTS on high K+-induced intracellular Ca2+ levels in our previous report, we did not present this data here (Kim et al., 2002). Inset, the peak Ca2+ fluorescence (FCa) was averaged and normalized as a ratio of the background fluorescence (F0) taken before in the presence or absence of GTS, *P<0.01 significantly different from carbachol or Ca2+ treatment alone.
Effect of ginsenosides on SOCE in hippocampal neurons
In order to characterize the effect of ginsenosides on SOCE in hippocampal neurons, we followed the intracellular Ca2+ imaging methods devised by Bouron (2000) but with slight modifications. Neurons were first depolarized with 50 mM KCl to load external Ca2+ into intracellular Ca2+ stores, necessary because the intracellular Ca2+ stores of cultured hippocampal neurons are empty at rest. Without prefilling Ca2+ by depolarization, treatment with the acetylcholine muscarinic receptor agonist, carbachol, does not induce intracellular Ca2+ releases in most neurons (Irving & Collingridge, 1998; Bouron, 2000). Thus, following high K+-induced depolarization, neurons were stimulated with carbachol to deplete the intracellular Ca2+ stores in the absence of extracellular Ca2+ and were then treated with external medium containing Ca2+ in order to induce SOCE. As shown in Figure 6b, treatment of hippocampal neurons with carbachol following previous stimulation with 50 mM KCl induced transient increases in cytosolic free Ca2+ in the absence of external Ca2+. After returning to basal Ca2+ levels, incubation with medium containing 10 mM Ca2+ induced sustained increases in intracellular Ca2+ levels. However, treatment with ginsenosides greatly attenuated both carbachol-stimulated intracellular Ca2+ release from intracellular Ca2+ stores, and SOCE by external Ca2+ influx (Figure 6b and inset). In this experiment, ginsenoside treatment did not affect the basal intracellular Ca2+ level in hippocampal neurons in normal medium as shown in a previous report by Kim et al. (2002).
Discussion
Although ginsenosides, the active ingredients of Panax ginseng, have been widely used as an invigorating agent for a long time, there have been few reports on ginsenoside-mediated signal transduction until now. Moreover, little is known about changes in intracellular Ca2+ signaling following exposure to ginsenosides. In previous studies, we demonstrated that ginsenosides interact with unidentified extracellular membrane components to induce a mobilization of Ca2+ from IP3-sensitive intracellular stores via activation of PTX-insensitive Gαq/11 proteins and the PLC pathway in Xenopus oocytes (Choi et al., 2001b, 2001c). The present study aimed to confirm that ginsenoside-induced intracellular Ca2+ mobilization from IP3-sensitive Ca2+ stores is coupled to the subsequent activation of the SOCE process. For this, we adapted the voltage clamp protocols of Parekh (1995) and Kuruma & Hartzell (1999), in which SOCE was indirectly monitored through measurement of biphasic inward currents. The biphasic current is composed of a transient Ca2+-activated Cl− current (activated by release of Ca2+ stores) followed by a sustained Ca2+-activated Cl− current (dependent upon external Ca2+ entry). In this study, we present four principal findings regarding ginsenoside action and the SOCE process. These are that (1) different time courses of biphasic inward Cl− current, (2) external Ca2+ dependency, (3) IP3 receptor activation, and (4) PLC activation are all involved in the main molecular mechanisms mediating ginsenoside-induced activation of the SOCE process in Xenopus oocytes.
First, in our hyperpolarizing voltage clamp protocol, ginsenosides induced a transient inward Cl− current that was rapidly inactivated 15 s following ginsenoside treatment, followed by a second inward Cl− current that appeared after the transient inward current was almost completely inactivated (2 min after ginsenoside treatment). The amplitude of the second inward Cl− current gradually increased and was much slower to reach the peak current than was the transient inward Cl− current (Figure 3). Thus, this difference in the timings of the appearance of the biphasic inward Cl− current following ginsenosides treatment might suggest that ginsenosides, in Xenopus oocytes, activate at least two types of Ca2+-activated Cl− current and that the first phase reflects Cl− current activated from release of Ca2+ from internal stores and the second phase reflects Cl− current induced by an influx of extracellular Ca2+ into the cell. Intraoocyte injection of IP3, or agonist stimulation of a receptor such as 5-HT2C, heterologously expressed in Xenopus oocytes, also exhibited similar patterns of inward Cl− currents (Parekh, 1995; Kuruma & Hartzell, 1999), supporting the theory that treatment with ginsenosides induces activation of the SOCE process as well as intracellular Ca2+ release.
Secondly, we could only observe a ginsenoside-induced sustained inward Cl− current when external Ca2+ was present. We also found that the magnitude of sustained inward Cl− current was dependent on external Ca2+ concentrations, whereas the transient inward Cl− current was independent of external Ca2+ levels. Moreover, pretreatment with the calcium channel blocker, La3+, blocked most of the sustained inward Cl− current and ISOC but had no effect on the transient inward Cl− current (Figure 4).
Evidence for a role for IP3 receptor activation in SOCE induction by ginsenosides is demonstrated by treatment with 2-APB, or intraoocyte injection of heparin, whereby ginsenoside-induced transient and sustained inward Cl− currents were both attenuated. It is known that 2-APB inhibits agonist-induced increases in intracellular free Ca2+ in platelets and neutrophils. 2-APB has been used extensively to inhibit the release of intracellular Ca2+ and 2-APB directly inhibits SOCE channels in human platelets (Dobrydneva & Blackmore, 2001). Heparin is a well-known IP3 receptor antagonist (Callamaras & Parker, 1994; Yao & Parker, 1994). Parekh (1995) carried out injection of heparin to confirm that the depletion-activated Ca2+ influx pathway was responsible for the sustained Cl− current. Thus, inhibition of intracellular Ca2+ mobilization by blocking of IP3 receptors is also coupled to the inhibition of SOCE, resulting in a subsequent inhibition of the sustained inward Cl− current in Xenopus oocytes.
The fourth piece of pharmacological evidence suggesting a role for PLC activation in ginsenoside-induced SOCE comes from experiments involving U73122, an active PLC inhibitor. The results of experiments blocking the ginsenoside effect of inducing the biphasic inward Cl− current by U73122, suggest that production of polyphosphoinositides by PLC activation is a key factor in the induction of the biphasic inward Cl− current by ginsenoside. Moreover, the present results are also consistent with the previous reports that U-73122 but not U73344 (inactive PLC inhibitor) blocked SOCE in epithelial cell and mast cell lines (Dobrydneva & Blackmore, 2001). These results suggest that PLC activation, which produces IP3 from the precursor PIP2, is required for ginsenoside-induced SOCE in Xenopus oocytes.
In addition, we studied the effects of protein phosphorylation on ginsenoside action in Xenopus oocytes. It was reported that SOCE, usually inactivated by 5-HT application after 15 min, was not inactivated but was rather prolonged by around two-fold when ATP-γS was injected into oocytes (Parekh & Terlau, 1996). Similarly, in our experiments, without intraoocyte injection of ATP-γS the sustained inward Cl− current induced by ginsenosides was inactivated after about 10 min (Figure 3c). However, following injection of ATP-γS, an ATP analogue that is readily used by protein kinases, the sustained but not transient Cl− current induced by ginsenosides was also prolonged more than 40 min (Figure 6a). What is the mechanism of the differential effect of ATP-γS on ginsenoside-induced biphasic Cl− current? One possibility is that thiophosphorylation of oocyte membrane protein(s) by protein kinases, in which thiol groups are not easily removed by phosphatases (Eckstein, 1985) that are involved in SOCE induction, might cause a persistent activation of SOCE resulting in prolonged opening of the sustained Cl− current without inactivation. The other possibility is that there might be two subtypes of Ca2+-activated Cl− channels in Xenopus oocytes. One is not affected by phosphorylation and dephosphorylation processes because it is transiently activated by intracellular Ca2+ release from ER. The other is slowly activated by extracellular Ca2+ influx via SOCE processes. The phosphorylation and dephosphorylation of this Cl− channel protein might affect the activation and inactivation process, respectively. Thus, ATP-γS-induced thiophosphorylation of Cl− channel proteins might cause a persistent activation of the sustained Cl− current as in oocytes expressing 5-HT1C receptors in the study by Parekh & Terlau (1996). However, we could not entirely explain how ATP-γS injected into oocytes induced a persistent activation of the ginsenoside-induced sustained Cl− current. Further studies will be needed to clarify the differential effect of ATP-γS on ginsenoside-induced biphasic Cl− currents.
In contrast to the effect of ginsenoside on intracellular Ca2+ release and SOCE induction in Xenopus oocytes shown in previous reports and in the present study (Choi et al., 2001b, 2001c), we have shown, using a Ca2+ imaging method, that ginsenosides inhibit both carbachol-stimulated intracellular Ca2+ release and intracellular Ca2+ depletion-induced SOCE in rat cultured hippocampal neurons (Figure 6b). Previous reports have shown that ginsenosides inhibit high threshold voltage-dependent Ca2+ channels via pertussis toxin-sensitive G proteins in several types of neuronal cells such as chromaffin cells and sensory neurons (Nah & McCleskey, 1994; Nah et al., 1995; Choi et al., 2001a; Rhim et al., 2002). Ginsenosides inhibited cation influx by blocking ligand-gated cation channel activity such as glutamate, 5-HT3A, and muscle and neuronal nicotinic acetylcholine receptors (Choi et al., 2002; Kim et al., 2002; Sala et al., 2002; Choi et al., 2003b). Moreover, Lee et al. (2003) showed that in cultured rat cortical neurons, ginsenosides inhibited carbachol-stimulated turnover of phosphoinositides like IP1, IP2, and IP3 which leads to intracellular [Ca2+]i mobilization. Thus, both previous studies and the present study support the concept that ginsenosides might not only block external Ca2+ influx by inhibition of channel activity but also inhibit carbachol-stimulated intracellular Ca2+ mobilization and intracellular Ca2+ depletion-activated SOCE in neuronal cells (Figure 6b).
In summary, ginsenosides induced intracellular Ca2+ release from IP3-sensitive internal stores and Ca2+ influx into the cell via the depletion-activated SOCE process in Xenopus laevis oocytes, whereas in neurons ginsenosides inhibited both intracellular acetylcholine muscarinic receptor-mediated Ca2+ release and SOCE. Further, these results highlight the possibility that ginsenosides may act as differential regulators of intracellular Ca2+ level in neurons and non-neuronal cells.
Acknowledgments
This work was supported by the Ministry of Science and Technology through the Bio-Food and Drug Research Center at Konkuk University, Chungju, Korea.
Abbreviations
- AMP-PCP
adenylyl 5′-(β,γ-methylene)-diphosphonate
- 2-APB
2-aminoethxydiphenyl borate
- ATP-γS
adenosine 5′-o-(3-Thiotriphosphate)
- BAPTA
bis (o-aminophenoxy) ethane-N,N,N′,N′-tetracetic acid
- GPCRs
G protein coupled receptors ginseng total saponins
- IP3
inositol 1,4,5-trisphosphate
- PLC
phospholipase C
- SOC
store-operated Ca2+ channel
- SOCE
store-operated Ca2+ entry
References
- BARISH M.E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. (London) 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE M.J., BOOTMAN M.D., LIPP P. Calcium – a life and death signal. Nature (London) 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., IRVINE R.F. Inositol triphosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- BOURON A. Activation of a capacitative Ca2+ entry pathway by store depletion in cultured hippocampal neurons. FEBS Lett. 2000;470:269–272. doi: 10.1016/s0014-5793(00)01340-5. [DOI] [PubMed] [Google Scholar]
- BROAD L.M., BRAUN F.-J., LIEVREMONT J.-P., BIRD G.S., KUROSAKI T., PUTNEY J.W., JR Role of the phospholipase C-inositol 1,4,5-triphosphate pathway in calcium release-activated calcium entry and capacitative calcium entry. J. Biol. Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- CALLAMARAS N., PARKER I. Inositol 1, 4, 5-triphosphate receptors in Xenopus laevis oocytes: localization and modulation by Ca2+ Cell Calcium. 1994;15:66–78. doi: 10.1016/0143-4160(94)90105-8. [DOI] [PubMed] [Google Scholar]
- CHOI S., JUNG S.Y., KIM C.H., KIM H.S., RHIM H., KIM S.C., NAH S.Y. Effect of ginsenosides on voltage-dependent Ca2+ channel subtypes in bovine chromaffin cells. J. Ethnopharmacol. 2001a;74:75–81. doi: 10.1016/s0378-8741(00)00353-6. [DOI] [PubMed] [Google Scholar]
- CHOI S., JUNG S.Y., LEE J.H., SALA F., ENGEL A.G., NAH S.Y. Effect of ginsenosides, active components of ginseng, on nicotinic acetylcholine receptor expressed in Xenopus oocytes. Eur. J Pharmacol. 2002;442:37–45. doi: 10.1016/s0014-2999(02)01508-x. [DOI] [PubMed] [Google Scholar]
- CHOI S., KIM H.J., KO Y.S., JEONG S.W., KIM Y.I., SIMONDS W.F., OH J.W., NAH S.Y. Gαq/11 coupled to mammalian phospholipase C β3-like enzyme mediates the ginsenoside effect on Ca2+-activated Cl− current in the Xenopus oocyte. J. Biol. Chem. 2001b;276:48797–48802. doi: 10.1074/jbc.M104346200. [DOI] [PubMed] [Google Scholar]
- CHOI S., LEE J.H., KIM Y.I., KANG M.J., RHIM H., LEE S.K., NAH S.Y. Effects of ginsenoside on G protein-coupled inwardly rectifying K+ channel activity expressed in Xenopus oocytes. Eur. J. Pharmacol. 2003a;468:83–92. doi: 10.1016/s0014-2999(03)01666-2. [DOI] [PubMed] [Google Scholar]
- CHOI S.E., LEE J.H., OH S., RHIM H., LEE S.M., NAH S.Y. Effects of ginsenoside Rg2 on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol. Cells. 2003b;15:108–113. [PubMed] [Google Scholar]
- CHOI S., RHO S.H., JUNG S.Y., KIM S.C., PARK C.S., NAH S.Y. A novel activation of Ca2+-activated Cl− channels in Xenopus oocytes by ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca2+ mobilization. Br. J. Pharmacol. 2001c;132:641–648. doi: 10.1038/sj.bjp.0703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DASCAL N. The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 1987;22:317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- DASCAL N., YEKUEL R., ORON Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. J. Exp. Zool. 1984;230:131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- DOBRYDNEVA Y., BLACKMORE P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol. Pharmacol. 2001;3:541–552. [PubMed] [Google Scholar]
- ECKSTEIN F. Nucleoside phosphorothioates. Annu. Rev. Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- HARTZELL H.C. Activation of different Cl− currents in Xenopus oocytes by Ca2+ liberation from stores and by capacitative Ca2+ influx. J. Gen. Physiol. 1996;108:157–175. doi: 10.1085/jgp.108.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVING A.J., COLLINGRIDGE G.L. A characterization of muscarinic receptor-mediated intracellular Ca2+ mobilization in cultured rat hippocampal neurons. J. Physiol. 1998;511:747–759. doi: 10.1111/j.1469-7793.1998.747bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S., AHN K., OH T.H., NAH S.Y., RHIM H. Inhibitory effect of ginsenosides on NMDA receptor mediated-signals in rat hippocampus neurons. Biochem. Biophys. Res. Comm. 2002;296:247–254. doi: 10.1016/s0006-291x(02)00870-7. [DOI] [PubMed] [Google Scholar]
- KURUMA A., HARTZELL H.C. Dynamics of calcium regulation of chloride currents in Xenopus oocytes. Am. J. Physiol. 1999;276:C161–C175. doi: 10.1152/ajpcell.1999.276.1.C161. [DOI] [PubMed] [Google Scholar]
- LECHLEITER J.D., CLAPHAM D.E. Molecular mechanisms of intracellular calcium excitability in Xenopus laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- LEE J.H., CHOI S., KIM J.H., KIM J.K., KIM J.I., NAH S.Y. Effects of ginsenosides on carbachol-stimulated formation of inositol phosphates in rat cortical cell cultures. Neurochem. Res. 2003;28:1307–1313. doi: 10.1023/a:1024979912161. [DOI] [PubMed] [Google Scholar]
- MACHACA K., HAUN S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J. Biol. Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R., PARKER I. Chloride current induced by injection of calcium into Xenopus oocytes. J. Physiol. (London) 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAH S.Y., MCCLESKEY E.W. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as μ-type opioids. J. Ethnopharmacol. 1994;42:45–51. doi: 10.1016/0378-8741(94)90022-1. [DOI] [PubMed] [Google Scholar]
- NAH S.Y., PARK H.J., MCCLESKEY E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8739–8743. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B. Interaction between capacitative Ca2+ influx and Ca2+-dependent Cl− currents in Xenopus oocytes. Pflugers Arch.-Eur. J. Physiol. 1995;430:954–963. doi: 10.1007/BF01837409. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., TERLAU H. Effects of protein phosphorylation on the regulation of capacitative calcium influx in Xenopus oocytes. Pflugers Arch.-Eur. J. Physiol. 1996;432:14–25. doi: 10.1007/s004240050100. [DOI] [PubMed] [Google Scholar]
- PARKER I., YAO Y. Relation between intracellular Ca2+ signals and Ca2+-activated Cl− current in Xenopus oocytes. Cell Calcium. 1994;15:276–288. doi: 10.1016/0143-4160(94)90067-1. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR Inositol phosphates and calcium entry. Adv. Second Messenger Phosphoprotein Res. 1992;26:143–160. [PubMed] [Google Scholar]
- RHIM H., KIM H., LEE D.Y., OH T.H., NAH S.Y. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur. J. Pharmacol. 2002;436:151–158. doi: 10.1016/s0014-2999(01)01613-2. [DOI] [PubMed] [Google Scholar]
- SALA F., MULET J., CHOI S., JUNG S.Y., NAH S.Y., RHIM H., VALOR L.M., CRIADO M., SALA S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J. Pharm. Exp. Ther. 2002;301:1052–1059. doi: 10.1124/jpet.301.3.1052. [DOI] [PubMed] [Google Scholar]
- THOMPSON A.K., MOSTAFAPOUR S.P., DENLIGER L.C., BLEASDALE J.E., FISHER S.K. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. J. Biol. Chem. 1991;266:23856–23862. [PubMed] [Google Scholar]
- YAO Y., PARKER I. Ca2+ influx modulation of temporal and spatial patterns of inositol triphosphate-mediated Ca2+ liberation in Xenopus oocytes. J. Physiol. 1994;476:17–28. [PMC free article] [PubMed] [Google Scholar]
- YAO Y., TSIEN R.Y. Calcium current activated by depletion of calcium stores in Xenopus oocytes. J. Gen. Physiol. 1997;109:703–715. doi: 10.1085/jgp.109.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]